Abstract
Transplantation of human pluripotent stem cell (hPSCs)-derived cardiomyocytes for the treatment of heart failure is a promising therapy. In order to implement this therapy requiring numerous cardiomyocytes, substantial production of hPSCs followed by cardiac differentiation seems practical. Conventional methods of culturing hPSCs involve using a 2D culture monolayer that hinders the expansion of hPSCs, thereby limiting their productivity. Advanced culture of hPSCs in 3D aggregates in the suspension overcomes the limitations of 2D culture and attracts immense attention. Although the hPSC production needs to be suitable for subsequent cardiac differentiation, many studies have independently focused on either expansion of hPSCs or cardiac differentiation protocols. In this review, we summarize the recent approaches to expand hPSCs in combination with cardiomyocyte differentiation. A comparison of various suspension culture methods and future prospects for dynamic culture of hPSCs are discussed in this study. Understanding hPSC characteristics in different models of dynamic culture helps to produce numerous cells that are useful for further clinical applications.
1. Introduction
Cardiovascular diseases are the leading cause of mortality worldwide. Angina and myocardial infarction known as heart attack is most frequent and occurs when the blood flow to the heart is obstructed, thereby damaging the heart muscles. Heart muscles cannot proliferate by themselves and their permanent loss results in cardiac injury. In order to restore the impaired cardiac function, engraftment of cardiomyocytes by transplantation is necessary [1,2]; however, a limited number of available donors is an obvious and crucial limitation for the transplantation. Recently, production of cardiomyocytes by differentiation from human pluripotent stem cells (hPSCs) has gained immense attention. hPSCs, including human embryonic stem cells (hESCs) [3] and human induced pluripotent stem cells (hiPSCs) [4], have the capability to differentiate into many cell lineages, such as cardiac progenitors, cardiomyocytes, and endothelial cells. These cells are reported to be necessary for formation of cardiac muscle tissue in vivo. Several studies reported the preclinical research using hPSC-derived cardiomyocytes on animal models [2,5,6,7,8,9], and the cardiology department of Osaka University (Japan) announced as the first research group that applied hiPSC-based therapy for human heart [10]. Cardiomyocytes derived from hPSCs can be transplanted to the patient’s body and also applied as a model for cardiac drug screening and disease modeling. Although mature cardiomyocytes are required to appropriately adapt for drug screening and disease modeling, immature cardiomyocytes are applicable for transplantation since the cardiomyocytes can mature in site after transplantation [11,12]. The transplanted cells improved the cardiac function by secreting certain growth factors and cytokines to induce heart repair [13,14]. In order to provide substantial number of cardiomyocytes for transplantation, a large-scale hPSC production system, which can easily be converted to cardiac differentiation, is required. That means hPSC expansion and cardiac differentiation has to be considered as connected processes for cardiomyocyte production. However, many review articles independently focused either on expansion of hPSCs [15,16] or cardiomyocyte differentiation [17,18] or maturation [19,20]. Although a recent review mentioned two-in-one systems that directly incorporate hPSCs expansion into cardiomyocytes differentiation [21], no studies have referred to these integrated systems in different culture formats of hPSCs. In this review, we summarize the methods for expansion of hPSCs in various culture formats and two-in-one systems for integrated hPSCs expansion and cardiomyocyte differentiation. We include discussing the pros and cons of each model and propose the future prospect toward achieving sustainable and scalable, highly efficient production of cardiomyocytes for use in clinical applications.
2. Large Scale Expansion Culture of Human Pluripotent Stem Cells
2.1. Adherent Culture of Human Pluripotent Stem Cells
Adherent cultures such as monolayers are made by using protein-coating materials such as fibronectin, vitronectin, and laminin [22,23,24] in order to promote cell adhesion on the treated surfaces of the culture dishes or flasks. Recently, synthetic peptides are developed as a cost-effective replacement for the expensive recombinant proteins [25,26]. Melkoumian et al. reported that the application of synthetic peptide-acrylate surfaces by conjugating peptides derived from active domain of vitronectin, laminin, fibronectin, or bone sialoprotein to acrylate can support self-renewal of hPSCs [25]. Notably, Synthemax (Corning), which is a synthetic surface comprising vitronectin-based peptides, is known to be capable of maintaining hiPSCs in chemically-defined medium in a long-term culture [26]. To further develop cost-effective materials, recent studies have also focused on polymer-based coating [27,28,29,30,31,32]. These polymer-based coating materials revealed the capability of maintaining hPSCs in an undifferentiated state; however, the mechanism underlying cell adhesion by using these polymers has not been elucidated. Future studies to clarify the influence of chemical parameter such as hydrophobicity, hydrophilicity, and stiffness on maintenance of multiple cell lines are recommended prior to commercialization of these products.
The simplest xenobiotic-free (xeno-free) medium for expansion of hPSCs is E8 media, which contains eight components: D-MEM/F-12, human insulin, human transferrin, selenium, ascorbic acid, sodium hydrogen carbonate, human recombinant fibroblast growth factor 2 (FGF-2 or bFGF), and transforming growth factor β1 (TGF-β1) [23]. This medium is commercialized such as TeSR-E8 medium (STEMCELL Technologies) and Essential 8 medium (Thermo Fisher); however, the high cost of using this medium creates a barrier for its further implementation in large-scale production. To develop a cost-effective medium, Yasuda et al. demonstrated that replacement of two growth factors in E8 medium (bFGF and TGF-β1) with chemical compounds, including Azakenpaullone, ID-8, and Tacrolimus, are capable of supporting long-term expansion of hPSCs [33]. This medium will be further optimized for cell growth capacity before being commercialized.
To facilitate cell expansion in a monolayer, a large surface area flask of 175 cm2 (T175) and its multilayer format with surface area up to 875 cm2 is developed (Corning, Falcon). An active gas ventilation system is included to maintain the CO2 concentration in all culture layers. Another modern cell culture system called CompacT SelecT was constructed by Sartorius to have a culture area up to 90 T175 along with 96 and 384-well plates for automated cell-based assay in the subsequent stage. In addition, the application of roller bottles (Integra Bioscience) or CellCube (Corning) is also considered to allow dynamic culture of 2D monolayer model in hPSCs scalable culture.
Monolayer culture has the advantage of being easily setup and is considered as a standard procedure in all laboratories. This method also produces a homogeneous effect since the cells are uniformly exposed to the medium components. Furthermore, this method allows easy assessment of cell quality by directly observing the cells and colonies under a microscope as well as facilitates medium change.
Despite its advantages as aforementioned, the 2D culture itself has the limitation of requiring a large surface area and hampers cell harvesting due to enzyme treatment. Moreover, 2D culture does not simulate the in vivo microenvironment. Therefore, replacement of 3D culture can be a suitable approach for large scale production of hPSCs.
2.2. Suspension Culture of Human Pluripotent Stem Cells
2.2.1. Application of Microcarriers
hPSCs are anchorage-dependent for survival and proliferation. Applications of cell supports, such as several types of microcarriers, hydrogels, and polymers, have been developed to aid the suspension culture of hPSCs. Microcarriers are supporting matrices for culturing adherent cells with a diameter varying from 10 μm up to 5 mm. Cell culture with microcarrier can increase the production capacity by allowing high density cell proliferation. Microcarriers are applicable for cell culturing in a stirrer bioreactor. Various shapes are available, but a round shape is popular. The size and shape of the microcarrier affects the sedimentation rate of cells in cell-microcarrier complexes. The ideal size for microporous microcarrier is about 100–300 µm in general [34]. Besides, the macroporous microcarrier has a large porous structure that increases the surface area with pore size up to 400 µm. Cells can form a confluent layer around the microporous microcarrier while they are entrapped inside the pores of the macroporous microcarriers. Although the macroporous microcarrier has the advantage of effectively protecting the cells from shear stress in the cell culture using a bioreactor, the cell yield was relatively low [35]. Microcarriers can be made from different materials such as plastic, glass, silica, dextran, or cellulose with the surface being coated by collagen or fibronectin to increase cell adhesion. The application of synthetic peptides for microcarrier surface coatings can help to reduce the cost in hPSC culture [36,37]. Among the popular microcarriers, the dextran-based Cytodex and cellulose-based Cytopore microcarriers, which are developed by Amersham Bioscience (GE Healthcare), are widely applied in culturing several cell lines [38,39,40].
