Abstract
Cyanobacteria are microorganisms that are well-adapted to sudden changes in their environment, namely to light conditions. This has allowed them to develop mechanisms for photoprotection, which encompass alteration in pigment composition. Therefore, light modulation appears to be a suitable strategy to enhance the synthesis of specific pigments (e.g., phycocyanin) with commercial interest, in addition to conveying a more fundamental perspective on the mechanisms of acclimatization of cyanobacterium species. In this study, Synechocystis salina was accordingly cultivated in two light phase stages: (i) white LED, and (ii) shift to distinct light treatments, including white, green, and red LEDs. The type of LED lighting was combined with two intensities (50 and 150 µmolphotons·m−2·s−1). The effects on biomass production, photosynthetic efficiency, chlorophyll a (chl a) content, total carotenoids (and profile thereof), and phycobiliproteins (including phycocyanin, allophycocyanin, and phycoerythrin) were assessed. White light (under high intensity) led to higher biomass production, growth, and productivity; this is consistent with higher photosynthetic efficiency. However, chl a underwent a deeper impact under green light (high intensity); total carotenoids were influenced by white light (high intensity); whilst red treatment had a higher effect upon total and individual phycobiliproteins. Enhanced PC productivities were found under modulation with red light (low intensities), and could be achieved 7 days earlier than in white LED (over 22 days); this finding is quite interesting from a sustainability and economic point of view. Light modulation accordingly appears to be a useful tool for supplementary studies pertaining to optimization of pigment production with biotechnological interest.
1. Introduction
Cyanobacteria are photosynthetic microorganisms that are widely adapted to environmental conditions across the globe. They can be divided into “sun” and “shade” species, depending on the light intensity required for growth thereof [1,2]. Said organisms are able to modify their photosynthetic apparatus to efficiently harvest light under fluctuating light conditions, and rapidly change from sub- to oversaturating light intensities. Therefore, cyanobacterial pigments have evolved to play an important role in the acclimation process, in particular to light. As major constituents of light-harvesting systems (LHS) and photosynthetic apparatuses—photosystem I (PSI), photosystem II (PSII), and phycobilisome, pigments are responsible to harvest light, and selectively absorbing at specific light wavelengths within the photosynthetic active radiation (PAR) range—a segment of the electromagnetic radiation spectrum, within 380–750 nm [3,4]. For this reason, cyanobacterial pigments, including chlorophyll a (chl a, λmax = 430 and 660), carotenoids (λ = 400–500 nm), and phycobiliproteins (PBPs), such as phycocyanin (PC, λmax = 620 nm), allophycocyanin (APC, λmax = 650 nm), and phycoerythrin (PE λmax= 560 nm) are highly organized into the photosynthetic apparatus [4,5]. In addition to functioning as light receptors and funneling energy to the photoreaction centers, (i.e., chl a and PBPs), they also play an important role in cyanobacterial adaptation to external factors (e.g., high light intensities and nutrient limitation). Therefore, light promotes rearrangement of composition and content of cyanobacterial pigments, and several mechanisms of photoacclimation (and photoprotection) can actually be triggered [6]. When adjustment of the spectrum of nearly complementary to available light—the so-called complementary chromatic adaptation (CCA)—is activated, the ratio of PC and PE composing the phycobilisome (i.e., photosynthetic structure that harbors PBPs) is changed [6,7]. Cyanobacteria possess four types of chromatic acclimation in response to red/green light, which differs between species and is highly dependent upon pigment composition. Type I acclimation does not change PC or PE levels in response to light; in Type II, a change occurs in PE levels by both green and red light, whilst PC remains constant; in Type III, the PC levels are positively impacted by red light, whilst PE is positively favored by green light and vice-versa; and in the Type IV acclimation process, PBP content is regulated by blue and green light [8].
On the other hand, when light stress by high light quanta induces accumulation of reactive oxygen species (ROS) in cyanobacterial cells, some of the first active cell defenses are activated, while mechanisms of carotenoid synthesis are provided for photoprotection [9]. Therefore, cyanobacteria are highly adaptable to quality (i.e., specific wavelength), quantity (i.e., intensity), and/or duration (i.e., photoperiod) of light under different environments [10]. This means that light fluctuations can physiologically influence synthesis of important bioactive pigments, including chl a, carotenoids, and PBPs, thus playing a vital role in cyanobacterial growth, and pigment accumulation in culture [11].
From a biotechnological perspective, marine natural pigments (e.g., from cyanobacteria) have been found to possess outstanding natural properties (e.g., antioxidant, antitumor, anti-inflammatory, antiviral). They are used as ingredients in formulation of drugs, food (e.g., natural colorants, antioxidants), feed, cosmetics (e.g., anti-ageing), and therapeutical diagnostic (e.g., fluorescent dyes), as well as nutraceutical formulae for human and animal welfare (e.g., aquaculture and pets) [12,13,14]. The global market of natural pigments is currently undergoing considerable expansion. For instance, the total market of PBPs was estimated in 2018 at ca. USD 60 million, and is expected to double by 2028, with a value of USD 124 million [15].
In the upcoming future, enhanced biotechnological production of cyanobacterial bioactive pigments is expected to resort to innovative and cost-effective industrial technologies—such as light emitting diodes (LED). In this regard, a better understanding of how abiotic factors (e.g., light, temperature, nutrients) influence biomass and pigment accumulation, particularly in photoautotrophic cultivation systems, becomes crucial. Therefore, light modulation emerges as a relevant strategy to improve both biomass and pigment production in cyanobacteria.
The use of new light technologies, such as LED, provides a sustainable alternative to conventional lamps (e.g., halogen, fluorescent, high pressure, sodium) [16,17]. Despite the higher cost of installation (but with declining prices throughout the years), LEDs have a long life and entail low energy usage compared to conventional lighting. This could compensate the initial investment to some extent, and provide a more environment-friendly solution [18]. Several studies employing LED technology have shown their efficiency towards cyanobacterial culture and pigment modulation (e.g., Arthrospira spp.) [17,19,20]. The intensity and light wavelength can be tuned toward production of specific bioactive pigments, in terms of quantity and quality, along with biomass production [17]. However, optimal conditions are always dependent on the species chosen for that purpose. For instance, both red and/or green light favor production of PC/PE, as well as biomass production, depending on the species and strain at stake [12,17]. Furthermore, white light under “optimum” intensity—lower than saturation and photo-inhibition points—has been reported to favor the accumulation of biomass. It is important to reach a compromise between biomass production and level of pigments of interest. Therefore, use of two-phase cultivation is a commonly explored technique—at a first stage to maximize biomass (preferentially under higher growth rates), and at a second phase to target compounds enhanced by stress conditions (i.e., high light intensities, different light spectra) [21].
The marine cyanobacterium Synechocystis salina LEGE 01655 (S. salina) was previously described as having a potential biotechnological value [22,23,24]. Few studies on Synechoscystis species have, however, focused on light preferences, especially Synechocystis sp. strain PCC 6803 [25,26]—a strain mainly composed of APC and PC [27]. S. salina LEGE 01655 was found to have PE as additional pigment in its phycobilisome [22], but a gap remains regarding how light influences its metabolic responses. The process of light modulation is indeed highly dependent on the pigment composition of each organism, with a strain-specific response [25].
