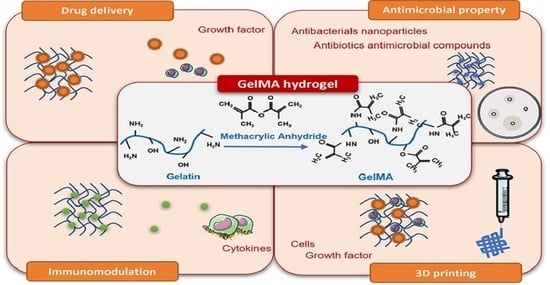
Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration
Abstract
:1. Introduction
2. The Synthesis of GelMA
2.1. The Effect of Reaction Conditions (Buffer, pH and Temperature) on DoM
2.2. The Effect of Gelatin Source on DoM
2.3. Crosslinking Agents for Stabilizing GelMA Hydrogels
2.4. GelMA Nanocomposites
3. Applications of GelMA with a Focus on Musculoskeletal Regeneration
3.1. Drug/Growth Factor Delivery

3.2. Antimicrobial Properties (Antibiotics/Antimicrobial Compounds/Antibacterial Nanoparticles)
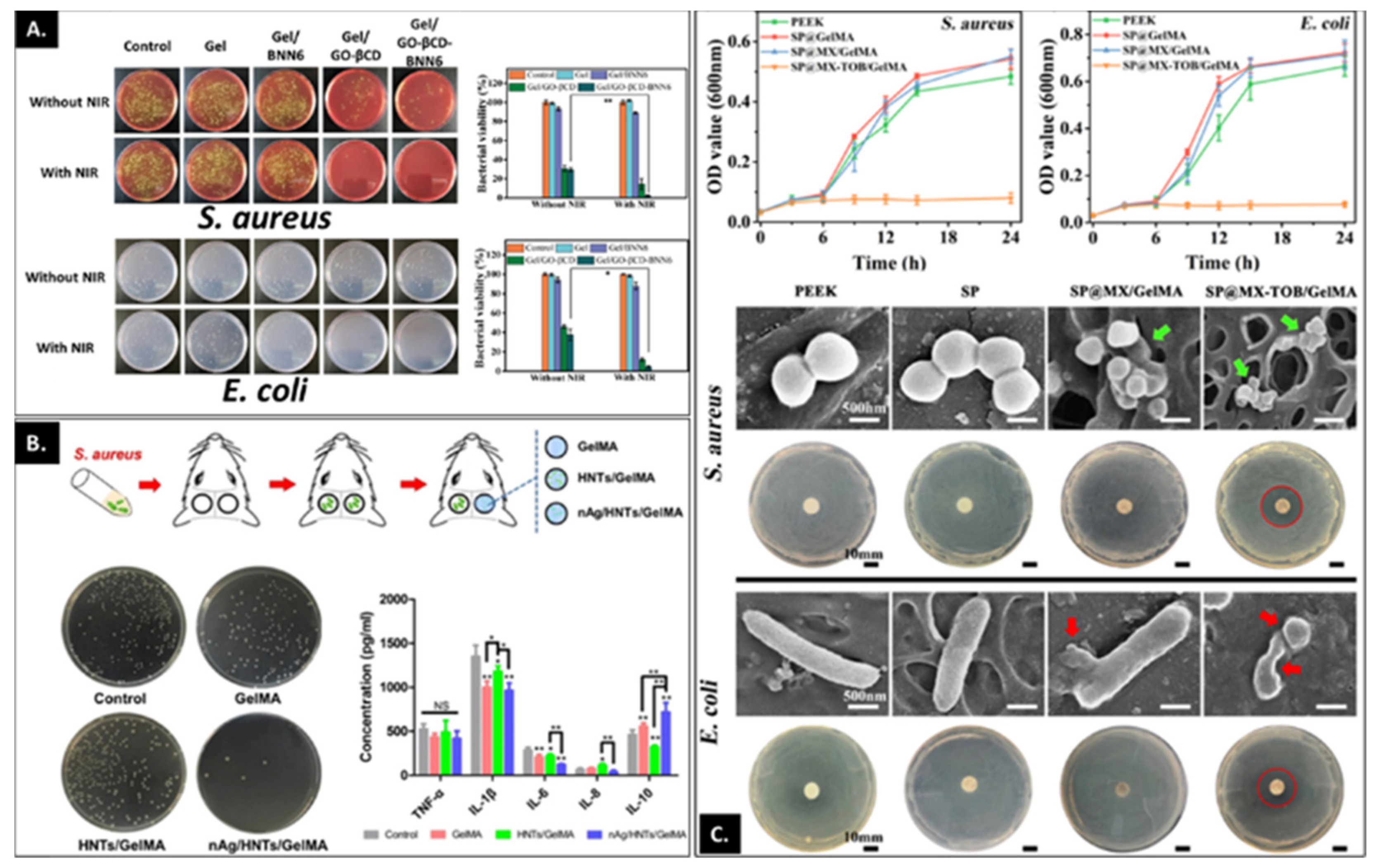
3.3. Modulation of Inflammation
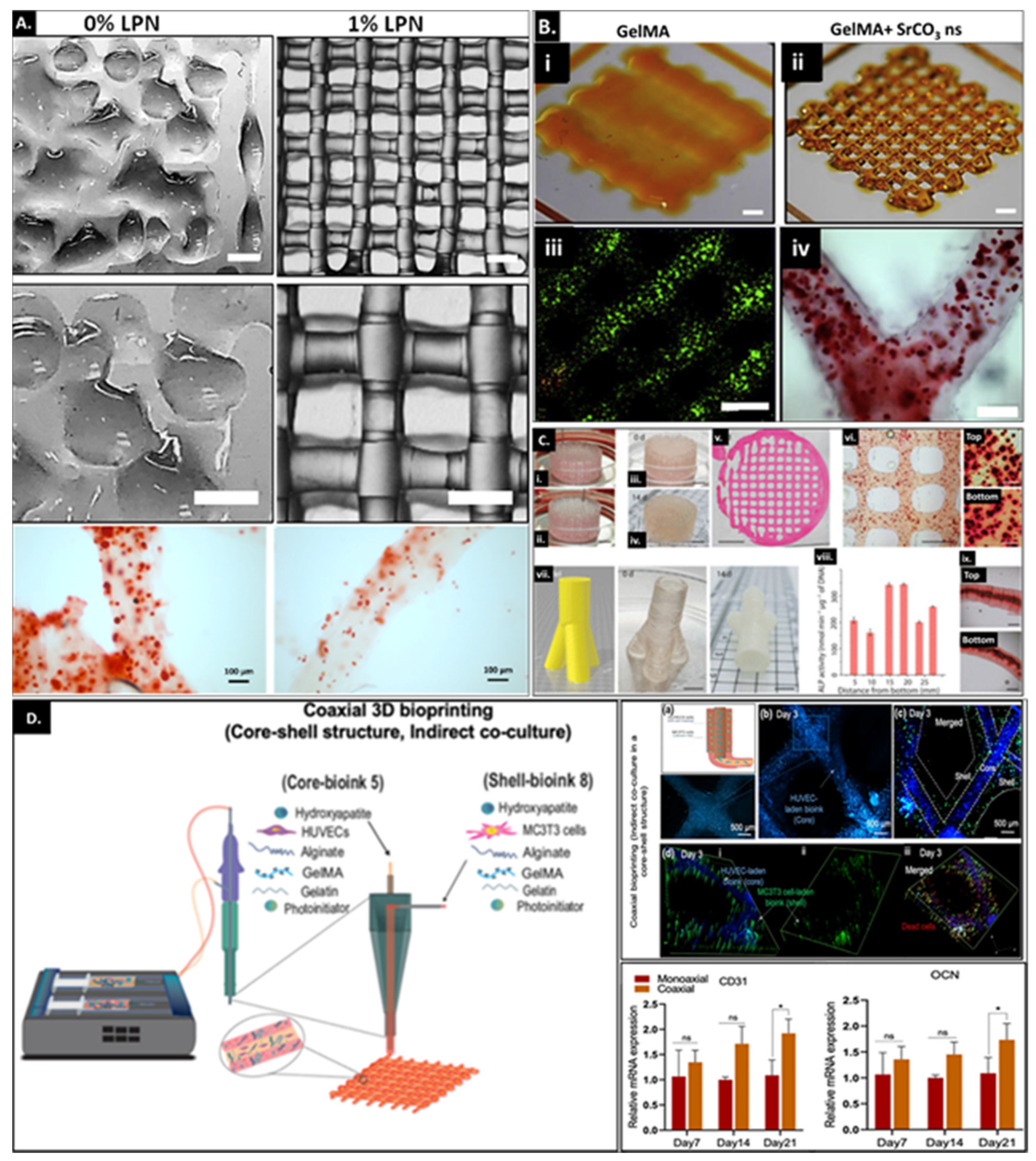
3.4. 3D Bioprinting and GelMA as a Cell Delivery Platform
| Additive | Cell Source | Photoinitiator Used for Crosslinking | Key Finding | Ref |
|---|---|---|---|---|
| Gelatin (Figure 6C) | Saos2 (human osteosarcoma cell line) | LAP | Inclusion of 5 wt% gelatin in 5 wt% GelMA to form a complementary bioink permits printability of complex structures. These include printing a bone-like geometry which was 4 cm long, 2 cm wide and 1 cm high and a 3 cm high and 1.5 cm wide trifurcated tube with hollow interior and overhanging walls. The different printed constructs displayed the same levels of ALP activity and matrix mineralization in different segments of the construct. | [113] |
| Gelatin, alginate and hydroxyapatite | MC3T3 (mouse pre-osteoblast cell line) and HUVECs (Human umbilical vein endothelial cells) | Irgacure I-2959 | Co-axial printing results in a 3D-printed construct with a core-shell structure with endothelial cells-laden ink forming the core and the MC3T3-laden ink forming the shell of the extruded fiber. Significant upregulation in osteogenic and angiogenic activity was observed for the osteon-like structures relative to the constructs printed via monoaxial 3D bioprinting. | [114] |
| Gelatin microgel (Figure 6D) | MC3T3/HUVECs | LAP | Combining sacrificial gelatin microgels with GelMA allows development of printed constructs with mesoscale pore networks for enhanced nutrient delivery and cell growth. The encapsulated cells demonstrate improved bioactivity within printed constructs ≥1 cm. The effect of the mesoscale porosity on cell functionality and tissue maturation still needs investigation. | [115] |
| Gellan gum (GG) and polylactic acid (PLA) microparticles as stem cell carriers | Rat MSCs | Irgacure I-2959 | Microcarrier MSCs (MCs) complexes were formed by utilizing PLA-based particles with MSCs adhered to their surface. The MCs containing GelMA-gellan gum bioink formed the bone compartment of the osteochondral construct. The inclusion of MCs provided mechanical reinforcement to the construct, whereas incorporation of GG improved viscosity and printability of the bioink. | [42] |
| Hydroxyapatite (HAp) and methacrylated hyaluronic acid (HAM) | hASCs (human adipose-derived stem cells) | LAP | HAp ink was prepared by incorporating HAp (5 wt%) within gelatin methacryloyl of different degrees of methacrylation and hyaluronic acid (7 wt% GM2, 5 wt% GM5 and 1 wt% HAM). HAp bioink demonstrated improved printability with printed structures remaining structurally intact over a 28-day period. Furthermore, the inclusion of HAp showed an osteo-supportive effect with upregulated osteogenic differentiation and matrix mineralization in osteogenic and control culture conditions. | [116] |
| Gelatin (G), acetylated gelatin methacryloyl (GMA), hydroxyapatite (HAp) and methacrylated hyaluronic acid (HAM) | ASCs (adipose derived stem cells) and HDMECs (human dermal microvascular endothelial cells) | LAP | Inclusion of GMA and G within GM for preparing the vascular bioink allowed improved materials properties with reduced crosslinking density and high swelling which allows capillary formation and maintenance. The combination of the vascularized bioink with the bone bioink (G, GM, HAP and HAM) demonstrated formation of a stable capillary-like network along with improved expression of bone-matrix-specific proteins relative to monoculture controls. | [117] |
