Simple Summary
A 9-week experiment aimed to investigate the effects of dietary cobalt levels on the growth performance, antioxidant capacity, and immunity of largemouth bass. According to the results of growth performance, whole-body composition, antioxidant parameters in the liver, gene expression of the Nrf2 signaling pathway, and NF-κB signaling pathway, it was found that there was an improvement in the growth performance and feed utilization of largemouth bass fed with optimal dietary cobalt levels. Furthermore, cobalt has an important function in improving the antioxidant status and immune response of largemouth bass. Quadratic regression analyses based on the SGR and FCR showed that the optimal requirement was 0.24 and 0.26 mg/kg of dietary cobalt, respectively.
Abstract
A 9-week experiment investigated the effects of dietary cobalt levels on the growth performance, antioxidant capacity, and immunity of largemouth bass. Six feed groups were designed and each group received different cobalt levels, including 0.129 mg/kg (control group), 0.192 mg/kg, 0.201 mg/kg, 0.233 mg/kg, 0.277 mg/kg, and 0.316 mg/kg. The results show that the control group (0.129 mg/kg diet) had the lowest final body weight (FBW), weight gain rate (WGR), and specific growth rate (SGR), and the highest feed conversion ratio (FCR), when compared to the cobalt supplementation groups. Dietary cobalt levels of 0.192 mg/kg increased the body protein content and decreased the body moisture content. Regarding antioxidant capacity, the highest catalase (CAT) activity was found in the 0.277 mg/kg dietary cobalt group, while the malondialdehyde (MDA) content was significantly diminished; the total antioxidant capacity (T-AOC) content and glutathione peroxidase (GSH-Px) activity exhibited the highest values in the 0.192 mg/kg and 0.233 mg/kg dietary cobalt groups, respectively. Regarding gene expression, compared with the control group, the mRNA expression of sod was upregulated in the 0.192 mg/kg, 0.233 mg/kg, and 0.277 mg/kg dietary cobalt groups, while the mRNA expression of gpx was diminished when dietary cobalt levels were below 0.233 mg/kg. In addition, the highest il-10 and tgf-β mRNA expression levels were observed in the 0.201 mg/kg and 0.233 mg/kg dietary cobalt groups, respectively. According to the quadratic regression analysis based on the SGR and FCR, the optimal requirement was 0.24 and 0.26 mg/kg of dietary cobalt, respectively.
1. Introduction
Largemouth bass (Micropterus salmoides), a member of the genus Micropterus in the sunfish family centrachidae of perciformes, is native to the Mississippi River system in California, United States []. It reaches sexual maturity at more than one year of age (around 131 g body weight) and reproduces once per year []. Largemouth bass was first introduced in mainland China in Guangdong province in 1980 [], and its production has expanded dramatically, reaching more than 880,000 tons by the year 2023 [].
As in other fish species, largemouth bass needs minerals (phosphorus, selenium, etc.) for different physiological activities and biochemical functions [,]. However, there is limited research thus far on the dietary cobalt requirements of aquatic species. Cobalt is an important component of cyanocobalamin (vitamin B12), which is implicated in nitrogen assimilation and the synthesis of muscle proteins, and has growth-promoting properties []. Furthermore, cobalt, as a trace mineral, is required by most animals for vitamin synthesis through gut microflora and bacteria [,], and it is commonly used as a feed additive in animal nutrition []. In the current study, cobalt chloride and cobalt nitrate were mainly used as cobalt sources to investigate the cobalt requirements of fish. In common carp (Cyprinus carpio), cobalt chloride is said to improve the growth rate and significantly increase the crude protein content when up to 1.5% is added to a supplementary diet [,]. In silver sea perch (Lates calcarifer) and Asian catfish (Clarias batrachus), a 2.5 mg/kg cobalt and 45 mg/kg zinc diet could improve growth performance []. Additionally, in juvenile white shrimp (Litopenaeus vannamei), a 10 mg/kg dietary supplementation of cobalt methionine enhanced growth performance []. However, the dietary cobalt requirement for largemouth bass has not yet been studied, and there is an urgent need to improve this database.
Previous studies have explored the roles of minerals on antioxidant capacity and immunity improvement in aquatic animal species. Other microminerals, such as selenium, are reportedly advantageous in aquaculture when used in an acceptable optimal range; selenium is vital for growth performance and immunity enhancement in certain fish, with varying dietary acceptability []. Similarly to the results of previous research, a study demonstrated that dietary cobalt yielded significantly higher values in antioxidant enzymes when added to the diet of Nile tilapia (Oreochromis niloticus) []. A previous study showed that appropriate levels of Co supplementation could improve the immune capacity of Golden mahseer (Tor putitora), whereas excessive Co additions could negatively affect the immune response, probably due to oxidative stress and the production of pro-inflammatory factors []. It is therefore of interest whether different dietary cobalt levels affect the antioxidant capacity and immune response of largemouth bass.