This work was accordingly aimed at assessing how light modulation under different wavelengths (white, green, and red) and intensities (high and dimmed light), in the two-phase cultivation of S. salina, can improve production of different pigments with added value (i.e., chl a, carotenoids, PBPs, and specifically PC—which were reported to high levels in this cyanobacterium [22]); and contributing to understand how this specie implements its chromatic acclimation.
2. Materials and Methods
2.1. Microorganism
Synechocystis salina strain LEGE 06155 (S. salina) was used for this study, and obtained from the Blue Biotechnology and Ecotoxicology Culture Collection (LEGE-CC)—CIIMAR (Centre of Marine and Environmental Research of University of Porto, Porto, Portugal).
2.2. Cyanobacterial Cultivation Conditions
The growth of S. salina strain LEGE 06155 was performed in 2 L-round flasks (working volume of 1.8 L) in BG11 culture medium [27], supplemented with 10 g·L−1 of NaCl, with pH 8.9 ± 0.1 previously adjusted with CHES buffer (PanReach, AppliChem, Chicago, IL, USA), with temperature maintained at 23 °C. Agitation was provided via continuous air bubbling at the bottom of the cultures, using an airflow of 0.75 Lair ·Lculture−1·min−1. Each run departed from an optical density (OD) of 0.1 (OD = λ680 nm − λ750 nm), which was kept in batch over 22 days, using the same conditions as for the pre-inoculum. The pre-inocula were maintained under a light intensity of 150 µmolphotons·m−2·s−1, with light provided by a white LED (GreenPowerReasearch module white, 15 W, Phillips, Amsterdam, The Netherlands) using a light/dark cycle (L:D) of 16 h:8 h.
2.3. Experimental Design
S. salina was subjected to a two-phase light treatment over 22 days. In the first phase, cultures were subjected to white (W) light, containing energy from different wavelengths (λmax = 450 nm, 540, and 580 nm), over 10 days for biomass growth. In the second phase, a light shock was imposed, with red (R) light (λmax = 660 nm) and green (G) light (λmax = 530 nm), both tested under 50 and 150 µmolphotons·m−2·s−1 for light intensities, over 12 days. The cultures were also tested under low light conditions for white light, and a complete curve with white light under 150 µmolphotons·m−2·s−1 was produced as control (Figure 1). Both green and red light were chosen for light shifts, since they have been reported to significantly influence PBP restructuration during cyanobacterial cell acclimatization and maintenance of photosynthetic efficiency [28,29]. The different light sources were placed below the cultures; the white LED (GreenPowerReasearch module deep red, 15 W, Phillips, The Netherlands) and red LED (GreenPowerReasearch module deep red, 10 W, Phillips, The Netherlands) consisted in a rack with 3 stripes of built-in lights, and the green LED (Systion, Portugal) consisted in a rack with 5 LED tubes. The light intensities were checked using a universal light meter with PAR sensor (UML-500 Universal, Walz, Effeltrich, Germany). Relative emission spectra of each LED (Figure 2) were measured with a spectroradiometer Lighting Passport Pro (model ALP-01, AsenseTek, Gatineau, QC, Canada), and the corresponding data were collected with software Spectrum Genius (AsenseTek, Canada, 2021).
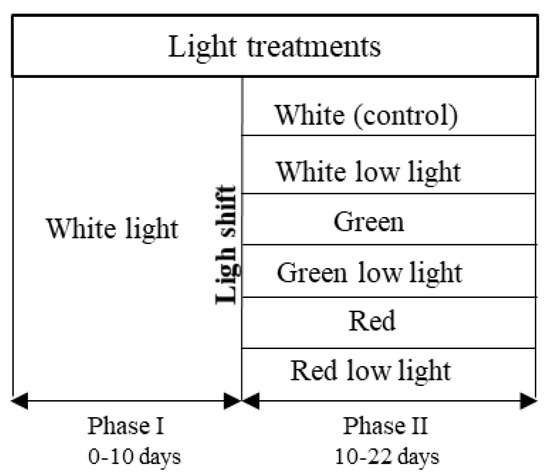
Figure 1.
Scheme of experimental design regarding light treatments applied to S. salina LEGE 06155 cultures: white (control, 150 μmolphotons·m–2·s–1), white low light (50 μmolphotons·m–2·s–1), green (150 μmolphotons·m–2·s–1), green low light (50 μmolphotons·m–2·s–1), red (150 μmolphotons·m–2·s–1), and red low light (50 μmolphotons·m–2·s–1).
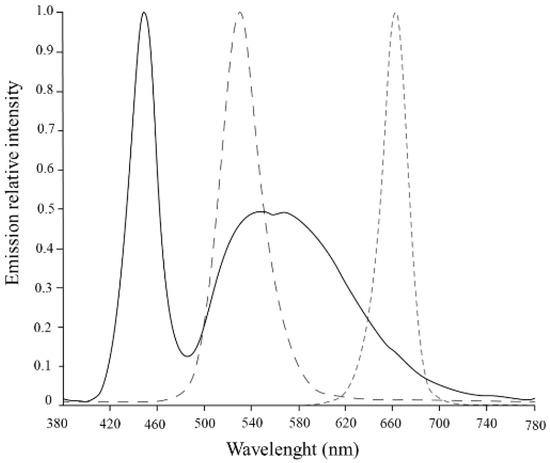
Figure 2.
Emission relative intensity of LED spectra used in this study: white ( ); green (
); green ( ) and red (
) and red ( ).
).
 ); green (
); green ( ) and red (
) and red ( ).
).
2.4. Determination of Cyanobacterial Biomass Growth and Production
The cyanobacterial growth and biomass production were determined via dry weight (DW), with samples collected at defined intervals—namely by 0, 1, 4, 6, 8, 10, 11, 15, 18, 20, and 22 days.
The DW was obtained by filtering aliquots of 2 mL of culture through preconditioned 0.45 µm (pore size) glass microfiber filter paper (Whatman GF/C, Maidstone, UK), and further dried at 100 °C until constant weight. Three biological replicates were performed, and DW analysis was performed in duplicate.
The maximum specific growth rate (µmax, d−1) was determined by numerical regression to experimental data. The maximum biomass productivity (Px, mg L−1 d−1) was determined using DW experimental data based on the following equation:
where X0 denotes biomass at the beginning of the experiment (time t0) and Xmax represents maximum biomass concentration achieved at time t. The pH was also regularly monitored with a pH meter (Hanna Instruments, model HI2210).
Px (t) = (Xt − X0)/(t − t0)
2.5. In Vivo Chlorophyll a Fluorescence Monitoring
Photosynthetic activity was evaluated by in vivo chlorophyll a fluorescence monitoring with pulse modulation (PAM), using a Junior-PAM/White fluorometer (Walz, Germany) [30,31]. Rapid light curves (RLC) were performed according to Figueroa et al. (2014) [32], in triplicate, with filtered circles of S. salina in contact with the fiber (90-degree angle) fixed at the center of the circle. RLC offers information about the overall photosynthetic efficiency state, as well as such other features as electron transport rates (i.e., saturation) of a photosynthetic organism. Maximal quantum yield of PSII—photosystem II-(Fv/Fm), maximum electron transport rates (ETR), and maximum non-photochemical quenching (NPQ) were duly calculated. Before measurement, samples were prepared by diluting a small volume of fresh culture with distilled water to an OD = 0.1, up to a final volume of 1 mL. This sample was filtered through a 0.45 nm filter paper Whatman (pore size 0.45 nm), and the biomass was then incubated in darkness for 15 min to assure that all photosynthetic reaction centers were closed. The samples were subjected to increased light pulses, from a basal state to a saturating pulse (up to 1500 µmolphotons·m−2·s−1) to estimate Fv/Fm [30,31].
