Consumption of Chlorogenic Acids through Coffee and Health Implications
Abstract
1. Introduction
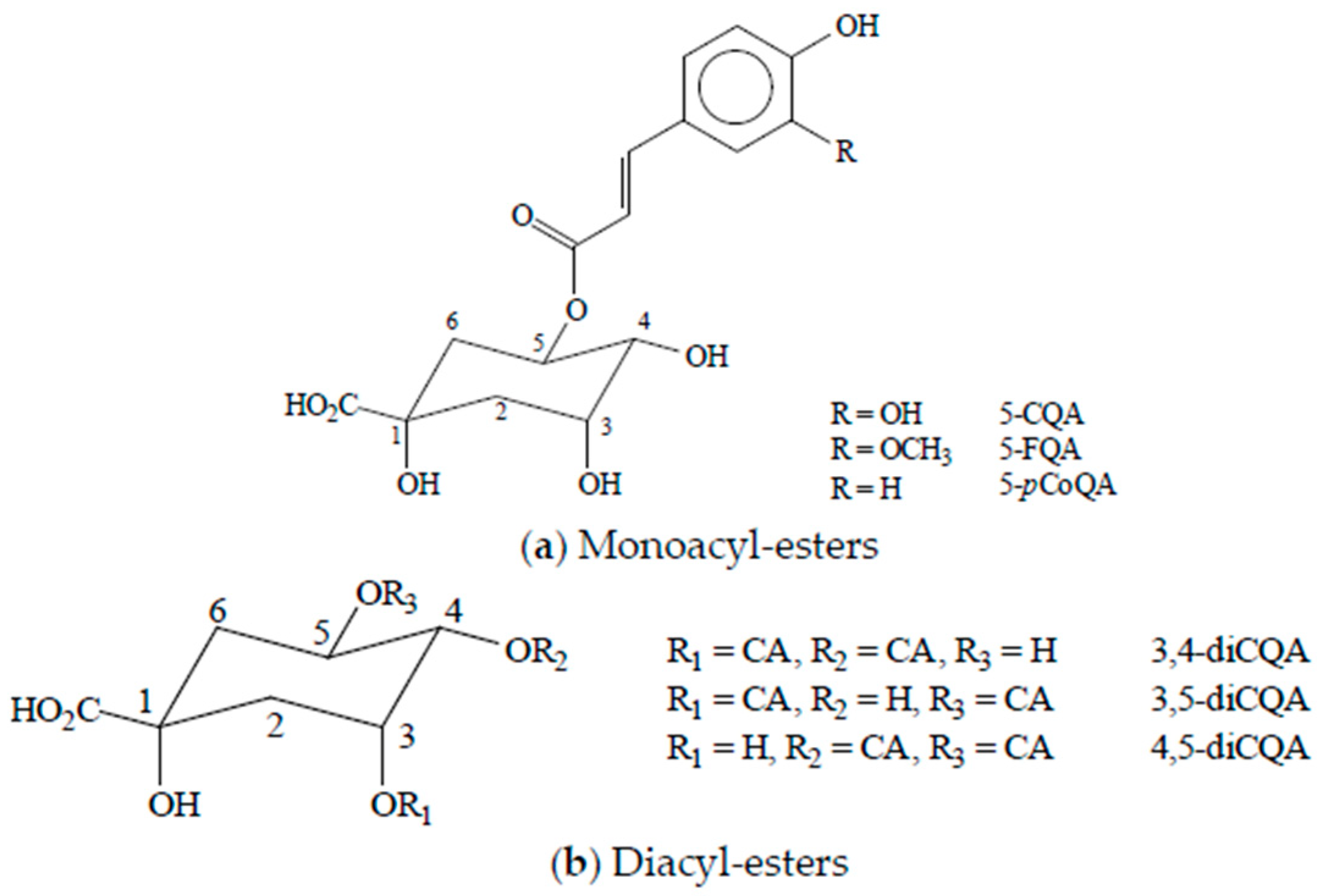
2. Chlorogenic Acids Levels in Coffee Beverages and Estimated Daily Consumption through Coffee
3. Bioavailability of Chlorogenic Acids and Lactones and Interaction with Other Food Components
4. Effects of Chlorogenic Acids on Human Body and Health
Antioxidant Activity
Anti-Inflammatory Effect and Wound Healing
Antimutagenic and Anticarcinogenic Effects
Hepatoprotective Effect
Anti-Diabetic Effect
Cardioprotective and Antihypertensive Effects
Antiobesity and Anti-Metabolic Syndrome Effects
Neuroprotective Effects
Antimicrobial Effect
Potential Prebiotic Effect
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Robiquet, P.J. Ueber den Kaffee. Ann. Pharm. 1837, 23, 93–95. [Google Scholar] [CrossRef]
- Barnes, H.M.; Feldman, J.R.; White, W.V. Isochlorogenic Acid. Isolation from Coffee and Structure Studies. J. Am. Chem. Soc. 1950, 72, 4178–4182. [Google Scholar] [CrossRef]
- IUPAC. Nomenclature of cyclitols. Biochem. J. 1976, 153, 23–31. [Google Scholar] [CrossRef]
- Moores, R.G.; McDermott, D.L.; Wood, T.R. Determination of Chlorogenic Acid in Coffee. Anal. Chem. 1948, 20, 620–624. [Google Scholar] [CrossRef]
- Kremr, D.; Bajer, T.; Bajerová, P.; Surmová, S.; Ventura, K. Unremitting problems with chlorogenic acid nomenclature: A review. Quim. Nova 2016, 39, 530–533. [Google Scholar] [CrossRef]
- Ukers, W.H. All About Coffee; The Tea and Coffee Trade Journal Company: New York, NY, USA, 1922; p. 796. [Google Scholar]
- Corse, J.R.E.; Lundin, A.C.; Wais, J.R. Identification of several components of isochlorogenic acid. Phytochemistry 1965, 4, 527–529. [Google Scholar] [CrossRef]
- Trugo, L.C. HPLC in Coffee Analysis. Ph.D. Thesis, University of Reading, Reading, UK, 1984. [Google Scholar]
- Wynne, K.N.; Familari, M.; Jaroslav, H.; Boublik, J.; Drummer, O.H.; Rae, I.D.; Funder, J.W. Isolation of opiate receptor ligands in coffee. Clin. Exp. Pharmacol. Physiol. 1987, 14, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Bottcher, B.M.; Maier, H.G. Isomers of quinic acid and quinides in roasted coffee: Indicators for the degree of roast’? In Proceedings of the International Conference on Coffee Science (ASIC), San Francisco, CA, USA, 14–19 July 1991.
