Abstract
Diabetes mellitus (DM) is a major global health concern, associated with both microvascular and macrovascular complications. Early identification of individuals at risk of hyperglycemia and diabetes progression is crucial for preventing long-term complications and improving patient outcomes. We investigated the association between neck circumference (NC) and hyperglycemia in non-diabetic individuals and in patients with uncontrolled DM, using data from the nationally representative Korea National Health and Nutrition Examination Survey (KNHANES) 2019–2021. Uncontrolled DM was defined as hemoglobin A1c (HbA1c) ≥ 7.0%, while hyperglycemia in non-diabetic individuals was defined as fasting blood glucose ≥ 126 mg/dL or HbA1c ≥ 6.5%. Logistic regression analyses were conducted to evaluate the association between NC and glycemic outcomes. NC was independently associated with hyperglycemia in non-diabetic individuals (Model 1: odds ratio (OR): 1.09; 95% confidence interval (CI): 1.05–1.13; Model 2: OR: 1.09; 95% CI: 1.05–1.13) and patients with uncontrolled DM (Model 1: OR: 1.10; 95% CI: 1.03–1.17; Model 2: OR: 1.11; 95% CI: 1.04–1.18) after adjusting for potential confounders. This study demonstrates that NC is a significant risk factor for hyperglycemia in the general population and for individuals with uncontrolled DM. NC may serve as a simple, non-invasive anthropometric marker to help identify individuals at elevated risk for poor glycemic control.
1. Introduction
Diabetes mellitus (DM) is a major global health concern, affecting approximately 537 million individuals worldwide in 2021, accounting for nearly 10.5% of the adult population [,,]. The economic burden of DM is substantial, with global healthcare expenditures totaling USD 966 billion in 2021 and projected to exceed USD 1054 billion by 2045 []. This increasing burden is attributed to lifestyle changes, increased obesity rates, urbanization, and aging populations [,,]. DM leads to various microvascular and macrovascular complications, such as diabetic retinopathy, diabetic nephropathy, and cardiovascular diseases (CVDs), all of which significantly impair the quality of life and increase healthcare costs [,,]. Uncontrolled diabetes, characterized by persistent hyperglycemia (glycated hemoglobin [HbA1c] ≥ 7%), significantly increases the risk and progression of diabetes-related complications [,]. A previous study showed that a 1% increase in HbA1c above the target level was associated with a 15–20% increased risk of cardiovascular complications and a 25–35% increased risk of microvascular complications []. The underlying mechanisms driving these complications include chronic systemic inflammation, oxidative stress, endothelial dysfunction, and insulin resistance, all of which contribute to vascular damage and metabolic dysregulation [,]. Early identification of individuals at risk of hyperglycemia and DM progression is critical for preventing long-term complications and improving patient outcomes.
Recently, the relationship between anthropometric measurements and metabolic disorders has been studied [,,]. Traditional measures such as body mass index (BMI), waist circumference (WC), and waist-to-hip ratio have long been established as indicators of metabolic risk [,]. Neck circumference (NC) is a novel and promising anthropometric marker of metabolic health. Furthermore, NC is strongly associated with central obesity, metabolic syndrome (MetS), and its components, such as hypertension (HTN), elevated triglycerides, and fasting blood sugar [,,]. Emerging evidence suggests that elevated NC is not only associated with MetS and its components but also strongly correlated with development and poor glycemic control in individuals with diabetes [,]. In addition, individuals with higher NC tend to exhibit increased fasting glucose levels, higher HbA1c levels, and reduced insulin sensitivity, highlighting the potential of NC as an early indicator of metabolic dysfunction [,]. Unlike BMI and WC, which require precise positioning and measurement protocols, NC can be measured quickly and with high reproducibility, making it an attractive option for large-scale screening and clinical practice. Given its simplicity, ease of measurement, and strong correlation with metabolic risk factors, NC is a practical tool for identifying individuals at risk of diabetes-related complications []. However, the relationship between NC and hyperglycemia across different populations, particularly Asian populations, remains to be fully elucidated. In this study, we demonstrated that NC is significantly associated with fasting hyperglycemia, not only in patients with DM but also in normal individuals in the general Korean population.
2. Materials and Methods
2.1. Study Population
This cross-sectional study used data from the Korea National Health and Nutrition Examination Survey (KNHANES) VIII, a nationally representative surveillance system that collects comprehensive health and nutritional data through standardized health interviews, examinations, and nutritional surveys. Data were collected between 2019 and 2021, with an initial enrollment of 22,559 individuals. Individuals younger than 20 years (n = 4048), pregnant women (n = 50), individuals with a history of cancer (n = 1174), and those with missing data (n = 7068) were excluded. After exclusion, the final study population consisted of 10,219 individuals (Figure 1).
