Integrated Analysis of an Innovative Composite Polycaprolactone Membrane and a Jason Membrane in Guided Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of nHAP
2.2.2. Production of Membranes
2.2.3. Physicochemical Characterization
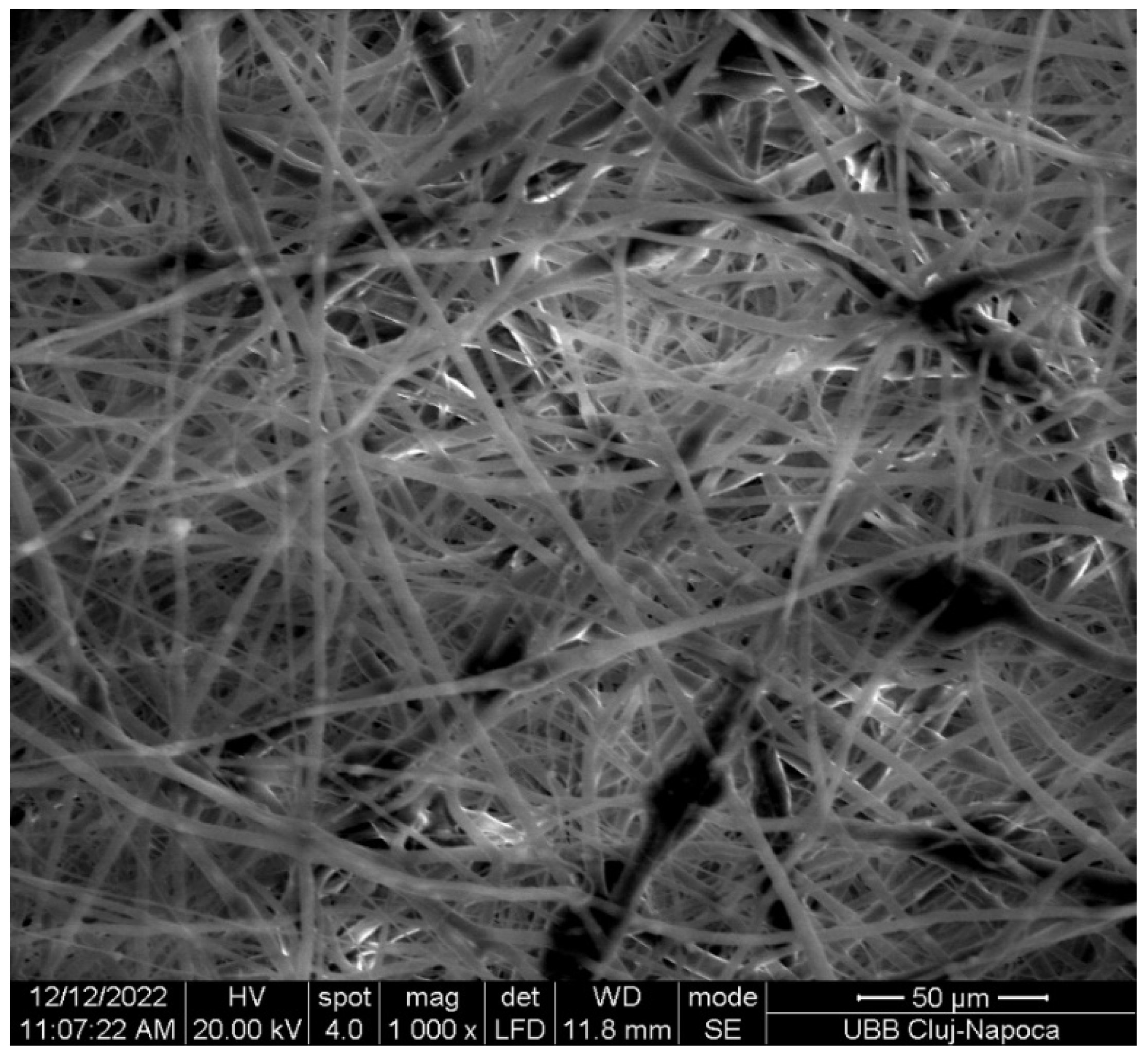
2.2.4. Structural Characterization
3. Results
4. Discussion
- Physicochemical Properties:
- ∘
- Mechanical Strength: PCL membranes typically exhibit superior mechanical stability compared to Jason membranes. This enhanced strength is beneficial for maintaining the structural integrity of the membrane during the healing process, particularly in complex or customized GBR applications. Conversely, while Jason membranes have adequate mechanical properties, they may not provide the same level of support in challenging scenarios.