While culturing of hPSCs, Phillips et al. succeeded in providing a proof-of-principle to attach hESCs on the polystyrene microcarriers for culturing in a spinner flask [41]. The continuous adherence and removal of cells from the uncoated microcarriers presumably results in reduced cell proliferation at each passage. Application of Matrigel (Corning)-coated [42] and vitronectin-coated microcarriers [43] increased the cell adhesion and enabled long-term culture. Notably, by applying polystyrene microcarriers coated with human vitronectin and E8 medium, Badenes et al. established a cost-effective xeno-free chemically-defined culture system for the suspension culture of hiPSCs [43]. Under the dynamic culture of this study, agitation rates around 30–70 rpm were optimal, which resulted in shear stress at 0.08 to 0.26 Pa. These values of shear stress are considerably lower than the predicted value of other cell types: 0.65 Pa for human embryonic kidney cells [44] and 0.78 Pa for murine embryonic stem cells [45]. Recently, by using the synthetic peptide-acrylate surface microcarriers, Badenes et al. demonstrated a scalable culture of hiPSCs by cell transfer from confluent beads to empty beads in a spinner flask. This method enables the long-term expansion of hiPSCs, which achieved 241-fold expansion in 15 days and yielded 3.3 × 108 cells in 15 mL culture medium [46].
The conventional applications of microcarriers require the separation of cells from microcarriers via enzymatic dissociation followed by a filtration step, which causes loss of viable cells. To further develop the applications of microcarriers in cell culture without the filtration step, biodegradable microcarriers are also developed [47,48,49]. Rodrigues et al. reported the utilization of dissolvable microcarriers (Corning), which can assist the cell proliferation in spinner-flasks up to 4-fold after 5 days. For harvesting, a solution containing EDTA and pectinase was used in combination with Accutase (Innovative Cell Technologies) to digest the cell-microcarrier complex into a single cell solution [49]. By using dissolvable microcarriers, a high recovery rate of 92% is obtained compared to 45% in conventional polystyrene microcarriers.
These studies demonstrated that microcarriers application is suitable for scalable culture of hPSCs in a bioreactor to obtain a substantial number of cells and derivatives for clinical purposes. Nevertheless, the disadvantage of dissociating the cells from microcarriers during cell harvesting can be a barrier for further differentiation applications.
2.2.2. Formation of Carrier-Free 3D Aggregates
Another approach in culturing hPSCs in the suspension culture is to form cell spheres by self-aggregation of hPSCs. Dissociation of cells into colonies [50], and then culturing them in absence of the coating material, results in formation of cell aggregates. To further improve the homogeneity of forming colonies, 3D aggregates of hPSCs can also be achieved by dissociation into single cells and culture in the presence of ROCK inhibitor [51,52,53,54]. In addition, a uniform population of aggregates can be accomplished by seeding the cells into fabricated microwells [54,55]. Cells can be cultured in static suspension at small scale by untreated culture dishes or dynamic suspension at larger scale in spinner flasks or bioreactor with agitation. Cell aggregates should be maintained over several passages in the static culture excluded from shear stress in order to enhance the adaptability to subsequent dynamic cultures [50,56]. By optimizing the agitation rates and concentration of shear protectants, Abbasalizadeh et al. were able to produce 3D size-controlled aggregates at clinically relevant numbers of hPSCs (~2 × 109 cells) in one month [52]. In this study, the frozen stock of suspension-adapted hPSCs for bioreactor was also prepared in advance without depending on adaptation in the static culture. Another group also reports the handy scalable stirred culture system to produce numerous hPSCs (2 × 109 cells) only in 14 days utilizing a ready-to-use and commercially-available 3 L bioreactor (Mobius 3 L Bioreactor, Merck Millipore) [57]. An hPSC culture platform conforming to GMP regulation has been developed by Chen et al. [53]. Furthermore, Wang et al. reported successful scalable expansion of hiPSCs as 3D aggregates in the defined xeno-free E8 medium [58]. Collectively, the future prospect of obtaining numerous cells for transplantation can be accomplished using this method.
Due to strong cell–cell interaction and extracellular matrix secretion of hPSCs [51,59], a compact cell sphere can be achieved, which is independent of the anchorage such as microcarriers. Thus, culture hiPSC as 3D aggregates facilitates cell harvesting and maintains the cell integrity for further differentiation applications. Nevertheless, in most cases, a ROCK inhibitor should be maintained not only during the time of sphere formation but also throughout the culture process to assist the cell’s proliferation in spheres. Long-time exposure to ROCK inhibitor was reported to affect the cell metabolism [60] and chromosomal aberration [61,62]. Furthermore, culturing cells in the presence of ROCK inhibitor reduced the expression of E-cadherin and promoted EMT toward mesendoderm differentiation [63], which may bias the direction of differentiation. These are potential disadvantages for maintaining the pluripotency and safety of hPSCs in a carrier-free suspension culture.
2.2.3. Hydrogels
Recently, application of hydrogel for suspension culture of hPSCs has received immense attention as it assists the cells in 3D organization while enhancing cell protection via encapsulation. Hydrogels are water-swollen polymers, which can be used to encapsulate cells into a 3D model. A highly applicable hydrogel is the thermal-responsive hydrogel, which is in liquid form at a low temperature and is solidified at higher temperature. During seeding, cells are mixed to form a complex with liquid hydrogels at 4 °C. The 3D complexes of cell–hydrogels are generally formed after incubation at 37 °C.
Hydrogels are classified as natural (collagen, fibrin), synthetic (polyethylene, poly acrylamide), and hybrid hydrogels comprising natural and synthetic polymers [64,65]. Hydrogels can be prepared as per the protocol mentioned in few previous studies [66,67]. Unlike the microcarriers that require a coating material to attach the cells on the surface, hydrogels encapsulate hPSCs in 3D organization, so that no coating is required. Moreover, addition of ROCK inhibitor is not necessary [68,69] or required only in few cases to increase the cell proliferation [70]. For cell harvesting, the hydrogel can be removed by changing the temperature to release cells from thermo-responsive hydrogel [70] or via enzymatic treatment to degrade cellulose-based hydrogel [69]. Although hydrogels facilitate cell harvest by simply changing the physical conditions, depending on materials, the difficulty in controlling the size of cell spheres in hydrogel encapsulation may hinder the reproducibility of further differentiation process.
2.2.4. Functional Polymers for Suspension Culture of hiPSCs without Agitation
To facilitate the hPSC expansion in the suspension with size-controlled aggregates, independent from the ROCK inhibitor, Otsuji et al. proposed the application of functional polymers including Gellan gum and methylcellulose [71]. Gellan gum allows the cells or spheres to be suspended in a 3D model without agitation while methylcellulose can protect the cell surface from fusion with other spheres to create a homogenous sphere size to support cell proliferation. Since these functional polymers can maintain spheres in 3D suspension, no agitation is required. Thus, the cells are maximally protected from damage by shear stress caused by agitation compared to the conventional 3D aggregates culture. The viscosity of these polymers was adjusted to a low level in order to unaffect the diffusion of soluble factors and no gelation was detected. For the culture method, cells are initially dissociated into colonies and cultured in ROCK inhibitor-containing medium for the first 24 h. After formation of cell aggregates on Day 1, ROCK inhibitor is withdrawn by changing the medium. For the passage, cells can be easily subcultured mechanically by passing through a 50–70 µm mesh and harvested via centrifugation. The withdrawal of ROCK inhibitors is advantageous in maintaining specific metabolism and facilitates the application of nonbiased differentiation capacity. This group was successful in applying this culture method for large scale cell expansion of hESCs in a 200-mL oxygen-permeable bag, which is specifically designed to improve gas exchange thereby promoting cell growth. Although agitation is not required to keep the cells in suspension, presumably, a gentle mixing is necessary to increase the oxygen aeration for large-scale cell production. Another group also reported successful establishment of a 3D culture system using Gellan gum and methylcellulose for culturing of cancer stem cells [72]. For details of culture method for expansion of hPSCs, please refer to Table 1.

Table 1.
Suspension culture methods for expansion human pluripotent stem cell (hPSCs).