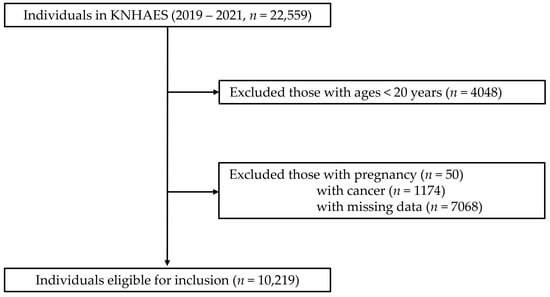
Figure 1.
Flow diagram of study population.
2.2. Demographic Data and Measurements
Trained personnel gathered participant information through questionnaires and one-on-one interviews and collected self-reported demographic information and medical backgrounds, including DM, HTN, and dyslipidemia. Smoking habits were classified into two categories: current smokers and non-smokers; the latter group included former smokers and individuals who had never smoked. Alcohol consumption was also categorized into two groups: non-drinkers, defined as those who had not consumed alcohol in the past year or drank alcohol less than once a month; and drinkers, defined as individuals who consumed alcohol more frequently than once a month. Physical activity levels were divided into two categories: regular exercise, defined as engaging in at least 150 min of moderate-intensity exercise per week, 75 min of high-intensity exercise per week, or an equivalent combination (where one minute of high-intensity exercise equates to two minutes of moderate-intensity exercise); and non-regular exercise, indicating activity levels below these thresholds. The NC measurement protocol required the participants to be properly positioned in a chair, maintaining contact between their back and the chair’s surface, while keeping their head upright and their arms relaxed alongside their body. For male participants, the measurement point was identified at the Adam’s apple, whereas for female participants, measurements were taken at the prominence of the thyroid cartilage. WC measurements were obtained at the intermediate point between the lowest rib margin and the superior border of the iliac crest along the right mid-axillary line. Height and weight measurements followed the standardized KNHANES protocols, and body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure readings were conducted by qualified nursing staff after participants rested for 5 min in a seated position with proper arm support at heart level. Three separate blood pressure measurements were obtained, including systolic (SBP) and diastolic (DBP) readings. Blood specimens were collected after a minimum of 8 h of fasting at night.
2.3. Definition of Uncontrolled DM, Hyperglycemia of Non-DM, and MetS
For this analysis, uncontrolled DM was defined as a hemoglobin A1c (A1c) ≥ 7.0% according to American Diabetes Association (ADA) guidelines []. In individuals without DM, hyperglycemia was defined as a fasting blood glucose level ≥ 126 mg/dL or A1c ≥ 6.5% []. The diagnosis of MetS was identified in individuals who met at least three of the following five criteria: (1) elevated fasting blood glucose (≥ 100 mg/dL), (2) increased blood pressure (DBP ≥ 85 mmHg or SBP ≥ 130 mmHg), (3) elevated triglyceride levels (TG ≥ 150 mg/dL), (4) reduced HDL cholesterol (< 50 mg/dL in women and < 40 mg/dL in men), and (5) abdominal obesity (WC ≥ 85 cm in women and ≥ 90 cm in men).
2.4. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation, while categorical variables are presented as frequencies and percentages. Baseline characteristics were analyzed by comparing normal versus hyperglycemic individuals without DM or with controlled DM versus individuals with diagnosed but uncontrolled DM. Univariate and multivariate logistic regression analyses were performed to examine the association between NC and both hyperglycemic individuals and patients with uncontrolled DM, with the results reported as odds ratios (ORs) and 95% confidence intervals (CIs). A multivariate analysis was conducted using two models with progressively adjusted confounders. The crude model is based on a simple model with only one variable. Model 1 was adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, HTN, chronic kidney disease (CKD), and dyslipidemia. Model 2 was further adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, SBP, DBP, estimated glomerular filtration rate, and total cholesterol. Statistical analyses were conducted using SPSS software version 29 (IBM Corporation, Armonk, NY, USA) and Prism version 10.1.2 (GraphPad). Figure 2 was created using Prism version 10.1.2 (GraphPad). Statistical significance was set at p < 0.05.