| Gelatin, polyethylene glycol and mesoporous calcium silicate nanostructure | rBMSCs (rat bone marrow stem cells) and RAW264.7 | LAP | Incorporation of 3% gelatin, 2% PEG and 0.4% MSN within 5% GelMA improved hydrogel physicomechanical properties and bioink printability. Additionally, inclusion of BMP4-loaded MSN supported M2 type polarization, osteogenic differentiation of rBMSCs in vitro and accelerated bone healing in the critical-sized calvarial defect in a diabetic mouse model. | [118] |
| Bone Particles (BP) | Cells native to BP | LAP | Inclusion of BP with 0–500 µm size distribution within 10% and 12.5% GelMA at the filler concentration of 15% w/v improved bioink printability and mechanical properties. Additionally, the cellular reserve from the viable BPs displayed cell migration and colonization of the hydrogel scaffold while retaining their osteogenic differentiation capability relative to scaffolds with BP in the size range of 150–500 µm. | [119] |
| Mesoporous silica nanoparticles (MSN) functionalized with calcium phosphate (CaP) and dexamethasone (Dex) (MSNCaPDex) | Human MSCs | Irgacure I-2959 | Inclusion of MSNCaPDex at 0.5% w/v concentration within 10% GelMA improved hydrogel printability and shape fidelity while supporting stem cell viability and osteogenic differentiation capability in the basal media without additional osteogenic factors included during culture conditions. | [120] |
| Laponite®XLG (Figure 6A) | Human MSCs | Ru/SPS | Inclusion of Laponite served multifold functionality where Laponite served as a growth factor reservoir, improved bioink printability and promoted osteogenic differentiation capability of encapsulated stem cells along with integration and vascularization of the implanted construct in the chick chorioallantoic membrane model. | [51] |
| SrCO3 (Figure 6B) | Human MSCs | Ru/SPS | Utilization of SrCO3 as a nanofiller within 5 wt% GelMA improved printability and shape fidelity of the printed construct over prolonged culture periods and enhanced osteogenic differentiation of encapsulated stem cells. | [52] |
4. Concluding Remarks and Future Perspectives
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 2006–2017. [Google Scholar] [CrossRef]
- Charalambides, C.; Beer, M.; Cobb, A.G. Poor results after augmenting autograft with xenograft (Surgibone) in hip revision surgery: A report of 27 cases. Acta Orthop. 2005, 76, 544–549. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef]
- Sisson, K.; Zhang, C.; Farach-Carson, M.; Chase, D.B.; Rabolt, J.F. Evaluation of Cross-Linking Methods for Electrospun Gelatin on Cell Growth and Viability. Biomacromolecules 2009, 10, 1675–1680. [Google Scholar] [CrossRef]
- Ofner, I.C.M.; Bubnis, W.A. Chemical and Swelling Evaluations of Amino Group Crosslinking in Gelatin and Modified Gelatin Matrices. Pharm. Res. 1996, 13, 1821–1827. [Google Scholar] [CrossRef]
- Sung, H.-W.; Huang, D.-M.; Chang, W.-H.; Huang, R.N.; Hsu, J.-C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives:In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, J.-Y.; Cheng, C.-Y.; Tsai, F.-J.; Yao, C.-H.; Liu, B.-S. An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 2005, 26, 3911–3918. [Google Scholar] [CrossRef]
- Bertozzi, N.; Simonacci, F.; Grieco, M.P.; Grignaffini, E.; Raposio, E. The biological and clinical basis for the use of adipose-derived stem cells in the field of wound healing. Ann. Med. Surg. 2017, 20, 41–48. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Yang, K.-C.; Wu, C.-C.; Yu, J.-H.; Lin, F.-H.; Sun, J.-S. Fabrication of large perfusable macroporous cell-laden hydrogel scaffolds using microbial transglutaminase. Acta Biomater. 2014, 10, 912–920. [Google Scholar] [CrossRef]
- Das, S.; Pati, F.; Choi, Y.-J.; Rijal, G.; Shim, J.-H.; Kim, S.W.; Ray, A.R.; Cho, D.-W.; Ghosh, S. Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2014, 11, 233–246. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin meth-acrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Hutson, C.B.; Nichol, J.W.; Aubin, H.; Bae, H.; Yamanlar, S.; Al-Haque, S.; Koshy, S.T.; Khademhosseini, A. Synthesis and char-acterization of tunable poly (ethylene glycol): Gelatin methacrylate composite hydrogels. Tissue Eng. Part A 2011, 17, 1713–1723. [Google Scholar] [CrossRef]
- Chen, M.B.; Srigunapalan, S.; Wheeler, A.R.; Simmons, C.A. A 3D microfluidic platform incorporating methacrylated gelatin hydrogels to study physiological cardiovascular cell–cell interactions. Lab Chip 2013, 13, 2591–2598. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chen, Y.-C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [Green Version]
