Confounding Factors and Their Mitigation in Measurements of Bioelectrical Impedance at the Skin Interface
Abstract
1. Overview of Physical Principles of Bioelectrical Impedance Techniques
1.1. Introduction
1.2. Basic Principles of Bioelectrical Impedance Techniques
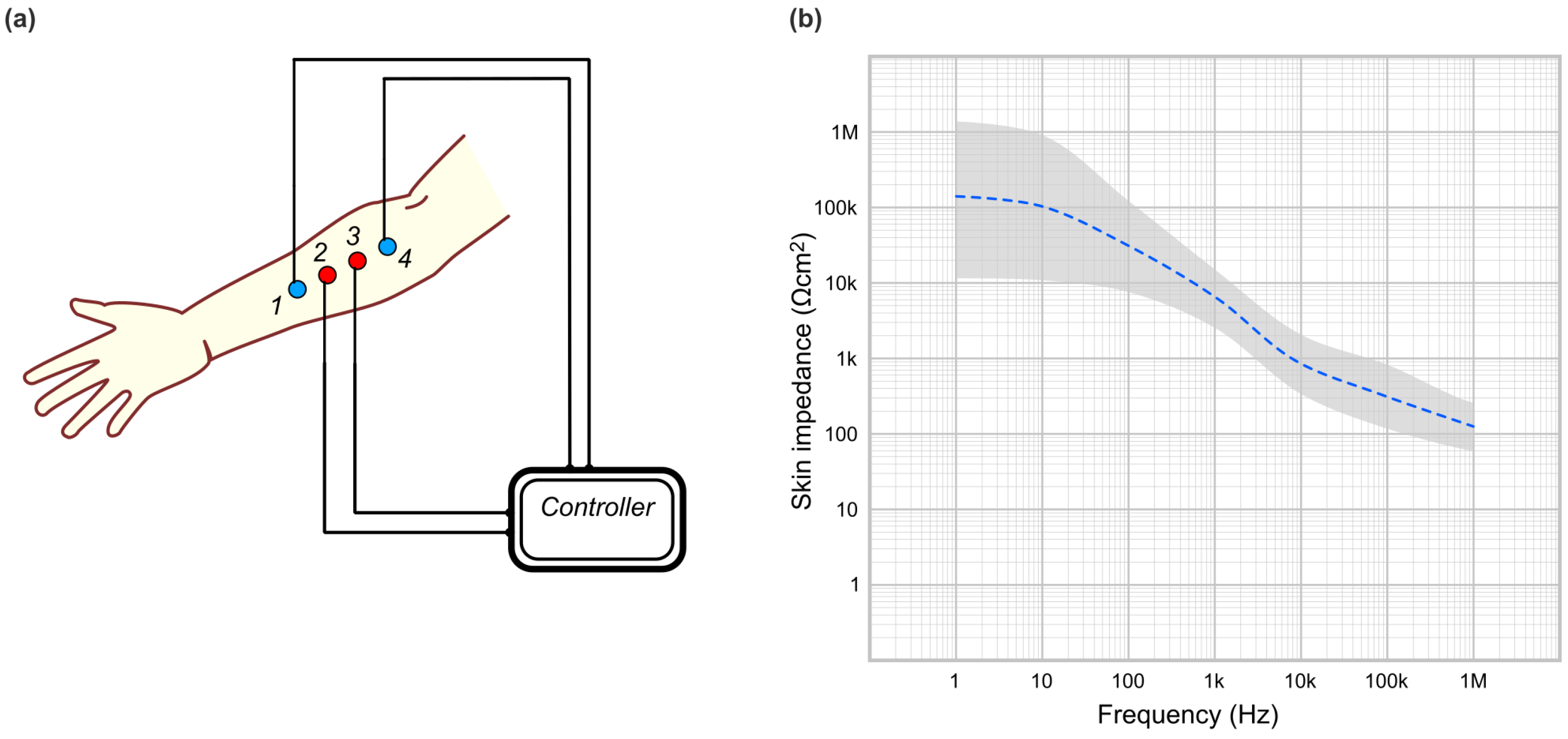
1.2.1. Electric Impedance Spectroscopy (EIS)
1.2.2. Electric Impedance Tomography (EIT)
1.3. General Considerations About the Skin
1.4. Challenges in Bioelectrical Impedance Measurements
2. Confounding Factors in Bioelectrical Impedance Measurements at the Skin Interface
2.1. Overall Physiological and Psychological Condition of the Participants
2.1.1. Health Status of Participants
2.1.2. Relevant Medical History
2.1.3. Core Temperature
2.1.4. Body Mass Index (BMI)
2.1.5. Age
2.1.6. Sex
2.1.7. Menstrual Cycle
2.1.8. Ethnicity
2.1.9. General Hydration Status
2.1.10. Feeding (Prandial) Status
2.1.11. Medication or Substance Use
2.1.12. Sleeping Status
2.1.13. Fitness
2.1.14. Exercise
2.1.15. Emotional Status
2.2. Environmental Conditions
2.2.1. Ambient Temperature
2.2.2. Ambient Relative Humidity
2.2.3. Stability of Ambient Conditions
2.2.4. Accommodation to Laboratory Conditions
2.2.5. Posture and Postural Accommodation
2.2.6. Season
2.3. Time Constraints
2.3.1. Time of Day
2.3.2. Timing of the Measurement: Immediate or Delayed After Electrode Placement
2.3.3. Measurement Repetitions in Time
2.4. Skin Condition
2.4.1. Tattoos, Piercings
2.4.2. Items in Contact with Skin: Jewelry and Conductive Clothing Accessories
2.4.3. Items in Contact with Skin: Clothing, Belts, etc.
2.4.4. Local Health of the Skin
2.5. Skin Preparation
2.5.1. Topical Hygiene Products
2.5.2. Skin Hygiene
- Water-and-soap: effective, but has several downsides. Harsh surfactants can cause skin dryness and irritation mainly due to damage to skin proteins [123]. As a recommendation, less irritating, “mild” surfactants exist, designed to alleviate this problem [124,125]. Additional care should be paid to the pH of the soap mixtures with water, as strong alkaline ones (pH ~ 10) increase SC thickness (by swelling and increasing lipid rigidity), even in the absence of surfactants [123]. Therefore, recommended soaps should have a neutral pH (~7) or close to the pH of SC (~5.5). Some soaps also have moisturizing agents (see above). Another downside is that it exposes the skin to water, which transiently increases the water content of the superficial layer (increases conductivity) or might leave a thin water film on the surface. Tap water might contain variable levels of oxidizers or reduced residues from water management (ozone, chlorine, potassium permanganate, polyphosphates, etc.) that might alter redox equilibrium at the electrode interface [126]. Countermeasures: (i) rinse effectively (maybe with normal saline instead of water); (ii) dry effectively (and include a timed interval between the skin preparation and electrode attachment).
- Rubbing alcohol (either ethanol or isopropyl alcohol in various concentrations): It cannot effectively remove proteinaceous residues that might clog sweat ducts, as proteins denature in concentrated alcohol solutions. Also, it removes skin oils in the hair follicles, which will slightly reduce the impedance temporarily [4] and might cause irritations. Therefore, cleaning with alcohol should be avoided if possible.
- Gel-based hygienic agents: These off-the-shelf products can contain various thickening agents that can attach to the SC as a thin additional layer; we recommend avoiding them.
- Single use cleaning wipes: These can be effective but check for the presence of moisturizing agents and pH; abrasive wipes are commercially available for slightly abrading the SC.
- Sterile woven cotton gauze (surgical cotton cloth) moistened in normal saline. When rubbed against the skin, this appears to be a safe option that we recommend, with the following observations. The texture appears to be effective for gentle, non-traumatic exfoliation, and the absorbent fibers of the cotton mop up oils and debris. We advise using normal saline (sterile 0.9% sodium chloride solution) and not water to further minimize osmotic water flux through microscopic cracks in the skin. The number of rubs per electrode location should be standardized in the research protocol (i.e., “each location was rubbed four times before with a woven cotton gauze moistened in normal saline”). For each electrode location, use a new cotton pad (to prevent cross-transfer of debris between locations).
