Abstract
Almost three million individuals suffer from multiple sclerosis (MS) throughout the world, a demyelinating disease in the nervous system with increased prevalence over the last five decades, and is now being recognized as one significant etiology of cognitive loss and dementia. Presently, disease modifying therapies can limit the rate of relapse and potentially reduce brain volume loss in patients with MS, but unfortunately cannot prevent disease progression or the onset of cognitive disability. Innovative strategies are therefore required to address areas of inflammation, immune cell activation, and cell survival that involve novel pathways of programmed cell death, mammalian forkhead transcription factors (FoxOs), the mechanistic target of rapamycin (mTOR), AMP activated protein kinase (AMPK), the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), and associated pathways with the apolipoprotein E (APOE-ε4) gene and severe acute respiratory syndrome coronavirus (SARS-CoV-2). These pathways are intertwined at multiple levels and can involve metabolic oversight with cellular metabolism dependent upon nicotinamide adenine dinucleotide (NAD+). Insight into the mechanisms of these pathways can provide new avenues of discovery for the therapeutic treatment of dementia and loss in cognition that occurs during MS.
Keywords:
APOE-ε4; apoptosis; autophagy; COVID-19; dementia; FoxO; mTOR; multiple sclerosis; nicotinamide; SIRT1 1. Increased Lifespan, Aging, Cellular Senescence, and Nervous System Disorders
Life expectancy is increasing throughout the globe, and individual lifespan is expected to reach 80 years of age [1,2,3,4,5,6]. There also is a one percent decrease in the age-adjusted death rate from the years 2000 through 2011 [7], and the number of individuals over the age of 65 has doubled during the last 50 years [8]. In large developing countries, such as China and India, the elderly population is expected to increase from five to ten percent in the coming years [9,10]. The observed increase in lifespan for individuals has several components that have resulted from measures focused on improving sanitation and reducing infection, allowing greater access to higher quality healthcare, the incorporation of early diagnostic and preventive measures, and quickly identifying individuals susceptible to both acute and chronic illnesses [5,11,12,13,14,15,16,17,18,19,20,21,22].
With the rise in longevity and lifespan, non-communicable diseases (NCDs) have increased in prevalence as well [2,5,23,24,25,26]. NCDs lead to seventy to eighty percent of annual deaths; that equals greater than forty million individuals dying each year [27,28]. Of the individuals affected by NCDs, fifteen million people at minimum are in the age range between thirty and sixty-nine years of age. With NCDs, there exists a disproportionate impact on lower-income countries [2,23,24,25]. In among ten to fifteen percent of people in high-net-worth nations, NCDs involve people that are below sixty years of age, but in low- and middle-net-worth nations, NCDs impact a larger segment of individuals that involves at least thirty-three percent of the population who are below sixty.
Nervous system diseases comprise a large portion of NCDs [2,29,30,31,32,33,34,35,36,37,38,39,40]. Neurodegenerative disorders impact more than fifteen percent of the global population and comprise greater than six hundred disease entities that can result in severe disability and death [10,37,41,42,43,44,45,46,47]. More than seven million individuals die each year from diseases of the nervous system [2,18]. A significant factor that plays a role in the onset and progression of neurodegenerative disorders is the cellular mechanisms of aging [21,40,48,49,50,51,52,53,54]. At the cellular level, telomeres (TLs), which are complexes of deoxyribonucleic acid (DNA), can affect aging, the onset of cell senescence, and the development of neurodegenerative disorders [55,56,57]. TLs reside at the end of chromosomes and can modulate cell replication, cellular lifespan, and protection for the DNA of the genome. TLs have greater than 2000 repetitions of non-coding double-stranded DNA with the sequence “TTAGGG”, and are completed with a guanine rich single-stranded DNA [55,58]. Several protein complexes are associated with TLs, which include telosome, shelterin, and CTC1-STN1-TEN1 (CST). These complexes are necessary to regulate the activity of TLs and provide stability for TLs. During cellular division, the telomerase protein can become active to maintain TL length through the addition of tandem repeat ribonucleic acid (RNA) templates, since a portion of the TLs length is lost in the amount of approximately 25–200 base pairs [59,60,61,62]. If telomerase function in somatic cells becomes no longer viable or the TLs become excessively short with less than 500 base pairs, cell proliferation is blocked and cell senescence results [6,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. If cell senescence does ensue, systems of the body cannot repair themselves, which leads to advancement of aging processes. As a result, an inability to remove cells that are senescent by the immune system also may stimulate tumorigenesis [6,40,63,64,67,68,72,75,77]. The release of reactive oxygen species (ROS) during oxidative stress can occur during the shortening of TLs and the onset of cell senescence. Oxidative stress exposure ultimately affects cell survival and mitochondrial organelle function through TL shortening and the onset of cell senescence [21,52,59,68,72,74,75,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94].
These processes related to ROS, mitochondrial injury, the shortening of TLs, and cell senescence can hasten aging, and also promote the onset of neurodegenerative disorders such as dementia and Alzheimer’s disease (AD) [55,58,67,69,71,73,95]. Individuals with shorter leukocyte TL lengths can have an increased risk of dementia and AD [56]. Leukocyte TL length has been tied to observations on cerebral magnetic resonance imaging (MRI). Longer TL lengths may be protective against dementia and nervous system injury due to increased hippocampal grey matter volumes, lower volumes of white matter hyperintensities, and lower presences of basal ganglia iron on MRI under these circumstances [57].
2. Cognitive Impairment in Neurodegeneration
All populations in all nations are affected by dementia, and it is the 7th leading cause of death per the World Health Organization [28]. Dementia is considered to be significantly underdiagnosed, with delays in recognition of cognitive loss in individuals. Evaluation and treatment of impaired cognition may not start until one or two years later following the performance of a correct investigation [96,97]. If one considers disorders such as AD, familial cases of AD (FAD) affect a much smaller proportion of the population. FAD is an autosomal dominant form of a mutated amyloid precursor protein (APP) gene, and occurs in approximately 200 families worldwide [71,95,98,99,100,101,102]. FAD occurs most often prior to age 55, and results from variable single-gene mutations on chromosomes 1, 14, and 21 [23,103,104]. In contrast, more than ten percent of the global population over the age of 65 is affected with the sporadic form of AD; the ε4 allele of the apolipoprotein E (APOE) gene results in heightened risk, and at least 60 percent of individuals with dementia have AD [73,98,105,106,107,108,109]. In the United States (US) alone, greater than 6 million individuals have AD, and an additional 4 million individuals are under treatment at an annual cost of 4 billion United States dollars (USD) [86,97,110,111,112]. As age and lifespan increases in the population, it is expected that the number of individuals with AD will increase to 30 million individuals over the next 15 to 20 years [69,105,113]. At present, fifty million people in the world, or five percent of the global population, have dementia [24,31,73,95,98,106,108,114]. When one considers the year 2030, 82 million people will endure disability from cognitive loss. In the year 2050, more than 155 million individuals will suffer from dementia [10,22,23,24].
In regard to the cost to care for cognitive impairment, dementia care has a cost factor of greater than $800 billion USD a year [28]. Social services and medical needs in the year 2030 will require 2 trillion USD every year in the US alone, as currently greater than 4 million individuals require resources of 3.8 billion USD total every year for care. Although possibly underestimated, it is predicted that market needs for AD will exceed 11 billion USD [18,105,115]. Additional expenses also come under consideration, such as the need to employ close to 70 million social care individuals and health care staff for the essential need of companion and adult behavior care, as well as outreach systems to address social needs [27,28,116]. Furthermore, in addition to AD, other neurodegenerative disorders may also have a complicated presentation with cognitive loss. Parkinson’s disease (PD) is ranked as a second nervous system disease that leads to dementia when compared to AD. PD is a movement disorder that leads to resting tremors, rigidity, and bradykinesia, and is characterized by the loss of dopaminergic neurons in the substantia nigra [22,29,69,111,117,118,119,120,121,122]. More than 10 million individuals suffer from PD in the world, which includes 50,000 new cases annually in the US, and many suffer from cognitive loss [117,123]. The number of individuals with PD is expected to double by the year 2030 [10,18,124,125]. More than $52 billion USD are spent in the US alone per year, with an annual cost per patient that approaches approximately $25,000 US dollars per year.
3. Cognitive Loss and Dementia That Can Occur in Multiple Sclerosis
Interestingly, individuals with the neurodegenerative disorder multiple sclerosis (MS) also are significantly impacted by cognitive loss and dementia (Figure 1). Demyelinating disorders, such as MS, affect a significant proportion of the world’s population [38,126,127,128,129,130,131,132,133,134,135] (Table 1). MS is considered to be the most common demyelinating disorder that affects the immune system and the central nervous system through the function of myelin-producing cells [117,127,134,136,137,138,139,140]. At least 2.5 million individuals suffer from MS around the world, and a continual increase in the prevalence of MS has occurred over the prior five decades. Women are affected more than men by MS [133].
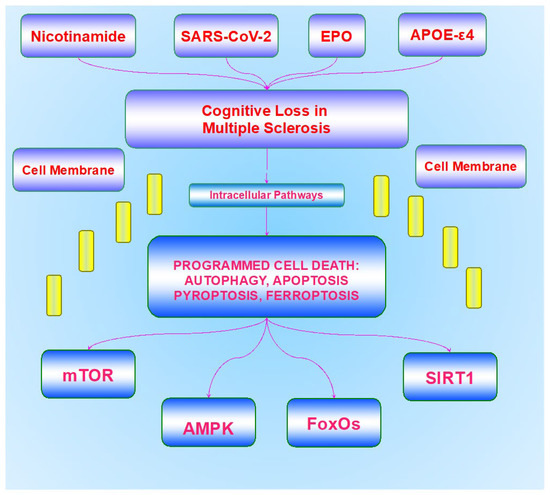
Figure 1.
Innovative pathways for the treatment of cognitive loss in multiple sclerosis. A number of new strategies can address multiple areas of cognitive loss and disability for multiple sclerosis that involve intracellular pathways inside the cell membrane (yellow bars), consisting of programmed cell death with autophagy, apoptosis, pyroptosis, and ferroptosis, mammalian forkhead transcription factors (FoxOs), the mechanistic target of rapamycin (mTOR), AMP activated protein kinase (AMPK), and the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1). External extracellular modulators outside of the cell membrane involve nicotinamide adenine dinucleotide (NAD+) through nicotinamide, trophic factor exposure such as with erythropoietin (EPO), and associated risk factor exposure with the apolipoprotein E-ε4 (APOE-ε4) gene and severe acute respiratory syndrome coronavirus (SARS-CoV-2).

Table 1.
Highlights.
While cognitive loss has not been previously considered as a primary disability in the MS patient group, it is increasingly becoming recognized. Early descriptions by Charcot noted that patients with MS were slow in formulation of their thoughts and had difficulty retrieving their memories [141]. Observations of cognitive loss have become more common and refined over the years. In some patient cohorts of MS that included both men and women, the prominent clinical phenotype was characterized by severe cognitive impairment that was progressive in nature [142]. In other recent studies, cognitive impairment may not be so severe, but definitely present with word finding difficulty and visual object naming [143]. The reduction in formation processing speed that is observed with cognitive loss in MS can be independent of mood disorders, and impairments in visual object meaning perception may appear uniquely in individuals with MS [143]. It is estimated that loss of cognitive function in MS occurs in approximately sixty-five percent of individuals and can affect processing of information, attention, memory recall, and other executive functions [144]. With cognitive loss in MS, cortical atrophy loss and abnormal cortical integrity occurs with cognitive behavior therapy programs, exercise treatment, and education programs still at an infancy stage to treat memory loss in MS [144]. Treatments based on LINGO-1 (LRR and Ig domain containing NOGO receptor interacting protein) antagonism and protein kinase B (Akt) activation in experimental models of cognitive loss in MS may assist to maintain memory function through the promotion of oligodendrocyte differentiation and myelination [145]. Mood disorders that include depression, apathy, and anxiety can accompany the cognitive loss in MS similar to presentations seen in AD [99,146,147,148,149,150,151]. The cognitive impairment in MS patients also may play a significant role in preventing individuals from returning to prior levels of functioning and the workforce [152]. Virus exposure also may contribute to neurodegenerative disorders such as AD and MS. In the case of MS, Epstein-Barr virus has an increased risk of leading to MS and cognitive loss that may occur up to 15 years after initial infection [43].
Risk factors for this cognitive loss in MS may share common ground with those of AD (Figure 1). For example, heightened risk of late-onset AD is present in conjunction with the ε4 allele of the apolipoprotein E (APOE-ε4) gene [105,153,154,155]. If someone has two ε4 alleles, they could potentially have a 20 times greater risk of suffering from AD compared to individuals without two ε4 alleles. The isoform APOE-ε4 cannot destroy β-amyloid (Aβ) in the brain, which may lead to an increased risk for the development of AD [153,154,156,157,158,159]. However, APOE in the body is important for cellular function. APOE originates in hepatic cells, is necessary for transporting cholesterol, triglycerides, and phospholipids in the body, as well as for regulating lipid homeostasis [160,161]. APOE in the central nervous system begins its production in astrocytes and facilitates the transfer of cholesterol to neuronal cells that are dependent upon APOE receptors [105,156]. APOE, at times, can destroy cerebral Aβ through apoptotic cellular pathways. Phosphatidylserine (PS) membrane exposure [162,163], an initial phase in apoptotic cell death, may be related to Aβ aggregation. Isoforms of APOE that are exclusive of APOE-ε4 have been suggested to block Aβ aggregation through the exposure of PS membranes [158]. These observations may be relevant for MS and cognitive loss in this disorder. APOE-ε4 may be associated with the onset and progression of cognitive impairment in patients with MS. Individuals with MS and APOE-ε4 experienced delayed rates to stimuli and difficulties with cognitive function [164]. In patients with optic neuritis [165,166], a common occurrence in almost half of patents with MS. APOE serum levels were significantly higher than in control patient groups and the APOE ε3/ε3 genotype may increase the risk of developing optic neuritis in males [167]. In addition to APOE-ε4, MS may share other cellular pathways with AD that lead to cognitive loss such as Aβ [37,102,168,169,170,171,172]. In studies that examine cerebrospinal fluid biomarkers, changes in Aβ42 as seen in AD can also predict early cognitive decline in MS [173]. Furthermore, the presence of tau seeding, which can lead to AD pathology [37,98,100,102,105,174,175,176], has been observed in the brains of patients with MS [177], and tau also may contribute to impaired oligodendrocyte maturation and pathological changes that may foster demyelination [178].
4. Innovative Therapeutic Strategies for Cognitive Loss in Multiple Sclerosis
MS can lead to multiple disabilities for individuals with the onset of cognitive impairment, behavioral difficulties, blindness, loss of motor function, sensory dysfunction, and loss of coordination. Given the spectrum of the presentations for MS, it should be no surprise that a vast array of cellular mechanisms may foster the onset and progression of MS. Pathways that involve inflammatory mediators, demyelination and remyelination pathways, oxidative stress, blood-brain barrier impairment, viral antigens, and cellular metabolism, which are dependent upon nicotinamide adenine dinucleotide (NAD+), have been tied to the underlying pathology of MS [10,29,31,33,39,43,72,77,85,86,99,100,115,162,171,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198]. Although new treatments that address MS, known as disease modifying therapies (DMTs), can limit the rate of relapse in patients with relapsing–remitting MS, DMTs cannot prevent disease progression. In addition, brain volume loss may be another component of dementia and further disability that may be independent of disease activity. Early initiation of DMTs may slow the progression of brain volume loss, but cognitive disability may continue to ensue [199]. In light of these considerations to address both disease onset and progression in MS, novel pathways of discovery are required (Table 1). Innovative avenues that can address underlying cellular disease mechanisms may provide fruitful options for future clinical care that involve programmed cell death pathways, mammalian forkhead transcription factors (FoxOs), the mechanistic target of rapamycin (mTOR), AMP activated protein kinase (AMPK), the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), and associated pathways with the apolipoprotein E (APOE-ε4) gene and severe acute respiratory syndrome coronavirus (SARS-CoV-2) (Figure 1).
5. Autophagy, Apoptosis, Pyroptosis, and Ferroptosis Involvement in Multiple Sclerosis
Recent studies have identified a significant role for pathways of programmed cell death that can ultimately control cell survival and cognitive impairment during MS [46,61,128,130,132,134,200,201,202] (Table 1). Programmed cell death pathways that involve autophagy, apoptosis, pyroptosis, and ferroptosis can influence cell survival during inflammation [34,39,66,73,74,85,87,203,204,205,206,207,208,209,210,211,212,213], oxidative stress [10,45,77,86,89,93,119,185,214,215,216,217,218,219], ischemia [219,220,221,222,223], and mitochondrial dysfunction [32,83,85,115,198,224,225,226,227,228,229,230,231,232] (Figure 1). Disorders of cellular metabolism that can lead to cognitive loss and other impairments, such as diabetes mellitus (DM), are also intimately tied to pathways of programmed cell death [24,72,115,208,214,216,219,224,233,234,235,236,237,238,239,240,241].
In general, autophagy consists of the recycling of cytoplasmic organelles and proteins that can lead to the remodeling and formation of new tissue structures [34,44,61,100,169,202,242,243,244,245]. Although the process of macroautophagy is usually described, other subsets of autophagy exist that include microautophagy and chaperone-mediated autophagy. Macroautophagy refers to the recycling of organelles by forming autophagosomes consisting of cytoplasmic proteins and organelles that can be combined with lysosomes for eventual degradation, which are then used during recycling processes [71,73,97,107,197,246]. Microautophagy employs the invagination of lysosomal membranes to sequester and consume components of the cell cytoplasm [105,229]. In relation to chaperone-mediated autophagy, this form of autophagy utilizes cytosolic chaperones that oversee the transportation of cytoplasmic structures across lysosomal membranes [10,124,198,246,247,248,249].
During MS, autophagy may have an important role in the regulation of oligodendrocyte development, myelination, and activation of microglia [130,134,201]. Autophagy in demyelinating diseases also has a vital role during oxidative stress and ROS generation [198,202,250,251,252,253,254]. The induction of ROS can result in alterations in mitochondrial function [2,79,85,87,255] that may also impact cognitive loss. In addition, during infections, the autophagy-lysosome pathway can increase inflammatory reactions. An example of this is the severe acute respiratory syndrome coronavirus (SARS-CoV-2) [85,110,115,169,239,251,256,257]. Recent work has shown that exposure to infectious agents, such as SARS-CoV-2 as part of coronavirus disease 2019 (COVID-19), may lead to increased death rates in patients with MS [258].