3. Application of Human Pluripotent Stem Cells for Cardiomyocyte Differentiation
3.1. Cardiomyocyte Differentiation as Monolayer Culture
Direct application of hPSCs culture in the monolayer for differentiation is a simple and effective way to induce cardiomyocytes, as all cells can be uniformly exposed to the media components and inducers. 2D culture is applicable for maintenance and proliferation of hPSCs as high and stable expression of pluripotency markers are reported [33,73]. Cardiomyocyte differentiation can be achieved by manipulating the Wnt signaling pathway in a biphasic manner. Specifically, the upregulation of Wnt signaling in the early stages (by small molecules CHIR99021 or BIO) is required to induce differentiation of mesendoderm and mesoderm. Then, downregulation of Wnt signaling by Wnt inhibitors (IWP2, XAV939, or Wnt-C59) is necessary to shape the direction of differentiation into cardiomyocytes. The typical protocol for cardiomyocyte differentiation using small molecules is reported by Lian et al. [74] (Figure 1). This protocol depends on B27 supplement containing bovine albumin serum (BSA), which should be replaced in xeno-free culture for clinical application. Further development toward xeno-free differentiation of cardiomyocytes gaining immense attention [75,76,77]. Minami et al. reported a defined and xeno-free system for cardiomyocyte differentiation independent from B27 by applying the IMDM medium supplemented with MEM nonessential amino acid, penicillin–streptomycin, L-glutamine, L-carnitine, 2-mercaptoethanol, and human serum albumin (HSA) [75]. By comparing the components of B27 supplements with other media beneficial for cardiac differentiation, Burridge et al. found that among several components of B27, only BSA is necessary for cardiomyocyte differentiation. After replacing BSA with HSA and optimizing it, this group reported a chemically defined and xeno-free protocol for cardiomyocyte differentiation with only three components, including RPMI 1640, L-ascorbic acid 2-phosphate, and rice-derived recombinant albumin [76]. In contrast, Lian et al. demonstrated that even BSA is not necessary and only three factors are required for addition in RPMI medium to effectively induce cardiomyocytes, including sodium selenite, progesterone, and putrescine [77]. This protocol introduces an albumin-free system to obtain cardiomyocytes in a cost-effective manner. Further reproduction in 2D culture as well as examining the application of this xeno-free medium in 3D culture is required to improve the efficiency of the differentiation process.
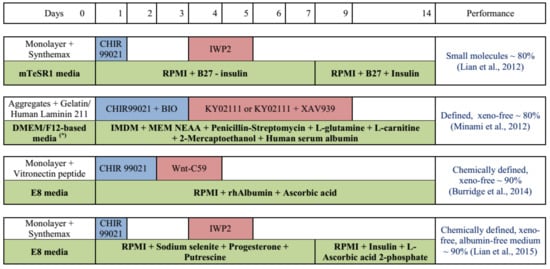
Figure 1.
Schematic representation of cardiomyocyte differentiation from hPSCs developed toward xeno-free culture [74,75,76,77]. Abbreviation: (*) DMEM/F12 + KSR + bFGF + MEM NEAA (non-essential amino acids) + 2-Mercaptoethanol. rhAlbumin: rice-derived recombinant human albumin. The blue and red boxes indicate the period of Wnt activation and inhibition, respectively.
For differentiating the hPSCs into cardiomyocytes at a large scale, Tohyama et al. reported the application of a system combining 4 or 10-layer culture plates with an area surface of 632 cm2 [78]. This system yielded 7.2 × 108 hiPSCs in 4-layer and 1.7 × 109 hiPSCs in 10-layer culture plates during the seeding of 1 × 106 hiPSCs per layer. Since the fluctuation of CO2 concentration causes mitochondrial dysfunction, which impairs cell proliferation [79], an active gas ventilation system was included to keep the CO2 concentration stable in every culture plate. A performance of 66–87% cardiomyocytes was obtained after 10 days of differentiation. In other reports, to increase the purity, a combination step for cardiomyocyte purification using lactate-supplemented media was applied. This process yields 6.2–7.0 × 108 cardiomyocytes in 4-layer culture plates with high efficiency up to 99%. In the 10-layer culture plates, 1.5–2.8 × 109 cells can be achieved, which are sufficient for transplantation in one patient [17,80,81].
Applying cardiomyocytes for drug screening and disease modeling requires not only a large number but also functional mature cells. In attempt to improve the cardiomyocyte maturation, co-differentiation with endothelial cells was approached since the endothelial cells are known to regulate cardiac development in vivo [82,83]. Giacomelli et al. simultaneously differentiated hPSCs into cardiomyocytes and endothelial cells in 2D culture for forming 3D micro cardiac tissue to mimic in vivo development [84]. This co-differentiation enabled yielding cardiac identity endothelial cells, which may be more appropriate for cardiomyocyte maturation than other sources of endothelial cells. In the study, a population of 50% cardiomyocytes and 20% endothelial cells was obtained. Consequently, the cardiomyocytes were enriched up to 80% by labeling with VCAM1 antibody, which is a cell surface adhesion molecule for cardiac cells in the early stage. After enrichment, these cells were mixed with the endothelial cells at a ratio of 85% cardiomyocytes: 15% endothelial cells to form 3D microtissue organization in a 96-well plate. Gene analysis revealed that prolong culture period of these microtissues up to 3 weeks significantly improved the expression of sarcomeric structure markers and Ca2+ handling and ion-channel genes. Furthermore, this model reduced the expression of fetal cardiomyocyte genes, suggesting crucial changes toward maturation of cardiomyocytes.
In the monolayer culture, hPSCs were conventionally plated at a high density and were maintained until confluence before differentiation induction was started [85,86,87]. The purpose of plating hPSCs at high density is to take advantage of the secretion signals from high density cells. Relying on cell density effect and its hidden signals may hinder the control of differentiation process. As a new approach, Le et al. proposed to commence differentiation at an initial low cell density to eliminate all the cell-to-cell or cell autonomous signals may present in high-density cells. Once the differentiation procedure is optimized, it enables the control of differentiation process without relying on cell density. That leads higher productivity even differentiation induction is commenced at 1% cell confluency [88].
Despite the monolayer culture of hPSCs providing advantages of evenly exposed cells to all medium components and inducers, this system presented difficulties in cell harvesting and hindered the monitoring of culture conditions. At a later stage, cardiomyocyte maturation by prolonging the culture period [89,90] may not be stably achieved in 2D culture, since the cardiomyocytes tend to detach from the culture surface due to strong contraction. The disadvantage of stability in the 2D culture should be considered for large scale production. Therefore, 3D culture approaches to facilitate scaling up and cell harvesting are good alternatives for large-scale production.
3.2. Cardiomyocyte Differentiation by Suspension Culture Using Microcarriers
Following the expansion of hPSCs using microcarriers, cardiac differentiation can be achieved by simply changing the hPSCs maintenance medium to cardiac differentiation medium [91]. After optimizing the type and concentration of microcarriers, Lecina et al. produced 80% beating aggregates with 20% cardiomyocytes; however, only a low yield of cardiomyocytes was obtained in the spinner culture due to effect of shear stress [38]. In recent years, these authors achieved high efficiency of 83.1% cardiomyocytes after differentiating and purifying them in lactate-supplemented medium in a 500-mL bioreactor scale. Removal of microcarriers by filter through 40–100 µm strainer following enzyme treatment has been proposed [38,42,43]. Utilization of the microcarriers as a matrix for cells is favorable for upscaling to use in the suspension culture except for optimizing the materials, size, shape, and concentration for the microcarriers. Moreover, dissociation of cardiomyocytes by enzyme treatment to detach microcarriers in the terminal stage results in poor survival of cardiomyocytes [18,92].
3.3. Cardiomyocyte Differentiation by Applying Carrier-Free Cell Aggregates
As an advanced approach, hPSCs can be expanded by forming carrier-free cell aggregates and can be directly applied in cardiomyocyte differentiation. In general, to form cell aggregates, hPSCs are dissociated into single cells by Accutase and cultured in the presence of ROCK inhibitor in a nontreated culture dish, an Erlenmeyer flask, or spinner flask bioreactors. Single cell dissociation enables to control the size of aggregates formed in the later process. Toward industrial production, cell number at the time of induction can be directly adjusted by this method. This approach overcomes the limitation of 2D model since the assessment and adjustment of cell density is easy and is monitored regularly during the culture process. Interestingly, ROCK inhibitor may be useful in priming hPSCs to differentiate into mesendoderm [63], which is the initial step toward cardiomyocyte formation [93]. Hence, addition of ROCK inhibitor during hPSC expansion may be preferred for cardiomyocyte differentiation; however, for versatility in application of cell aggregate culture, addition of ROCK inhibitor may need to be diminished.
Differentiation of hPSC aggregates into cardiomyocytes was reported by several researchers [94,95]. Kempf et al. reported the successful establishment of a suspension culture system in 125 mL Erlenmeyer flasks (20 mL working volume) and 100 mL bioreactor, achieving 60% and 84% cell population expression cardiac Troponin T (cTnT), respectively [94]. Notably, in a similar manner of 3D cardiac differentiation of carrier-free cell aggregates, Chen et al. succeeded in establishing a GMP compliant process to obtain up to 90% cardiomyocytes with a yield of 1.5–2 × 109 cardiomyocytes/L in 1 L spinner flask [95].
