Effect of Deferoxamine on Post-Transfusion Iron, Inflammation, and In Vitro Microbial Growth in a Canine Hemorrhagic Shock Model: A Randomized Controlled Blinded Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
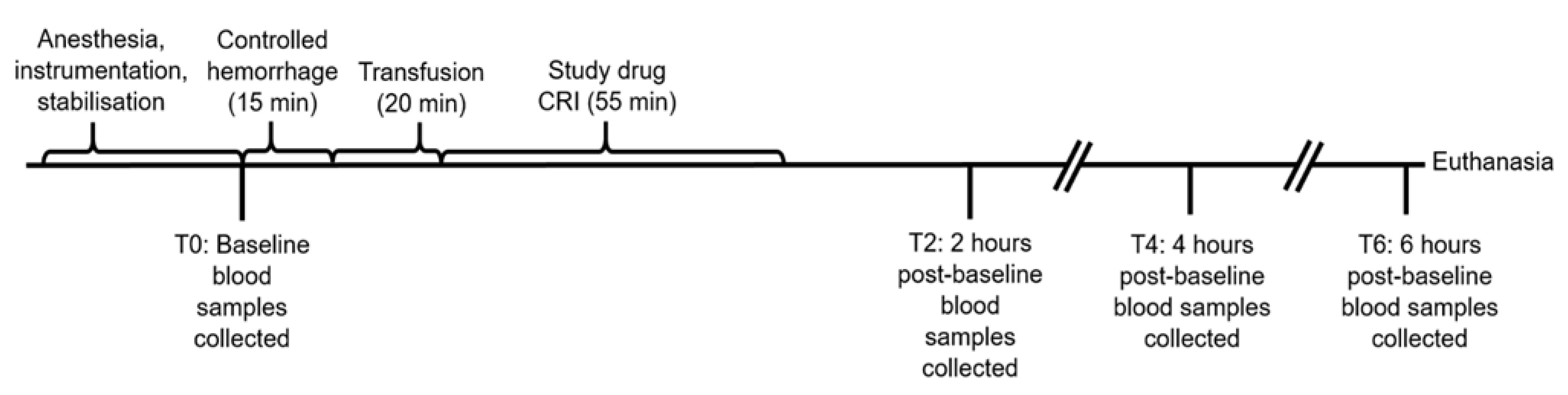
2.2. Study Protocol
2.3. Sample Collection and Analysis
2.4. In Vitro E. coli Growth
2.5. Statistical Analysis
3. Results
3.1. Pre-Transfusion Characteristics
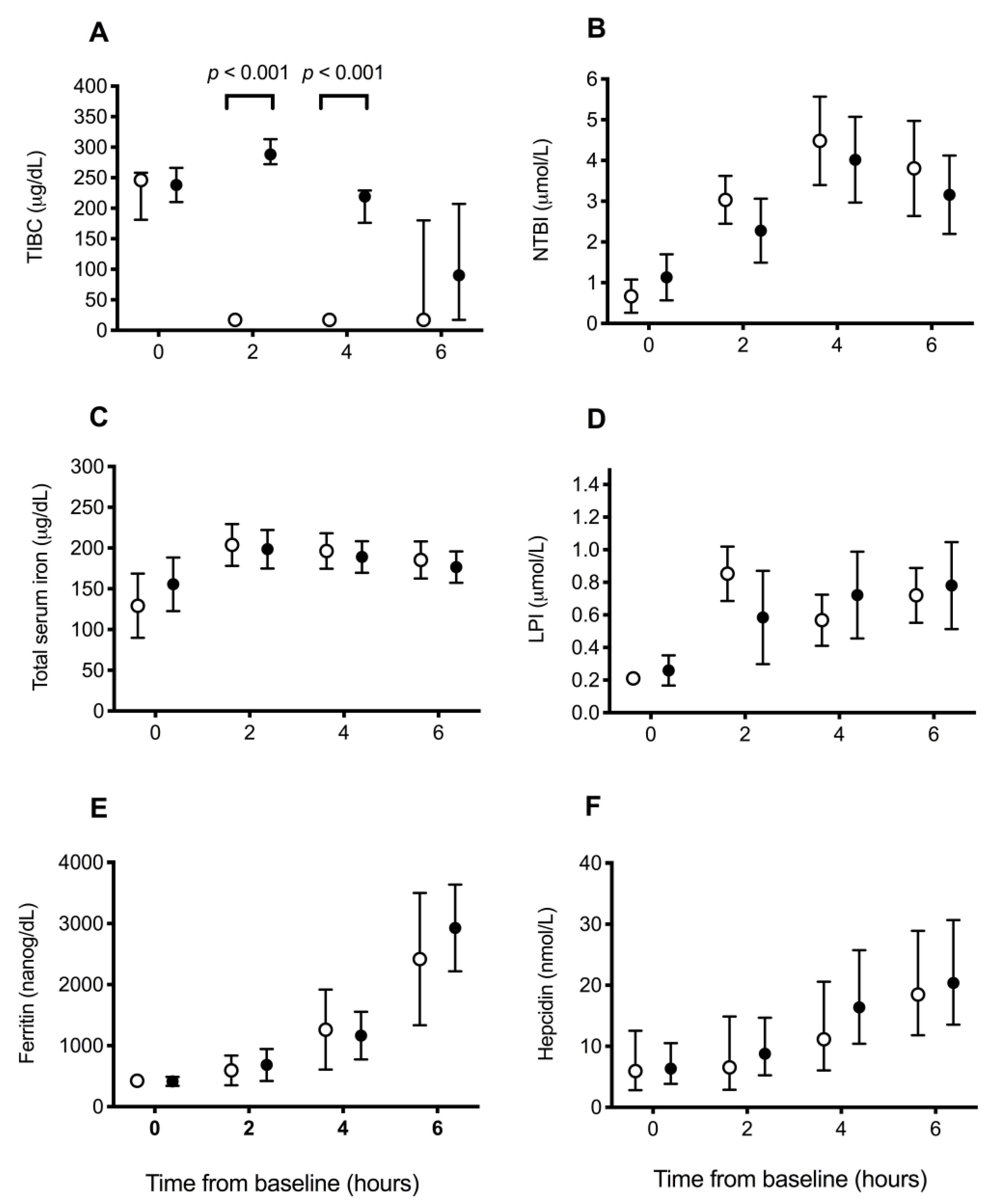
3.2. Iron Parameters
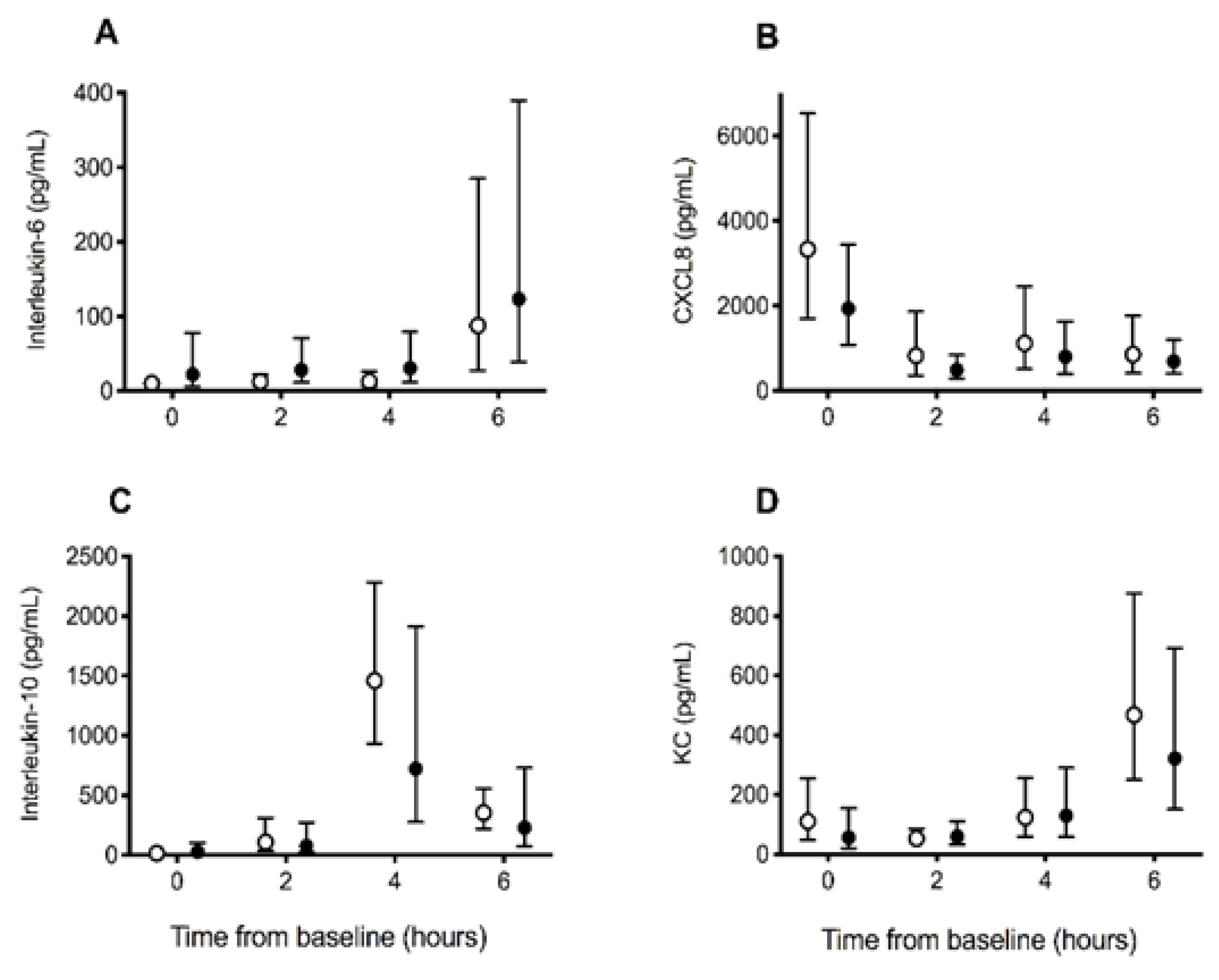
3.3. Inflammation Biomarkers
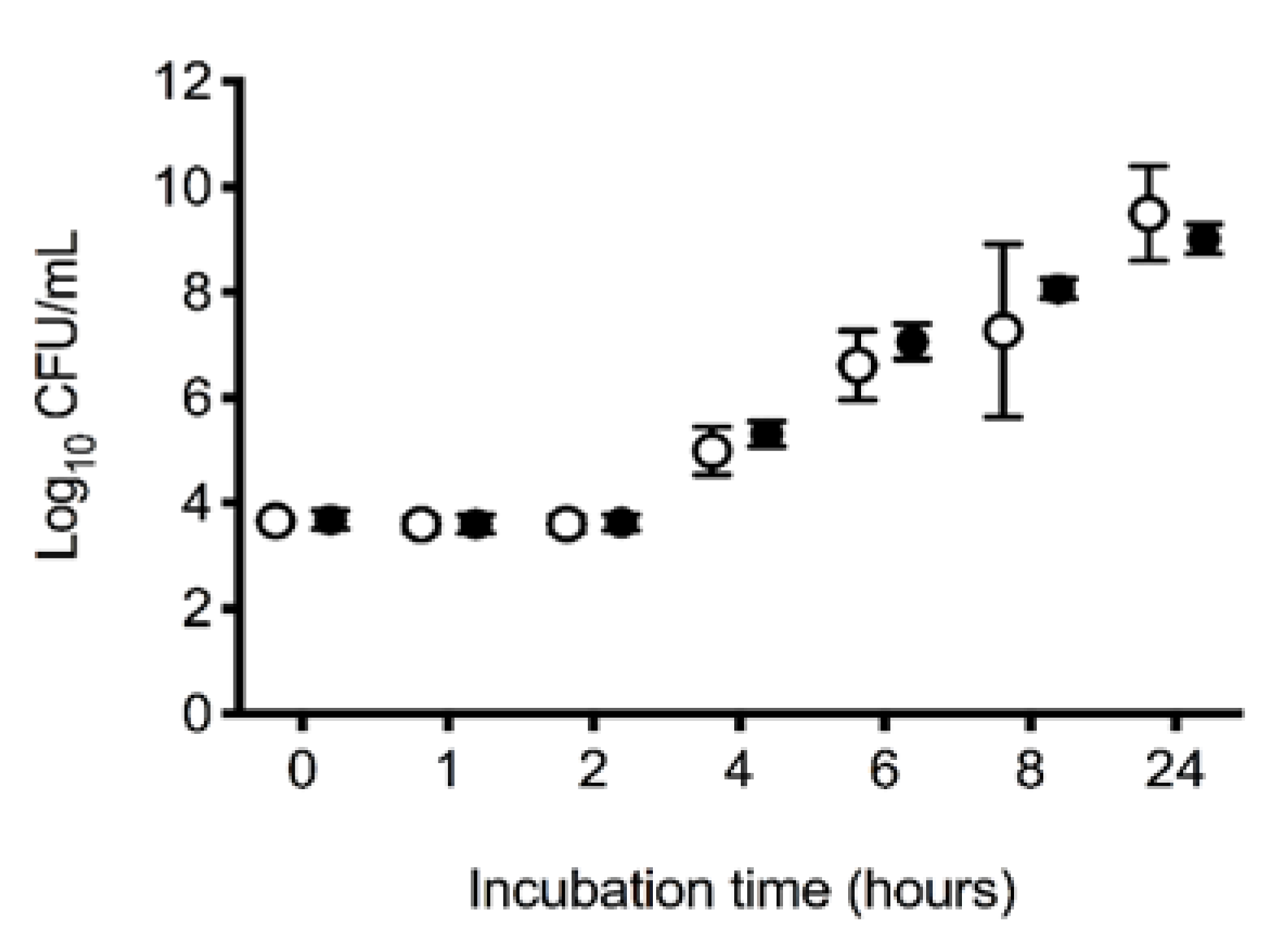
3.4. In Vitro E. coli Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marik, P.E.; Corwin, H.L. Efficacy of red blood cell transfusion in the critically ill: A systematic review of the literature. Crit. Care Med. 2008, 36, 2667–2674. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Baron, J.-F.; Reinhart, K.; Gattinoni, L.; Thijs, L.; Webb, A.; Meier-Hellmann, A.; Nollet, G.; Peres-Bota, D.; for the ABC Investigators. Anemia and blood transfusion in critically ill patients. JAMA 2002, 288, 1499–1507. [Google Scholar] [CrossRef]
- Corwin, H.L.; Gettinger, A.; Pearl, R.G.; Fink, M.P.; Levy, M.M.; Abraham, E.; MacIntyre, N.R.; Shabot, M.M.; Duh, M.-S.; Shapiro, M.J. The CRIT Study: Anemia and blood transfusion in the critically ill—Current clinical practice in the United States. Crit. Care Med. 2004, 32, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.M.; Dimcheff, D.E.; Blumberg, N.; Saint, S.; Langa, K.M.; Kuhn, L.; Hickner, A.; Rogers, M.A. Health care–associated infection after red blood cell transfusion: A systematic review and meta-analysis. JAMA 2014, 311, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Carlucci, C.; Isgrò, G.; Boncilli, A.; De Benedetti, D.; De la Torre, T.; Brozzi, S.; Frigiola, A. Duration of red blood cell storage and outcomes in pediatric cardiac surgery: An association found for pump prime blood. Crit. Care 2009, 13, R207. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.G.; Li, L.; Sessler, D.I.; Figueroa, P.; Hoeltge, G.A.; Mihaljevic, T.; Blackstone, E.H. Duration of red-cell storage and complications after cardiac surgery. N. Engl. J. Med. 2008, 358, 1229–1239. [Google Scholar] [CrossRef]