- Bennat, C.; Engelhardt, U.H.; Kiehne, A.; Wirries, F.M.; Maier, H.G.Z. HPLC Analysis of chlorogenic acid lactones in roasted coffee. Zeitschrift für Lebensmittel-Untersuchung und Forschung 1994, 199, 17–21. [Google Scholar] [CrossRef]
- Schrader, K.; Kiehne, A.; Engelhardt, U.H.; Maier, H.G. Determination of chlorogenic acids with lactones in roasted coffee. J. Sci. Food Agric. 1996, 71, 392–398. [Google Scholar] [CrossRef]
- De Paulis, T.; Commers, P.; Farah, A.; Zhao, J.; McDonald, M.P.; Galici, R.; Martin, P.R. 4-Caffeoyl-1,5-quinide in roasted coffee inhibits [3H]naloxone binding and reverses anti-nociceptive effects of morphine in mice. Psychopharmacology 2004, 176, 146–153. [Google Scholar] [CrossRef]
- De Paulis, T.; Schmidt, D.E.; Bruchey, A.K.; Kirby, M.T.; McDonald, M.P.; Commers, P.; Lovinger, D.M.; Martin, P.R. Dicinnamoylquinides in roasted coffee inhibit the human adenosine transporter. Eur. J. Pharmacol. 2002, 442, 215–223. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S. The cinnamoyl–amino acid conjugates of green robusta coffee beans. Food Chem. 2004, 87, 457–463. [Google Scholar] [CrossRef]
- Clifford, M.N.; Marks, S.; Knight, S.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. J. Agric. Food Chem. 2006, 54, 4095–4101. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Surucu, B.; Kuhnert, N. Characterization by LC-MS(n) of four new classes of chlorogenic acids in green coffee beans: Dimethoxycinnamoylquinic acids, diferuloylquinic acids, caffeoyl-dimethoxycinnamoylquinic acids, and feruloyl-dimethoxycinnamoylquinic acids. J. Agric. Food Chem. 2006, 54, 1957–1969. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Hierarchical scheme for liquid chromatography/multi-stage spectrometric identification of 3,4,5-triacyl chlorogenic acids in green Robusta coffee beans. Rapid Commun. Mass Spectrom. 2010, 24, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MS(n): Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Karaköse, H.; Jaiswal, R. Analysis of chlorogenic acids and other hydroxycinnamates in food, plants, and pharmacokinetic studies. In Handbook of Analysis of Active Compounds in Functional Foods; Nollet, L.M.L., Toldrae, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 461–506. ISBN 9781439815885. [Google Scholar]
- Matei, M.F.; Jaiswal, R.; Kuhnert, N.J. Investigating the chemical changes of chlorogenic acids during coffee brewing: Conjugate addition of water to the olefinic moiety of chlorogenic acids and their quinides. J. Agric. Food Chem. 2012, 60, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef]
- Abrankó, L.; Clifford, M.N. An unambiguous nomenclature for the acyl-quinic acids commonly known as chlorogenic acids. J. Agric. Food Chem. 2017, 65, 3602–3608. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Lima, J.P. Major chlorogenic acids’ contents and distribution in coffees. In Coffee: Production, Quality and Chemistry; Royal Society of Chemistry: London, UK, 2018; in press; ISBN 978-1782620044. [Google Scholar]
- Tfouni, S.A.V.; Carreiro, L.B.; Teles, C.R.A.; Furlani, R.P.Z.; Cipolli, K.M.V.A.B.; Camargo, M.C.R. Caffeine and chlorogenic acids intake from coffee brew: Influence of roasting degree and brewing procedure. Int. J. Food Sci. Technol. 2014, 49, 747–752. [Google Scholar] [CrossRef]
- Niseteo, T.; Komes, D.; Belščak-Cvitanović, A.; Horžić, D.; Budeč, M. Bioactive composition and antioxidant potential of different commonly consumed coffee brews affected by their preparation technique and milk addition. Food Chem. 2012, 134, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Crozier, T.W.M.; Stalmach, A.; Lean, M.E.J.; Crozier, A. Espresso coffees, caffeine and chlorogenic acid intake: Potential health implications. Food Funct. 2012, 3, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Leand, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res. Int. 2018, in press. [Google Scholar] [CrossRef]
- Bastos, I.P.N. Influência dos métodos de preparo e granulometria do café sobre os teores de ácidos clorogênicos e lactonas na bebida e sobre a preferência do consumidor. Master’s Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Farah, A.; Lima, J.P. Chlorogenic acids: Daily consumption through coffee, metabolism and potential health effects. In Coffee: Consumption and Health Implications; Royal Society of Chemistry: London, UK, 2018; in press; ISBN 978-1782620044. [Google Scholar]
- Farah, A. Nutritional and health effects of coffee. In Achieving Sustainable Cultivation of Coffee; Lashermes, P., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 1–31. ISBN 978-1-78676-152-1. [Google Scholar]
- Folmer, B.; Farah, A.; Jones, L.; Fogliano, V. Human Wellbeing, Sociability, Performance and Health. In The Craft and Science of Coffee; Folmer, B., Ed.; Elsevier: Cambridge, UK, 2017; pp. 493–520. [Google Scholar]
- Trugo, L.C.; Macrae, R. Chlorogenic acid composition of instant coffees. Analyst 1984, 109, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chemical and physical aspects of green coffee and coffee products. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Chroom Helm: Sydney, Australia, 1987; pp. 305–374. ISBN 978-1-4615-6657-1. [Google Scholar]
- Fujioka, K.; Shibamoto, T. Quantitation of volatiles and nonvolatile acids in an extract from coffee beverages: Correlation with antioxidant activity. J. Agric. Food Chem. 2006, 54, 6054–6058. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.S.; Kim, H.T.; Jeong, I.H.; Hong, S.R.; Oh, M.S.; Park, K.H.; Shim, J.H.; Abd El-Aty, A.M. Determination of chlorogenic acids and caffeine in homemade brewed coffee prepared under various conditions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1064, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Juániz, I.; Monente, C.; Caemmererb, B.; Krohb, L.W.; Paz De Peña, M.; Cid, C. Evaluation of spent coffee obtained from the most common coffeemakers as a source of hydrophilic bioactive compounds. J. Agric. Food Chem. 2012, 60, 12565–12573. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Rocha, L.; Alves, A. Quantification of caffeoylquinic acids in coffee brews by HPLC-DAD. J. Anal. Methods Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.; Rao, N.Z. The effect of time, roasting temperature, and grind size on caffeine and chlorogenic acid concentrations in cold brew coffee. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.Z.; Fuller, M. Acidity and antioxidant activity of cold brew coffee. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Duarte, G. Metabolism and bioavailability of coffee chlorogenic acids in humans. In Coffee and Health Disease Prevention; Preedy, V.R., Ed.; Elsevier: Cambridge, UK, 2015; pp. 789–812. ISBN 9780124167162. [Google Scholar]
- Nogueira, M.; Trugo, L.C. Distribuição de isômeros de ácido clorogênico e teores de cafeína e trigonelina em cafés solúveis brasileiros. Ciênc. Tecnol. Aliment. 2003, 23, 296–299. [Google Scholar] [CrossRef]
- Duarte, G.S.; Farah, A. Chlorogenic acids and lactones in Brazilian commercial coffee. In Proceedings of the International Conference on Coffee Science (ASIC), Trieste, Italy, 14–18 May 2009. [Google Scholar]
- Sanchez-Bridge, B.; Renouf, M.; Sauser, J.; Beaumont, M.; Actis-Goretta, L. The roasting process does not influence the extent of conjugation of coffee chlorogenic and phenolic acids. Biofactors 2016, 42, 259–267. [Google Scholar] [CrossRef]
- Lima, J.P.; Farah, A. Contribution of foods for habitual daily intake of methylxanthines in Rio de Janeiro. In Proceedings of the International Conference on Coffee Science (ASIC), Armenia, Colombia, 8–13 September 2014. [Google Scholar]
- Mills, C.E.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. The effect of processing on chlorogenic acid content of commercially available coffee. Food Chem. 2013, 141, 3335–3340. [Google Scholar] [CrossRef]
- Floegel, A.; Pischon, T.; Bergmann, M.M.; Teucher, B.; Kaaks, B.; Boeing, H. Coffee consumption and risk of chronic disease in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Am. J. Clin. Nutr. 2012, 95, 901–908. [Google Scholar] [CrossRef]
- Torres, T.; Farah, A. Coffee, maté, açaí and beans are the main contributors to the antioxidant capacity of Brazilian’s diet. Eur. J. Nutr. 2017, 56, 1523–1533. [Google Scholar] [CrossRef]
- International Coffee Organization. World Coffee Consumption. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on November 18th 2018).