The ETRmax was calculated based on ETR = Y(II) × EPAR × fAQPSII, where Y(II) is the effective quantum yield, EPAR is the photosynthetic active radiation (PAR), A is the absorbance such that A = 1–10−ODPAR, with ODPAR being the OD used to measure the chlorophyll a fluorescence in the PAR region (0.1); finally, fAQPSII is the fraction of absorbed quanta to PSII in the PAR region of the spectrum, with a typical value of 0.36 for cyanobacteria [33]. Non-photochemical quenching (NPQ) was calculated using (Fm − Fm′)/Fm′, where Fm is the maximal fluorescence following application of saturation light pulse after 15 min incubation in darkness, and Fm′ is the maximal fluorescence upon application of saturation light pulse after 20 s incubation at each light intensity [34]. The RLC was fitted to a mathematical function to find the maximal ETR (ETR), expressed in μmolelectrons·m−2·s−1, and the maximum non-photochemical quenching (NPQ).
2.6. Pigment Extraction and Quantification
2.6.1. Pigment Extract Preparation
Extraction of chl a, total carotenoid, and PBPs were performed with ethanol and water, respectively, in sequential extraction (at 0, 1, 4, 6, 8, 10, 11, 15, 18, 20, and 22 days). To analyze carotenoid total content, aliquotes of 3 mL of fresh culture were collected and centrifuged (2744 g × 10 min); the supernatant was discarded, and 3 mL of ethanol was added to the remaining pellet with glass beads. Extraction was performed via bead beater in a Precellys homogenizer (Bertin Technologies, Montigny-le-Bretonneux, France), using a 6 min cycle at 8000 rpm (30 s homogenization with 40 s pause). The extracts were centrifuged (2744 g × 10 min), and the supernatant was kept under dimmed light and transferred to 5 ml Eppendorf’s for storage at −4 °C until pigment content determination. In extraction of PBPs, 3 mL of distilled water was added to the remaining pellet, and then vortex stirred over 20 s. Extracts were centrifuged at 2000 g over 8 min, and kept in dark for further content determination.
2.6.2. Determination of Pigment Content
The chl a and total carotenoid content were assessed via spectrophotometry (Shimadzu UV-1800, USA), according to Lichtenthaler and Buschmann (2001) [35]. Absorbance was read at λ = 470, λ = 652 and λ = 665 nm, and the content calculated as follows:
Results were expressed as concentration (mg·L−1) and maximum productivity (mgcar·Lculture−1·d−1).
Chl a (μg·mL−1) = (16.72 A664) − (9.16 A648)
Total carotenoids (μg·mL−1) = (1000 A470) − (1.63 Chl a) / 209
For PBPs quantification, namely phycocyanin (PC), allophycocyanin (APC), and phycoerythrin (PE), the aqueous extracts were spectrophotometrically read at λ = 562, 615 and 652 nm; Bennett and Bogorad’s equations [36] were then applied as follows:
The PBP content was expressed as concentration (mg·L−1) and in terms of maximum productivity (mg·L−1·d−1).
PC (mg·mL−1) = (A615) − 0.474 A652)/5.34
APC (mg·mL−1) = [(A652) − (0.208 A615)]/5.09
PE (mg·mL−1) = [(A562) − 2.41(PC) − 0.849 (APC)]/9.62
2.7. Carotenoid Profile and Quantification
To ascertain whether there was a change in carotenoid profile between before and after the light treatments, a more detailed analysis was performed on the 10th day (before the light shock treatment) and on the last day (22nd day) of culture, for the sake of comparison. The samples were prepared by collecting 10 mL of fresh culture, and then centrifuged (2744× g × 10 min). The supernatant was discarded, and 5 mL of pure acetone was added to the remaining pellet, as well as 100 µL of internal standard solution of trans-β-Apo-8′-carotenal (170 mg·L−1; Sigma-Aldrich ≥ 96.0% (UV)]. Glass beads were also added to the samples for further Precellys-mediated homogenization (8000 rpm, 6 min cycle over 40 s homogenization, 30 s pause). The extracts were centrifuged (2744 g × 10 min) and kept at −20 °C until further analysis. The identification and quantification of carotenoids by HPLC was performed by a method described elsewhere [37]. The extracts were accordingly evaporated in a vacuum rotary evaporator, and resuspended in 400 µL of acetone:ethyl acetate (9:1) following previous filtration with a PTFE filter syringe (Membrane solutions, 0.22 µm) before injection. The HPLC used (Waters Alliance 2695, Milford, MA, USA) was equipped with LichoCart 250–4 C18 reverse-phase column (250 × 4 mm, 5-μm bondapack) (Merck, Darmstadt, Germany) (stationary phase), an FLR detector (Waters, Milford, MA, USA), a photodiode array (PDA) (Waters, USA) set to spectrum scanning within the range of λ = 250–750 nm, and a column heater (Waters, USA) to maintain a constant temperature throughout the analysis.
Ethyl acetate (solvent A) and acetonitrile:water 9:1 (v/v) served as mobile phase (solvent B); a gradient with these solvents was established over time as follows: 0–31 min (0–60% A); 31–36 min (60% A); 36–38 min (60–100% A); 38–43 min (100% A); 43–50 min (100-0% A); and 50–55 min (0% A). During the elution process, the column was maintained at 25 ± 2 °C under a steady pressure of 3000 bar, with an overall flow rate of 1 mL·min−1. Spectrum data from all peaks were collected within the range 250–750 nm.
The pigments were identified by the extracts UV–Vis spectra, and by comparison of retention times (RT) with standards of zeaxanthin (Extrasynthese, ≥98.0% (UV) with RT = 13.2 min, chl a (Sigma, ≥96.0% (UV)) with RT = 24.6 min, echinenone with RT = 25.2 min, and β-carotene (Extrasynthese, ≥98.0% (UV)) with RT = 32.8 min; and quantified via calibration curves encompassing said standards. Three biological replicates were analyzed, and results were expressed as µg·gDw−1. Total carotenoid productivities were expressed as mgcar·L−1·d−1.
2.8. Statistical Analysis
The experimental data were analyzed using software GraphPad Prism, version 8 for Windows (GraphPad Software, San Diego, CA, USA). Data analyzed were submitted to Shapiro–Wilk test, to confirm the normal distribution of residuals. Then, a one-way ANOVA with Tukey’s multiple comparison tests was used to assess variance between total content of chl a, total carotenoids, and PBPs.
3. Results
3.1. Growth and Biomass Production
S. salina was found to grow under all light treatments applied, after 10 days with white light treatment. At early growth, some lag phase became apparent, which rapidly entered the exponential phase between 1–4 days, with μmax = 0.18 ± 0.00 d−1 (Figure 3). The curve of the white treatment presented an average productivity of 0.18 ± 0.01 mg·L−1·d−1 within 0–22 days. No significant growth patterns regarding green and low green light treatments were recorded (p > 0.05); yet white light, low white, red, and red under low light intensity seemed to affect positively the biomass, with μaverage = 0.17 ± 0.01 d−1. Biomass productivities were hereby considered as the average productivities, taking into account the period 0–22 d; no significant differences were recorded among them (p > 0.05), with an average value of Px = 0.17 ± 0.01 g·L−1·d−1, except for green and low green treatments that presented (a lower) Px = 0.13 ± 0.00 g·L−1·d−1 (Table 1).