- Leal-Noval, S.R.; Jara-López, I.; García-Garmendia, J.L.; Marín-Niebla, A.; Herruzo-Avilés, A.; Camacho-Laraña, P.; Loscertales, J. Influence of erythrocyte concentrate storage time on postsurgical morbidity in cardiac surgery patients. Anesthesiology 2003, 98, 815–822. [Google Scholar] [CrossRef]
- Karam, O.; Tucci, M.; Bateman, S.T.; Ducruet, T.; Spinella, P.C.; Randolph, A.G.; Lacroix, J. Association between length of storage of red blood cell units and outcome of critically ill children: A prospective observational study. Crit. Care 2010, 14, R57. [Google Scholar] [CrossRef]
- Zallen, G.; Offner, P.J.; Moore, E.E.; Blackwell, J.; Ciesla, D.J.; Gabriel, J.; Denny, C.; Silliman, C.C. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am. J. Surg. 1999, 178, 570–572. [Google Scholar] [CrossRef]
- Ng, M.S.; David, M.; Middelburg, R.A.; Ng, A.S.; Suen, J.Y.; Tung, J.-P.; Fraser, J.F. Transfusion of packed red blood cells at the end of shelf life is associated with increased risk of mortality—A pooled patient data analysis of 16 observational trials. Haematologica 2018, 103, 1542–1548. [Google Scholar] [CrossRef]
- Tinmouth, A.; Fergusson, D.; Yee, I.C.; Hébert, P.C.; ABLE Investigators and the Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion 2006, 46, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Wurlod, V.A.; Smith, S.A.; McMichael, M.A.; O’Brien, M.; Herring, J.; Swanson, K.S. Iron metabolism following intravenous transfusion with stored versus fresh autologous erythrocyte concentrate in healthy dogs. Am. J. Vet. Res. 2015, 76, 996–1004. [Google Scholar] [CrossRef]
