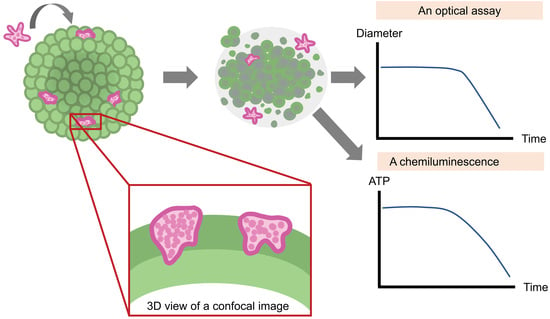
An Optical and Chemiluminescence Assay for Assessing the Cytotoxicity of Balamuthia mandrillaris against Human Neurospheroids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture of B. mandrillaris from the Biopsied Brain of a Human Subject
2.2. Culture of Human Neuroblastoma SH-SY5Y Cells
2.3. Formation of Human Neurospheroids
2.4. Culture of B. mandrillaris and Cocultures with Human Neurospheroids
2.5. Cell Labeling with Fluorescent Dye and Confocal Microscopy
2.6. Reverse-Transcription PCR
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
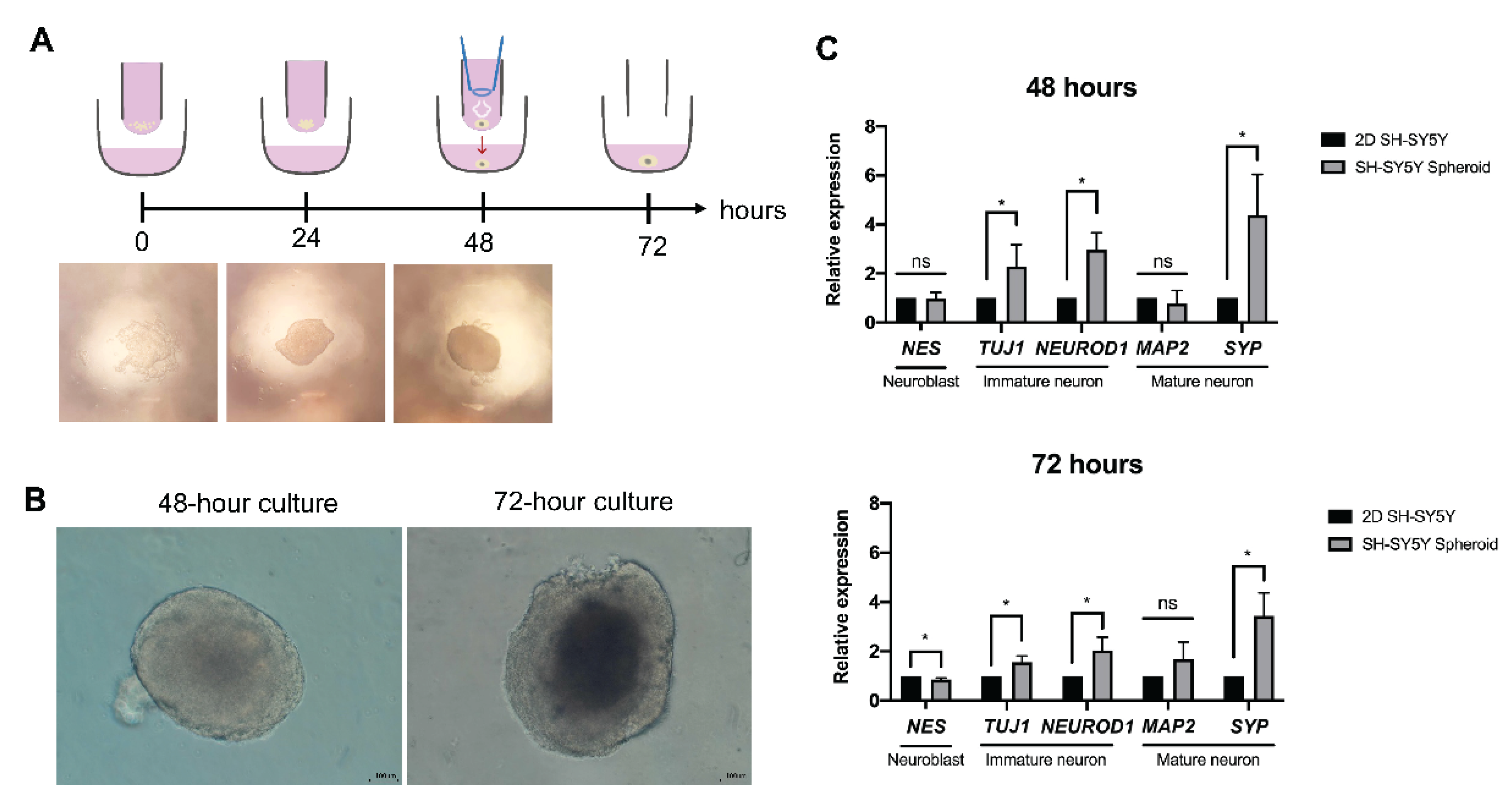
3.1. Microscopic Observation of Human Neurospheroids
3.2. Neuron-Specific Transcript of Human Neurospheroid
3.3. Decrease in the Size of Neurospheroids Post Coculture with B. mandrillaris Trophozoites
3.4. Cytotoxicity Assay
3.5. Properties of the Neurospheroid at the Molecular Level
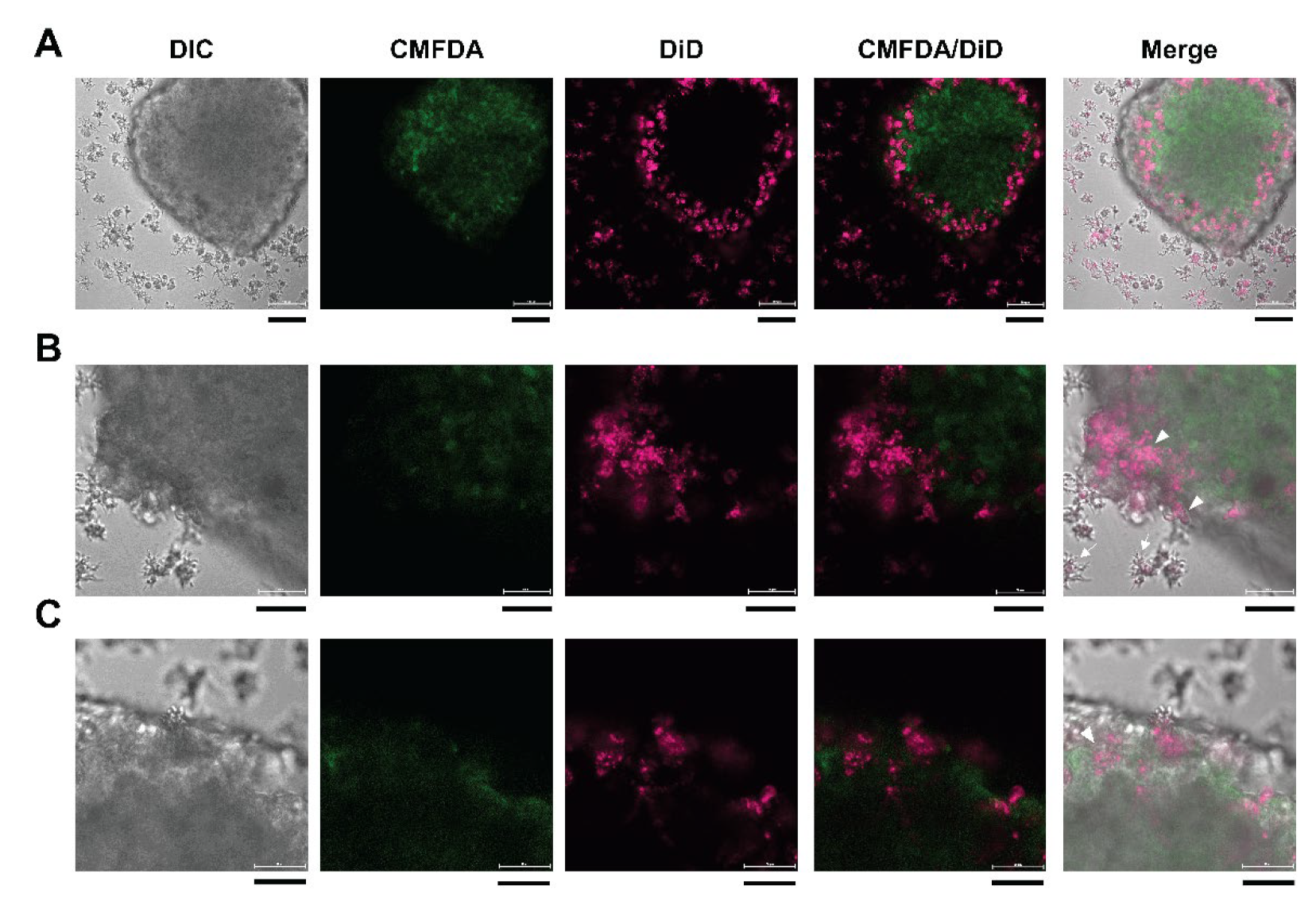
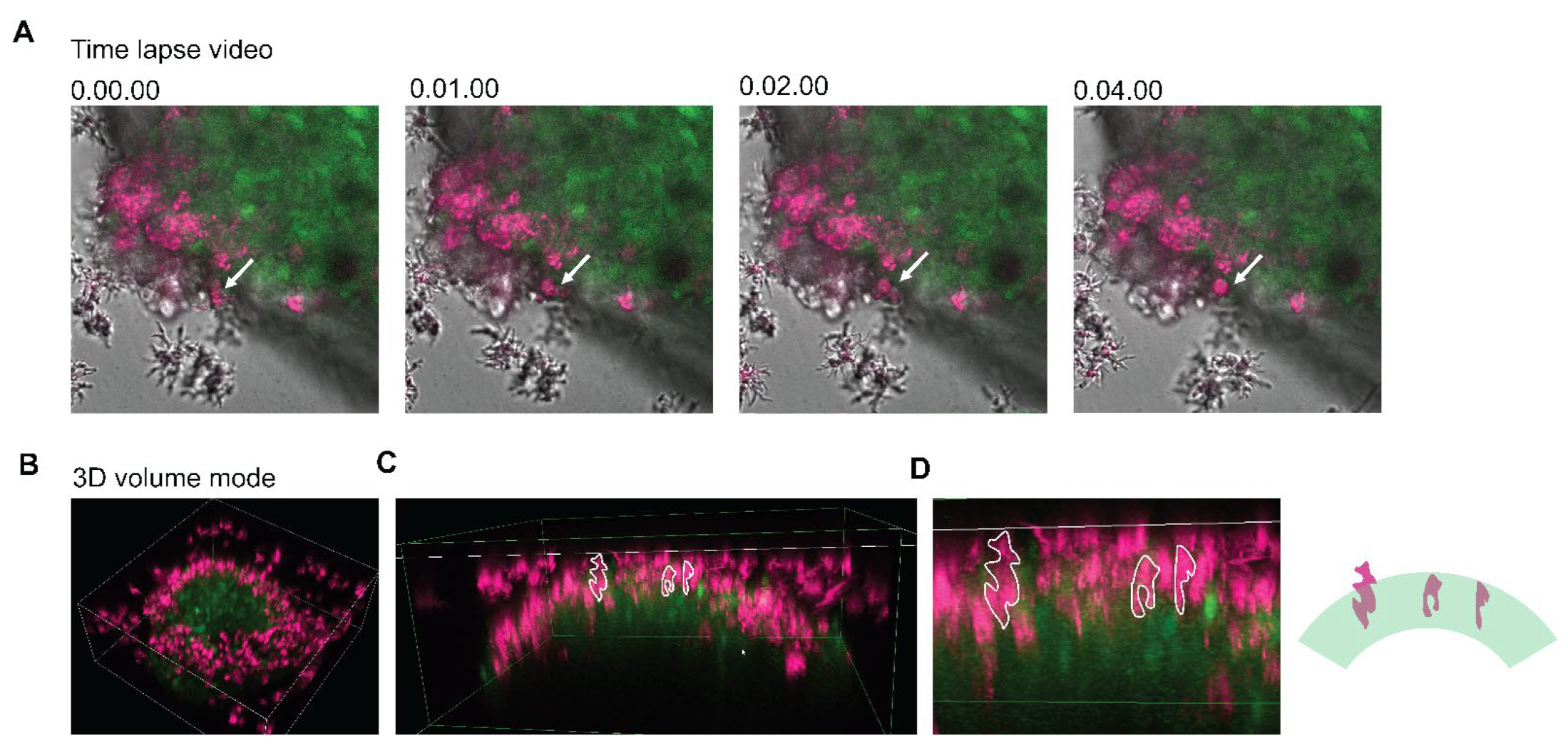
3.6. 3D Imaging of Neurospheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3d scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Lagies, S.; Schlimpert, M.; Neumann, S.; Waldin, A.; Kammerer, B.; Borner, C.; Peintner, L. Cells grown in three-dimensional spheroids mirror in vivo metabolic response of epithelial cells. Commun. Biol. 2020, 3, 246. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Lin, C.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [Green Version]
- Mittler, F.; Obeid, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-content monitoring of drug effects in a 3d spheroid model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Han, S.; Sanny, A.; Chan, D.L.; van Noort, D.; Lim, W.; Tan, A.H.; Park, S. 3d hanging spheroid plate for high-throughput car t cell cytotoxicity assay. J. Nanobiotechnol. 2022, 20, 30. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Kapalczynska, M.; Kolenda, T.; Przybyla, W.; Zajaczkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Blizniak, R.; Luczewski, L.; Lamperska, K. 2d and 3d cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar]
