Abstract
Micronutrient deficiencies are of great public health and socioeconomic importance. Food fortification has been widely used as a simple low-cost resource to increase mineral intake. Considering that coffee is the most consumed food product worldwide, in this study, C. arabica and C. canephora seeds were roasted, ground, and fortified with three salts of iron, zinc, and calcium as part of the selection of appropriate mineral vehicles for fortification. After ranking the performance through a test by a trained tasters’ panel, only two salts for each mineral remained. Mineral recoveries were evaluated by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) in filtered (paper and nylon filters) and espresso brews. The best mean recoveries for each mineral in espresso brew prepared from fortified coffees were: 80.8% of iron as ferrous bisglycinate chelate, 75.4% of zinc as zinc lactate, and 72.1% of calcium as calcium lactate. These better ranked salts by the tasters’ panel. In filtered brews, mean recovery values of 51.1%, 47.6%, and 51.6% were obtained for the same mineral salts, respectively. No difference or very small differences were observed between species and types of filter. The results implications are discussed.
1. Introduction
Micronutrient deficiencies are of great public health and socioeconomic importance, affecting about 2 billion people worldwide [1,2]. These are problems that mostly affect developing countries, causing great impact on health and well-being and contributing to the increased risk of morbidity and mortality in populations [3]. Additionally, influenced by economic and income growth, urbanization, and globalization, a significant shift in the quality and quantity of human diets and nutrition-related epidemiology has occurred in the past few decades. Even at moderate levels, micronutrient deficiencies can exert serious detrimental effects on the human body. In addition to health effects, they have implications for economic and social development, especially with high costs of public health [4].
Iron deficiency is one of the main factors that lead to anemia, which affects 27% of the population (1.97 billion people). It is estimated that roughly 38% of pregnant women, 29% of non-pregnant women, and 29% of all women of reproductive age (including all social classes) have anemia globally, corresponding to 496 million non-pregnant women and 32 million pregnant women [5]. Iron plays an integral role in a wide range of physiological functions; therefore, the health consequences of iron deficiency and iron deficiency anemia in women are extensive and potentially serious if left untreated [6]. Symptoms are often nonspecific but can include fatigue, irritability, hair loss, poor concentration, palpitations, and dizziness. In severe cases of iron deficiency anemia, tachycardia, ankle edema, and heart failure may arise [7].
Zinc is one of the most important trace elements in living organisms and has three major biological functions: catalytic, structural, and regulatory. The human body mass contains 2–3 g of zinc, and approximately 57% and 29% of total body zinc exist in skeletal muscle and bone, respectively; heart and blood plasma are known to contain 0.4% and 0.1% of body zinc, respectively [8]. This is a multifunctional metal compatible with satisfactory growth, health, and well-being. It is essential for the structure and activity of various proteins and cellular components and plays an important role in human physiology from involvement in the proper function of the immune system to its importance in cellular growth, cell proliferation, and cell apoptosis, as well as in the activity of numerous zinc-binding proteins [9]. Based on the estimated prevalence of zinc deficiency, the global population at risk for inadequate zinc intake is up to 17%, while in South Asia, up to 30% of the inhabitants may be deficient [10].
Calcium as a nutrient is most commonly associated with the formation and metabolism of bone. Over 99% of total body calcium is found as calcium hydroxyapatite in bones and teeth, where it provides hard tissue with its strength. Calcium in the circulatory system, extracellular fluid, muscle, and other tissues is critical for mediating vascular contraction and vasodilatation, muscle function, nerve transmission, intracellular signaling, and hormonal secretion [11]. In developing countries, such as South Africa and Nigeria, for example, calcium deficiency is considered an important factor in the etiology of rickets [12]. Additionally, all over the world, inadequate calcium intake has been correlated with increased prevalence of diseases such as osteoporosis, systemic arterial hypertension (SAH), and colon cancer, regardless of social class [13].
Food fortification has been widely used by the food industry in high-, middle-, and low-income countries as a simple low-cost resource to increase mineral intake and prevent and/or correct nutritional deficiencies [14,15]. The foods chosen as fortification vehicles should be regularly consumed by the population and be easily accessible to them. Coffee meets these criteria. It is the most consumed beverage and food product in the world after water. According to the International Coffee Organization, the world consumption of coffee was about 9 million tons in 2016 (latest report). This volume represents an average annual growth rate of 1.6% since 2012 [16]. In the last decade, science has offered a whole new perspective on the use of coffee that is now considered by many as a functional food. A number of caffeine-related benefits of coffee drinking, such as enhancement of mental performance, including alertness [17], memory [18,19], mood [20,21], cognitive functions [22,23,24], and physical performance [25], are well known. Furthermore, studies have demonstrated the ability of coffee polyphenols, caffeine, and other coffee compounds to promote antioxidant and anti-inflammatory effects protecting the body against degenerative and chronic diseases such as type 2 diabetes and Alzheimer’s, cancer and liver diseases, in addition to other diseases [26,27].
Coffee’s beneficial health properties, together with its high consumption due to its relatively low cost, high accessibility, and high acceptance by populations, make it an excellent candidate for use as a micronutrient fortification vehicle. Additionally, in a previous study [28], we have shown that coffee can be considered an appropriate iron and zinc fortification vehicle, since the salts, ferrous fumarate, and zinc gluconate added to soluble coffee brews presented reasonable bioavailability, 58% and 78% for iron and zinc, respectively, when expressed relative to that of the mineral salts ingested with water. These bioavailability values are comparable to those observed for other foods fortified with such minerals [29,30]. However, in the referred study [28], the type of coffee used was soluble, which is not consumed by all segments of the world populations. Ground roasted coffee is predominantly consumed worldwide, representing about 77.4% of total consumption compared to 22.6% for soluble coffee in importing countries [31]. In Brazil, which is the largest coffee producer and exporter country in the world [16], only about 12% of the population consumes soluble coffee [32]. The same occurs worldwide, especially in those segments that most require fortification. Among the preparation methods, filtered and espresso are the two most used worldwide by different segments of populations [32].
There are a number of possible salts used as vehicles to increase mineral stability and bioavailability and they present different degrees of solubility. In view of the fact that the various existing coffee preparation methods have shown different efficiencies in extracting coffee solids, in order to develop a fortified ground roasted coffee, the efficiency of mineral salts extraction during the brew’s preparation must be considered. Therefore, in order to select the most suitable mineral for this purpose, the present study aimed to evaluate the recovery of iron, zinc, and calcium elements in filtered and espresso brews made from ground roasted coffees fortified with different mineral salts.