- Schuurman, W.; Levett, P.A.; Pot, M.W.; van Weeren, P.R.; Dhert, W.J.A.; Hutmacher, D.W.; Melchels, F.P.W.; Klein, T.J.; Malda, J. Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs. Macromol. Biosci. 2013, 13, 551–561. [Google Scholar] [CrossRef]
- Kaemmerer, E.; Melchels, F.P.; Holzapfel, B.M.; Meckel, T.; Hutmacher, D.W.; Loessner, D. Gelatine methacrylamide-based hydrogels: An alternative three-dimensional cancer cell culture system. Acta Biomater. 2014, 10, 2551–2562. [Google Scholar] [CrossRef]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Greene, T.; Lin, T.-Y.; Andrisani, O.M.; Lin, C.-C. Comparative study of visible light polymerized gelatin hydrogels for 3D culture of hepatic progenitor cells. J. Appl. Polym. Sci. 2016, 134, 44585. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Cui, F.Z.; Hu, K.; Zhu, X.D.; Fan, D.D. Bone regeneration by using scaffold based on mineralized recombinant collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 86, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mazaki, T.; Shiozaki, Y.; Yamane, K.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; Ito, Y.; et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci. Rep. 2014, 4, 4457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, H.; Lin, C.-C. Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator. Macromol. Rapid Commun. 2013, 34, 269–273. [Google Scholar] [CrossRef]
- Lim, K.S.; Klotz, B.J.; Lindberg, G.C.J.; Melchels, F.P.W.; Hooper, G.J.; Malda, J.; Gawlitta, D.; Woodfield, T.B.F. Visible Light Cross-Linking of Gelatin Hydrogels Offers an Enhanced Cell Microenvironment with Improved Light Penetration Depth. Macromol. Biosci. 2019, 19, e1900098. [Google Scholar] [CrossRef]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.; Dhert, W.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Hoch, E.; Schuh, C.; Hirth, T.; Tovar, G.E.M.; Borchers, K. Stiff gelatin hydrogels can be photo-chemically synthesized from low viscous gelatin solutions using molecularly functionalized gelatin with a high degree of methacrylation. J. Mater. Sci. Mater. Med. 2012, 23, 2607–2617. [Google Scholar] [CrossRef]
- Sutter, M.; Siepmann, J.; Hennink, W.E.; Jiskoot, W. Recombinant gelatin hydrogels for the sustained release of proteins. J. Control. Release 2007, 119, 301–312. [Google Scholar] [CrossRef]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Shirahama, H.; Lee, B.H.; Tan, L.P.; Cho, N.-J. Precise Tuning of Facile One-Pot Gelatin Methacryloyl (GelMA) Synthesis. Sci. Rep. 2016, 6, 31036. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, H.; Sakthivel, K.; Mohamed, M.G.A.; Boras, E.; Shin, S.R.; Kim, K. Designing Gelatin Methacryloyl (GelMA)-Based Bioinks for Visible Light Stereolithographic 3D Biofabrication. Macromol. Biosci. 2020, 21, e2000317. [Google Scholar] [CrossRef] [PubMed]
- Young, A.T.; White, O.C.; Daniele, M.A. Rheological Properties of Coordinated Physical Gelation and Chemical Crosslinking in Gelatin Methacryloyl (GelMA) Hydrogels. Macromol. Biosci. 2020, 20, 2000183. [Google Scholar] [CrossRef] [PubMed]
- Sakr, M.A.; Sakthivel, K.; Hossain, T.; Shin, S.R.; Siddiqua, S.; Kim, J.; Kim, K. Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering. J. Biomed. Mater. Res. Part A 2021, 110, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tian, Z.; Menard, F.; Kim, K. Comparative study of gelatin methacrylate hydrogels from different sources for bio-fabrication applications. Biofabrication 2017, 9, 44101. [Google Scholar] [CrossRef]
- Sewald, L.; Claaßen, C.; Götz, T.; Claaßen, M.H.; Truffault, V.; Tovar, G.E.M.; Southan, A.; Borchers, K. Beyond the modification degree: Impact of raw material on physicochemical properties of gelatin type A and type B methacryloyls. Macromol. Biosci. 2018, 18, 1800168. [Google Scholar] [CrossRef]
- Lee, B.H.; Lum, N.; Seow, L.Y.; Lim, P.Q.; Tan, L.P. Synthesis and Characterization of Types A and B Gelatin Methacryloyl for Bioink Applications. Materials 2016, 9, 797. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Health Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Sani, E.S.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef]