The main purpose of this study was, thus, to determine the optimal cobalt requirement for juvenile largemouth bass, and assess the effect of dietary cobalt levels on growth performance, antioxidant capacity, and immune status.
2. Materials and Methods
2.1. Experimental Diet
Six feed groups were formulated at the Freshwater Fisheries Research Center (FFRC). Six cobalt addition levels of 0, 0.1, 0.2, 0.3, 0.4, and 0.5 mg/kg in the diets were prepared with reference to the feed formula of largemouth bass reported by Song et al. [] by adding cobalt chloride to the base formulation (Table 1). Through measurement and analysis, the following concentrations of dietary cobalt were determined: 0.129 (control group), 0.192, 0.201, 0.233, 0.277, and 0.316 mg/kg, respectively. All six isonitrogenous and iso-lipidic diets were weighed according to the formulation table, the ingredients were mixed, and water was added; the mix was used to prepare 1.5 mm caliber pellets using a pelleting machine (F-26(II); South China University of Science and Technology, Guangzhou, China), and finally, the feeds were ventilated, dried, and bagged in a cool place outdoors, and stored at −20 °C until their subsequent use.

Table 1.
Experimental basic formula (%, dry matter).
2.2. Feeding Management
The feeding practice system used was a series of recirculation aquaculture system (RAS) tanks at FFRC (Yixing base). Six treatments were set, each having triplicate samples. Each treatment used 3 tanks, comprising a total of 18 tanks with a volume of 270 L each. Juvenile largemouth bass were provided by Chia Tai Aquatic Products Co. Ltd. (Huzhou, China). Twenty fish (average weight: 1.67 ± 0.03 g) were stocked in each tank. The fish were acclimatized for seven days and thereafter fed twice a day for nine weeks to achieve significant satiety. During the feeding trial, the water quality indicators were detected by the Octadem Multi-Parameter Water Quality Analyser (Type OCT-A) (Wuxi Octadem Biotechnology Co., LTD, Wuxi, China) daily, the pH ranged from 7.5 to 8.0, the dissolved oxygen content was larger than 6.0 mg/L, and the water temperature was maintained at 30 ± 2 °C.
2.3. Sample Collection
After a 9-week feed, the fish were starved for 24 h before sampling. All fish from each tank were taken to record the total number and weight of the fish. Then eight fish were randomly selected, and liver samples were taken from three of the fish and stored at −80 °C before antioxidant parameters and qPCR analysis. In addition, another five fish per tank were stored at −20 °C for the whole-body proximate analysis.
2.4. Proximate Chemical Composition Analyses
The approximate dietary composition and body composition were determined according to the AOAC method []. The moisture determination was carried out by oven drying at 105 °C. The crude lipid content was determined by using Soxhlet extraction, and the crude protein content was analyzed via the Kjeldahl method. The ash content was obtained using a muffle furnace at 550 °C.
2.5. Liver Antioxidant Parameters
Catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and malondialdehyde (MDA) were analyzed by using assay kits from Jian Cheng Bioengineering Institute, Nanjing []. The CAT activity was measured by the ammonium molybdenum acid method, the activity of SOD was determined with the WST-1 method, the microplate method was used for the determination of GSH, the GSH-Px activity was measured by the colorimetric method, the T-AOC was determined by the ABTS method, and the MDA content was measured by the TBA method.
2.6. qPCR Analysis
RNA extraction of liver tissues was performed using the RNAiso Plus reagent (Vazyme Biotech Co., Nanning, China), and its concentration was determined using a Nanodrop 2000 spectrophotometer (Waltham, MA, USA). The concentration of the RNA samples was adjusted to 60 ng/µL, with A260/A280 ranging between 1.8 and 2.0. All gene expressions were determined using an SYBR Green Kit (Vazyme Biotech Co., Nanning, China), and quantification was performed using a PCR machine (CFX96 Real time system, Bio-Rad, Singapore). gapdh was used as the reference gene; it has previously been used in a largemouth bass study [], and no significant changes were found in this study or the previous study. Table 2 presents the primer sequences for the genes; the relative standard curve method was used to quantify mRNA expression.

Table 2.
Primer sequences for real-time PCR analysis in this work.