- Piper, K.J.; Foster, H.; Susanto, D.; Maree, C.L.; Thornton, S.D.; Cobbs, C.S. Fatal balamuthia mandrillaris brain infection associated with improper nasal lavage. Int. J. Infect. Dis. 2018, 77, 18–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gompf, S.G.; Garcia, C. Lethal encounters: The evolving spectrum of amoebic meningoencephalitis. IDCases 2019, 15, e00524. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L.; Visvesvara, G.S. Opportunistic amoebae: Challenges in prophylaxis and treatment. Drug Resist. Updates 2004, 7, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.S.; Campbell, E.; Fuller, A.; Spelman, D.W.; Cameron, R.; Malham, G.; Gin, D.; Lewin, S.R. Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. J. Neurosurg. 2011, 114, 458–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuoco, J.A.; Klein, B.J.; LeBel, D.P.; Faulhaber, J.; Apfel, L.S.; Witcher, M.R. Successful treatment of a balamuthia mandrillaris cerebral abscess in a pediatric patient with complete surgical resection and antimicrobial therapy. Pediatr. Infect. Dis. J. 2022, 41, e54–e57. [Google Scholar] [CrossRef] [PubMed]
- Laurie, M.T.; White, C.V.; Retallack, H.; Wu, W.; Moser, M.S.; Sakanari, J.A.; Ang, K.; Wilson, C.; Arkin, M.R.; De Risi, J.L. Functional assessment of 2177 u.S. And international drugs identifies the quinoline nitroxoline as a potent amoebicidal agent against the pathogen balamuthia mandrillaris. MBio 2018, 9, e02051–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, C.A.; Colon, B.L.; Chen, E.; Hull, M.V.; Kyle, D.E. Discovery of repurposing drug candidates for the treatment of diseases caused by pathogenic free-living amoebae. PLoS Negl. Trop. Dis. 2020, 14, e0008353. [Google Scholar] [CrossRef]