2.5.3. Stratum Corneum: Modifying or Not
- For stripping, the most used method seems to be the use of adhesive tape (taped to the skin, lifted rapidly), several times (one to four times most common). Some authors specify the type of tape used (generic plastic based/cellulose based), others do not; also, there are commercially available tapes specifically marketed for the purpose of stripping the SC. Other authors used other stripping methods (for instance, fine-grained sandpaper rubbed on the skin). Mechanical abrasion seems to be the most effective method to reduce impedance [127].
- Other authors prefer not to use stripping, for the following reasons: because it is unpleasant, cannot be applied on hairy skin easily, reduces participation, modifies the natural state of the skin, severe stripping (~20 times) denudes completely the skin protection and increases the risk of infections. Accordingly, lack of stripping will induce a variability between subjects. We want to note that even if the study authors did not use stripping, it might be done inadvertently by the participants, if they, in their hygienic habits at home, used cosmetic exfoliating soaps or gels or hard sponges or brushes (that can have a stripping effect). If the choice of the researcher is to avoid stripping, the participants should perhaps be instructed to refrain from using exfoliating cosmetics for the duration of the study (especially if the study design has repeated measures in time). This could be an easy to miss confounding factor in longer duration studies.
- Another mechanical abrasion recommended by some researchers involves the use of an abrasive electrolytic gel, rubbed on the skin [95]; the application should be carefully performed, not to spill the gel into adjacent areas (it could create an electrical shorting between neighboring electrodes).
- Other modifications could be chemical exfoliation with different agents or microperforation with microneedles [127], but these appear to be less effective than stripping.
- “Penetration enhancers” are substances that increase the permeability of the skin for certain species. Surfactants (like sodium lauryl sulfate, commonly used in shampoos, soaps, etc), solvents (like dimethylsulphoxide), esters of organic acids, and aromatic compounds seem effective in reducing the parallel resistance of SC [95], but their use is severely restricted by their toxicity, or risks of irritations and allergies.
2.5.4. Shaving
2.6. Electrodes
2.6.1. “Wet” Electrodes
- Advantages: The presence of the liquid/gel media increases the effective surface area by eliminating random tiny air pockets, resulting in a stabler impedance signal over time. The initial electrical contact is usually stable and reliable. The electrodes based on adhesive gels are very easy to apply and are used extensively in hospitals in electrocardiography (ECG) recordings; their ubiquity made them a popular choice for many impedance research projects.
- Disadvantages: The presence of a gel/conductive saline solution moistens the skin, thus the skin impedance is lowered. This process is not stable because over time the conductive liquid can infiltrate between the tiny cracks in the stratum corneum, making spurious conductive bridges with the underlying layer. Also, the outer layers of the gel can dry, modifying conductance; thus, the gel–skin interface can actually generate an unwanted noise over longer recording [80]. Most of these electrodes are single use; some participants might be allergic to a particular gel formulation.
2.6.2. “Dry” and Other Contact Electrodes
- Advantages: Most types are designed to overcome the problems of wet electrodes (easy to use, reusable, comfortable) and can be used easily in any location of the body, but they come with their own challenges. Flexible contact electrodes have the advantage of high conformability to skin shape (even on hairy regions) and if they are elastic are able to maintain the interface even in motion (like breathing or exercising) [138]. In general, because of the lack of a contact liquid or gel, the absolute impedance values recorded are generally higher than those recorded with wet electrodes. Newer conformal electrodes appear to maintain stable electrical contact for longer durations in EIT applications [141].
- Disadvantages: Even if they are not “wet” in a chemical sense, an electrical double layer forms in time at the contact with the skin (from natural sweat/oils that the underlying skin might secrete during the usage). This results in a variable contact impedance that “drifts” over time from the initial value [136]. There are attempts to minimize this effect via porous mesh-like electrodes [142]. Dry contact electrodes (especially solid ones) are highly sensitive to motion artifacts [143]. The motion artifacts have a large variation between subjects and appear with overt motion and also with involuntary movements (like breathing) [22].
2.6.3. “Non-Contact” Electrodes
2.6.4. “Inserted” Electrodes
- Advantages: They avoid the variability of the contact between the applied electrode and the skin surface, discussed above. They appear to have a better signal-to-noise ratio than the rest of the electrodes [147].
- Disadvantages: (i) The geometrical arrangement of the needle electrode surface influences the recordings: at lower frequencies of the electrical signal, they have a high, significant electrode polarization impedance; the impedance of the surrounding tissue was thus best recorded at higher frequencies above 10 kHz [148]. (ii) As an additional source of error, most of these electrodes have to be “activated” before measurement with different procedures (typically by dipping them in saline solution with wetting agents or on-site electrolytical treatments). This allows a stabilization of the electrode surface area, reducing the variation of electrode impedance observed during the in vivo recording. This phase must be followed carefully before the measurement; the results (from the same electrode) are markedly different with or without this pre-treatment [148]. (iii) Needle insertion is a medical procedure that breaks the integrity of the skin barrier, and proper sanitary precautions must be followed to avoid infections or medical complications. It might be painful and less likely to be accepted by the participants/patients than alternatives. A promising solution to these problems appears to be “microneedles” arranged in an array that can penetrate the nonconducting stratum corneum of the skin without pain and can even be arranged in multichannel setups [149].
2.6.5. Electrode Surface Area Considerations
- “Small” electrode area. Advantage: It leads to higher density current lines below the electrode, which has a higher chance to intercept the interest area (for instance, an unknown low impedance anatomical structure that was to be investigated in an impedance spectroscopy study or impedance tomography study). Disadvantage: Higher current density means a higher chance for the sudden appearance of burned-through low impedance paths in the epidermal layer (and thus a false low impedance reading) [99].
- “Larger” electrode area. Advantage: It is easier to use and attach. Lower current density beneath the electrode reduces the chances that the tissue is adversely affected by the measurement, but also lowers the signal-to-noise ratio. Also, a larger perimeter of the electrode is a source of other confounding factors (noise at the electrode–skin interface [150] and, especially in the case of wet electrodes, a greater surface area of evaporation, so the gel will desiccate faster).Newer generations of “compound electrodes” [151,152] that have a large outer electrode area to inject current and a smaller inner area to sense voltage are actively designed to work around the above described problems. “Active electrodes” are a newer generation of electrodes that incorporate parts of the controlling electronics for better signal-to-noise ratio [153,154].
2.7. Electrode Placement
2.7.1. Pressure on Electrodes
2.7.2. Electrode Location
2.8. General Problems Related to Hardware
2.8.1. Wires
2.8.2. Equipment Location
2.9. Sources of Bias in Human Trials
2.10. Data Modeling
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Alternating Current |
| BIA | Bioelectric Impedance Analysis |
| BMI | Body Mass Index |
| EIS | Electric Impedance Spectroscopy |
| EIT | Electric Impedance Tomography |
| GAM | General Additive Modeling |
| GLMM | General Linear Mixed Models |
| LED | Light Emitting Diodes |
| PCA | Principal Component Analysis |
| SC | Stratum Corneum |
| UPS | Uninterruptible Power Supply |
References
- Lee, C.I.; Elmore, J.G. Radiation-Related Risks of Imaging; Givens, J., D’Aronson, M., Eds.; UpToDate, Wolters Kluwer: Philadelphia, PA, USA, 2025; Available online: https://www.uptodate.com/contents/radiation-related-risks-of-imaging (accessed on 3 June 2025).