- Hod, E.A.; Brittenham, G.M.; Billote, G.B.; Francis, R.O.; Ginzburg, Y.Z.; Hendrickson, J.E.; Jhang, J.; Schwartz, J.; Sharma, S.; Sheth, S. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non–transferrin-bound iron. Blood 2011, 118, 6675–6682. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef] [PubMed]
- Collard, K.J.; White, D.L. On the source of the non-transferrin-bound iron which accumulates in packed red blood cell units during storage. Blood Transfus. 2014, 12, 527. [Google Scholar] [PubMed]
- Brissot, P.; Ropert, M.; Le Lan, C.; Loréal, O. Non-transferrin bound iron: A key role in iron overload and iron toxicity. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 403–410. [Google Scholar] [CrossRef]
- Cabantchik, Z.I.; Breuer, W.; Zanninelli, G.; Cianciulli, P. LPI-labile plasma iron in iron overload. Best Pract. Res. Clin. Haematol. 2005, 18, 277–287. [Google Scholar] [CrossRef]
- Fleming, R.E.; Ponka, P. Iron overload in human disease. N. Engl. J. Med. 2012, 366, 348–359. [Google Scholar] [CrossRef]
- Schreck, R.; Albermann, K.; Baeuerle, P.A. Nuclear factor kB: An oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free. Radic. Res. Commun. 1992, 17, 221–237. [Google Scholar] [CrossRef]
- Lowy, F.D. How Staphylococcus aureus adapts to its host. N. Engl. J. Med. 2011, 364, 1987–1990. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and iron homeostasis. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Keberle, H. The biochemistry of desferrioxamine and its relation to iron metabolism. Ann. N. Y. Acad. Sci. 1964, 119, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Elfenbein, J.; Giguère, S.; Meyer, S.; Javsicas, L.; Farina, L.; Zimmel, D.; Sanchez, L. The effects of deferoxamine mesylate on iron elimination after blood transfusion in neonatal foals. J. Vet. Intern. Med. 2010, 24, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Hod, E.A.; Zhang, N.; Sokol, S.A.; Wojczyk, B.S.; Francis, R.O.; Ansaldi, D.; Francis, K.P.; Della-Latta, P.; Whittier, S.; Sheth, S. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 2010, 115, 4284–4292. [Google Scholar] [CrossRef] [PubMed]