- ∘
- Resorption Rate: Jason membranes generally resorb more quickly than PCL membranes, which are designed for longer-term stability. This rapid resorption allows for quicker tissue integration but may limit their application in situations where prolonged support is necessary.
- Biological Behavior:
- ∘
- Biocompatibility: The Jason membrane, being derived from natural collagen, is known for its high biocompatibility, promoting better integration with surrounding tissues. In contrast, while PCL is biocompatible, it is a synthetic polymer, and its natural integration may not match the rapidity seen with Jason membranes.
- ∘
- Integration Speed: Jason membranes often integrate rapidly into the biological environment, facilitating quicker healing. This rapid integration can be advantageous in clinical scenarios requiring fast recovery. In contrast, PCL membranes, although slower to integrate, provide sustained mechanical support that can be crucial for long-term regeneration.
- Durability and Stability:
- ∘
- Long-Term Performance: PCL membranes demonstrate durability over extended periods, making them particularly suitable for complex or customized GBR applications. This long-term stability is vital in situations where bone regeneration may take considerable time. In contrast, Jason membranes, while effective for certain applications, may not offer the same level of long-term support.
- Clinical Applicability:
- ∘
- Indications for Use: The choice between PCL and Jason membranes may depend on the specific clinical scenario. For straightforward defects, Jason membranes may be preferred due to their rapid integration and biocompatibility. However, for more complex defects requiring custom solutions and extended support, PCL membranes may be the better choice.