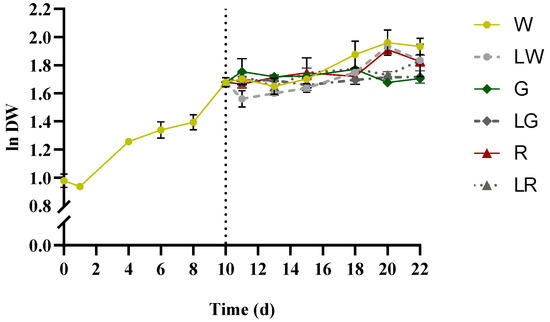
Figure 3.
Growth curves of S. salina LEGE 06155, expressed as logarithm of dry weight (ln DW) versus time (per day). The vertical dotted line denotes the separation between phase I: treatment of W (white, 150 μmolphotons·m−2·s−1) (control), from 0 to 10th days (left) and phase II: LW (low white, 50 μmolphotons·m−2·s−1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons·m−2·s−1), and LR (low red, 50 μmolphotons·m−2·s−1) from 10th to 22nd days (right). The data points show average ± SD, n = 3.

Table 1.
Kinetic parameters determined for growth of phase II of S. salina LEGE 06155 cultivation: µaverage (average specific growth rate), expressed in d−1, and Px (average productivity), expressed in g·L−1·d−1 for each applied light treatment: W (white, 150 μmolphotons·m−2·s−1) (control), LW (low white, 50 μmolphotons·m−2·s−1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons·m−2·s−1), and LR (low red, 50 μmolphotons·m−2·s−1). The lowercase letters denote statistically significant differences across columns (p < 0.05).
3.2. Photosynthetic Efficiency
S. salina was evaluated regarding its photosynthetic efficiency, in terms of Fv/Fm, ETRmax, and NPQ (Figure 4). Results were presented as box and whiskers plots (Figure 4), owing to the high variance in data points. Figure 4A depicts the distribution of results regarding Fv/Fm for each light treatment. It unfolded significantly lower trends of Fv/Fm values (i.e., median = 0.26), for S. salina grown under red light (high intensity), than in other treatments (p < 0.05). A higher distribution trend was observed for the white and green treatment (p < 0.05), in the order of 0.36.
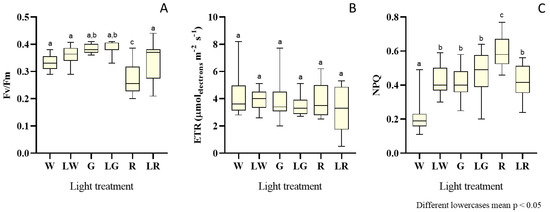
Figure 4.
Box and whisker plots of the data of photosynthetic efficiency, for each light treatment: W (white, 150 μmolphotons·m−2·s−1) (control), LW (low white, 50 μmolphotons·m−2·s–1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons·m–2·s–1), and LR (low red, 50 μmolphotons·m−2·s−1) applied to S. salina LEGE 06155, considering Fv/Fm (maximum quantum yield in PSII) (A), ETR (electron transport rate) expressed in μmolelectrons·m−2·s−1 (B), and NPQ (non-photochemical quenching) (C). The horizontal line within the box indicates the median, boundaries of the box indicate the 25th and 75th quartile, and whiskers indicate the lowest and the highest values of the results. The lowercase letters denote statistically significant differences (p < 0.05).
Regarding ETR or electron transport rate (Figure 4B) for the photosynthetic apparatus in the PSII (or rate at which electrons move through the photosynthetic chain), the trends are quite similar for all treatments–except for the white treatment under low intensities, which exhibited a slightly higher median (median = 4 μmolelectrons·m−2·s−1); however, this does not materialize a significant difference relative to the other treatments (p > 0.05).
The results in terms of NPQ, or non-photochemical quenching, are presented in Figure 4C. The lowest NPQ values (median = 0.19) were found for S. salina treated with white light under high intensity. The highest value for this parameter was observed for the treatment with high red light (median = 0.58). NPQ for the other light treatments yielded similar median values (i.e., 0.43), without significant differences between them (p > 0.05).
3.3. Effect of Light Treatment on Pigment Content and Profile
3.3.1. General Pigment Content
As the fluctuations of general pigment content over time were quite pronounced, the results are presented in the form of box and whiskers plots (Figure 5). In general, distinct light wavelengths and combined light intensities significantly impacted the content of different pigments, including chl a, total carotenoids, and phycobiliproteins.
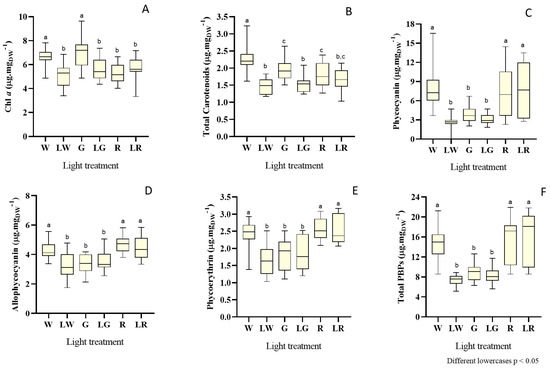
Figure 5.
Box and whisker plots of pigment content, expressed in mg gDW−1, for each light treatment: W (white, 150 μmolphotons·m−2·s−1) (control), LW (low white, 50 μmolphotons·m−2·s−1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons.m−2.s−1), and LR (low red, 50 μmolphotons·m−2·s−1) applied to S. salina LEGE 06155, considering chl a (A), total carotenoids (B), phycocyanin (C), allophycocyanin (D), phycoerythrin (E), and total phycobiliproteins (F). The horizontal line within the box indicates the median, the boundaries of the box indicate the 25th and 75th quartiles, and the whiskers indicate the lowest and the highest values of the results. The lowercase letters denote statistically significant differences (p < 0.05).
Considering the content of chl a, one of the most important pigments in photosynthesis, the highest values were observed for treatment under green light, at high intensities (median = 7.31 mg·gDW−1, day 13). However, chl a presented lower content trends when red light treatments (both under low and high intensities) and green light treatment (with low intensity) were applied (Figure 5A) (median = 5.32 mg·gDW−1).
Results on total carotenoids showed that white, green, and red treatments, under higher intensities, tend to influence the accumulation of these pigments to higher concentrations, when compared with the same treatments under low light intensities (Figure 5B). The highest concentration recorded was under white light treatment for high intensity, with a median of 2.18 mg·gDW−1 and a major peak of 3.23 mg·gDW−1 at day 15.
Concerning phycobiliproteins content, the first remark should focus on the order of magnitude of the values for the content of each PBP individually—at least for the treatment with white light (under high intensities) and red light (both under low and high intensities). PC produced high peaks, with values ranging in 13.59–16.54 mg·gDW−1, with the best value obtained for the white treatment under high intensity on day 16 (Figure 5C); APC took values between 4.18–5.84 mg·gDW−1 (the maximum under red treatment for low intensity) (Figure 5D), and PE reached a maximum of 3.17 mg·gDW−1 (in the red treatment under low intensity) on day 20 (Figure 5E). For total PBP, both red treatments (under low and high intensities) impacted positively the content of PBPs, without significant differences between them (p > 0.05), and a maximum value of 21.89 mg·gDW−1 on day 22. The difference between treatments with green light (irrespective of light intensity) and also white treatment (low intensity) is apparent, as the latter followed a negative trend of accumulation of the whole of these pigments.