2.7. Statistical Analysis
All data were subjected to normality and homogeneity tests using Levene’s test where necessary, and then the experimental data (means ± SE) were analyzed using SPSS v. 20.0 (IBM Corp., Armonk, NY, USA) statistical software for one-way analysis of variance (ANOVA). Mean testing within the groups was performed using Duncan’s test with a p < 0.05 significance level, and the results were reported as mean ± standard error (SE) values. The quadratic regression model was selected to determine the optimum dietary cobalt requirement by comparing the estimation coefficient (R2) among the linear regression model (SGR, 0.641; FCR, 0.457), quadratic regression model (SGR, 0.952; FCR, 0.957), and broken-linear regression model (SGR, 0.911; FCR, 0.956).
3. Results
3.1. Growth Performance and Feed Utilization
Table 3 shows that the weight gain rate (WGR) and specific growth rate (SGR) were affected by dietary cobalt levels. The control group (0.129 mg/kg diet) showed significantly lower WGR and SGR than all other groups (p < 0.05), unlike the feed conversion ratio (FCR), where it showed significantly higher values than all other groups (p < 0.05). In addition, dietary cobalt levels did not affect the survival rate (SR) within groups (p > 0.05). Quadratic regression analyses based on SGR (R2 = 0.952) and FCR (R2 = 0.957) showed that the optimal dietary cobalt requirement was 0.24 and 0.26 mg/kg of diet, respectively (Figure 1).

Table 3.
Growth performance and feed utilization.
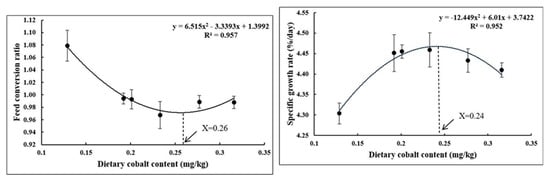
Figure 1.
Optimal cobalt requirement of juvenile largemouth bass.
3.2. Whole-Body Composition
Table 4 shows that dietary cobalt levels did not have effects on the lipid and ash contents of the whole body (p > 0.05). The crude protein content of the whole body showed a higher value when the dietary cobalt level was 0.192 mg/kg as opposed to moisture content values, which were significantly higher than the control group (p < 0.05).

Table 4.
Whole-body composition of largemouth bass fed with different levels of dietary cobalt.
3.3. Antioxidant Indicators in Liver
The results in Table 5 show that when the dietary cobalt level was 0.192 mg/kg, the level of T-AOC was significantly higher than that in other groups (p < 0.05), and the level of CAT in the 0.277 mg/kg dietary cobalt level group was significantly higher than that in the control group (p < 0.05). When the dietary cobalt level was 0.233 mg/kg, the level of GSH-Px was significantly higher than that in the control group (p < 0.05), and the MDA content in the 0.277 mg/kg and 0.316 mg/kg dietary cobalt level groups was significantly lower than that in the 0.201 mg/kg dietary cobalt level group (p < 0.05). The levels of SOD and GSH were not affected by dietary cobalt levels (p > 0.05).

Table 5.
Antioxidant enzymatic parameters of largemouth bass fed with different levels of dietary cobalt.
3.4. Gene Expression of Nrf2 Signaling Pathway
Figure 2 shows that dietary cobalt levels did not affect the nrf2 and keap1 mRNA expression levels (p > 0.05). In addition, the sod mRNA levels were markedly upregulated at the 0.201 mg/kg, 0.233 mg/kg, and 0.277 mg/kg dietary cobalt levels compared with the control group (p < 0.05). Interestingly, this phenomenon was apparent when the dietary cobalt level reached 0.233 mg/kg, and the mRNA levels of gpx were upregulated compared with the control group (p < 0.05).
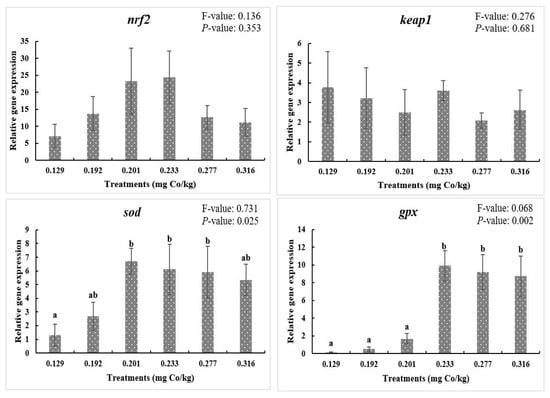
Figure 2.