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Papuc, A.; Bran, S.; Moldovan, M.; Lucaciu, O.; Armencea, G.; Baciut, G.; Dinu, C.; Onișor, F.; Kretschmer, W.; Baciut, M. How Is Bone Regeneration Influenced by Polymer Membranes? Insight into the Histological and Radiological Point of View in the Literature. Membranes 2024, 14, 193. [Google Scholar] [CrossRef]
- Vijayalekha, A.; Anandasadagopan, S.K.; Pandurangan, A.K. An Overview of Collagen-Based Composite Scaffold for Bone Tissue Engineering. Appl. Biochem. Biotechnol. 2023, 195, 4617–4636. [Google Scholar] [CrossRef]
- Fan, L.; Ren, Y.; Emmert, S.; Vučković, I.; Stojanovic, S.; Najman, S.; Schnettler, R.; Barbeck, M.; Schenke-Layland, K.; Xiong, X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 3744. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Laude, M.; Oyebanji, Z.; Birur, R.; Martinez, A.L.; Gilbert, I.; Baek, P.; Longbottom, N.; McCullough, N.; Horn, S.J.; et al. Co-Electrospinning Extracellular Matrix with Polycaprolactone Enables a Modular Approach to Balance Bioactivity and Mechanics of a Multifunctional Bone Wrap. Adv. Healthc. Mater. 2025, e02020. [Google Scholar] [CrossRef]
- MegaGen. Jason® Membrane & Collprotect® Membrane; MegaGen: Daegu, Republic of Korea, 2015. [Google Scholar]
- Garimella, A.; Ghosh, S.B.; Bandyopadhyay-Ghosh, S. Biomaterials for bone tissue engineering: Achievements to date and future directions. Biomed. Mater. 2024, 20, 012001. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, Y.; Wang, L.; Liu, X.; Wang, S.; Xu, Z.; Ma, Y. Research on 3D-printed scaffolds with microstructure bio-inspired optimization for orbital bone defect repair. J. Mater. Sci. Mater. Med. 2024, 36, 95. [Google Scholar] [CrossRef]
- Qin, T.; Lian, X.; Ullah, A.; Liu, Z.; Fu, T.; Hao, R.; Zhao, L.; Huang, D. The multi-scale porous and hydrophilic 3D printed polycaprolactone/silk fibroin/β-tricalcium phosphate bone scaffolds effect on femoral defect repair. Int. J. Biol. Macromol. 2025, 330, 148230. [Google Scholar] [CrossRef]