With the activation of apoptosis, myelin injury, recovery, and cell death can be affected in MS. This may be mediated through the generation of ROS and oxidative stress that leads to mitochondrial dysfunction, demyelination, and neuronal axonal loss [21,53,87,198,200,232,241,259]. Apoptosis is a cell death pathway that consists of an early phase and a later phase [22,45,61,202,260]. During the early phase, phosphatidylserine (PS) membrane asymmetry loss occurs on the plasma membrane [261,262,263,264,265]. With the loss of membrane PS asymmetry during cell injury, inflammatory cells that reside in the nervous system, such as microglia, are attracted to injured cells and can remove them from the nervous system, resulting in nervous system dysfunction [261,266,267,268,269]. However, the loss of membrane PS asymmetry is reversible, and if membrane asymmetry is restored, injured cells are then given the ability to recover, and not be engulfed by inflammatory cells [197,270,271,272]. With the later phase that involves deoxyribonucleic acid (DNA) degradation in the cell [34,40,77,226,273,274,275], caspase activation plays a prominent role [15,77,194,206,210,273,276,277,278]. This process of caspase activation and DNA destruction is irreversible [259]. Limiting apoptotic cell death may minimize memory loss and cognition during both acute and chronic insults [34,93,96,169,172,185,276,279]. This may occur during measures that reduce inflammatory pathways and affect neuronal and oligodendrocyte survival [9,108,132,187,252,276,279,280,281,282], as well as cognitive performance [25,283,284,285].
Pyroptosis also may have an important function in MS and the treatment of patients. Increased inflammasome expression has been associated with potential treatment failures in MS patients [286], as well as the increased inflammatory activity of cytokine release and immune cell activity in experimental models [134]. Pyroptosis is a programmed cell death pathway that can oversee inflammatory cell activation in the nervous system [34,66,169,212,213]. Pyroptosis is initiated with the production of a supramolecular complex, known as the pyroptosome or the inflammasome. It should be noted that inflammasomes are cytosolic oligomers of multiple proteins, and the inflammasome family with NLRs (nucleotide-binding oligomerization domain and leucine-rich repeat-containing receptors) contains NLRP1, NLRP3, NLRP6, and NLRC4. Inflammasome activation can occur through pattern recognition receptors responding to the damage-associated molecular pattern (DAMP) of host cells or pathogen-associated molecular pattern (PAMP) in the microbial family [34,66,134,185,212,213,286,287,288,289,290]. In pyroptosis, the inflammasome is responsible for caspase 5, caspase 4, and caspase 1 activation. Pyroptosis also results in plasma membrane permeabilization involving protein family members of gasdermin. Gasdermin proteins consist of an N-terminal domain with intrinsic pore-forming properties and a C-terminal domain that inhibit N-terminal domain pore formation. Plasma pores result from fragmentation of the N-terminal domain during breakage of the association of the N-terminal and the C-terminal domains. At this point, interleukin-1 family members and other cytokines are released through cell membranes that can either assist or obstruct cell survival, which requires gasdermin. Gasdermin proteins are necessary to generate membrane pores since family members of interleukin-1 family members are absent of peptides in the membrane to generate membrane pores [24,34,220]. Once the cell membranes are open, DAMP entities involving DNA and adenosine triphosphate (ATP) are released. In the canonical inflammasome path, DAMPs activate the NLR family pyrin domain containing 3 (NLRP3) inflammasome. In contrast, caspase 4 and caspase 5 are activated, such as in Gram-negative bacterial infections, with noncanonical inflammasomes involving lipopolysaccharide proteins. Pyroptosis in conjunction with necroptosis and apoptosis can result in inflammation that generates elevated cytokine release and cell injury [212,291]. In the setting of ROS release and oxidative stress, pyroptosis can generate a severe inflammatory response that affects both neuronal and vascular cells, impairing cognition [5,85,86,94,106,185,292].
Ferroptosis, also an important mediator of the programmed cell death pathway, has been recently linked to MS and cognitive loss [169,293,294]. Ferroptosis results in intracellular iron accumulation and loss of glutathione homeostasis [295]. With the failure of glutathione-dependent oxidative stress defenses, ferroptosis leads to excessive lipid peroxidation and subsequent cell death. Under some conditions, it is believed that ferroptosis during MS results in the development of pathogenic T lymphocytes that impair the function of both neuronal and glial cells [296]. Ferroptosis is also involved in tumorigenesis [297] and cardiomyocyte injury as well [298].
6. Mammalian Forkhead Transcription Factors and Multiple Sclerosis
Given that mammalian forkhead transcription factors (FoxOs) can have an important relationship to cell death pathways during neurodegenerative disorders [2,5,49,259,260,299,300,301,302,303,304], they are increasingly being recognized as potential therapeutic targets for MS (Figure 1). In particular, the mammalian FOXO proteins of the O class can lead to neuronal cell death through apoptosis and autophagy activation [5,49,50,68,128,203,250,301,305,306,307,308,309,310,311,312,313,314,315]. Other studies suggest that the progressive course of MS may be associated with epigenetic changes of DNA methylation that are dependent upon genetic variations of FOXOs, such as FoxO1 and FoxO3a [128] (Table 1).
FoxOs are especially interesting since they can influence behavior and memory loss [18,203,309,316]. More than one hundred genes in the forkhead family and nineteen human subgroups have been described. They consist of FOXA to FOXS following the discovery of the Drosophila melanogaster gene forkhead. Forkhead proteins are also termed forkhead in rhabdomyosarcoma (FKHR) (FOXO1), FKHRL1 (forkhead in rhabdomyosarcoma like protein 1) (FOXO3a), the Drosophila gene fork head (fkh), Forkhead Related Activator (FREAC)-1 and -2, and the acute leukemia fusion gene located in chromosome X (AFX) (FOXO4) [231,259]. Numbers of Arabic origin are employed for the nomenclature with “Fox”, then a subclass or subgroup letter is listed, and then the member number is presented within the subclass [186,259]. Letters are capitalized for human Fox proteins. Only the initial letter is listed as uppercase for the mouse; for all other chordates, the initial and subclass letters are in uppercase [259,317,318]. Given that FoxO proteins are transcription factors, they bind to deoxyribonucleic acid (DNA) through the FoxO-recognized element in the C-terminal basic region of the forkhead DNA binding domain. In the α-helix H3 recognition region, fourteen protein-DNA contacts modulate the gene expression of targets [319]. A number of factors control DNA and forkhead interactions that involve FoxO protein phosphorylation or acetylation, FoxO protein compartmentalization in the nucleus, and alteration of electrostatic changes [259,277,306,307,320,321].
FoxO proteins are expressed throughout the body. In the nervous system, mammalian FOXO proteins of the O class that consist of FOXO1, FOXO3, FOXO4, and FOXO6 can lead to nervous system disorders [5,49,50,317,322,323]. FoxO proteins are also linked to metabolic function that can affect neurodegenerative disorders [17,24,169]. The function of FoxO proteins is conserved among multiple species, which include Caenorhabditis elegans, Drosophila melanogaster, and mammals. FoxO proteins are homologous to the transcription factor Dauer Formation-16 (DAF-16) in the worm Caenorhabditis elegans, affecting metabolic insulin signaling, cell cycle regulation, cell survival, and may also oversee lifespan extension [324,325,326]. FoxO proteins appear to have a selective expression in the nervous system, which may offer insight into the biology for specific FoxO proteins. For example, FoxO3 may affect auditory synaptic transmission [327], cerebral endothelial vascular cell survival [269,328], cerebral traumatic injury [316], cell survival during oxidative stress [53,329], and hippocampal degeneration [330,331]. FoxO6 modulates gluconeogenesis [332] and memory consolidation [322], and is present in several regions of the brain, such as the hippocampus, the amygdala, and the nucleus accumbens [333,334]. FoxO1 can have a more diverse role in gastric cancer [335], glaucoma [313], renal disease [315], astrocyte survival [336], motor and memory pathways in the striatum and sub-regions of the hippocampus [333], ischemic brain injury [337], and attenuation of Aβ accumulation and tau phosphorylation in the brain [338].
The structure of FoxOs is interesting. The forkhead box (FOX) family of genes have a butterfly-like appearance on X-ray crystallography and nuclear magnetic resonance imaging with a conserved forkhead domain (the “forkhead box”), described as a “winged helix”. Three α-helices, three β-sheets, and two loops make up the “winged helix” that appears to be unique for the forkhead family, since other winged helix domains do not fall under the Fox protein family.
FoxOs are modulated by epigenetic and post-translation protein modifications that involve phosphorylation [232,259,339], ubiquitylation [331], and acetylation [306,320,321]. Phosphorylation of FoxOs is controlled by Akt [72,340] to prevent translation to the nucleus through association with 14-3-3 proteins, inhibit gene transcription, and block apoptosis [259,312,330]. Once FoxO proteins such as FoxO3a are activated, cytochrome c release can occur with caspase-induced apoptotic death [262,341,342,343]. Akt also has a secondary regulatory mechanism that controls FoxO proteins to prevent caspase activity. Although FoxO3a is phosphorylated in the presence of oxidative stress, cleavage of FoxO3a does not occur during Akt inhibition of caspase 3 activity, preventing apoptosis [344] and the generation of “pro-apoptotic” amino-terminal (Nt) fragments following FoxO3a cleavage [345]. Akt also results in the ubiquitination and degradation of FoxOs through the 26S proteasome. In regard to acetylation, FoxOs are acetylated by histone acetyltransferases that include the CREB-binding protein (CBP), the CBP-associated factor, and p300. Once FoxOs undergo acetylation, FoxO proteins are able to transfer to the cell nucleus, but FoxO protein activity and DNA binding is somewhat inhibited by acetylation of lysine residues on FoxO proteins [306,320,321,346], and acetylation of FoxOs also leads to phosphorylation of FoxOs by Akt [347,348].
FoxOs are intimately tied to the pathways of programmed cell death. Blockade of FoxO transcription factor activity can inhibit microglial cell apoptotic death during ROS and Aβ exposure [343,344,349,350,351], foster the protective effects of metabotropic glutamate receptors [259,352], and prevent neuronal apoptotic cell loss through nicotinamide adenine dinucleotide (NAD+) precursors [86,162,198,213,353,354,355,356,357,358]. For example, nicotinamide can block FoxO protein activity [329,359] and is protective through two mechanisms of post-translational modification of FoxO3a [50,186,317]. Nicotinamide can not only maintain phosphorylation of FoxO3a and inhibit its activity to potentially block caspase 3 activity [329], but also it can reduce caspase activity and preserve the integrity of the FoxO3a protein to block FoxO3a proteolysis; that would normally lead to the generation of “pro-apoptotic” amino-terminal (Nt) fragments. Furthermore, growth factors such as erythropoietin (EPO) [119,169,187,340,360,361,362,363,364,365] are also dependent upon FoxOs to prevent apoptotic cell loss. Through post-translational changes, EPO phosphorylates FoxO3a [366] to sequester FoxO3a in the cell cytoplasm through the association with 14-3-3 protein [269,367]. EPO can also remove FOXO3a and FOXO1a acetylation [368], and decrease the transcriptional activity of FoxO1 [369].
Although phosphorylation and prevention of nuclear trafficking of FoxOs can potentially promote anti-aging pathways [331], FoxOs also have a beneficial side that can be linked to autophagy pathways [18,309]. Atherosclerosis can be lessened with FoxO1 and autophagy activity [259,370]. In experimental studies with Huntington’s disease (HD), increased activity of autophagy with FoxO1 can limit neuronal Huntington (mHtt) protein deposition [371]. Exercise-induced activation of autophagy results in the down-regulation of FoxO3a and suppression of sarcopenia [232]. Autophagy induction in association with modulation of FoxO signaling also results in decreased renal tubulointerstitial fibrosis [315] and protection against cardiotoxicity during ferroptosis [298].
In relation to MS, transcription factors, such as FoxO1, can impact brain myelination and support oligodendrocyte growth [372]. In the presence of enhanced activation of FoxO3a, inflammation in the brain tissue can ensue with cytokine release and apoptosis activation [373]. In older individuals, neuronal apoptosis and DNA destruction has been associated with nuclear transcription of target genes by FoxO3a [374]. As a result, therapeutic strategies would consider inhibition of FoxO DNA transcription through post-translational phosphorylation and exclusion from nuclear trafficking. However, autophagy activation may have protective effects during MS. Scenarios exist that can enhance neuronal and vascular survival through combined autophagy induction and activation of FoxOs. Autophagy with FoxO activity, such as during HD, can remove cellular deposits that would otherwise result in cell death [74,375]. In addition, the absence of FoxO3a may be detrimental and represent a lost checkpoint, since relapse in MS may occur with osteopontin and T cell activation under such conditions [376].
Other studies suggest that FoxOs in combination with silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) may lead to immune dysregulation and neuronal inflammation during MS [377] (Figure 1). SIRT1 is a member of the sirtuin family (sirtuin 1) and is a histone deacetylase [2,5,98,106,195,299,308,378,379,380,381,382]. SIRT1 oversees DNA transcription by transferring acetyl groups from ε-N-acetyl lysine amino acids to the histones of DNA. FoxO proteins are deacetylated by SIRT1 as well as other histone deacetylases [18,299,313,321,383,384,385]. SIRT1 decreases oxidative stress, offers protection to neurons, and can preserve memory function [5,76,78,98,106,380,386,387]. SIRT1 maintains mitochondrial function in conjunction with other pathways in experimental models of neurodegeneration [388]. SIRT1 also prevents memory loss during oxidative stress in murine experimental models [389]. In part, SIRT1 activity can block FoxO to prevent cell injury [22,378]. However, SIRT1 is also controlled at times by FoxO proteins in feedback pathways. FoxOs bind to the SIRT1 promoter region to alter forkhead transcription. This promoter region contains a cluster of five putative core binding repeat motifs (IRS-1) and a forkhead-like consensus-binding site (FKHD-L). As an example, FoxO proteins are necessary for pre-implantation embryo development and control SIRT1 protein expression through autofeedback pathways [390]. FoxO proteins, such as FoxO1, also can modulate SIRT1 transcription and increase SIRT1 expression [391]. FoxOs and SIRT1 work synergistically to increase cell survival. SIRT1 and FoxO3a have been shown to limit Aβ injury that affects mitochondria and reduce oxidative stress toxicity [392].
7. The Mechanistic Target of Rapamycin and Multiple Sclerosis
MS and demyelinating disease can be significantly impacted by the mechanistic target of rapamycin (mTOR) pathways (Figure 1). mTOR is a 289-kDa serine/threonine protein kinase that is encoded by a single gene, FRAP1 [13,46,71,73,98,124,243,244,393] (Table 1). mTOR is also known as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 [22,98,229]. Initially, mTOR was reported in Saccharomyces cerevisiae with TOR1 and TOR2 genes [124]. Both mTOR and TOR are inhibited by rapamycin, a macrolide antibiotic in Streptomyces hygroscopicus [229]. mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) use mTOR as a central component [71,96,100,308,394]. mTORC1 consists of the proline-rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8), and Raptor [71,98]. mTOR oversees Raptor, which rapamycin can inhibit. Rapamycin can associate with immunophilin FK-506-binding protein 12 (FKBP12), which connects to the FKBP12 -rapamycin-binding domain (FRB) at the carboxy (C) -terminal of mTOR to block activity of the FRB domain of mTORC1 [22]. However, other possibilities for inhibition of mTORC1 activity exist that consist of Akt and p70 ribosomal S6 kinase (p70S6K) inhibitory phosphorylation, and catalytic domain allosteric alterations [395]. It is believed that mTORC2 disassembly is necessary with long-term administration of rapamycin to achieve activity inhibition equal to rapamycin inhibition of mTORC1. Deptor associates with both ataxia-telangiectasia (ATM), the transactivation/transformation domain-associated protein of mTOR, and the FAT domain (FKBP12 -rapamycin-associated protein) (FRAP) to block mTOR and mTORC1 activity. PRAS40 interferes with binding of p70S6K, mTORC1, and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor to block the activity of mTORC1 [22,98,219,396,397]. Acting as a checkpoint in this pathway, Akt influences mTORC1 activity to phosphorylate PRAS40, and inhibits it to increase mTORC1 activity [15,340,365]. PRAS40 is released from Raptor, and PRAS40 is subsequently maintained in the cytoplasm associated to the 14-3-3 protein [398,399,400]. mLST8 can enhance mTOR activation [22]. mLST8 facilitates Raptor in binding to 4EBP1 and p70S6K [401]. In contrast to mTORC1, mTORC2 consists of Rictor, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), mLST8, and the protein observed with Rictor-1 (Protor-1) [22,98,382,402,403]. mTORC2 controls changes in the cytoskeleton that involves protein kinase C-α (PKC-α) and migratory effects of cells with the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2, as well as Rho signaling. A protein kinase A/protein kinase G/protein kinase C (AGC) family member, glucocorticoid induced protein kinase 1 (SGK1) activity is fostered by mTORC2. Activity of SGK1 can also be enhanced by Protor-1 [404,405]. mTORC2 assembly and the subsequent phosphorylation of Akt is controlled by mSin1 [406]. Cell survival can be enhanced during mSIN1 and Rictor phosphorylation of Akt at serine473, and lead to threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1).
mTOR can influence programmed cell death pathways through multiple mechanisms. mTOR activity can block apoptotic cell death in the nervous system [15,69,71,73,85,115,172,276,407], prevent oxidative stress injury [399,408,409,410], modulate the progression of infectious agents [85,411,412,413,414,415,416], and oversee metabolic homeostasis [72,115,417,418]. In the presence of mTOR activity, Aβ toxicity can be blocked [154,172,399,409,419,420,421], vascular cell death is prevented [105,422], and neonatal and adult central nervous system hypoxic injury is prevented [276,423]. Furthermore, neuronal differentiation is promoted [424], microglia survival is increased during oxidative stress exposure and Aβ toxicity [117,399,409,420,425,426], and neuroplasticity is fostered [48,310,427,428].