- Khalil, S.; Mohktar, M.; Ibrahim, F. The Theory and Fundamentals of Bioimpedance Analysis in Clinical Status Monitoring and Diagnosis of Diseases. Sensors 2014, 14, 10895–10928. [Google Scholar] [CrossRef]
- Lawler, J.C.; Davis, M.J.; Griffith, E.C. Electrical Characteristics of the Skin. The impedance of the surface sheath and deep tissues. J. Investig. Dermatol. 1960, 34, 301–308. [Google Scholar] [CrossRef]
- Tregear, R.T. Interpretation of Skin Impedance Measurements. Nature 1965, 205, 600–601. [Google Scholar] [CrossRef]
- Gersing, E. Impedance spectroscopy on living tissue for determination of the state of organs. Bioelectrochem. Bioenerg. 1998, 45, 145–149. [Google Scholar] [CrossRef]
- Bodenstein, M.; David, M.; Markstaller, K. Principles of electrical impedance tomography and its clinical application. Crit. Care Med. 2009, 37, 713–724. [Google Scholar] [CrossRef]
- Lu, F.; Wang, C.; Zhao, R.; Du, L.; Fang, Z.; Guo, X.; Zhao, Z. Review of Stratum Corneum Impedance Measurement in Non-Invasive Penetration Application. Biosensors 2018, 8, 31. [Google Scholar] [CrossRef]
- Tomicic, V.; Cornejo, R. Lung monitoring with electrical impedance tomography: Technical considerations and clinical applications. J. Thorac. Dis. 2019, 11, 3122–3135. [Google Scholar] [CrossRef]
- Mansouri, S.; Alharbi, Y.; Haddad, F.; Chabcoub, S.; Alshrouf, A.; Abd-Elghany, A.A. Electrical Impedance tomography – recent applications and developments. J. Electr. Bioimpedance 2021, 12, 50–62. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Xie, F.; Ren, S.; Xu, S. The Research Progress of Electrical Impedance Tomography for Lung Monitoring. Front. Bioeng. Biotechnol. 2021, 9, 726652. [Google Scholar] [CrossRef]
- Abasi, S.; Aggas, J.R.; Garayar-Leyva, G.G.; Walther, B.K.; Guiseppi-Elie, A. Bioelectrical Impedance Spectroscopy for Monitoring Mammalian Cells and Tissues under Different Frequency Domains: A Review. ACS Meas. Sci. Au 2022, 2, 495–516. [Google Scholar] [CrossRef]
- Youssef Baby, L.; Bedran, R.S.; Doumit, A.; El Hassan, R.H.; Maalouf, N. Past, present, and future of electrical impedance tomography and myography for medical applications: A scoping review. Front. Bioeng. Biotechnol. 2024, 12, 1486789. [Google Scholar] [CrossRef]
- Baidillah, M.R.; Riyanto, R.; Busono, P.; Karim, S.; Febryarto, R.; Astasari, A.; Sangaji, D.; Taruno, W.P. Electrical impedance spectroscopy for skin layer assessment: A scoping review of electrode design, measurement methods, and post-processing techniques. Measurement 2024, 226, 114111. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance spectroscopy. Ann. Biomed. Eng. 1992, 20, 289–305. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Coster, H.G.; Chilcott, T.C.; Coster, A.C. Impedance spectroscopy of interfaces, membranes and ultrastructures. Bioelectrochem. Bioenerg. 1996, 40, 79–98. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098. [Google Scholar] [CrossRef]
- Stupin, D.D.; Kuzina, E.A.; Abelit, A.A.; Emelyanov, A.K.; Nikolaev, D.M.; Ryazantsev, M.N.; Koniakhin, S.V.; Dubina, M.V. Bioimpedance Spectroscopy: Basics and Applications. ACS Biomater. Sci. Eng. 2021, 7, 1962–1986. [Google Scholar] [CrossRef]
- Bora, D.J.; Dasgupta, R. Various skin impedance models based on physiological stratification. IET Syst. Biol. 2020, 14, 147–159. [Google Scholar] [CrossRef]
- Heaney, M.B. Electrical Conductivity and Resistivity. In The Measurement, Instrumentation and Sensors Handbook; CRC Press LLC: Boca Raton, FL, USA, 1999; pp. 1331–1344. [Google Scholar]
- Birgersson, U.; Birgersson, E.; Ollmar, S. Estimating electrical properties and the thickness of skin with electrical impedance spectroscopy: Mathematical analysis and measurements. J. Electr. Bioimpedance 2012, 3, 51–60. [Google Scholar] [CrossRef]
- Cömert, A.; Hyttinen, J. Impedance spectroscopy of changes in skin-electrode impedance induced by motion. BioMed. Eng. OnLine 2014, 13, 149. [Google Scholar] [CrossRef]
- Mitchell, M.; Muftakhidinov, B.; Winchen, T.; Wilms, A.; van Schaik, B.; Jędrzejewski-Szmek, Z.; Mitchell, C.; Fantino, W.; Megner, J.; Richter, T.; et al. Engauge Digitizer Software. 2023. Available online: https://sourceforge.net/projects/digitizer/ (accessed on 15 January 2025). [CrossRef]
- Rosell, J.; Colominas, J.; Riu, P.; Pallas-Areny, R.; Webster, J. Skin impedance from 1 Hz to 1 MHz. IEEE Trans. Biomed. Eng. 1988, 35, 649–651. [Google Scholar] [CrossRef]
- Webster, J.G. Skin impedance. J. Electr. Bioimpedance 2014, 5, 1. [Google Scholar] [CrossRef]
- Walter-Kroker, A.; Kroker, A.; Mattiucci-Guehlke, M.; Glaab, T. A practical guide to bioelectrical impedance analysis using the example of chronic obstructive pulmonary disease. Nutr. J. 2011, 10, 35. [Google Scholar] [CrossRef]
- Buffa, R.; Mereu, E.; Comandini, O.; Ibanez, M.E.; Marini, E. Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur. J. Clin. Nutr. 2014, 68, 1234–1240. [Google Scholar] [CrossRef]
- Bora, D.J.; Dasgupta, R. Estimation of skin impedance models with experimental data and a proposed model for human skin impedance. IET Syst. Biol. 2020, 14, 230–240. [Google Scholar] [CrossRef]
- Pennati, F.; Angelucci, A.; Morelli, L.; Bardini, S.; Barzanti, E.; Cavallini, F.; Conelli, A.; Di Federico, G.; Paganelli, C.; Aliverti, A. Electrical Impedance Tomography: From the Traditional Design to the Novel Frontier of Wearables. Sensors 2023, 23, 1182. [Google Scholar] [CrossRef]
- Xu, G.; Wang, R.; Zhang, S.; Yang, S.; Justin, G.A.; Sun, M.; Yan, W. A 128-Electrode Three Dimensional Electrical Impedance Tomography System. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007. [Google Scholar] [CrossRef]
- Adler, A.; Boyle, A. Electrical Impedance Tomography: Tissue Properties to Image Measures. IEEE Trans. Biomed. Eng. 2017, 64, 2494–2504. [Google Scholar] [CrossRef]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2016, 72, 83–93. [Google Scholar] [CrossRef]
- Rabbani, K.S.; Kabir, A.M.B.H. Studies on the effect of the third dimension on a two-dimensional electrical impedance tomography system. Clin. Phys. Physiol. Meas. 1991, 12, 393–402. [Google Scholar] [CrossRef]
- Putensen, C.; Hentze, B.; Muenster, S.; Muders, T. Electrical Impedance Tomography for Cardio-Pulmonary Monitoring. J. Clin. Med. 2019, 8, 1176. [Google Scholar] [CrossRef]
- Agnelli, J.P.; Kolehmainen, V.; Lassas, M.J.; Ola, P.; Siltanen, S. Simultaneous Reconstruction of Conductivity, Boundary Shape, and Contact Impedances in Electrical Impedance Tomography. SIAM J. Imaging Sci. 2021, 14, 1407–1438. [Google Scholar] [CrossRef]
- Chitturi, V.; Farrukh, N. Spatial resolution in electrical impedance tomography: A topical review. J. Electr. Bioimpedance 2017, 8, 66–78. [Google Scholar] [CrossRef]