- Sani, E.S.; Lara, R.P.; Aldawood, Z.; Bassir, S.H.; Nguyen, D.; Kantarci, A.; Intini, G.; Annabi, N. An Antimicrobial Dental Light Curable Bioadhesive Hydrogel for Treatment of Peri-Implant Diseases. Matter 2019, 1, 926–944. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276. [Google Scholar] [CrossRef] [PubMed]
- Levato, R.; Visser, J.; Planell, J.A.; Engel, E.; Malda, J.; Timoneda, M.A.M. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication 2014, 6, 035020. [Google Scholar] [CrossRef]
- O’Connell, C.D.; Zhang, B.; Onofrillo, C.; Duchi, S.; Blanchard, R.; Quigley, A.; Bourke, J.; Gambhir, S.; Kapsa, R.; Di Bella, C.; et al. Tailoring the mechanical properties of gelatin methacryloyl hydrogels through manipulation of the photocrosslinking conditions. Soft Matter 2018, 14, 2142–2151. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Melchels, F.P.W.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J. Biomed. Mater. Res. Part A 2013, 102, 2544–2553. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Stratesteffen, H.; Köpf, M.; Kreimendahl, F.; Blaeser, A.; Jockenhoevel, S.; Fischer, H. GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication 2017, 9, 045002. [Google Scholar] [CrossRef]
- De Moor, L.; Minne, M.; Tytgat, L.; Vercruysse, C.; Dubruel, P.; Van Vlierberghe, S.; Declercq, H. Tuning the Phenotype of Cartilage Tissue Mimics by Varying Spheroid Maturation and Methacrylamide-Modified Gelatin Hydrogel Characteristics. Macromol. Biosci. 2021, 21, 2000401. [Google Scholar] [CrossRef]
- Xu, H.; Casillas, J.; Krishnamoorthy, S.; Xu, C. Effects of Irgacure 2959 and lithium phenyl-2,4,6-trimethylbenzoylphosphinate on cell viability, physical properties, and microstructure in 3D bioprinting of vascular-like constructs. Biomed. Mater. 2020, 15, 055021. [Google Scholar] [CrossRef]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; Franca, C.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Pahoff, S.; Meinert, C.; Bas, O.; Nguyen, L.; Klein, T.J.; Hutmacher, D.W. Effect of gelatin source and photoinitiator type on chondrocyte redifferentiation in gelatin methacryloyl-based tissue-engineered cartilage constructs. J. Mater. Chem. B 2019, 7, 1761–1772. [Google Scholar] [CrossRef]
- Cidonio, G.; Alcala-Orozco, C.R.; Lim, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.-H.; Dawson, J.I.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Kumar, D.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Design and characterisation of multi-functional strontium-gelatin nanocomposite bioinks with improved print fidelity and osteogenic capacity. Bioprinting 2020, 18, e00073. [Google Scholar] [CrossRef]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B. Hybrid biofabrication of 3D osteoconductive constructs comprising Mg-based nanocomposites and cell-laden bioinks for bone repair. Bone 2021, 154, 116198. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.-I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl–Riboflavin (GelMA–RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Bai, S.; Li, B.; Liu, H.; Wu, G.; Liu, S. Fabrication of gelatin methacrylate/nanohydroxyapatite microgel arrays for periodontal tissue regeneration. Int. J. Nanomed. 2016, 11, 4707–4718. [Google Scholar] [CrossRef] [Green Version]
- Sadat-Shojai, M.; Khorasani, M.-T.; Jamshidi, A. 3-Dimensional cell-laden nano-hydroxyapatite/protein hydrogels for bone regeneration applications. Mater. Sci. Eng. C 2015, 49, 835–843. [Google Scholar] [CrossRef]
- Pu, X.; Tong, L.; Wang, X.; Liu, Q.; Chen, M.; Li, X.; Lu, G.; Lan, W.; Li, Q.; Liang, J.; et al. Bioinspired Hydrogel Anchoring 3DP GelMA/HAp Scaffolds Accelerates Bone Reconstruction. ACS Appl. Mater. Interfaces 2022, 14, 20591–20602. [Google Scholar] [CrossRef]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.; Camci-Unal, G. Hydroxyapatite-Incorporated Composite Gels Improve Mechanical Properties and Bioactivity of Bone Scaffolds. Macromol. Biosci. 2020, 20, e2000176. [Google Scholar] [CrossRef]
- Wang, H.; Hu, B.; Li, H.; Feng, G.; Pan, S.; Chen, Z.; Li, B.; Song, J. Biomimetic Mineralized Hydroxyapatite Nanofiber-Incorporated Methacrylated Gelatin Hydrogel with Improved Mechanical and Osteoinductive Performances for Bone Regeneration. Int. J. Nanomed. 2022, 17, 1511–1529. [Google Scholar] [CrossRef]