- McCown, J.L.; Specht, A.J. Iron homeostasis and disorders in dogs and cats: A review. J. Am. Anim. Hosp. Assoc. 2011, 47, 151–160. [Google Scholar] [CrossRef]
- Callan, M.B.; Patel, R.T.; Rux, A.H.; Bandyopadhyay, S.; Sireci, A.N.; O’Donnell, P.A.; Ruane, T.; Sikora, T.; Marryott, K.; Sachais, B.S. Transfusion of 28-day-old leucoreduced or non-leucoreduced stored red blood cells induces an inflammatory response in healthy dogs. Vox Sang. 2013, 105, 319–327. [Google Scholar] [CrossRef]
- Tenenbein, M. Benefits of parenteral deferoxamine for acute iron poisoning. J. Toxicol. Clin. Toxicol. 1996, 34, 485–489. [Google Scholar] [CrossRef]
- Howland, M.A. Risks of parenteral deferoxamine for acute iron poisoning. J. Toxicol. Clin. Toxicol. 1996, 34, 491–497. [Google Scholar] [CrossRef]
- Zhang, D.; Okada, S.; Kawabata, T.; Yasuda, T. An improved simple colorimetric method for quantitation of non-transferrin-bound iron in serum. Biochem. Mol. Biol. Int. 1995, 35, 635–641. [Google Scholar]
- Esposito, B.P.; Breuer, W.; Sirankapracha, P.; Pootrakul, P.; Hershko, C.; Cabantchik, Z.I. Labile plasma iron in iron overload: Redox activity and susceptibility to chelation. Blood 2003, 102, 2670–2677. [Google Scholar] [CrossRef]
- Mead, M.K.; Claus, M.; Litton, E.; Smart, L.; Raisis, A.; Rossi, G.; Trengove, R.D. Gummer JPA: Identification of The Canidae Iron Regulatory Hormone Hepcidin. Sci. Rep. 2019, 9, 19400. [Google Scholar] [CrossRef]
- Novosad, S.A.; Sapiano, M.R.; Grigg, C.; Lake, J.; Robyn, M.; Dumyati, G.; Felsen, C.; Blog, D.; Dufort, E.; Zansky, S.; et al. Vital signs: Epidemiology of sepsis: Prevalence of health care factors and opportunities for prevention. Morb. Mortal. Wkly. Rep. 2016, 65, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.J.F.B. Iron uptake by Escherichia coli. Front. Biosci. 2003, 8, 1409–1421. [Google Scholar] [CrossRef]
