Rapid Differential Detection of Wild-Type Classical Swine Fever Virus and Hog Cholera Lapinized Virus Vaccines by TaqMan MGB-Based Dual One-Step Real-Time RT-PCR
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. The Strains Used in This Study
2.2. Main Reagents and Instruments
2.3. Preparation of Nucleic Acids from Pathogenic Microorganisms
2.4. Primer and Probe Design
2.5. Preparation of Standard Positive Plasmids
2.6. Optimization of Dual TaqMan-MGB RT-qPCR
2.7. Establishment of the Standard Curve
2.8. Analytic Specificity
2.9. Analytic Sensitivity
2.10. Inclusiveness of the Test
2.11. Precision of the Test
2.12. Detection in Biological Samples Collected from CSFV-Vaccinated Pigs
3. Results
3.1. Optimization of Dual TaqMan-MGB qPCR for CSFV and HCLV
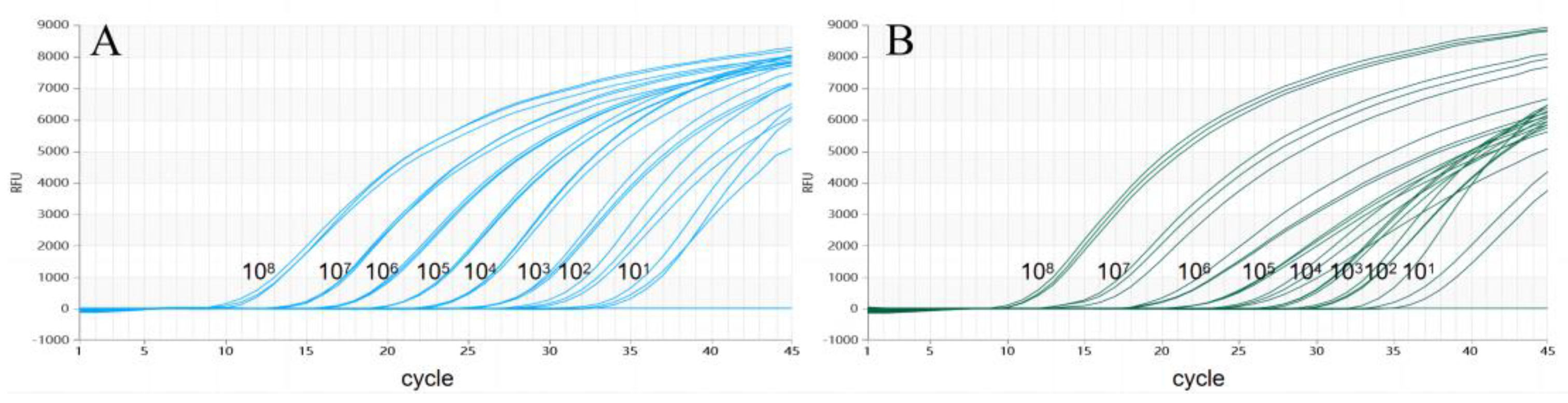
3.2. Preparation of the Standard Curve
3.3. Analytic Specificity
3.4. Analytic Sensitivity
3.5. Inclusiveness of the Test
3.6. Precision of the Test
3.7. Detection in Biological Samples Collected from CSFV-Vaccinated Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-An Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Guo, Z.; Ding, N.Z.; He, C.Q. Studying classical swine fever virus: Making the best of a bad virus. Virus Res. 2015, 197, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeier, H.; Strebelow, G.; Depner, K.; Hoffmann, B.; Beer, M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J. Gen. Virol. 2004, 85, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Moennig, V. The control of classical swine fever in wild boar. Front. Microbiol. 2015, 6, 160779. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.; Avalos Ramirez, R.; Orlich, M.; Cedillo Rosales, S.; Konig, M.; Schweizer, M.; Stalder, H.; Schirrmeier, H.; Thiel, H.J. Genetic and antigenic characterization of novel pestivirus genotypes: Implications for classification. Virology 2003, 311, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.R.; Bevins, S.N. A Review of Classical Swine Fever Virus and Routes of Introduction into the United States and the Potential for Virus Establishment. Front. Vet. Sci. 2018, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ganges, L.; Crooke, H.R.; Bohórquez, J.A.; Postel, A.; Sakoda, Y.; Becher, P.; Ruggli, N. Classical swine fever virus: The past, present and future. Virus Res. 2020, 289, 198151. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Schmeiser, S.; Bernau, J.; Meindl-Boehmer, A.; Pridotkas, G.; Dirbakova, Z.; Mojzis, M.; Becher, P. Improved strategy for phylogenetic analysis of classical swine fever virus based on full-length E2 encoding sequences. Vet. Res. 2012, 43, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B. Classical Swine Fever in China-An Update Minireview. Front. Vet. Sci. 2019, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liao, Y.; Zhang, M.; Liu, C.; Li, Z.; Li, Y.; Li, X.; Wu, K.; Yi, L.; Ding, H.; et al. Anti-Classical Swine Fever Virus Strategies. Microorganisms 