- Mirică, I.C.; Furtos, G.; Lucaciu, O.; Pascuta, P.; Vlassa, M.; Moldovan, M.; Campian, R.S. Electrospun membranes based on polycaprolactone, nano-hydroxyapatite and metronidazole. Materials 2021, 14, 931. [Google Scholar] [CrossRef]
- Lucaciu, P.O.; Repciuc, C.C.; Matei, I.A.; Fiț, N.I.; Andrei, S.; Marica, R.; Petrescu, B.N.; Crișan, B.; Aghiorghiesei, O.; Mirică, I.C.; et al. In Vivo Validation of a Nanostructured Electrospun Polycaprolactone Membrane Loaded with Gentamicin and Nano-Hydroxyapatite for the Treatment of Periodontitis. Membranes 2024, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Mirica, I.C.; Furtos, G.; Moldovan, M.; Prodan, D.; Petean, I.; Campian, R.-S.; Pall, E.; Lucaciu, O. Morphology, Cytotoxicity, and Antimicrobial Activity of Electrospun Polycaprolactone Biomembranes with Gentamicin and Nano-Hydroxyapatite. Membranes 2023, 14, 10. [Google Scholar] [CrossRef]
- Wang, B.; Xie, X.; Jiang, W.; Zhan, Y.; Zhang, Y.; Guo, Y.; Wang, Z.; Guo, N.; Guo, K.; Sun, J. Osteoinductive micro-nano guided bone regeneration membrane for in situ bone defect repair. Stem Cell Res. Ther. 2014, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Cady, C.; Nair, K.; Rodriguez, H.C.; Rust, B.; Ghandour, S.; Potty, A.; Gupta, A. Optimization of Polycaprolactone and Type I Collagen Scaffold for Tendon Tissue Regeneration. Cureus 2024, 16, e56930. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, Z.; Khakbiz, M.; Davari, N.; Bonakdar, S.; Mohammadi, J.; Shokrgozar, M.A.; Derhambakhsh, S. Fabrication and multiscale modeling of polycaprolactone/amniotic membrane electrospun nanofiber scaffolds for wound healing. Artif. Organs 2023, 47, 1267–1284. [Google Scholar] [CrossRef]
