Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Sample Collection
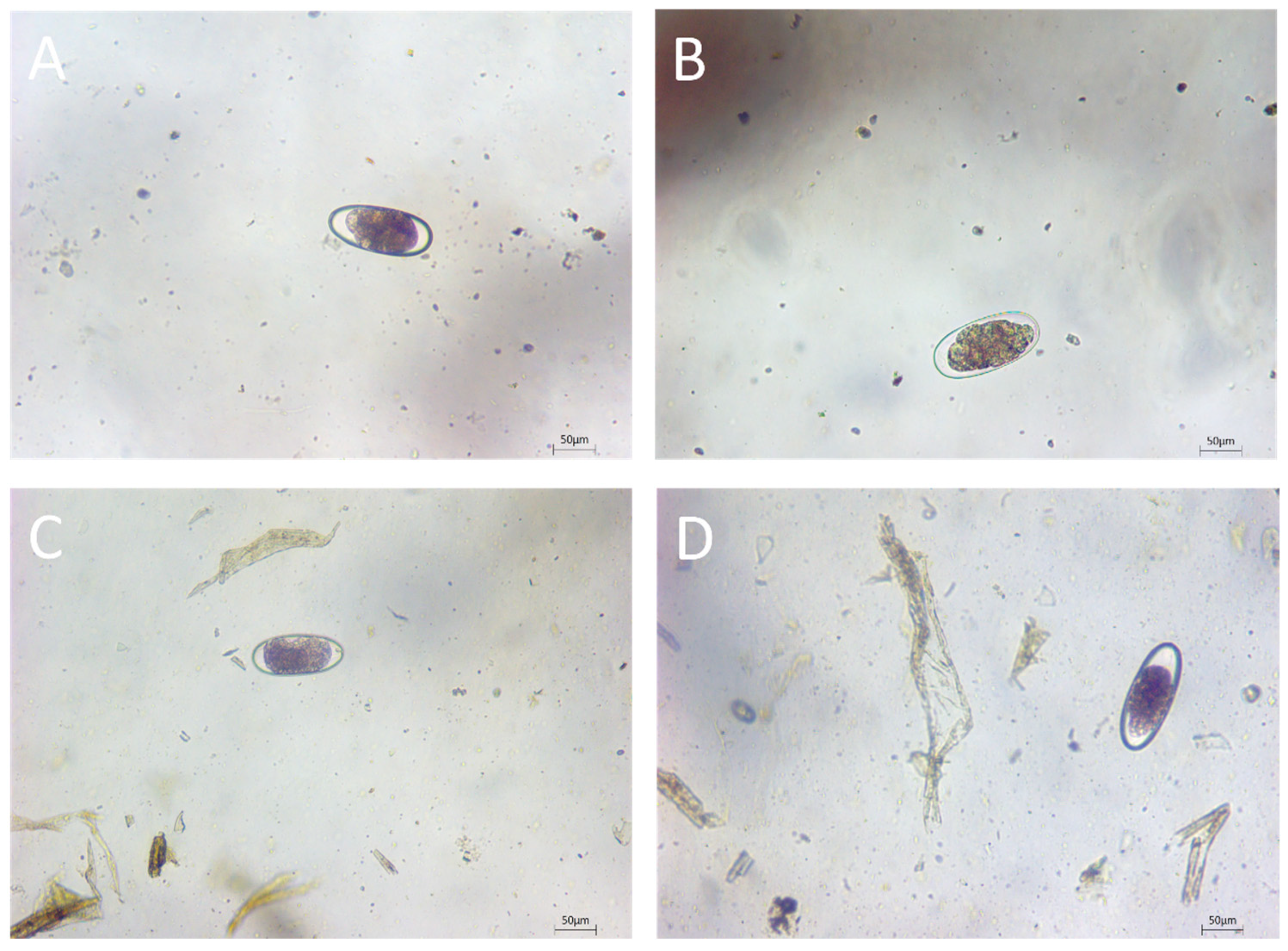
2.3. Morphological Examination
2.4. Molecular Identification
2.5. Statistical Analyses
3. Results
3.1. Descriptive Data Analysis
3.2. Infection Status in Different Regions, Age Groups, and Sex
3.3. PCR Detection of Nematode Eggs Distribution and Co-Infection Patterns of Gastrointestinal Helminths in Camels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadim, I.T.; Mahgoub, O.; Purchas, R.W. A review of the growth, and of the carcass and meat quality characteristics of the one-humped camel (Camelus dromedaries). Meat Sci. 2008, 80, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Bekele, J.T.; Aregawi, W.G.; Wegi, F.G.; Geletu, A.S.; Tesfamariam, W. Epidemiological Investigation of Gastrointestinal Parasites of Dromedary Camels in Administrative Zone Three of Afar Region, Ethiopia. Vet. Med. Int. 2022, 2022, 8433997. [Google Scholar] [CrossRef] [PubMed]
- Sazmand, A.; Joachim, A.; Otranto, D. Zoonotic parasites of dromedary camels: So important, so ignored. Parasites Vectors 2019, 12, 610. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hilali, M.; Van Wilpe, E.; Calero-Bernal, R.; Verma, S.K.; Abbas, I.E. A review of sarcocystosis in camels and redescription of Sarcocystis cameli and Sarcocystis ippeni sarcocysts from the one-humped camel (Camelus dromedarius). Parasitology 2015, 142, 1481–1492. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schuster, R.K. A review of coccidiosis in Old World camels. Vet. Parasitol. 2018, 262, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Schuster, R.K.; Kinne, J. Gametogony of Eimeria cameli in the small intestine of one-humped camel (Camelus dromedarius). Parasitol. Res. 2018, 117, 3633–3638. [Google Scholar] [CrossRef]
- Ederli, N.B.; de Oliveira, F.C. Gastrointestinal nematodes in ostriches, Struthio camelus, in different regions of the state of Rio de Janeiro, Brazil. Rev. Bras. Parasitol. Vet. 2015, 24, 168–173. [Google Scholar] [CrossRef][Green Version]
- Saad, N.M.; Hussein, A.A.A.; Ewida, R.M. Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in Upper Egypt. Vet. World 2018, 11, 1262–1265. [Google Scholar] [CrossRef]
- Sazmand, A.; Joachim, A. Parasitic diseases of camels in Iran (1931–2017)—A literature review. Parasite 2017, 24, 21. [Google Scholar] [CrossRef]
- Guowu, Z.; Kai, Z.; Xifeng, W.; Chunhui, J.; Chengcheng, N.; Yue, Z.; Jun, Q.; Qingling, M.; Xingxing, Z.; Kuojun, C.; et al. Occurrence of Gastrointestinal Parasites in Camels in the Tianshan Mountains Pastoral Area in China. J. Vet. Res. 2020, 64, 509–515. [Google Scholar] [CrossRef]