2. Methods
2.1. Preparation of Coffee Matrices
High quality raw Coffea arabica beans (Cooxupé, Minas Gerais, Brazil), classified as “soft beverage” or specialty coffee by the Brazilian classification system (COB), and a high quality Coffea canephora (Cooabriel, Espírito Santo, Brazil), were roasted separately in a laboratory-scale fluidized bed roaster (I-Roast® Model No. 40009, Heartware Home Products, Gurnee, IL, USA) at a maximum temperature of 245 °C for 6 min to reach medium roast degree (#55—Roast Color Classification System Agtron—SCAA, 1995). Samples were ground in a disk mill (Gourmet M-50, LEOGAP, Curitiba, PR, Brazil) to pass a 20 mesh (0.85 mm) sieve (medium grid). For a preliminary ranking sensory test, a blend containing 80% and 20% of the aforementioned C. arabica and C. canephora samples, respectively, was used. Ground samples were packaged, vacuum sealed, and kept at −20 °C until fortification and brewing. For the sensory test and evaluation of minerals recovery (item 2.4), fortification with each of the nine tested salts was performed separately.
2.2. Mineral Salts
When choosing mineral salts to be used in food fortification, their solubility, bioavailability, and sensory modifications in the food matrix must be considered [33]. Three types of salts of iron, zinc, and calcium were initially selected based on bioavailability and sensory aspects reported in previous fortification studies using various types of food matrixes [28,29,30,34,35,36,37,38,39]: ferrous bisglycinate chelate (Infiniti, São Paulo, Brazil), ferrous sulfate (Quimibras, Rio de Janeiro, Brazil), ferrous fumarate (Synth, São Paulo, Brazil), zinc lactate (Purac, Rio de Janeiro, Brazil), zinc bisglycinate chelate (Infiniti, São Paulo, Brazil), zinc sulfate heptahydrate (Synth, São Paulo, Brazil), calcium lactate (Purac, Rio de Janeiro, Brazil), tricalcium phosphate (Solutech, São Paulo, Brazil), and calcium citrate (Synth, São Paulo, Brazil). Salts were analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (item 2.6) for verification of compliance with salts labels information and they were all in conformity.
2.3. Fortification of Ground Roasted Coffee
Based on their the salts chemical structures and on the preliminary results from ICP-OES analyses, iron, zinc and calcium salts were weighted to obtain the concentration of each mineral corresponding to 30% of the National Agency for Sanitary Surveillance (ANVISA) Dietary Reference Intake (DRI) for adults (4.2 mg of iron, 2.1 mg of zinc and 300.0 mg of calcium per 100 g of ground roasted coffee, as the DRI for adults is 14 mg for iron, 7 mg for zinc, and 1000 mg for calcium) [40]. Considering the The United States Department of Agriculture (USDA) Nutrient Database for Standard Reference, the amount of salts added to 100 g of ground roasted coffee would correspond to 23.4% and 52.4% of iron RDI for women and men respectively, 26.2% and 19.0% of zinc, respectively, and 30% of calcium RDI for men and women equally. Fortification of ground roasted coffees was performed in the following way: after roasting and grinding coffee beans, 100 g were separately fortified with ferrous bisglycinate chelate, ferrous sulfate, zinc lactate, zinc bisglycinate chelate, calcium lactate, or tricalcium phosphate, in the amounts aforementioned, using geometric dilution, which is a technique used to obtain an equal distribution of low quantity particles (salts) within the blend with a large portion of powder (coffee) [41]. Six aliquots of each fortified coffee were collected randomly at different occasions for spectrometric analysis. The final mean SD was <2% of results per 100 g indicated that fortification was well performed.
2.4. Preliminary Sensory Test
A preliminary sensory test (ranking test) was carried out according to Meilgaard et al. [42] and Choi [43] to select two salts of iron, zinc, and calcium, out of three, to be used in the recovery tests considering their attributes of visual appearance and taste. Solubility was also considered when preparing the brews. The salts were diluted separately in hot water and in brewed coffee prepared in an electric coffee dripper, using the ground roasted blend described in item 2.1, in the proportion of 10 g of coffee per 100 mL of water at 95 °C. Coffee was served at about 60–65 °C, under white light. Water with mineral salts was served at room temperature (25 °C). For mouth wash between samples, water and crackers were served at room temperature. The brews, containing one mineral at a time, were offered to 10 trained coffee tasters (students and teachers) from the Federal University of Rio de Janeiro in randomly numbered plastic cups and they were asked to list attributes regarding appearance and taste for each brew. Based on taste, they were also asked to rank the three samples containing the different salts of the same mineral and the worse among them was excluded from the study.
2.5. Brews Preparation
The two most commonly used brewing methods worldwide, dripping (or filtered) and espresso [32], were used for recovery evaluation. Brews were prepared in duplicate for each sample. Unfortified (control) and fortified coffee brews were prepared at 10% (10 g of coffee per 100 mL of ultra-pure water at 90–95 °C) [32] using an electric coffee dripper (Britânia CB30, São Paulo, Brazil), with a paper filter (Mellita#103) or with a nylon filter (Britânia CB30). For filtered coffees, the average extraction time was 110 s for both filters. New paper filters were used and discarded after each extraction, while the nylon filter was thoroughly washed with boiling water. For espresso preparation, an espresso coffee maker (Royal Cappuccino, Saeco, Italy), operating in no infusion mode, a pressure of about 9 bar, and water temperature of 90 °C was used. Average extraction time for espresso was 40 s.
2.6. Minerals Analyses
For the determination of mineral contents in coffee powder, 250 mg of coffee powder were digested with 2.5 mL of 65% nitric acid (VETEC, Rio de Janeiro, RJ, Brazil), in a 90 °C water bath for 4 h. One milliliter of ultra-pure hydrogen peroxide 30–32% (VETEC) was added to stop the reaction. For brews, 1 mL of brew was digested with 1 mL of nitric acid, with no addition of hydrogen peroxide [44].