- Litany, R.I.J.; Praseetha, P.K. Tiny tots for a big-league in wound repair: Tools for tissue regeneration by nanotechniques of today. J. Control. Release 2022, 349, 443–459. [Google Scholar] [CrossRef]
- Rahmatabadi, D.; Aberoumand, M.; Soltanmohammadi, K.; Soleyman, E.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Bodaghi, M.; Baghani, M. 4D Printing-Encapsulated Polycaprolactone–Thermoplastic Polyurethane with High Shape Memory Performances. Adv. Eng. Mater. 2022, 25, 2201309. [Google Scholar] [CrossRef]
- Karalashvili, L.; Kakabadze, A.; Uhryn, M.; Vyshnevska, H.; Ediberidze, K.; Kakabadze, Z. Bone Grafts for Reconstruction of Bone Defects (Review). Georgian Med. News 2018, 282, 44–49. [Google Scholar]
- Huang, J.; Best, S.M.; Bonfield, W.; Brooks, R.A.; Rushton, N.; Jayasinghe, S.N.; Edirisinghe, M.J. In vitro assessment of the biological response to nano-sized hydroxyapatite. J. Mater. Sci. Mater. Med. 2004, 15, 441–444. [Google Scholar] [CrossRef]
- Inam, H.; Sprio, S.; Tavoni, M.; Abbas, Z.; Pupilli, F.; Tampieri, A. Magnetic Hydroxyapatite Nanoparticles in Regenerative Medicine and Nanomedicine. Int. J. Mol. Sci. 2024, 25, 2809. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Park, S.; Choi, J.; Vu, T.T.H.; Doan, V.H.M.; Vo, T.T.; Lee, B.; Oh, J. Hydroxyapatite: A journey from biomaterials to advanced functional materials. Adv. Colloid Interface Sci. 2023, 321, 103013. [Google Scholar] [CrossRef]
- Rogowska-Tylman, J.; Locs, J.; Salma, I.; Woźniak, B.; Pilmane, M.; Zalite, V.; Wojnarowicz, J.; Kędzierska-Sar, A.; Chudoba, T.; Szlązak, K.; et al. In vivo and in vitro study of a novel nanohydroxyapatite sonocoated scaffolds for enhanced bone regeneration. Mater. Sci. Eng. 2019, 99, 669–684. [Google Scholar] [CrossRef]
- Linh, N.V.L.; Du, N.T.; My, N.T.N.; Tuyen, N.N.; Phu, H.D.; Tram, N.X.T. Electrospun Polycaprolactone/Hydroxyapatite (PCL/HAp) microfibers as potential biomaterials for tissue engineering. Materials 2022, 66, 2895–2899. [Google Scholar] [CrossRef]