According to the Kempf group, a Wnt signal activator (GSK3 inhibitor) CHIR99201 concentration is critical while cell aggregate size is not a prevailing factor for high efficiency of cardiac differentiation across different culture-platforms of 12-well plate, Erlenmeyer and bioreactor. In static 12-well plate, by using NKX2.5-GFP reporter cell line (transgenic cell line carrying green fluorescent protein (GFP) gene under cardiac linage-specific NKX2.5 gene promotor), this group proved that aggregates of all sizes (around 100–400 µm) and shapes differentiated into contracting GFP+ colonies at 7.5 µM CHIR99021. However, at other CHIR99021 concentrations, low GFP expression was observed in all aggregate sizes, suggesting that the aggregate size is not important. In their speculation, the method for 3D aggregates formation in bioreactor is a predominant factor deciding the differentiation efficiency. Aggregates formed by cyclic perfusion feeding are efficient to form cardiomyocytes when batch-feeding is inefficient. Considering this issue, Chen et al. claimed that both CHIR99021 concentration and aggregate size are the dominant factors determining cardiac differentiation in the suspension culture [95]. They noticed that the aggregates obtained on Day 2 after cell inoculation revealed higher cTnT expression, whereas cells on Day 3 presented lower efficiency, thereby suggesting that the larger size of aggregates reduced the differentiation efficiency. They noted that for cardiac differentiation, the optimal concentration of CHIR99021 depends on the aggregate size. Higher CHIR99021 concentrations are needed to effectively promote cardiac differentiation from larger aggregates. Presumably, optimal concentration of CHIR99021 induction for the total number of cell population or depth of cell layers may be the key factor deciding cardiac differentiation efficiency. In addition, the homogeneity in the aggregate size would be required for good reproducibility of differentiation process.
Regarding the homogeneity in cell population of the 2D monolayer culture, several groups noticed that more beating cells can be obtained in the peripheral side of the culture dishes, so-called the rim effect [88,96,97]. Laco et al. demonstrated that the peripheral cells tend to have higher level of cell proliferation, thus promoting cell contraction earlier and stronger than the center cells [98]. Increase in the G1 cell cycle phase and loss in G2/M restrict the cell expansion at the center part of the culture dish [98,99]. Therefore, to enhance the differentiation efficiency in the central part of cell aggregates in 3D culture, small-sized and uniform cell aggregates may be favorable [95].
Nguyen et al. demonstrated that improvement in cardiomyocyte maturation and enrichment can also be obtained by producing a 3D cardiosphere even from 2D differentiated cardiomyocytes [100]. By harvesting the cardiomyocytes on culture plates after 14 days of differentiation and replating into the microwells (Aggrewell 400; StemCell Technologies), a population containing 80–100% beating aggregates can be obtained, with the initial population as little as 10% cardiomyocytes. By applying the same technique of microwell to make aggregates from hPSCs-derived cardiac progenitor, Correia et al. demonstrated that the 3D aggregate culture improves the cardiomyocyte purity and metabolic maturation. Compared to the 2D culture, the 3D culture revealed increased gene expression associated with mitochondrial oxidative phosphorylation and decreased gene expression of glycolysis and lipid synthesis [101]. Collectively, these works proved that 3D aggregates culture indeed results in cardiomyocyte enrichment and maturation.
3.4. Cardiomyocyte Differentiation by Applying Hydrogels
A novel approach to directly obtain different culture formats of cardiomyocytes such as microisland, macrotissues, or microsphere by applying hydrogels, is developed [102]. hPSCs are expanded in the 3D model in hydrogel and continuously transferred for cardiac differentiation in 3D model while achieves cardiomyocyte sheet-like structures. As mentioned previously, hydrogel is a hydrophilic polymer which can swell in liquid to form a 3D network and retain its structure until it changes in external physical/chemical conditions [66]. Kerscher’s group used polyethylene glycol (PEG)-fibrinogen hydrogels to encapsulate the hPSCs and form 3D structure for expansion and cardiac differentiation continuously. To form 3D structure from single cell dissociation, ROCK inhibitor was applied only for the first 24 h culture in mTeSR1 medium (StemCell Technologies). Removal of ROCK inhibitor during cell expansion helps to reduce the change in hPSCs metabolism [60]. A population of 75% cardiomyocytes was obtained on Day 20 and could be sustained longer for maturity. T-tubule structure, which is a key component of functional mature cardiomyocytes and has only been seen in postnatal and adult cardiomyocytes, was obtained in this culture model. In this study, only cardiac tissue created on coverslip by micro-island method for drug treatment was reported. As a future prospect, application of several hydrogel models to form macrotissue or microsphere of hPSCs is considered for large scale production of various patterns of cardiomyocytes, which can be used for different biomedical purposes. For details of the culture method for hPSC expansion and cardiomyocyte differentiation, please refer to Table 2.

Table 2.
Two-in-one system for hPSCs expansion and cardiomyocytes (CMs) differentiation.
4. Strategies for Cardiomyocyte Maturation
As cardiomyocytes differentiated from hPSCs have similar properties as that of fetal cardiac cell fate [20], these cells should be cultured until they mature in order to be suitable for application in drug screening and disease modeling. Required maturity may be highly variable and depended on target diseases, function, and molecules. Toxicology studies may need most matured cells similar to adult cardiomyocytes. Maturation of cardiomyocytes can be enhanced by co-culturing with endothelial cells [82,83], prolonging the culture time [89,90], controlling the surrounding extracellular matrix [104,105], as well as by applying mechanical and electrical stimulation [106,107,108].
Since cardiomyocytes are subjected to electrical stimulation from the early stage of tubular heart formation, this approach is helpful in increasing cardiomyocyte maturation in terms of improving myofibril ultrasound organization, increasing conduction speed, and promoting both Ca2+ handling and electrophysiological properties [106]. Besides, addition of carbon nanofibers to hydrogels to increase the conductivity of hydrogels can help promote cardiomyocyte growth and enhance cardiac function [109,110]. Notably, the application of mechanical stimulation is crucial for cardiomyocyte maturation since the main function of the heart is the mechanical pump that responds to mechanical stimulation throughout its lifetime. By applying cyclic stretch, Mihic et al. improved functional maturation and viability of cardiac tissues [107]. Ruan et al. demonstrated that applying mechanical stimulation revealed improvement in sarcomere alignment and formed stiffer constructs; while addition effect of electrical stimulation did not change the cell morphology but resulted in improved contractility [108]. These data reveal a promising approach of combining multiple stimuli in improving cardiomyocyte maturation. Notably, Shen et al. developed a bioreactor system using cyclic strain and pulsatile flow that can enhance the expression of cardiac-related proteins and genes as well as cardiac ion channel genes [111]. This bioreactor comprises a fluid and an air chamber separated by silicon membrane to ensure a sterile environment. By applying this dynamic culture system, the improvement in SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) activity was obtained while maturation level was similar to the primary cardiomyocytes by Raman fingerprint analysis. Combination of dynamic culture and by prolonging the culture time to 20 days exhibited significant change in cardiomyocyte maturation [111]. In the absence of dynamic culture, prolonging culture to several months is required for hPSC-derived cardiomyocytes to achieve the phenotype of adult cardiomyocytes [89]. Thus, dynamic culture plays a pivotal role in promoting cardiomyocyte maturation.
Several dynamic culture systems were applied for suspension culture of hPSCs and cardiomyocyte differentiation such as the spinner flask (Corning) [95], rotary orbital suspension culture [100], DASGIP, or DASbox Bioreactor System (Eppendorf) [94,112,113]. Application of dynamic culture in the bioreactor to obtain sufficient amount of hPSCs and application for cardiomyocyte differentiation would be an appropriate direction for large-scale production. Dynamic culture has an advantage in not only scaling up but also significantly increasing the functional genes and contractile proteins expression in cardiomyocyte production [114,115,116]. The functional improvement of hPCS-derived cardiomyocytes in a dynamic culture is driven via mTOR signaling pathway [116] and ERK1/2 signaling pathway [114]; however, in a dynamic culture, it is necessary to consider the influence of shear stress on the cell viability and proliferation through physical damage and cell death. Shear stress can also affect the gene expression via mechanotransduction, in which the physical signals are perceived at the periphery of the cell and then converted into biochemical signals in the cell [117]. Although suboptimal values of shear stress resulted in low efficiency of cardiomyocyte differentiation [95,103], appropriate application of shear stress to enhance cell signaling cascade by optimizing agitation rate in 3D culture is an effective approach to improve cardiac differentiation efficiency and maturity [114,118].