- Zhao, S.S.; Li, Y.H.; Zhang, Y.; Zhou, Q.; Jing, B.; Xu, C.Y.; Zhang, L.X.; Song, J.K.; Qi, M.; Zhao, G.H. Multilocus genotyping of Giardia duodenalis in Bactrian camels (Camelus bactrianus) in China. Parasitol. Res. 2020, 119, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Liu, J.; Wang, J.; Jia, D.; Liu, A.; He, Z.; Guan, G.; Liu, Z.; Liu, G.; et al. First Report of Theileria Infection of Bactrian Camels (Camelus bactrianus) in Xinjiang, China. Acta Parasitol. 2019, 64, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Ai, J.; Yang, J.; Zhu, H.; Zhou, Y.; Zhu, Y.; Zhang, H.; Qin, Q.; Kang, M.; Sun, Y.; et al. Seroepidemiology of Neosporosis in Various Animals in the Qinghai-Tibetan Plateau. Front. Vet. Sci. 2022, 9, 953380. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, H.L.; Luo, X.P.; Li, J.Y.; Wang, R.; Yang, B.; Wang, P.L.; Zhai, B.T.; Yang, X.Y.; Yang, L.R. Parabronema skrjabini (Nematoda: Habronematidae) infection and development in the intermediate host-Haematobia irritans (Linnaeus, 1758) in Inner Mongolia, China. Vet. Parasitol. 2021, 291, 109326. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M. Camel Clinical Biochemistry and Hematology. Clin. Enzymol. 2018, 9, 123–172. [Google Scholar] [CrossRef]
- Yang, D.A.; Laven, R.A.; Heuer, C.; Vink, W.D.; Chesterton, R.N. Farm level risk factors for bovine digital dermatitis in Taranaki, New Zealand: An analysis using a Bayesian hurdle model. Vet. J. 2018, 234, 91–95. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing Vienna, Austria; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Allah, D. The types and characteristics of grassland vegetation in China. Resour. Data J. 2023, 2, 50–57. [Google Scholar]
- Ma, L.; Zheng, J.; Pen, J.; Xiao, X.; Liu, Y.; Liu, L.; Han, W.; Li, G.; Zhang, J. Monitoring and influencing factors of grassland livestock overload in Xinjiang from 1982 to 2020. Front. Plant Sci. 2024, 15, 1340566. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Wan, H.; Wang, Y.; Sun, C.; Zhou, H. Analyzing the relationship between animal diversity and the remote sensing vegetation parameters: The case of Xinjiang, China. Sustainability 2021, 13, 9897. [Google Scholar] [CrossRef]
- Miao, J.; Xiao, S.; Wang, J. Comparative study of camel milk from different areas of Xinjiang province in China. Food Sci. Anim. Resour. 2023, 43, 674. [Google Scholar] [CrossRef]
- Parsani, H.; Singh, V.; Momin, R. Common parasitic diseases of camel. Vet. World 2008, 1, 317–318. [Google Scholar]