- Kalpana, M.; Nagalakshmi, R. Nano Hydroxyapatite for Biomedical Applications Derived from Chemical and Natural Sources by Simple Precipitation Method. Appl. Biochem. Biotechnol. 2023, 195, 3994–4010. [Google Scholar] [CrossRef] [PubMed]
- Alshahrani, A.A.; Alorabi, A.Q.; Hassan, M.S.; Amna, T.; Azizi, M. Chitosan-functionalized hydroxyapatite-cerium oxide heterostructure: An efficient adsorbent for dyes removal and antimicrobial agent. Nanomaterials 2022, 12, 2713. [Google Scholar] [CrossRef]
- Wüster, J.; Neckel, N.; Sterzik, F.; Xiang-Tischhauser, L.; Barnewitz, D.; Genzel, A.; Koerdt, S.; Rendenbach, C.; Müller-Mai, C.; Heiland, M.; et al. Effect of a synthetic hydroxyapatite-based bone grafting material compared to established bone substitute materials on regeneration of critical-size bone defects in the ovine scapula. Regen. Mater. 2024, 11, rbae041. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic Material for Bone, Periodontal, and Dental Tissue Regeneration: Where Are We Now, and Where Are We Heading Next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef] [PubMed]
- Sharon V, M.; Malaiappan, S. Biocompatibility and periodontal regenerative potential of hydroxyapatite nanoparticles from Portunus sanguinolentus Shells: A crystallographic, morphological, and molecular gene expression analysis. J. Dent. 2025, 157, 105762. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Jiménez-Rosado, M.; Romero, A.; Guerrero, A. Polymer-based scaffolds for soft-tissue engineering. Polymers 2020, 12, 1566. [Google Scholar] [CrossRef]
- Shang, L.; Shao, J.; Ge, S. Immunomodulatory Properties: The Accelerant of Hydroxyapatite-Based Materials for Bone Regeneration. Tissue Eng. C Methods 2022, 28, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Diacerein-loaded novel gastroretentive nanofiber system using PLLA: Development and in vitro characterization. Artif. Cell Nanomed. Biotechnol. 2016, 44, 928–936. [Google Scholar]