In regard to cognitive pathways, mTOR can oversee cellular metabolism to improve cognition. mTOR may provide protection through an improved nutritional balance and Mediterranean dietary regimen. mTOR can limit Aβ-induced astrocyte and non-neuronal cell injury through enhanced Akt activity during consumption of polyphenol of olives and olive oil that may be linked to the prevention of AD [429]. mTOR can modulate insulin physiology in neurodegenerative studies to increase survival of astrocytes [429], block hyperglycemic endothelial cell injury [430], and preserve metabolic regulation [431]. As a component of the mTOR pathway, the AMP-activated protein kinase (AMPK) modulates cellular metabolism [106,169,198,237,378,381,382,394,432,433], and the activation of AMPK reduces cognitive loss in studies of DM and AD [434,435], removes cerebral Aβ [436] and tau [437], limits Aβ neurotoxicity [392], diminishes long-term inflammation in in the nervous system [10,71,438,439], and fosters pathways for healthy aging [6,440,441].
Autophagy pathways are also critical for impacting neurodegeneration and usually involve the blockade of mTOR activity for neuroprotective pathways [17,22,44,70,100,105,169,242,243,244,259,293]. The induction of autophagy, which may require an mTOR blockade, can protect neuronal and non-neuronal cells [10,24,73,89,106,172,224,442]. For example, diseases of the retina may require mTOR inhibition with rapamycin to prevent retinal degeneration during MS DM [46]. Inflammatory pathways that involve peripheral blood mononuclear cells in MS also may require inhibition of mTOR pathways with rapamycin [443]. In experimental MS models, rapamycin with mTOR inhibition can prevent the clinical course of both relapsing-remitting and chronic experimental autoimmune encephalomyelitis, suggesting important clinical applications for the treatment of MS [201]. Rapamycin, through the blockade of cytokine release and the inhibition of a microglia immune response, has been shown to reduce clinical symptomatology and inflammatory responses in models of experimental autoimmune encephalomyelitis [134]; it has been suggested that inhibition of mTOR pathways may be necessary to reduce the risk of MS development [135]. Autophagy activation during decreased mTOR activity can maintain mitochondrial function [444], prevent injury to dopamine-dependent cells [445], provide neuroprotection with glutamine-dependent mechanisms [446], and decrease ROS release [447]. The improvement of memory function and cognition also may be linked to the maintenance of cellular glucose homeostasis. In the presence of limited mTOR activity, autophagy activation can lead to removal of Aβ, reduction of cognitive loss, and enhanced insulin-glucose metabolism [448]. Reductions in memory loss can be promoted through nutritional changes that focus on calorie reduction to reduce mTOR, increase autophagy activation [449], and foster microglial cell activity that can be altered by serum glucose changes [450]. Cognition may be improved with autophagy induction and reduced mTOR to remove tau [437], while loss of a necessary autophagy balance can contribute to dementia [156].
However, it is important to recognize that cognitive function as well as neuroprotection relies upon a careful balance between the activity of apoptosis and autophagy. mTOR and autophagy blockades are required for brain interneuron progenitor development [451]. Autophagy activation during high serum glucose levels can result in oxidative stress through mitochondrial dysfunction [94,380,441,452,453,454], lead to progenitor endothelial cell injury, and prevent new blood vessel growth [455]. Under some circumstances, autophagy can result in neuronal cell death [375,456,457]. Dysfunction or loss of mTOR signaling may at times result in cognitive impairment [13,18,73,105,117,458]. Furthermore, trophic factors such as EPO lead to enhanced neurovascular cell survival through activation of mTOR and reduction in autophagy [187,273,340]. EPO controls Akt and PRAS40 as well fosters neuronal cell health [137,398,459,460].
In regard to MS, mTOR can importantly impact inflammatory pathways that lead to neurodegeneration [3,73,85,211,244,285,461,462]. In clinical studies, mTOR pathway molecules may play an important role in determining the onset and progression of MS in patients [135]. Current therapies exist with metformin and biguanides that can impact neurodegenerative disease, and include demyelinating disease and cognitive loss [85,115,132,463]. Metformin blocks mTOR activity to foster autophagy. However, it is known that metformin can also act independent of AMPK pathways [464]. Inhibition of mTOR activity with AMPK activation during metformin treatment can support myelin growth through the reduction of oxidative stress in oligodendrocytes [132]. These observations with metformin also appear to promote activity of oligodendrocytes that can lead to myelination and repair in the nervous system [132]. In regard to risk factors for MS, metformin can limit impaired function in overweight individuals or those suffering from DM when exposed to COVID-19 [465,466]. In models of autoimmune encephalomyelitis, the modulation of mTOR and autophagy activity can affect activated microglia, reduce the release of cytokines, and potentially modulate inflammation and demyelination in the nervous system [134]. It is important to note that since the loss of SIRT1 activity may be involved in immune dysregulation during MS [377], mTOR has a complex relationship with SIRT1 [22]. SIRT1 can require limited mTOR activity to support neuronal development in the presence of low nutritional circumstances [467]. During ROS release and oxidative stress, a combination of SIRT1 activation, autophagy induction, and reduced mTOR is necessary for the function of embryonic stem cells and organelles such as mitochondria [468]. Inhibition of mTOR with SIRT1 activation can increase photoreceptor cell survival [469] and limit cell senescence [470]. However, at certain times, SIRT1, mTOR, and FoxOs may be necessary for cell survival, since protection of neurons in the dopamine system requires complementary activities for SIRT1, FoxOs, and mTOR [471].
8. Conclusions and Future Considerations
Lifespan expectancy is rising throughout the world. As a result, the prevalence of neurodegenerative disorders that affect more than fifteen percent of the global population and comprise greater than six hundred disease entities is increasing. With the increased age of the population and underlying cellular mechanisms such as cell senescence and TL impairment, dementia has now become the 7th leading cause of death in the world. Given this knowledge, increased focus is now directed to individuals with MS, a disorder that affects a significant proportion of the world’s population. Individuals with MS are now recognized as being significantly impacted by cognitive loss and dementia. It is believed that loss of cognitive function in MS occurs in approximately sixty-five percent of individuals and can affect processing of information, attention, and memory recall. Multiple cellular mechanisms may lead to onset and progression of MS, such as inflammatory mediators, demyelination and remyelination pathways, oxidative stress, blood-brain barrier impairment, viral antigens, and cellular metabolism dependent upon nicotinamide adenine dinucleotide (NAD+). At present, DMTs can only limit the rate of relapse in MS patients, but cannot prevent disease progression. Of further concern, early initiation of DMTs may slow the progression of brain volume loss, but cognitive disability may continue to progress. New and innovative avenues for the investigation and treatment of demyelinating disorders are required that involve autophagy, apoptosis, FoxOs, mTOR, AMPK, SIRT1, and related systems with the APOE-ε4 gene and SARS-CoV-2.
For the pursuance of new strategies that can address cognitive loss in MS, APOE and infection with SARS-CoV-2 may be significant risk factors for MS. Interestingly, risk factors for cognitive loss in MS share similarities with other cognitive disorders such as AD. In patients with optic neuritis, APOE serum levels are markedly higher than in control patient groups, and the APOE ε3/ε3 genotype may increase the risk of developing optic neuritis in males. Other risk factors such as SARS-CoV-2 may be a risk factor for developing cognitive loss in MS. Memory loss can develop after infection with SARS-CoV-2 [85,115,283,472,473,474]. APOE-ε4 is associated with long-COVID disability and loss in cognition [110,155,475]. Two ε4 alleles of APOE-ε4 confers a loss in gene activity that can defend against viral infections leading to disruption of cerebral blood vessels and increased inflammatory activity [155,192]. As a result, individuals with APOE may experience demyelination, cognitive loss, and increased death rates during a SARS-CoV-2 infection [10,15,22,33,38,51,258,277,433,476,477].
Pathways with programmed cell death in MS have intricate relationships with FoxOs, mTOR, and SIRT1. Autophagy, apoptosis, pyroptosis, and ferroptosis may have an important role in the regulation of oligodendrocyte development, myelination, inflammasome expression, intracellular iron accumulation, activation of microglia, and cell survival during oxidative stress. However, these relationships are complex and may require a fine balance. For example, although enhanced FoxO3 activity alone may foster disease progression in MS by resulting in inflammation, cytokine activation, and neuronal cell apoptosis, FoxO activation in combination with autophagy during these circumstances may be protective during MS. FoxO3a loss also may be detrimental and represent a lost checkpoint, since MS recurrence may ensue with osteopontin and T cell activation under these conditions. These studies suggest that activation of FoxO with complementary autophagy induction may be necessary for protective pathways in MS. FoxO proteins are also dependent upon SIRT1, and function through autofeedback mechanisms to regulate SIRT1 activity. FoxOs and SIRT1 can work synergistically to increase cell survival and reduce oxidative stress toxicity. In a similar vein, mTOR inhibition with autophagy activation can provide neuroprotection, decrease ROS release, increase astrocyte viability, and preserve glucose homeostasis. However, mTOR and autophagy blockades are required for brain interneuron progenitor development, and the dysfunction or loss of mTOR signaling can lead to cognitive impairment. Furthermore, SIRT1, mTOR, AMPK, and FoxOs may be necessary for cell survival that involves agents such as nicotinamide and EPO, as well as for dopaminergic neuronal cell survival that requires complementary activities for SIRT1, FoxOs, and mTOR. The pathways of programmed cell death, FoxOs, mTOR, AMPK, and SIRT1 offer great promise for the understanding and treatment of cognitive loss in MS, but future investigations will be necessary to further understand the complexity of these pathways to achieve long-lasting beneficial outcomes.
Funding
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, NS053956, and NIH ARRA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Maiese, K. Cutting through the complexities of mTOR for the treatment of stroke. Curr. Neurovasc. Res. 2014, 11, 177–186. [Google Scholar] [CrossRef]
- Maiese, K. Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural Regen. Res. 2021, 16, 448–455. [Google Scholar] [CrossRef]
- Geier, C.; Perl, A. Therapeutic mTOR blockade in systemic autoimmunity: Implications for antiviral immunity and extension of lifespan. Autoimmun. Rev. 2021, 20, 102984. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front. Genet. 2021, 12, 688526. [Google Scholar] [CrossRef]
- Jalgaonkar, M.P.; Parmar, U.M.; Kulkarni, Y.A.; Oza, M.J. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol. Res. 2021, 175, 106014. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, H.; Wang, B.; Zhang, Y.; Zheng, X.; Shao, B.; Zhuge, Q.; Jin, K. Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells 2021, 10, 660. [Google Scholar] [CrossRef]
- Minino, A.M. Death in the United States, 2011. NCHS Data Brief; Centers for Disease Control and Prevention Nation Center for Health Statistics: Hyattsville, MD, USA, 2013; pp. 1–8. [Google Scholar]
- Hayutin, A. Global demographic shifts create challenges and opportunities. PREA Q. 2007, Fall, 46–53. [Google Scholar]
- Maiese, K. SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J. Stem Cells 2015, 7, 235–242. [Google Scholar] [CrossRef]
- Maiese, K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr. Neurovasc. Res. 2017, 14, 299–304. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Hsieh, C.-C.; Chu, P.-M.; Chen, J.-Y.; Huang, Y.-C.; Chen, C.-Y. Roles of protein tyrosine phosphatases in hepatocellular carcinoma progression (Review). Oncol. Rep. 2023, 49, 48. [Google Scholar] [CrossRef]
- Jiang, W.; Ding, K.; Yue, R.; Lei, M. Therapeutic effects of icariin and icariside II on diabetes mellitus and its complications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-B.; Hu, X.-Y.; Chen, M.-W.; Xiong, C.-H.; Na Zhao, N.; Ge, Y.-H.; Wang, H.; Gao, X.-L.; Xu, N.-J.; Zhao, L.-X.; et al. p85S6K sustains synaptic GluA1 to ameliorate cognitive deficits in Alzheimer’s disease. Transl. Neurodegener. 2023, 12, 1. [Google Scholar] [CrossRef]
- Kahmini, F.R.; Ghaleh, H.D.; Shahgaldi, S. Sirtuins: Subtle Regulators Involved in Convoluted Mechanisms of Pregnancy. Cell. Physiol. Biochem. 2022, 56, 644–662. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, M.; Tian, J.; Gao, M.; Liu, M.; Fu, X.; Jin, T.; Pan, J.; Chen, F.; An, F. WNT1-inducible signalling pathway protein 1 stabilizes atherosclerotic plaques in apolipoprotein-E-deficient mice via the focal adhesion kinase/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase pathway. J. Hypertens. 2022, 40, 1666–1681. [Google Scholar] [CrossRef]
- Hacioglu, C.; Kar, F.; Kanbak, G. Reproductive Effects of Nicotinamide on Testicular Function and Structure in Old Male Rats: Oxidative, Apoptotic, Hormonal, and Morphological Analyses. Reprod. Sci. 2021, 28, 3352–3360. [Google Scholar] [CrossRef]
- Maiese, K. Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways. Biomolecules 2021, 11, 1002. [Google Scholar] [CrossRef]
- Maiese, K. Neurodegeneration, memory loss, and dementia: The impact of biological clocks and circadian rhythm. Front. Biosci. 2021, 26, 614–627. [Google Scholar] [CrossRef]
- Patocka, J.; Kuca, K.; Oleksak, P.; Nepovimova, E.; Valis, M.; Novotny, M.; Klimova, B. Rapamycin: Drug Repurposing in SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 217. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Zhang, Z.; Kang, G.; Pastor-Alonso, O.; Biagiotti, S.; Page, C.E.; Sandoval, K.; Knox, A.; Connolly, A.; et al. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J. Neurosci. 2021, 41, 2554–2565. [Google Scholar] [CrossRef]
- Odnokoz, O.; Nakatsuka, K.; Wright, C.; Castellanos, J.; Klichko, V.I.; Kretzschmar, D.; Orr, W.C.; Radyuk, S.N. Mitochondrial Redox Signaling Is Critical to the Normal Functioning of the Neuronal System. Front. Cell Dev. Biol. 2021, 9, 613036. [Google Scholar] [CrossRef]
- Maiese, K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): Oversight for neurodegenerative disorders. Biochem. Soc. Trans. 2018, 46, 351–360. [Google Scholar] [CrossRef]
- Maiese, K. Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr. Neurovasc. Res. 2019, 15, 367–371. [Google Scholar] [CrossRef]
- Maiese, K. Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int. Rev. Neurobiol. 2020, 155, 1–35. [Google Scholar] [CrossRef]
- Schell, M.; Wardelmann, K.; Kleinridders, A. Untangling the effect of insulin action on brain mitochondria and metabolism. J. Neuroendocr. 2021, 33, e12932. [Google Scholar] [CrossRef]
- Speer, H.; D’cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health—A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- World Health Organization. Description of the Global Burden of NCDs, Their Risk Factors and Determinants. In Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011; pp. 1–176. [Google Scholar]
- World Health Organization. Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switzerland, 2017; pp. 1–44. [Google Scholar]
- Wang, Q.; Zheng, J.; Pettersson, S.; Reynolds, R.; Tan, E.-K. The link between neuroinflammation and the neurovascular unit in synucleinopathies. Sci. Adv. 2023, 9, eabq1141. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, L.; Chen, Q.; Huang, Y.; Chen, X.; Qiao, D. The Role of Non-coding RNAs in Methamphetamine-Induced Neurotoxicity. Cell Mol. Neurobiol. 2023, 43, 2415–2436. [Google Scholar] [CrossRef]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol. Neurobiol. 2022, 60, 923–959. [Google Scholar] [CrossRef] [PubMed]