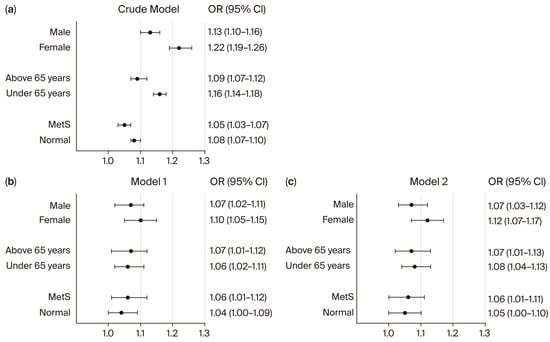
Figure 2.
Subgroup analysis for the association between neck circumference and hyperglycemia in non-diabetic individuals. Forest plots show the odds ratios (ORs) for NC and hyperglycemia in univariate (a) and multivariate (b,c) logistic regression analyses. ORs and 95% confidence intervals (CIs) were calculated using univariate and multivariate logistic regression models. Model 1 was adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, HTN, CKD, and dyslipidemia. Model 2 was adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, SBP, DBP, eGFR, and total cholesterol. Abbreviation: MetS: metabolic syndrome.
3. Results
3.1. Baseline Characteristics of the Study Participants
The baseline characteristics of the study participants are summarized in Table 1. A total of 10,219 individuals were included and categorized into a non-DM group (n = 8203) and a DM group (n = 2016). The non-DM group was further divided into two subgroups: normal glucose (n = 5181) and hyperglycemia (n = 3022). Hyperglycemia in non-diabetic individuals was defined as a fasting blood glucose level ≥ 126 mg/dL or an A1c ≥ 6.5%. The DM group was stratified into controlled DM (n = 1156) and uncontrolled DM (n = 860), with uncontrolled DM defined as an HbA1c level ≥ 7.0%.

Table 1.
Baseline characteristics of study population.
Males represented a significantly higher proportion in the hyperglycemic subgroup compared to the normal glycemic subgroup (51.2% vs. 37.1%; p < 0.01) among non-diabetic participants. Similarly, male predominance was observed in the uncontrolled DM subgroup compared to the controlled DM subgroup (52.8% vs. 52.0%; p = 0.72), although this difference was not statistically significant. The hyperglycemic subgroup was significantly older than the normal glycemic subgroup (60.0 ± 11.3 vs. 57.5 ± 11.7 years; p < 0.01), while participants with uncontrolled DM were significantly younger than those with controlled DM (63.5 ± 10.6 vs. 65.3 ± 10.4 years; p < 0.01). NC showed significant differences across glycemic status groups. Participants with normal glucose levels had the lowest NC (34.3 ± 3.1 cm), whereas hyperglycemic individuals had higher values (35.7 ± 3.3 cm, p < 0.01). Among DM patients, NC was greater in the uncontrolled group (36.6 ± 3.4 cm) compared to the controlled group (36.2 ± 3.3 cm, p < 0.01).
Triglyceride levels were significantly elevated in both the hyperglycemic and uncontrolled DM subgroups compared to their counterparts (hyperglycemic: 151.6 ± 125.5 vs. normal: 119.6 ± 86.2 mg/dL; uncontrolled DM: 173.0 ± 153.1 vs. controlled DM: 141.1 ± 98.9 mg/dL; both p < 0.01). Conversely, HDL cholesterol levels showed an inverse relationship with worsening glycemic status, with the lowest levels observed in the uncontrolled DM subgroup (46.0 ± 11.0 mg/dL). The prevalence of MetS was notably higher in hyperglycemic individuals (50.9%) compared to those in the normal glucose subgroup (12.0%) (p < 0.01). Similarly, among DM patients, the uncontrolled group had a higher prevalence of MetS (66.7%) than the controlled group (53.5%) (p < 0.01). CKD was also more common in the hyperglycemic group (5.0% vs. 3.2%, p < 0.01), although among DM patients, the difference in CKD prevalence between the uncontrolled and controlled groups was not significant (9.0% vs. 10.4%; p = 0.29).
3.2. Associations of NC and Hyperglycemia and Uncontrolled DM
To validate the association between NC and both hyperglycemia and uncontrolled DM, we conducted univariate and multivariate logistic regression analyses (Table 2). In the crude model, NC showed significant associations with hyperglycemia in non-diabetic individuals (OR: 1.15; 95% CI: 1.14–1.17; p < 0.01) and patients with uncontrolled DM (OR: 1.04; 95% CI: 1.02–1.07; p < 0.01). In the adjusted models, these associations remained robust. In Model 1, NC was independently associated with hyperglycemia in non-diabetic individuals (OR: 1.09; 95% CI: 1.05–1.13; p < 0.01). Among DM patients, NC was also significantly associated with uncontrolled DM (OR: 1.10; 95% CI: 1.03–1.17; p < 0.01). Model 2, which incorporated additional adjustments for SBP, DBP, eGFR, and total cholesterol, confirmed the significance of these associations. NC remained significantly associated with hyperglycemia in non-diabetic individuals (OR: 1.09; 95% CI: 1.05–1.13; p < 0.01) and those with uncontrolled DM (OR: 1.11; 95% CI: 1.04–1.18; p < 0.01). We also evaluated the relationship between NC and DM prevalence (Table 3). The results demonstrated that increased NC was significantly associated with DM prevalence in the crude model (OR: 1.15; 95% CI: 1.13–1.17; p < 0.01). This association persisted after adjustments in both Model 1 and Model 2, with NC remaining a significant predictor of DM prevalence (Model 1: OR: 1.19; 95% CI: 1.15–1.24; p < 0.01; Model 2: OR: 1.23; 95% CI: 1.18–1.28; p < 0.01).