- He, Y.; Zhu, T.; Liu, L.; Shi, X.; Lin, Z. Modifying collagen with alendronate sodium for bone regeneration applications. RSC Adv. 2018, 8, 16762–16772. [Google Scholar] [CrossRef] [PubMed]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Philippe Dumas, S.B.F. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–383. [Google Scholar] [CrossRef]
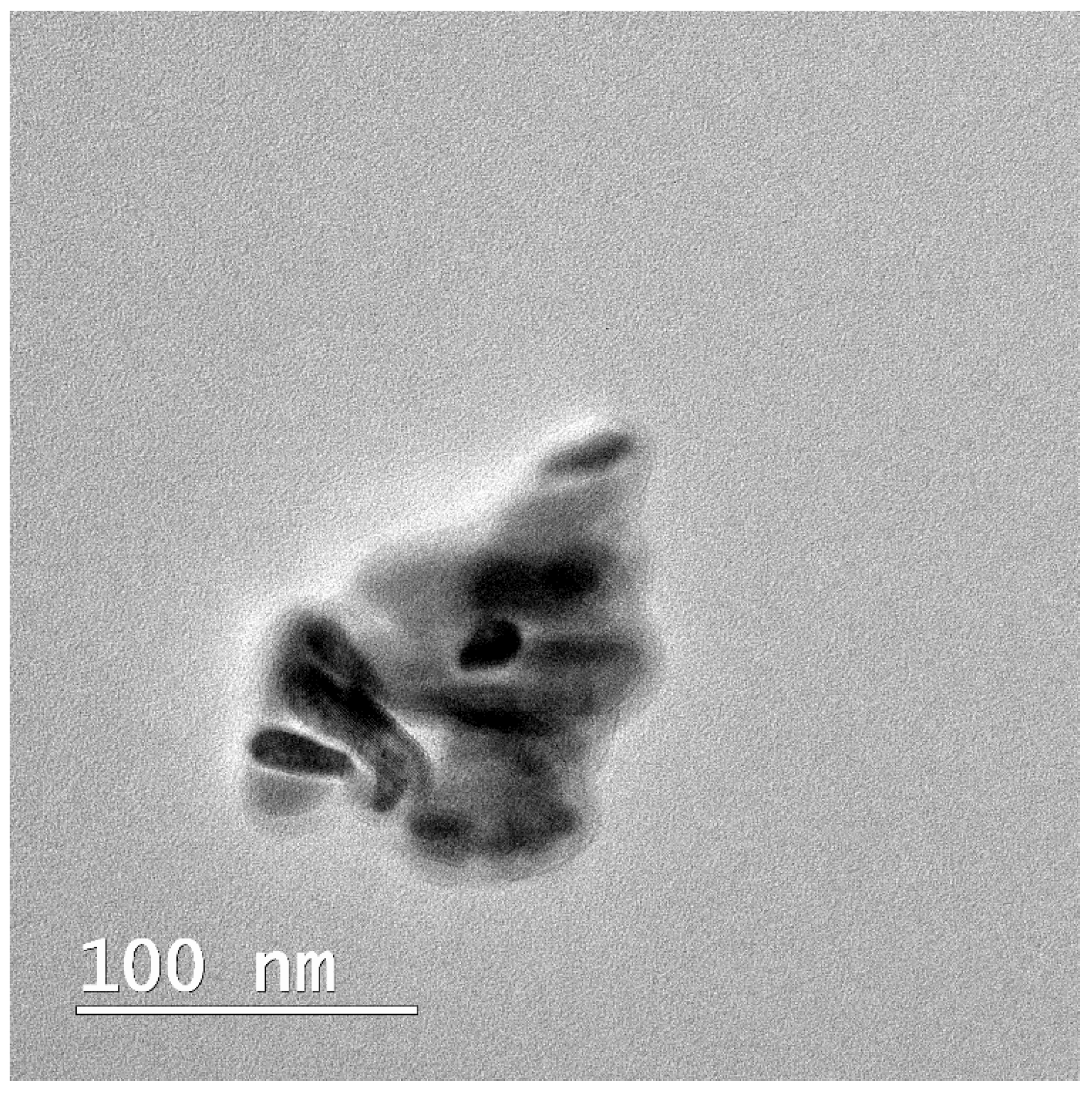
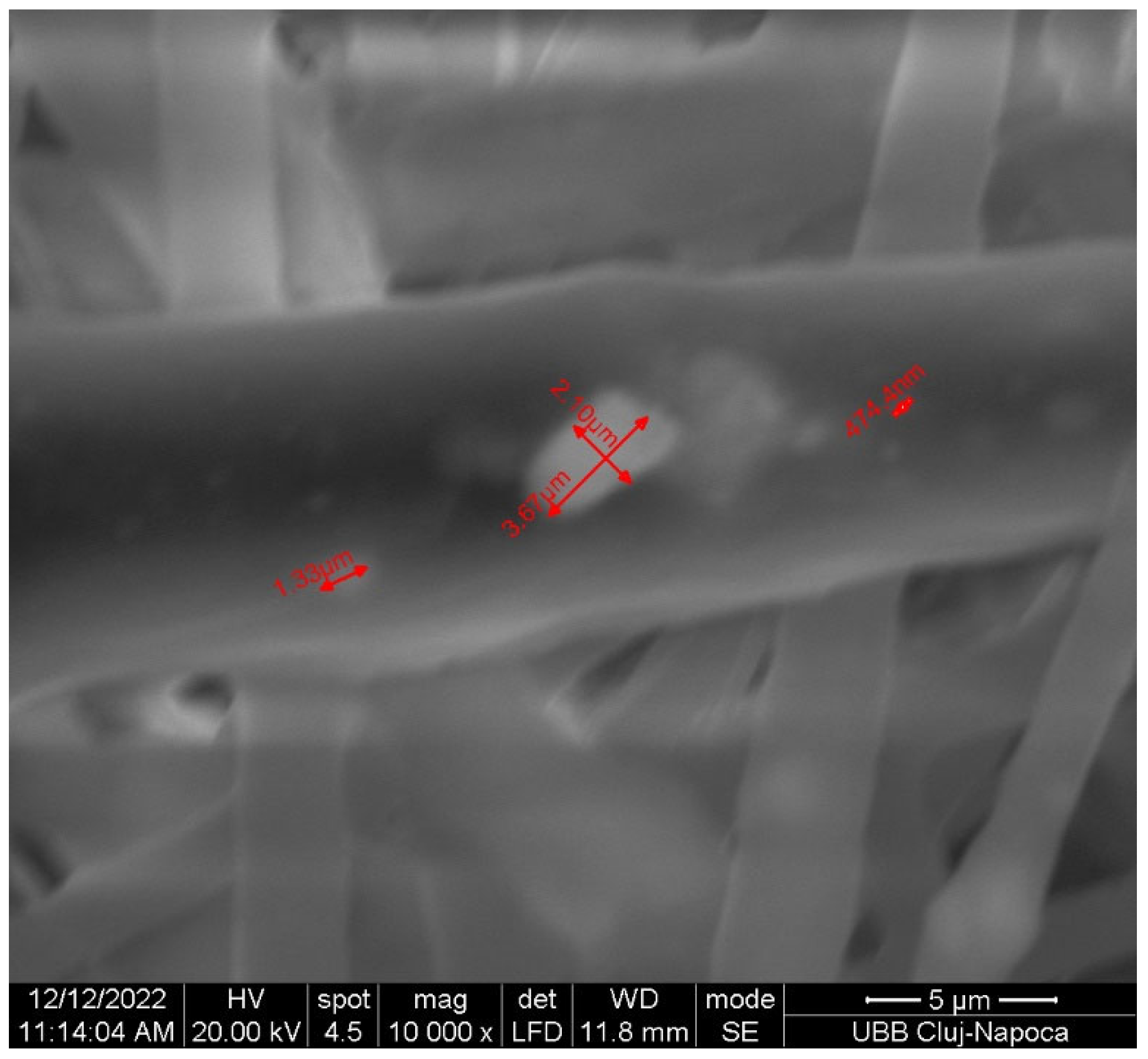
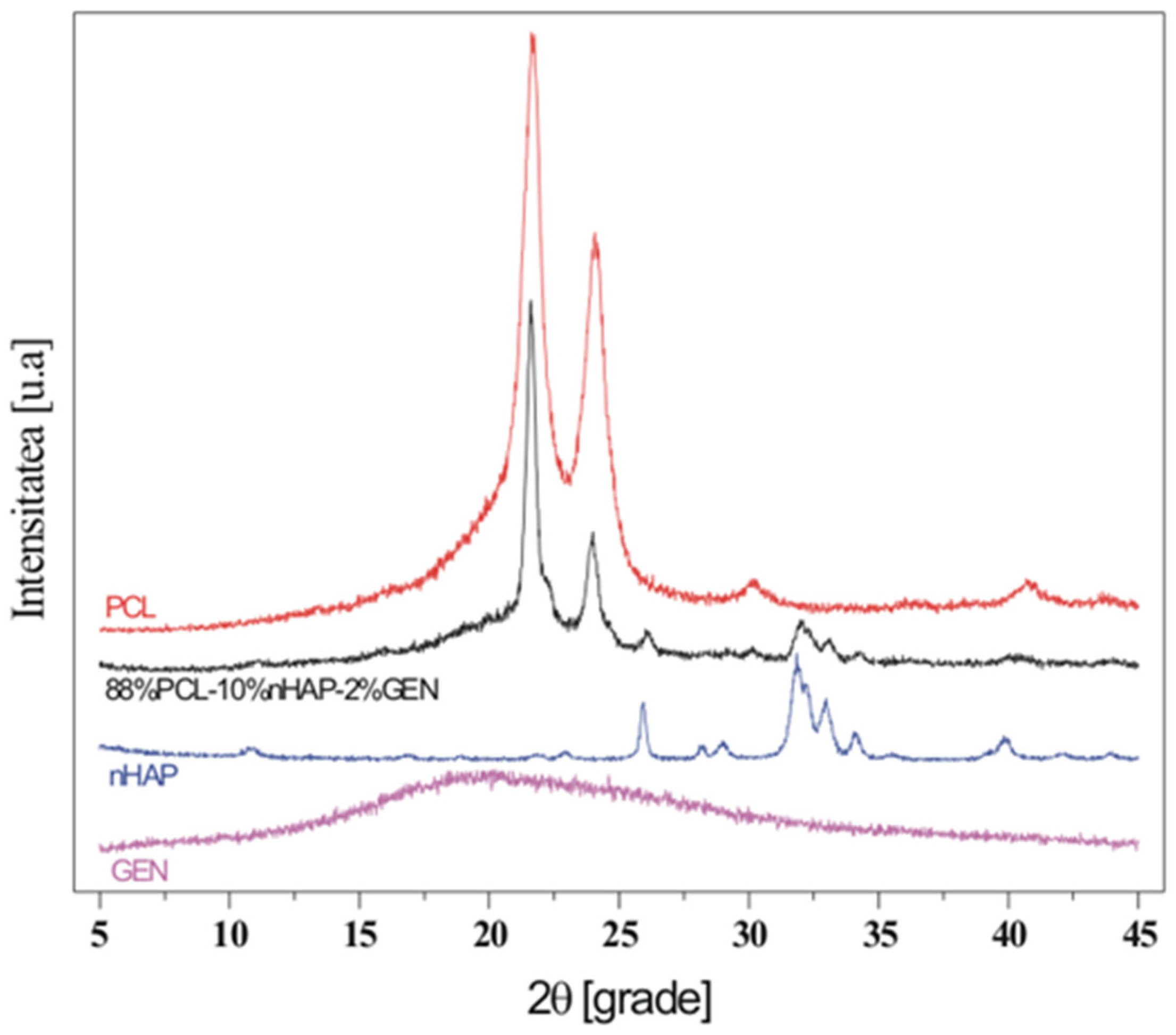
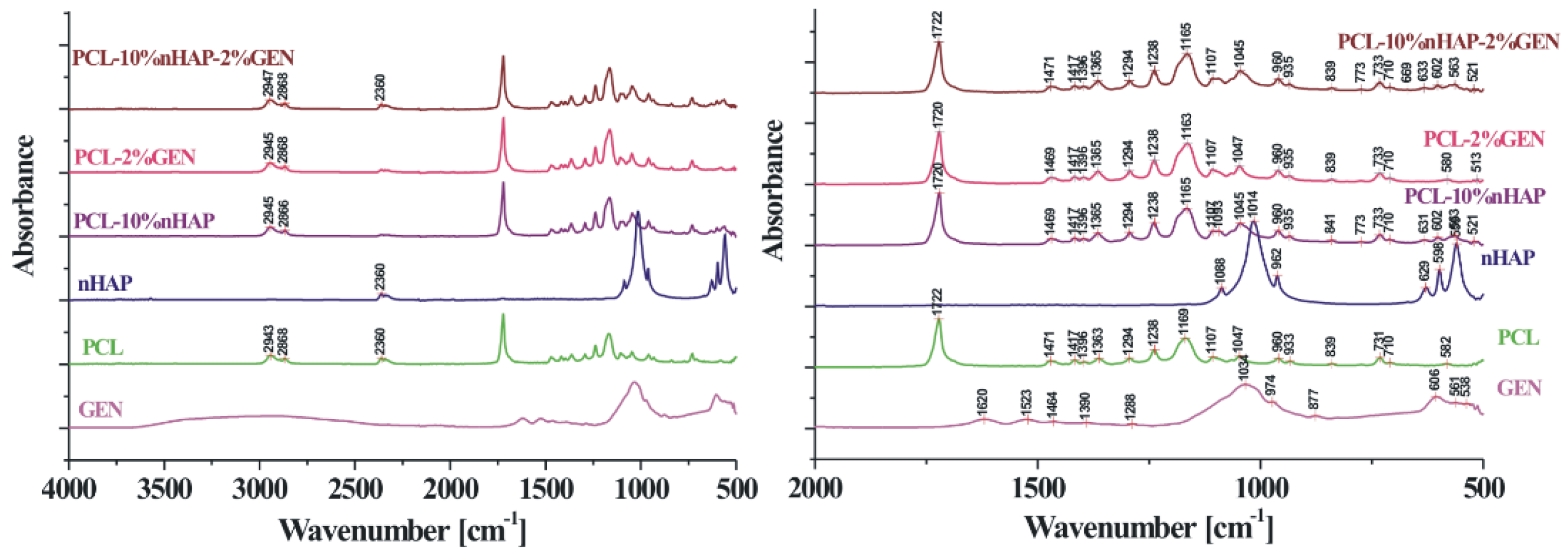
| Characteristic | Jason Membrane | PCL Membrane |
|---|---|---|
| Origin | Natural collagen types I and III (porcine pericardium) | Synthetic aliphatic polyester |
| Structure | Multilayered, with dense collagen fibers | Semicrystalline, with controllable porosity |
| Degradation Method | Enzymatic (collagenases) | Hydrolysis (esters → caproic acid) |
| Resorption Time | 3–6 months | 6–24 months (depending on design and thickness) |
| Degradation Products | Physiological amino acids | Fatty acids with potential local irritative effects |
| Parameter | Jason | PCL |
|---|---|---|
| Tensile Strength | High for collagen | Superior, supports volumetric stability |
| Flexibility | High, allows for easy adaptation | Rigid/semi-rigid, requiring pre-shaping |
| Manipulability | Easy to handle, non-sticky | Requires special handling or thermal pre-forming |
| Fixation | Pins, screws, or sutures | Often requires specific screws or pins |
| Parameter | Jason | PCL |
|---|---|---|
| Biological Integration | Very rapid | Slow |
| Dimensional Stability | Limited (3–6 months) | Excellent (6–24 months) |
| Cost | Moderate | High (especially for 3D printed variations) |
| Customization | No | Yes—CAD/CAM, 3D printing |