Analyses were performed in triplicate by an inductively coupled plasma optical emission spectrometer (ICP-OES), model 4300 DV (Perkin Elmer-Sciex, Norwalk, CT, USA). The simultaneous operation mode was applied and the optimized parameters for the quantification of Fe, Zn, and Ca elements were: plasma generator power 1.5 kW, auxiliary air flow 0.2 L/min, cooling air flow 15.0 L/min, air mist flow 0.45 L/min, and pump speed 1.50 mL/min. The wavelengths (λ) applied for readings were 259.94 nm for Fe, 206.20 nm for Zn, and 317.93 nm for Ca [44]. Quantitative calibration mode was used. Analytical curves were built using suitable dilutions of a multi-element aqueous standard solution of 1000 mg/L (Merck-IV; 23 elements, Merck, Darmstadt, Germany). Four calibration solutions with the following concentrations were used: 0.050, 0.100, 0.200, 0.500 mg/L (r = 0.99999 for Fe, 0.99999 for Zn, and 0.99998 for Ca). Samples were introduced through a conical concentric nebulizator with a cyclonic chamber (Glass Expansion, Melbourne, Vic, Australia) without previous filtration. Readings were performed in the automatic background correction mode. All reagents were of analytical grade. Ultra-pure water (resistivity of 18.2 MΩ, Milli Q system, Millipore, Bedford, MA, USA) was used for solutions preparation. The multi-element aqueous standard solution was also used for spectral interference tests. Limits of detection (LOD) calculated as 3 times the sample SD of ten blank readings of the calibration curve (ultra-pure water acidified with nitric acid) were 0.00014 mg/L for Fe, 0.0009 mg/L for Zn, and 0.0048 mg/mL for Ca. The limits of quantification (LOQ), calculated as 3.3 times the LOD value for the respective elements, were: 0.00046 mg/mL for Fe; 0.00297 mg/mL for Zn, and 0.01584 mg/mL for Ca.
2.7. Statistical Analyses
For statistical analysis, the Statistica software, version 12.0 (StatSoft, Tulsa, OK, USA) was used. Analysis of variance (ANOVA) followed by a Fisher test were used to compare mineral contents in ground roasted coffees and mineral concentrations in the unfortified (control) and fortified brews. Differences were considered when p ≤ 0.001.
3. Results and Discussion
3.1. Preliminary Sensory Test
Regarding the appearance of ground roasted coffees, after the addition of calcium lactate and calcium citrate, small whitish spots were observed in the coffee mixture, despite adequate homogenization. This fact was not observed when tricalcium phosphate was used for fortification. However, this salt showed low solubility, as precipitation was observed in the brew. No iron or zinc salts caused changes in the appearance of ground roasted coffees after fortification. Regarding the brews’ appearance, however, the use of iron salts altered their coloration to a greenish tone in the bottom of the cup. This fact may have occurred due to the iron oxidation, which can cause undesirable alterations associated with taste and appearance, including product color changes [45]. Color is reported to be one of the main parameters considered in iron fortification studies. Depending on the iron salt and concentration used, the food may darken, affecting its appearance and acceptance by consumers [46,47].
Attributes given by tasters to all salts were similar in water and coffee, but coffee seemed to slightly improve the sensory responses to mineral salts. According to all ten trained tasters, among the three evaluated salts for each mineral, the most well accepted ones (ranked first) when dissolved in both water and coffee were ferrous bisglycinate chelate, zinc lactate, and calcium lactate, although a slight metallic taste for ferrous bisclycinate, and slight astringency for zinc and calcium lactate were perceived, both in water and coffee. Ferrous fumarate, zinc sulfate heptahydrate, and calcium citrate salts were eliminated from the study due to their intense metallic (ferrous fumarate and zinc sulfate heptahydrate) or astringent (calcium citrate) tastes (Table 1). Ferrous fumate also presented low solubility. Despite the apparently lower solubility compared to ferrous bisglycinate and ferrous sulfate, zinc bisglycine chelate and tricalcium phosphate proceeded in the study to be compared with other salts in the recovery tests. Figure 1 illustrates the study design for the evaluation of minerals recovery in coffee brews after the preliminary sensory test.

Table 1.
Ranking and taste attributes described in preliminary screening evaluating mineral salts approval by trained tasters #.
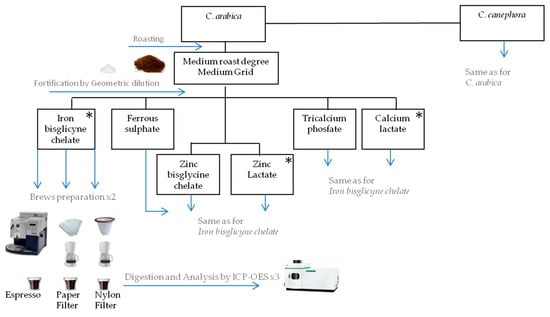
Figure 1.
Scheme for the evaluation of minerals recovery in coffee brews after preliminary sensory test. Salts indicated with stars were the most well accepted among salts remaining in the study.
3.2. Mineral Contents in Unfortified and Fortified Ground Roasted Coffees
Table 2 presents the mean contents of iron, zinc, and calcium in the unfortified and fortified ground roasted C. arabica and C. canephora samples used for the mineral recovery evaluation in the brews.

Table 2.
Mean contents of iron, zinc, and calcium, measured using ICP-OES, in the unfortified (control) and fortified ground roasted coffees used for recovery evaluation in brews §.
3.2.1. Unfortified (Control) Coffees
Despite the statistically significant differences between mineral contents of C. arabica and C. canephora (Table 2), such differences were very small (equal to or lower than 2.6%). This similarity between both species has been previously reported [48,49], although in a few studies, they have not occurred, possibly due to differences in soil composition, plant age, climatic differences, use of fertilizer during plant cultivation, and possibly, to different analytical methods [50,51].
Iron contents in C. arabica and C. canephora control samples (Table 2, mean of 5.68 ± 0.02 mg/100 g) were below those described in the Brazilian Food Composition Table (TACO) (8.10 mg/100 g) [52], also obtained using ICP-OES. On the other hand, Gogoasa et al. [49] analyzed five blends of C. arabica and C. canephora species and obtained a mean iron content of 3.63 ± 0.05 mg/100 g.
Zinc values found in the present study for C. arabica and C. canephora species (Table 2, mean of 0.83 ± 0.03 mg/100 g) were in the same magnitude than those reported in TACO (0.50 mg/100 g) [52] by Gogoasa et al. [49] (mean of 0.62 ± 0.02 mg/100 g for C. arabica and C. canephora species) and by Malik et al. [53] (mean of 0.70 ± 0.16 mg/100 g for C. arabica and C. canephora species).