Table 2.
Association between neck circumference and hyperglycemia and uncontrolled DM.

Table 3.
Association between neck circumference and DM.
3.3. Subgroup Analysis of NC and Both Hyperglycemia and Uncontrolled DM Association
We next performed subgroup analyses to evaluate whether the association between NC and hyperglycemia in non-diabetic individuals, as well as those with uncontrolled DM, was consistent across different populations (Figure 2 and Figure 3). In non-diabetic individuals (Figure 2), NC was significantly associated with hyperglycemia in both males and females in the crude model (male: OR 1.13; 95% CI: 1.10–1.16; female: OR 1.22; 95% CI: 1.19–1.26). These associations remained significant after adjustment (Model 2: male: OR 1.07; 95% CI: 1.03–1.12; female: OR 1.12; 95% CI: 1.07–1.17). When stratified by age, the association between NC and hyperglycemia was observed in both younger and older non-diabetic participants in the crude model and remained significant after adjustment for multiple confounders (Figure 2). Among non-diabetic individuals with metabolic syndrome (MetS), NC was significantly associated with hyperglycemia in both crude (OR 1.05; 95% CI: 1.03–1.07) and adjusted models (Model 1: OR 1.06; 95% CI: 1.01–1.12; Model 2: OR 1.06; 95% CI: 1.01–1.11). In contrast, among non-DM individuals without MetS, NC was not significantly associated with hyperglycemia in adjusted models (Model 1: OR 1.04, 95% CI: 1.00–1.09; Model 2: OR 1.05, 95% CI: 1.00–1.10).
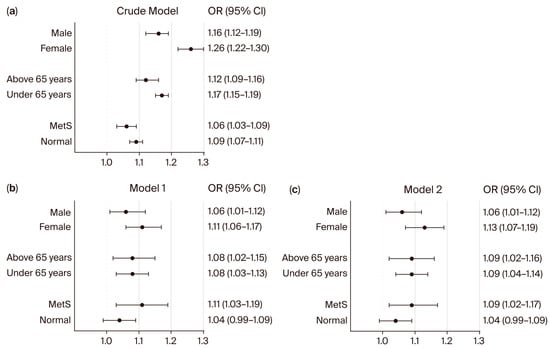
Figure 3.
Subgroup analysis for the association between neck circumference and patients with uncontrolled DM. Forest plots show the odds ratios (ORs) for NC and patients with uncontrolled DM in univariate (a) and multivariate (b,c) logistic regression analyses. ORs and 95% confidence intervals (CIs) were calculated using univariate and multivariate logistic regression models. Model 1 was adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, HTN, CKD, and dyslipidemia. Model 2 was adjusted for sex, age, BMI, WC, smoking status, alcohol consumption, regular exercise, SBP, DBP, eGFR, and total cholesterol. Abbreviation: MetS: metabolic syndrome.
In DM patients (Figure 3), NC was also significantly associated with uncontrolled diabetes in both males (OR 1.16; 95% CI: 1.12–1.19) and females (OR 1.26; 95% CI: 1.22–1.30) in the crude model, with persistent associations in adjusted models (Model 2: male: OR 1.06; 95% CI: 1.01–1.12; female: OR 1.13; 95% CI: 1.07–1.19). For age groups among DM patients, significant associations were observed in both patients ≥65 years (crude OR: 1.12; 95% CI: 1.09–1.16; Model 2: OR 1.09; 95% CI: 1.02–1.16) and <65 years (crude OR: 1.17; 95% CI: 1.15–1.19; Model 2: OR 1.09; 95% CI: 1.04–1.14). Among DM patients with MetS, the association between NC and uncontrolled DM remained significant (crude OR: 1.06; 95% CI: 1.03–1.09; Model 1: OR 1.11; 95% CI: 1.03–1.19; Model 2: OR 1.09; 95% CI: 1.02–1.17). However, in DM patients without MetS, NC was not significantly associated with uncontrolled diabetes in adjusted models (Model 1: OR 1.04; 95% CI: 0.99–1.09; Model 2: OR 1.04; 95% CI: 0.99–1.09).