- Boone, K.G.; Holder, D.S. Effect of skin impedance on image quality and variability in electrical impedance tomography: A model study. Med. Biol. Eng. Comput. 1996, 34, 351–354. [Google Scholar] [CrossRef]
- Moore, K.L.; Dalley, A.F.; Agur, A.M. Integumentary System. In Clinical Oriented Anatomy; Wolters Kluwer: Philadelphia, PA, USA, 2018; pp. 104–115. [Google Scholar]
- McAdams, E.; Jossinet, J. Problems in equivalent circuit modelling of the electrical properties of biological tissues. Bioelectrochem. Bioenerg. 1996, 40, 147–152. [Google Scholar] [CrossRef]
- Martinsen, Ø.G.; Grimnes, S.; Sveen, O. Dielectric properties of some keratinised tissues. Part 1:Stratum corneum and nailin situ. Med. Biol. Eng. Comput. 1997, 35, 172–176. [Google Scholar] [CrossRef]
- Poblet, E.; Jiménez, F.; Ortega, F. The contribution of the arrector pili muscle and sebaceous glands to the follicular unit structure. J. Am. Acad. Dermatol. 2004, 51, 217–222. [Google Scholar] [CrossRef]
- Vogt, A.; Mandt, N.; Lademann, J.; Schaefer, H.; Blume-Peytavi, U. Follicular Targeting–A Promising Tool in Selective Dermatotherapy. J. Investig. Dermatol. Symp. Proc. 2005, 10, 252–255. [Google Scholar] [CrossRef]
- Pearson, S.; Colbert, A.P.; McNames, J.; Baumgartner, M.; Hammerschlag, R. Electrical Skin Impedance at Acupuncture Points. J. Altern. Complement. Med. 2007, 13, 409–418. [Google Scholar] [CrossRef]
- Sha, N.; Kenney, L.P.; Heller, B.W.; Barker, A.T.; Howard, D.; Moatamedi, M. A Finite Element Model to Identify Electrode Influence on Current Distribution in the Skin. Artif. Organs 2008, 32, 639–643. [Google Scholar] [CrossRef]
- Abe, Y.; Nishizawa, M. Electrical aspects of skin as a pathway to engineering skin devices. APL Bioeng. 2021, 5, 041509. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- de Groot, J.H.B.; Smeets, M.A.M.; Semin, G.R. Rapid Stress System Drives Chemical Transfer of Fear from Sender to Receiver. PLoS ONE 2015, 10, e0118211. [Google Scholar] [CrossRef]
- de Groot, J.H.B.; Kirk, P.A.; Gottfried, J.A. Encoding fear intensity in human sweat. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190271. [Google Scholar] [CrossRef]
- Hu, Y.; Converse, C.; Lyons, M.; Hsu, W. Neural control of sweat secretion: A review. Br. J. Dermatol. 2018, 178, 1246–1256. [Google Scholar] [CrossRef]
- Charkoudian, N. Skin Blood Flow in Adult Human Thermoregulation: How It Works, When It Does Not, and Why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef]
- Johnson, J.M. Nonthermoregulatory control of human skin blood flow. J. Appl. Physiol. 1986, 61, 1613–1622. [Google Scholar] [CrossRef]
- Gunnar Wallin, B. Neural control of human skin blood flow. J. Auton. Nerv. Syst. 1990, 30, S185–S190. [Google Scholar] [CrossRef] [PubMed]
- Bau, J.; Wang, Y.; Wang, W. Reduction of skin impedance by the improvement of the blood circulation. In Proceedings of the 2001 Conference Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001. [Google Scholar] [CrossRef]
- Ward, L.C. Electrical bioimpedance: From the past to the future. J. Electr. Bioimpedance 2021, 12, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, F.A.A.; Prins, N. Psychophysics, 2nd ed.; Elsevier Science and Technology Books; Elsevier Academic Press: London, UK, 2016. [Google Scholar]
- Pabst, O.; Martinsen, Ø.G.; Chua, L. The non-linear electrical properties of human skin make it a generic memristor. Sci. Rep. 2018, 8, 15806. [Google Scholar] [CrossRef]
- Pabst, O.; Martinsen, Ø.G.; Chua, L. Information can be stored in the human skin memristor which has non-volatile memory. Sci. Rep. 2019, 9, 19260. [Google Scholar] [CrossRef] [PubMed]
- Pabst, O.; Sørebø, Ø.M.; Andersen, K.S.; Ousdal, E.L.; Bråthen, S.W.; Rehman, B.U.; Gholami, H.; Zhou, Z.; Takahashi, K.; Dumesso, D.T.; et al. Storing information electrically in human skin. J. Electr. Bioimpedance 2021, 12, 73–81. [Google Scholar] [CrossRef]
- Rinaldi, A.O.; Korsfeldt, A.; Ward, S.; Burla, D.; Dreher, A.; Gautschi, M.; Stolpe, B.; Tan, G.; Bersuch, E.; Melin, D.; et al. Electrical impedance spectroscopy for the characterization of skin barrier in atopic dermatitis. Allergy 2021, 76, 3066–3079. [Google Scholar] [CrossRef]
- Brunsgaard, E.K.; Sanchez, B.; Grossman, D. Electrical Impedance Dermography: Background, Current State, and Emerging Clinical Opportunities. Dermatol. Res. Pract. 2024, 2024. [Google Scholar] [CrossRef]
- González-Correa, C.H.; Caicedo-Eraso, J.C. Bioelectrical impedance analysis (BIA): A proposal for standardization of the classical method in adults. J. Phys. Conf. Ser. 2012, 407, 012018. [Google Scholar] [CrossRef]
- Steihaug, O.M.; Bogen, B.; Kristoffersen, M.H.; Ranhoff, A.H. Bones, blood and steel: How bioelectrical impedance analysis is affected by hip fracture and surgical implants. J. Electr. Bioimpedance 2017, 8, 54–59. [Google Scholar] [CrossRef]
- Wagner, D.R. Case study: Effect of surgical metal implant on single frequency bioelectrical impedance measures of an athlete. Physiol. Rep. 2020, 8, e14464. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Coratella, G.; Cerullo, G.; Noriega, Z.; Francisco, R.; Charrier, D.; Irurtia, A.; Lukaski, H.; Silva, A.M.; Paoli, A. High-standard predictive equations for estimating body composition using bioelectrical impedance analysis: A systematic review. J. Transl. Med. 2024, 22, 515. [Google Scholar] [CrossRef]
- Berger, P.; Perot, V.; Desbarats, P.; Tunon-de Lara, J.M.; Marthan, R.; Laurent, F. Airway Wall Thickness in Cigarette Smokers: Quantitative Thin-Section CT Assessment. Radiology 2005, 235, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.; Huvanandana, J.; Nguyen, C.; Duncan, J.M.; Chapman, D.G.; Tonga, K.O.; Zimmermann, S.C.; Slattery, A.; King, G.G.; Thamrin, C. Time-based pulmonary features from electrical impedance tomography demonstrate ventilation heterogeneity in chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 127, 1441–1452. [Google Scholar] [CrossRef]
- Gonzalez-Correa, C.; Evans, J.; Smye, S. Variables affecting bia measurement of body water. Med. Biol. Eng. Comput. 1999, 37, 106–107. [Google Scholar]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin changes in the obese patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef]
- Guida, B.; Nino, M.; Perrino, N.; Laccetti, R.; Trio, R.; Labella, S.; Balato, N. The impact of obesity on skin disease and epidermal permeability barrier status. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Deurenberg, P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am. J. Clin. Nutr. 1996, 64, 449S–452S. [Google Scholar] [CrossRef]
- Pailler-Mattei, C.; Debret, R.; Vargiolu, R.; Sommer, P.; Zahouani, H. In vivo skin biophysical behaviour and surface topography as a function of ageing. J. Mech. Behav. Biomed. Mater. 2013, 28, 474–483. [Google Scholar] [CrossRef]