- Barthels, D.; Prateeksha, P.; Nozohouri, S.; Villalba, H.; Zhang, Y.; Sharma, S.; Anderson, S.; Howlader, S.I.; Nambiar, A.; Abbruscato, T.J.; et al. Dental Pulp-Derived Stem Cells Preserve Astrocyte Health during Induced Gliosis by Modulating Mitochondrial Activity and Functions. Cell Mol. Neurobiol. 2022, 43, 2105–2127. [Google Scholar] [CrossRef]
- González-Fernández, C.; González, P.; González-Pérez, F.; Rodríguez, F.J. Characterization of Ex Vivo and In Vitro Wnt Transcriptome Induced by Spinal Cord Injury in Rat Microglial Cells. Brain Sci. 2022, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Pyroptosis, Apoptosis, and Autophagy: Critical Players of Inflammation and Cell Demise in the Nervous System. Curr. Neurovasc. Res. 2022, 19, 241–244. [Google Scholar] [CrossRef]
- Pettigrew, D.B.; Singh, N.; Kirthivasan, S.; Crutcher, K.A. The Role of Tissue Geometry in Spinal Cord Regeneration. Medicina 2022, 58, 542. [Google Scholar] [CrossRef]
- Salemi, M.; Mogavero, M.P.; Lanza, G.; Mongioì, L.M.; Calogero, A.E.; Ferri, R. Examples of Inverse Comorbidity between Cancer and Neurodegenerative Diseases: A Possible Role for Noncoding RNA. Cells 2022, 11, 1930. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Zeng, W.; Song, L.L.; Wang, H.M.; Qu, L.Q.; Lo, H.H.; Yu, L.; Wu, A.G.; Wong, V.K.W.; Law, B.Y.K. Extracellular Vesicle Delivery of Neferine for the Attenuation of Neurodegenerative Disease Proteins and Motor Deficit in an Alzheimer’s Disease Mouse Model. Pharmaceuticals 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Y.; Liu, R.; Li, W.; Hua, B.; Bao, Y. Wnt Signaling Pathways: A Role in Pain Processing. NeuroMol. Med. 2022, 24, 233–249. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, L.; Liang, Y.; Peng, D.; Aschner, M.; Jiang, Y. Signal transduction associated with lead-induced neurological disorders: A review. Food Chem. Toxicol. 2021, 150, 112063. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef]
- Savu, D.I.; Moisoi, N. Mitochondria-Nucleus communication in neurodegenerative disease. Who talks first, who talks louder? Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2022, 1863, 148588. [Google Scholar] [CrossRef] [PubMed]
- Yalçin, M.; Mundorf, A.; Thiel, F.; Amatriain-Fernández, S.; Kalthoff, I.S.; Beucke, J.-C.; Budde, H.; Garthus-Niegel, S.; Peterburs, J.; Relógio, A. It’s About Time: The Circadian Network as Time-Keeper for Cognitive Functioning, Locomotor Activity and Mental Health. Front. Physiol. 2022, 13, 873237. [Google Scholar] [CrossRef]
- Levine, K.S.; Leonard, H.L.; Blauwendraat, C.; Iwaki, H.; Johnson, N.; Bandres-Ciga, S.; Ferrucci, L.; Faghri, F.; Singleton, A.B.; Nalls, M.A. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron 2023, 111, 1086–1093.e2. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Rao, K.R.; Manjunath, M.; Saiswaroop, R.; Patnana, D.P.; Phalguna, K.S.; Choudhary, B.; Sivaramakrishnan, V. Vitamin B6, B12 and folate modulate deregulated pathways and protein aggregation in yeast model of Huntington disease. 3 Biotech 2023, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, M.; Liu, J.; Wei, Z.; Yuan, J.; Wu, W.; Wu, Z.; Lai, Y.; Zhao, Z.; Chen, H.; et al. Neuropilin-1 promotes mitochondrial structural repair and functional recovery in rats with cerebral ischemia. J. Transl. Med. 2023, 21, 297. [Google Scholar] [CrossRef] [PubMed]
- Casciano, F.; Zauli, E.; Rimondi, E.; Mura, M.; Previati, M.; Busin, M.; Zauli, G. The role of the mTOR pathway in diabetic retinopathy. Front. Med. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Radulovic, J.; Gabbay, V. PFC mTOR signaling as a biological signature for cognitive deficits in bipolar disorder without psychosis. Cell Rep. Med. 2021, 2, 100282. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.; Abd-Elrahman, K.S.; Derksen, A.; Rowe, E.M.; Thompson, A.M.; Rudyk, C.A.; Prowse, N.A.; Dwyer, Z.; Bureau, S.C.; Fortin, T.; et al. mGluR5 Allosteric Modulation Promotes Neurorecovery in a 6-OHDA-Toxicant Model of Parkinson’s Disease. Mol. Neurobiol. 2019, 57, 1418–1431. [Google Scholar] [CrossRef]
- Ji, J.S.; Liu, L.; Zeng, Y.; Yan, L.L. Effect of FOXO3 and Air Pollution on Cognitive Function: A Longitudinal Cohort Study of Older Adults in China from 2000 to 2014. J. Gerontol. Ser. A 2022, 77, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C. OutFOXOing disease and disability: The therapeutic potential of targeting FoxO proteins. Trends Mol. Med. 2008, 14, 219–227. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z.; Shang, Y.C. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol. Ther. 2008, 118, 58–81. [Google Scholar] [CrossRef]
- Oyefeso, F.A.; Muotri, A.R.; Wilson, C.G.; Pecaut, M.J. Brain organoids: A promising model to assess oxidative stress-induced central nervous system damage. Dev. Neurobiol. 2021, 81, 653–670. [Google Scholar] [CrossRef]
- Sooknual, P.; Pingaew, R.; Phopin, K.; Ruankham, W.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis and neuroprotective effects of novel chalcone-triazole hybrids. Bioorganic Chem. 2020, 105, 104384. [Google Scholar] [CrossRef]
- Yalçin, M.; Malhan, D.; Basti, A.; Peralta, A.R.; Ferreira, J.J.; Relógio, A. A Computational Analysis in a Cohort of Parkinson’s Disease Patients and Clock-Modified Colorectal Cancer Cells Reveals Common Expression Alterations in Clock-Regulated Genes. Cancers 2021, 13, 5978. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.Y.; Webster, C.; Kumari, S.; Gallacher, J.E.J.; Sarkar, C. The associations of socioeconomic status with incident dementia and Alzheimer’s disease are modified by leucocyte telomere length: A population-based cohort study. Sci. Rep. 2023, 13, 6163. [Google Scholar] [CrossRef] [PubMed]
- Topiwala, A.; Nichols, T.E.; Williams, L.Z.J.; Robinson, E.C.; Alfaro-Almagro, F.; Taschler, B.; Wang, C.; Nelson, C.P.; Miller, K.L.; Codd, V.; et al. Telomere length and brain imaging phenotypes in UK Biobank. PLoS ONE 2023, 18, e0282363. [Google Scholar] [CrossRef]
- Kuan, X.-Y.; Fauzi, N.S.A.; Ng, K.Y.; Bakhtiar, A. Exploring the Causal Relationship between Telomere Biology and Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 4169–4183. [Google Scholar] [CrossRef]
- Cardoso, S.; López, I.P.; Piñeiro-Hermida, S.; Pichel, J.G.; Moreira, P.I. IGF1R Deficiency Modulates Brain Signaling Pathways and Disturbs Mitochondria and Redox Homeostasis. Biomedicines 2021, 9, 158. [Google Scholar] [CrossRef]
- De Bonis, M.L.; Ortega, S.; Blasco, M.A. SIRT1 Is Necessary for Proficient Telomere Elongation and Genomic Stability of Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 690–706. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Shafi, O. Inverse relationship between Alzheimer’s disease and cancer, and other factors contributing to Alzheimer’s disease: A systematic review. BMC Neurol. 2016, 16, 236. [Google Scholar] [CrossRef]
- Begum, M.K.; Konja, D.; Singh, S.; Chlopicki, S.; Wang, Y. Endothelial SIRT1 as a Target for the Prevention of Arterial Aging: Promises and Challenges. J. Cardiovasc. Pharmacol. 2021, 78, S63–S77. [Google Scholar] [CrossRef]
- Cai, J.; Qi, H.; Yao, K.; Yao, Y.; Jing, D.; Liao, W.; Zhao, Z. Non-Coding RNAs Steering the Senescence-Related Progress, Properties, and Application of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 650431. [Google Scholar] [CrossRef]
- Dorvash, M.; Farahmandnia, M.; Tavassoly, I. A Systems Biology Roadmap to Decode mTOR Control System in Cancer. Interdiscip. Sci. Comput. Life Sci. 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gao, C.; Pu, Y.; Luo, L.; Xu, Y.; Xu, Y. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Front. Pharmacol. 2021, 12, 653940. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Luo, B. Current perspective on the regulation of FOXO4 and its role in disease progression. Cell Mol. Life Sci. 2019, 77, 651–663. [Google Scholar] [CrossRef]
- Maiese, K. Driving neural regeneration through the mammalian target of rapamycin. Neural Regen. Res. 2014, 9, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Stem cell guidance through the mechanistic target of rapamycin. World J. Stem Cells 2015, 7, 999–1009. [Google Scholar]
- Maiese, K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br. J. Clin. Pharmacol. 2015, 82, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr. Neurovasc. Res. 2020, 17, 327–331. [Google Scholar] [CrossRef]
- Rapaka, D.; Bitra, V.R.; Challa, S.R.; Adiukwu, P.C. mTOR signaling as a molecular target for the alleviation of Alzheimer’s disease pathogenesis. Neurochem. Int. 2022, 155, 105311. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. 2021, 26, 50–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Z.; Deng, Y.-J.; Xie, Q.-Q.; Ren, E.-H.; Ma, Z.-J.; He, X.-G.; Gao, Y.-C.; Kang, X.-W. Sirtuins and intervertebral disc degeneration: Roles in inflammation, oxidative stress, and mitochondrial function. Clin. Chim. Acta 2020, 508, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, H.; Wang, Q.; Chen, S.; Wang, R.; Wang, Z.; Yang, C.; Chen, A.; Zhao, J.; Zhou, Z.; et al. Sirt1 overexpression improves senescence-associated pulmonary fibrosis induced by vitamin D deficiency through downregulating IL-11 transcription. Aging Cell 2022, 21, e13680. [Google Scholar] [CrossRef]
- Maiese, K. The bright side of reactive oxygen species: Lifespan extension without cellular demise. J. Transl. Sci. 2016, 2, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; He, Y.; Hu, F.; Li, M.; Yao, Y. Genkwanin Alleviates Mitochondrial Dysfunction and Oxidative Stress in a Murine Model of Experimental Colitis: The Participation of Sirt1. Ann. Clin. Lab. Sci. 2022, 52, 301–313. [Google Scholar]
- Fields, C.R.; Bengoa-Vergniory, N.; Wade-Martins, R. Targeting Alpha-Synuclein as a Therapy for Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 299. [Google Scholar] [CrossRef]
- Gallyas, F., Jr.; Sumegi, B.; Szabo, C. Role of Akt Activation in PARP Inhibitor Resistance in Cancer. Cancers 2020, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Groen, C.M.; Podratz, J.L.; Pathoulas, J.; Staff, N.; Windebank, A.J. Genetic Reduction of Mitochondria Complex I Subunits is Protective against Cisplatin-Induced Neurotoxicity in Drosophila. J. Neurosci. 2021, 42, 922–937. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of α-synuclein in gastrointestinal microbiome dysbiosis-related Parkinson’s disease progression (Review). Mol. Med. Rep. 2021, 24, 73. [Google Scholar] [CrossRef]
- Li, N.; Yue, L.; Wang, J.; Wan, Z.; Bu, W. MicroRNA-24 alleviates isoflurane-induced neurotoxicity in rat hippocampus via attenuation of oxidative stress. Biochem. Cell Biol. 2020, 98, 208–218. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wang, X.-X.; Truong, D.; Wu, Y.-C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Curr. Neurovasc. Res. 2020, 17, 332–337. [Google Scholar] [CrossRef]
- Maiese, K. Nicotinamide as a Foundation for Treating Neurodegenerative Disease and Metabolic Disorders. Curr. Neurovasc. Res. 2021, 18, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Marón, F.J.M.; Ferder, L.; Reiter, R.J.; Manucha, W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J. Steroid Biochem. Mol. Biol. 2020, 199, 105595. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.L.; Santos, G.G.L.; Espirito-Santo, R.F.; Silva, G.S.A.; Evangelista, A.F.; Silva, D.N.; Soares, M.B.P.; Villarreal, C.F. Reestablishment of Redox Homeostasis in the Nociceptive Primary Afferent as a Mechanism of Antinociception Promoted by Mesenchymal Stem/Stromal Cells in Oxaliplatin-Induced Chronic Peripheral Neuropathy. Stem Cells Int. 2021, 2021, 8815206. [Google Scholar] [CrossRef] [PubMed]
- Perluigi, M.; Di Domenico, F.; Barone, E.; Butterfield, D. mTOR in Alzheimer disease and its earlier stages: Links to oxidative damage in the progression of this dementing disorder. Free Radic. Biol. Med. 2021, 169, 382–396. [Google Scholar] [CrossRef]
- Piao, S.; Lee, I.; Jin, S.-A.; Kim, S.; Nagar, H.; Choi, S.-J.; Jeon, B.H.; Kim, C.-S. SIRT1 Activation Attenuates the Cardiac Dysfunction Induced by Endothelial Cell-Specific Deletion of CRIF1. Biomedicines 2021, 9, 52. [Google Scholar] [CrossRef]
- Prasuhn, J.; Brüggemann, N. Genotype-driven therapeutic developments in Parkinson’s disease. Mol. Med. 2021, 27, 42. [Google Scholar] [CrossRef]
- Xiong, J.; Bonney, S.; Gonçalves, R.V.; Esposito, D. Brassinosteroids control the inflammation, oxidative stress and cell migration through the control of mitochondrial function on skin regeneration. Life Sci. 2022, 307, 120887. [Google Scholar] [CrossRef]
- Zhuang, X.; Ma, J.; Xu, G.; Sun, Z. SHP-1 knockdown suppresses mitochondrial biogenesis and aggravates mitochondria-dependent apoptosis induced by all trans retinal through the STING/AMPK pathways. Mol. Med. 2022, 28, 125. [Google Scholar] [CrossRef]
- Raut, S.K.; Khullar, M. Oxidative stress in metabolic diseases: Current scenario and therapeutic relevance. Mol. Cell Biochem. 2022, 478, 185–196. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Y.; Qin, L.-F.; Xu, Z.-G.; Gao, X.-R.; Liu, C.-B.; Xu, G.-T.; Chen, Y.-Z. Comprehensive Bibliometric Analysis of Stem Cell Research in Alzheimer’s Disease from 2004 to 2022. Dement. Geriatr. Cogn. Disord. 2023, 52, 47–73. [Google Scholar] [CrossRef]
- Maiese, K. Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen. Res. 2016, 11, 372–385. [Google Scholar] [CrossRef]
- Maiese, K. Impacting dementia and cognitive loss with innovative strategies: Mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen. Res. 2019, 14, 773–774. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Cognitive impairment with diabetes mellitus and metabolic disease: Innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev. Clin. Pharmacol. 2020, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; Garcez, M.L.; Kim, Y.-K. The shared molecular mechanisms underlying aging of the brain, major depressive disorder, and Alzheimer’s disease: The role of circadian rhythm disturbances. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 123, 110721. [Google Scholar] [CrossRef] [PubMed]
- Amini, J.; Sanchooli, N.; Milajerdi, M.-H.; Baeeri, M.; Haddadi, M.; Sanadgol, N. The interplay between tauopathy and aging through interruption of UPR/Nrf2/autophagy crosstalk in the Alzheimer’s disease transgenic experimental models. Int. J. Neurosci. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Lathe, R.; Clair, D.S. Programmed ageing: Decline of stem cell renewal, immunosenescence, and Alzheimer’s disease. Biol. Rev. 2023, 98, 1424–1458. [Google Scholar] [CrossRef]
- Rani, S.; Dhar, S.B.; Khajuria, A.; Gupta, D.; Jaiswal, P.K.; Singla, N.; Kaur, M.; Singh, G.; Barnwal, R.P. Advanced Overview of Biomarkers and Techniques for Early Diagnosis of Alzheimer’s Disease. Cell Mol. Neurobiol. 2023, 43, 2491–2523. [Google Scholar] [CrossRef]
- Filley, C.M.; Rollins, Y.D.; Anderson, C.A.; Arciniegas, D.B.; Howard, K.L.; Murrell, J.R.; Boyer, P.J.; Kleinschmidt-DeMasters, B.K.; Ghetti, B. The Genetics of Very Early Onset Alzheimer Disease. Cogn. Behav. Neurol. 2007, 20, 149–156. [Google Scholar] [CrossRef]
- Torres, A.A.; Sollhuber, M.; Fernandez, M.; Sanchez-Montero, J. Multi-Target-Directed Ligands and other Therapeutic Strategies in the Search of a Real Solution for Alzheimer’s Disease. Curr. Neuropharmacol. 2014, 12, 2–36. [Google Scholar] [CrossRef]
- Maiese, K. Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann. Med. 2014, 46, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.-R.; Qu, Y.-J.; Hu, B.; An, H.-M. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed. Pharmacother. 2022, 152, 113208. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, M.; Ahmadi, M.; Afshar, S.; Lorzadeh, S.; Adlimoghaddam, A.; Jalal, N.R.; West, R.; Dastghaib, S.; Igder, S.; Torshizi, S.R.N.; et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol. Ther. 2022, 237, 108171. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, A.; Reynolds, R. Diverse pathways to neuronal necroptosis in Alzheimer’s disease. Eur. J. Neurosci. 2022, 56, 5428–5441. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.R. Blood based biomarkers for movement disorders. Acta Neurol. Scand. 2022, 146, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Apolipoprotein-ε4 allele (APOE-ε4) as a Mediator of Cognitive Loss and Dementia in Long COVID-19. Curr. Neurovasc. Res. 2022, 19, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Ullah, H.; Hussain, A.; Asif, M.; Nawaz, F. Natural products as bioactive agents in the prevention of dementia. CNS Neurol. Disord.-Drug Targets 2022, 22, 466–476. [Google Scholar] [CrossRef]
- Zhu, G.; Tong, Q.; Ye, X.; Li, J.; Zhou, L.; Sun, P.; Liang, F.; Zhong, S.; Cheng, R.; Zhang, J. Phototherapy for Cognitive Function in Patients with Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 14, 936489. [Google Scholar] [CrossRef]