- Elyasi, L.; Jahanshahi, M.; Jameie, S.B.; Hamid Abadi, H.G.; Nikmahzar, E.; Khalili, M.; Jameie, M.; Jameie, M. 6-ohda mediated neurotoxicity in sh-sy5y cellular model of parkinson disease suppressed by pretreatment with hesperidin through activating l-type calcium channels. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Peng, W.N.; Deng, Y.; Li, J.J.; Tian, X.R. Action of trichostatin a on alzheimer’s disease-like pathological changes in sh-sy5y neuroblastoma cells. Neural. Regen. Res. 2020, 15, 293–301. [Google Scholar] [CrossRef]
- Ho, B.X.; Pek, N.M.Q.; Soh, B.S. Disease modeling using 3d organoids derived from human induced pluripotent stem cells. Int. J. Mol. Sci. 2018, 19, 936. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [Green Version]
- Pengsart, W.; Tongkrajang, N.; Whangviboonkij, N.; Sarasombath, P.T.; Kulkeaw, K. Balamuthia mandrillaris trophozoites ingest human neuronal cells via a trogocytosis-independent mechanism. Parasit. Vectors 2022, 15, 232. [Google Scholar] [CrossRef]
- Almeida, A.S.; Soares, N.L.; Vieira, M.; Gramsbergen, J.B.; Vieira, H.L. Carbon monoxide releasing molecule-a1 (corm-a1) improves neurogenesis: Increase of neuronal differentiation yield by preventing cell death. PLoS ONE 2016, 11, e0154781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piras, S.; Furfaro, A.L.; Brondolo, L.; Passalacqua, M.; Marinari, U.M.; Pronzato, M.A.; Nitti, M. Differentiation impairs bach1 dependent ho-1 activation and increases sensitivity to oxidative stress in sh-sy5y neuroblastoma cells. Sci. Rep. 2017, 7, 7568. [Google Scholar] [CrossRef] [PubMed]