- Park, S.Y.; Freedman, N.D.; Haiman, C.A.; Marchand, L.L.; Wilkens, L.R.; Setiawan, V.W. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann. Intern. Med. 2017, 167, 228–235. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Bhatti, S.K.; Patil, H.R.; DiNicolantonio, J.J.; Lucan, S.C.; Lavie, C.J. Effects of habitual coffee consumption on cardiometabolic disease, cardiovascular health, and all-cause mortality. J. Am. Coll. Cardiol. 2013, 62, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Scientific American. How Is Caffeine Removed to Produce Decaffeinated Coffee? Available online: https://www.scientificamerican.com/article/how-is-caffeine-removed-t/ (accessed on 18 November 2018).
- Saura-Calixto, F.; Goni, I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006, 94, 442–447. [Google Scholar] [CrossRef]
- Brazilian Institute of Geography and Statistics. 2017. Available online: https://www.ibge.gov.br/apps/populacao/projecao/ (accessed on 18 November 2018).
- Grosso, G.; Stepaniak, U.; Topor-Madry, R.; Szafraniec, K.; Pajak, A. Estimated dietary intake and major food sources of polyphenols in the Polish arm of the HAPIEE study. Nutrition 2010, 30, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- The Environment and Food Agency of Iceland. Caffeine Consumption in Iceland in 2002; The Environment and Food Agency of Iceland, Office of Food: Reykjavík, Iceland, 2004.
- Svilaas, A.; Sakhi, A.K.; Andersen, L.F.; Svilaas, T.; Ström, E.C.; Jacobs, D.R.; Blomhoff, R. Intakes of antioxidants in coffee, wine, and vegetables are correlated with plasma carotenoids in humans. J. Nutr. 2004, 134, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Ruusunen, A.; Lehto, S.M.; Tolmunen, T.; Mursu, J.; Kaplan, G.A.; Voutilainen, S. Coffee, tea and caffeine intake and the risk of severe depression in middle-aged Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J. Nutr. 2004, 134, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Bizzo, M.L.G.; Farah, A.; Kemp, J.A.; Scancetti, L.B. Highlights in the History of Coffee Science Related to Health. In Coffee and Health Disease Prevention; Preedy, V.R., Ed.; Elsevier: Cambridge, UK, 2015; pp. 11–17. ISBN 9780124167162. [Google Scholar]
- Booth, A.N.; Emerson, O.H.; Jones, F.T.; Deeds, F. Urinary metabolites of caffeic and chlorogenic acids. J. Biol. Chem. 1957, 229, 51–59. [Google Scholar] [PubMed]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Bourne, L.C.; Rice-Evans, C.A. Urinary detection of hydroxycinnamates and flavonoids in humans after high dietary intake of fruit. Free Radic. Res. 1998, 28, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Dupas, C.; Baglieri, A.M.; Ordonaud, C.; Tom, D.; Maillard, M. Chlorogenic acid is poorly absorbed, independently of the food matrix: A Caco-2 cells and rat chronic absorption study. Mol. Nutr. Food Res. 2006, 50, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007, 137, 2196–2221. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.; Marmet, C.; Longet, K.; Fraering, A.; Moulin, J.; Barron, D.; Dionisi, F.; Cavin, C.; Steiling, H.; et al. Plasma appearance and correlation between coffee and green tea metabolites in human subjects. Br. J. Nutr. 2010, 104, 1635–1640. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Williamson, G.; Renouf, M.; Marmer, C.; Stiling, H.; Dionisi, F.; Barron, D.; Melcher, R.; Richling, E. Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol. Nutr. Food Res. 2012, 56, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Williamson, G.; Crozier, A. Impact of dose on the bioavailability of coffee chlorogenic acids in humans. Food Funct. 2014, 5, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Marmet, C.; Giuffrida, F.; Lepage, M.; Barron, D.; Beaumont, M.; Williamson, G.; Dionisi, F. Dose-response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol. Nutr. Food Res. 2014, 58, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Nakamura, S.; Kondou, N.; Takasu, Y.; Ochiai, R.; Masukawa, Y. Liquid chromatography-electrospray ionization-tandem mass spectrometry for simultaneous analysis of chlorogenic acids and their metabolites in human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 858, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic acids are absorbed from the rat stomach with different absorption rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef]
- Farrel, T.L.; Dew, T.P.; Poquet, L.; Hanson, P.; Williamson, G. Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab. Dispos. 2011, 39, 2338–2346. [Google Scholar] [CrossRef]
- Konishi, Y.; Shimizu, M. Transepithelial transport of ferulic acid by monocarboxylic acid transporter in Caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2003, 67, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, M.A.; Goldman, P. Caffeic acid metabolism by bacteria of the human gastrointestinal tract. J. Bacteriol. 1971, 108, 996–1000. [Google Scholar] [PubMed]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, 48–66. [Google Scholar] [CrossRef]
- Reduil, K.; Smarrito-Menozzi, C.; Guy, P.; Rezzi, S.; Dionisi, F.; Williamson, G.; Nagy, K.; Renouf, M. Identification of novel circulating coffee metabolites in human plasma by liquid chromatography-mass spectrometry. J. Cromatogr. A 2011, 1218, 4678–5688. [Google Scholar] [CrossRef]
- Gomez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of hydroxycinnamates in an instant green/roasted coffee blend in humans. Identification of novel colonic metabolites. Food Funct. 2018, 9, 331–343. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kroll, J.; Hohl, U.C. Model studies on reactions of plant phenols with whey proteins. Nahrung 2001, 45, 72–81. [Google Scholar] [CrossRef]
- Sinisi, V.; Forzato, C.; Cefarin, N.; Navarini, L.; Berti, F. Interaction of chlorogenic acids and quinides from coffee with human serum albumin. Food Chem. 2015, 168, 332–340. [Google Scholar] [CrossRef]
- Hollman, C.; Van Het Hof, K.H.; Tijburg, L.B.; Katan, M.B. Addition of milk does not affect the absorption of flavonols from tea in man. Free Radic. Res. 2001, 34, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Andres-Lacueva, C.; Estruch, R.; Mata-Bilbao, M.L.; Izquierdo-Pulido, M.; Waterhouse, A.L.; Lamuela-Raventos, R.M. Milk does not affect the bioavailability of cocoa powder flavonoid in healthy human. Ann. Nutr. Metab. 2007, 51, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Duarte, G.S.; Farah, A. Effect of simultaneous consumption of milk and coffee on chlorogenic acids’ bioavailability in humans. J. Agric. Food Chem. 2011, 59, 7925–7931. [Google Scholar] [CrossRef]