Relative gene expressions of Nrf2 signaling pathway. F-value, Homogeneity of Variance Test. Results are shown as mean values and standard error (±SE) (n = 9), significant differences between the six treatments are denoted by different letters (p < 0.05).
3.5. Gene Expression of NF-κB Signaling Pathway
As shown in Figure 3, dietary cobalt levels did not affect the nf-kb, il-8, and tnf-α mRNA expression levels (p > 0.05). Moreover, the mRNA levels of il-10 at the 0.201 mg/kg dietary cobalt level exhibited a higher value than the control group (p < 0.05). In addition, the mRNA levels of tgf-β were also higher at the 0.233 mg/kg dietary cobalt level than all the remaining levels (p < 0.05).

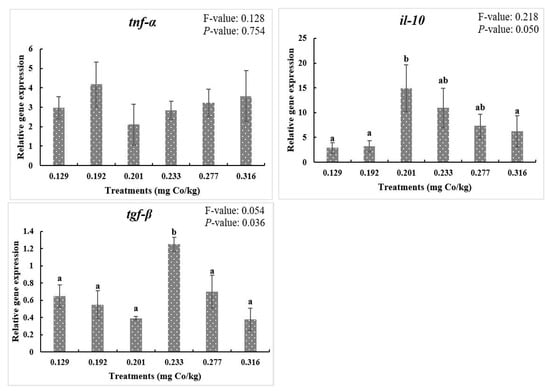
Figure 3.
Relative gene expressions of NF-κB signaling pathway. F-value, Homogeneity of Variance Test. Results are shown as mean values and standard error (±SE) (n = 9), significant differences between the six treatments are denoted by different letters (p < 0.05).
4. Discussion
In aquatic organisms, specifically fish species, it has been reported that there is a positive correlation between the mineral content in a diet and growth performance parameters. Although studies on trace minerals are limited, research in this field is ongoing. Previous studies on the importance of trace minerals in aquaculture nutrition have shown that dietary cobalt has an impact on growth in carp and rainbow trout as well as an improvement in survival rates in gold spot mullet (Liza parsia) []. Furthermore, in Golden mahseer, the addition of 3 mg/kg dietary cobalt significantly affected the growth performance with a 37.52% weight gain compared to the control group []. In our study, dietary cobalt levels (0.192 mg/kg–0.316 mg/kg) were shown to improve the growth parameters and reduce the FCR as compared to the control group (0.129 mg/kg diet). Furthermore, it was recommended that the supplementation of cobalt should not exceed 1 mg/kg of complete feed for all animal species except fish []. The range for the optimal cobalt requirement for different fish species is 0.05–5 mg/kg diet []. In this study, the requirement of cobalt for largemouth bass is 0.24–0.26 mg/kg diet based on FCR and SGR. These results fully demonstrate the importance of optimal dietary cobalt levels in promoting fish growth.
Furthermore, it was shown that the body protein content increased and the moisture content decreased at 0.192 mg/kg cobalt in the diet compared to the control. In this study, the increased protein content in fish body composition was assisted by the supplementation of dietary cobalt, which facilitates amino acid incorporation into the fish body []. Similarly, another study showed an increase in crude protein content in carp fed cobalt supplements []. In addition, dietary cobalt levels did not affect the composition of crude ash and crude lipid contents in this study. These results were slightly perplexing as we expected to observe an increased ash content due to the addition of cobalt as a trace mineral. It has also been found that the level of the mineral magnesium does not make a difference in body ash content [], which is similar to our findings. The probable reason for these results is that under specific conditions of appropriate mineral content and balanced ratios in the feed, fish can effectively absorb these minerals without affecting the ash content of the fish. In addition, there are many reasons for differences in body composition, and differences in breeding environment and individual size are all potential influencing factors.