- Paul, A.; Manoharan, V.; Krafft, D.; Assmann, A.; Uquillas, J.; Shin, S.R.; Hasan, A.; Hussain, M.A.; Memic, A.; Gaharwar, A.K.; et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J. Mater. Chem. B 2016, 4, 3544–3554. [Google Scholar] [CrossRef] [Green Version]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive Nanoengineered Hydrogels for Bone Tissue Engineering: A Growth-Factor-Free Approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, F.; Zhang, W.; Mo, Y.; Zeng, L.; Li, X.; Chen, X. Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Mater. Sci. Eng. C 2018, 89, 119–127. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, S.; Lin, Z.; Li, E.N.; Yagi, H.; Cao, G.; Yocum, L.; Li, L.; Hao, T.; Bruce, K.K.; et al. Graphene oxide-functionalized nanocomposites promote osteogenesis of human mesenchymal stem cells via enhancement of BMP-SMAD1/5 signaling pathway. Biomaterials 2021, 277, 121082. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.-K.; Bae, M.S.; Lee, J.B.; Lee, D.-W.; Byun, W.; Lee, C.H.; Kim, E.-C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle–hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Kurian, A.G.; Singh, R.K.; Lee, J.-H.; Kim, H.-W. Surface-Engineered Hybrid Gelatin Methacryloyl with Nanoceria as Reactive Oxygen Species Responsive Matrixes for Bone Therapeutics. ACS Appl. Bio Mater. 2022, 5, 1130–1138. [Google Scholar] [CrossRef]
- Comeau, P.; Willett, T. Triethyleneglycol dimethacrylate addition improves the 3D-printability and construct properties of a GelMA-nHA composite system towards tissue engineering applications. Mater. Sci. Eng. C 2020, 112, 110937. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Lauer-Fields, J.L.; Schmoekel, H.G.; Metters, A.T.; Weber, F.E.; Fields, G.B.; Hubbell, J.A. Synthetic matrix metallo-proteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. USA 2003, 100, 5413–5418. [Google Scholar] [CrossRef] [Green Version]
- Um, S.H.; Lee, J.B.; Park, N.; Kwon, S.Y.; Umbach, C.C.; Luo, D. Enzyme-catalysed assembly of DNA hydrogel. Nat. Mater. 2006, 5, 797–801. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.M.; Krijgsveld, J.; Zaat, S.A.J.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. Ed. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Samorezov, J.E.; Headley, E.B.; Everett, C.R.; Alsberg, E. Sustained presentation of BMP-2 enhances osteogenic differentiation of human adipose-derived stem cells in gelatin hydrogels. J. Biomed. Mater. Res. Part A 2016, 104, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.H.; McKinney, J.; Miller, T.; Bongiorno, T.; McDevitt, T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater. 2014, 13, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhou, X.; Sun, W.; Zhang, Z.; Teng, W.; Wang, F.; Sun, H.; Zhang, W.; Wang, J.; Yu, X.; et al. Vascular Derived ECM Improves Therapeutic Index of BMP-2 and Drives Vascularized Bone Regeneration. Small 2022, 2107991. [Google Scholar] [CrossRef]
- Wei, B.; Wang, W.; Liu, X.; Xu, C.; Wang, Y.; Wang, Z.; Xu, J.; Guan, J.; Zhou, P.; Mao, Y. Gelatin methacrylate hydrogel scaffold carrying resveratrol-loaded solid lipid nanoparticles for enhancement of osteogenic differentiation of BMSCs and effective bone regeneration. Regen. Biomater. 2021, 8, rbab044. [Google Scholar] [CrossRef]
- Pacelli, S.; Maloney, R.; Chakravarti, A.R.; Whitlow, J.; Basu, S.; Modaresi, S.; Gehrke, S.; Paul, A. Controlling Adult Stem Cell Behavior Using Nanodiamond-Reinforced Hydrogel: Implication in Bone Regeneration Therapy. Sci. Rep. 2017, 7, 6577. [Google Scholar] [CrossRef] [Green Version]
- Van Tienderen, G.S.; Berthel, M.; Yue, Z.; Cook, M.; Liu, X.; Beirne, S.; Wallace, G.G. Advanced fabrication approaches to controlled delivery systems for epilepsy treatment. Expert Opin. Drug Deliv. 2018, 15, 915–925. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, J.; Chen, R.; Huang, Y.; Liu, Y.; Bai, J.; Ge, G.; Shi, X.; Chen, Y.; Shi, J.; et al. Sustained Release of Melatonin from GelMA Liposomes Reduced Osteoblast Apoptosis and Improved Implant Osseointegration in Osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 6797154. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Y.; Liu, H.; Cheng, R.; Ni, Q.; Ye, T.; Cui, W. Local release of gemcitabine via in situ UV-crosslinked lipid-strengthened hydrogel for inhibiting osteosarcoma. Drug Deliv. 2018, 25, 1642–1651. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Tabata, Y. Dual-controlled release system of drugs for bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 28–40. [Google Scholar] [CrossRef]