- Pires, T.; Azambuja, A.P.; Horimoto, A.; Veroneze, M.; Alvim, R.; Krieger, J.E.; Pereira, A. A population-based study of the stratum corneum moisture. Clin. Cosmet. Investig. Dermatol. 2016, 9, 79–87. [Google Scholar] [CrossRef]
- Cua, A.B.; Wilhelm, K.P.; Maibach, H.I. Elastic properties of human skin: Relation to age, sex, and anatomical region. Arch. Dermatol. Res. 1990, 282, 283–288. [Google Scholar] [CrossRef]
- Thieulin, C.; Pailler-Mattei, C.; Abdouni, A.; Djaghloul, M.; Zahouani, H. Mechanical and topographical anisotropy for human skin: Ageing effect. J. Mech. Behav. Biomed. Mater. 2020, 103, 103551. [Google Scholar] [CrossRef]
- Frerichs, I.; Händel, C.; Becher, T.; Schädler, D. Sex differences in chest electrical impedance tomography findings. Physiol. Meas. 2024, 45, 075005. [Google Scholar] [CrossRef] [PubMed]
- Coulombe, N.; Gagnon, H.; Marquis, F.; Skrobik, Y.; Guardo, R. A parametric model of the relationship between EIT and total lung volume. Physiol. Meas. 2005, 26, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Wesley, N.O.; Maibach, H.I. Racial (Ethnic) Differences in Skin Properties: The Objective Data. Am. J. Clin. Dermatol. 2003, 4, 843–860. [Google Scholar] [CrossRef]
- Girardeau-Hubert, S.; Deneuville, C.; Pageon, H.; Abed, K.; Tacheau, C.; Cavusoglu, N.; Donovan, M.; Bernard, D.; Asselineau, D. Reconstructed Skin Models Revealed Unexpected Differences in Epidermal African and Caucasian Skin. Sci. Rep. 2019, 9, 7456. [Google Scholar] [CrossRef]
- Goyal, K.; Borkholder, D.A.; Day, S.W. Dependence of Skin-Electrode Contact Impedance on Material and Skin Hydration. Sensors 2022, 22, 8510. [Google Scholar] [CrossRef]
- Li, D.; Pu, Z.; Liang, W.; Liu, T.; Wang, R.; Yu, H.; Xu, K. Non-invasive measurement of normal skin impedance for determining the volume of the transdermally extracted interstitial fluid. Measurement 2015, 62, 215–221. [Google Scholar] [CrossRef]
- Wilke, K.; Martin, A.; Terstegen, L.; Biel, S.S. A short history of sweat gland biology. Int. J. Cosmet. Sci. 2007, 29, 169–179. [Google Scholar] [CrossRef]
- Liu, J.C.; Verhulst, S.; Massar, S.A.; Chee, M.W. Sleep Deprived and Sweating It Out: The Effects of Total Sleep Deprivation on Skin Conductance Reactivity to Psychosocial Stress. Sleep 2015, 38, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Dewasmes, G.; Bothorel, B.; Nicolas, A.; Candas, V.; Libert, J.P.; Ehrhart, J.; Muzet, A. Local sweating responses during recovery sleep after sleep deprivation in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Coratella, G.; Campa, F.; Matias, C.N.; Toselli, S.; Koury, J.C.; Andreoli, A.; Sardinha, L.B.; Silva, A.M. Generalized bioelectric impedance-based equations underestimate body fluids in athletes. Scand. J. Med. Sci. Sport. 2021, 31, 2123–2132. [Google Scholar] [CrossRef]
- Bessa, A.L.; Oliveira, V.N.; Agostini, G.; Oliveira, R.J.; Oliveira, A.C.; White, G.E.; Wells, G.D.; Teixeira, D.N.; Espindola, F.S. Exercise Intensity and Recovery: Biomarkers of Injury, Inflammation, and Oxidative Stress. J. Strength Cond. Res. 2016, 30, 311–319. [Google Scholar] [CrossRef]
- Nunes, J.P.; Araújo, J.P.M.; Ribeiro, A.S.; Campa, F.; Schoenfeld, B.J.; Cyrino, E.S.; Trindade, M.C.C. Changes in Intra-to-Extra-Cellular Water Ratio and Bioelectrical Parameters from Day-Before to Day-Of Competition in Bodybuilders: A Pilot Study. Sports 2022, 10, 23. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092. [Google Scholar] [CrossRef]
- Grimnes, S. Skin impedance and electro-osmosis in the human epidermis. Med. Biol. Eng. Comput. 1983, 21, 739–749. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, Y. Non-linear electrical properties of skin in the low frequency range. Med. Biol. Eng. Comput. 1981, 19, 302–310. [Google Scholar] [CrossRef]
- Johnsen, G.K.; Lütken, C.A.; Martinsen, Ø.G.; Grimnes, S. Memristive model of electro-osmosis in skin. Phys. Rev. E 2011, 83, 031916. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191. [Google Scholar] [CrossRef]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Singh, Y.N.; Singh, S.K.; Ray, A.K. Bioelectrical Signals as Emerging Biometrics: Issues and Challenges. ISRN Signal Process. 2012, 2012, 712032. [Google Scholar] [CrossRef]
- McAdams, E.T.; Jossinet, J.; Lackermeier, A.; Risacher, F. Factors affecting electrode-gel-skin interface impedance in electrical impedance tomography. Med. Biol. Eng. Comput. 1996, 34, 397–408. [Google Scholar] [CrossRef]
- Gao, D.; Yue, K.; Wang, Z.; Wang, T. Effects of Temperature and Humidity on Skin Resistance, Capacitance and Threshold Current in Electrotactile Stimulation; Elsevier BV: Amsterdam, The Netherlands, 2024. [Google Scholar] [CrossRef]
- Oh, S.; Leung, L.; Bommannan, D.; Guy, R.; Potts, R. Effect of current, ionic strength and temperature on the electrical properties of skin. J. Control. Release 1993, 27, 115–125. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Iftime, A.; Troumpata, L.; Periferakis, A.T.; Maier, C.; Costache, D.O. Detection of Acupuncture Points and Meridians Based on their Electrical Properties: Current Evidence and Future Research Perspectives. Rom. J. Mil. Med. 2025, 128, 378–400. [Google Scholar] [CrossRef]
- Ahn, A.C.; Martinsen, Ø.G. Electrical Characterization of Acupuncture Points: Technical Issues and Challenges. J. Altern. Complement. Med. 2007, 13, 817–824. [Google Scholar] [CrossRef]
- Jayaraman, A.; Kaczmarek, K.A.; Tyler, M.E.; Okpara, U.O. Effect of localized ambient humidity on electrotactile skin resistance. In Proceedings of the 2007 IEEE 33rd Annual Northeast Bioengineering Conference, Long Island, NY, USA, 10–11 March 2007; pp. 110–111. [Google Scholar]
- Gibson, A.L.; Beam, J.R.; Alencar, M.K.; Zuhl, M.N.; Mermier, C.M. Time course of supine and standing shifts in total body, intracellular and extracellular water for a sample of healthy adults. Eur. J. Clin. Nutr. 2014, 69, 14–19. [Google Scholar] [CrossRef]
- Allison, G.T.; Singer, K.P.; Marshall, R.N. The effect of body position on bioelectrical resistance in individuals with spinal cord injury. Disabil. Rehabil. 1995, 17, 424–429. [Google Scholar] [CrossRef]
- Lyons-Reid, J.; Ward, L.C.; Tint, M.T.; Kenealy, T.; Godfrey, K.M.; Chan, S.Y.; Cutfield, W.S. The influence of body position on bioelectrical impedance spectroscopy measurements in young children. Sci. Rep. 2021, 11, 10346. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Riedel, T. Electrical impedance tomography: Amplitudes of cardiac related impedance changes in the lung are highly position dependent. PLoS ONE 2017, 12, e0188313. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamamoto, Y. Analysis for the change of skin impedance. Med. Biol. Eng. Comput. 1977, 15, 219–227. [Google Scholar] [CrossRef]
- Suwabe, H.; Serizawa, A.; Kajiwara, H.; Ohkido, M.; Tsutsumi, Y. Degenerative processes of elastic fibers in sun-protected and sun-exposed skin: Immunoelectron microscopic observation of elastin, fibrillin-1, amyloid P component, lysozyme and alpha1-antitrypsin. Pathol. Int. 1999, 49, 391–402. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef]