4. Discussion
In this study, using a nationwide, cross-sectional, population-based dataset, we demonstrated that NC is a significant risk factor for hyperglycemia in the general population, as well as for participants with uncontrolled DM. The associations remained even after adjusting for several confounding factors, suggesting that NC could serve as an independent anthropometric marker for identifying individuals at risk of metabolic dysregulation.
The global prevalence of DM has significantly increased, posing a major public health burden [,,]. This trend is observed across various regions and demographic groups and is driven by factors such as lifestyle changes, urbanization, aging populations, and dietary transitions [,,]. Maintaining optimal glycemic control is critical for preventing diabetes-related complications [], including microvascular conditions such as diabetic retinopathy and nephropathy, and macrovascular complications such as CVDs [,,,].
Glycemic control in individuals with diabetes is typically assessed using fasting glucose and A1c levels [,,]. A1c is a well-established marker of long-term glycemic control, and fasting glucose provides insight into short-term glucose regulation; both require laboratory testing, making them less accessible in resource-limited settings [,]. However, the accuracy of HbA1c values can be significantly compromised in patients with anemia, CKD, and vitamin B12 and folate deficiencies [,,,]. These conditions alter hemoglobin metabolism, leading to misleading HbA1c results due to changes in red blood cell lifespan [,]. Additionally, these tests involve blood collection, specialized equipment, and associated costs, which may not be feasible for large-scale screening of certain populations. In contrast, NC is a simple, non-invasive, and cost-effective anthropometric measure that can be easily obtained without the need for laboratory testing. Its strong association with hyperglycemia and metabolic dysfunction suggests that NC could serve as an alternative screening tool for identifying individuals at risk of poor glycemic control, particularly in primary care settings where routine A1c testing may not be available.
Our findings suggest that NC could be a valuable tool for the early identification and management of hyperglycemia, potentially reducing the progression of DM and its associated complications. Previous studies have identified NC as an important anthropometric index associated with obesity, MetS, DM, CVDs, and CKD [,,,]. In addition, NC has been associated with markers of insulin resistance, such as the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) []. Consistently, our study found that increased NC was associated with DM prevalence in both the crude and adjusted models (Table 3). Baseline characteristics (Table 1) revealed that individuals with hyperglycemia in the non-DM group were older, whereas those with uncontrolled DM in the DM group were relatively younger. Additionally, both hyperglycemic individuals in the non-DM group and those with uncontrolled DM were more likely to be smokers and alcohol drinkers, and had higher BMI and WC compared to individuals with normal glucose levels in the non-DM group and those with controlled diabetes in the DM group. However, despite these confounders, our adjusted models demonstrated that NC remained significantly associated with hyperglycemia in individuals without DM and with those with uncontrolled diabetes. To validate these associations further, we used two adjustment models. Model 1 included categorical confounding variables such as the prevalence of HTN, CKD, and dyslipidemia, whereas Model 2 incorporated continuous confounding variables, including SBP, DBP, eGFR, and total cholesterol. The consistent association between NC and hyperglycemia across both models highlights the potential of NC as an independent anthropometric marker of metabolic risk.
Subgroup analysis revealed that the association between NC and both hyperglycemia in non-diabetic individuals and those with uncontrolled DM was significant only among participants with MetS (Figure 2 and Figure 3). In individuals without MetS, the association between NC and glycemic outcomes was not statistically significant after adjustment, suggesting that the predictive value of NC may be particularly relevant in individuals with an unhealthy metabolic profile. These findings support the hypothesis that NC reflects upper-body adiposity and its associated metabolic burden, which is more pronounced in individuals with MetS. This also underscores the importance of considering overall metabolic context when evaluating the clinical relevance of anthropometric markers such as NC. While traditional anthropometric measures such as BMI and WC remain important tools in clinical assessment, NC has emerged as a promising predictor of metabolic disorders, offering unique insights into upper-body fat distribution [,]. This anatomical location is particularly relevant because upper body adiposity plays a crucial role in the pathophysiology of insulin resistance and systemic inflammation [,]. The metabolically active fat in this region secretes proinflammatory cytokines such as IL-6 and adipokines, which impair glucose metabolism and elevate cardiovascular risk by promoting hepatic gluconeogenesis, dyslipidemia, and endothelial dysfunction [,]. NS is also a recognized risk factor for obstructive sleep apnea, which contributes to insulin resistance and systemic inflammation via intermittent hypoxia-induced oxidative stress and sympathetic activation [,].