- Kanczler, J.; Ginty, P.J.; White, L.; Clarke, N.M.; Howdle, S.M.; Shakesheff, K.; Oreffo, R. The effect of the delivery of vascular endothelial growth factor and bone morphogenic protein-2 to osteoprogenitor cell populations on bone formation. Biomaterials 2010, 31, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, J.; Sun, H.; Wang, F.; Wang, Y.; Zhang, Z.; Teng, W.; Ye, Y.; Huang, D.; Zhang, W.; et al. Spatiotemporal regulation of angiogenesis/osteogenesis emulating natural bone healing cascade for vascularized bone formation. J. Nanobiotechnol. 2021, 19, 420. [Google Scholar] [CrossRef] [PubMed]
- Barati, D.; Shariati, S.R.P.; Moeinzadeh, S.; Melero-Martin, J.M.; Khademhosseini, A.; Jabbari, E. Spatiotemporal release of BMP-2 and VEGF enhances osteogenic and vasculogenic differentiation of human mesenchymal stem cells and endothelial colony-forming cells co-encapsulated in a patterned hydrogel. J. Control. Release 2015, 223, 126–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Leeuwenburgh, S.C. Sustained delivery of biomolecules from gelatin carriers for applications in bone regeneration. Ther. Deliv. 2014, 5, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kretlow, J.D.; Spicer, P.P.; Tabata, Y.; Demian, N.; Wong, M.E.; Kasper, F.; Mikos, A.G. Antibiotic-releasing porous polymethylmethacrylate/gelatin/antibiotic constructs for craniofacial tissue engineering. J. Control. Release 2011, 152, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhang, Q.; Ren, W.; Yi, X.; Zhou, Z.; Peng, X.; Yu, X.; Lang, M. Controlled release of gentamicin from gelatin/genipin reinforced beta-tricalcium phosphate scaffold for the treatment of osteomyelitis. J. Mater. Chem. B 2013, 1, 3304–3313. [Google Scholar] [CrossRef]
- Yaylaoğlu, M.B. Development of a calcium phosphate–gelatin composite as a bone substitute and its use in drug release. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Sezer, U.A.; Aksoy, E.A.; Durucan, C.; Hasirci, N. Gentamicin loaded β-tricalcium phosphate/gelatin composite microspheres as biodegradable bone fillers. Polym. Compos. 2012, 33, 1644–1651. [Google Scholar] [CrossRef]
- Kim, H.-W.; Knowles, J.C.; Kim, H.-E. Porous scaffolds of gelatin-hydroxyapatite nanocomposites obtained by biomimetic approach: Characterization and antibiotic drug release. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005, 74, 686–698. [Google Scholar] [CrossRef]
- Sezer, U.A.; Aksoy, E.A.; Hasirci, V.; Hasirci, N. Poly (ε-caprolactone) composites containing gentamicin-loaded β-tricalcium phosphate/gelatin microspheres as bone tissue supports. J. Appl. Polym. Sci. 2013, 127, 2132–2139. [Google Scholar] [CrossRef]
- Changez, M.; Koul, V.; Dinda, A.K. Efficacy of antibiotics-loaded interpenetrating network (IPNs) hydrogel based on poly(acrylic acid) and gelatin for treatment of experimental osteomyelitis: In vivo study. Biomaterials 2005, 26, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, H.; Liao, K.; Hu, Q.; Guo, R.; Deng, K. Functionalized GO Nanovehicles with Nitric Oxide Release and Photothermal Activity-Based Hydrogels for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 28952–28964. [Google Scholar] [CrossRef]
- Cheng, H.; Yue, K.; Kazemzadeh-Narbat, M.; Liu, Y.; Khalilpour, A.; Li, B.; Zhang, Y.S.; Annabi, N.; Khademhosseini, A. Mussel-Inspired Multifunctional Hydrogel Coating for Prevention of Infections and Enhanced Osteogenesis. ACS Appl. Mater. Interfaces 2017, 9, 11428–11439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, Q.; Huang, K.; Fu, C.; Huang, C.; Fang, Y.; Gu, Z.; Wu, J.; Wang, Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogel with osteoimmunomodulatory and antibacterial activity for bone regeneration. Chem. Eng. J. 2019, 382, 123019. [Google Scholar] [CrossRef]