- Wang, D.; Cortés-Puch, I.; Sun, J.; Solomon, S.B.; Kanias, T.; Remy, K.E.; Feng, J.; Alimchandani, M.; Quezado, M.; Helms, C. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion 2014, 54, 1712–1724. [Google Scholar] [CrossRef]
- Porter, J.; Abeysinghe, R.; Marshall, L.; Hider, R.; Singh, S. Kinetics of removal and reappearance of non-transferrin-bound plasma iron with deferoxamine therapy. Blood 1996, 88, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Ozment, C.P.; Turi, J.L. Iron overload following red blood cell transfusion and its impact on disease severity. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Kushner, J.P.; Porter, J.P.; Olivieri, N.F. Secondary iron overload. ASH Educ. Program Book 2001, 2001, 47–61. [Google Scholar] [CrossRef]
- Kell, D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009, 2, 2. [Google Scholar]
- Cabantchik, Z.I. Labile iron in cells and body fluids: Physiology, pathology, and pharmacology. Front. Pharmacol. 2014, 5, 45. [Google Scholar] [CrossRef]
- Eid, R.; Arab, N.T.; Greenwood, M.T. Iron mediated toxicity and programmed cell death: A review and a re-examination of existing paradigms. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 399–430. [Google Scholar] [CrossRef]
- Lin, M.; Rippe, R.A.; Niemela, O.; Brittenham, G.; Tsukamoto, H.; Physiology, L. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am. J. Physiol.-Gastrointest. 1997, 272, G1355–G1364. [Google Scholar] [CrossRef]
- Xiong, S.; She, H.; Takeuchi, H.; Han, B.; Engelhardt, J.F.; Barton, C.; Zandi, E.; Giulivi, C.; Tsukamoto, H. Signaling role of intracellular iron in NF-κB activation. J. Biol. Chem. 2003, 278, 17646–17654. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986, 201, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Nemeth, E. Iron homeostasis in host defence and inflammation. Nat. Rev. Immunol. 2015, 15, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Litton, E.; Lim, J. Iron metabolism: An emerging therapeutic target in critical illness. Crit. Care 2019, 23, 81. [Google Scholar] [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014, 46, 678. [Google Scholar] [CrossRef]