- Jung, G.S.; Lee, K.M.; Park, J.K.; Choi, S.K.; Jeon, W.B. Morphogenetic and neuronal characterization of human neuroblastoma multicellular spheroids cultured under undifferentiated and all-trans-retinoic acid-differentiated conditions. BMB Rep. 2013, 46, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.I.; Lee, J.; Sung, Y.H.; Oh, J.; Hyeon, D.Y.; Kim, Y.; Lee, S.; Devkota, S.; Kim, H.J.; Park, B.; et al. Impaired akt signaling and lung tumorigenesis by pierce1 ablation in kras-mutant non-small cell lung cancer. Oncogene 2020, 39, 5876–5887. [Google Scholar] [CrossRef] [PubMed]
- Anada, T.; Fukuda, J.; Sai, Y.; Suzuki, O. An oxygen-permeable spheroid culture system for the prevention of central hypoxia and necrosis of spheroids. Biomaterials 2012, 33, 8430–8441. [Google Scholar] [CrossRef]
- Leek, R.; Grimes, D.R.; Harris, A.L.; McIntyre, A. Methods: Using three-dimensional culture (spheroids) as an in vitro model of tumour hypoxia. Adv. Exp. Med. Biol. 2016, 899, 167–196. [Google Scholar]
- Biedler, J.L.; Roffler-Tarlov, S.; Schachner, M.; Freedman, L.S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978, 38, 3751–3757. [Google Scholar]
- Gilany, K.; Van Elzen, R.; Mous, K.; Coen, E.; Van Dongen, W.; Vandamme, S.; Gevaert, K.; Timmerman, E.; Vandekerckhove, J.; Dewilde, S.; et al. The proteome of the human neuroblastoma cell line sh-sy5y: An enlarged proteome. Biochim. Biophys. Acta 2008, 1784, 983–985. [Google Scholar] [CrossRef]
- Kodet, R.; Nohynkova, E.; Tichy, M.; Soukup, J.; Visvesvara, G.S. Amebic encephalitis caused by balamuthia mandrillaris in a czech child: Description of the first case from europe. Pathol. Res. Pract. 1998, 194, 423–429. [Google Scholar] [CrossRef]
- Stidd, D.A.; Root, B.; Weinand, M.E.; Anton, R. Granulomatous amoebic encephalitis caused by balamuthia mandrillaris in an immunocompetent girl. World Neurosurg. 2012, 78, 715.e7–715.e12. [Google Scholar] [CrossRef]