Besides, extracellular matrix effects, including its composition, alignment, and stiffness, are also important in deciding the cardiomyocyte behavior such as contraction and calcium handling [20,119]. Application of polyacrylamide hydrogel as the stiffness-controllable substrate for cardiomyocytes culture reveals that high degree of maturation can be achieved at intermediate stiffness of 5–10 kPa [120]. Interestingly, Noor et al. recently demonstrated autologous hydrogels produced by processing patient cell-derived extracellular matrix can be applied as a scaffold for cardiomyocyte differentiation [121]. By applying the autologous hydrogels as bioink and 3D printing techniques, they could print vascularized and perfusable heart patches which are completely consistent with the patient’s immunological characteristic. This group of authors really paves the way for engineering hearts in appropriate structure to apply in transplantation, drug screening, and disease modeling.
5. Conclusions and Future Perspective
Cardiomyocytes derived from hPSCs are a promising cell source for cell transplantation therapy in heart failures as well as drug discovery and disease modeling for cardiac diseases; however, as discussed in this review, there are several technical barriers to achieve necessary number and quality of cardiomyocytes.
For transplantation, it may be adequate to use numerous immature cardiomyocytes. To date, the efficient methods for cardiomyocyte differentiation from large-scale expansion of hPSCs may include culturing hiPSC as 3D aggregates and hydrogel application to form a 3D structure. For avoiding cellular damage caused by shear stress in the production, encapsulation by hydrogels or macroporous microcarrier will be efficient [35,122]. Applying hiPSC as 3D aggregates independent from the ROCK inhibitor is ideal; however, 3D aggregates along with the ROCK inhibitor is also favorable for cell commitment toward mesendoderm lineage including cardiomyocytes [63,123]. For hPSC expansion and cardiac differentiation in suspension with size-controlled aggregates without depending on carrier and agitation, application of functional polymers as proposed by Otsuji et al. [71] is recommended although the system needs to be improved on oxygen supply in large reactor for industrial-scale cell production. While further studies to examine the effect of viscosity on diffusion of soluble factors are necessary, application of functional polymers for hPSC culture may serve as a future prospect for hPSC expansion at industrial scale and cardiomyocyte differentiation (Figure 2).
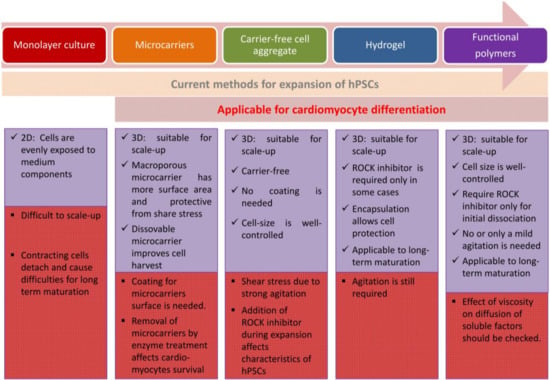
Figure 2.
Integrated methods for hPSCs expansion and cardiomyocytes differentiation.
In order to improve cardiomyocyte maturation, co-culture with mesenchymal stem cells or exposure to soluble factors from mesenchymal stem cells may be useful as reported by Yoshida et al. [124]. As the future perspective, the first step to enhance cardiomyocyte maturation can be addressed by co-culture with endothelial cells and mesenchymal stem cells. The identification of factors and mechanisms lead advantage in co-culture system and replacement of the endothelial cells and mesenchymal stem cells with the factors may be necessary for large scale cardiomyocyte production. For the second step, prolonging time in dynamic culture using 3D aggregates in suspension can be applied to achieve further maturation. Application of stiffness-controllable polyacrylamide hydrogels as scaffold in the later stage to mimic the stiffness of native cardiomyocytes can be a good consideration to modulate the extracellular matrix for further enhancing cardiac maturation [125]. Although the 3D culture system has not yet been established, application of polymers to control stiffness of the surface such as polyacrylamide [126] or poly(vinyl alcohol-co-vinyl acetate-co-itaconic acid) [127] may be a good approach to enhance the cardiomyocyte maturation in future.
In this review, we introduced the culture systems for large scale expansion of hPSCs integrated with methods for cardiomyocyte differentiation and further discussed methods for maturation of the cardiomyocytes. Dynamic cell culture system would be the most practical and productive method for large scale production of cardiomyocytes, which are required for transplantation therapy; however, several issues need to be addressed in each method, such as avoiding dependence on ROCK inhibitors in cell aggregates dissociation, requirement of fine tuning of shear stress, purity and maturation, establishment of xeno-free culture system compatible for various hPSC lines, and verification according to GMP regulation. We hope these issues will be resolved promptly and patients will be able to undergo cardiomyocyte transplantation to cure their disease in the near future.
Author Contributions
M.N.T.L. wrote the manuscript. K.H. revised and completed the work.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript.
References
- Wang, H. Pluripotent Stem Cells and Repair of Myocardial Infarction. Trop. Med. Surg. 2015. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.J.; Martinez-Fernandez, A.; Yamada, S.; Perez-Terzic, C.; Ikeda, Y.; Terzic, A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 2009, 120, 408–416. [Google Scholar] [CrossRef]
- Singla, D.K.; Long, X.; Glass, C.; Singla, R.D.; Yan, B. Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium. Mol. Pharm. 2011, 8, 1573–1581. [Google Scholar] [CrossRef]
- Ahmed, R.P.; Ashraf, M.; Buccini, S.; Shujia, J.; Haider, H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lalit, P.A.; Hei, D.J.; Raval, A.N.; Kamp, T.J. Induced pluripotent stem cells for post-myocardial infarction repair: Remarkable opportunities and challenges. Circ. Res. 2014, 114, 1328–1345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A.; et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Osaka University to Begin World’s First iPS Cell-Based Therapy for the Heart. Available online: http://www.med.osaka-u.ac.jp/eng/archives/3739 (accessed on 20 April 2019).
- Halbach, M.; Krausgrill, B.; Hannes, T.; Wiedey, M.; Peinkofer, G.; Baumgartner, S.; AliSahito, R.G.; Pfannkuche, K.; Pillekamp, F.; Reppel, M.; et al. Time-course of the electrophysiological maturation and integration of transplanted cardiomyocytes. J. Mol. Cell. Cardiol. 2012, 53, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Li, T.S.; Cheng, K.; Malliaras, K.; Smith, R.R.; Zhang, Y.; Sun, B.; Matsushita, N.; Blusztajn, A.; Terrovitis, J.; Kusuoka, H.; et al. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J. Am. Coll. Cardiol. 2012, 59, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell-Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Chen, K.G.; Mallon, B.S.; McKay, R.D.; Robey, P.G. Human pluripotent stem cell culture: Considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014, 14, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Dakhore, S.; Nayer, B.; Hasegawa, K. Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int. 2018, 2018, 7396905. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.E.; Dai, D.F.; Laflamme, M.A. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv. Drug Deliv. Rev. 2016, 96, 3–17. [Google Scholar] [CrossRef]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A.; et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 2016, 1863, 1728–1748. [Google Scholar] [CrossRef]
- Aigha, I.; Raynaud, C. Maturation of pluripotent stem cell derived cardiomyocytes: The new challenge. Glob. Cardiol. Sci. Pract. 2016, 2016, e201606. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.J.; Butcher, J. Naturally Engineered Maturation of Cardiomyocytes. Front. Cell Dev. Biol. 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Andree, B.; Zweigerdt, R. Large-scale production of human pluripotent stem cell derived cardiomyocytes. Adv. Drug Deliv. Rev. 2016, 96, 18–30. [Google Scholar] [CrossRef]
- Tsutsui, H.; Valamehr, B.; Hindoyan, A.; Qiao, R.; Ding, X.; Guo, S.; Witte, O.N.; Liu, X.; Ho, C.M.; Wu, H. An optimized small molecule inhibitor cocktail supports long-term maintenance of human embryonic stem cells. Nat. Commun. 2011, 2, 167. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Miyazaki, T.; Isobe, T.; Nakatsuji, N.; Suemori, H. Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Sci. Rep. 2017, 7, 41165. [Google Scholar] [CrossRef]
- Melkoumian, Z.; Weber, J.L.; Weber, D.M.; Fadeev, A.G.; Zhou, Y.; Dolley-Sonneville, P.; Yang, J.; Qiu, L.; Priest, C.A.; Shogbon, C.; et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yao, H.; Weber, J.L.; Melkoumian, Z.K.; Ye, K. A synthetic, xeno-free peptide surface for expansion and directed differentiation of human induced pluripotent stem cells. PLoS ONE 2012, 7, e50880. [Google Scholar] [CrossRef] [PubMed]
- Brafman, D.A.; Chang, C.W.; Fernandez, A.; Willert, K.; Varghese, S.; Chien, S. Long-term human pluripotent stem cell self-renewal on synthetic polymer surfaces. Biomaterials 2010, 31, 9135–9144. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Krishanu, S.; Bogatyrev, S.R.; Yang, J.; Hook, A.L.; Kalcioglu, Z.I.; Cho, S.W.; Mitalipova, M.; Pyzocha, N.; Roja, F.; et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat. Mater. 2010, 9, 768–778. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Nandivada, H.; Ding, J.; Nogueira-de-Souza, N.C.; Krebsbach, P.H.; O’Shea, K.S.; Lahann, J.; Smith, G.D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 581–583. [Google Scholar] [CrossRef]
- Irwin, E.F.; Gupta, R.; Dashti, D.C.; Healy, K.E. Engineered polymer-media interfaces for the long-term self-renewal of human embryonic stem cells. Biomaterials 2011, 32, 6912–6919. [Google Scholar] [CrossRef]
- Ross, A.M.; Nandivada, H.; Ryan, A.L.; Lahann, J. Synthetic substrates for long-term stem cell culture. Polymer 2012, 53, 2533–2539. [Google Scholar] [CrossRef]
- Celiz, A.D.; Smith, J.G.; Patel, A.K.; Hook, A.L.; Rajamohan, D.; George, V.T.; Flatt, L.; Patel, M.J.; Epa, V.C.; Singh, T.; et al. Discovery of a Novel Polymer for Human Pluripotent Stem Cell Expansion and Multilineage Differentiation. Adv. Mater. 2015, 27, 4006–4012. [Google Scholar] [CrossRef]
- Yasuda, S.-y.; Ikeda, T.; Shahsavarani, H.; Yoshida, N.; Nayer, B.; Hino, M.; Vartak-Sharma, N.; Suemori, H.; Hasegawa, K. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nat. Biomed. Eng. 2018, 2, 173–182. [Google Scholar] [CrossRef]
- Microcarrier Cell Culture—Principles and Methods. Available online: http://www.gelifesciences.co.kr/wp-content/uploads/2016/07/023.8_Microcarrier-Cell-Culture.pdf (accessed on 20 April 2019).
