Migration of Bisphenol A from Can Coatings into Beverages at the End of Shelf Life Compared to Regulated Test Conditions
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Material
2.2. Migration Contact Experiments
2.3. Preparation of Food Samples
2.4. Extraction of Samples for the Determination of the Residual Content of BPA
2.5. Quantification of Bisphenol A
2.6. Recovery Experiments
2.7. Migration Modeling
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids). Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Executive summary. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Gehring, C.; Welle, F. Migration testing of polyethylene terephthalate: Comparison of regulated test conditions with migration into real food at the end of shelf life. Packag. Technol. Sci. 2018, 31, 771–780. [Google Scholar] [CrossRef]
- Regulation (EU) No 10/2011. Commission Regulation (EU) on Plastic Materials and Articles Intended to Come into Contact with Food; European Union: Brussel, Belgium, 2011. [Google Scholar]
- Commission Regulation (EU) 2018/213 of 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food and Amending Regulation (EU) No 10/2011 as Regards the Use of That Substance in Plastic Food Contact Materials; European Union: Brussel, Belgium, 2018.
- LOI no 2012-1442 du 24 Décembre 2012 Visant à la Suspension de la Fabrication, de L’importation, de L’exportation et de la Mise sur le Marché de Tout Conditionnement à Vocation Alimentaire Contenant du Bisphénol A (Act No. 2012-1442 of 24 December 2012 to Suspend the Manufacture, Import, Export and Placing on the Market of Any Food Packaging Containing Bisphenol A); Legifrance: Paris, France, 2012.
- European Standard EN 13130-1. Materials and Articles in Contact with Foodstuffs—Plastics Substances Subject to Limitation Part 1: Guide to Test Methods for the Specific Migration of Substances from Plastics to Foods and Food Simulants and the Determination of Substances in Plastics and the Selection of Conditions of Exposure to Food Simulants; European Committee for Standardisation (CEN): Brussel, Belgium, 2004. [Google Scholar]
- Rodiut, B.; Borgeat, C.H.; Cavin, S.; Fragniere, C.; Dudler, V. Application of finite element analysis (FEA) for the simulation of release of additives from multilayer polymeric packaging structures. Food Addit. Contam. 2005, 22, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Welle, F. A new method for the prediction of diffusion coefficients in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 129, 1845–1851. [Google Scholar] [CrossRef]