- Renoulf, M.; Marmet, C.; Guy, P.; Fraering, A.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Cavin, C.; Dionisi, F.; et al. Nondairy creamer, but not milk, delays the appearance of coffee phenolic acid equivalents in human plasma. J. Nutr. 2010, 140, 259–263. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Scherbl, D.; Renouf, M.; Marmet, C.; Poquet, L.; Cristiani, I.; Dahbane, S.; Emandy-Azar, S.; Sauser, J.; Galan, J.; Dionisi, F.; et al. Breakfast consumption induces retarded release of chlorogenic acid metabolites in humans. Eur. Food Res. Technol. 2017, 243, 791–806. [Google Scholar] [CrossRef]
- Felberg, I.; Farah, A.; Monteiro, M.C.; Godoy, R.L.O.; Pacheco, S.; Calado, V.; Donangelo, C.M.J. Effect of simultaneous consumption of soymilk and coffee on the urinary excretion of isoflavones, chlorogenic acids and metabolites in healthy adults. Func. Foods 2015, 19, 688–699. [Google Scholar] [CrossRef]
- Budryn, G.; Pałecz, B.; Rachwał-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Belica, S.; Navarro-González, I.; Meseguer, J.M.; Pérez-Sánchez, H. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in β-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Marques, V.; Farah, A. Chlorogenic acids from green and roasted coffees are equally absorbed and metabolized by humans. FASEB J. 2010, 24, 922. [Google Scholar]
- Marques, V.X.; Farah, A. Urinary excretion of chlorogenic acids and metabolites in humans after green mate (I. paraguariensis) consumption. FASEB J. 2010, 24, 922. [Google Scholar]
- Bravo, X. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Gutnisky, A.; Rizzo, N.; Castro, M.E.; Garbossa, G. The inhibitory action of chlorogenic acid on the intestinal iron absorption in rats. Acta Physiol. Pharmacol. Ther. Latinoam. 1992, 42, 139–146. [Google Scholar]
- Brune, M.; Rossander, L.; Hallberg, L. Iron absorption and phenolic compounds: Importance of different phenolic structures. Eur. J. Clin. Nutr. 1989, 43, 547–557. [Google Scholar] [PubMed]
- Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef]
- Freedman, N.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1894. [Google Scholar] [CrossRef]
- Liu, J.; Sui, X.; Lavie, C.J.; Hebert, J.R.; Earnest, C.P.; Zhang, J.; Blair, S.N. Association of coffee consumption with all-cause and cardiovascular disease mortality. Mayo Clin. Proc. 2013, 88, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Crippa, A.; Discacciati, A.; Larsson, S.C.; Wolk, A.; Orsini, N. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: A dose-response meta-analysis. Am. J. Epidemiol. 2014, 180, 763–765. [Google Scholar] [CrossRef]
- Loftfield, E.; Freedman, N.D.; Graubard, B.I.; Guertin, K.A.; Black, A.; Huang, W.-Y.; Shebl, F.M.; Mayne, S.T.; Sinha, R. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am. J. Epidemiol. 2015, 182, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willet, W.; van Dam, R.M.; Hu, F.A. Association of coffee consumption with total and cause-specific mortality in 3 large prospective cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef]
- USDA National Nutrient Database for Standard Reference (Release 28, Released September 2015, Slightly Revised May 2016) United States Department of Agriculture. Available online: https://ndb.nal.usda.gov/ndb (accessed on 18 November 2018).
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enk, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazic, V.; Abbasa, M.; Kambohd, A.A.; Khane, G.J.; Shumzaidf, M.; Ahmadg, F.; Babazadehh, D.; FangFangi, X.; Modarresi-Ghazanij, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bian, H.; Liu, Z.; Wang, Y.; Dai, J.; He, W.; Liao, X.; Liu, R.; Luo, J. Chlorogenic acid protects MSCs against oxidative stress by altering FOXO family genes and activating intrinsic pathway. Eur. J. Pharmacol. 2012, 674, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Chen, W.; Gu, X.; Shan, R.; Zou, J.; Liu, G.; Shahid, M.; Gao, J.; Han, B. Cytoprotective effect of chlorogenic acid against hydrogen peroxide-induced oxidative stress in MC3T3-E1 cells through PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Oncotarget 2017, 8, 14680–14692. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. Effects of coffee on type 2 diabetes mellitus. Nutrition 2014, 30, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Laranjinha, J.A.N.; Almeida, L.M.; Madeira, V.C.M. Reactivity of dietary phenolic acids with peroxyl radicals: Antioxidant activity upon low density lipoprotein peroxidation. Biochem. Pharmacol. 1994, 48, 487–494. [Google Scholar] [CrossRef]
- Gordon, M.H.; Wishart, K.J. Effects of chlorogenic acid and bovine serum albumin on the oxidative stability of low-density lipoproteins in vitro. J. Agric. Food Chem. 2010, 58, 5828–5833. [Google Scholar] [CrossRef] [PubMed]
- Cinkilic, N.; Cetintas, S.K.; Zorlu, T.; Vatan, O.; Yilmaz, D.; Cavas, T.; Tunc, S.; Ozkan, L.; Bilaloglu, R. Radioprotection by two phenolic compounds: Chlorogenic and quinic acid, on X-ray induced DNA damage in human blood lymphocytes in vitro. Food Chem. Toxicol. 2013, 53, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Vallyathan, V.; Castranova, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–2010. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.W.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Zheng, J.; Kim, S.M.; Hyun, C.L.; Ahn, Y.S.; Hyun, J.W. The polyphenol chlorogenic acid attenuates UVB-mediated oxidative stress in human HaCaT keratinocytes. Biomol. Ther. 2014, 22, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. BBA Gen. Subj. 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P.; Liu, Y.J. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Marković, S.; Tošović, J. Comparative study of the antioxidative activities of caffeoylquinic and caffeic acids. Food Chem. 2016, 210, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Shim, J.; Kim, H.W.; Kim, J.; Jang, Y.J.; Yang, H.; Park, J.; Choi, S.H.; Yoon, J.H.; et al. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H₂O₂-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012, 60, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. Isolation and quantification of major chlorogenic acids in three major instant coffee brands and their potential effects on H2O2-induced mitochondrial membrane depolarization and apoptosis in PC-12 cells. Food Funct. 2013, 4, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Baeza, G.; Amigo-Benavent, M.; Sarria, B.; Goya, L.; Mateos, R.; Bravo, L. Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food. Res. Int. 2014, 62, 1038–1046. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V.P. Protective effect of tetrahydrocurcumin and chlorogenic acid against streptozotocin-nicotinamide generated oxidative stress induced diabetes. J. Funct. Foods 2010, 2, 134–142. [Google Scholar] [CrossRef]