Biologically, antioxidants are compounds or substances that inhibit damage to body cells by free radicals as a result of oxidation []. Antioxidant substances actively fight against free radicals to prevent cell damage, and they are named antioxidants due to their ability to break down the process of oxidation [,]. In previous studies, minerals such as selenium, zinc, manganese, and iron have been found to play an important role in enzymatic reactions [], have antioxidant effects, and can help reduce free radical damage and cellular oxidative stress [,,,]. In our study, appropriate levels of cobalt were shown to improve antioxidant capacity, which is supported by the results of relevant antioxidant indicators. As reported, cobalt is an essential trace element for organisms and is associated with a variety of enzyme activities []. In our experiment, higher CAT and GSH-Px activities were observed at 0.277 mg/kg and 0.233 mg/kg dietary cobalt levels, respectively. In addition, the T-AOC content showed a significant increase when the dietary cobalt level was 0.192 mg/kg, which was considerably more significant than the control group. Similar results for some parameters were obtained in previous studies, whereby cobalt exposure in Japanese flounder (Paralichthys olivaceus) resulted in significantly higher CAT activity when the cobalt level was 5 mg/L than that in the control group []. Liu et al. [] reported that appropriate dietary cobalt levels could significantly increase the hepatic T-AOC content and GSH-PX activity in pearl gentian grouper (Epinephelus lanceolatus♂ × E. fuscoguttatus♀). In addition, MDA is considered a lipid peroxidation marker used to determine lipid peroxidation as a result of increased oxidative stress [,]. Our study demonstrated that dietary cobalt levels (0.277 mg/kg–0.316 mg/kg) could significantly reduce hepatic MDA levels and could help to reduce the potential for oxidative stress, similar to the findings of Liu et al. [], who showed that moderate amounts of cobalt could reduce liver MDA levels. This study thus revealed that dietary cobalt supplementation improved the antioxidant capacity of largemouth bass.
The Nrf2-Keap1 signaling pathway is one of the most classical pathways regulating the antioxidant system in animals, which can be induced by external oxidative stress conditions to maintain intracellular redox homeostasis and reduce cellular sensitivity to death signals []. In this study, although nrf2 and keap1 did not change significantly among the treatment groups, their downstream antioxidant factors (sod and gpx) were both modulated by dietary cobalt levels. In its normal state, Nrf2 is present in the cytoplasm and is maintained in a relatively stable state by the ubiquitination system [], which, as explained here, in the absence of stimulation by external or internal conditions, may not cause changes in nrf2 and keap1. However, sod and gpx are not only regulated by the Nrf2 signaling pathway but are also present in several signaling pathways (PI3K-Akt, MAPK, JAK-STAT, etc.) [,,], which allows them to undergo an important antioxidative stress effect. In this study, the sod mRNA levels were significantly upregulated in the 0.201, 0.233, and 0.277 mg/kg dietary cobalt levels, and when the cobalt level reached 0.233 mg/kg, the gpx mRNA expression levels were upregulated. In mineral studies, dietary cobalt added to feeds has also been found to activate the antioxidant systems in organisms []. The expression levels of downstream genes sod and gpx were elevated in common carp (Cyprinus carpio) when Doum Palm Fruit Powder (DPFP) containing cobalt was added at 200 mg/kg []. Therefore, the addition of cobalt to the feed at a certain level can also improve antioxidant capacity by promoting the expression of antioxidant-related genes.
NF-κB has multiple biological functions and assists in complex biological processes; there are multiple mechanisms of action of NF-κB in the stress response, and it is involved in the inflammatory response and the development of stress-related diseases []. It has been demonstrated that mineral supplementation can activate the NF-κB signaling pathway [,]. In this study, the levels of some pro-inflammatory factors (nfκb, tnfα, il-8) did not change significantly, while anti-inflammatory factors (tgf-β and il-10) appeared to be upregulated. The NF-κB family of transcription factors is essential for the regulation of systemic immune pro-inflammatory responses, and the stimuli that initiate NF-κB activation are diverse but are usually attributed to pro-inflammatory cytokines and chemokines []. The non-significant changes in pro-inflammatory factors (tnfα and il-8) in this experiment might have led to the non-significant differences in nf-κb. Conversely, tgf-β, which is responsible for controlling cell proliferation and differentiation and the healing of wounds, is part of the immune system [], and the highest expression level was observed in the 0.233 mg/kg dietary cobalt level, while il-10 mRNA, which increased at the 0.201 mg/kg dietary cobalt level, is encoded by the IL-10 gene located on chromosome 1, and its expression implies its role in guarding the host against pathogens due to its anti-inflammatory properties [,]. The activity of these genes shows how dietary cobalt levels contributed to the improvement in the immune status of largemouth bass.
5. Conclusions
This study’s results present an improvement in the growth performance and feed utilization of largemouth bass fed with optimal dietary cobalt levels. Furthermore, cobalt has an important function in improving the antioxidant status and immune response of largemouth bass by regulating the Nrf2-Keap1 and NF-κB signaling pathways. Quadratic regression analyses based on the SGR and FCR showed that the optimal requirement was 0.24 and 0.26 mg/kg of dietary cobalt, respectively.
Author Contributions
Formal analysis, J.D.J. and D.H.; writing—original draft preparation, D.H. and J.D.J.; writing—review and editing, L.Z., M.R. and H.L.; project administration, L.Z. and H.L.; methodology, H.L.; investigation, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Central Public-interest Scientific Institution Basal Research Fund, Freshwater Fisheries Research Center, CAFS (NO. 2024JBFR01), the earmarked fund for CARS (CARS-46), National Natural Science Foundation of China (32102806).