- Schluesener, J.K.; Zhu, X.; Schluesener, H.J.; Wang, G.; Ao, P. Key network approach reveals new insight into Alzheimer’s disease. IET Syst. Biol. 2014, 8, 169–175. [Google Scholar] [CrossRef]
- Mavroidi, B.; Kaminari, A.; Matiadis, D.; Hadjipavlou-Litina, D.; Pelecanou, M.; Tzinia, A.; Sagnou, M. The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro. Brain Sci. 2022, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Nicotinamide: Oversight of Metabolic Dysfunction through SIRT1, mTOR, and Clock Genes. Curr. Neurovasc. Res. 2021, 17, 765–783. [Google Scholar] [CrossRef]
- World Health Organization. Dementia: A Public Health Priority; World Health Organization: Geneva, Switzerland, 2012; pp. 1–4. [Google Scholar]
- Maiese, K. The Challenges for Drug Development: Cytokines, Genes, and Stem Cells. Curr. Neurovasc. Res. 2012, 9, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Badavath, V.N.; ul Hassan, S.S.; Hasan, M.M.; Bhatia, S.; Al-Harassi, A.; et al. Unravelling the potential neuroprotective facets of erythropoietin for the treatment of Alzheimer’s disease. Metab. Brain Dis. 2021, 37, 1–16. [Google Scholar] [CrossRef]
- Hu, G.; Wang, T.; Ma, C. EPO activates PI3K-IKKα-CDK1 signaling pathway to promote the proliferation of Glial Cells under hypoxia environment. Genet. Mol. Biol. 2022, 45, 0210249. [Google Scholar] [CrossRef]
- Movahedpour, A.; Vakili, O.; Khalifeh, M.; Mousavi, P.; Mahmoodzadeh, A.; Taheri-Anganeh, M.; Razmeh, S.; Shabaninejad, Z.; Yousefi, F.; Behrouj, H.; et al. Mammalian target of rapamycin (mTOR) signaling pathway and traumatic brain injury: A novel insight into targeted therapy. Cell Biochem. Funct. 2022, 40, 232–247. [Google Scholar] [CrossRef]
- Shkodina, A.D.; Tan, S.C.; Hasan, M.M.; Abdelgawad, M.; Chopra, H.; Bilal, M.; Boiko, D.I.; Tarianyk, K.A.; Alexiou, A. Roles of clock genes in the pathogenesis of Parkinson’s disease. Ageing Res. Rev. 2021, 74, 101554. [Google Scholar] [CrossRef]
- Unni, S.; Deshmukh, P.; Krishnappa, G.; Bharath, M.M.S.; Padmanabhan, B. Chlorhexidine as a Keap1-Nrf2 inhibitor: A new target for an old drug for Parkinson’s disease therapy. J. Biomol. Struct. Dyn. 2022, 41, 5367–5381. [Google Scholar] [CrossRef]
- Kuiper, M.A.; Visser, J.J.; Bergmans, P.L.; Scheltens, P.; Wolters, E.C. Decreased cerebrospinal fluid nitrate levels in Parkinson’s disease, Alzheimer’s disease and multiple system atrophy patients. J. Neurol. Sci. 1994, 121, 46–49. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. mTOR: On target for novel therapeutic strategies in the nervous system. Trends Mol. Med. 2012, 19, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Vallée, J.-N.; Lecarpentier, Y. Parkinson’s Disease: Potential Actions of Lithium by Targeting the WNT/β-Catenin Pathway, Oxidative Stress, Inflammation and Glutamatergic Pathway. Cells 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Shang, Y.C.; Mu, Y.; Cui, S.; Yao, Q.; Maiese, K. Targeting erythropoietin for chronic neurodegenerative diseases. Expert Opin. Ther. Targets 2013, 17, 707–720. [Google Scholar] [CrossRef]
- Gaindh, D.; Choi, Y.-B.; Marchese, M.; Dowling, P.; Cook, S.; Blumberg, B.; Park, J.H.; Lu, W. Prolonged Beneficial Effect of Brief Erythropoietin Peptide JM4 Therapy on Chronic Relapsing EAE. Neurotherapeutics 2020, 18, 401–411. [Google Scholar] [CrossRef]
- Edgünlü, T.G.; Ünal, Y.; Çelik, S.K.; Genç, Ö.; Emre, U.; Kutlu, G. The effect of FOXO gene family variants and global DNA metylation on RRMS disease. Gene 2019, 726, 144172. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. No effects without causes: The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. 2018, 93, 1518–1557. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Novel Insights for Multiple Sclerosis and Demyelinating Disorders with Apoptosis, Autophagy, FoxO, and mTOR. Curr. Neurovasc. Res. 2021, 18, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Oktelik, F.B.; Yilmaz, V.; Turkoglu, R.; Akbayir, E.; Tuzun, E.; Deniz, G.; Cinar, S. Expression of Akt1 and p-Akt1 in peripheral T cell subsets of multiple sclerosis patients. Acta Neurol. Belg. 2020, 121, 1777–1782. [Google Scholar] [CrossRef]
- Sanadgol, N.; Barati, M.; Houshmand, F.; Hassani, S.; Clarner, T.; Shahlaei, M.; Golab, F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol. Rep. 2019, 72, 641–658. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; Jiang, N.; He, D.; Bai, Y.; Xin, Y. Rapamycin combined with MCC950 to treat multiple sclerosis in experimental autoimmune encephalomyelitis. J. Cell. Biochem. 2018, 120, 5160–5168. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Fan, K.-Y.; Wang, Q.; Hu, J.-X.; Wang, Q.; Zhang, H.-Y.; Song, S.; Zhao, R.; Qiao, J.; Zhang, S.-X. Genetically Determined Levels of mTOR-Dependent Circulating Proteins and Risk of Multiple Sclerosis. Neurol. Ther. 2023, 12, 751–762. [Google Scholar] [CrossRef]
- Hemmer, B.; Cepok, S.; Zhou, D.; Sommer, N. Multiple Sclerosis—A Coordinated Immune Attack Across the Blood Brain Barrier. Curr. Neurovasc. Res. 2004, 1, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Erythropoietin: New Directions for the Nervous System. Int. J. Mol. Sci. 2012, 13, 11102–11129. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Tegla, C.A.; Cudrici, C.D.; Kruszewski, A.M.; Azimzadeh, P.; Boodhoo, D.; Mekala, A.P.; Rus, V.; Rus, H. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol. Res. 2014, 61, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, A.; Rodi, M.; Dimisianos, N.; Emmanuil, A.; Kalavrizioti, D.; Lagoudaki, R.; Grigoriadis, N.C.; Papathanasopoulos, P. Immune Parameters That Distinguish Multiple Sclerosis Patients from Patients with Other Neurological Disorders at Presentation. PLoS ONE 2015, 10, e0135434. [Google Scholar] [CrossRef]
- Rodi, M.; Dimisianos, N.; De Lastic, A.-L.; Sakellaraki, P.; Deraos, G.; Matsoukas, J.; Papathanasopoulos, P.; Mouzaki, A. Regulatory Cell Populations in Relapsing-Remitting Multiple Sclerosis (RRMS) Patients: Effect of Disease Activity and Treatment Regimens. Int. J. Mol. Sci. 2016, 17, 1398. [Google Scholar] [CrossRef]
- Charcot, J.M. Lectures on the Diseases of the Nervous System; New Sydenham Society: London, UK, 1877. [Google Scholar]
- Staff, N.P.; Lucchinetti, C.F.; Keegan, B.M. Multiple Sclerosis with Predominant, Severe Cognitive Impairment. Arch. Neurol. 2009, 66, 1139–1143. [Google Scholar] [CrossRef]
- Yap, S.M.; Davenport, L.; Cogley, C.; Craddock, F.; Kennedy, A.; Gaughan, M.; Kearney, H.; Tubridy, N.; De Looze, C.; O’keeffe, F.; et al. Word finding, prosody and social cognition in multiple sclerosis. J. Neuropsychol. 2022, 17, 32–62. [Google Scholar] [CrossRef]
- Jongen, P.J.; Ter Horst, A.T.; Brands, A.M. Cognitive impairment in multiple sclerosis. Minerva Med. 2012, 103, 73–96. [Google Scholar]
- Sun, J.-J.; Ren, Q.-G.; Xu, L.; Zhang, Z.-J. LINGO-1 antibody ameliorates myelin impairment and spatial memory deficits in experimental autoimmune encephalomyelitis mice. Sci. Rep. 2015, 5, srep14235. [Google Scholar] [CrossRef]
- An, X.; Yao, X.; Li, B.; Yang, W.; Cui, R.; Zhao, G.; Jin, Y. Role of BDNF-mTORC1 Signaling Pathway in Female Depression. Neural Plast. 2021, 2021, 6619515. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Z.; Wang, D.; Xiao, J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta Biochim. Et Biophys. Sin. 2020, 52, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhu, J. Roles of Exosomes and Exosomal MicroRNAs in Postoperative Sleep Disturbance. Nat. Sci. Sleep 2021, 13, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, G.; Jin, L.; Shi, J. Nicotinamide Improves Cognitive Function in Mice with Chronic Cerebral Hypoperfusion. Front. Neurol. 2021, 12, 596641. [Google Scholar] [CrossRef]
- Sakai, M.; Yu, Z.; Hirayama, R.; Nakasato, M.; Kikuchi, Y.; Ono, C.; Komatsu, H.; Nakanishi, M.; Yoshii, H.; Stellwagen, D.; et al. Deficient Autophagy in Microglia Aggravates Repeated Social Defeat Stress-Induced Social Avoidance. Neural Plast. 2022, 2022, 7503553. [Google Scholar] [CrossRef] [PubMed]
- Su, L.-D.; Wang, N.; Han, J.; Shen, Y. Group 1 Metabotropic Glutamate Receptors in Neurological and Psychiatric Diseases: Mechanisms and Prospective. Neuroscientist 2021, 28, 453–468. [Google Scholar] [CrossRef]
- Ruet, A.; Deloire, M.; Hamel, D.; Ouallet, J.-C.; Petry, K.; Brochet, B. Cognitive impairment, health-related quality of life and vocational status at early stages of multiple sclerosis: A 7-year longitudinal study. J. Neurol. 2012, 260, 776–784. [Google Scholar] [CrossRef]
- A Margrett, J.; Schofield, T.; Martin, P.; Poon, L.W.; Masaki, K.; A Donlon, T.; Kallianpur, K.J.; Willcox, B.J. Novel Functional, Health, and Genetic Determinants of Cognitive Terminal Decline: Kuakini Honolulu Heart Program/Honolulu-Asia Aging Study. J. Gerontol. Ser. A 2021, 77, 1525–1533. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Maes, M.; Puri, B.K. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol. Neurobiol. 2018, 56, 406–434. [Google Scholar] [CrossRef]
- Lord, J.S.; Rezaiezadeh, J.S.; Yekaninejad, M.S.; Izadi, P. The association of APOE genotype with COVID-19 disease severity. Sci. Rep. 2022, 12, 13483. [Google Scholar] [CrossRef]
- Caberlotto, L.; Nguyen, T.-P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M.; et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3935. [Google Scholar] [CrossRef]
- Cacabelos, R.; Carril, J.C.; Cacabelos, N.; Kazantsev, A.G.; Vostrov, A.V.; Corzo, L.; Cacabelos, P.; Goldgaber, D. Sirtuins in Alzheimer’s Disease: SIRT2-Related GenoPhenotypes and Implications for PharmacoEpiGenetics. Int. J. Mol. Sci. 2019, 20, 1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Pollard, H.B.; Arispe, N. Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-beta-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides 2002, 23, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Jia, L.; Liu, C.-C.; Rong, Z.; Zhong, L.; Yang, L.; Chen, X.-F.; Fryer, J.D.; Wang, X.; Zhang, Y.-W.; et al. TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway. J. Neurosci. 2017, 37, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Jiao, R.; Wang, P.; Zhu, Y.; Zhao, J.; De Jager, P.; Bennett, D.A.; Jin, L.; Xiong, M. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Sci. Rep. 2020, 10, 4107. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. New Strategies for Alzheimer Disease and Cognitive Impairment. Oxidative Med. Cell. Longev. 2009, 2, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z. Nicotinamide: Necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol. Sci. 2003, 24, 228–232. [Google Scholar] [CrossRef]
- Maiese, K.; Vincent, A.M. Membrane asymmetry and DNA degradation: Functionally distinct determinants of neuronal programmed cell death. J. Neurosci. Res. 2000, 59, 568–580. [Google Scholar] [CrossRef]
- Naseri, A.; Baghernezhad, K.; Seyedi-Sahebari, S.; Alhoseini, S.A.; Gholipour-Khalili, E.; Zafarani, F.; Talebi, M. The association of apolipoprotein E (ApoE) genotype and cognitive outcomes in multiple sclerosis; a systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2022, 65, 104011. [Google Scholar] [CrossRef]
- Bastakis, G.G.; Ktena, N.; Karagogeos, M.; Savvaki, M. Models and treatments for traumatic optic neuropathy and demyelinating optic neuritis. Dev. Neurobiol. 2019, 79, 819–836. [Google Scholar] [CrossRef]
- Ding, S.L.S.; Leow, S.N.; Munisvaradass, R.; Koh, E.H.; Bastion, M.L.C.; Then, K.Y.; Kumar, S.; Mok, P.L. Revisiting the role of erythropoietin for treatment of ocular disorders. Eye 2016, 30, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Momkute, L.; Vilkeviciute, A.; Gedvilaite, G.; Dubinskaite, G.; Kriauciuniene, L.; Liutkeviciene, R. Association of APOE Serum Levels and APOE ε2, ε3, and ε4 Alleles with Optic Neuritis. Genes 2022, 13, 1188. [Google Scholar] [CrossRef]
- Coelho, T.; Cruz, M.W.; Chao, C.-C.; Parman, Y.; Wixner, J.; Weiler, M.; Barroso, F.A.; Dasgupta, N.R.; Jung, S.W.; Schneider, E.; et al. Characteristics of Patients with Hereditary Transthyretin Amyloidosis-Polyneuropathy (ATTRv-PN) in NEURO-TTRansform, an Open-label Phase 3 Study of Eplontersen. Neurol. Ther. 2022, 12, 267–287. [Google Scholar] [CrossRef]
- Maiese, K. Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System. Biomolecules 2023, 13, 816. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Huang, Y.; Guo, X.-R.; Zhang, M.-Q.; Yuan, X.-S.; Zu, H.-B. DHCR24 reverses Alzheimer’s disease-related pathology and cognitive impairment via increasing hippocampal cholesterol levels in 5xFAD mice. Acta Neuropathol. Commun. 2023, 11, 102. [Google Scholar] [CrossRef]
- Adhikari, U.K.; Khan, R.; Mikhael, M.; Balez, R.; David, M.A.; Mahns, D.; Hardy, J.; Tayebi, M. Therapeutic anti-amyloid β antibodies cause neuronal disturbances. Alzheimer’s Dement. 2022, 19, 2479–2496. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, Z.; Peng, Y.; Gao, J.; Zheng, F.; Tan, J.; Xu, J.; Wang, T. Neuroprotection of Triptolide against Amyloid-Beta1-42-induced toxicity via the Akt/mTOR/p70S6K-mediated Autophagy Pathway. An. Acad. Bras. Cienc. 2022, 94, e20210938. [Google Scholar] [CrossRef]
- Tiu, V.E.; Popescu, B.O.; Enache, I.I.; Tiu, C.; Terecoasa, E.; Panea, C.A. Serum and CSF Biomarkers Predict Active Early Cognitive Decline Rather Than Established Cognitive Impairment at the Moment of RRMS Diagnosis. Diagnostics 2022, 12, 2571. [Google Scholar] [CrossRef]
- Lio, C.T.; Kacprowski, T.; Klaedtke, M.; Jensen, L.R.; Bouter, Y.; Bayer, T.A.; Kuss, A.W. Small RNA Sequencing in the Tg4–42 Mouse Model Suggests the Involvement of snoRNAs in the Etiology of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 87, 1671–1681. [Google Scholar] [CrossRef]
- Schubert, C.R.; Paulsen, A.J.; Pinto, A.A.; Merten, N.; Cruickshanks, K.J. Effect of Long-Term Storage on the Reliability of Blood Biomarkers for Alzheimer’s Disease and Neurodegeneration. J. Alzheimer’s Dis. 2022, 85, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Shiravandi, A.; Yari, F.; Tofigh, N.; Ashtiani, M.K.; Shahpasand, K.; Ghanian, M.-H.; Shekari, F.; Faridbod, F. Earlier Detection of Alzheimer’s Disease Based on a Novel Biomarker cis P-tau by a Label-Free Electrochemical Immunosensor. Biosensors 2022, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, M.S.; Mirbaha, H.; Shang, P.; Zandee, S.; Foong, C.; Prat, A.; White, C.L.; Stuve, O.; Diamond, M.I. Tau seeding in cases of multiple sclerosis. Acta Neuropathol. Commun. 2022, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Miyamoto, Y.; Nakata, R.; Higashi, Y.; Shinmyo, Y.; Kawasaki, H.; Miyasaka, T.; Misonou, H. Identification of Tau protein as a novel marker for maturation and pathological changes of oligodendrocytes. Glia 2022, 71, 1002–1017. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Buhadily, A.K.; Al-Gareeb, A.I.; Alorabi, M.; Al-Harcan, N.A.H.; El-Bouseary, M.M.; Batiha, G.E.-S. Citicoline and COVID-19: Vis-à-vis conjectured. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Maiahy, T.J.; Alexiou, A.; Mukerjee, N.; Batiha, G.E.-S. Prostaglandins and non-steroidal anti-inflammatory drugs in COVID-19. Biotechnol. Genet. Eng. Rev. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Qahtani, A.A.; Pantazi, I.; Alhamlan, F.S.; Alothaid, H.; Matou-Nasri, S.; Sourvinos, G.; Vergadi, E.; Tsatsanis, C. SARS-CoV-2 modulates inflammatory responses of alveolar epithelial type II cells via PI3K/AKT pathway. Front. Immunol. 2022, 13, 1020624. [Google Scholar] [CrossRef]
- Assogna, M.; Di Lorenzo, F.; Martorana, A.; Koch, G. Synaptic Effects of Palmitoylethanolamide in Neurodegenerative Disorders. Biomolecules 2022, 12, 1161. [Google Scholar] [CrossRef]
- Beegum, F.; Anuranjana, P.V.; George, K.T.; Divya, K.P.; Begum, F.; Krishnadas, N.; Shenoy, R.R. Sirtuins as therapeutic targets for improving delayed wound healing in diabetes. J. Drug Target. 2022, 30, 911–926. [Google Scholar] [CrossRef]
- Braun, S.; Zaucke, F.; Brenneis, M.; Rapp, A.E.; Pollinger, P.; Sohn, R.; Jenei-Lanzl, Z.; Meurer, A. The Corpus Adiposum Infrapatellare (Hoffa’s Fat Pad)—The Role of the Infrapatellar Fat Pad in Osteoarthritis Pathogenesis. Biomedicines 2022, 10, 1071. [Google Scholar] [CrossRef]
- Feng, H.; Xue, M.; Deng, H.; Cheng, S.; Hu, Y.; Zhou, C. Ginsenoside and Its Therapeutic Potential for Cognitive Impairment. Biomolecules 2022, 12, 1310. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Hou, J. FoxO proteins: Cunning concepts and considerations for the cardiovascular system. Clin. Sci. 2009, 116, 191–203. [Google Scholar] [CrossRef]