However, this study had several limitations. First, its cross-sectional design inherently limited its ability to establish causality between NC and uncontrolled DM. While our findings demonstrate strong associations, longitudinal research is necessary to determine whether changes in NC over time can effectively predict the diabetes control status. Second, despite our comprehensive adjustment for confounding variables, the potential influence of unmeasured factors, such as dietary patterns and family history, cannot be completely eliminated. Finally, NC was assessed at a single time point, and variations in NC over time were not examined, which may have affected its long-term predictive value. Despite these limitations, this study had several strengths. One of its key strengths is the use of a large nationwide population-based dataset, which allows for robust subgroup analysis and enhances the generalizability of the findings. Comprehensive adjustments for confounding variables, including BMI, WC, and other metabolic risk factors, further strengthened the validity of the results. Additionally, this study provides valuable insights into the differential associations between NC and hyperglycemia across sex, age, and MetS subgroups.
5. Conclusions
Our findings highlight the clinical significance of NC as an independent predictor of hyperglycemia in individuals without DM and those with uncontrolled diabetes. Given its strong association with metabolic risk factors, NC has the potential to be a simple yet effective screening tool for identifying individuals at an increased risk of glycemic dysregulation and diabetes. Future research should focus on longitudinal studies to establish a temporal relationship between changes in NC and metabolic outcomes. Additionally, further investigations of population-specific NC thresholds and the potential impact of targeted interventions aimed at reducing neck adiposity may enhance its clinical utility. The integration of NC measurements into routine clinical practice may improve early detection and risk stratification, ultimately contributing to improved diabetes prevention and management strategies.
Author Contributions
Conceptualization: M.S. and Y.Y.; formal analysis: Y.Y., H.S., W.Y., J.H.K., H.L.K., B.C.S. and M.S.; writing—original draft preparation: Y.Y., H.S. and W.Y.; writing—review and editing: M.S.; visualization: M.S.; supervision: M.S.; funding acquisition: Y.Y. and J.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by research funds from Chosun University Hospital, 2023-21. This research was supported by the Global-Learning & Academic research institution for Master’s/PhD students, and Postdocs (LAMP) Program of the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (No. RS-2023-00285353).
Institutional Review Board Statement
All participants provided informed consent for their voluntary involvement, and the KNHANES was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention. The study protocol was approved by the Institutional Review Board of Chosun University Hospital and was in compliance with the Declaration of Helsinki (2024-09-028).
Informed Consent Statement
All individuals participating in the KNHANES study provided informed consent.
Data Availability Statement
The information was obtained from the Korea National Health and Nutrition Examination Survey (KNHANES), organized by the Korea Centers for Disease Control and Prevention (KCDCP), and can be freely obtained from the KCDCP website (https://knhanes.cdc.go.kr accessed on 29 March 2025).
Acknowledgments
We thank you for the MDPI English and figure editing service.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DM | Diabetes mellitus |
| CVD | Cardiovascular disease |
| BMI | Body mass index |
| WC | Waist circumference |
| NC | Neck circumference |
| MetS | Metabolic syndrome |
| CKD | Chronic kidney disease |
| HTN | Hypertension |
References
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Jarcho, J.A. Increase in the incidence of diabetes and its implications. N. Engl. J. Med. 2017, 376, 1473–1474. [Google Scholar] [CrossRef]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Cannon, A.; Handelsman, Y.; Heile, M.; Shannon, M. Burden of illness in type 2 diabetes mellitus. J. Manag. Care Spec. Pharm. 2018, 24, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, B.K.; Srivastava, A.K. Diabetes mellitus: Complications and therapeutics. Med. Sci. Monit. 2006, 12, 130–147. [Google Scholar]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Rao Kondapally Seshasai, S.; Kaptoge, S.; Thompson, A.; Di Angelantonio, E.; Gao, P.; Sarwar, N.; Whincup, P.H.; Mukamal, K.J.; Gillum, R.F.; Holme, I.; et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011, 364, 829–841. [Google Scholar]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef]