Institutional Review Board Statement
The study was approved by the Laboratory Animal Ethics Committee of the Freshwater Fisheries Research Center (LAECFFRC-2023-04-10).
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the manuscript, tables, and figures.
Conflicts of Interest
Authors Lu Zhang are employed by Tongwei Agricultural Development Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. Lu Zhang played a major role in the design of the study, writing—review and editing and project administration.
References
- Lorenzoni, M.; DoErr, A.J.M.; Giovinazzo, G.; Mearelli, M.; Selvi, S. Growth and reproduction of largemouth bass (Micropterus salmoides Lacépède, 1802) in Lake Trasimeno (Umbria, Italy). Fish. Res. 2002, 56, 89–95. [Google Scholar] [CrossRef]
- Hussein, G.H.G.; Chen, M.; Qi, P.P.; Cui, Q.K.; Yu, Y.; Hu, W.H.; Tian, Y.; Fan, Q.X.; Gao, Z.X.; Feng, M.W.; et al. Aquaculture industry development, annual price analysis and out-of-season spawning in largemouth bass Micropterus salmoides. Aquaculture 2020, 519, 734901. [Google Scholar] [CrossRef]
- Jiang, B.; Lu, G.; Du, J.; Wang, J.; Hu, Y.; Su, Y.; Li, A. First report of trypanosomiasis in farmed largemouth bass (Micropterus salmoides) from China: Pathological evaluation and taxonomic status. Parasitol. Res. 2019, 118, 1731–1739. [Google Scholar] [CrossRef] [PubMed]
- C.F.S. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Wang, P.; Li, X.; Xu, Z.; Ji, D.; He, M.; Dang, J.Y.; Leng, X.J. The digestible phosphorus requirement in practical diet for largemouth bass (Micropterus salmoides) based on growth and feed utilization. Aquac. Fish. 2022, 7, 632–638. [Google Scholar] [CrossRef]
- Kohshahi, A.J.; Sourinejad, I.; Sarkheil, M.; Johari, S.A. Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): Influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2019, 45, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Hertz, Y.; Madar, Z.; Hepher, B.; Gertler, A. Glucose metabolism in the common carp (Cyprinus carpio L.): The effects of cobalt and chromium. Aquaculture 1989, 76, 255–267. [Google Scholar] [CrossRef]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Younus, N.; Zuberi, A.; Rashidpour, A.; Metón, I. Dietary cobalt supplementation improves growth and body composition and induces the expression of growth and stress response genes in Tor putitora. Fish Physiol. Biochem. 2020, 46, 371–381. [Google Scholar] [CrossRef]
- Aquilina, G.; Bories, G.; Brantom, P. Scientific Opinion on the use of cobalt compounds as additives in animal nutrition. EFSA J. 2009, 7, 1–45. [Google Scholar] [CrossRef]
- Al-ghanem, K.A. Effect of cobalt-supplemented diets on bioaccumulation, digestive enzyme activities and growth of Cyprinus carpio. Toxicol. Environ. Chem. 2011, 93, 985–995. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kaviraj, A. Evaluation of growth and bioaccumulation of cobalt in different tissues of common carp, Cyprinus carpio (Actinopterygii: Cypriniformes: Cyprinidae), fed cobalt-supplemented diets. Acta Ichthyol. Et Piscat. 2009, 39, 87–93. [Google Scholar] [CrossRef]
- Sapkale, P.H.; Singh, R.K. Dietary Zinc and Cobalt Requirements of Fry of Seabass (Lates calcarifer) and Catfish (Clarias batrachus). Isr. J. Aquac. Bamidgeh 2011, 63, 434–447. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Zhu, Z.; Ray, G.W.; Zhou, S.; Yang, Q.; Tan, B. Effects of cobalt sources and levels on growth performance, serum biochemistry, metabolic activities, and cobalt contents in the tissue of juvenile Litopenaeus vannamei. N. Am. J. Aquac. 2022, 84, 336–344. [Google Scholar] [CrossRef]
- Sumana, S.L.; Chen, H.; Shui, Y.; Zhang, C.; Yu, F.; Zhu, J.; Su, S. Effect of Dietary Selenium on the Growth and Immune Systems of Fish. Animals 2023, 13, 2978. [Google Scholar] [CrossRef] [PubMed]