2021, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, H.; Everett, H.; Sosan, O.; Crooke, H.; Meindl-Bohmer, A.; Qiu, H.J.; Moennig, V.; Belak, S.; Widen, F. A generic real-time TaqMan assay for specific detection of lapinized Chinese vaccines against classical swine fever. J. Virol. Methods 2011, 175, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hoffmann, B.; Baule, C.; Beer, M.; Belak, S.; Widen, F. Two real-time RT-PCR assays of classical swine fever virus, developed for the genetic differentiation of naturally infected from vaccinated wild boars. J. Virol. Methods 2009, 159, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Jamnikar Ciglenecki, U.; Grom, J.; Toplak, I.; Jemersic, L.; Barlic-Maganja, D. Real-time RT-PCR assay for rapid and specific detection of classical swine fever virus: Comparison of SYBR Green and TaqMan MGB detection methods using novel MGB probes. J. Virol. Methods 2008, 147, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, H.; Belak, S.; Widen, F. Development of a primer-probe energy transfer real-time PCR assay for improved detection of classical swine fever virus. J. Virol. Methods 2009, 160, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.L.; Fonseca Júnior, A.A.; Oliveira, A.M.; Sales, E.B.; Alves, B.R.; Dorella, F.A.; Camargos, M.F. Validation of a real time PCR for classical Swine Fever diagnosis. Vet. Med. Int. 2014, 2014, 171235. [Google Scholar] [CrossRef] [PubMed]
- Haines, F.J.; Hofmann, M.A.; King, D.P.; Drew, T.W.; Crooke, H.R. Development and validation of a multiplex, real-time RT PCR assay for the simultaneous detection of classical and African swine fever viruses. PLoS ONE 2013, 8, e71019. [Google Scholar] [CrossRef] [PubMed]


| Primer/Probe Name | Sequences (5′-3′) |
|---|---|
| CSFV-F | 5′-ATAGGGGAGATGAAGGAAGG-3′ |
| CSFV-R | 5′-ACCCGCAGGTTCTCTGA-3′ |
| CSFV-P | 5′-6-FAM-ATCTGTGGATCTTCCCCATGA-MGB-3′ |
| HCLV-P | 5′-VIC-ATCTGTGAATCTTTCCCATGA-MGB-3′ |
| Optimization Project | ---- | Average Ct Values (107/106/105) | R2 |
|---|---|---|---|
| Annealing temperature (CSFV) | 58 °C | 17.77/20.65/24.08 | R2 = 0.99747 |
| 60 °C | 16.86/20.39/23.41 | R2 = 0.99798 | |
| 62 °C | 16.73/21.86/23.07 | R2 = 0.88697 | |
| Annealing temperature (HCLV) | 58 °C | 18.52/22.25/24.32 | R2 = 0.97342 |
| 60 °C | 18.42/22.12/24.24 | R2 = 0.97602 | |
| 62 °C | 18.22/22.95/24.03 | R2 = 0.88374 | |
| Primer concentration (CSFV) | 100 nM | 16.95/19.76/21.92 | R2 = 0.99433 |
| 200 nM | 18.36/20.63/23.30 | R2 = 0.99782 | |
| 300 nM | 17.22/19.54/23.87 | R2 = 0.97045 | |
| 400 nM | 16.64/19.53/24.05 | R2 = 0.98413 | |
| Primer concentration (HCLV) | 100 nM | 16.43/20.20/23.48 | R2 = 0.99839 |
| 200 nM | 18.65/21.28/25.01 | R2 = 0.99013 | |
| 300 nM | 17.65/21.78/25.03 | R2 = 0.99528 | |
| 400 nM | 18.30/21.14/25.92 | R2 = 0.97885 | |
| Probe concentration | 200 nM | 17.43/20.20/26.42 | R2 = 0.95321 |
| 400 nM | 19.35/22.82/24.37 | R2 = 0.95351 | |
| 600 nM | 16.31/21.00/24.18 | R2 = 0.98788 |