- Bando, Y.; Takahashi, T.; Uehara, H.; Kagegi, T.; Nagahiro, S.; Izumi, K. Autopsy case of amebic granulomatous meningoencephalitis caused by balamuthia mandrillaris in japan. Pathol. Int. 2012, 62, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Krasaelap, A.; Prechawit, S.; Chansaenroj, J.; Punyahotra, P.; Puthanakit, T.; Chomtho, K.; Shuangshoti, S.; Amornfa, J.; Poovorawan, Y. Fatal balamuthia amebic encephalitis in a healthy child: A case report with review of survival cases. Korean J. Parasitol. 2013, 51, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Tweedell, R.E.; Tao, D.; Hamerly, T.; Robinson, T.M.; Larsen, S.; Gronning, A.G.B.; Norris, A.M.; King, J.G.; Law, H.C.H.; Baumbach, J.; et al. The selection of a hepatocyte cell line susceptible to plasmodium falciparum sporozoite invasion that is associated with expression of glypican-3. Front. Microbiol. 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca-Berzal, C.; Aran, V.J.; Escario, J.A.; Gomez-Barrio, A. Experimental models in chagas disease: A review of the methodologies applied for screening compounds against trypanosoma cruzi. Parasitol. Res. 2018, 117, 3367–3380. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 2020, 27, 937–950.e939. [Google Scholar] [CrossRef]
- Yi, S.A.; Nam, K.H.; Yun, J.; Gim, D.; Joe, D.; Kim, Y.H.; Kim, H.J.; Han, J.W.; Lee, J. Infection of brain organoids and 2d cortical neurons with sars-cov-2 pseudovirus. Viruses 2020, 12, 1004. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.T.; Lee, J.S.; Shin, J.; Cui, B.; Yang, K.; Choi, Y.S.; Choi, N.; Lee, S.H.; Lee, J.H.; et al. Fungal brain infection modelled in a human-neurovascular-unit-on-a-chip with a functional blood-brain barrier. Nat. Biomed. Eng. 2021, 5, 830–846. [Google Scholar] [CrossRef]

| Target Gene | Primer Sequence (5′ to 3′) | Reference |
|---|---|---|