With regard to calcium, the mean content obtained in the present study for C. arabica and C. canephora species (Table 2, mean of 131.74 ± 0.03 mg/100 g) was also in the same magnitude of those reported by TACO [52] for C. arabica species (107.00 mg/100 g), and of mean values reported by Malik et al. [53] (144.30 ± 24.9 mg/100 g) and Gogoasa et al. [49] (149.60 ± 0.03 mg/100 g) for C. arabica and C. canephora.
3.2.2. Fortified Coffees
The mean concentrations of iron, zinc, and calcium obtained in ground roasted coffees after fortification (Table 3) were consistent with the amount of minerals present in the salts used for fortification.

Table 3.
Mean concentrations of iron, zinc and calcium in brews from ground roasted, unfortified (control), and fortified C. arabica and C. canephora coffee species (mg/100 mL) §.
3.3. Mineral Concentrations in Unfortified and Fortified Coffee Brews
The mean concentrations of iron, zinc, and calcium in filtered and espresso coffee brews prepared from the unfortified and fortified ground roasted coffees are shown in Table 3 and Figure 2.
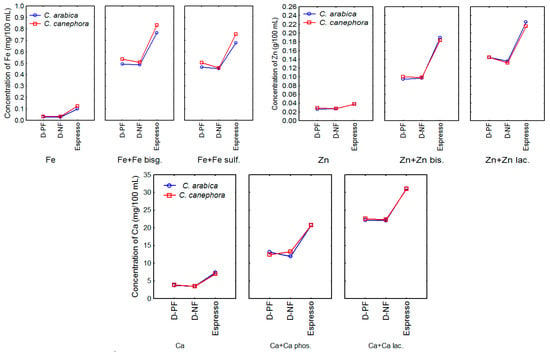
Figure 2.
Means plot considering the influence of different coffee species, brewing methods (dripper paper filter (D-PF), dripper nylon paper (D-NF) and espresso), and minerals. Fe bis—ferrous bisglycinate chelate, Fe sulf—ferrous sulfate; Zn bis—zinc bisglycinate chelate; Zn lac—zinc lactate; Ca phos—tricalcium phosphate; Ca lac—calcium lactate.
3.3.1. Brews from Unfortified Coffee Grounds
Results reported in Table 3 are in agreement with our previous results obtained by Costa [38] who reported a mean iron content of 0.109 mg/100 mL and zinc content of 0.033 mg/100 mL for coffees prepared using the espresso method. The calcium content was not evaluated in the referred study.
Regarding filtered brews, Malik et al. [53] reported mean values of 0.009, 0.002, and 0.914 mg/100 mL for iron, zinc, and calcium, respectively, in filtered brews from ground roasted coffees, lower than those found in the present study. Gillies and Birkbeck [54], on the other hand, reported mean concentrations of 0.033, 0.018, and 2.78 mg/100 mL, respectively, in paper-filtered coffee brews. Such differences among studies are common and can be attributed to the different ways of brewing coffees, including parameters applied in the extraction process, such as the proportion of coffee and water, temperature, water characteristics, and extraction time, as well as different characteristics of ground coffees and analytical methods [51,55].
In general, among the unfortified coffees, no difference was observed between mineral extraction from C. arabica and C. canephora species. Also, there was no difference or a very small difference in iron and zinc extractions using paper and nylon filters. On the other hand, the difference between filtered and espresso methods was remarkable, especially in the fortified coffees. Considering both species and filters, espresso method extracted 19.9%, 46.1%, and 54.6% of iron, zinc, and calcium, respectively, while dripping extracted 5.68%, 36.7%, and 27.7%, respectively. The differences in the extractability among the minerals existing in the coffee matrix, according to Donangelo [48], may be related to the nature and strength of the complexes that their ions form with the constituents of the matrix, such as polyphenols, caffeine, and other compounds, while higher extraction by espresso method can be attributed to the higher pressure (9 bar) applied to the brewing process compared to all other extraction methods, including the filtered coffee (little more than 1 bar) [51].
3.3.2. Brews from Fortified Ground Coffees
In order to calculate the mineral percent recovery in fortified coffees, both the coffee matrix mineral composition and the mineral amount used for fortification were considered. In fortified coffees, all variables contributed to differences in the results, including species, type of mineral salt, and extraction method, although only small differences were observed between results for C. arabica and C. canephora species and for paper and nylon filters (Table 3, Figure 2). In general, there was a tendency for higher values for paper filters, but in practical life, this difference would probably have no significance. Also, the difference in espresso method compared to filtered methods was considerably higher in fortified coffees (Figure 2).
The mean percentages of iron recovery in the espresso and filtered brews obtained from fortified coffees were 76.6% and 49.3%, respectively (Figure 3A). For zinc, the mean percentages recovered were 69.6% and 40.4%, respectively (Figure 3B), while for calcium, they were 60.1% and 40.5%, respectively (Figure 3C).
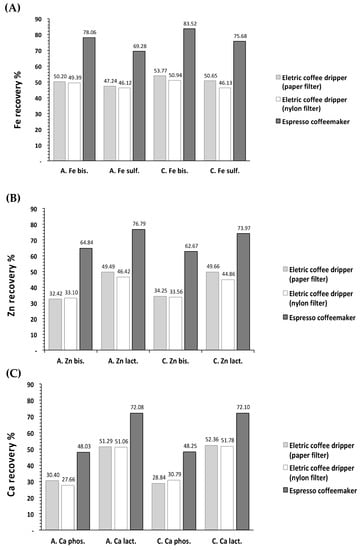
Figure 3.
Mean percent recovery of iron (A), zinc (B), and calcium (C) in brews prepared from fortified ground roasted C. arabica and C. canephora using an electric dripper (paper and nylon filters) and espresso machine. § Note: Recovery was calculated based on the sum of the amount of mineral existing in the matrix and the added amount of salt. A Fe bis—C. arabica, ferrous bisglycinate chelate; A. Fe sulf.—C. arabica, ferrous sulfate; C. Fe bis—C. canephora, ferrous bisglycinate chelate; C. Fe sulf.—C. canephora, ferrous sulfate.; A Zn bis—C. arabica, zinc bisglycinate chelate; A. Zn lact.—C. arabica, zinc lactate; C. Zn bis—C. canephora, zinc bisglycinate chelate; C. Zn lact.—C. canephora, zinc lactate; A Ca phos—C. arabica, calcium phosfate; C Ca phos—C. canephora, calcium phosfate; A Ca lact—C. arabica, calcium lactate; C Ca lact—C. canephora, calcium lactate.