- Le Fur, I.; Reinberg, A.; Lopez, S.; Morizot, F.; Mechkouri, M.; Tschachler, E. Analysis of Circadian and Ultradian Rhythms of Skin Surface Properties of Face and Forearm of Healthy Women. J. Investig. Dermatol. 2001, 117, 718–724. [Google Scholar] [CrossRef]
- Mayrovitz, H.N. Intraday Variations in Skin Water Parameters. Skin Pharmacol. Physiol. 2024, 37, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Fujimura, T.; Imokawa, G. Dermal fluid translocation is an important determinant of the diurnal variation in human skin thickness. Br. J. Dermatol. 2001, 145, 590–596. [Google Scholar] [CrossRef]
- Sousa, A.; Noites, A.; Vilarinho, R.; Santos, R. Long-Term Electrode–Skin Impedance Variation for Electromyographic Measurements. Sensors 2023, 23, 8582. [Google Scholar] [CrossRef]
- Serup, J.; Alsing, K.K.; Olsen, O.; Koch, C.B.; Hansen, R.H. On the mechanism of painful burn sensation in tattoos on magnetic resonance imaging (MRI). Magnetic substances in tattoo inks used for permanent makeup (PMU) identified: Magnetite, goethite, and hematite. Skin Res. Technol. 2023, 29, e13281. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y. Dielectric Properties of Textile Materials: Analytical Approximations and Experimental Measurements—A Review. Textiles 2022, 2, 50–80. [Google Scholar] [CrossRef]
- Mustata, F.S.C.; Mustata, A. Dielectric Behaviour of Some Woven Fabrics on the Basis of Natural Cellulosic Fibers. Adv. Mater. Sci. Eng. 2014, 2014, 216548. [Google Scholar] [CrossRef]
- Gonzalez, J.; Rizvi, S.; Crown, E. Mathematical Modeling Of Electrostatic Propensity Of Protective Clothing Systems. In Proceedings of the Electrical Overstress/Electrostatic Discharge Symposium, Santa Clara, CA, USA, 23–25 September 1997; pp. 153–162. [Google Scholar] [CrossRef]
- Al-Qaham, Y.; Mohamed, M.; Ali, W. Electric static charge generated from the friction of textiles. J. Egypt. Soc. Tribol. 2013, 10, 46–56. [Google Scholar]
- Kathirgamanathan, P.; Toohey, M.J.; Haase, J.; Holdstock, P.; Laperre, J.; Schmeer-Lioe, G. Measurements of incendivity of electrostatic discharges from textiles used in personal protective clothing. J. Electrost. 2000, 49, 51–70. [Google Scholar] [CrossRef]
- Nuccitelli, R.; Nuccitelli, P.; Li, C.; Narsing, S.; Pariser, D.M.; Lui, K. The electric field near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Repair Regen. 2011, 19, 645–655. [Google Scholar] [CrossRef]
- Spaulding, K.; Ahn, A.; Colbert, A.P. Acupuncture Needle Stimulation Induces Changes in Bioelectric Potential. Med. Acupunct. 2013, 25, 141–148. [Google Scholar] [CrossRef]
- Clar, E.; Her, C.; Sturelle, C. Skin impedance and moisturization. J. Soc. Cosmet. Chem. 1975, 26, 337–353. [Google Scholar]
- Roy, A.; Bhattacharjee, S.; Podder, S.; Ghosh, A. Measurement of bioimpedance and application of Cole model to study the effect of moisturizing cream on human skin. AIMS Biophys. 2020, 7, 362–379. [Google Scholar] [CrossRef]
- Martinsen, Ø.G.; Grimnes, S. Long-Term Effect of Some Skin Moisturizers. Open Dermatol. J. 2008, 2, 87–89. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Draelos, Z.D. The science behind skin care: Cleansers. J. Cosmet. Dermatol. 2017, 17, 8–14. [Google Scholar] [CrossRef]
- Percival, S.L.; Chen, R.; Mayer, D.; Salisbury, A. Mode of action of poloxamer-based surfactants in wound care and efficacy on biofilms. Int. Wound J. 2018, 15, 749–755. [Google Scholar] [CrossRef]
- Bard, A.J.; Abruna, H.D.; Chidsey, C.E.; Faulkner, L.R.; Feldberg, S.W.; Itaya, K.; Majda, M.; Melroy, O.; Murray, R.W. The electrode/electrolyte interface—A status report. J. Phys. Chem. 1993, 97, 7147–7173. [Google Scholar] [CrossRef]
- Murphy, B.B.; Scheid, B.H.; Hendricks, Q.; Apollo, N.V.; Litt, B.; Vitale, F. Time Evolution of the Skin–Electrode Interface Impedance under Different Skin Treatments. Sensors 2021, 21, 5210. [Google Scholar] [CrossRef]
- Lodén, M.; Hagforsen, E.; Lindberg, M. The presence of body hair influences the measurement of skin hydration with the Corneometer. Acta Derm.-Venereol. 1995, 75, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Özgün, N.; Kondziela, D.J.; Bennewitz, R. Role of Hair Coverage and Sweating for Textile Friction on the Forearm. Tribol. Lett. 2020, 68, 100. [Google Scholar] [CrossRef]
- Acar, G.; Ozturk, O.; Golparvar, A.J.; Elboshra, T.A.; Böhringer, K.; Yapici, M.K. Wearable and Flexible Textile Electrodes for Biopotential Signal Monitoring: A review. Electronics 2019, 8, 479. [Google Scholar] [CrossRef]
- Cesarelli, G.; Donisi, L.; Coccia, A.; Amitrano, F.; D’Addio, G.; Ricciardi, C. The E-Textile for Biomedical Applications: A Systematic Review of Literature. Diagnostics 2021, 11, 2263. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, G.; Ehrmann, A. Textile-Based Sensors for Biosignal Detection and Monitoring. Sensors 2021, 21, 6042. [Google Scholar] [CrossRef]
- Vidhya, C.M.; Maithani, Y.; Singh, J.P. Recent Advances and Challenges in Textile Electrodes for Wearable Biopotential Signal Monitoring: A Comprehensive Review. Biosensors 2023, 13, 679. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, X.; Ullah, M.W.; Li, S.; Wang, Q.; Yang, G. Electroconductive natural polymer-based hydrogels. Biomaterials 2016, 111, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Meziane, N.; Webster, J.G.; Attari, M.; Nimunkar, A.J. Dry electrodes for electrocardiography. Physiol. Meas. 2013, 34, R47–R69. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Kusche, R.; Kaufmann, S.; Ryschka, M. Dry electrodes for bioimpedance measurements—Design, characterization and comparison. Biomed. Phys. Eng. Express 2018, 5, 015001. [Google Scholar] [CrossRef]
- Huang, S.; Liu, Y.; Zhao, Y.; Ren, Z.; Guo, C.F. Flexible Electronics: Stretchable Electrodes and Their Future. Adv. Funct. Mater. 2018, 29. [Google Scholar] [CrossRef]
- Park, S.; Ban, S.; Zavanelli, N.; Bunn, A.E.; Kwon, S.; Lim, H.r.; Yeo, W.H.; Kim, J.H. Fully Screen-Printed PI/PEG Blends Enabled Patternable Electrodes for Scalable Manufacturing of Skin-Conformal, Stretchable, Wearable Electronics. ACS Appl. Mater. Interfaces 2023, 15, 2092–2103. [Google Scholar] [CrossRef]
- Mirbakht, S.S.; Golparvar, A.; Umar, M.; Kuzubasoglu, B.A.; Irani, F.S.; Yapici, M.K. Highly Self-Adhesive and Biodegradable Silk Bioelectronics for All-In-One Imperceptible Long-Term Electrophysiological Biosignals Monitoring. Adv. Sci. 2025, 12, 2405988. [Google Scholar] [CrossRef]
- Waldmann, A.D.; Wodack, K.H.; März, A.; Ukere, A.; Trepte, C.J.; Böhm, S.H.; Reuter, D.A. Performance of Novel Patient Interface for Electrical Impedance Tomography Applications. J. Med. Biol. Eng. 2017, 37, 561–566. [Google Scholar] [CrossRef]