- Chen, A.K.; Chen, X.; Choo, A.B.; Reuveny, S.; Oh, S.K. Critical microcarrier properties affecting the expansion of undifferentiated human embryonic stem cells. Stem Cell Res. 2011, 7, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Rodrigues, A.F.; Correia, C.; Sousa, M.F.; Brito, C.; Coroadinha, A.S.; Serra, M.; Alves, P.M. Robust Expansion of Human Pluripotent Stem Cells: Integration of Bioprocess Design with Transcriptomic and Metabolomic Characterization. Stem Cells Transl. Med. 2015, 4, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.A.V.; Silva, T.P.; Nogueira, D.E.S.; Fernandes, T.G.; Hashimura, Y.; Wesselschmidt, R.; Diogo, M.M.; Lee, B.; Cabral, J.M.S. Scalable culture of human induced pluripotent cells on microcarriers under xeno-free conditions using single-use vertical-wheel™ bioreactors. J. Chem. Technol. Biotechnol. 2018, 93, 3597–3606. [Google Scholar] [CrossRef]
- Lecina, M.; Ting, S.; Choo, A.; Reuveny, S.; Oh, S. Scalable platform for human embryonic stem cell differentiation to cardiomyocytes in suspended microcarrier cultures. Tissue Eng. Part C Methods 2010, 16, 1609–1619. [Google Scholar] [CrossRef]
- Merten, O.W. Advances in cell culture: Anchorage dependence. Philos. Trans. R. Soc. London. Ser. Bbiol. Sci. 2015, 370, 20140040. [Google Scholar] [CrossRef]
- Farrell, C.J.; Cicalese, S.M.; Davis, H.B.; Dogdas, B.; Shah, T.; Culp, T.; Hoang, V.M. Cell confluency analysis on microcarriers by micro-flow imaging. Cytotechnology 2016, 68, 2469–2478. [Google Scholar] [CrossRef]
- Phillips, B.W.; Horne, R.; Lay, T.S.; Rust, W.L.; Teck, T.T.; Crook, J.M. Attachment and growth of human embryonic stem cells on microcarriers. J. Biotechnol. 2008, 138, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.K.; Chen, A.K.; Mok, Y.; Chen, X.; Lim, U.M.; Chin, A.; Choo, A.B.; Reuveny, S. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009, 2, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Fernandes, T.G.; Cordeiro, C.S.; Boucher, S.; Kuninger, D.; Vemuri, M.C.; Diogo, M.M.; Cabral, J.M. Defined Essential 8 Medium and Vitronectin Efficiently Support Scalable Xeno-Free Expansion of Human Induced Pluripotent Stem Cells in Stirred Microcarrier Culture Systems. PLoS ONE 2016, 11, e0151264. [Google Scholar] [CrossRef]
- Stathopoulos, N.A.; Hellums, J.D. Shear stress effects on human embryonic kidney cells in Vitro. Biotechnol. Bioeng. 1985, 27, 1021–1026. [Google Scholar] [CrossRef]
- Cormier, J.T.; Nieden, N.I.Z.; Rancourt, D.E.; Kallos, M.S. Expansion of Undifferentiated Murine Embryonic Stem Cells as Aggregates in Suspension Culture Bioreactors. Tissue Eng. 2006, 12, 3233–3245. [Google Scholar] [CrossRef] [PubMed]
- Badenes, S.M.; Fernandes, T.G.; Miranda, C.C.; Pusch-Klein, A.; Haupt, S.; Rodrigues, C.A.V.; Diogo, M.M.; Brüstle, O.; Cabral, J.M.S. Long-term expansion of human induced pluripotent stem cells in a microcarrier-based dynamic system. J. Chem. Technol. Biotechnol. 2017, 92, 492–503. [Google Scholar] [CrossRef]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.P.; Chan, J.K.Y.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, X.; Yu, K.; Meng, H.; Zheng, Y.; Peng, J.; Lu, S.; Liu, X.; Xie, Y.; Qiao, K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials 2018, 183, 171–172. [Google Scholar] [CrossRef]
- Rodrigues, A.L.; Rodrigues, C.A.V.; Gomes, A.R.; Vieira, S.F.; Badenes, S.M.; Diogo, M.M.; Cabral, J.M.S. Dissolvable Microcarriers Allow Scalable Expansion And Harvesting Of Human Induced Pluripotent Stem Cells Under Xeno-Free Conditions. Biotechnol. J. 2019, 14, e1800461. [Google Scholar] [CrossRef]
- Amit, M.; Chebath, J.; Margulets, V.; Laevsky, I.; Miropolsky, Y.; Shariki, K.; Peri, M.; Blais, I.; Slutsky, G.; Revel, M.; et al. Suspension Culture of Undifferentiated Human Embryonic and Induced Pluripotent Stem Cells. Stem Cell Rev. Rep. 2010, 6, 248–259. [Google Scholar] [CrossRef]
- Olmer, R.; Haase, A.; Merkert, S.; Cui, W.; Palecek, J.; Ran, C.; Kirschning, A.; Scheper, T.; Glage, S.; Miller, K.; et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010, 5, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizadeh, S.; Larijani, M.R.; Samadian, A.; Baharvand, H. Bioprocess Development for Mass Production of Size-Controlled Human Pluripotent Stem Cell Aggregates in Stirred Suspension Bioreactor. Tissue Eng. Part C 2012, 18. [Google Scholar] [CrossRef]
- Chen, V.C.; Couture, S.M.; Ye, J.; Lin, Z.; Hua, G.; Huang, H.I.; Wu, J.; Hsu, D.; Carpenter, M.K.; Couture, L.A. Scalable GMP compliant suspension culture system for human ES cells. Stem Cell Res. 2012, 8, 388–402. [Google Scholar] [CrossRef]
- Hookway, T.A.; Butts, J.C.; Lee, E.; Tang, H.; McDevitt, T.C. Aggregate formation and suspension culture of human pluripotent stem cells and differentiated progeny. Methods 2016, 101, 11–20. [Google Scholar] [CrossRef]
- Bauwens, C.L.; Toms, D.; Ungrin, M. Aggregate Size Optimization in Microwells for Suspension-based Cardiac Differentiation of Human Pluripotent Stem Cells. J. Vis. Exp. JOVE 2016. [Google Scholar] [CrossRef] [PubMed]
- Amit, M.; Laevsky, I.; Miropolsky, Y.; Shariki, K.; Peri, M.; Itskovitz-Eldor, J. Dynamic suspension culture for scalable expansion of undifferentiated human pluripotent stem cells. Nat. Protoc. 2011, 6, 572–579. [Google Scholar] [CrossRef]
- Kwok, C.K.; Ueda, Y.; Kadari, A.; Gunther, K.; Ergun, S.; Heron, A.; Schnitzler, A.C.; Rook, M.; Edenhofer, F. Scalable stirred suspension culture for the generation of billions of human induced pluripotent stem cells using single-use bioreactors. J. Tissue Eng. Regen. Med. 2017, 12, e1076–e1087. [Google Scholar] [CrossRef]
- Wang, Y.; Chou, B.K.; Dowey, S.; He, C.; Gerecht, S.; Cheng, L. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res. 2013, 11, 1103–1116. [Google Scholar] [CrossRef]
- Kim, M.H.; Takeuchi, K.; Kino-Oka, M. Role of cell-secreted extracellular matrix formation in aggregate formation and stability of human induced pluripotent stem cells in suspension culture. J. Biosci. Bioeng. 2018, 127, 372–380. [Google Scholar] [CrossRef]
- Vernardis, S.; Terzoudis, K.; Panoskaltsis, N.; Mantalaris, A. Human embryonic and induced pluripotent stem cells maintain phenotype but alter their metabolism after exposure to ROCK inhibitor. Sci. Rep. 2017, 7, 42138. [Google Scholar] [CrossRef]
- Bai, Q.; Ramirez, J.M.; Becker, F.; Pantesco, V.; Lavabre-Bertrand, T.; Hovatta, O.; Lemaitre, J.M.; Pellestor, F.; De Vos, J. Temporal analysis of genome alterations induced by single-cell passaging in human embryonic stem cells. Stem Cells Dev. 2015, 24, 653–662. [Google Scholar] [CrossRef]