| Nestin (Neural progenitor marker) | Fw: CTGTGAGTGTCAGTGTCCCC Rv: CTCTAGAGGGCCAGGGACTT | [21] Almeida et al., 2016 |
| Tuj1 (Neuron-specific class III beta-tubulin; Early stage neural differentiation marker) | Fw: GCAAGGTGCGTGAGGAGTAT Rv: GTCTGACACCTTGGGTGAGG | [21] Almeida et al., 2016 |
| NeuroD1 (Mature neuronal marker) | Fw: CCCTTCCTTTGATGGACCCC Rv: AAATGGTGAAACTGGCGTGC | [22] Piras et al., 2017 |
| MAP2 (Mature neuronal marker) | Fw: GGAGCTGAGTGGCTTGTCAT Rv: CTAGCTCCAGACAGACGCAG | [21] Almeida et al., 2016 |
| SYP (Synaptophysin; differentiated postmitotic neuronal cell marker) | Fw: TCCTCGTCAGCCGAATTCTTT Rv: CTCGCTACTTGTTCTGCAGGAA | [23] Jung et al., 2012 |
| Bcl-2 (Anti-apoptotic gene marker) | Fw: TCATGTGTGTGGAGAGCGTC Rv: TCAGTCATCCACAGGGCGAT | [21] Almeida et al., 2016 |
| Bax (Apoptotic gene marker) | Fw: TCAGGATGCGTCCACCAAGAAG Rv: TGTGTCCACGGCGGCAATCATC | [24] Roh et al., 2022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pengsart, W.; Kulkeaw, K. An Optical and Chemiluminescence Assay for Assessing the Cytotoxicity of Balamuthia mandrillaris against Human Neurospheroids. Bioengineering 2022, 9, 330. https://doi.org/10.3390/bioengineering9070330
Pengsart W, Kulkeaw K. An Optical and Chemiluminescence Assay for Assessing the Cytotoxicity of Balamuthia mandrillaris against Human Neurospheroids. Bioengineering. 2022; 9(7):330. https://doi.org/10.3390/bioengineering9070330
Chicago/Turabian StylePengsart, Worakamol, and Kasem Kulkeaw. 2022. "An Optical and Chemiluminescence Assay for Assessing the Cytotoxicity of Balamuthia mandrillaris against Human Neurospheroids" Bioengineering 9, no. 7: 330. https://doi.org/10.3390/bioengineering9070330
APA StylePengsart, W., & Kulkeaw, K. (2022). An Optical and Chemiluminescence Assay for Assessing the Cytotoxicity of Balamuthia mandrillaris against Human Neurospheroids. Bioengineering, 9(7), 330. https://doi.org/10.3390/bioengineering9070330