- Franz, R.; Welle, F. Migration measurement and modelling from poly(ethylene terephthalate) (PET) into softdrinks and fruit juices in comparison with food simulants. Food Addit. Contam. 2008, 25, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Franz, R.; Gmeiner, M.; Gruner, A.; Kemmer, D.; Welle, F. Diffusion behaviour of the acetaldehyde scavenger 2-aminobenzamide in polyethylene terephthalate for beverage bottles. Food Addit. Contam. 2016, 33, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ewender, J.; Welle, F. Determination of the activation energies of diffusion of organic molecules in poly(ethylene terephthalate). J. Appl. Polym. Sci. 2013, 128, 3885–3892. [Google Scholar] [CrossRef]
| Sample No | Description |
|---|---|
| 1a | empty cans, BPA-based epoxy coating, filling volume 250 mL |
| 1b | cans filled with energy drink, BPA-based epoxy coating, filling volume 250 mL |
| 2a | empty cans, BPA-based epoxy coating, filling volume 250 mL |
| 2b | cans filled with energy drink, BPA-based epoxy coating, filling volume 250 mL |
| 3a | empty cans, BPA-free epoxy coating, filling volume 330 mL |
| 3b | cans filled with coke, BPA-free epoxy coating, filling volume 330 mL |
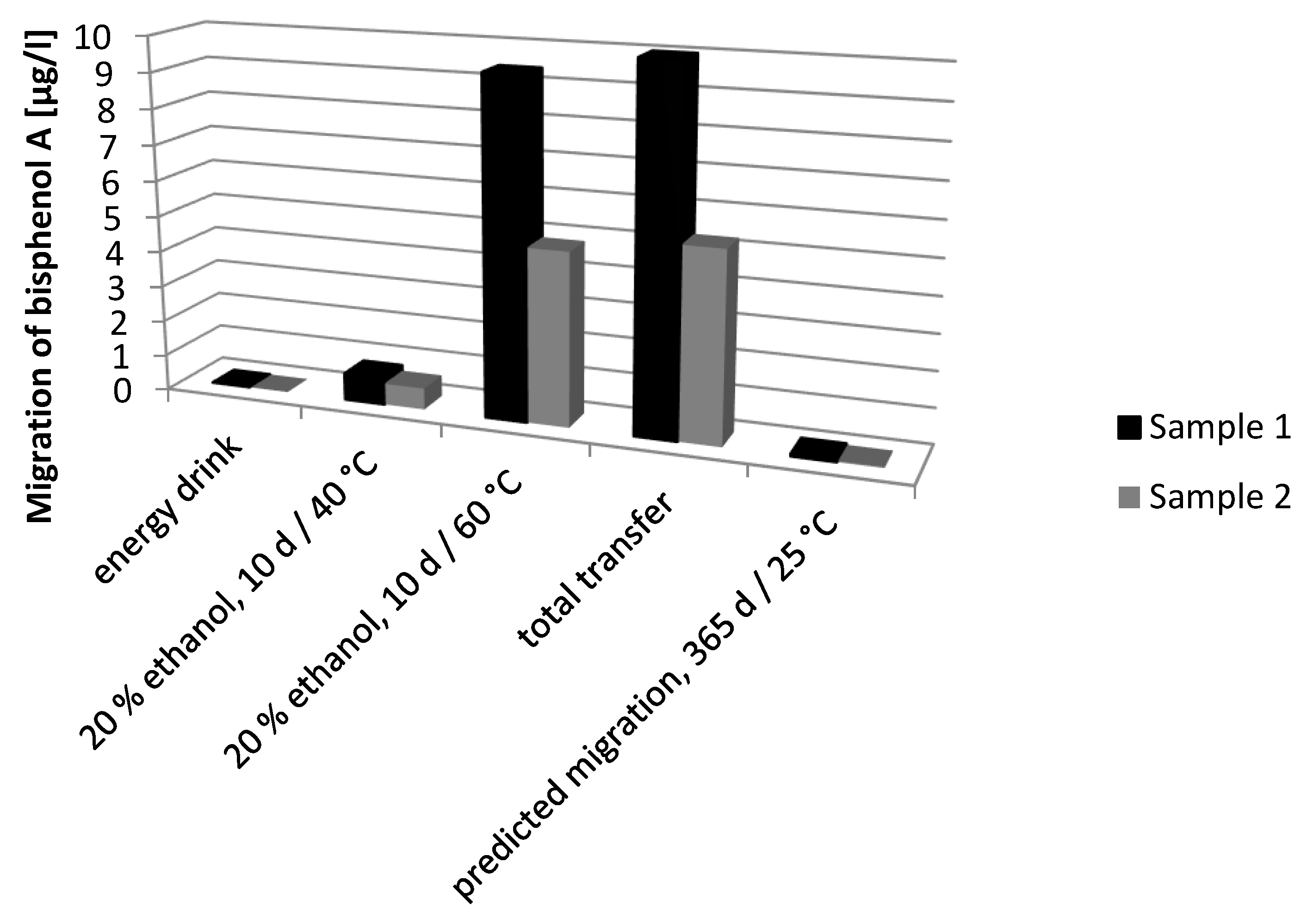
| Sample | Storage Conditions | Migration [µg/L] |
|---|---|---|
| 1a | 10 d at 60 °C | 9.40 ± 1.40 |
| 2a | 10 d at 60 °C | 4.84 ± 0.84 |
| 3a | 10 d at 60 °C | <0.99 |
| 1a | 10 d at 40 °C | 0.76 ± 0.22 |
| 2a | 10 d at 40 °C | 0.60 ± 0.07 |
| 3a | 10 d at 40 °C | <0.99 |
| 1b | end of shelf life, energy drink | <0.14 |
| 2b | end of shelf life, energy drink | <0.14 |
| 3b | end of shelf life, coke | <0.14 |
| Sample | Migration Potential (µg/dm2) | Calculated Total Transfer [µg/L] |
|---|---|---|
| 1 | 1.00 | 10.2 |
| 2 | 0.52 | 5.3 |
| 3 | <0.16 | <1.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stärker, C.; Welle, F. Migration of Bisphenol A from Can Coatings into Beverages at the End of Shelf Life Compared to Regulated Test Conditions. Beverages 2019, 5, 3. https://doi.org/10.3390/beverages5010003
Stärker C, Welle F. Migration of Bisphenol A from Can Coatings into Beverages at the End of Shelf Life Compared to Regulated Test Conditions. Beverages. 2019; 5(1):3. https://doi.org/10.3390/beverages5010003
Chicago/Turabian StyleStärker, Carina, and Frank Welle. 2019. "Migration of Bisphenol A from Can Coatings into Beverages at the End of Shelf Life Compared to Regulated Test Conditions" Beverages 5, no. 1: 3. https://doi.org/10.3390/beverages5010003
APA StyleStärker, C., & Welle, F. (2019). Migration of Bisphenol A from Can Coatings into Beverages at the End of Shelf Life Compared to Regulated Test Conditions. Beverages, 5(1), 3. https://doi.org/10.3390/beverages5010003