- El, M.F.; Teiba, I.I.; Zaki, M.A.A.; Alabssawy, A.N.; El-hais, A.M.; Gabr, A.A.; Dawood, M.A.O.; Zaineldin, A.I.; Mzengereza, K.; Shadrack, R.S.; et al. Assessing the effectiveness of CoQ10 dietary supplementation on growth performance, digestive enzymes, blood health, immune response, and oxidative-related genes expression of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 98, 420–428. [Google Scholar] [CrossRef]
- Younus, N.; Zuberi, A. Significance of extrinsic factors for the optimization of dietary cobalt supplementation in Tor putitora fingerlings. Fish Physiol. Biochem. 2022, 48, 883–897. [Google Scholar] [CrossRef]
- Song, B.W.; Yang, H.; Li, X.Q.; Wang, P.; He, M.; Xu, Z.; Yang, P.X.; Leng, X.J. Dietary zinc requirement of juvenile largemouth bass (Micropterus salmoides). J. Fish. China 2021, 45, 1715–1725. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2003; Volume 1. [Google Scholar]
- Zhang, L.M.; Zhang, L.; Liang, H.L.; Huang, D.Y.; Ren, M.C. Effects of Taurine and Vitamin C on the Improvement of Antioxidant Capacity, Immunity and Hypoxia Tolerance in Gibel Carp (Carrassius auratus gibeilo). Antioxidants 2024, 13, 1169. [Google Scholar] [CrossRef]
- Kayiira, J.C.; Mi, H.F.; Liang, H.L.; Ren, M.C.; Huang, D.Y.; Zhang, L. Effect of Dietary Copper on Growth Performance, Antioxidant Capacity, and Immunity in Juvenile Largemouth Bass (Micropterus salmoides). Fishes 2024, 9, 369. [Google Scholar] [CrossRef]
- Gu, J.Z.; Liang, H.L.; Ge, X.P.; Xia, D.; Pan, L.K.; Mi, H.F.; Ren, M.C. A study of the potential effect of yellow mealworm (Tenebrio molitor) substitution for fish meal on growth, immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2022, 120, 214–221. [Google Scholar] [CrossRef]
- Yang, P.; Wang, W.Q.; Chi, S.Y.; Mai, K.S.; Song, F.; Wang, L. Effects of dietary lysine on regulating GH-IGF system, intermediate metabolism and immune response in largemouth bass (Micropterus salmoides). Aquac. Rep. 2020, 17, 100323. [Google Scholar] [CrossRef]
- Paul, B.N.; Mukhopadhyay, P.K. Importance of Trace Minerals in Aquaculture Nutrition. Fish. Chimes 2001, 21, 34–36. Available online: https://www.researchgate.net/publication/299367144 (accessed on 23 March 2016).
- Pati, P.; Mondal, K. A Review on the Dietary Requirements of Trace Minerals in Freshwater Fish. J. Environ. Sociobiol. 2019, 16, 171–206. Available online: https://www.researchgate.net/publication/347464039 (accessed on 18 December 2020).
- Mukherjee, S.; Kaviraj, A. Ecotoxicological assessment of cobalt used as supplement in the diet of common carp Cyprinus carpio. Bull. Environ. Contam. Toxicol. 2011, 87, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.Y.; Xie, P.; Luo, Z.; Lin, H.Z.; Zhao, Y.H.; Xi, W.Q. Dietary manganese requirement of juvenile yellow catfish Pelteobagrus fulvidraco, and effects on whole body mineral composition and hepatic intermediary metabolism. Aquaculture 2012, 326, 68–73. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Shukla, A.; Mossman, B.T. Oxidants and Antioxidants | Oxidants. In Encyclopedia of Respiratory Medicine; Academic Press: Cambridge, MA, USA, 2006; Volume 1–4, pp. 271–278. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Glutathione Peroxidases. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 399–404. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
- Mclaughlin, Q.R.; Gunderson, M.P. Effects of selenium treatment on endogenous antioxidant capacity in signal crayfish (Pacifastacus leniusculus). Comp. Biochem. Physiology. Toxicol. Pharmacol. 2022, 256, 109324. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.D.; Bu, X.Y.; Huang, Q.C.; Qiao, F.; Chen, X.F.; Shi, Q.C.; Qin, J.G.; Chen, L.Q. A comparation between different iron sources on growth performance, iron utilization, antioxidant capacity and non-specific immunity in Eriocheir sinensis. Anim. Feed. Sci. Technol. 2022, 288, 115300. [Google Scholar] [CrossRef]
- Yu, L.Y.; Li, L.Y.; Yu, H.R.; Yuan, Z.Y.; Ma, C.Y.; Wu, X.J.; Kong, W.G. Effects of dietary manganese supplementation on the growth performance, tissue manganese content and antioxidant capacity of coho salmon (Oncorhynchus kisutch) post-smolts. Aquac. Rep. 2024, 34, 101924. [Google Scholar] [CrossRef]