3.3.2. Carotenoid Profile
The carotenoid profile was assessed before application of each light treatment during cultivation (10th day), and on the last day of cultivation (22nd day) to assess differences across all light treatments (Table 2). Overall, the carotenoid profile did not change across all light treatments, but the content did generally increase. The highest differences were observed for white treatment (under high intensity), in particular for zeaxanthin and β-carotene (p < 0.05). The green treatment also stood out, doubling the content of zeaxanthin, echinenone, and β-carotene. The content in lycopene was outstanding, with almost 2.6-fold following application of the red treatment.

Table 2.
Carotenoid profile and content for light treatment applied on the 10th day of cultivation of S. salina LEGE 06155: W (T10) (white, 150 μmolphotons·m−2·s−1) and for the 22nd day of cultivation, for the light treatments applied: W (white, 150 μmolphotons·m−2·s−1), LW (low white, 50 μmolphotons·m−2·s−1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons·m−2·s−1), and LR (low red, 50 μmolphotons·m−2·s−1). The results are reported as average ± SD (n = 3) and expressed in μg·gDW−1. For each row, different upper lowercase indicates p < 0.05. * Quantification was in trans-β-Apo-8′-carotenal (internal standard) equivalents.
3.4. PC/PE Ratio
As light influences directly the ratio between PC and PE in cyanobacteria, as a response to acclimatization under different light conditions, the ratio between these two pigments was analyzed right before application of the light shifts (phase I, 10th day), during treatment (phase II, 15th day) and by the end of light treatment (phase II, 22nd day) (Table 3). An increasing trend was observed with cultivation time for all treatments, except the low green treatment. The most prominent increase was found under the low red treatment, in particular from the 10th day on, and for both days 15 and 20. On the other hand, the lowest increase was recorded under low green treatment, with a significant increase in phycoerythrin by the 22nd day, when compared to both 10th and 15th day (p < 0.05).

Table 3.
Phycocyanin/phycoerythrin (PC/PE) ratio on 10th (phase I), 15th and 22nd days (phase II) of cultivation of S. salina LEGE 06155, for the light treatments: W (white, 150 μmolphotons.m−2.s−1) (control), LW (low white, 50 μmolphotons·m−2.·s−1), G (green, 150 μmolphotons·m−2·s−1), LW (low green, 50 μmolphotons·m−2·s−1), R (red, 150 μmolphotons·m−2·s−1), and LR (low red, 50 μmolphotons·m−2·s−1). The results are reported as average ± SD (n = 3) and expressed as %. For each row, different upper lowercase indicates p < 0.05.
3.5. Pigment Productivities
Pigment productivities were assessed for all treatments applied to S. salina; maximum peaks of productivity varied to a significant extent (p < 0.05) (Table 4). In addition, those peaks were not always recorded on the same days for some treatments. The highest productivity of chl a was obtained for white treatment (control), on the 8th day of cultivation. On the other hand, productivities for total carotenoids were achieved at later stages (i.e., stationary phase) of cultivation, with the highest value obtained for white treatment, 0.32 ± 0.01 mg·g−1·L−1 (i.e., more than double when compared to the same treatment under low intensities). For PC, both red and white treatments (high intensity) were ≈17-fold the lowest productivity achieved, under white treatment and low intensity. However, the highest productivity of PC took 22 days under white light treatment, whereas the second highest value (under low red treatment) attained the highest productivities earlier, i.e., on the 15th day. Both APC and PE exhibited higher productivities for white light treatment (high intensity), but for distinct periods of time (6th and 22nd days, respectively). For total PBPs, white and red treatments tend to increase the production of these pigments without significant differences (p > 0.05). The maximum productivity achieved for red treatment (high intensity) (15th day) was almost 7-fold that of the lowest value achieved for white treatment under low intensity.

Table 4.
Maximum productivities of pigments for light treatments applied to S. salina LEGE 06155: White (150 μmolphotons·m−2·s−1) (control), low white (50 μmolphotons·m−2·s−1), green (150 μmolphotons·m−2·s−1), low green (50 μmolphotons·m−2·s−1), red (150 μmolphotons·m−2·s−1), and low red (50 μmolphotons·m−2·s−1) applied. The lowercase letters denote statistically significant differences (p < 0.05) for each parameter analyzed (in column). Results are reported as average ± SD (n = 3) and expressed in mg·g−1·L−1.
4. Discussion
Light quality and intensity play an important role in cyanobacterial biomass and photosynthetic pigment production [15]. In this study, S. salina was subjected to different treatments with LEDs (white, green, and red, combined with two different intensities) in two distinct phases. The effect of light modulation upon bioactive pigment productivities, and how this cyanobacterium responds to different light treatments applied were evaluated.
4.1. Biomass Production and Photosynthetic Efficiency
Regarding growth and biomass production (and apart from the control), emission spectra of red LED (irrespective of light intensity) were shown to positively impact biomass production by S. salina. Several studies have demonstrated red light to be an external stimulator of increased growth in some species of cyanobacteria, (e.g., Arthrospira platensis, Cyanobium sp.), possibly correlated with a peak absorption of chl a and PBP increased production [38,39,40,41,42]. However, chl a content did not stand out for red light treatment, and no linear correlation between biomass concentration and this pigment was observed (except for the white treatment under high intensity, data not shown). Similar results were previously reported by Mohsenpour et al. (2012) [43] for microalga Chlorella vulgaris and cyanobacterium Gloethece membranacea subjected to violet, green, orange, or red light treatments. For cyanobacteria, due to presence of PBPs, this relationship cannot be so straightforwardly established. The red treatment leads indeed to a higher content in total PBPs, which could relate to the complementary chromatic adaptation or CCA–according to which the ratio of PC and PE is altered, and can produce a more efficient photosynthetic process [6]. Results for photosynthetic efficiency were, nevertheless, inconsistent with these data—at least for the red treatment under high light intensity. Fv/Fm considers the ratio between variable and maximum fluorescence of chl a, which indicates the maximum quantum yield of PSII—and an indicator of “good health” of the photosynthetic apparatus in the organism at stake; the higher its value, the best their maximum quantum yield, and probably the higher the growth and biomass productivities. This value was high for all treatments, except red light under high intensity, thus indicating poor photosynthetic performance–probably induced by saturation of the photosystem. Additionally, NPQ values exhibited an elevated value that unfolds a loss of efficiency in the photosynthetic apparatus, meaning that energy is not harnessed for photochemical reactions, but probably turns out dissipated as heat [30]. NPQ is a photoprotective mechanism present in cyanobacteria, but also in other photosynthetic organisms (e.g., plants, algae), which relies on the conversion and dissipation of the excess excitation energy to heat. In cyanobacteria, this mechanism involves conformational changes of an orange carotenoid protein (OCP) that binds to the phycobilisome and leads to energy quenching in the phycobiliproteins–thus significantly decreasing the amount of energy reaching the PSII reaction centers [44]. This mechanism is usually activated under blue-green light; however, OCP was demonstrated to play an important role in photoprotection under strong orange-red light, by decreasing the singlet oxygen concentration, potentially causing oxidative stress of the cells [44,45]. Possibly under the red treatment, the dissipation of heat by NPQ is promoted by other routes, including carotenoids (e.g., β-carotene, zeaxanthin) present near the highly excited chl a at the core of the photosynthetic apparatus. Moreover, the ETR did not show significant alterations, as the electron transfer rate can be maintained if this extra energy is freed through the NPQ mechanism; hence, the photosynthetic system is probably saturated [46].