- Pari, L.; Karthikesan, K.; Menon, V.P. Comparative and combined effect of chlorogenic acid and tetrahydrocurcumin on antioxidant disparities in chemical induced experimental diabetes. Mol. Cell. Biochem. 2010, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M.; Soliman, R.E. Chlorogenic and caftaric acids in liver toxicity and oxidative stress induced by methamphetamine. J. Toxicol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Cachofeiro, V.; Goicochea, M.; Garcia de Vinuesa, S.; Oubina, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Ambade, A.; Mandrekar, P. Oxidative stress and inflammation: Essential partners in alcoholic liver disease. Int. J. Hepatol. 2012. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopes de Faria, J.B. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic. Res. 2007, 41, 216–224. [Google Scholar] [CrossRef]
- Cotran, R.S.; Kumar, V.; Robbins, S.L.; Schoen, F.J. Cellular injury and cellular death. In Robbins Pathologic Basis of Disease; WB Saunders Company: Philadelphia, PA, USA, 1994; pp. 1–34. ISBN 978-1455726134. [Google Scholar]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.H.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H.; Sałaga, M.; Zielin’ska, M.; Piechota-Polan’czyk, A.; Owczarek, K.; Kordek, R.; Lewandowska, U.; Chen, C.; Fichna, J. Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2015, 388, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, N.Y.; Topal, A. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Etoz, B.C.; Gul, Z.; Ziyanok, S.; Inan, S.; Turacozen, O.; Gul, N.Y.; Topal, A.; Cinkilic, N.; Tas, S.; et al. In vivo systemic chlorogenic acid therapy under diabetic conditions: Wound healing effects and cytotoxicity/genotoxicity profile. Food Chem. Toxicol. 2015, 81, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical composition, antioxidant activity, and the effect of the aqueous extract of coffee (Coffea arabica L.) bean residual press cake on the skin wound healing. Oxid. Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Oxidative stress as an iceberg in carcinogenesis and cancer biology. Arch. Biochem. Biophys. 2016, 595, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Nkondjock, A. Coffee consumption and the risk of cancer: An overview. Cancer Lett. 2009, 277, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ya-Min, L.; Peng, J.; Le-Zhi, L. Coffee consumption associated with reduced risk of oral cancer: A meta-analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, 381–389. [Google Scholar] [CrossRef]
- Mishra, M.; Panta, R.; Miyares, M. Influence of coffee and its components on breast cancer: A review. Asian Pac. J. Trop. Dis. 2016, 6, 827–831. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef] [PubMed]
- Jurkowska, R.Z.; Jurkowski, T.P.; Jeltsch, A. Structure and function of mammalian DNA methyltransferases. Eur. J. Chem. Biol. 2011, 12, 206–222. [Google Scholar] [CrossRef]
- Kasai, H.; Fukada, S.; Yamaizumi, Z.; Sugie, S.; Mori, H. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000, 38, 467–471. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Boettler, U.; Volz, N.; Pahlke, G.; Teller, N.; Kotyczka, C.; Somoza, V.; Stiebitz, H.; Bytof, G.; Lantz, I.; Lang, R. Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo. Mol. Nutr. Food Res. 2011, 55, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Honjo, S.; Kono, S.; Coleman, M.P.; Shinchi, K.; Sakurai, Y.; Todoroki, I.; Umeda, T.; Wakabayashi, K.; Imanishi, K.; Nishikawa, H. Coffee consumption and serum aminotransferases in middle-aged Japanese men. J. Clin. Epidemiol. 2001, 54, 823–829. [Google Scholar] [CrossRef]
- La Vecchia, C. Coffee, liver enzymes, cirrhosis and liver cancer. J. Hepatol. 2005, 42, 444–446. [Google Scholar] [CrossRef]
- Wadhawan, M.; Anand, A.C. Coffee and Liver Disease. J. Clin. Exp. Hepatol. 2016, 6, 40–46. [Google Scholar] [CrossRef]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Larsson, C.; Wolk, A. Coffee consumption and risk of liver cancer: A meta-analysis. Gastroenterology 2007, 132, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Bosetti, C.; Tavoni, A.; Gallus, S.; La Vecchia, C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1929. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.R.; Parise, E.R.; Carvalho, L. Coffee has hepatoprotective benefits in Brazilian patients with chronic hepatitis C even in lower daily consumption than in American and European populations. Braz. J. Infect. Dis. 2014, 18, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Matsushige, K.; Hase, K.; Kadota, S.; Namba, T. Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol. Pharm. Bull. 1996, 19, 1479–1484. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, J.; Yu, X.; Tao, W.; Jiang, F.; Yin, Z.; Liu, C. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm. Res. 2010, 59, 871–877. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Bai, Y.; Zhao, J.; Zhang, Y.; Zhang, L. Chlorogenic acid against carbon tetrachloride-induced liver fibrosis in rats. Eur. J. Pharmacol. 2009, 623, 119–124. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef]
- Zhang, W.; Lopez-Garcia, E.; Li, T.Y.; Hu, F.B.; van Dam, R.M. Coffee consumption and risk of cardiovascular diseases and all-cause mortality among men with type 2 diabetes. Diabetes Care 2009, 32, 1043–1045. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Pi-Sunyer, F.X.; Chen, C.C.; Davidson, L.E.; Liu, C.S.; Li, T.C.; Wu, M.F.; Li, C.I.; Chen, W.; Lin, C.C. Coffee consumption is inversely associated with type 2 diabetes in Chinese. Eur. J. Clin. Investig. 2011, 41, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Boehm, N.; Janzowski, C.; Lang, R.; Hofmann, T.; Stockis, J.-P.; Albert, F.W.; Stiebitz, H.; Bytof, G.; Lantz, I.; et al. Antioxidant-rich coffee reduces DNA damage, elevates glutathione status and contributes to weight control: Results from an intervention study. Mol. Nutr. Food Res. 2011, 55, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Lee, C.M.Y.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sandwall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Shearer, J.; Sellars, E.; Farah, A.; Graham, T.E.; Wasserman, D.H. Effects of chronic coffee consumption on glucose kinetics in the conscious rat. Can. J. Physiol. Pharmacol. 2007, 85, 823–830. [Google Scholar] [CrossRef]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef]
- Simon, C.; Herling, A.W.; Preibisch, G.; Burger, H.J. Upregulation of hepatic glucose 6-phosphatase gene expression in rats treated with an inhibitor of glucose-6-phosphate translocase. Arch. Biochem. Biophys. 2000, 373, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Waite, M. Treatment for Alzheimer’s disease: Has anything changed? Austr. Prescr. 2015, 38, 60–63. [Google Scholar] [CrossRef]
- Shearer, J.; Farah, A.; de Paulis, T.; Bracy, D.P.; Pencek, R.R.; Graham, T.E.; Wasserman, D.H. Quinides of roasted coffee enhance insulin action in conscious rats. J. Nutr. 2003, 133, 3529–3532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—WHO. The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 18 November 2018).