- American Diabetes Association. 6. Glycemic targets: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.; Mikolajczyk, T. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Evans, J.L.; Maddux, B.A.; Goldfine, I.D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 2005, 7, 1040–1052. [Google Scholar] [CrossRef]
- Głuszek, S.; Ciesla, E.; Głuszek-Osuch, M.; Kozieł, D.; Kiebzak, W.; Wypchło, Ł.; Suliga, E. Anthropometric indices and cut-off points in the diagnosis of metabolic disorders. PLoS ONE 2020, 15, e0235121. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, R.; Li, S.; Cai, R.; Ni, F.; Zheng, H.; Hu, R.; Sun, T. Association between four anthropometric indexes and metabolic syndrome in US adults. Front. Endocrinol. 2022, 13, 889785. [Google Scholar] [CrossRef]
- Ramesh, N.; Kumar, P.; Sweta, S.; Prasad, A.; Tiwari, L.K. Correlation of anthropometric measurements with body mass index and estimation of the proportion of metabolic syndrome among overweight and obese children: A hospital-based cross-sectional study. BMJ Paediatr. Open 2024, 8, e002354. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Surkan, P.; Azadbakht, L. The association of neck circumference with risk of metabolic syndrome and its components in adults: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 657–674. [Google Scholar] [CrossRef]
- Onat, A.; Hergenç, G.; Yüksel, H.; Can, G.; Ayhan, E.; Kaya, Z.; Dursunoğlu, D. Neck circumference as a measure of central obesity: Associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin. Nutr. 2009, 28, 46–51. [Google Scholar] [CrossRef]
- Yang, G.-R.; Yuan, S.-Y.; Fu, H.-J.; Wan, G.; Zhu, L.-X.; Bu, X.-L.; Zhang, J.-D.; Du, X.-P.; Li, Y.-L.; Ji, Y. Neck circumference positively related with central obesity, overweight, and metabolic syndrome in Chinese subjects with type 2 diabetes: Beijing Community Diabetes Study 4. Diabetes Care 2010, 33, 2465–2467. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Shahdadian, F.; Moradi, S.; Ghavami, A.; Mohammadi, H.; Rouhani, M.H. Neck circumference in relation to glycemic parameters: A systematic review and meta-analysis of observational studies. Diabetol. Metab. Syndr. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Cho, N.H.; Oh, T.J.; Kim, K.M.; Choi, S.H.; Lee, J.H.; Park, K.S.; Jang, H.C.; Kim, J.Y.; Lee, H.K.; Lim, S. Neck circumference and incidence of diabetes mellitus over 10 years in the Korean Genome and Epidemiology Study (KoGES). Sci. Rep. 2015, 5, 18565. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Y.; Xiang, Q.; Fang, S.; Chen, Y.; Chen, C.; Zhang, W.; Zhang, H.; Xia, F.; Wang, N. Associations between abdominal obesity indices and diabetic complications: Chinese visceral adiposity index and neck circumference. Cardiovasc. Diabetol. 2020, 19, 1–12. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 6. Glycemic targets: Standards of medical care in diabetes—2022. Diabetes Care 2022, 45, S83–S96. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhao, L.; Chen, C.; Ren, Z.; Jing, Y.; Qiu, J.; Liu, D. National burden and risk factors of diabetes mellitus in China from 1990 to 2021: Results from the Global Burden of Disease study 2021. J. Diabetes 2024, 16, e70012. [Google Scholar] [CrossRef]
- He, K.-J.; Wang, H.; Xu, J.; Gong, G.; Liu, X.; Guan, H. Global burden of type 2 diabetes mellitus from 1990 to 2021, with projections of prevalence to 2044: A systematic analysis across SDI levels for the global burden of disease study 2021. Front. Endocrinol. 2024, 15, 1501690. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Animaw, W.; Seyoum, Y. Increasing prevalence of diabetes mellitus in a developing country and its related factors. PLoS ONE 2017, 12, e0187670. [Google Scholar] [CrossRef]
- Nordwall, M.; Arnqvist, H.J.; Bojestig, M.; Ludvigsson, J. Good glycemic control remains crucial in prevention of late diabetic complications–the Linköping Diabetes Complications Study. Pediatr. Diabetes 2009, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.E.; Bornfeldt, K.E. Impact of diabetes mellitus. Arter. Thromb. Vasc. Biol. 2016, 36, 1049–1053. [Google Scholar] [CrossRef]