- Litton, E.; Baker, S.; Erber, W.; Farmer, S.; Ferrier, J.; French, C.; Gummer, J.; Hawkins, D.; Higgins, A.; Hofmann, A. Hepcidin predicts response to IV iron therapy in patients admitted to the intensive care unit: A nested cohort study. J. Intensive Care 2018, 6, 60. [Google Scholar] [CrossRef]
- Litton, E.; Latham, P.; Inman, J.; Luo, J.; Allan, P. Safety and efficacy of erythropoiesis-stimulating agents in critically ill patients admitted to the intensive care unit: A systematic review and meta-analysis. Intensive Care Med. 2019, 45, 1190–1199. [Google Scholar] [CrossRef]
- Leuenberger, N.; Barras, L.; Nicoli, R.; Robinson, N.; Baume, N.; Lion, N.; Barelli, S.; Tissot, J.D.; Saugy, M. Hepcidin as a new biomarker for detecting autologous blood transfusion. Am. J. Hematol. 2016, 91, 467–472. [Google Scholar] [CrossRef]
- Lorenz, L.; Müller, K.F.; Poets, C.F.; Peter, A.; Olbina, G.; Westerman, M.; Franz, A.R. Short-term effects of blood transfusions on hepcidin in preterm infants. Neonatology 2015, 108, 205–210. [Google Scholar] [CrossRef]
- Herzlich, J.; Litmanovitz, I.; Regev, R.; Bauer, S.; Sirota, G.; Steiner, Z.; Arnon, S. Iron homeostasis after blood transfusion in stable preterm infants—An observational study. J. Perinat. Med. 2016, 44, 919–923. [Google Scholar] [CrossRef]
- Stripeli, F.; Kapetanakis, J.; Gourgiotis, D.; Drakatos, A.; Tsolia, M.; Kossiva, L. Post-transfusion changes in serum hepcidin and iron parameters in preterm infants. Pediatr. Int. 2018, 60, 148–152. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ye, X. The effect of red blood cell transfusion on plasma hepcidin and growth differentiation factor 15 in gastric cancer patients: A prospective study. Ann. Transl. Med. 2019, 7, 466. [Google Scholar] [CrossRef]