- Garitaonandia, I.; Amir, H.; Boscolo, F.S.; Wambua, G.K.; Schultheisz, H.L.; Sabatini, K.; Morey, R.; Waltz, S.; Wang, Y.C.; Tran, H.; et al. Increased risk of genetic and epigenetic instability in human embryonic stem cells associated with specific culture conditions. PLoS ONE 2015, 10, e0118307. [Google Scholar] [CrossRef]
- Maldonado, M.; Luu, R.J.; Ramos, M.E.P.; Nam, J. ROCK inhibitor primes human induced pluripotent stemcells to selectively differentiate towardsmesendodermal lineage via epithelial-mesenchymal transition-likemodulation. Stem Cell Res. 2016, 17, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Khoneisser, J.; Huang, P.C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef]
- Siti-Ismail, N.; Bishop, A.E.; Polak, J.M.; Mantalaris, A. The benefit of human embryonic stem cell encapsulation for prolonged feeder-free maintenance. Biomaterials 2008, 29, 3946–3952. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Urtti, A.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef]
- Lei, Y.; Schaffer, D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, E5039–E5048. [Google Scholar] [CrossRef]
- Otsuji, T.G.; Bin, J.; Yoshimura, A.; Tomura, M.; Tateyama, D.; Minami, I.; Yoshikawa, Y.; Aiba, K.; Heuser, J.E.; Nishino, T.; et al. A 3D Sphere Culture System Containing Functional Polymers for Large-Scale Human Pluripotent Stem Cell Production. Stem Cell Rep. 2014, 2, 734–745. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Chen, L.; Liu, J.X.; Huang, J.W.; Wu, G.; Zheng, Y.F.; Yao, K.T. A novel three-dimensional tumorsphere culture system for the efficient and low-cost enrichment of cancer stem cells with natural polymers. Exp. Ther. Med. 2018, 15, 85–92. [Google Scholar] [CrossRef]
- Miyazaki, T.; Futaki, S.; Hasegawa, K.; Kawasaki, M.; Sanzen, N.; Hayashi, M.; Kawase, E.; Sekiguchi, K.; Nakatsuji, N.; Suemori, H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008, 375, 27–32. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Minami, I.; Yamada, K.; Otsuji, T.G.; Yamamoto, T.; Shen, Y.; Otsuka, S.; Kadota, S.; Morone, N.; Barve, M.; Asai, Y.; et al. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep. 2012, 2, 1448–1460. [Google Scholar] [CrossRef] [PubMed]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014, 11, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Bao, X.; Zilberter, M.; Westman, M.; Fisahn, A.; Hsiao, C.; Hazeltine, L.B.; Dunn, K.K.; Kamp, T.J.; Palecek., S.P. Chemically defined, albumin-free human cardiomyocyte generation. Nat. Methods 2015, 12, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, S.; Fujita, J.; Fujita, C.; Yamaguchi, M.; Kanaami, S.; Ohno, R.; Sakamoto, K.; Kodama, M.; Kurokawa, J.; Kanazawa, H.; et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep. 2017, 9, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Vohwinkel, C.U.; Lecuona, E.; Sun, H.; Sommer, N.; Vadasz, I.; Chandel, N.S.; Sznajder, J.I. Elevated CO2 levels cause mitochondrial dysfunction and impair cell proliferation. J. Biol. Chem. 2011, 286, 37067–37076. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, S.; Sovalat, H.; Henon, P.; Bischoff, N.; Arkam, Y.; Ojeda-Uribe, M.; Bouar, R.; Rimelen, V.; Brink, I.; Dallemand, R.; et al. Long-term benefit of intracardiac delivery of autologous granulocyte-colony-stimulating factor-mobilized blood CD34+ cells containing cardiac progenitors on regional heart structure and function after myocardial infarct. Cytotherapy 2009, 11, 1002–1015. [Google Scholar] [CrossRef]
- Ogasawara, T.; Okano, S.; Ichimura, H.; Kadota, S.; Tanaka, Y.; Minami, I.; Uesugi, M.; Wada, Y.; Saito, N.; Okada, K.; et al. Impact of extracellular matrix on engraftment and maturation of pluripotent stem cell-derived cardiomyocytes in a rat myocardial infarct model. Sci. Rep. 2017, 7, 8630. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Davis, M.E.; Lisowski, L.K.; Lee, R.T. Endothelial-cardiomyocyte interactions in cardiac development and repair. Annu. Rev. Physiol. 2006, 68, 51–66. [Google Scholar] [CrossRef]
- Chen, K.; Bai, H.; Arzigian, M.; Gao, Y.X.; Bao, J.; Wu, W.S.; Shen, W.F.; Wu, L.; Wang, Z.Z. Endothelial cells regulate cardiomyocyte development from embryonic stem cells. J. Cell. Biochem. 2010, 111, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.; Orlova, V.V.; Mummery, C.L. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Paige, S.L.; Osugi, T.; Afanasiev, O.K.; Pabon, L.; Reinecke, H.; Murry, C.E. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 2010, 5, e11134. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Estarás, C.; Hsu, H.; Huang, L.; Jones, K. YAP repression of the WNT3 gene controls hESC differentiation along the cardiac mesoderm lineage. Genes Dev. 2017, 31, 2250–2263. [Google Scholar] [CrossRef]
- Le, M.N.T.; Takahi, M.; Maruyama, K.; Kurisaki, A.; Ohnuma, K. Cardiac differentiation at an initial low density of human-induced pluripotent stem cells. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 513–522. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.Z.; Regnier, M.; Laflamme, M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef]
- Kamakura, T.; Makiyama, T.; Sasaki, K.; Yoshida, Y.; Wuriyanghai, Y.; Chen, J.; Hattori, T.; Ohno, S.; Kita, T.; Horie, M.; et al. Ultrastructural Maturation of Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Long-Term Culture. Circ. J. 2013, 77, 1307–1314. [Google Scholar] [CrossRef]
- Oh, S.; Chen, A.; Ting, S.; Wei, H.; Reuveny, S. Unified microcarrier process for human pluripotent stem cell expansion, and cardiomyocyte differentiation and purification in a 500 mL bioreactor. Cytotherapy 2017, 19, e22. [Google Scholar] [CrossRef]
- Braam, S.R.; Nauw, R.; Oostwaard, D.W.; Mummery, C.; Passier, R. Inhibition of ROCK improves survival of human embryonic stem cell–derived cardiomyocytes after dissociation. Ann. N. Y. Acad. Sci. 2010, 1188, 52–57. [Google Scholar] [CrossRef]
- Rao, J.; Pfeiffer, M.J.; Frank, S.; Adachi, K.; Piccini, I.; Quaranta, R.; Arauzo-Bravo, M.; Schwarz, J.; Schade, D.; Leidel, S.; et al. Stepwise Clearance of Repressive Roadblocks Drives Cardiac Induction in Human ESCs. Cell Stem Cell 2016, 18, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kempf, H.; Olmer, R.; Kropp, C.; Ruckert, M.; Jara-Avaca, M.; Robles-Diaz, D.; Franke, A.; Elliott, D.A.; Wojciechowski, D.; Fischer, M.; et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 2014, 3, 1132–1146. [Google Scholar] [CrossRef]
- Chen, V.C.; Ye, J.; Shukla, P.; Hua, G.; Chen, D.; Lin, Z.; Liu, J.C.; Chai, J.; Gold, J.; Wu, J.; et al. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Hartung, S.; Schwanke, K.; Haase, A.; David, R.; Franz, W.M.; Martin, U.; Zweigerdt, R. Directing cardiomyogenic differentiation of human pluripotent stem cells by plasmid-based transient overexpression of cardiac transcription factors. Stem Cells Dev. 2013, 22, 1112–1125. [Google Scholar] [CrossRef]