- Tao, B.; Chen, M.; Lin, C.; Lu, L.; Yuan, Z.; Liu, J.; Liao, Q.; Xia, Z.; Peng, Z.; Cai, K. Zn-incorporation with graphene oxide on Ti substrates surface to improve osteogenic activity and inhibit bacterial adhesion. J. Biomed. Mater. Res. Part A 2019, 107, 2310–2326. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; He, Y.; Yuan, Z.; Tao, B.; Li, K.; Lin, C.; Xu, K.; Chen, M.; Dai, L.; Li, X.; et al. Enzymatically-degradable hydrogel coatings on titanium for bacterial infection inhibition and enhanced soft tissue compatibility via a self-adaptive strategy. Bioact. Mater. 2021, 6, 4670–4685. [Google Scholar] [CrossRef]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 45891–45903. [Google Scholar] [CrossRef]
- Stevens, K.R.; Einerson, N.J.; Burmania, J.A.; Kao, W.J. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J. Biomater. Sci. Polym. Ed. 2002, 13, 1353–1366. [Google Scholar] [CrossRef]
- Yu, T.; Wang, W.; Nassiri, S.; Kwan, T.; Dang, C.; Liu, W.; Spiller, K.L. Temporal and spatial distribution of macrophage phenotype markers in the foreign body response to glutaraldehyde-crosslinked gelatin hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 721–742. [Google Scholar] [CrossRef]
- Donaldson, A.R.; Tanase, C.E.; Awuah, D.; Bathrinarayanan, P.V.; Hall, L.; Nikkhah, M.; Khademhosseini, A.; Rose, F.; Alexander, C.; Ghaemmaghami, A.M. Photocrosslinkable Gelatin Hydrogels Modulate the Production of the Major Pro-inflammatory Cytokine, TNF-α, by Human Mononuclear Cells. Front. Bioeng. Biotechnol. 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Zhang, Y.; Sun, S.; Li, Q.; Chen, K.; An, C.; Wang, L.; Beucken, J.J.J.P.V.D.; Wang, H. Control of Matrix Stiffness Using Methacrylate–Gelatin Hydrogels for a Macrophage-Mediated Inflammatory Response. ACS Biomater. Sci. Eng. 2020, 6, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.; Shin, S.R.; Leijten, J.; Li, Y.; Singh, S.; Liu, J.C.; Annabi, N.; Abdi, R.; Dokmeci, M.R.; Vrana, N.E. Integrin-mediated inter-actions control macrophage polarization in 3D hydrogels. Adv. Healthc. Mater. 2017, 6, 1700289. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Oreffo, R.O.; Dawson, J.I. From hurdle to springboard: The macrophage as target in biomaterial-based bone regeneration strategies. Bone 2022, 159, 116389. [Google Scholar] [CrossRef]
- Rajabi, N.; Rezaei, A.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Luo, H.; RamaKrishna, S.; Berto, F. Recent Advances on Bioprinted Gelatin Methacrylate-Based Hydrogels for Tissue Repair. Tissue Eng. Part A 2021, 27, 679–702. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Naniz, M.A.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2020, 9, 535–573. [Google Scholar] [CrossRef]
- Abdulghani, S.; Morouço, P.G. Biofabrication for osteochondral tissue regeneration: Bioink printability requirements. J. Mater. Sci. Mater. Med. 2019, 30, 20. [Google Scholar] [CrossRef]
- Freeman, F.E.; Burdis, R.; Kelly, D.J. Printing New Bones: From Print-and-Implant Devices to Bioprinted Bone Organ Precursors. Trends Mol. Med. 2021, 27, 700–711. [Google Scholar] [CrossRef]
- Ying, G.; Jiang, N.; Yu, C.; Zhang, Y.S. Three-dimensional bioprinting of gelatin methacryloyl (GelMA). Bio-Des. Manuf. 2018, 1, 215–224. [Google Scholar] [CrossRef]
- Arumugasaamy, N.; Baker, H.B.; Kaplan, D.S.; Kim, P.C.W.; Fisher, J.P. Fabrication and Printing of Multi-material Hydrogels. In 3D Printing and Biofabrication; Ovsianikov, A., Yoo, J., Mironov, V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–34. [Google Scholar] [CrossRef]
- Qiao, Z.; Lian, M.; Han, Y.; Sun, B.; Zhang, X.; Jiang, W.; Li, H.; Hao, Y.; Dai, K. Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials 2020, 266, 120385. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; Elizondo, M.E.; Koons, G.L.; Diba, M.; Chim, L.K.; Cosgriff-Hernandez, E.; Melchiorri, A.J.; Mikos, A.G. Fiber engraving for bioink bioprinting within 3D printed tissue engineering scaffolds. Bioprinting 2020, 18, e00076. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Armstrong, J.P.K.; Lin, Y.; Wojciechowski, J.P.; Lee-Reeves, C.; Hachim, D.; Zhou, K.; Burdick, J.A.; Stevens, M.M. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks. Sci. Adv. 2020, 6, eabc5529. [Google Scholar] [CrossRef] [PubMed]