On the other hand, the white control was shown to be the best for biomass growth and productivity. This is consistent with data regarding the highest chl a and total PBPs content, concomitant with the pertaining to photosynthetic efficiency with increased Fv/Fm, increasing trend of ETR, and low NPQ. The efficient photosynthetic performance relates to the absorption spectra of pigments overlapping LED wavelengths (i.e., 450 and 540 nm). Therefore, PBPs efficiently funnel the energy to chl a that is directly transferred to the PSII reaction centers, with minimal losses in energy efficiency (i.e., reduced NPQ).
Significant differences in kinetic parameters, in terms of light intensities, were not found. The photosynthetic rate is directly proportional to light irradiance; light intensity below light saturation point leads to increased photosynthetic performance, along with higher biomass productivities and rates of CO2 uptake [47]. If light overcomes the critical point of light saturation, then cultures enter a state of photooxidation– which potentially leads to damage in the photosystem apparatus and would possibly compromise overall photosynthetic efficiency, thus jeopardizing the growth of those microorganisms. For this study, it seems that the light intensities chosen did not reach the level that is common for light saturation, as photoinhibition was observed in none of those light treatments. This observation is also corroborated by the content of total carotenoids and chl a. Cyanobacterial accumulation of chl a and carotenoids constitutes an important factor in the regulation of photosynthesis and growth [48,49]. In general, under high light intensities—and usually when the saturation point is overpassed—chl a tends to substantially decline, (i.e. Car/Chl a increases) due to photoinhibition [50]. In this work, both chl a and carotenoids underwent a positive response under all tested wavelengths at high light intensities (150 μphotons·m−2·s−1); it seems that light curves did not attain a plateau level or photoinhibition, and that both chl a and carotenoids accumulation are favored, as cells tend to optimize the capture of available light.
4.2. Acclimatization and Pigment Content and Profile
In this study, all pigments evaluated were influenced by the quality (and intensity) of light; and S. salina was able to respond to the environmental alteration by readapting its photosynthetic pigment antenna. On the one hand, green light (high intensity) favored accumulation of chl a, whilst total carotenoids were produced under white light (control); on the other hand, white treatment (control) and red light (both intensities) stimulated production of both PBPs, and each one individually. The accumulation of chl a under green light was also previously observed for cyanobacterium Gloeothece membranacea, which showed an increase of 1.12- and 1.6-fold when compared to the control (white light) [43]. Cyanobium sp. subjected to five different light conditions (white, green, red, blue, and UV) also preferred green light for chl a accumulation [40]. In fact, green light has been reported to be the light less efficiently absorbed [51]; however, its supplementation can help deepen penetration into dense cyanobacterial cultures, and become more absorbed than other wavelengths [52].
Total carotenoids were favored by the high light intensity in the white treatment; the effects of green treatment and red light under high intensities (in addition to white treatment) upon total carotenoid content are also noteworthy, including some carotenoids individually: results of lycopene (red), zeaxanthin (green), echinenone (green), and β-carotene (green) can be explained as part of the strategy for acclimatization. In cyanobacteria, carotenoids have the main role in energy dissipation and protection against oxidative damage [15,53]. Some studies show that, depending on the species, some cyanobacteria are more impacted by green or red light in terms of enhanced carotenoid production. For instance, Pseudanabaena sp. increased its content of carotenoids by 30%, under green light when compared to white light [54]; this contradicts the results for Cyanobium sp. with two-phase cultivation, using white and red LEDs–with a 50% increase in carotenoids under the red phase when compared to the white phase [21]. These differences can be related not only to the PBP composition of each species, but also to the possible existence of a green-red photoreceptor involved in carotenoid production by certain cyanobacteria [53].
The total PBP production and each PBP individually were chiefly promoted by red light treatment (apart from control, p > 0.05). These pigments tend to absorb under a yellow-red range, and several studies point at the red light as a stimulator of biomass growth and increased production of PBPs (in particular PC) [11,40,55,56]. For instance, in Arthrospira platensis subjected to different light qualities using LED (red, white, green, and yellow), PC was preferentially stimulated under the red spectrum [55]. These pigments have massive importance for the mechanisms of adaptation and regulation to different types of light; most cyanobacteria possess CCA mechanisms that help in transient reconfiguration of their phycobilisome, with an alteration of PC/PE ratios, in order to better adapt the organism to the quality and quantity of incident radiation from the environment [6]. In addition, green/red light modulation produces a response in PC/PE levels, and an alteration in the corresponding absorption properties of the phycobilisome [6,57]. S. salina seems to fit in Type III acclimatization. The application of light shock supported increase in the PC/PE ratios under red treatments (particularly at low intensity); whereas the green treatment led to a slight increase in PE over time. PE absorbs under the green range, and this realization agrees with said results. Moreover, literature reports have it that PBP production is preferentially obtained under low to medium intensities of light, since high light intensities can cause photoinhibition [12]. However, the results are somehow contradictory, because the effect of light intensity was not relevant across the red treatments—it instead stood out for white light under high intensity. Possibly for both white and red lights, S. salina can stand higher intensities as the maximum photoinhibition threshold has not yet been reached. Recall that the overall process of chromatic adaptation is not straightforward, and is highly strain-dependent. In addition, it depends on the physiological age of the cultures, and the genetic/molecular regulation of the chromatic adaptation itself [6,57].
4.3. Pigment Productivities and Biotechnological Perspectives
In light of a biotechnological perspective, chromatic light effects could be used as a tool to enhance production of certain natural pigments in cyanobacterial cultures. Therefore, this study provided a few hints about the strategy to follow with LED treatment/modulation for this species of cyanobacterium, depending on the target pigment. The use of LEDs should also meet requirements in terms of growth and biomass accumulation; in this work, both specific growth rate and biomass productivities seemed quite low when compared to the literature, even under the first stage of cultivation used for control. For instance, S. salina is able to achieve maximum growth rates of 0.3 d−1 under fluorescent light conditions (100 μmolphotons·m−2·s−1), with productivities of ca. 1.5 g·L−1·d−1 (data not shown). LED quality does not seem to be limiting to S. salina growth, but the chosen intensities were apparently low. In fact, it has been reported that Synechocystis (strain PCC 6803) can stand up to 500 μmolphotons·m−2·s−1 with high growth and photosynthetic performance (under a specific temperature of 30 °C and pH 8); however, photoinhibition occurs when light intensity exceeds 800 μmolphotons·m−2·s−1 [38]. Therefore, we believe that higher growth rates, biomass production, and possibly enhanced pigment production could be obtained by increasing the light intensity of LED; unfortunately, the selected intensities could not be further increased, due to LED equipment limitations.