The mineral salts with higher recoveries in both tested brewing methods were: ferrous bisglycinate chelate, zinc lactate, and calcium lactate, with mean recovery percentages of 80.8%, 75.4%, and 72.1%, respectively, in espresso brews, and of 51.1%, 47.6%, and 51.6%, respectively, in filtered brews (Figure 3A–C). These were the same salts that presented better attributes and ranking positions in the preliminary sensory test. Although ferrous sulfate presented mean recoveries (72.5% in the espresso coffees and 47.5% in filtered coffees) similar to ferrous bisglycinate chelate, it presented a more intense metallic flavor in the preliminary sensory test when compared to ferrous bisglycinate chelate, therefore it is not recommended.
Several studies on food fortification have shown that ferrous bisglycinate chelate is a recommended source of iron for food fortification as it is a soluble salt with high bioavailability, safety and stability in foods like milk, yogurt, and wheat flour [56,57,58,59].
Considering that Brazilians consume, on average, about 220 mL of coffee per day [60], it can be estimated that the consumption of 220 mL of the iron-fortified filtered brew would provide 1.336 mg, or about 9.5% of iron recommended daily intake (RDI), which is 14 mg according to ANVISA [40]. This is equivalent to 16.7% of men and 7.4% of women USDA Nutrient Database for Standard Reference (NDSR) [61] RDI for iron. Comparing iron content in iron-fortified coffee brews with those of other plant food sources, 247 mL of the fortified brew from this study is equivalent to 100 g of cooked black beans, containing about 1.5 mg of this mineral [52]. According to the Family Budget Survey (POF) published by the Brazilian Institute of Geography and Statistics and the Ministry of Health in 2008/2009, the mean habitual per capita consumption of wheat flour by Brazilian families is 75 g/day, where such flour theoretically contains about 3.1 mg/iron (about 2.5 times the amount contained in 220 mL filtered fortified coffee). Comparing coffee and fortified wheat flour, despite a higher amount of iron, unlike the fortified coffee brew, wheat flour presents in its composition phytates, which may considerably inhibit the absorption of iron in the intestinal lumen [62]. On the other hand, coffee polyphenols are also able to chelate iron, although to a lesser extent [63].
It has been previously reported that zinc lactate has higher solubility, stability, bioavailability, and a more neutral taste when compared to other zinc salts used in food fortification [64,65]. Zinc lactate added to strawberry jam in an amount equivalent to 50% of the RDI for Brazilian adults (3.5 mg Zn) tasted similar to conventional strawberry jam and presented good stability six months after jelly production [66]. Two hundred milliliters of filtered brew prepared with the present coffee fortified with zinc would provide, on average, 0.368 mg, or about 5.3% of zinc RDI [40]. Taking into account the USDA-NDSR [60] RDI for zinc, the provided amount of zinc would represent 3.3% RDI for men and 4.6% RDI for women. According to Araujo et al. [67] the inadequate consumption of this mineral can reach up to 30.0% of the Brazilian population. Zinc deficiency is also highly prevalent in other low- and middle-income countries. Thus, affected populations are at increased risk of growth retardation, diarrheal diseases, and respiratory tract infections. Micronutrient supplements, such as zinc, are therefore potentially important interventions in the context of reaching the United Nations Millennium Development Goals [68].
Calcium lactate has better solubility when compared to tricalcium phosphate. Calcium lactate is known to have good solubility, bioavailability, low cost, and neutral taste when compared to other calcium salts being widely used in food fortification [69,70]. In addition, Haro et al. [71] fortified juices with different calcium salts and observed that calcium lactate showed good stability in beverages over a period of 12 months. The calcium-enriched mango yogurt prepared after fortification of pasteurized yogurt mix with calcium lactate corresponding to 50 mg Ca/100 mL did not show significant differences in flavor, color, body, and texture scores when compared to the control [72]. Taking into account the consumption of 220 mL of filtered and espresso coffees, the calcium fortified coffee brews in the present study would provide, on average, 56.0 mg, corresponding to 5.6% of the Brazilian and American RDI of 1000 mg for adults (considering both ANVISA and USDA-NDSR) [40,61]. This content is equivalent to other plant food sources of calcium, such as kale, with a mean content of 65.5 mg of calcium per 50 g [52]. According to Araujo et al. [67], the inadequate consumption of this mineral can reach percentages up to 90.0% for both Brazilian men and women. Regarding the prevalence of calcium inadequacy in Europe, in most nutritional surveys reviewed by Viñas et al. [73], more than 20% of individuals presented intake below the estimated average requirement (EAR) defined by the Institute of Medicine of the United States. According to World Health Organization (WHO)/Food and Agriculture Organization (FAO), [2], the best indication of calcium adequacy especially for developing countries is probably provided by comparing dietary intakes with recommended nutrient intakes (RNI), despite the variability; and uncertainty in the recommended intakes for calcium. On the basis of the fact that intakes of dairy products are low, it is highly likely that low or very low calcium intakes are very common in developing countries and therefore they would benefit from coffee fortification.
Still regarding calcium, it has been reported in different studies that acute consumption of caffeine can increase the urinary excretion of this mineral and reduce bone formation, especially in those who do not consume coffee regularly. However, due to body mechanisms for adaptation, in regular coffee consumers, this increase in calcium excretion is lowered. Nawrot et al., [74] concluded in a review that caffeine intakes lower than 400 mg/day (3 to 4 100 mL coffee cups) does not have significant effects on bone status or calcium balance in individuals ingesting at least 800 mg calcium/day (this are the latest recommendations on the subject) [75]. Therefore, calcium fortification would help increase daily intake and help making up for eventual losses due to caffeine.
4. Conclusions and Final Considerations
Ferrous bisglycinate chelate, zinc lactate, and calcium lactate presented better recoveries in both dripping and espresso extraction methods compared to the other tested minerals. As ferrous sulphate, zinc bisglycinate chelate, and tricalcium phosphate presented very low recoveries during brewing, probably due to low solubility, these salts would not be recommended for fortification of ground roasted coffees. Among the salts with better recovery, zinc bisglycinate chelate and tricalcium phosphate presented very promising sensorial results when offered to tasters in the amount of 30% of Brazilian DRI, while ferrous bisglycinate chelate did not present satisfactory results. Therefore, the effect of lowering the amount of this salt, as well as new iron salts, should be evaluated in sensory tests. Also, the positive or negative effects of the salts’ association on the sensorial attributes and acceptance of coffee brews should be evaluated.