- Shahabipour, F.; Tavafoghi, M.; Ii, G.E.A.; Bonakdar, S.; Oskuee, R.K.; Shokrgozar, M.A.; Potyondy, T.; Alambeigi, F.; Ahadian, S. Coaxial 3D bioprinting of tri-polymer scaffolds to improve the osteogenic and vasculogenic potential of cells in co-culture models. J. Biomed. Mater. Res. Part A 2022, 110, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Gao, Q.; Xie, C.; Fu, J.; Xiang, M.; Liu, Z.; Xiang, L.; He, Y. Sacrificial microgel-laden bioink-enabled 3D bioprinting of mesoscale pore networks. Bio-Des. Manuf. 2020, 3, 30–39. [Google Scholar] [CrossRef]
- Wenz, A.; Borchers, K.; Tovar, G.E.M.; Kluger, P.J. Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting. Biofabrication 2017, 9, 044103. [Google Scholar] [CrossRef]
- Leucht, A.; Volz, A.-C.; Rogal, J.; Borchers, K.; Kluger, P.J. Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents. Sci. Rep. 2020, 10, 5330. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Ma, Z.; Zhao, X.; Jin, W.; Zhang, C.; Ma, J.; Qiang, L.; Wang, W.; Deng, Q.; Yang, H.; et al. Three-dimensional bioprinting of multicell-laden scaffolds containing bone morphogenic protein-4 for promoting M2 macrophage polarization and accelerating bone defect repair in diabetes mellitus. Bioact. Mater. 2020, 6, 757–769. [Google Scholar] [CrossRef]
- Ratheesh, G.; Vaquette, C.; Xiao, Y. Patient-Specific Bone Particles Bioprinting for Bone Tissue Engineering. Adv. Health Mater. 2020, 9, e2001323. [Google Scholar] [CrossRef]
- Tavares, M.T.; Gaspar, V.M.; Monteiro, M.V.; Farinha, J.P.S.; Baleizão, C.; Mano, J.F. GelMA/bioactive silica nanocomposite bioinks for stem cell osteogenic differentiation. Biofabrication 2021, 13, 035012. [Google Scholar] [CrossRef]



| Cross-Linking System | Biological Response | Ref. |
|---|---|---|
| APS/TEMED | Encapsulated chondrocytes showed >80% viability after 24 h. | [26] |
| Eosin Y (photosensitizer), Triethanolamine (TEA; initiator) and Vinylcaprolactam (VC; co-monomers) | Viability both in 2D and 3D cultures is dependent on hydrogel formulation (concentration of macromer, Eosin Y, TEA and VC and crosslinking time) along with in vivo biocompatibility and bone-forming capability. | [39,40,41] |
| Irgacure I-2959 | Cell viability was dependent on the concentration of Irgacure and duration of crosslinking. The system has been extensively investigated in the literature. however there is a gradual drift towards crosslinking systems using visible light due to the associated negative effect on the cytotoxicity and cell functionality with the UV-light source. | [16,25,42,43,44,45,46,47] |
| Lithium phenyl-2 4 6-trimethylbenzoylphosphinate (LAP) | Cell viability of >75% which is dependent on crosslinking conditions including macromer concentration, LAP concentration and time of crosslinking; good cytocompatibility especially at high photo-initiator concentrations (0.7% w/v) during prolonged bioprinting conditions (60 min) with small pore size and low swelling ratio and slower degradation. | [48,49,50] |
| Ruthenium/sodium persulfate (Ru/SPS) | Superior cell viability (>80% over long-term cultures) and support cell differentiation capabilities (osteogenesis, chondrogenesis). | [25,51,52,53] |
| Riboflavin | Improved viability and expression of late osteogenic markers such as osteocalcin of KUSA-1 (murine bone marrow-derived MSCs committed towards osteocyte differentiation) in 20% GelMA crosslinked with riboflavin relative to hydrogels crosslinked using Irgacure I-2959. | [54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Dawson, J.I.; Oreffo, R.O.C.; Tabata, Y.; Kumar, D.; Aparicio, C.; Mutreja, I. Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration. Bioengineering 2022, 9, 332. https://doi.org/10.3390/bioengineering9070332
Kim Y-H, Dawson JI, Oreffo ROC, Tabata Y, Kumar D, Aparicio C, Mutreja I. Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration. Bioengineering. 2022; 9(7):332. https://doi.org/10.3390/bioengineering9070332
Chicago/Turabian StyleKim, Yang-Hee, Jonathan I. Dawson, Richard O. C. Oreffo, Yasuhiko Tabata, Dhiraj Kumar, Conrado Aparicio, and Isha Mutreja. 2022. "Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration" Bioengineering 9, no. 7: 332. https://doi.org/10.3390/bioengineering9070332
APA StyleKim, Y.-H., Dawson, J. I., Oreffo, R. O. C., Tabata, Y., Kumar, D., Aparicio, C., & Mutreja, I. (2022). Gelatin Methacryloyl Hydrogels for Musculoskeletal Tissue Regeneration. Bioengineering, 9(7), 332. https://doi.org/10.3390/bioengineering9070332