- Yao, H.; Liu, M.; Ma, W.; Yue, H.; Su, Z.; Song, R.; Ma, Q.; Li, L.; Wu, Z.; Ma, Y. Prevalence and pathology of Cephalopina titillator infestation in Camelus bactrianus from Xinjiang, China. BMC Vet. Res. 2022, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Zheng, S.; Chang, Y.; Zhang, K.; Zhang, S.; Zhang, L. Molecular identification and biological characterization of Cryptosporidium muris from camels (Camelus bactrianus) in China. Parasites Vectors 2021, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Meng, P.; Ye, Q.; Zhang, D. Toxoplasma gondii infection in Bactrian camel (Camelus bactrianus) in China. Vet. Parasitol. 2013, 192, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Zhen, L.; Xinyan, Z.; Weilong, T.; Junzhen, C.; Yanting, G.; Xinyan, H.; Shengnan, L.; Ruixin, T.; Wenli, D.; Zhanqiang, S.; et al. Investigation on the infection of gastrointestinal parasites in Bactrian camels in some areas of Xinjiang. Heilongjiang Anim. Sci. Vet. Med. 2020, 15, 98–100. [Google Scholar]
- Ibrahim, A.M.; Kadle, A.A.; Yusuf, A.A. Gastro-intestinal parasites of camels (Camelus dromedarius) from Mogadishu, Somalia. Open J. Vet. Med. 2016, 6, 112–118. [Google Scholar] [CrossRef][Green Version]
- Metwally, D.M.; Al-Otaibi, T.T.; Albasyouni, S.A.; El-Khadragy, M.F.; Alajmi, R.A. Prevalence of eimeriosis in the one-humped camels (Camelus dromedarius) from Riyadh and Al-Qassim, Saudi Arabia. PeerJ 2020, 8, e10347. [Google Scholar] [CrossRef]
- Kassahun, A.; Wubishet, Z.; Getachew, D.; Gashaw, B.; Geda, S.; Kassahun, G.A. Study on the prevalence and associated risk factors of camel gastro-intestinal parasites in southern Ethiopiaz. J. Vet. Med. Res. 2020, 7, 1184. [Google Scholar]
- Wakil, Y.; Lawal, J.R.; Gazali, Y.A.; Bello, A.; Mshelia, E.; Ayomikun, A.M. Prevalence of gastrointestinal parasites in one humped camels (Camelus dromedarius) slaughtered at the Maiduguri metropolitan abattoir, Borno State, Nigeria. J. Vet. Med. Anim. Sci. 2017, 2, 96–101. [Google Scholar] [CrossRef]
- Bouragba, M.; Laatamna, A.; Cheddad, F.E.; Baroudi, D.; Houali, K.; Hakem, A. Gastrointestinal parasites of dromedary camel (Camelus dromedarius) in Algeria. Vet. World 2020, 13, 1635–1640. [Google Scholar] [CrossRef]
| Variable | Positives | Total | Prevalence (%) | 95% Confidence Interval | Χ2 (DF) 1 | p-Value | |
|---|---|---|---|---|---|---|---|
| Region | 44.172 (1) | <0.0001 | |||||
| Urumqi | Urumqi | 71 | 245 | 29 | 23.4–35.1 | ||
| Other regions | Aksu | 2 | 44 | 4.5 | 0.6–15.5 | ||
| Altay | 6 | 37 | 16.2 | 6.2–32 | |||
| Hetian | 0 | 33 | 0 | NA | |||
| Ili | 0 | 76 | 0 | NA | |||
| Sub-total 2 | 8 | 190 | 4.2 | 1.8–8.1 | |||
| Age | 26.484 (1) | <0.0001 | |||||
| ≤3 years old | 59 | 211 | 28 | 22–24.5 | |||
| >3 years old | 20 | 224 | 8.9 | 5.5–13.5 | |||
| Sex | 2.4454 (1) | 0.1179 | |||||
| Female | 40 | 186 | 21.5 | 15.8–28.1 | |||
| Male | 39 | 249 | 15.7 | 11.4–20.8 | |||
| Total | 79 | 435 | 18.2 | 14.7–22.2 |