As previously mentioned coffee largely meets the prerequisites for use as a food fortification vehicle, being considered a popular food, widely consumed by the populations in general. Ground roasted coffee was selected as an option for fortification due to the lower cost compared to soluble coffee. Considering the loss of minerals observed in filtered coffee, which is most used by the mineral deficient segments of populations, and aiming the preparation of large quantities of the beverage for popular restaurants and governmental institutions, the fortification of soluble coffee could be an option since it does not present a loss of salts. However, given the very low cost of these mineral salts and the higher cost and lower acceptability of soluble coffee by this segment of the populations, fortification of ground roasted coffee is still a promising option. The higher extraction observed in espresso coffees indicates that fortification can be also performed in coffees targeting espresso coffee consumers of different social classes and countries who are also in need for diet supplementation. For example, women in their menopausal period could largely benefit from calcium fortification.
Regarding the possibility of toxicity, the tolerable upper intake level (UL) can be defined as the highest average daily nutrient intake that is likely to pose no risk of adverse health effects to almost all individuals in the general population [76]. For iron, zinc, and calcium, these limits would be 45 mg, 40 mg, and 2500 mg, respectively. Considering salts that obtained the best recovery results, ferrous bisglycinate chelate, zinc lactate, and calcium lactate, heavy coffee consumers (for example, 500 mL/day) would consume, on average, 2.52 mg of iron, 0.70 mg of zinc, and 111.44 mg of calcium through filtered brew from fortified coffee, or about 3.99 mg of iron, 1.10 mg of zinc, and 155.63 mg of calcium in the espresso brew. Therefore, neither of both methods would reach the UL for these minerals [77].
With the development of micro- and nano-encapsulation technologies, one may ask why traditional salts were used for ground coffee fortification (especially iron) since they have the disadvantage of possibly interacting with the food matrix and alter its sensory properties. However, considering filtering coffee is the most used preparation method by those who are in need of fortification, the authors hypothesized that in addition to being costlier, such particles, especially microparticles, could present higher retention in the paper or nylon filter. However, new experiments should be performed using such technologies for coffee fortification, especially nanoparticles, which can be very fine and may pass through these filters.
Considering that product development must include consumer acceptance, stability during storage, and microbiological safety analyses, these aspects will be approached in future studies.
Author Contributions
A.S. and A.F. wrote the manuscript; N.M.B., J.D.P.L., C.V., T.D.S.P., and A.F. were involved in the chemical and statistical analyses. A.F. and C.M.D. planned the study.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq, Brazil grant number 78118/2013-9) and the Rio de Janeiro State Research Support Foundation (FAPERJ: grant number E-34/2014#204810).
Acknowledgments
The authors would like to thank the coffee producers’ cooperatives COOXUPÉ (Minas Gerais, Brazil) and COOABRIEL (Espirito Santo, Brazil) for generously providing the C. arabica and C. canephora samples, respectively. The authors acknowledge scholarships provided by the National Council for Scientific and Technological Development (CNPq, Brazil reg.#309091/2016-0) and the Rio de Janeiro State Research Support Foundation (FAPERJ: E-02/2017#234092).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tulchinsky, T.H. Micronutrient Deficiency conditions: Global health issues. Public Health Rev. 2010, 32, 243–255. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines on Food Fortification with Micronutrients; WHO: Geneva, Switzerland, 2006; Available online: http://www.who.int/nutrition/publications/guide_food_fortification_micronutrients.pdf (accessed on 20 August 2018).
- Piccoli, N.B.; Grede, N.; Pee, S.; Singhkumarwong, A.; Roks, E.; Moench-Pfanner, R.; Bloem, M.W. Rice fortification: Its potential for improving micronutrient intake and steps required for implementation at scale. Food Nutr. Bull. 2012, 33, S360–S372. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Double Burden of Malnutrition—Policy Brief; WHO: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/handle/10665/255413/WHO-NMH-NHD-17.3-eng.pdf?ua=1 (accessed on 5 August 2018).
- Metas Mundiales de Nutritión 2025: Documento Normativo sobre Anemia; WHO: Geneva, Switzerland, 2017. Available online: https://www.who.int/nutrition/publications/globaltargets2025_policybrief_anaemia/es/ (accessed on 29 July 2018).
- McLean, E.; Cogswel, M.; Egli, I.; Wojdyla, D.; Benoist, B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.G.; Kadir, R.A.; Breymann, C.; Fraser, I.S.; Taher, I. Impact and management of iron deficiency and iron deficiency anemia in women’s health. Expert Rev. Hematol. 2018, 11, 727–736. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.C.; Loutsidou, A.S.; Chara, S.M. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, L.; Crane, J.S. Zinc Deficiency. StatPearls Publishing LLC: Florida, FL, USA, 2018. [Google Scholar]
- Ross, C.; Taylor, C.L.; Yaktine, A.L.; Del Valle, H.B. Dietary Reference Intakes for Calcium and Vitamin D; Institute of Medicine (US) Committee; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Prentice, A. Nutritional rickets around the world. J. Steroid Biochem. Mol. Biol. 2013, 136, 201–206. [Google Scholar] [CrossRef]
- US Department of Health & Human Services. National Institutes of Health, 2018. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/ (accessed on 16 October 2018).
- Marques, M.F.; Marques, M.M.; Xavier, E.R.; Gregório, E.L. Fortificação de alimentos: Uma alternativa para suprir as necessidades de micronutrientes no mundo contemporâneo. HU Revista 2012, 38, 79–86. [Google Scholar]
- De Pee, S. Proposing Nutrients and Nutrient Levels for Rice Fortification; Nutrition Advisory Office, World Food Programme: Rome, Italy, 2014. [Google Scholar]
- International Coffee Organization (ICO). Coffee Market Report; International Coffee Organization: London, UK, 2017. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 15 November 2018).