- Maiese, K. New Avenues of Exploration for Erythropoietin. JAMA 2005, 293, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.; Vieira, M.; Delerue-Matos, C.; Grosso, C.; Soares, C. Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review. Mar. Drugs 2022, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, L.; Zhang, C.; Xu, Q.; Sun, Y. Targeting protein phosphatases for the treatment of inflammation-related diseases: From signaling to therapy. Signal Transduct. Target. Ther. 2022, 7, 177. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, P.; Clements, T.; Venø, M.; Abrahams, V.M.; Holder, B. Distinct non-coding RNA cargo of extracellular vesicles from M1 and M2 human primary macrophages. J. Extracell. Vesicles 2022, 11, 12293. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, L.; Cao, W.; Wu, J.; Chen, X. Identification of Inhibitors and Drug Targets for Human Adenovirus Infections. Viruses 2022, 14, 959. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Hou, Y.; Leverenz, J.B.; Kallianpur, A.; Mehra, R.; Liu, Y.; Yu, H.; Pieper, A.A.; Jehi, L.; et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimer’s Res. Ther. 2021, 13, 110. [Google Scholar] [CrossRef]
- Zhuang, X.; Tsukuda, S.; Wrensch, F.; Wing, P.A.; Schilling, M.; Harris, J.M.; Borrmann, H.; Morgan, S.B.; Cane, J.L.; Mailly, L.; et al. The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. bioRxiv 2021. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar] [CrossRef]
- du Toit, W.L.; Kruger, R.; Gafane-Matemane, L.F.; Schutte, A.E.; Louw, R.; Mels, C.M.C. Markers of arterial stiffness and urinary metabolomics in young adults with early cardiovascular risk: The African-PREDICT study. Metabolomics 2023, 19, 1–14. [Google Scholar] [CrossRef]
- Maiese, K. Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen. Res. 2015, 10, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Front. Biosci. 2020, 25, 1925–1973. [Google Scholar] [CrossRef] [PubMed]
- Slezáková, D.; Kadlic, P.; Jezberová, M.; Boleková, V.; Valkovič, P.; Minar, M. Brain volume loss in multiple sclerosis is independent of disease activity and might be prevented by early disease-modifying therapy. Neurol. I Neurochir. Pol. 2023, 57, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2015, 277, 58–67. [Google Scholar] [CrossRef]
- Dello Russo, C.; Lisi, L.; Feinstein, D.L.; Navarra, P. mTOR kinase, a key player in the regulation of glial functions: Relevance for the therapy of multiple sclerosis. Glia 2012, 61, 301–311. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin. Ther. Targets 2012, 16, 1203–1214. [Google Scholar] [CrossRef]
- Cheema, P.S.; Nandi, D.; Nag, A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021, 11, 210069. [Google Scholar] [CrossRef]
- Guo, P.-W.; Huang, H.-T.; Ma, J.; Zuo, Y.; Huang, D.; He, L.-L.; Wan, Z.-M.; Chen, C.; Yang, F.-F.; You, Y.-W. Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling. Mol. Med. 2021, 27, 113. [Google Scholar] [CrossRef]
- Hajializadeh, Z.; Khaksari, M. The protective effects of 17-β estradiol and SIRT1 against cardiac hypertrophy: A review. Heart Fail. Rev. 2021, 27, 725–738. [Google Scholar] [CrossRef]
- Mansour, R.M.; El Sayed, N.S.; Ahmed, M.A.E.; El-Sahar, A.E. Addressing Peroxisome Proliferator-Activated Receptor-gamma in 3-Nitropropionic Acid-Induced Striatal Neurotoxicity in Rats. Mol. Neurobiol. 2022, 59, 4368–4383. [Google Scholar] [CrossRef]
- Najjar, R.S.; Turner, C.G.; Wong, B.J.; Feresin, R.G. Berry-Derived Polyphenols in Cardiovascular Pathologies: Mechanisms of Disease and the Role of Diet and Sex. Nutrients 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Ran, D.; Hong, W.; Yan, W.; Mengdie, W. Properties and molecular mechanisms underlying geniposide-mediated therapeutic effects in chronic inflammatory diseases. J. Ethnopharmacol. 2021, 273, 113958. [Google Scholar] [CrossRef] [PubMed]
- Ren, L. Circular RNA PIP5K1A act as microRNA-552-3p sponge to regulates inflammation, oxidative damage in glucolipotoxicity-induced pancreatic INS-1 β-cells via Janus kinase 1. Bioengineered 2022, 13, 5724–5736. [Google Scholar] [CrossRef]
- Sabzali, M.; Eidi, A.; Khaksari, M.; Khastar, H. Anti-inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox. Res. 2022, 40, 605–613. [Google Scholar] [CrossRef]
- Sharma, V.K.; Singh, T.G.; Singh, S.; Garg, N.; Dhiman, S. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem. Res. 2021, 46, 3103–3122. [Google Scholar] [CrossRef]
- Farahani, M.; Niknam, Z.; Amirabad, L.M.; Amiri-Dashatan, N.; Koushki, M.; Nemati, M.; Pouya, F.D.; Rezaei-Tavirani, M.; Rasmi, Y.; Tayebi, L. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed. Pharmacother. 2022, 145, 112420. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhao, Y.; Wang, Y.; Xie, R.; Tong, Y.; Sauer, J.-D.; Gong, S. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat. Nanotechnol. 2022, 17, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.; Zeng, C.; Xia, Z.; Zhao, B. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. FASEB J. 2021, 35, 1–16. [Google Scholar] [CrossRef]
- Maiese, K. Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr. Neurovasc. Res. 2017, 14, 184–189. [Google Scholar] [CrossRef]
- Pabel, S.; Hamdani, N.; Luedde, M.; Sossalla, S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure—Has the Mystery Been Unravelled? Curr. Heart Fail. Rep. 2021, 18, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dou, S.; Zhu, J.; Shao, Z.; Wang, C.; Cheng, B. Regulatory effects of ghrelin on endoplasmic reticulum stress, oxidative stress, and autophagy: Therapeutic potential. Neuropeptides 2020, 85, 102112. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Ye, W.; Liu, J.; Zhou, L.; Song, Y. The Emerging Key Role of Klotho in the Hypothalamus–Pituitary–Ovarian Axis. Reprod. Sci. 2020, 28, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, S.; Zhang, X.; Chen, L. Targeting PRAS40: A novel therapeutic strategy for human diseases. J. Drug Target. 2021, 29, 703–715. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef]
- Zhao, Y.; Lützen, U.; Gohlke, P.; Jiang, P.; Herdegen, T.; Culman, J. Neuroprotective and antioxidative effects of pioglitazone in brain tissue adjacent to the ischemic core are mediated by PI3K/Akt and Nrf2/ARE pathways. J. Mol. Med. 2021, 99, 1073–1083. [Google Scholar] [CrossRef]
- Li, W.; Zhu, L.; Ruan, Z.-B.; Wang, M.-X.; Ren, Y.; Lu, W. Nicotinamide protects chronic hypoxic myocardial cells through regulating mTOR pathway and inducing autophagy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5503–5511. [Google Scholar]
- Xu, G.; Shen, H.; Nibona, E.; Wu, K.; Ke, X.; Al Hafiz, A.; Liang, X.; Zhong, X.; Zhou, Q.; Qi, C.; et al. Fundc1 is necessary for proper body axis formation during embryogenesis in zebrafish. Sci. Rep. 2019, 9, 18910. [Google Scholar] [CrossRef]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Chen, G.; Zeng, L.; Yan, F.; Liu, J.; Qin, M.; Wang, F.; Zhang, X. Long-term oral administration of naringenin counteracts aging-related retinal degeneration via regulation of mitochondrial dynamics and autophagy. Front. Pharmacol. 2022, 13, 919905. [Google Scholar] [CrossRef]
- Cui, L.; Weiyao, J.; Chenghong, S.; Limei, L.; Xinghua, Z.; Bo, Y.; Xiaozheng, D.; Haidong, W. Rheumatoid arthritis and mitochondrial homeostasis: The crossroads of metabolism and immunity. Front. Med. 2022, 9, 1017650. [Google Scholar] [CrossRef] [PubMed]
- Ojo, J.O.; Reed, J.M.; Crynen, G.; Vallabhaneni, P.; Evans, J.; Shackleton, B.; Eisenbaum, M.; Ringland, C.; Edsell, A.; Mullan, M.; et al. APOE genotype dependent molecular abnormalities in the cerebrovasculature of Alzheimer’s disease and age-matched non-demented brains. Mol. Brain 2021, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagy, A.E.-F.B.M.; Saleh, A.M.; Attaallah, A.; Gahnem, R.A. Therapeutic role of Azadirachta indica leaves ethanolic extract against diabetic nephropathy in rats neonatally induced by streptozotocin. Ultrastruct. Pathol. 2021, 45, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J. Diabetes 2015, 6, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Programming Apoptosis and Autophagy with Novel Approaches for Diabetes Mellitus. Curr. Neurovasc. Res. 2015, 12, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss. Curr. Neurovasc. Res. 2018, 14, 415–420. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, J.; Wu, L.; Zhang, H.; Lv, J.; Chen, N. Exercise-Induced Autophagy Suppresses Sarcopenia through Akt/mTOR and Akt/FoxO3a Signal Pathways and AMPK-Mediated Mitochondrial Quality Control. Front. Physiol. 2020, 11, 583478. [Google Scholar] [CrossRef]
- Li, L.; Sun, Y.; Zhang, Y.; Wang, W.; Ye, C. Mutant Huntingtin Impairs Pancreatic β-cells by Recruiting IRS-2 and Disturbing the PI3K/AKT/FoxO1 Signaling Pathway in Huntington’s Disease. J. Mol. Neurosci. 2021, 71, 2646–2658. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Wu, C.; Li, D.; Wu, Y.; Ye, L.; Ye, L.; Chen, X.; Li, P.; Yuan, Y.; et al. Acidic fibroblast growth factor attenuates type 2 diabetes-induced demyelination via suppressing oxidative stress damage. Cell Death Dis. 2021, 12, 107. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Wang, S.; Shang, Y.C. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. Int. J. Mol. Sci. 2012, 13, 13830–13866. [Google Scholar] [CrossRef]
- Zarneshan, S.N.; Fakhri, S.; Farzaei, M.H.; Khan, H.; Saso, L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem. Toxicol. 2020, 145, 111714. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, H.; Yu, P.; Qian, T.; Xu, X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front. Med. 2021, 8, 644121. [Google Scholar] [CrossRef]
- Maiese, K. Erythropoietin and diabetes mellitus. World J. Diabetes 2015, 6, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Swain, O.; Romano, S.K.; Miryala, R.; Tsai, J.; Parikh, V.; Umanah, G.K.E. SARS-CoV-2 Neuronal Invasion and Complications: Potential Mechanisms and Therapeutic Approaches. J. Neurosci. 2021, 41, 5338–5349. [Google Scholar] [CrossRef]
- Yamashima, T.; Ota, T.; Mizukoshi, E.; Nakamura, H.; Yamamoto, Y.; Kikuchi, M.; Yamashita, T.; Kaneko, S. Intake of ω-6 Polyunsaturated Fatty Acid-Rich Vegetable Oils and Risk of Lifestyle Diseases. Adv. Nutr. Int. Rev. J. 2020, 11, 1489–1509. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Suo, H.; Song, J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2020, 61, 3857–3875. [Google Scholar] [CrossRef]
- Ciesielska, K.; Gajewska, M. Fatty Acids as Potent Modulators of Autophagy Activity in White Adipose Tissue. Biomolecules 2023, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.D.; Jha, N.K.; Ojha, S.; Sadek, B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules 2023, 28, 1889. [Google Scholar] [CrossRef]
- Zhao, W.; Xie, C.; Zhang, X.; Liu, J.; Liu, J.; Xia, Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023, 13, e2995. [Google Scholar] [CrossRef]
- Tramutola, A.; Lanzillotta, S.; Aceto, G.; Pagnotta, S.; Ruffolo, G.; Cifelli, P.; Marini, F.; Ripoli, C.; Palma, E.; Grassi, C.; et al. Intranasal Administration of KYCCSRK Peptide Rescues Brain Insulin Signaling Activation and Reduces Alzheimer’s Disease-like Neuropathology in a Mouse Model for Down Syndrome. Antioxidants 2023, 12, 111. [Google Scholar] [CrossRef]
- Corti, O.; Blomgren, K.; Poletti, A.; Beart, P.M. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J. Neurochem. 2020, 154, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Matalonga, L.; Gort, L.; Ribes, A. Small molecules as therapeutic agents for inborn errors of metabolism. J. Inherit. Metab. Dis. 2016, 40, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Moors, T.E.; Hoozemans, J.J.M.; Ingrassia, A.; Beccari, T.; Parnetti, L.; Chartier-Harlin, M.-C.; van de Berg, W.D.J. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol. Neurodegener. 2017, 12, 11. [Google Scholar] [CrossRef]
- Zhou, Z.D.; Selvaratnam, T.; Lee, J.C.T.; Chao, Y.X.; Tan, E.-K. Molecular targets for modulating the protein translation vital to proteostasis and neuron degeneration in Parkinson’s disease. Transl. Neurodegener. 2019, 8, 6. [Google Scholar] [CrossRef]
- Ali, T.; Rahman, S.U.; Hao, Q.; Li, W.; Liu, Z.; Shah, F.A.; Murtaza, I.; Zhang, Z.; Yang, X.; Liu, G.; et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020, 69, e12667. [Google Scholar] [CrossRef]
- Boga, J.A.; Coto-Montes, A. ER stress and autophagy induced by SARS-CoV-2: The targets for melatonin treatment. Melatonin Res. 2020, 3, 346–361. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Beiram, R.; Azimullah, S.; Mf, N.M.; Ojha, S.K.; Adem, A.; Jalal, F.Y. Valeric Acid Protects Dopaminergic Neurons by Suppressing Oxidative Stress, Neuroinflammation and Modulating Autophagy Pathways. Int. J. Mol. Sci. 2020, 21, 7670. [Google Scholar] [CrossRef]
- Qi, X.; Mitter, S.K.; Yan, Y.; Busik, J.V.; Grant, M.B.; E Boulton, M. Diurnal Rhythmicity of Autophagy Is Impaired in the Diabetic Retina. Cells 2020, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Q.; Kumar, A.V.; Mills, J.; Lapierre, L.R. C. elegans to model autophagy-related human disorders. Prog. Mol. Biol. Transl. Sci. 2020, 172, 325–373. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.-T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- He, W.; Gao, Y.; Zhou, J.; Shi, Y.; Xia, D.; Shen, H.-M. Friend or Foe? Implication of the autophagy-lysosome pathway in SARS-CoV-2 infection and COVID-19. Int. J. Biol. Sci. 2022, 18, 4690–4703. [Google Scholar] [CrossRef]
- Maity, S.; Saha, A. Therapeutic Potential of Exploiting Autophagy Cascade Against Coronavirus Infection. Front. Microbiol. 2021, 12, 675419. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, U.; Amidei, C.B.; Avossa, F.; Schievano, E.; Kingwell, E. Association of multiple sclerosis-related mortality with COVID-19 and other common infections: A multiple causes of death analysis. Eur. J. Neurol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Maiese, K. FoxO Proteins in the Nervous System. Anal. Cell. Pathol. 2015, 2015, 569392. [Google Scholar] [CrossRef]
- Zhao, T.; Miao, H.; Song, Z.; Li, Y.; Xia, N.; Zhang, Z.; Zhang, H. Metformin alleviates the cognitive impairment induced by benzo[a]pyrene via glucolipid metabolism regulated by FTO/FoxO6 pathway in mice. Environ. Sci. Pollut. Res. 2023, 30, 69192–69204. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr. Neurovasc. Res. 2010, 7, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal 2010, 22, 1317–1329. [Google Scholar] [CrossRef]
- Taveira, G.B.; Mello, É.O.; Souza, S.B.; Monteiro, R.M.; Ramos, A.C.; Carvalho, A.O.; Rodrigues, R.; Okorokov, L.A.; Gomes, V.M. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of caspases and extracellular H+ flux. Biosci. Rep. 2018, 38, BSR20180119. [Google Scholar] [CrossRef]
- Almasieh, M.; Catrinescu, M.-M.; Binan, L.; Costantino, S.; Levin, L.A. Axonal Degeneration in Retinal Ganglion Cells Is Associated with a Membrane Polarity-Sensitive Redox Process. J. Neurosci. 2017, 37, 3824–3839. [Google Scholar] [CrossRef]
- Viola, G.; Bortolozzi, R.; Hamel, E.; Moro, S.; Brun, P.; Castagliuolo, I.; Ferlin, M.G.; Basso, G. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem. Pharmacol. 2012, 83, 16–26. [Google Scholar] [CrossRef]
- Bailey, T.J.; Fossum, S.L.; Fimbel, S.M.; Montgomery, J.E.; Hyde, D.R. The inhibitor of phagocytosis, O-phospho-l-serine, suppresses Müller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp. Eye Res. 2010, 91, 601–612. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr. Neurovasc. Res. 2009, 6, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, C.; Lei, M.; Li, G.; Yi, L.; Luo, F.; Li, Y.; Ding, L.; Liu, Z.; Li, S.; et al. Activation of Wnt/β-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J. Mol. Neurosci. 2012, 49, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Wang, S.; Shang, Y.C.; Chong, Z.Z.; Maiese, K. Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity during Oxidant Stress. Curr. Neurovasc. Res. 2011, 8, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, I.-H.; Nam, J.-B.; Cho, Y.; Chung, D.-Y.; Kim, S.-H.; Kim, J.-S.; Cho, Y.-D.; Hong, E.-K.; Sohn, N.-W.; et al. Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules 2015, 20, 1904–1921. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.-J.; Yuan, B.; Yu, B.; Wang, Y.-Q.; Wu, J.-J.; Zhou, W.-H.; Qiu, Z. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci. Rep. 2015, 5, 7645. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, L.; Chen, T.; Liu, Z.; Liu, H.; Li, Z. Erythropoietin Attenuates Advanced Glycation Endproducts-Induced Toxicity of Schwann Cells In Vitro. Neurochem. Res. 2015, 40, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.Z.; Kang, J.-Q.; Maiese, K. Erythropoietin Is a Novel Vascular Protectant through Activation of Akt1 and Mitochondrial Modulation of Cysteine Proteases. Circulation 2002, 106, 2973–2979. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; Jin, H.; Ullah, M.; Wang, X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am. J. Cancer Res. 2021, 11, 5233–5248. [Google Scholar]