- Taguchi, K.; Hida, M.; Matsumoto, T.; Ikeuchi-Takahashi, Y.; Onishi, H.; Kobayashi, T. Effect of short-term polyphenol treatment on endothelial dysfunction and thromboxane A2 levels in streptozotocin-induced diabetic mice. Biol. Pharm. Bull. 2014, 37, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- McDowell, I.F.; Lang, D. Homocysteine and endothelial dysfunction: A link with cardiovascular disease. J. Nutr. 2000, 130, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B. 5-Caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J. Nutr. Biochem. 2009, 20, 800–805. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kanegae, M.P.; da Fonseca, L.M.; Brunetti, L.L.; Silva, S.O.; Ximenes, V.F. The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: Contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. Biochem. Pharmacol. 2007, 74, 457–464. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: An epidemiological study in a general Japanese population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Mure, K.; Maeda, S.; Mukoubayashi, C.; Mugitani, K.; Iwane, M.; Kinoshita, F.; Mohara, O.; Takeshita, T. Habitual coffee consumption inversely associated with metabolic syndrome-related biomarkers involving adiponectin. Nutrition 2013, 29, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; van Dam, R.M.; Rajpathak, S.; Willett, W.C.; Manson, J.E.; Hu, F.B. Changes in caffeine intake and long-term weight change in men and women. Am. J. Clin. Nutr. 2006, 83, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.; Axen, K.; Schnoll, R.; Boozer, C. Coffee, tea and diabetes: The role of weight loss and caffeine. Int. J. Obes. 2005, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Thom, E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on Regulating Glucose and Lipids Metabolism: A Review. Evid. Based Complement. Alternat. Med. 2013. [Google Scholar] [CrossRef]
- de Sotillo, D.V.R.; Hadley, M.; Sotillo, J.E. Insulin receptor exon 11+/- is expressed in Zucker (fa/fa) rats, and chlorogenic acid modifies their plasma insulin and liver protein and DNA. J. Nutr. Biochem. 2006, 17, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Bily, A.; Rolland, Y.; Roller, M. Lipolytic activity of Svetol®, a decaffeinated green coffee bean extract. Phytother. Res. 2014, 28, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, X.C.; Zhong, Y.L.; He, W.Y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Rosso, A.; Mossey, J.; Lippa, C.F. Caffeine: Neuroprotective functions in cognition and Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Demen. 2008, 23, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Cao, C. Caffeine and Coffee as Therapeutics against Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Pathak, L.; Agrawal, Y.; Dhir, A. Natural polyphenols in the management of major depression. Expert Opin. Investig. Drugs 2013, 22, 863–880. [Google Scholar] [CrossRef]
- Esposito, E.; Rotilio, D.; Di Matteo, V.; Di Giulio, C.; Cacchio, M.; Algeri, S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging 2002, 23, 719–735. [Google Scholar] [CrossRef]
- Ramassamy, C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: A review of their intracellular targets. Eur. J. Pharmacol. 2006, 545, 51–64. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Berrocal, R.; Rao, K.S.; Durant, A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2015, 51, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Rammal, H.; Dicko, A. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.F.; Brown, P.H.; Lyle, B.J.; Chen, Y.; Richard, B.M.; Williams, C.E.; Lin, Y.C.; Hsu, C.W.; Cheng, I.H. Roasted coffees high in lipophilic antioxidants and chlorogenic acid lactones are more neuroprotective than green coffees. J. Agric. Food Chem. 2009, 57, 9801–9808. [Google Scholar] [CrossRef] [PubMed]
- Antônio, A.G.; Moraes, R.S.; Perrone, D.; Maia, L.C.; Santos, K.R.N.; Iorio, N.L.P.; Farah, A. Species, roasting degree and decaffeination influence the antibacterial activity of coffee against Streptococcus mutans. Food Chem. 2010, 118, 782–788. [Google Scholar] [CrossRef]
- Almeida, A.A.P.; Naghetini, C.C.; Santos, V.R.; Antonio, A.G.; Farah, A.; Gloria, M.B.A. Influence of natural coffee compounds, coffee extracts and increased levels of caffeine on the inhibition of Streptococcus mutans. Food Res. Int. 2012, 49, 459–461. [Google Scholar] [CrossRef]
- Karunanidhi, A.; Thomas, R.; Van Belkum, A.; Neela, V. In vitro antibacterial and antibiofilm activities of chlorogenic acid against clinical isolates of Stenotrophomonas maltophilia including the trimethoprim/sulfamethoxazole resistant strain. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Farzaei, M.H.; Abdollahi, M.; Rahimi, R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015, 21, 6499–6517. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.; Zhou, X. Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: Persimmon, guava, and sweetsop. BioMed Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Bajko, E.; Kalinowska, M.; Borowski, P.; Siergiejczyk, L.; Lewandowski, W. Uinic acid: A spectroscopic study and biological screening for antimicrobial activity. LWT-Food Sci. Technol. 2016, 65, 471–479. [Google Scholar] [CrossRef]
- Kabir, F.; Katayama, S.; Tanji, N.; Nakamura, S. Antimicrobial effects of chlorogenic acid and related compounds. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 359–365. [Google Scholar] [CrossRef]