- Dal Canto, E.; Ceriello, A.; Rydén, L.; Ferrini, M.; Hansen, T.B.; Schnell, O.; Standl, E.; Beulens, J.W. Diabetes as a cardiovascular risk factor: An overview of global trends of macro and micro vascular complications. Eur. J. Prev. Cardiol. 2019, 26, 25–32. [Google Scholar] [CrossRef]
- Mishra, B.K.; Raghav, A.; Jeong, G.-B.; Jain, M.; Shukla, P.; Sharma, S. Estimation of HbA1c and Impact of Continuous Glucose Monitoring in Hypoglycemic States. Glucose Insul. Homeost. 2024, 63. [Google Scholar] [CrossRef]
- Jovanovic, L.; Peterson, C.M. The clinical utility of glycosylated hemoglobin. Am. J. Med. 1981, 70, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.S. Pitfalls in hemoglobin A1c measurement: When results may be misleading. J. Gen. Intern. Med. 2014, 29, 388–394. [Google Scholar] [CrossRef]
- Inoue, K.; Goto, A.; Kishimoto, M.; Tsujimoto, T.; Yamamoto-Honda, R.; Noto, H.; Kajio, H.; Terauchi, Y.; Noda, M. Possible discrepancy of HbA1c values and its assessment among patients with chronic renal failure, hemodialysis and other diseases. Clin. Exp. Nephrol. 2015, 19, 1179–1183. [Google Scholar] [CrossRef]
- Gram-Hansen, P.; Eriksen, J.; Mourits-Andersen, T.; Olesen, L. Glycosylated haemoglobin (HbA1c) in iron-and vitamin B12 deficiency. J. Intern. Med. 1990, 227, 133–136. [Google Scholar] [CrossRef]
- Alzahrani, B.A.; Salamatullah, H.K.; Alsharm, F.S.; Baljoon, J.M.; Abukhodair, A.O.; Ahmed, M.E.; Malaikah, H.; Radi, S. The effect of different types of anemia on HbA1c levels in non-diabetics. BMC Endocr. Disord. 2023, 23, 24. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, Y.-m.; Lee, S.; Shin, B.-C.; Kim, H.-L.; Chung, J.-H.; Son, M. Association between Neck Circumference and Chronic Kidney Disease in Korean Adults in the 2019–2021 Korea National Health and Nutrition Examination Survey. Nutrients 2023, 15, 5039. [Google Scholar] [CrossRef] [PubMed]
- Aswathappa, J.; Garg, S.; Kutty, K.; Shankar, V. Neck circumference as an anthropometric measure of obesity in diabetics. N. Am. J. Med. Sci. 2013, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Díaz, D.A.; Lera, L.; Márquez, C.; Valenzuela, A.; Saguez, R.; Weisstaub, G.; Albala, C. Neck Circumference Cut-Off Points for Identifying Adiposity: Association with Chronic Metabolic Diseases in Older People. J. Pers. Med. 2024, 14, 710. [Google Scholar] [CrossRef]
- Laurindo, L.F.; Cruz Camargo, F.; Perfeito, A.; Benedito Ciano, B.; Tainá Coelho, C.; Assis Apolinário, G.; do Nascimento Vicentin, I.; Cambui Andreasi, J.; Leme Boaro, B.; José Tofano, R. Exploring the Associations of Neck Circumference, Blood Pressure, CRP, and Insulin Resistance on the Visceral Adiposity Index: Insights from a Cross-Sectional Study. Endocrines 2024, 5, 538–546. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Moon, H.-R.; Yun, J.-M. Neck circumference as a predictor of metabolic syndrome in koreans: A cross-sectional study. Nutrients 2021, 13, 3029. [Google Scholar] [CrossRef]
- Son, D.-H.; Han, J.H.; Lee, J.-H. Neck Circumference as a Predictor of Insulin Resistance in People with Non-alcoholic Fatty Liver Disease. J. Obes. Metab. Syndr. 2023, 32, 214. [Google Scholar] [CrossRef]
- Jensen, M.D. Role of body fat distribution and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 2008, 93, s57–s63. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Krishnaswami, S.; Harris, T.B.; Katsiaras, A.; Kritchevsky, S.B.; Simonsick, E.M.; Nevitt, M.; Holvoet, P.; Newman, A.B. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch. Intern. Med. 2005, 165, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Williams, C.; Vega, G.L. Upper body fat predicts metabolic syndrome similarly in men and women. Eur. J. Clin. Investig. 2018, 48, e12941. [Google Scholar] [CrossRef] [PubMed]
- Ahbab, S.; Ataoğlu, H.E.; Tuna, M.; Karasulu, L.; Çetin, F.; Temiz, L.Ü.; Yenigün, M. Neck circumference, metabolic syndrome and obstructive sleep apnea syndrome; evaluation of possible linkage. Med. Sci. Monit. 2013, 19, 111. [Google Scholar] [PubMed]
- Kim, S.E.; Park, B.S.; Park, S.H.; Shin, K.J.; Ha, S.Y.; Park, J.; Park, K.M. Predictors for presence and severity of obstructive sleep apnea in snoring patients: Significance of neck circumference. Sleep Med. 2015, 12, 34–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).