- Pang, Y.; Qin, M.; Hu, P.; Ji, K.; Xiao, R.; Sun, N.; Pan, X.; Zhang, X. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int. J. Mol. Med. 2020, 46, 1707–1720. [Google Scholar] [CrossRef]
- Lan, T.; Xu, Y.; Li, S.; Li, N.; Zhang, S.; Zhu, H. Cornin protects against cerebral ischemia/reperfusion injury by preventing autophagy via the PI3K/Akt/mTOR pathway. BMC Pharmacol. Toxicol. 2022, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, S.; Li, P.; Li, L. A novel adipokine WISP1 attenuates lipopolysaccharide-induced cell injury in 3T3-L1 adipocytes by regulating the PI3K/Akt pathway. Obes. Res. Clin. Pract. 2022, 16, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. WISP1: Clinical insights for a proliferative and restorative member of the CCN family. Curr. Neurovasc. Res. 2014, 11, 378–389. [Google Scholar] [CrossRef]
- He, C.; Xu, Y.; Sun, J.; Li, L.; Zhang, J.H.; Wang, Y. Autophagy and Apoptosis in Acute Brain Injuries: From Mechanism to Treatment. Antioxid. Redox Signal. 2023, 38, 234–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Liu, Y.; Qian, Z.-M.; Luo, Q.-Q.; Ke, Y. CX3CL1/CX3CR1 Axis Plays a Key Role in Ischemia-Induced Oligodendrocyte Injury via p38MAPK Signaling Pathway. Mol. Neurobiol. 2015, 53, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhao, Y.; Zhu, Y.; Wang, W.; Liu, X.; Lu, F. Interfering TUG1 Attenuates Cerebrovascular Endothelial Apoptosis and Inflammatory injury after Cerebral Ischemia/Reperfusion via TUG1/miR-410/FOXO3 ceRNA Axis. Neurotox. Res. 2021, 40, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Zheng, V.; Chen, L.; Ma, M.; Shen, S.; Qu, J.; Zhang, H.; Gurney, M.E.; O’donnell, J.M.; et al. A Novel PDE4D Inhibitor BPN14770 Reverses Scopolamine-Induced Cognitive Deficits via cAMP/SIRT1/Akt/Bcl-2 Pathway. Front. Cell Dev. Biol. 2020, 8, 599389. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A.; Hu, D.; Chereshnev, V. Problems of Pathogenesis and Pathogenetic Therapy of COVID-19 from the Perspective of the General Theory of Pathological Systems (General Pathological Processes). Int. J. Mol. Sci. 2021, 22, 7582. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Kiasalari, Z.; Rahmani, T.; Sanaierad, A.; Afshin-Majd, S.; Naderi, G.; Baluchnejadmojarad, T.; Roghani, M. Diosgenin Attenuates Cognitive Impairment in Streptozotocin-Induced Diabetic Rats: Underlying Mechanisms. Neuropsychobiology 2020, 80, 25–35. [Google Scholar] [CrossRef]
- Xu, T.; Liu, J.; Li, X.-R.; Yu, Y.; Luo, X.; Zheng, X.; Cheng, Y.; Yu, P.-Q.; Liu, Y. The mTOR/NF-κB Pathway Mediates Neuroinflammation and Synaptic Plasticity in Diabetic Encephalopathy. Mol. Neurobiol. 2021, 58, 3848–3862. [Google Scholar] [CrossRef]
- Malhotra, S.; Hurtado-Navarro, L.; Pappolla, A.; Villar, L.M.M.; Río, J.; Montalban, X.; Pelegrin, P.; Comabella, M. Increased NLRP3 Inflammasome Activation and Pyroptosis in Patients with Multiple Sclerosis with Fingolimod Treatment Failure. Neurol.-Neuroimmunol. Neuroinflammation 2023, 10, e200100. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (CoV-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331. [Google Scholar] [CrossRef]
- Crespo, I.; Fernández-Palanca, P.; San-Miguel, B.; Álvarez, M.; González-Gallego, J.; Tuñón, M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell. Mol. Med. 2020, 24, 7625–7636. [Google Scholar] [CrossRef]
- Park, M.H.; Gutiérrez-García, A.K.; Choudhury, M. Mono-(2-ethylhexyl) Phthalate Aggravates Inflammatory Response via Sirtuin Regulation and Inflammasome Activation in RAW 264.7 Cells. Chem. Res. Toxicol. 2019, 32, 935–942. [Google Scholar] [CrossRef]
- Vaamonde-García, C.; López-Armada, M.J. Role of mitochondrial dysfunction on rheumatic diseases. Biochem. Pharmacol. 2019, 165, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Yan, W.-T.; Lu, S.; Yang, Y.-D.; Ning, W.-Y.; Cai, Y.; Hu, X.-M.; Zhang, Q. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural Regen. Res. 2021, 16, 1628–1637. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Maiese, K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: Diversified control of cell growth, inflammation, and injury. Histol. Histopathol. 2007, 22, 1251–1267. [Google Scholar] [CrossRef]
- Qin, D.; Li, D.; Wang, C.; Guo, S. Ferroptosis and central nervous system demyelinating diseases. J. Neurochem. 2023, 165, 759–771. [Google Scholar] [CrossRef]
- Maiese, K. The Implications of Telomere Length: Advanced Aging, Cell Senescence, MRI Phenotypes, Stem Cells and Alzheimer’s Disease. Curr. Neurovasc. Res. 2023; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Ferroptosis, Iron Metabolism, and Forkhead Transcription Factors (FoxOs). Curr. Neurovasc. Res. 2023; ahead of print. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Meuth, S.G.; Peixoto, C.A. The role of iron metabolism in the pathogenesis and treatment of multiple sclerosis. Front. Immunol. 2023, 14, 1137635. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Ai, R.; Mu, S.; Niu, X.; Guo, Z.; Liu, L. MiR-19a suppresses ferroptosis of colorectal cancer cells by targeting IREB2. Bioengineered 2022, 13, 12021–12029. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, Y.; Chen, J.; Zou, P.; Li, J. Transcriptional activation of ENPP2 by FoxO4 protects cardiomyocytes from doxorubicin-induced toxicity. Mol. Med. Rep. 2021, 24, 12307. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Korićanac, G.; Tepavčević, S.; Stanišić, J.; Romić, S.; Ćulafić, T.; Ivković, T.; Stojiljković, M. Low-Intensity Exercise Affects Cardiac Fatty Acid Oxidation by Increasing the Nuclear Content of PPARα, FOXO1, and Lipin1 in Fructose-Fed Rats. Metab. Syndr. Relat. Disord. 2023, 21, 122–131. [Google Scholar] [CrossRef] [PubMed]
- E Sierra-Pagan, J.; Dsouza, N.; Das, S.; A Larson, T.; Sorensen, J.R.; Ma, X.; Stan, P.; Wanberg, E.J.; Shi, X.; Garry, M.G.; et al. FOXK1 regulates Wnt signalling to promote cardiogenesis. Cardiovasc. Res. 2023, 119, 1728–1739. [Google Scholar] [CrossRef]
- Jain, S. A Computational Model for Detection of Lung Diseases Due to Forkhead Transcription Factors. In Emergent Converging Technologies and Biomedical Systems; Lecture Notes in Electrical Engineering; Springer: Singapore, 2022; pp. 71–81. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Xue, K.; Yu, R.; Li, C.; Fei, W.; Ning, X.; Han, Y.; Wang, Z.; Shu, J.; et al. Deficiency of two-pore segment channel 2 contributes to systemic lupus erythematosus via regulation of apoptosis and cell cycle. Chin. Med. J. 2022, 135, 447–455. [Google Scholar] [CrossRef]
- O’donnell, B.T.; Monjure, T.A.; Al-Ghadban, S.; Ives, C.J.; L’ecuyer, M.P.; Rhee, C.; Romero-Lopez, M.; Li, Z.; Goodman, S.B.; Lin, H.; et al. Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors. Cells 2022, 11, 2367. [Google Scholar] [CrossRef]
- Maiese, K. Forkhead Transcription Factors: Vital Elements in Biology and Medicine; Advances in Experimental Medicine and Biology; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010; p. 665. [Google Scholar]
- Beretta, G.L.; Corno, C.; Zaffaroni, N.; Perego, P. Role of FoxO Proteins in Cellular Response to Antitumor Agents. Cancers 2019, 11, 90. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol suppresses acetaminophen-induced liver damage by upregulation/activation of SIRT1. Pharm. Biol. 2021, 59, 144–154. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Yu, M. MicroRNA-4722-5p and microRNA-615-3p serve as potential biomarkers for Alzheimer’s disease. Exp. Ther. Med. 2022, 23, 241. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Z.; Yu, Y.; Jia, Y.; Li, L.; Shi, X.; Wang, F. Exploring the potential mechanism of action of ursolic acid against gastric cancer and COVID-19 using network pharmacology and bioinformatics analysis. Curr. Pharm. Des. 2023, 29, 1274–1292. [Google Scholar] [CrossRef]
- Maiese, K. Forkhead transcription factors: New considerations for alzheimer’s disease and dementia. J. Transl. Sci. 2016, 2, 241–247. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. Oxidative stress: Biomarkers and novel therapeutic pathways. Exp. Gerontol. 2010, 45, 217–234. [Google Scholar] [CrossRef]
- Razzaghi, A.; Choobineh, S.; Gaeini, A.; Soori, R. Interaction of exercise training with taurine attenuates infarct size and cardiac dysfunction via Akt–Foxo3a–Caspase-8 signaling pathway. Amino Acids 2023, 1–12. [Google Scholar] [CrossRef]
- Sanphui, P.; Das, A.K.; Biswas, S.C. Forkhead Box O3a requires BAF57, a subunit of chromatin remodeler SWI/SNF complex for induction of p53 up-regulated modulator of apoptosis (Puma) in a model of Parkinson’s disease. J. Neurochem. 2020, 154, 547–561. [Google Scholar] [CrossRef]
- Yaman, D.; Takmaz, T.; Yüksel, N.; Dinçer, S.A.; Şahin, F.I. Evaluation of silent information regulator T (SIRT) 1 and Forkhead Box O (FOXO) transcription factor 1 and 3a genes in glaucoma. Mol. Biol. Rep. 2020, 47, 9337–9344. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, Q.; Bi, X.-J. MiRNA-96 accelerates the malignant progression of ovarian cancer via targeting FOXO3a. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 65–73. [Google Scholar] [PubMed]
- Zhao, H.Y.; Li, H.Y.; Jin, J.; Jin, J.Z.; Zhang, L.Y.; Xuan, M.Y.; Jin, X.M.; Jiang, Y.J.; Zheng, H.L.; Jin, Y.S.; et al. L-carnitine treatment attenuates renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. Korean J. Intern. Med. 2021, 36, S180–S195. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Gao, C.-C.; Qi, M.; Han, Y.-L.; Zhou, M.-L.; Zheng, L.-R. Expression of FOXO transcription factors in the brain following traumatic brain injury. Neurosci. Lett. 2021, 753, 135882. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Hou, J. A “FOXO” in sight: Targeting Foxo proteins from conception to cancer. Med. Res. Rev. 2008, 29, 395–418. [Google Scholar] [CrossRef]
- Maiese, K.; Hou, J.; Chong, Z.Z.; Shang, Y.C. A Fork in the Path: Developing Therapeutic Inroads with FoxO Proteins. Oxidative Med. Cell. Longev. 2009, 2, 119–129. [Google Scholar] [CrossRef]
- Clark, K.L.; Halay, E.D.; Lai, E.; Burley, S.K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 1993, 364, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Chen, H.; Liu, X.; Wang, Y.; Fan, A.; Qi, L.; Pan, L.; Bai, W.; Zhang, Y.; Sun, Y. ID1 inhibits foot-and-mouth disease virus replication via targeting of interferon pathways. FEBS J. 2021, 288, 4364–4381. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; El-Kott, A.F. Acylated ghrelin protects against doxorubicin-induced nephropathy by activating silent information regulator 1. Basic Clin. Pharmacol. Toxicol. 2021, 128, 805–821. [Google Scholar] [CrossRef]
- Salih, D.A.; Rashid, A.J.; Colas, D.; de la Torre-Ubieta, L.; Zhu, R.P.; Morgan, A.A.; Santo, E.E.; Ucar, D.; Devarajan, K.; Cole, C.J.; et al. FoxO6 regulates memory consolidation and synaptic function. Genes Dev. 2012, 26, 2780–2801. [Google Scholar] [CrossRef] [PubMed]
- Salcher, S.; Spoden, G.; Hagenbuchner, J.; Führer, S.; Kaserer, T.; Tollinger, M.; Huber-Cantonati, P.; Gruber, T.; Schuster, D.; Gust, R.; et al. A drug library screen identifies Carbenoxolone as novel FOXO inhibitor that overcomes FOXO3-mediated chemoprotection in high-stage neuroblastoma. Oncogene 2019, 39, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Dorman, J.B.; Rodan, A.; Kenyon, C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Sangaletti, R.; D’amico, M.; Grant, J.; Della-Morte, D.; Bianchi, L. Knock-out of a mitochondrial sirtuin protects neurons from degeneration in Caenorhabditis elegans. PLoS Genet. 2017, 13, e1006965. [Google Scholar] [CrossRef]
- Gilels, F.; Paquette, S.T.; Zhang, J.; Rahman, I.; White, P.M. Mutation of Foxo3 Causes Adult Onset Auditory Neuropathy and Alters Cochlear Synapse Architecture in Mice. J. Neurosci. 2013, 33, 18409–18424. [Google Scholar] [CrossRef]
- Maiese, K.; Li, F.; Chong, Z.Z. Erythropoietin in the brain: Can the promise to protect be fulfilled? Trends Pharmacol. Sci. 2004, 25, 577–583. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Lin, S.-H.; Maiese, K. The NAD+ Precursor Nicotinamide Governs Neuronal Survival during Oxidative Stress through Protein Kinase B Coupled to FOXO3a and Mitochondrial Membrane Potential. J. Cereb. Blood Flow Metab. 2004, 24, 728–743. [Google Scholar] [CrossRef]
- Wang, S.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. WISP1 neuroprotection requires FoxO3a post-translational modulation with autoregulatory control of SIRT1. Curr. Neurovasc. Res. 2013, 10, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Zeldich, E.; Chen, C.-D.; Colvin, T.A.; Bove-Fenderson, E.A.; Liang, J.; Zhou, T.B.T.; Harris, D.A.; Abraham, C.R. The Neuroprotective Effect of Klotho is Mediated via Regulation of Members of the Redox System. J. Biol. Chem. 2014, 289, 24700–24715. [Google Scholar] [CrossRef] [PubMed]
- Calabuig-Navarro, V.; Yamauchi, J.; Lee, S.; Zhang, T.; Liu, Y.-Z.; Sadlek, K.; Coudriet, G.M.; Piganelli, J.D.; Jiang, C.-L.; Miller, R.; et al. Forkhead Box O6 (FoxO6) Depletion Attenuates Hepatic Gluconeogenesis and Protects against Fat-induced Glucose Disorder in Mice. J. Biol. Chem. 2015, 290, 15581–15594. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, M.F.; Jacobs, F.M.; Smidt, M.P.; Burbach, J.P.H. Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr. Patterns 2006, 6, 134–140. [Google Scholar] [CrossRef] [PubMed]
- van der Heide, L.P.; Jacobs, F.M.J.; Burbach, J.P.H.; Hoekman, M.F.M.; Smidt, M.P. FoxO6 transcriptional activity is regulated by Thr26 and Ser184, independent of nucleo-cytoplasmic shuttling. Biochem. J. 2005, 391, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Guo, Y.; Lou, S. LncRNA ANCR Promotes Invasion and Migration of Gastric Cancer by Regulating FoxO1 Expression to Inhibit Macrophage M1 Polarization. Dig. Dis. Sci. 2020, 65, 2863–2872. [Google Scholar] [CrossRef]
- Lee, S.-J.; Seo, B.-R.; Choi, E.-J.; Koh, J.-Y. The role of reciprocal activation of cAbl and Mst1 in the Oxidative death of cultured astrocytes. Glia 2014, 62, 639–648. [Google Scholar] [CrossRef]
- Xiong, X.; Xie, R.; Zhang, H.; Gu, L.; Xie, W.; Cheng, M.; Jian, Z.; Kovacina, K.; Zhao, H. PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol. Dis. 2014, 66, 43–52. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, S.; Yang, J.; Zhang, Y.; Liu, Y.; Nie, J.; Meng, D.; Shi, R.; Yao, Z.; Wang, M.; et al. FoxO1 overexpression reduces Aβ production and tau phosphorylation in vitro. Neurosci. Lett. 2020, 738, 135322. [Google Scholar] [CrossRef]
- Peng, S.; Li, W.; Hou, N.; Huang, N. A Review of FoxO1-Regulated Metabolic Diseases and Related Drug Discoveries. Cells 2020, 9, 184. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Regeneration in the nervous system with erythropoietin. Front. Biosci. 2016, 21, 561–596. [Google Scholar] [CrossRef]
- Hou, J.; Chong, Z.Z.; Shang, Y.C.; Maiese, K. FOXO3a governs early and late apoptotic endothelial programs during elevated glucose through mitochondrial and caspase signaling. Mol. Cell. Endocrinol. 2010, 321, 194–206. [Google Scholar] [CrossRef]
- Qi, X.-F.; Li, Y.-J.; Chen, Z.-Y.; Kim, S.-K.; Lee, K.-J.; Cai, D.-Q. Involvement of the FoxO3a pathway in the ischemia/reperfusion injury of cardiac microvascular endothelial cells. Exp. Mol. Pathol. 2013, 95, 242–247. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, J.; Leng, S.; Long, D.; Luo, X. Mitochondrial FOXO3a is involved in amyloid β peptide-induced mitochondrial dysfunction. J. Bioenerg. Biomembr. 2016, 48, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Lee, S.; Park, S.H.; Lee, J.H.; Han, S.Y.; Kim, S.T.; Kim, Y.-K.; Jeon, S.; Koo, B.-S.; Cho, K.S. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2012, 419, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Charvet, C.; Alberti, I.; Luciano, F.; Jacquel, A.; Bernard, A.; Auberger, P.; Deckert, M. Proteolytic regulation of Forkhead transcription factor FOXO3a by caspase-3-like proteases. Oncogene 2003, 22, 4557–4568. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Wang, H.; Gaur, U.; Little, P.; Xu, J.; Zheng, W. FOXO Signaling Pathways as Therapeutic Targets in Cancer. Int. J. Biol. Sci. 2017, 13, 815–827. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Daitoku, H.; Hatta, M.; Aoyama, H.; Yoshimochi, K.; Fukamizu, A. Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation. Proc. Natl. Acad. Sci. USA 2005, 102, 11278–11283. [Google Scholar] [CrossRef]
- Maiese, K. FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr. Neurovasc. Res. 2015, 12, 404–413. [Google Scholar] [CrossRef]
- Czubowicz, K.; Jęśko, H.; Wencel, P.; Lukiw, W.J.; Strosznajder, R.P. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol. Neurobiol. 2019, 56, 5436–5455. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; North, B.J.; Wei, W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2017, 1868, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Hou, J.; Maiese, K. The Forkhead Transcription Factor FOXO3a Controls Microglial Inflammatory Activation and Eventual Apoptotic Injury through Caspase 3. Curr. Neurovasc. Res. 2009, 6, 20–31. [Google Scholar] [CrossRef]