- Einöther, S.J.; Giesbrecht, T. Caffeine as an attention enhancer: Reviewing existing assumptions. Psychopharmacology 2013, 225, 251–274. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimers Dis. 2010, 20, S85–S94. [Google Scholar] [CrossRef]
- Borota, D.; Murray, E.; Keceli, G.; Chang, A.; Watabe, J.M.; Ly, M.; Toscano, J.P.; Yassa, M.A. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 2014, 17, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Sutherland, D.; Christopher, G. Effects of repeated doses of caffeine on mood and performance of alert and fatigued volunteers. J. Psychopharmacol. 2005, 19, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Thornton, J.A.; Adam, G.E.; Lieberman, H.R. Effects of 2 adenosine antagonists, quercetin and caffeine, on vigilance and mood. J. Clin. Psychopharmacol. 2010, 30, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Khan, F.; Lam, H. Epidemiologic evidence of a relationship between tea, coffee, or caffeine consumption and cognitive decline. Adv. Nutr. 2013, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K.; Carrière, I.; De Mendonça, A.; Portet, F.; Dartigues, J.F.; Rouaud, O.; Barberger-Gateau, P.; Ancelin, M.L. The neuroprotective effects of caffeine: A prospective population study (the three city study). Neurology 2007, 69, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Corley, J.; Jia, X.; Kyle, J.A.; Gow, A.J.; Brett, C.E.; Starr, J.M.; Mcneill, G.; Deary, I.J. Caffeine consumption and cognitive function at age 70: The Lothian Birth Cohort 1936 Study. Psychosom. Med. 2010, 72, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Taylor, L.; Willoughby, D.; Stout, J.; Graves, B.S.; et al. International society of sports nutrition position stand: Caffeine and performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef]
- Figueiredo, R.C. Enriquecimento de Café Solúvel com Ferro, Zinco e Ácido Fólico: Avaliação da Biodisponibilidade em Mulheres Adultas [Dissertação de Mestrado]; Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Giorgini, E.; Fisberg, M.; De Paula, R.A.C.; Ferreira, A.M.A.; Valle, J.; Braga, J.A.P. The use of sweet rolls fortified with iron bis-glycinate chelate in the prevention of iron deficiency anemia in preschool children. Archivos Latinoamericanos de Nutrición 2001, 1, 48–53. [Google Scholar]
- Rodrigues, J.E.F.G.; Pineda, O.; Name, J.J.; Sanchez, J.G. Effectiveness of iron bis-glycine chelate in chocolate drink in the control of iron deficiency in preschool children. Nutrire 2006, 31, 43–52. [Google Scholar]
- International Coffee Organization (ICO). Annual Review, 2011/2012. London, UK. Available online: http://www.ico.org/documents/cy2012-13/annual-review-2011-12e.pdf (accessed on 20 September 2018).
- Associação Brasileira da Indústria de Café. Tendências do Mercado de Café. 2016. Rio de Janeiro, Brasil. Available online: http://abic.com.br/src/uploads/2018/05/2016.pdf (accessed on 8 October 2018).
- Monteiro, M.A.; Minim, V.P.; Silva, A.F.; Chaves, J.B. Influência da torra sobre a aceitação da bebida café. Rev. Ceres 2010, 57, 145–150. [Google Scholar] [CrossRef]
- Umbelino, D.C.; Rossi, E.A.; Cardello, H.M.; Lepera, J.S. Aspectos tecnológicos e sensoriais do “iogurte” de soja enriquecido com cálcio. Ciência Tecnologia Alimentos 2001, 21, 276–280. [Google Scholar] [CrossRef]
- Tuma, R.B.; Yuyama, L.K.; Aguiar, J.; Marques, H. Impacto da farinha de mandioca fortificada com ferro aminoácido quelato no nível de hemoglobina de pré-escolares. Revista Nutrição 2003, 16, 29–39. [Google Scholar] [CrossRef]
- Casé, F.; Deliza, R.; Rosenthal, A.; Mantovani, D.; Felberg, I. Produção de “leite” de soja enriquecido com cálcio. Ciência Tecnologia Alimentos 2005, 25, 86–91. [Google Scholar] [CrossRef]
- Gonçalves, A.A.; Leindecker, T.; Biedrzycki, A. Suco de uva em pó fortificado com ferro. Alimentos Nutrição Araraquara 2008, 19, 177–181. [Google Scholar]
- Costa, L. Café Torrado e Moído Fortificado: Avaliação da Eficiência de Extração dos Minerais Adicionados e Análise Sensorial do Produto Desenvolvido [Dissertação de Mestrado]; Universidade Federal do Rio de Janeiro: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Lima, E.C.; Cardoso, M.H. Bebida de soja (Glycine Max) e acerola (Malpighia Punicifolia) enriquecida com cálcio. Alim. Nutr Araraquara 2012, 23, 549–553. [Google Scholar]
- Brasil Ministério da Saúde. Agência Nacional De Vigilância Sanitária. Resolução RDC nº 269, 22 de setembro de 2005; Regulamento técnico sobre a ingestão diária recomendada (IDR) de proteína, vitaminas e minerais; Ministério da Saúde: Brasília (DF), 2005.
- Alyami, H.; Dahmash, E.; Bowen, J.; Mohammed, A.R. An investigation into the effects of excipient particle size, blending techniques and processing parameters on the homogeneity and content uniformity of a blend containing low-dose model drug. PLoS ONE 2017, 12, e0178772. [Google Scholar] [CrossRef] [PubMed]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999; 387p. [Google Scholar]
- Choi, S.E. Sensory Evaluation. In Food Science: An Ecological Approach, 1st ed.; Edelstein, S., Ed.; Jones and Bartlett Learning LLC: Burlington, MA, USA, 2014. [Google Scholar]
- Wrobel, K. Determination of total aluminum, chromium, copper, iron, manganese, and nickel and their fractions leached to the infusions of black tea, green tea, Hibiscus sabdariffa, and Ilex paraguariensis (mate) by ETA-AAS. Biol. Trace Elem. Res. 2000, 78, 271–280. [Google Scholar] [CrossRef]
- Mehansho, H. Iron fortification technology development: New approaches. J. Nutr. 2006, 136, 1059–1063. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.R.; Ferreira, S.M.; Canniatti-Brazaca, S.G. Perfil sensorial e aceitabilidade de barras de cereais fortificadas com ferro. Alim. Nutr. 2009, 20, 95–106. [Google Scholar]
- Sousa, C.; Fernandes, B.C.; Fernandes, P.H. Characterization of lactic drink pasteurized with added iron. Rev. Teccen 2015, 8, 1–32. [Google Scholar]
- Donangelo, C.M. Minerals. In Coffee: Production, Quality and Chemistry; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 505–516. [Google Scholar]
- Gogoasa, L.; Pirvu, A.; Alda, M.; Ariana, V.; Maria, R.; Maria, B.D.; Diana, M.; Simion, B.; Gergen, L. The mineral contentofdifferentcoffeebrands. J. HFB 2013, 17, 68–71. [Google Scholar]
- Farnezi, M.M.; Silva, E.B.; Guimarães, P.T. Nutritional diagnosis of coffee plantations in the Upper Jequitinhonha Valley, Minas Gerais State, Brazil: DRIs norms and critical nutrient ranges. Revista Brasileira de Ciência do Solo 2009, 33, 969–978. [Google Scholar] [CrossRef]
- Farah, A. Coffee Constituents. In Coffee: Emerging Health Effects and Disease Prevention, 1st ed.; Chu, Y., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 21–57. [Google Scholar]
- TACO—Tabela Brasileira de Composição de Alimentos/NEPA—UNICAMP, 4th ed.NEPA-UNICAMP: Campinas, Brazil, 2011.