| Clinical Situation | Jason | PCL |
|---|---|---|
| Socket Preservation | Ideal | Rarely used |
| Sinus Lift | Frequently utilized | Used in customized variations (e.g., 3D print) |
| Horizontal Augmentation | Suitable for moderate defects | Recommended for long-term stability |
| Vertical Augmentation | Limited | Indicated (due to rigidity) |
| Peri-implant Defects | Yes | Yes, in customized forms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Papuc, A.; Bran, S.; Moldovan, M.; Armencea, G.; Crisan, B.; Crisan, L.; Baciut, G.; Dinu, C.; Onișor, F.; Kretschmer, W.; et al. Integrated Analysis of an Innovative Composite Polycaprolactone Membrane and a Jason Membrane in Guided Bone Regeneration. Bioengineering 2026, 13, 23. https://doi.org/10.3390/bioengineering13010023
Papuc A, Bran S, Moldovan M, Armencea G, Crisan B, Crisan L, Baciut G, Dinu C, Onișor F, Kretschmer W, et al. Integrated Analysis of an Innovative Composite Polycaprolactone Membrane and a Jason Membrane in Guided Bone Regeneration. Bioengineering. 2026; 13(1):23. https://doi.org/10.3390/bioengineering13010023
Chicago/Turabian StylePapuc, Alexandra, Simion Bran, Marioara Moldovan, Gabriel Armencea, Bogdan Crisan, Liana Crisan, Grigore Baciut, Cristian Dinu, Florin Onișor, Winfried Kretschmer, and et al. 2026. "Integrated Analysis of an Innovative Composite Polycaprolactone Membrane and a Jason Membrane in Guided Bone Regeneration" Bioengineering 13, no. 1: 23. https://doi.org/10.3390/bioengineering13010023
APA StylePapuc, A., Bran, S., Moldovan, M., Armencea, G., Crisan, B., Crisan, L., Baciut, G., Dinu, C., Onișor, F., Kretschmer, W., & Baciut, M. (2026). Integrated Analysis of an Innovative Composite Polycaprolactone Membrane and a Jason Membrane in Guided Bone Regeneration. Bioengineering, 13(1), 23. https://doi.org/10.3390/bioengineering13010023