In terms of pigments, the exposure of S. salina to green light (high intensity) positively influenced the production of chl a, whilst white light enhanced carotenoid productivities in addition to positively influencing production of zeaxanthin and β-carotene; these are important pigments for use by the food and aquaculture industries as nutraceuticals or colorants [15,53]. Focusing on PC, one of the most important pigments produced by S. salina and important for their market value (noting that these pigments are expanding in the market, due to their unique colorant and antioxidant properties [5]), red shock treatment could be chosen to enhance their productivities. This selection seems more suitable than white light, because the latter takes 5 more days to reach a similar productivity. A study by Pagels et al. (2020) [21] also unfolded the positive effect of the two-phase, white-red light treatment strategy; PC productivities were improved within 14 days (10 days of white + 4 days of red), instead of needing 21 days of cultivation.
Our option for low intensity may also be regarded as a cost-saving one. The energy consumption by the process is a relevant parameter that affects the whole microalgal production costs. In view of this, low energy consumption techniques might be preferred over high energy consumption regarding artificial light [58]. In addition, the use of LED-tailored lighting will probably be elected in the near future as the light source for microalgal and cyanobacterial biomass cultivation at large scale, and concomitant production of specific pigments and other value-added compounds (at least indoors). They entail indeed lower energy consumption, lower maintenance requirements, and wider lifespan than common fluorescent light. A few studies conducted with light modulation for cyanobacteria have emerged in recent years [20,21,55,59,60], as LED quality and efficiency have been steadily increasing [20].
Finally, fundamental studies pertaining to light modulation are quite important to pinpoint and understand which optimization strategies are to be followed toward optimization of the content of desired pigments. This study, in particular, showed the capacity of S. salina to acclimate to different light conditions, fitting in type III of acclimatization. From a biotechnological perspective, the modulation with white and red light (10 days of white + 5 days of red) proved a relevant strategy to enhance PC productivity. There is no need to cultivate this cyanobacterium over 22 days, because similar productivities can be achieved 7 days earlier when compared to white light. In an commercial microalgal bioprocess, this strategy may have significant impacts regarding financial and energy savings, a deed quite appealing from a biotechnological perspective.
Author Contributions
A.C.G., F.P. and J.A., conceived and designed experiments. J.A. conducted experiments. J.A., F.P., and T.T. performed analytical measurements. J.A. wrote the manuscript draft. F.X.M. and A.C.G. were responsible for supervision (including revision of manuscript) and funding acquirement. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the following: national funds through FCT, within the scope of UIDB/04423/2020 and UIDP/04423/2020 granted to Interdisciplinary Centre of Marine and Environmental Research (CIIMAR); and national funds through FCT/MCTES (PIDDAC), within the scope of LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDB/00511/2020 (LEPABE), granted to Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE). A PhD fellowship (PD/BD/137887/2018) for author JA, under the joint supervision of authors FXM and ACG, was granted by FCT, under the auspices of Programa Operacional Capital Humano (POCH) supported by the European Social Fund (ESF) and Portuguese funds (MECTES).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgments
We thank Professor Isabel Sousa Pinto, team leader of the Laboratory of Coastal Biodiversity (CIIMAR), for making available the facilities and some equipment to perform the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khajepour, F.; Hosseini, S.A.; Ghorbani Nasrabadi, R.; Markou, G. Effect of light intensity and photoperiod on growth and biochemical composition of a local isolate of Nostoc calcicola. Appl. Biochem. Biotechnol. 2015, 176, 2279–2289. [Google Scholar] [CrossRef]
- Schagerl, M.; Müller, B. Acclimation of chlorophyll a and carotenoid levels to different irradiances in four freshwater cyanobacteria. J. Plant Physiol. 2006, 163, 709–716. [Google Scholar] [CrossRef]
- Porra, R.J.; Pfundel, E.E.; Endel, N. Metabolism and function of photosynthetic pigments. In Phytoplankton Pigments in Oceanography: Guidelines to Modern Methods; Jeffrey, S.W., Mantoura, R.F.C., Wright, S.W., Eds.; UNESCO Publishing: Paris, France, 1997; pp. 85–126. [Google Scholar]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Chanu, N.K.; Chaurasia, N. Cyanobacterial pigments and their fluorescence characteristics: Applications in research and industry. In Advances in Cyanobacterial Biology; Singh, P.K., Kumar, A., Singh, V.K., Shrivastava, A.K., Eds.; Academic Press: New York, NY, USA, 2020; pp. 55–72. [Google Scholar]
- Kehoe, D.M. Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 9029–9030. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Sonani, R.R.; Prasad Rastogi, R.; Madamwar, D. The phycobilisomes: An early requisite for efficient photosynthesis in cyanobacteria. EXCLI J. 2015, 14, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Gutu, A.; Kehoe, D.M. Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol. Plant 2012, 5, 1–13. [Google Scholar] [CrossRef]
- Brunet, C.; Johnsen, G.; Lavaud, J.; Roy, S. Pigments and photoacclimation processes. In Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: New York, NY, USA, 2011. [Google Scholar]
- Heydarizadeh, P.; Poirier, I.; Loizeau, D.; Ulmann, L.; Mimouni, V.; Schoefs, B.; Bertrand, M. Plastids of marine phytoplankton produce bioactive pigments and lipids. Mar. Drugs 2013, 11, 3425–3471. [Google Scholar] [CrossRef]
- Ojit, S.K.; Indrama, T.; Gunapati, O.; Avijeet, S.O.; Subhalaxmi, S.A.; Silvia, C.; Indira, D.W.; Romi, K.; Thadoi, D.A.; Tiwari, O.N.; et al. The response of phycobiliproteins to light qualities in Anabaena circinalis. J. Appl. Biol. Biotechnol. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Lau, N.; Matsui, M.; Abdullah, A.A. Cyanobacteria: Photoautotrophic microbial factories for the sustainable synthesis of industrial products. Biomed Res. Int. 2015, 2015, 754934. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef] [PubMed]
- Assunção, J.; Amaro, H.M.; Malcata, F.X.; Guedes, A.C. Cyanobacterial pigments: Photosynthetic function and biotechnological purposes. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Elsevier Academic Press: London, UK, 2022; ISBN 9780128214916. [Google Scholar]
- Milia, M.; Corrias, F.; Addis, P.; Zitelli, G.C.; Cicchi, B.; Torzillo, G.; Andreotti, V.; Angioni, A. Influence of Different Light Sources on the Biochemical Composition of Arthrospira spp. Grown in Model Systems. Foods 2022, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Niangoran, N.U.F.; Buso, D.; Zissis, G.; Prudhomme, T. Influence of light intensity and photoperiod on energy efficiency of biomass and pigment production of Spirulina (Arthrospira platensis). OCL 2021, 28, 37. [Google Scholar] [CrossRef]