- Sales, A.; Miguel, M.A.L.; Farah, A. Effect of coffee aqueous extracts and bioactive compounds on probiotic bacteria growth. In Proceedings of the International Conference on Coffee Science (ASIC), Portland, OR, USA, 16–20 September 2018. [Google Scholar]
- Sung, W.S.; Lee, D.G. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl. Chem. 2010, 82, 219–226. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Ather, A.; Thompson, K.D.; Gambari, R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005, 67, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Akioka, T.; Ueno, K.; Chujyo, T.; Okazaki, K.I.; King, P.J.; Robinson, W.E. Anti-human immunodeficiency virus activity of 3,4,5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol. Nutr. Food Res. 2006, 50, 396–400. [Google Scholar] [CrossRef]
- Chiang, L.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C. C Antiviral activity of Plantago major extracts and related compounds in vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Esposito, F.; Sanna, C.; Del Vecchio, C.; Cannas, V.; Venditti, A.; Corona, A.; Bianco, A.; Serrilli, A.M.; Guarcini, L.; Parolin, C. Hypericum hircinum L. components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathog. Dis. 2013, 68, 116–124. [Google Scholar] [CrossRef]
- Pleško, S.; Volk, H.; Lukšič, M.; Podlipnik, Č. In silico study of plant polyphenols’ interactions with VP24-Ebola virus membrane-associated protein. Acta Chim. Slov. 2015, 62, 555–564. [Google Scholar] [CrossRef]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Ovarzábal, I.S.; Gill, C.; Rowland, I. An exploratory study into the putative prebiotic activity of fructans isolated from Agave angustifolia and the associated anticancer activity. Anaerobe 2013, 22, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.C.; Reimer, R.A. Long-term intake of a high prebiotic fiber diet but not high protein reduces metabolic risk after a high fat challenge and uniquely alters gut microbiota and hepatic gene expression. Nutr. Res. 2014, 34, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Quagliana, K.A.; Nelson, P.D.; Buddington, R.K. Dietary oligofructose and inulin modulate immune functions in mice. Nutr. Res. 2003, 23, 257–267. [Google Scholar] [CrossRef]
- Kumar, V.P.; Prashanth, K.V.H.; Venkatesh, Y.P. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa). Carbohydr. Polym. 2015, 117, 115–122. [Google Scholar] [CrossRef]
- Munjal, U.; Glei, M.; Pool-Zobel, B.L.; Scharlau, D. Fermentation products of inulin-type fructans reduce proliferation and induce apoptosis in human colon tumour cells of different stages of carcinogenesis. Br. J. Nutr. 2009, 102, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Griffin, I.J.; Hicks, P.M.D.; Heaney, R.P.; Abrams, S.A. Enriched chicory inulin increases calcium absorption mainly in girls with lower calcium absorption. Nutr. Res. 2003, 23, 901–909. [Google Scholar] [CrossRef]
- Fernandes, R.; Beserra, B.T.S.; Mocellin, M.C.; Kuntz, M.G.F.; da Rosa, J.S.; de Miranda, R.C.D.; Schreiber, C.S.O.; Fröde, T.S.; Nunes, E.A.; Trindade, E.B.S.M. Effects of prebiotic and synbiotic supplementation on inflammatory markers and anthropometric indices after roux-en-Y gastric bypass: A randomized, triple-blind, placebo-controlled pilot study. J. Clin. Gastroenterol. 2015, 50, 208–217. [Google Scholar] [CrossRef]
- Cluny, N.L.; Eller, L.K.; Keenan, C.M.; Reimer, R.A.; Sharkey, K.A. Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity 2015, 23, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Griffin, I.J.; Hawthorne, K.M.; Ellis, K.J. Effect of prebiotic supplementation and calcium intake on body mass index. J. Pediatr. 2007, 151, 293–298. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D.; Everard, A.; Neyrinck, A.M.; Bindels, L.B. Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 2015, 58, 2206–2217. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Savige, G.S.; Reid, C.M. Effect of dietary prebiotic supplementation on advanced glycation, insulin resistance and inflammatory biomarkers in adults with pre-diabetes: A study protocol for a double-blind placebo-controlled randomised crossover clinical trial. BMC Endocr. Disord. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Rechner, A.R.; Spencer, J.P.E.; Kuhnle, G.; Hahn, U.; Rice-Evans, C.A. Novel biomarkers of the metabolism of caffeic acid derivatives in vivo. Free Radic. Biol. Med. 2001, 30, 1213–1222. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
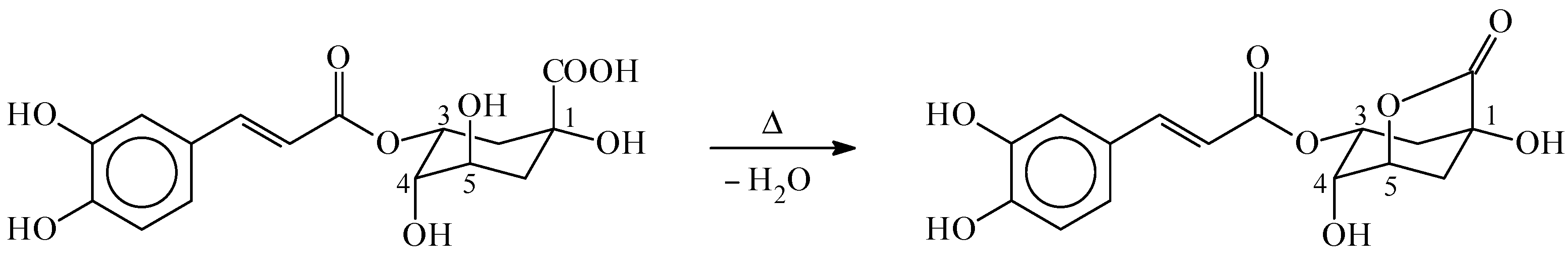
| Species | Country | N | Roast Degree | Amount of Powder to Water | Water Temperature | Brewing Time | 5-CQA | Other CGA (CQA, FQA, diCQA, CQL) | Total CGA | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| per 100 mL | mg/100 mL | |||||||||
| Manual drip | ||||||||||
| Arabica | Colombia | 1 | M | 10 | 100 °C | 2 min | 75.1 | 81.7 | 156.8 | [40] |
| Arabica | Indonesia | 1 | M | 10 | 100 °C | 2 min | 73.4 | 80.4 | 153.8 | [40] |
| Arabica | Kenya | 1 | MD | 10 | 100 °C | 2 min | 31.2 | 34.2 | 65.4 | [40] |
| Arabica | Costa Rica | 1 | MD | 10 | 100 °C | 2 min | 10.9 | 62.4 | 73.3 | [40] |
| Arabica | USA | 1 | M | 7 | 98 °C | ~3 min | 35.4 | 48.3 | 83.7 | [34]* |
| Blend | Brazil | 1 | ML | 10 | 95 °C | ~2.5 min | 39.1 | 41.5 | 80.6 | [33]* |
| Blend | Brazil | 1 | MD | 10 | 95 °C | ~2.5 min | 9.8 | 14.4 | 24.2 | [33]* |
| Electric dripper | ||||||||||
| Arabica | Guatemala | 1 | Nr | 6 | 90 °C | 6 min | 74.2 | 92.6 | 166.8 | [41] |