- Matsukawa, R.; Miyamoto, A.; Yokota, T.; Someya, T. Skin Impedance Measurements with Nanomesh Electrodes for Monitoring Skin Hydration. Adv. Healthc. Mater. 2020, 9, 2001322. [Google Scholar] [CrossRef] [PubMed]
- Searle, A.; Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 2000, 21, 271–283. [Google Scholar] [CrossRef]
- Spinelli, E.; Haberman, M. Insulating electrodes: A review on biopotential front ends for dielectric skin–electrode interfaces. Physiol. Meas. 2010, 31, S183–S198. [Google Scholar] [CrossRef]
- Gow, B.J.; Cheng, J.L.; Baikie, I.D.; Martinsen, Ø.G.; Zhao, M.; Smith, S.; Ahn, A.C. Electrical Potential of Acupuncture Points: Use of a Noncontact Scanning Kelvin Probe. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Kwon, H.; Di Cristina, J.F.; Rutkove, S.B.; Sanchez, B. Recording characteristics of electrical impedance-electromyography needle electrodes. Physiol. Meas. 2018, 39, 055005. [Google Scholar] [CrossRef]
- Xia, W.; Song, D.; Fu, H.; Lei, T.; Wang, K.; Zeng, Y. Comparison of subdermal needle and surface adhesive electrodes for intraoperative neuromonitoring during spine surgeries. J. Orthop. Surg. Res. 2025, 20, 490. [Google Scholar] [CrossRef] [PubMed]
- Kalvøy, H.; Tronstad, C.; Nordbotten, B.; Grimnes, S.; Martinsen, Ø.G. Electrical Impedance of Stainless Steel Needle Electrodes. Ann. Biomed. Eng. 2010, 38, 2371–2382. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, X.; Huang, S.; Huang, X.; Liu, Z.; Yao, C.; He, M.; Chen, J.; Chen, H.j.; Liu, J.; et al. Multichannel microneedle dry electrode patches for minimally invasive transdermal recording of electrophysiological signals. Microsyst. Nanoeng. 2024, 10, 72. [Google Scholar] [CrossRef]
- Huigen, E.; Peper, A.; Grimbergen, C.A. Investigation into the origin of the noise of surface electrodes. Med. Biol. Eng. Comput. 2002, 40, 332–338. [Google Scholar] [CrossRef]
- Hua, P.; Woo, E.; Webster, J.; Tompkins, W. Using compound electrodes in electrical impedance tomography. IEEE Trans. Biomed. Eng. 1993, 40, 29–34. [Google Scholar] [CrossRef]
- Yan, W.; Hong, S.; Chaoshi, R. Optimum design of electrode structure and parameters in electrical impedance tomography. Physiol. Meas. 2006, 27, 291–306. [Google Scholar] [CrossRef]
- Gaggero, P.O.; Adler, A.; Brunner, J.; Seitz, P. Electrical impedance tomography system based on active electrodes. Physiol. Meas. 2012, 33, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, D.; Bardill, A.; Bayford, R.; Demosthenous, A. A 122 fps, 1 MHz Bandwidth Multi-Frequency Wearable EIT Belt Featuring Novel Active Electrode Architecture for Neonatal Thorax Vital Sign Monitoring. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 927–937. [Google Scholar] [CrossRef]
- Yang, L.; Gan, L.; Zhang, Z.; Zhang, Z.; Yang, H.; Zhang, Y.; Wu, J. Insight into the Contact Impedance between the Electrode and the Skin Surface for Electrophysical Recordings. ACS Omega 2022, 7, 13906–13912. [Google Scholar] [CrossRef] [PubMed]
- Mascia, A.; Collu, R.; Makni, N.; Concas, M.; Barbaro, M.; Cosseddu, P. Impedance Characterization and Modeling of Gold, Silver, and PEDOT:PSS Ultra-Thin Tattoo Electrodes for Wearable Bioelectronics. Sensors 2025, 25, 4568. [Google Scholar] [CrossRef]
- Colachis, M.; Schlink, B.R.; Colachis, S.; Shqau, K.; Huegen, B.L.; Palmer, K.; Heintz, A. Benchtop Performance of Novel Mixed Ionic–Electronic Conductive Electrode Form Factors for Biopotential Recordings. Sensors 2024, 24, 3136. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, L.; Zhang, Z.; Yang, H.; Zhang, Y.; Wu, J. The Feature, Performance, and Prospect of Advanced Electrodes for Electroencephalogram. Biosensors 2023, 13, 101. [Google Scholar] [CrossRef]
- Xiong, F.; Fan, M.; Feng, Y.; Li, Y.; Yang, C.; Zheng, J.; Wang, C.; Zhou, J. Advancements in dry and semi-dry EEG electrodes: Design, interface characteristics, and performance evaluation. AIP Adv. 2025, 15, 040703. [Google Scholar] [CrossRef]
- Li, H.; Tan, P.; Rao, Y.; Bhattacharya, S.; Wang, Z.; Kim, S.; Gangopadhyay, S.; Shi, H.; Jankovic, M.; Huh, H.; et al. E-Tattoos: Toward Functional but Imperceptible Interfacing with Human Skin. Chem. Rev. 2024, 124, 3220–3283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ko, S.H. Tattoo electrodes in bioelectronics: A pathway to next-generation wearable systems. Nanoscale Horiz. 2025, 10, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Galliani, M.; Greco, F.; Ismailova, E.; Ferrari, L.M. On the Breathability of Epidermal Polymeric-Printed Tattoo Electrodes. ACS Appl. Electron. Mater. 2025, 7, 1408–1414. [Google Scholar] [CrossRef]
- Abd-Elbaki, M.K.M.; Ragab, T.M.; Ismael, N.E.R.; Khalil, A.S.G. Robust, self-adhesive and anti-bacterial silk-based LIG electrodes for electrophysiological monitoring. RSC Adv. 2023, 13, 31704–31719. [Google Scholar] [CrossRef]
- Fernandes, S.; Ramos, A.; Vega-Barbas, M.; García-Vázquez, C.; Seoane, F.; Pau, I. Smart Textile Technology for the Monitoring of Mental Health. Sensors 2025, 25, 1148. [Google Scholar] [CrossRef]
- Chang, L.; Jing, H.; Liu, C.; Qiu, C.; Ling, X. High-Entropy Materials for Prospective Biomedical Applications: Challenges and Opportunities. Adv. Sci. 2024, 11, 2406521. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, Z.; Xiang, Z.; Zhou, J.; Wang, X.; Liu, Q.; Gan, L.; Shi, S.; Yang, W.; Zhang, Y.; et al. A high-entropy electrode material for electrobiochemical and eletrophysiological signals detection. Chem. Eng. J. 2024, 499, 156209. [Google Scholar] [CrossRef]
- Xu, P.; Challa, S.; Zhang, Z.; Wu, X.; Wang, S.; Pan, P.; Liu, X. Ultrasoft, elastic, and ionically conductive polyethylene glycol/ionic liquid bottlebrush ionogels. Materials Horiz. 2025. [Google Scholar] [CrossRef]
- Ho, M.; Ramirez, A.B.; Akbarnia, N.; Croiset, E.; Prince, E.; Fuller, G.G.; Kamkar, M. Direct Ink Writing of Conductive Hydrogels. Adv. Funct. Mater. 2025, 35, 2415507. [Google Scholar] [CrossRef]
- Baran, I.; Iftime, A.; Popescu, A. Diffusion–convection effects on drug distribution at the cell membrane level in a patch-clamp setup. Biosystems 2010, 102, 134–147. [Google Scholar] [CrossRef]
- Najjar, H.; Khoury, F.; Saleh, S.; Khraiche, M. Optimizing Wrist-Based Bioimpedance: The Role of Electrode Type, Positioning and Signal Frequency in Health Monitoring. In Proceedings of the 2023 IEEE 4th International Multidisciplinary Conference on Engineering Technology (IMCET), Beirut, Lebanon, 12–14 December 2023; pp. 209–213. [Google Scholar] [CrossRef]
- Taji, B.; Chan, A.D.C.; Shirmohammadi, S. Effect of Pressure on Skin-Electrode Impedance in Wearable Biomedical Measurement Devices. IEEE Trans. Instrum. Meas. 2018, 67, 1900–1912. [Google Scholar] [CrossRef]
- González-Correa, C.A.; Brown, B.H.; Smallwood, R.H.; Walker, D.C.; Bardhan, K.D. Electrical bioimpedance readings increase with higher pressure applied to the measuring probe. Physiol. Meas. 2005, 26, S39–S47. [Google Scholar] [CrossRef]