- Malik, J.; Szakova, J.; Drabek, O.; Balik, J.; Kokoska, L. Determination of certain micro and macro elements in plant stimulants and their infusions. Food Chem. 2008, 111, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.E.; Birkbeck, J.A. Tea and coffee as sources of some minerals in the New Zealand diet. Am. J. Clin. Nutr. 1983, 38, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Flaten, T.P. Aluminium in tea—Concentrations, speciation and bioavailability. Coord. Chem. Rev. 2002, 228, 385–395. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Ferrous glycinate (processed with citric acid). Chem. Tech. Assess. 2004. [Google Scholar]
- Drago, S.R.; Valencia, M.E. Effect of fermentation on iron, zinc, and calcium availability from iron-fortified dairy products. J. Food Sci. 2002, 67, 3130–3134. [Google Scholar] [CrossRef]
- Osman, A.K.; Al-Othaimeen, A. Experience with ferrous bis-glycinechelate as an iron fortificant in milk. Int. J. Nutr. Res. 2002, 72, 257–263. [Google Scholar] [CrossRef]
- Marchi, R.P.; Szarfarc, S.C.; Rodrigues, J.E. Consumption of fortified rice in prophylaxis of iron deficiency. Nutrire 2004, 28, 53–64. [Google Scholar]
- Cecafé, Brazilian Coffee Exporters Council. Available online: Https://www.cecafe.com.br/en/about-coffee/consumption/ (accessed on 9 October 2018).
- USDA Nutrient Database for Standard Reference. US Department of Agriculture, Agricultural Research Service. Nutrient Data Laboratory Home Page. Available online: http://www.nal.usda. gov/fnic/foodcomp (accessed on 19 November 2018).
- Mazariegos, M.; Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Lei, S.; Grunwald, G.K. Zinc absorption in Guatemalan school children fed normal or low-phytate maize. Am. J. Clin. Nutr. 2006, 83, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin. Nutr. 1989, 43, 547–557. [Google Scholar] [PubMed]
- Lohmann, P. Minerais na alimentação. Food Ingred. Brasil 2008, 4, 48–65. [Google Scholar]
- Brown, K.H.; Hambidge, M.; Ranum, P. Zinc fortification of cereal flours: Current recommendations and research needs. Food Nutr. Bull. 2010, 31, S62–S74. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B. Desenvolvimento de Produtos Alimentares Adicionados de ferro, Cálcio, Zinco e Carotenoides (Alfacaroteno e Betacaroteno) Como Proposta de Alimentos Enriquecidos ou Fontes Destes Nutrientes [Dissertação de Mestrado]; Universidade Federal Rural do Rio de Janeiro: Rio de Janeiro, Brazil, 2008. [Google Scholar]
- Araujo, M.C.; Bezerra, I.N.; Barbosa, F.S.; Junger, W.L.; Yokoo, E.M.; Pereira, R.A.; Sichieri, R. Macronutrient consumption and inadequate micronutrient intake in adults. Revista Saúde Pública 2013, 47, 177s–189s. [Google Scholar] [CrossRef]
- World Health Organization. World Health Report; WHO: Geneva, Switzerland, 2013. Available online: https://www.who.int/gho/publications/world_health_statistics/EN_WHS2013_Full.pdf (accessed on 10 November 2018).
- Gerstner, G. How to fortify beverages with calcium. Food Market. Technol. 2003, 10, 16–19. [Google Scholar]
- Elian, M.; Srianta, I.; Trisnawati, C.; Arisasmita, J.H. Effects of calcium fortification (calcium lactate gluconate) on the physicochemical and sensory properties of soy-corn milk. Int. J. Food Nutr. Public Health 2012, 5, 91–104. [Google Scholar]
- Haro, J.F.; Martínez, C.; Ros, G.; Vidal, M.L. Stability of calcium bioaccessibility and sensory parameters during the storage of fortified juices. Food Sci. Technol. Int. 2006, 12, 281–285. [Google Scholar] [CrossRef]
- Singh, G.; Mukumarappan, K. Influence of calcium fortification on sensory, physical and rheological characteristics of fruit yogurt. Food Sci. Technol. 2008, 41, 1145–1152. [Google Scholar] [CrossRef]
- Viñas, B.R.; Barba, L.R.; Ngo, J.; Gurinovic, M.; Novakovic, R.; Cavelaars, A.; de Groot, L.C.P.G.M.; van’t Veer, P.; Matthys, C.; Majem, L.S. Prevalence of Inadequate Nutrient Intakes in Europe. Ann. Nutr. Metab. 2011, 59, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef] [PubMed]
- De Paula-Lima, J.; Farah, A. Potential Negative Effects of Caffeine Consumption on Health. In Coffee: Consumption and Health Implications; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 493–512. [Google Scholar]
- Otten, J.; Hellwig, J.P.; Meyers, L.D. (Eds.) The National Academy of Sciences, Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academy of Sciences: Washington, D.C., WA, USA, 2006. [Google Scholar]
- Padovani, R.M.; Amaya-Farfán, J.; Colugnati, F.A.; Domene, S.M. Dietary reference intakes: Application of tables in nutritional studies. Rev. Nutr. 2006, 19, 741–760. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).