- Pasricha, S.-R.; Frazer, D.M.; Bowden, D.K.; Anderson, G.J. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with β-thalassemia major: A longitudinal study. Blood 2013, 122, 124–133. [Google Scholar] [CrossRef]
- Kitsati, N.; Liakos, D.; Ermeidi, E.; Mantzaris, M.D.; Vasakos, S.; Kyratzopoulou, E.; Eliadis, P.; Andrikos, E.; Kokkolou, E.; Sferopoulos, G. Rapid elevation of transferrin saturation and serum hepcidin concentration in hemodialysis patients after intravenous iron infusion. Haematologica 2015, 100, e80. [Google Scholar] [CrossRef] [PubMed]
- Daba, A.; Gkouvatsos, K.; Sebastiani, G.; Pantopoulos, K. Differences in activation of mouse hepcidin by dietary iron and parenterally administered iron dextran: Compartmentalization is critical for iron sensing. J. Mol. Med. 2013, 91, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Roberts, W.L.; Smith, P.T.; Martin, W.J.; Rainey, P.M. Performance characteristics of three serum iron and total iron-binding capacity methods in acute iron overdose. Am. J. Clin. Pathol. 1999, 112, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.B. Deferoxamine pharmacokinetics. Semin. Hematol. 2001, 38, 63–68. [Google Scholar] [CrossRef]
| Baseline | 2 Hours 1 | 4 h | 6 h | p Value * | |
|---|---|---|---|---|---|
| Hematocrits (L/L) | |||||
| Control | 0.44 (0.41–0.47) | 0.44 (0.42–0.46) | 0.41 (0.39–0.43) | 0.40 (0.38–0.42) | 0.95 |
| Treatment | 0.46 (0.44–0.48) | 0.47 (0.45–0.50) | 0.43 (0.41–0.45) | 0.42 (0.40–0.45) | |
| White blood cells (×109/L) | |||||
| Control | 4.73 (3.54–5.92) | 4.97 (3.68–6.26) | 3.96 (2.95–4.97) | 4.58 (2.67–6.49) | 0.23 |
| Treatment | 4.03 (3.35–4.71) | 4.52 (3.50–5.54) | 4.83 (3.98–5.68) | 5.50 (4.50–6.50) | |
| Neutrophils (×109/L) | |||||
| Control | 3.43 (2.46–4.40) | 3.73 (2.71–4.76) | 2.98 (2.14–3.82) | 3.89 (2.10–5.70) | 0.18 |
| Treatment | 2.81 (2.21–3.40) | 3.48 (2.56–4.41) | 3.91 (3.16–4.66) | 4.86 (3.90–5.83) | |
| Lymphocytes (×109/L) | |||||
| Control | 0.99 (0.68–1.29) | 0.90 (0.71–1.09) | 0.70 (0.55–0.85) | 0.50 (0.36–0.63) | 0.89 |