- Kempf, H.; Olmer, R.; Haase, A.; Franke, A.; Bolesani, E.; Schwanke, K.; Robles-Diaz, D.; Coffee, M.; Gohring, G.; Drager, G.; et al. Bulk cell density and Wnt/TGFbeta signalling regulate mesendodermal patterning of human pluripotent stem cells. Nat. Commun. 2016, 7, 13602. [Google Scholar] [CrossRef] [PubMed]
- Laco, F.; Woo, T.L.; Zhong, Q.; Szmyd, R.; Ting, S.; Khan, F.J.; Chai, C.L.L.; Reuveny, S.; Chen, A.; Oh, S. Unraveling the Inconsistencies of Cardiac Differentiation Efficiency Induced by the GSK3beta Inhibitor CHIR99021 in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 10, 1851–1866. [Google Scholar] [CrossRef]
- Jacobs, K.; Zambelli, F.; Mertzanidou, A.; Smolders, I.; Geens, M.; Nguyen, H.T.; Barbe, L.; Sermon, K.; Spits, C. Higher-Density Culture in Human Embryonic Stem Cells Results in DNA Damage and Genome Instability. Stem Cell Rep. 2016, 6, 330–341. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Hookway, T.A.; Wu, Q.; Jha, R.; Preininger, M.K.; Chen, X.; Easley, C.A.; Spearman, P.; Deshpande, S.R.; Maher, K.; et al. Microscale generation of cardiospheres promotes robust enrichment of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2014, 3, 260–268. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Carido, M.; Sebastião, M.J.; Gomes-Alves, P.; Elliott, D.A.; Domian, I.J.; Teixeira, A.P.; et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2018, 115, 630–644. [Google Scholar] [CrossRef]
- Kerscher, P.; Turnbull, I.C.; Hodge, A.J.; Kim, J.; Seliktar, D.; Easley, C.J.; Costa, K.D.; Lipke, E.A. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials 2016, 83, 383–395. [Google Scholar] [CrossRef]
- Ting, S.; Chen, A.; Reuveny, S.; Oh, S. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Res. 2014, 13, 202–213. [Google Scholar] [CrossRef]
- Horning, M.; Kidoaki, S.; Kawano, T.; Yoshikawa, K. Rigidity matching between cells and the extracellular matrix leads to the stabilization of cardiac conduction. Biophys. J. 2012, 102, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhi Hirata, T.Y. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater. 2018, 65, 44–52. [Google Scholar] [CrossRef]
- Nunes, S.S.; Miklas, J.W.; Liu, J.; Aschar-Sobbi, R.; Xiao, Y.; Zhang, B.; Jiang, J.; Masse, S.; Gagliardi, M.; Hsieh, A.; et al. Biowire: A platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods 2013, 10, 781–787. [Google Scholar] [CrossRef]
- Mihic, A.; Li, J.; Miyagi, Y.; Gagliardi, M.; Li, S.H.; Zu, J.; Weisel, R.D.; Keller, G.; Li, R.K. The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials 2014, 35, 2798–2808. [Google Scholar] [CrossRef]
- Ruan, J.L.; Tulloch, N.L.; Razumova, M.V.; Saiget, M.; Muskheli, V.; Pabon, L.; Reinecke, H.; Regnier, M.; Murry, C.E. Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 2016, 134, 1557–1567. [Google Scholar] [CrossRef]
- Stout, D.A.; Yoo, J.; Santiago-Miranda, A.N.; Webster, T.J. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular application. Int. J. Nanomed. 2012, 7, 5653–5669. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Knopf, A.; Westendorf, C.; Kraushaar, U.; Riedl, J.; Bauer, H.; Poschel, S.; Layland, S.L.; Holeiter, M.; Knolle, S.; et al. Steps toward Maturation of Embryonic Stem Cell-Derived Cardiomyocytes by Defined Physical Signals. Stem Cell Rep. 2017, 9, 122–135. [Google Scholar] [CrossRef]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef]
- Kempf, H.; Kropp, C.; Olmer, R.; Martin, U.; Zweigerdt, R. Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Nat. Protoc. 2015, 10, 1345–1361. [Google Scholar] [CrossRef]
- Dvir, T.; Levy, O.; Shachar, M.; Granot, Y.; Cohen, S. Activation of the ERK1/2 cascade via pulsatile interstitial fluid flow promotes cardiac tissue assembly. Tissue Eng. 2007, 13, 2185–2193. [Google Scholar] [CrossRef]
- Cheng, M.; Moretti, M.; Engelmayr, G.C.; Freed, L.E. Insulin-like growth factor-I and slow, bi-directional perfusion enhance the formation of tissue-engineered cardiac grafts. Tissue Eng. Part A 2009, 15, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Jackman, C.P.; Carlson, A.L.; Bursac, N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials 2016, 111, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shafa, M.; Krawetz, R.; Zhang, Y.; Rattner, J.B.; Godollei1, A.; Duff, H.J.; Rancourt, D.E. Impact of stirred suspension bioreactor culture on the differentiation of murine embryonic stem cells into cardiomyocytes. BMC Cell Biol. 2011, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Mogi, A.; Takei, S.; Shimizu, H.; Miura, H.; Tomotsune, D.; Sasaki, K. Effects of Fluid Dynamic Forces Created by Rotary Orbital Suspension Culture on Cardiomyogenic Differentiation of Human Embryonic Stem Cells. J. Med. Biol. Eng. 2014, 34, 101. [Google Scholar] [CrossRef]
- Takahashi, K.; Kakimoto, Y.; Toda, K.; Naruse, K. Mechanobiology in cardiac physiology and diseases. J. Cell. Mol. Med. 2013, 17, 225–232. [Google Scholar] [CrossRef]
- Tallawi, M.; Rai, R.; Boccaccini, A.R.; Aifantis, K.E. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng. Part B Rev. 2015, 21, 157–165. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019. [Google Scholar] [CrossRef]
- Jing, D.; Parikh, A.; Tzanakakis, E.S. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant. 2010, 19, 1397–1412. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.T.; Yeih, D.F.; Liang, S.M.; Chien, C.Y.; Yu, Y.L.; Ko, B.S.; Jan, Y.J.; Kuo, C.C.; Sung, L.Y.; Shyue, S.K.; et al. Rho-associated kinase inhibitors promote the cardiac differentiation of embryonic and induced pluripotent stem cells. Int. J. Cardiol. 2015, 201, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Miyagawa, S.; Fukushima, S.; Kawamura, F.; Kashiyama, N.; Ohashi, F.; Toyofuku, T.; Toda, K.; Sawa, Y. Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes by Soluble Factors from Human Mesenchymal Stem Cells. Mol. Ther. 2018, 26, 2681–2695. [Google Scholar] [CrossRef]
- Ribeiro, A.J.; Ang, Y.S.; Fu, J.D.; Rivas, R.N.; Mohamed, T.M.; Higgs, G.C.; Srivastava, D.; Pruitt, B.L. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 12705–12710. [Google Scholar] [CrossRef] [PubMed]
- Horning, M.; Nakahata, M.; Linke, P.; Yamamoto, A.; Veschgini, M.; Kaufmann, S.; Takashima, Y.; Harada, A.; Tanaka, M. Dynamic Mechano-Regulation of Myoblast Cells on Supramolecular Hydrogels Cross-Linked by Reversible Host-Guest Interactions. Sci. Rep. 2017, 7, 7660. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Kao, S.H.; Ling, Q.D.; Chen, Y.M.; Li, H.F.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Chang, S.C.; Lee, H.C.; et al. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep. 2015, 5, 18136. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).