| Arabica | USA | 1 | M | 7 | 95 °C | 4 min | 41.3 | 54.8 | 96.1 | [34]* |
| Arabica | Portugal | 1 | Nr | 13.3 | 100 °C | 2.5 min | 16.9 | 46.8 | 63.7 | [42] |
| Robusta | Portugal | 1 | Nr | 13.3 | 100 °C | 2.5 min | 16.4 | 45.9 | 62.4 | [42] |
| Robusta | Vietnam | 1 | Nr | 6 | 90 °C | 6 min | 43.8 | 56.6 | 100.4 | [41] |
| Blend | Brazil | 1 | ML | 10 | Nr | ~3 min | 44.9 | 85.1 | 126.6 | [33]* |
| Blend | Brazil | 1 | MD | 10 | Nr | ~3 min | 12.4 | 21.8 | 34.2 | [33]* |
| Espresso | ||||||||||
| Arabica | USA | 1 | M | 7 | 90 °C | 28 s | 40.8 | 54.6 | 95.4 | [34]* |
| Arabica | Ethiopia | 1 | Nr | 14 | 92 °C | 30 s | 446.0 | 584 | 1030.0 | [32] |
| Arabica | Guatemala | 1 | Nr | 17.5 | 90 °C | 24 s | 68.9 | 150.1 | 150.1 | [41] |
| Arabica | Ethiopia | 1 | Nr | 18 | 92 °C | 25 s | 480.0 | 661.0 | 1141.0 | [32] |
| Robusta | Vietnam | 1 | Nr | 17.5 | 90 °C | 24 s | 37.0 | 49.5 | 86.5 | [32] |
| Blend | Portugal | 1 | Nr | 5.3 | 90 °C | 21 s | 33.2 | 88.8 | 122.0 | [42] |
| Blend | Scotland-coffee shops | 20 | Nr | Nr | Nr | Nr | 45.2–468.2 | 43.6–449.2 | 88.8–918.1 | [30] |
| Blend | Brazil | 1 | ML | 10 | 90 °C | <1 min | 26.0 | 60.5 | 86.5 | [33]* |
| Blend | Brazil | 1 | MD | 10 | 90 °C | <1 min | 10.3 | 27.8 | 38.2 | [33]* |
| Moka | ||||||||||
| Arabica | USA | 1 | M | 7 | 95 °C | 10 min | 47.0 | 64.2 | 111.2 | [34]* |
| Arabica | Guatemala | 1 | Nr | 8 | 93 °C | 10 min | 107.2 | 140.2 | 247.4 | [41] |
| Arabica | Ethiopia | 1 | Nr | 10 | 90 °C | Nr | 122.0 | 145.0 | 267.0 | [32] |
| Arabica | Portugal | 1 | Nr | 13 | Nr | Nr | 18.8 | 55.5 | 74.3 | [42] |
| Robusta | Portugal | 1 | Nr | 13 | Nr | Nr | 22.5 | 64.7 | 87.2 | [42] |
| Robusta | Vietnam | 1 | Nr | 8 | 93 °C | 10 min | 62.5 | 90.4 | 152.9 | [41] |
| Blend | Brazil | 1 | ML | 10 | Nr | ~7 min | 58.8 | 88.0 | 146.7 | [33]* |
| Blend | Brazil | 1 | MD | 10 | Nr | ~7 min | 19.0 | 35.8 | 54.8 | [33]* |
| Infusion bag | ||||||||||
| Arabica | Japan | 4 | Nr | 2.5 | 95 °C | 5 min | 8.9/10.2 | 22.6/28.6 | 31.5–38.6 | [34]* |
| French press | ||||||||||
| Arabica | Guatemala | 1 | Nr | 8 | 98 °C | 5 min | 85.7 | 110.8 | 196.5 | [41] |
| Arabica | Hawaii | 2 | M | 10 | 98 °C | 6 min | Nr | 46.0/51.0 | 46.0/51.0 | [43] |
| Arabica | USA | 1 | M | 7 | 90 °C | 5 min | 38.8 | 49.3 | 88.1 | [34]* |
| Arabica | Portugal | 1 | Nr | 13 | 100 °C | 2.5 min | 16.3 | 48.1 | 64.4 | [43] |
| Arabica | Brazil | 1 | L | 10 | 100 °C | 6 min | 126.1 | 124.0 | 250.1 | [32] |
| Arabica | Mexico | 1 | L | 10 | 100 °C | 6 min | 147.6 | 133.2 | 280.8 | [44] |
| Arabica | Ethiopia | 1 | Nr | 10 | 95 °C | 5 min | 53.0 | 67.0 | 120.0 | [32] |
| Robusta | Portugal | 1 | Nr | 13 | 100 °C | 2.5 min | 17.3 | 49.3 | 66.6 | [42] |
| Robusta | Vietnam | 1 | Nr | 8 | 98 °C | 5 min | 36.3 | 57.6 | 93.9 | [42] |
| Aeropress | ||||||||||
| Arabica | Ethiopia | 1 | Nr | 6.6 | 93 °C | 1 min | 72.0 | 82 | 154.0 | [32] |
| Cold brewing | ||||||||||
| Arabica | USA | 1 | M | 7 | 95 °C followed by 10 °C | 12 hr | 30.3 | 33 | 63.3 | [34]* |
| Arabica | USA | 1 | M | 7 | 10 °C | 12 hr | 28.6 | 30.6 | 59.2 | [34]* |
| Arabica | Hawaii | 2 | M | 10 | 25 °C | 24 hr | Nr | 51.0/52.0 | 51.0/52.0 | [43] |
| Arabica | Hawaii | 2 | D | 10 | 25 °C | 24 hr | Nr | 36.0/39.0 | 36.0/39.0 | [43] |
| Arabica | Brazil | 1 | L | 10 | 25 °C | 7 hr | 112.4 | 107.7 | 220.1 | [44] |
| Arabica | Mexico | 1 | L | 10 | 25 °C | 7 hr | 85.7 | 75.9 | 161.6 | [44] |
| Arabica | Ethiopia | 1 | Nr | 10 | 25 °C | 6 hr | 139.0 | 180.0 | 319.0 | [32] |
| Turkish coffee | ||||||||||
| Arabica | USA | 1 | M | 7 | 90 °C | 5 min | 46.1 | 64 | 110.1 | [34]* |
| Blend | Croatia—Local manufacturer | 3 | Nr | 8 | 98 °C | 5 min | 79.5–101.3 | 74.6–99.8 | 154.1–201.1 | [29] |
| Boiled coffee | ||||||||||
| Arabica | USA | 1 | M | 7 | 95 °C | 5 min | 45.2 | 59.7 | 104.9 | [34]* |
| Arabica | Brazil | 1 | L | 10 | 100 °C | Nr | 126.5 | 169.1 | 295.6 | [28] |
| Arabica | Brazil | 1 | M | 10 | 100 °C | Nr | 30.7 | 51.2 | 81.9 | [28] |
| Arabica | Brazil | 1 | D | 10 | 100 °C | Nr | 8.7 | 17.4 | 26.1 | [28] |
| Arabica | Portugal | 1 | Nr | 13.3 | 100 °C | 2 min | 19.4 | 54.9 | 74.3 | [42] |
| Blend | Brazil | 1 | ML | 10 | 100 °C | ~4 min | 96.8 | 133.8 | 230.6 | [33]* |
| Blend | Brazil | 1 | MD | 10 | 100 °C | ~4 min | 23.6 | 47.5 | 71.13 | [33]* |
| Soluble or instant coffee beverage | ||||||||||
| Nr | Portugal | 3 | Nr | 1.3 | 100 °C | - | 6.0–6.8 | 11.0–15.2 | 17.0–22.0 | [42] |
| Nr | UK | 2 | L | 0.9 | 100 °C | - | 5.7/7.2 | 9.0/10.8 | 14.7/18.0 | [50] |
| Nr | UK | 2 | M | 0.9 | 100 °C | - | 7.1/7.5 | 10.3/11.7 | 17.4/19.2 | [50] |
| Nr | UK | 1 | Green and roasted | 0.9 | 100 °C | - | 21.5 | 22.5 | 44.0 | [50] |
| Ready to drink cold coffee beverage | ||||||||||
| Nr | Japan | 4 | Nr | - | - | 65.5–74.8 | 141.0–169.0 | 206.5–243.8 | [34]* | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farah, A.; de Paula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. https://doi.org/10.3390/beverages5010011
Farah A, de Paula Lima J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages. 2019; 5(1):11. https://doi.org/10.3390/beverages5010011
Chicago/Turabian StyleFarah, Adriana, and Juliana de Paula Lima. 2019. "Consumption of Chlorogenic Acids through Coffee and Health Implications" Beverages 5, no. 1: 11. https://doi.org/10.3390/beverages5010011
APA StyleFarah, A., & de Paula Lima, J. (2019). Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages, 5(1), 11. https://doi.org/10.3390/beverages5010011