| Region/Sex | Age | Sex | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≤3 Years Old | >3 Years Old | Female-Total | Male-Total | ||||||
| Female | Male | Age-Total | Female | Male | Age-Total | ||||
| Urumqi | Urumqi | 60 | 59 | 119 | 30 | 96 | 126 | 90 | 155 |
| Other regions | Sub-total 1 | 72 | 20 | 92 | 24 | 74 | 98 | 96 | 94 |
| Aksu | 0 | 0 | 0 | 24 | 20 | 44 | 24 | 20 | |
| Altay | 17 | 20 | 37 | 0 | 0 | 0 | 17 | 20 | |
| Hetian | 14 | 0 | 14 | 0 | 19 | 19 | 14 | 19 | |
| Ili | 41 | 0 | 41 | 0 | 35 | 35 | 41 | 35 | |
| 186 | 249 | ||||||||
| Total | 132 | 79 | 211 | 54 | 170 | 224 | 435 | ||
| Variable | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Region | Urumqi | 4.62 | 2.63–8.41 | <0.0001 |
| Other regions | ref | |||
| Age | ≤3 years old | 10.53 | 5.12–24.65 | <0.0001 |
| >3 years old | ref | |||
| Infection Number | Urumqi | Aksu | Altay | Male | Female | |
|---|---|---|---|---|---|---|
| H. contortus | 11 | 6 | 1 | 4 | 7 | 4 |
| Trichostrongylus spp. | 20 | 17 | 1 | 2 | 11 | 9 |
| C. ovina | 18 | 16 | 0 | 2 | 12 | 6 |
| Ostertagia spp | 3 | 3 | 0 | 0 | 2 | 1 |
| Bunostomum spp. | 2 | 2 | 0 | 0 | 2 | 0 |
| H. contortus and Trichostrongylus spp. | 4 | 4 | 0 | 0 | 3 | 1 |
| H. contortus and C. ovina | 3 | 2 | 0 | 1 | 3 | 0 |
| H. contortus and Ostertagia spp. | 1 | 1 | 0 | 0 | 1 | 0 |
| Trichostrongylus spp. and C. ovina | 9 | 8 | 0 | 1 | 6 | 3 |
| Trichostrongylus spp. and Ostertagia spp. | 1 | 1 | 0 | 0 | 1 | 0 |
| Trichostrongylus spp. and Bunostomum spp. | 1 | 1 | 0 | 0 | 1 | 0 |
| C. ovina and Ostertagia spp. | 2 | 2 | 0 | 0 | 2 | 0 |
| H. contortus, Trichostrongylus spp. and C. ovina | 1 | 1 | 0 | 0 | 1 | 0 |
| H. contortus, C. ovina, and Ostertagia spp. | 1 | 1 | 0 | 0 | 1 | 0 |
| Trichostrongylus spp., C. ovina, and Ostertagia spp. | 1 | 1 | 0 | 0 | 1 | 0 |
| Total | 36 | 28 | 2 | 6 | 20 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, D.A.; Yang, M.; Pi, M.; Zhang, Y.; Su, Z. Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China. Vet. Sci. 2024, 11, 503. https://doi.org/10.3390/vetsci11100503
Zhang Y, Yang DA, Yang M, Pi M, Zhang Y, Su Z. Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China. Veterinary Sciences. 2024; 11(10):503. https://doi.org/10.3390/vetsci11100503
Chicago/Turabian StyleZhang, Yi, Danchen Aaron Yang, Min Yang, Mengjie Pi, Yang Zhang, and Zhanqiang Su. 2024. "Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China" Veterinary Sciences 11, no. 10: 503. https://doi.org/10.3390/vetsci11100503
APA StyleZhang, Y., Yang, D. A., Yang, M., Pi, M., Zhang, Y., & Su, Z. (2024). Prevalence and Associated Risk Factors of Helminth Infections in the Digestive Tract of Camels in Xinjiang, China. Veterinary Sciences, 11(10), 503. https://doi.org/10.3390/vetsci11100503