| Ct-Value (CSFV/HCLV) | Standard Deviation | Relative Standard Deviation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gentier 96R | StepOne Plus | ||||||||
| Dates | Concentration | Lot No.20240608 | Lot No.20240610 | ||||||
| 06/11 | 103 copies/µL | 24.5/27.0 | 24.1/26.9 | 24.7/26.9 | 25.1/27.1 | 25.0/27.3 | 25.1/27.4 | 0.40/0.21 | 1.61%/0.77% |
| 06/12 | 25.2/27.4 | 25.0/27.1 | 25.3/27.4 | 24.9/27.2 | 25.1/27.4 | 25.3/27.1 | 0.16/0.15 | 0.65%/0.55% | |
| 06/13 | 25.5/27.7 | 24.9/27.3 | 25.1/27.3 | 24.7/27.6 | 24.9/27.5 | 25.2/27.7 | 0.28/0.18 | 1.12%/0.67% | |
| 06/14 | 24.9/27.2 | 25.2/26.8 | 25.1/27.5 | 24.7/27.4 | 25.4/27.6 | 25.3/26.8 | 0.26/0.35 | 1.03%/1.28% | |
| 06/15 | 25.5/27.3 | 25.6/27.1 | 25.1/26.9 | 26.4/27.8 | 24.9/27.6 | 25.4/27.9 | 0.52/0.40 | 1.45%/2.03% | |
| 06/11 | 101 copies/µL | 32.5/34.4 | 32.4/34.3 | 32.4/34.4 | 32.2/34.6 | 32.4/33.9 | 32.8/34.1 | 0.20/0.25 | 0.60%/0.72% |
| 06/12 | 32.6/34.4 | 32.8/34.6 | 32.2/34.1 | 32.6/34.3 | 32.9/33.8 | 32.4/34.1 | 0.25/0.278 | 0.78%/0.81% | |
| 06/13 | 32.2/34.4 | 32.1/34.3 | 32.9/34.5 | 32.6/33.9 | 32.7/34.3 | 32.6/34.4 | 0.30/0.20 | 0.94%/0.61% | |
| 06/14 | 31.9/34.4 | 32.6/34.2 | 31.9/34.1 | 31.9/34.8 | 32.3/34.5 | 32.4/34.6 | 0.31/0.26 | 0.96%/0.75% | |
| 06/15 | 32.5/34.4 | 32.8/34.1 | 32.4/34.6 | 32.8/34.9 | 33.3/35.1 | 33.0/34.8 | 0.33/0.36 | 1.00%/1.04% | |
| Categories | Dual Real-Time PCR | Commercial Kit | |
|---|---|---|---|
| CSFV | HCLV | CSFV | |
| Lymph node | 4 | 0 | 4 |
| Meat | 2 | 2 | 3 |
| Pharyngeal swab | 7 | 0 | 7 |
| Whole blood | 7 | 3 | 11 |
| Feces | 11 | 0 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, M.; Sun, Y.; Xiao, X.; Wang, S.; Li, P.; Liu, Y.; Zhao, H.; Meng, Y.; Yin, R. Rapid Differential Detection of Wild-Type Classical Swine Fever Virus and Hog Cholera Lapinized Virus Vaccines by TaqMan MGB-Based Dual One-Step Real-Time RT-PCR. Vet. Sci. 2024, 11, 289. https://doi.org/10.3390/vetsci11070289
Zhang Y, Wang M, Sun Y, Xiao X, Wang S, Li P, Liu Y, Zhao H, Meng Y, Yin R. Rapid Differential Detection of Wild-Type Classical Swine Fever Virus and Hog Cholera Lapinized Virus Vaccines by TaqMan MGB-Based Dual One-Step Real-Time RT-PCR. Veterinary Sciences. 2024; 11(7):289. https://doi.org/10.3390/vetsci11070289
Chicago/Turabian StyleZhang, Yongzhe, Meiqi Wang, Yajuan Sun, Xingyu Xiao, Songsong Wang, Peng Li, Yansong Liu, Hongri Zhao, Yan Meng, and Rui Yin. 2024. "Rapid Differential Detection of Wild-Type Classical Swine Fever Virus and Hog Cholera Lapinized Virus Vaccines by TaqMan MGB-Based Dual One-Step Real-Time RT-PCR" Veterinary Sciences 11, no. 7: 289. https://doi.org/10.3390/vetsci11070289
APA StyleZhang, Y., Wang, M., Sun, Y., Xiao, X., Wang, S., Li, P., Liu, Y., Zhao, H., Meng, Y., & Yin, R. (2024). Rapid Differential Detection of Wild-Type Classical Swine Fever Virus and Hog Cholera Lapinized Virus Vaccines by TaqMan MGB-Based Dual One-Step Real-Time RT-PCR. Veterinary Sciences, 11(7), 289. https://doi.org/10.3390/vetsci11070289