- Wong, Y.M. Is An Acupuncture Point Identifiable with Skin Electrical Resistance Measurement? Acupunct. Med. 2014, 32, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Karsten, J.; Stueber, T.; Voigt, N.; Teschner, E.; Heinze, H. Influence of different electrode belt positions on electrical impedance tomography imaging of regional ventilation: A prospective observational study. Crit. Care 2016, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Chichester, UK, 2023. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Mansournia, M.A.; Higgins, J.P.T.; Sterne, J.A.C.; Hernán, M.A. Biases in Randomized Trials: A Conversation Between Trialists and Epidemiologists. Epidemiology 2017, 28, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Li, X.; Zhao, Z.; Yang, L. Reduction of Spike-like Noise in Clinical Practice for Thoracic Electrical Impedance Tomography Using Robust Principal Component Analysis. Bioengineering 2025, 12, 402. [Google Scholar] [CrossRef]
- Nejadgholi, I.; Bolic, M. A comparative study of PCA, SIMCA and Cole model for classification of bioimpedance spectroscopy measurements. Comput. Biol. Med. 2015, 63, 42–51. [Google Scholar] [CrossRef]
- Stasiak, M.; Sikora, J.; Filipowicz, S.F.; Nita, K. Principal component analysis and artificial neural network approach to electrical impedance tomography problems approximated by multi-region boundary element method. Eng. Anal. Bound. Elem. 2007, 31, 713–720. [Google Scholar] [CrossRef]
- Zagar, T.; Krizaj, D. Multivariate analysis of electrical impedance spectra for relaxed and contracted skeletal muscle. Physiol. Meas. 2008, 29, S365–S372. [Google Scholar] [CrossRef]
- Brown, V.A. An Introduction to Linear Mixed-Effects Modeling in R. Adv. Methods Pract. Psychol. Sci. 2021, 4, 251524592096035. [Google Scholar] [CrossRef]
- Cheung, T.; Nuño, M.; Hoffman, M.; Katz, M.; Kilbane, C.; Alterman, R.; Tagliati, M. Longitudinal Impedance Variability in Patients with Chronically Implanted DBS Devices. Brain Stimul. 2013, 6, 746–751. [Google Scholar] [CrossRef] [PubMed]
- NIH. Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. Am. J. Clin. Nutr. 1996, 64, 524S–532S. [Google Scholar] [CrossRef]
- Brantlov, S.; Ward, L.C.; Jødal, L.; Rittig, S.; Lange, A. Critical factors and their impact on bioelectrical impedance analysis in children: A review. J. Med. Eng. Technol. 2016, 41, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Colclough, A. Two theories of experimental error. J. Res. Natl. Bur. Stand. 1987, 92, 167. [Google Scholar] [CrossRef]
- Hon, G. Towards a typology of experimental errors: An epistemological view. Stud. Hist. Philos. Sci. Part A 1989, 20, 469–504. [Google Scholar] [CrossRef]
- Brakenhoff, T.B.; Mitroiu, M.; Keogh, R.H.; Moons, K.G.; Groenwold, R.H.; van Smeden, M. Measurement error is often neglected in medical literature: A systematic review. J. Clin. Epidemiol. 2018, 98, 89–97. [Google Scholar] [CrossRef]
- Lima-Oliveira, G.; Volanski, W.; Lippi, G.; Picheth, G.; Guidi, G.C. Pre-analytical phase management: A review of the procedures from patient preparation to laboratory analysis. Scand. J. Clin. Lab. Investig. 2017, 77, 153–163. [Google Scholar] [CrossRef]
- Narayanan, S. The Preanalytic Phase. Am. J. Clin. Pathol. 2000, 113, 429–452. [Google Scholar] [CrossRef] [PubMed]

| Confounding Factor | Accounted for and Mitigated in the Experimental Design? | |||
|---|---|---|---|---|
| Yes | No | Partially | N/A | |
| Overall physiological and psychological condition of the participants | ||||
| Health status of participants | □ | □ | □ | □ |
| Relevant medical history | □ | □ | □ | □ |
| Core temperature | □ | □ | □ | □ |
| Body mass index (BMI) | □ | □ | □ | □ |
| Age | □ | □ | □ | □ |
| Sex | □ | □ | □ | □ |
| Menstrual cycle | □ | □ | □ | □ |
| Ethnicity | □ | □ | □ | □ |
| General hydration status | □ | □ | □ | □ |
| Feeding (prandial) status | □ | □ | □ | □ |
| Medication or substance use | □ | □ | □ | □ |
| Sleeping status | □ | □ | □ | □ |
| Fitness | □ | □ | □ | □ |
| Exercise | □ | □ | □ | □ |
| Emotional status | □ | □ | □ | □ |
| Environmental conditions | ||||
| Ambient temperature | □ | □ | □ | □ |
| Ambient relative humidity | □ | □ | □ | □ |
| Stability of ambient conditions | □ | □ | □ | □ |
| Accommodation to laboratory conditions | □ | □ | □ | □ |
| Posture and Postural accommodation | □ | □ | □ | □ |
| Season | □ | □ | □ | □ |
| Time constraints | ||||
| Time of day | □ | □ | □ | □ |
| Timing of the measurement: immediate or delayed after electrode placement | □ | □ | □ | □ |
| Measurement repetitions in time | □ | □ | □ | □ |
| Skin condition | ||||
| Tattoos, piercings | □ | □ | □ | □ |
| Items in contact with skin: jewelry and conductive clothing accessories | □ | □ | □ | □ |
| Items in contact with skin: clothing, belts, etc. | □ | □ | □ | □ |
| Local health of the skin | □ | □ | □ | □ |
| Skin preparation | ||||
| Topical hygiene products | □ | □ | □ | □ |
| Skin hygiene | □ | □ | □ | □ |
| Stratum corneum: modifying or not | □ | □ | □ | □ |
| Shaving | □ | □ | □ | □ |
| Electrodes | ||||
| Electrode type | □ | □ | □ | □ |
| Electrode surface area considerations | □ | □ | □ | □ |
| Electrode placement | ||||
| Pressure on electrodes | □ | □ | □ | □ |
| Electrode location | □ | □ | □ | □ |
| General problems related to hardware | ||||
| Wires | □ | □ | □ | □ |
| Equipment location | □ | □ | □ | □ |
| Sources of bias in human trials | ||||
| Check for potential biases | □ | □ | □ | □ |
| Data modeling | ||||
| Check for appropriate modeling | □ | □ | □ | □ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iftime, A.; Scheau, C.; Babeș, R.-M.; Ionescu, D.; Periferakis, A.; Călinescu, O. Confounding Factors and Their Mitigation in Measurements of Bioelectrical Impedance at the Skin Interface. Bioengineering 2025, 12, 926. https://doi.org/10.3390/bioengineering12090926
Iftime A, Scheau C, Babeș R-M, Ionescu D, Periferakis A, Călinescu O. Confounding Factors and Their Mitigation in Measurements of Bioelectrical Impedance at the Skin Interface. Bioengineering. 2025; 12(9):926. https://doi.org/10.3390/bioengineering12090926
Chicago/Turabian StyleIftime, Adrian, Cristian Scheau, Ramona-Madalina Babeș, Diana Ionescu, Argyrios Periferakis, and Octavian Călinescu. 2025. "Confounding Factors and Their Mitigation in Measurements of Bioelectrical Impedance at the Skin Interface" Bioengineering 12, no. 9: 926. https://doi.org/10.3390/bioengineering12090926
APA StyleIftime, A., Scheau, C., Babeș, R.-M., Ionescu, D., Periferakis, A., & Călinescu, O. (2025). Confounding Factors and Their Mitigation in Measurements of Bioelectrical Impedance at the Skin Interface. Bioengineering, 12(9), 926. https://doi.org/10.3390/bioengineering12090926