| Treatment | 0.99 (0.87–1.10) | 0.79 (0.62–0.95) | 0.62 (0.51–0.73) | 0.46 (0.35–0.57) | |
| Monocytes (×109/L) | |||||
| Control | 0.17 (0.11–0.23) | 0.22 (0.10–0.34) | 0.18 (0.12–0.24) | 0.13 (0.06–0.21) | 0.10 |
| Treatment | 0.12 (0.10–0.14) | 0.14 (0.11–0.17) | 0.21 (0.18–0.25) | 0.14 (0.09–0.19) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claus, M.A.; Smart, L.; Raisis, A.L.; Sharp, C.R.; Abraham, S.; Gummer, J.P.A.; Mead, M.K.; Bradley, D.L.; Van Swelm, R.; Wiegerinck, E.T.G.; et al. Effect of Deferoxamine on Post-Transfusion Iron, Inflammation, and In Vitro Microbial Growth in a Canine Hemorrhagic Shock Model: A Randomized Controlled Blinded Pilot Study. Vet. Sci. 2023, 10, 121. https://doi.org/10.3390/vetsci10020121
Claus MA, Smart L, Raisis AL, Sharp CR, Abraham S, Gummer JPA, Mead MK, Bradley DL, Van Swelm R, Wiegerinck ETG, et al. Effect of Deferoxamine on Post-Transfusion Iron, Inflammation, and In Vitro Microbial Growth in a Canine Hemorrhagic Shock Model: A Randomized Controlled Blinded Pilot Study. Veterinary Sciences. 2023; 10(2):121. https://doi.org/10.3390/vetsci10020121
Chicago/Turabian StyleClaus, Melissa A., Lisa Smart, Anthea L. Raisis, Claire R. Sharp, Sam Abraham, Joel P. A. Gummer, Martin K. Mead, Damian L. Bradley, Rachel Van Swelm, Erwin T. G. Wiegerinck, and et al. 2023. "Effect of Deferoxamine on Post-Transfusion Iron, Inflammation, and In Vitro Microbial Growth in a Canine Hemorrhagic Shock Model: A Randomized Controlled Blinded Pilot Study" Veterinary Sciences 10, no. 2: 121. https://doi.org/10.3390/vetsci10020121
APA StyleClaus, M. A., Smart, L., Raisis, A. L., Sharp, C. R., Abraham, S., Gummer, J. P. A., Mead, M. K., Bradley, D. L., Van Swelm, R., Wiegerinck, E. T. G., & Litton, E. (2023). Effect of Deferoxamine on Post-Transfusion Iron, Inflammation, and In Vitro Microbial Growth in a Canine Hemorrhagic Shock Model: A Randomized Controlled Blinded Pilot Study. Veterinary Sciences, 10(2), 121. https://doi.org/10.3390/vetsci10020121