- Assunção, J.; Malcata, F.X. Enclosed “non-conventional” photobioreactors for microalga production: A review. Algal Res. 2020, 52, 102107. [Google Scholar] [CrossRef]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef]
- Glemser, M.; Heining, M.; Schmidt, J.; Becker, A.; Garbe, D.; Buchholz, R.; Brück, T. Application of light-emitting diodes (LEDs) in cultivation of phototrophic microalgae: Current state and perspectives. Appl. Microbiol. Biotechnol. 2016, 100, 1077–1088. [Google Scholar] [CrossRef]
- Pagels, F.; Lopes, G.; Vasconcelos, V.; Guedes, A.C. White and red LEDs as two-phase batch for cyanobacterial pigments production. Bioresour. Technol. 2020, 307, 123105. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Synechocystis salina: Potential bioactivity and combined extraction of added-value metabolites. J. Appl. Phycol. 2021, 33, 3731–3746. [Google Scholar] [CrossRef]
- Afonso, T.B.; Costa, M.S.; Rezende De Castro, R.; Freitas, S.; Silva, A.; Schneider, M.P.C.; Martins, R.; Leão, P.N. Bartolosides E-K from a Marine coccoid cyanobacterium. J. Nat. Prod. 2016, 79, 2504–2513. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Bland, E.; Angenent, L.T. Pigment-targeted light wavelength and intensity promotes efficient photoautotrophic growth of Cyanobacteria. Bioresour. Technol. 2016, 216, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Pojidaeva, E.; Zinchenko, V.; Shestakov, S.V.; Sokolenko, A. Involvement of the SppA1 peptidase in acclimation to saturating light intensities in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2004, 186, 3991–3999. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef]
- Montgomery, B.L. Mechanisms and fitness implications of photomorphogenesis during chromatic acclimation in cyanobacteria. J. Exp. Bot. 2016, 67, 4079–4090. [Google Scholar] [CrossRef]
- Palenik, B. Chromatic Adaptation in Marine. Microbiology 2001, 67, 991–994. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Jerez, C.G.; Korbee, N. Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat. Am. J. Aquat. Res. 2013, 41, 801–819. [Google Scholar] [CrossRef]
- Pagels, F.; Barufi, J.B.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light regulating metabolic responses of cyanobium sp. (cyanobacteria). Fundam. Appl. Limnol. 2020, 193, 285–297. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Domínguez-González, B.; Korbee, N. Vulnerability and acclimation to increased UVB radiation in three intertidal macroalgae of different morpho-functional groups. Mar. Environ. Res. 2014, 97, 30–38. [Google Scholar] [CrossRef]
- Johnsen, G.; Sakshaug, E. Biooptical characteristics of PSII and PSI in 33 species (13 pigment groups) of marine phytoplankton, and the relevance for pulseamplitude-modulated and fast-repetition-rate fluorometry. J. Phycol. 2007, 43, 1236–1251. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1994, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Pereira, R.D.; Malcata, F.X. Effects of temperature and pH on growth and antioxidant content of the microalga Scenedesmus obliquus. Biotechnol. Prog. 2011, 27, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Cordara, A.; Re, A.; Pagliano, C.; Van Alphen, P.; Pirone, R.; Saracco, G.; dos Santos, F.B.; Hellingwerf, K.; Vasile, N. Analysis of the light intensity dependence of the growth of Synechocystis and of the light distribution in a photobioreactor energized by 635 nm light. PeerJ 2018, 6, e5256. [Google Scholar] [CrossRef]
- Chen, H.B.; Wu, J.Y.; Wang, C.F.; Fu, C.C.; Shieh, C.J.; Chen, C.I.; Wang, C.Y.; Liu, Y.C. Modeling on chlorophyll a and phycocyanin production by Spirulina platensis under various light-emitting diodes. Biochem. Eng. J. 2010, 53, 52–56. [Google Scholar] [CrossRef]
- Pagels, F.; Bonomi-Barufi, J.; Vega, J.; Abdala-Díaz, R.; Vasconcelos, V.; Guedes, A.C.; Figueroa, F.L. Light quality triggers biochemical modulation of Cyanobium sp.—photobiology as tool for biotechnological optimization. J. Appl. Phycol. 2020, 32, 2851–2861. [Google Scholar] [CrossRef]
- Da Fontoura Prates, D.; Radmann, E.M.; Duarte, J.H.; de Morais, M.G.; Costa, J.A.V. Spirulina cultivated under different light emitting diodes: Enhanced cell growth and phycocyanin production. Bioresour. Technol. 2018, 256, 38–43. [Google Scholar] [CrossRef]
- Tian, F.; Buso, D.; Wang, T.; Lopes, M.; Niangoran, U.; Zissis, G. Effect of Red and Blue LEDs on the Production of Phycocyanin by Spirulina Platensis Based on Photosynthetically Active Radiation. J. Sci. Technol. Light. 2018, 41, 148–152. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Kirilovsky, D. Changing Color for Photoprotection: The Orange Carotenoid Protein. Trends Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Sedoud, A.; López-Igual, R.; Rehman, A.U.; Wilson, A.; Perreau, F.; Boulay, C.; Vass, I.; Krieger-Liszkay, A.; Kirilovsky, D. The cyanobacterial photoactive orange carotenoid protein is an excellent singlet oxygen quencher. Plant Cell 2014, 26, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, M.Y.; Kuzminov, F.I.; Fadeev, V.V.; Kim, J.D.; Falkowski, P.G. A kinetic model of non-photochemical quenching in cyanobacteria. Biochim. Biophys. Acta Bioenerg. 2011, 1807, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Ravelonandro, P.H.; Ratianarivo, D.H.; Joannis-cassan, C. Influence of light quality and intensity in the cultivation of Spirulina platensis from Toliara (Madagascar) in a closed system. J. Chem. Tecnol. Biotechnol. 2008, 848, 842–848. [Google Scholar] [CrossRef]
- Paerl, H.W. Cyanobacterial carotenoids: Their roles in maintaining optimal photosynthetic production among aquatic bloom forming genera. Oecologia 1984, 61, 143–149. [Google Scholar] [CrossRef]
- Kehoe, D.M.; Gutu, A. Responding to color: The regulation of complementary chromatic adaptation. Annu. Rev. Plant Biol. 2006, 57, 127–150. [Google Scholar] [CrossRef]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum—A prospective phycobiliprotein-producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 328. [Google Scholar] [CrossRef]
- Mattos, E.R.; Singh, M.; Cabrera, M.L.; Das, K.C. Enhancement of biomass production in Scenedesmus bijuga high-density culture using weakly absorbed green light. Biomass Bioenergy 2015, 81, 473–478. [Google Scholar] [CrossRef]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Maurya, R.R.; Patidar, S.K.; Haldar, S.; Mishra, S. Effect of light quality on the C-phycoerythrin production in marine cyanobacteria Pseudanabaena sp. isolated from Gujarat coast, India. Protein Expr. Purif. 2012, 81, 5–10. [Google Scholar] [CrossRef]
- Rivera, C.; Niño, L.; Gelves, G. Modeling of phycocyanin production from Spirulina platensis using different light-emitting diodes. S. Afr. J. Chem. Eng. 2021, 37, 167–178. [Google Scholar] [CrossRef]
- Keithellakpam, O.S.; Nath, T.O.; Oinam, A.S.; Thingujam, I.; Oinam, G.; Dutt, S.G. Effect of external pH on cyanobacterial phycobiliproteins production and ammonium excretion. J. Appl. Biol. Biotechnol. 2015, 3, 38–42. [Google Scholar] [CrossRef]
- Johnson, E.M.; Kumar, K.; Das, D. Physicochemical parameters optimization, and purification of phycobiliproteins from the isolated Nostoc sp. Bioresour. Technol. 2014, 166, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Fu, C.C.; Liu, Y.C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Van Hieu, H.; Quang-Tuong, L.; Cong Doan, B. The Production of High Phycocyanin by Applications of Red Light-Emitting Diodes (LEDs) In Vitro Algae Growth on Spirulina platensis. J. Nano-Electron. Phys. 2021, 13, 03034. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).