- Bergeron, N.; Guay, F. Impact of zinc and arginine on antioxidant status of weanling piglets raised under commercial conditions. Anim. Nutr. 2019, 3, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Gong, C.; Ren, J.; Zhang, X.; Wang, G.; Liu, Y.; Ren, Y.; Zhao, Y.; Yu, Q.; Wang, Y.; et al. Toxicity of nickel and cobalt in Japanese flounder. Environ. Pollut. 2020, 263, 114516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.Y.; Li, B.S.; Qiao, H.J.; Liu, X.D.; Hao, T.T.; Wang, X.Y. Effects of dietary cobalt methionine on growth performance, mineral deposition, and hepatic enzyme activities in juvenile pearl gentian grouper (Epinephelus lanceolatus♂ × E. fuscoguttatus♀). J. Fish. Sci. China 2016, 23, 574–583. (In Chinese) [Google Scholar] [CrossRef]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2010, 70, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.; Kim, S.; Kang, D. The role of hydrogen peroxide and peroxiredoxins throughout the cell cycle. Antioxidants 2020, 9, 280. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291. [Google Scholar] [CrossRef]
- Mann, G.E.; Forman, H.J. Introduction to Special Issue on ′Nrf2 Regulated Redox Signaling and Metabolism in Physiology and Medicine. Free. Radic. Biol. Med. 2015, 88, 91–92. [Google Scholar] [CrossRef]
- Khedr, N.F.; Talkan, O.F.A. New insights into arsenic, lead, and iron neurotoxicity: Activation of MAPK signaling pathway and oxidative stress. J. Biochem. Mol. Toxicol. 2022, 36, e23040. [Google Scholar] [CrossRef]
- Gao, C.; Fei, X.; Wang, M.; Chen, Q.; Zhao, N. Cardamomin protects from diabetes-induced kidney damage through modulating PI3K/AKT and JAK/STAT signaling pathways in rats. Int. Immunopharmacol. 2022, 107, 108610. [Google Scholar] [CrossRef]
- Silvestre, F. Signaling pathways of oxidative stress in aquatic organisms exposed to xenobiotic. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 436–448. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Khalil, A.A.; Amer, S.A.; Shalaby, S.I.; Badr, H.A.; Farag, M.F.M.; Altohamy, D.E.; Abdel Rahman, A.N. Effects of dietary doum palm fruit powder on growth, antioxidant capacity, immune response, and disease resistance of African catfish, Clarias gariepinus (B.). Animals 2020, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Dadar, M.; Khalili, M.; Cerezuela, R.; Esteban, M.Á. Effect of dietary supplementation of palm fruit extracts on the transcriptomes of growth, antioxidant enzyme and immune-related genes in common carp (Cyprinus carpio) fingerlings. Aquacult. Res. 2017, 48, 3684–3692. [Google Scholar] [CrossRef]
- Xia, Z.B.; Meng, F.R.; Fang, Y.X.; Wu, X.; Zhang, C.W.; Liu, Y.; Liu, D.; Li, G.Q.; Feng, F.B.; Qiu, H.Y. Inhibition of NF-κB signaling pathway induces apoptosis and suppresses proliferation and angiogenesis of human fibroblast-like synovial cells in rheumatoid arthritis. Medicine 2018, 97, e10920. [Google Scholar] [CrossRef]
- Guo, Y.L.; Wu, P.; Jiang, W.D.; Liu, Y.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q. The impaired immune function and structural integrity by dietary iron deficiency or excess in gill of fish after infection with Flavobacterium columnare: Regulation of NF-κB, TOR, JNK, p38MAPK, Nrf2 and MLCK signalling. Fish Shellfish Immunol. 2018, 74, 593–608. [Google Scholar] [CrossRef]
- Guo, Y.L.; Feng, L.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhou, X.Q. Dietary iron deficiency impaired intestinal immune function of on-growing grass carp under the infection of Aeromonas hydrophila: Regulation of NF-kappa B and TOR signaling. Fish Shellfish Immunol. 2019, 93, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Anikumar, S.; Jin, E.W. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit. Rev. Immunol. 2013, 32, 23–63. [Google Scholar] [CrossRef]
- Robert, S.; Gerald, G.; Katarzyna, W.; Stefan, K.; Ellen, W.; Kerstin, W.; Jens, G. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).