- Li, F.; Maiese, K.; Chong, Z.Z. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and β-Catenin during Oxidative Stress. Curr. Neurovasc. Res. 2006, 3, 107–117. [Google Scholar] [CrossRef]
- AlSaleh, A.; Shahid, M.; Farid, E.; Bindayna, K. The Effect of Ascorbic Acid and Nicotinamide on Panton–Valentine Leukocidin Cytotoxicity: An Ex Vivo Study. Toxins 2023, 15, 38. [Google Scholar] [CrossRef]
- Kumar, A.; Ou, Y. From bench to behaviour: The role of lifestyle factors on intraocular pressure, neuroprotection, and disease progression in glaucoma. Clin. Exp. Ophthalmol. 2023, 51, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.C.; Silva, A.; James, P.F.; Wu, S.S.X.; Howitt, J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD + activity in recipient cells. Aging Cell 2022, 21, e13647. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef]
- Rehman, I.U.; Khan, A.; Ahmad, R.; Choe, K.; Park, H.Y.; Lee, H.J.; Atiq, A.; Park, J.; Hahm, J.R.; Kim, M.O. Neuroprotective Effects of Nicotinamide against MPTP-Induced Parkinson’s Disease in Mice: Impact on Oxidative Stress, Neuroinflammation, Nrf2/HO-1 and TLR4 Signaling Pathways. Biomedicines 2022, 10, 2929. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, K.J.; Zhang, F.Y.; Xiang, B. Nicotinamide mitigates radiation injury in submandibular gland by protecting mitochondrial structure and functions. J. Oral Pathol. Med. 2022, 51, 801–809. [Google Scholar] [CrossRef]
- Maiese, K.; Chong, Z.Z.; Hou, J.; Shang, Y.C. The Vitamin Nicotinamide: Translating Nutrition into Clinical Care. Molecules 2009, 14, 3446–3485. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Supplementary Pharmacotherapy for the Behavioral Abnormalities Caused by Stressors in Humans, Focused on Post-Traumatic Stress Disorder (PTSD). J. Clin. Med. 2023, 12, 1680. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Cure of Alzheimer’s Dementia Requires Addressing All of the Affected Brain Cell Types. J. Clin. Med. 2023, 12, 2049. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, L.; Chen, W.; Cheng, J.; Zhao, X.; Zhang, Y.; Li, R.; Zhou, M.; Yao, Y.; Li, Y.; et al. Expression of EPO and related factors in the liver and kidney of plain and Tibetan sheep. Histol. Histopathol. 2023; epub ahead of print. [Google Scholar] [CrossRef]
- Govindappa, P.K.; Elfar, J.C. Erythropoietin promotes M2 macrophage phagocytosis of Schwann cells in peripheral nerve injury. Cell Death Dis. 2022, 13, 245. [Google Scholar] [CrossRef]
- Senousy, M.A.; Hanafy, M.E.; Shehata, N.; Rizk, S.M. Erythropoietin and Bacillus Calmette–Guérin Vaccination Mitigate 3-Nitropropionic Acid-Induced Huntington-like Disease in Rats by Modulating the PI3K/Akt/mTOR/P70S6K Pathway and Enhancing the Autophagy. ACS Chem. Neurosci. 2022, 13, 721–732. [Google Scholar] [CrossRef]
- Sergio, C.-M.; Rolando, C.-A. Erythropoietin regulates signaling pathways associated with neuroprotective events. Exp. Brain Res. 2022, 240, 1303–1315. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Hou, J.; Shang, Y.C.; Wang, S.; Maiese, K. EPO Relies upon Novel Signaling of Wnt1 that Requires Akt1, FoxO3a, GSK-3β, and β-Catenin to Foster Vascular Integrity during Experimental Diabetes. Curr. Neurovasc. Res. 2011, 8, 103–120. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Maiese, K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br. J. Pharmacol. 2007, 150, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, D.L.; G-Amlak, M.; Deb, D.K.; Platanias, L.C.; Uddin, S.; Wickrema, A. Phosphorylation of forkhead transcription factors by erythropoietin and stem cell factor prevents acetylation and their interaction with coactivator p300 in erythroid progenitor cells. Oncogene 2002, 21, 1556–1562. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Wu, X.; Liang, J.; Qi, Z.; Liu, X.; Min, L.; Ji, X.; Luo, Y. Erythropoietin Delivered via Intra-Arterial Infusion Reduces Endoplasmic Reticulum Stress in Brain Microvessels of Rats Following Cerebral Ischemia and Reperfusion. J. Neuroimmune Pharmacol. 2015, 10, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Weikel, K.A.; Cacicedo, J.M.; Ruderman, N.B.; Ido, Y. Knockdown of GSK3β increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells. Biosci. Rep. 2016, 36, e00382. [Google Scholar] [CrossRef]
- Vidal, R.L.; Figueroa, A.; Court, F.A.; Thielen, P.; Molina, C.; Wirth, C.; Caballero, B.; Kiffin, R.; Segura-Aguilar, J.; Cuervo, A.M.; et al. Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum. Mol. Genet. 2012, 21, 2245–2262. [Google Scholar] [CrossRef]
- Palazuelos, J.; Klingener, M.; Aguirre, A. TGF Signaling Regulates the Timing of CNS Myelination by Modulating Oligodendrocyte Progenitor Cell Cycle Exit through SMAD3/4/FoxO1/Sp1. J. Neurosci. 2014, 34, 7917–7930. [Google Scholar] [CrossRef]
- Fernandez, A.M.; Hervas, R.; Dominguez-Fraile, M.; Garrido, V.N.; Gomez-Gutierrez, P.; Vega, M.; Vitorica, J.; Perez, J.J.; Aleman, I.T. Blockade of the Interaction of Calcineurin with FOXO in Astrocytes Protects Against Amyloid-β-Induced Neuronal Death. J. Alzheimer’s Dis. 2016, 52, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Fluteau, A.; Ince, P.G.; Minett, T.; Matthews, F.E.; Brayne, C.; Garwood, C.J.; Ratcliffe, L.E.; Morgan, S.; Heath, P.R.; Shaw, P.J.; et al. The nuclear retention of transcription factor FOXO3a correlates with a DNA damage response and increased glutamine synthetase expression by astrocytes suggesting a neuroprotective role in the ageing brain. Neurosci. Lett. 2015, 609, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Biswas, S.C. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-β-induced Neuronal Death. J. Biol. Chem. 2017, 292, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.M.; Youssef, S.; Haws, M.E.; Zhang, S.Y.; Sobel, R.A.; Steinman, L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2006, 8, 74–83. [Google Scholar] [CrossRef]
- Sharma, N.; Shandilya, A.; Kumar, N.; Mehan, S. Dysregulation of SIRT-1 Signaling in Multiple Sclerosis and Neuroimmune Disorders: A Systematic Review of SIRTUIN Activators as Potential Immunomodulators and their Influences on other Dysfunctions. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 1845–1868. [Google Scholar] [CrossRef]
- Maiese, K. Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr. Neurovasc. Res. 2017, 14, 82–88. [Google Scholar] [CrossRef]
- Jahan, R.; Yousaf, M.; Khan, H.; Shah, S.A.; Khan, A.A.; Bibi, N.; Javed, F.; Ijaz, M.; Ali, A.; Wei, D.-Q. Zinc Ortho Methyl Carbonodithioate Improved Pre and Post-Synapse Memory Impairment via SIRT1/p-JNK Pathway against Scopolamine in Adult Mice. J. Neuroimmune Pharmacol. 2023, 1–12. [Google Scholar] [CrossRef]
- Sun, C.; Bai, S.; Liang, Y.; Liu, D.; Liao, J.; Chen, Y.; Zhao, X.; Wu, B.; Huang, D.; Chen, M.; et al. The role of Sirtuin 1 and its activators in age-related lung disease. Biomed. Pharmacother. 2023, 162, 114573. [Google Scholar] [CrossRef]
- Guimera, A.M.; Clark, P.; Wordsworth, J.; Anugula, S.; Rasmussen, L.J.; Shanley, D.P. Systems modelling predicts chronic inflammation and genomic instability prevent effective mitochondrial regulation during biological ageing. Exp. Gerontol. 2022, 166, 111889. [Google Scholar] [CrossRef] [PubMed]
- Sadria, M.; Seo, D.; Layton, A.T. The mixed blessing of AMPK signaling in Cancer treatments. BMC Cancer 2022, 22, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Song, X.; Zhang, D.; Guo, N.; Wu, C.; Chen, K.; Liu, Y.; Yuan, L.; Chen, X.; Huang, X. Resveratrol improves left ventricular remodeling in chronic kidney disease via Sirt1-mediated regulation of FoxO1 activity and MnSOD expression. Biofactors 2019, 46, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K. Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr. Neurovasc. Res. 2018, 15, 81–91. [Google Scholar] [CrossRef]
- Rong, Y.; Ren, J.; Song, W.; Xiang, R.; Ge, Y.; Lu, W.; Fu, T. Resveratrol Suppresses Severe Acute Pancreatitis-Induced Microcirculation Disturbance through Targeting SIRT1-FOXO1 Axis. Oxidative Med. Cell. Longev. 2021, 2021, 8891544. [Google Scholar] [CrossRef]
- Fangma, Y.; Wan, H.; Shao, C.; Jin, L.; He, Y. Research Progress on the Role of Sirtuin 1 in Cerebral Ischemia. Cell. Mol. Neurobiol. 2022, 43, 1769–1783. [Google Scholar] [CrossRef]
- Zhang, R.-B.; Ren, L.; Ding, D.-P.; Wang, H.-D.; Peng, J.; Zheng, K. Protective Effect of the SIRT1-Mediated NF-κB Signaling Pathway against Necrotizing Enterocolitis in Neonatal Mice. Eur. J. Pediatr. Surg. 2022. [Google Scholar] [CrossRef]
- Sayed, N.H.; Fathy, N.; Kortam, M.A.; Rabie, M.A.; Mohamed, A.F.; Kamel, A.S. Vildagliptin Attenuates Huntington’s Disease through Activation of GLP-1 Receptor/PI3K/Akt/BDNF Pathway in 3-Nitropropionic Acid Rat Model. Neurotherapeutics 2019, 17, 252–268. [Google Scholar] [CrossRef]
- Khan, M.; Ullah, R.; Rehman, S.U.; Shah, S.A.; Saeed, K.; Muhammad, T.; Park, H.Y.; Jo, M.H.; Choe, K.; Rutten, B.P.; et al. 17β-Estradiol Modulates SIRT1 and Halts Oxidative Stress-Mediated Cognitive Impairment in a Male Aging Mouse Model. Cells 2019, 8, 928. [Google Scholar] [CrossRef]
- Kuscu, N.; Gungor-Ordueri, N.E.; Sozen, B.; Adiguzel, D.; Celik-Ozenci, C. FoxO transcription factors 1 regulate mouse preimplantation embryo development. J. Assist. Reprod. Genet. 2019, 36, 2121–2133. [Google Scholar] [CrossRef]
- Xiong, S.; Salazar, G.; Patrushev, N.; Alexander, R.W. FoxO1 Mediates an Autofeedback Loop Regulating SIRT1 Expression. J. Biol. Chem. 2011, 286, 5289–5299. [Google Scholar] [CrossRef]
- Lin, C.-L.; Huang, W.-N.; Li, H.-H.; Huang, C.-N.; Hsieh, S.; Lai, C.; Lu, F.-J. Hydrogen-rich water attenuates amyloid β-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem. Interact. 2015, 240, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Yao, M.; Chang, D.; Liu, J. mTOR (Mammalian Target of Rapamycin): Hitting the Bull’s Eye for Enhancing Neurogenesis after Cerebral Ischemia? Stroke 2023, 54, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.; Li, T.; He, Y.; Guan, A.; Chen, L.; Gao, Y.; Xu, Q.; Wang, H.; Luo, R.; Zhao, L.; et al. Resistin secreted by porcine alveolar macrophages leads to endothelial cell dysfunction during Haemophilus parasuis infection. Virulence 2023, 14, 2171636. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Nagy, J.A.; Manseau, E.J.; Phung, T.L.; Dvorak, H.F.; Benjamin, L.E. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1172–1178. [Google Scholar] [CrossRef]
- Chen, G.; Li, Z.; Chen, C.; Liu, J.; Zhu, W.; She, L.; Huang, H.; Qin, Y.; Liu, G.; Wang, J.; et al. The Molecular Landscape and Biological Alterations Induced by PRAS40-Knockout in Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2021, 10, 565669. [Google Scholar] [CrossRef]
- Paudel, S.; Liu, B.; Cummings, M.J.; E Quinn, K.; Bazer, F.W.; Caron, K.M.; Wang, X. Temporal and spatial expression of adrenomedullin and its receptors in the porcine uterus and peri-implantation conceptuses. Biol. Reprod. 2021, 105, 876–891. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE 2012, 7, e45456. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr. Neurovasc. Res. 2012, 9, 239–249. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Wen, Q.; Zheng, Y.; Lazarovici, P.; Jiang, H.; Lin, J.; Zheng, W. Proline-rich Akt substrate of 40kDa (PRAS40): A novel downstream target of PI3k/Akt signaling pathway. Cell. Signal. 2012, 24, 17–24. [Google Scholar] [CrossRef]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GβL, a Positive Regulator of the Rapamycin-Sensitive Pathway Required for the Nutrient-Sensitive Interaction between Raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.S.; Mitra, K.; Akter, S.; Ramproshad, S.; Mondal, B.; Khan, I.N.; Islam, M.T.; Sharifi-Rad, J.; Calina, D.; Cho, W.C. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer Cell Int. 2022, 22, 284. [Google Scholar] [CrossRef]
- Katsianou, M.A.; Papavassiliou, K.A.; Gargalionis, A.N.; Agrogiannis, G.; Korkolopoulou, P.; Panagopoulos, D.; Themistocleous, M.S.; Piperi, C.; Basdra, E.K.; Papavassiliou, A.G. Polycystin-1 regulates cell proliferation and migration through AKT/mTORC2 pathway in a human craniosynostosis cell model. J. Cell. Mol. Med. 2022, 26, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.M.; Alessi, D.R. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem. J. 2008, 416, 375–385. [Google Scholar] [CrossRef]
- Pearce, L.R.; Sommer, E.M.; Sakamoto, K.; Wullschleger, S.; Alessi, D.R. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem. J. 2011, 436, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Frias, M.A.; Thoreen, C.C.; Jaffe, J.D.; Schroder, W.; Sculley, T.; Carr, S.A.; Sabatini, D.M. mSin1 Is Necessary for Akt/PKB Phosphorylation, and Its Isoforms Define Three Distinct mTORC2s. Curr. Biol. 2006, 16, 1865–1870. [Google Scholar] [CrossRef]
- Carter, C.C.; Mast, F.D.; Olivier, J.P.; Bourgeois, N.M.; Kaushansky, A.; Aitchison, J.D. Dengue activates mTORC2 signaling to counteract apoptosis and maximize viral replication. Front. Cell. Infect. Microbiol. 2022, 12, 979996. [Google Scholar] [CrossRef]
- Cheng, J.; North, B.J.; Zhang, T.; Dai, X.; Tao, K.; Guo, J.; Wei, W. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell 2018, 17, e12801. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging 2012, 4, 187–201. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Sun, S.; Chen, S. Neuroprotective Effects of Salidroside in a Mouse Model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2020, 40, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Ghasemnejad-Berenji, M. mTOR inhibition: A double-edged sword in patients with COVID-19? Hum. Cell 2021, 34, 698–699. [Google Scholar] [CrossRef]
- Hasbal, N.B.; Turgut, D.; Oguz, E.G.; Ulu, S.; Gungor, O. Effect of Calcineurin Inhibitors and Mammalian Target of Rapamycin Inhibitors on the Course of COVID-19 in Kidney Transplant Recipients. Ann. Transplant. 2021, 26, e929279-1–e929279-6. [Google Scholar] [CrossRef] [PubMed]
- Philips, A.M.; Khan, N. Amino acid sensing pathway: A major check point in the pathogenesis of obesity and COVID-19. Obes. Rev. 2021, 22, e13221. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, S.; Mansouri, R.; Ali-Hassanzadeh, M.; Mojtahedi, Z.; Shafiei, R.; Savardashtaki, A.; Hamidizadeh, N.; Karimazar, M.; Nguewa, P.; Manzano-Román, R. The host mTOR pathway and parasitic diseases pathogenesis. Parasitol. Res. 2021, 120, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; The ARIA Group; Cristol, J.-P.; Czarlewski, W.; Anto, J.M.; Martineau, A.; Haahtela, T.; Fonseca, S.C.; Iaccarino, G.; Blain, H.; et al. Nrf2-interacting nutrients and COVID-19: Time for research to develop adaptation strategies. Clin. Transl. Allergy 2020, 10, 58. [Google Scholar] [CrossRef]
- Teixeira, L.B.; Santos, W.C. The mTOR pathway as a target for SARS-COV-2: Rapamycin as a possible alternative pharmacological therapeutic for COVID-19. Act. Farma Terap. 2020, 18, 102–108. [Google Scholar]
- Saenwongsa, W.; Nithichanon, A.; Chittaganpitch, M.; Buayai, K.; Kewcharoenwong, C.; Thumrongwilainet, B.; Butta, P.; Palaga, T.; Takahashi, Y.; Ato, M.; et al. Metformin-induced suppression of IFN-α via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Sci. Rep. 2020, 10, 3229. [Google Scholar] [CrossRef]
- Tian, Y.; Xiao, Y.; Geng, T.; Sun, C.; Gu, J.; Tang, K.; Liu, B.; Liu, Y.; Sun, F. Clusterin suppresses spermatogenic cell apoptosis to alleviate diabetes-induced testicular damage by inhibiting autophagy via the PI3K/AKT/mTOR axis. Biol. Cell 2020, 113, 14–27. [Google Scholar] [CrossRef]
- Bellozi, P.M.Q.; Lima, I.V.D.A.; Dória, J.G.; Vieira, É.L.M.; Campos, A.C.; Candelario-Jalil, E.; Reis, H.J.; Teixeira, A.L.; Ribeiro, F.M.; de Oliveira, A.C.P. Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-β 1–42 induced neurotoxicity and memory impairment. Sci. Rep. 2016, 6, 25226. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr. Neurovasc. Res. 2013, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Liu, T.; Law, P.-Y.; Loh, H.H.; Qiu, Y.; Chen, H.-Z. μ-Opioid Receptor Attenuates AβOligomers-Induced Neurotoxicity through mTOR Signaling. CNS Neurosci. Ther. 2014, 21, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-A.; Lee, C.-H. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. J. Veter-Sci. 2017, 18, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.-S.; Wang, Y.-F.; Long, X.-X.; Ma, Y. Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling. Experiment 2018, 24, 7459–7468. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shen, S.; Cai, M.; Jin, L.; Lu, J.; Xu, K.; Zhang, J.; Feng, G.; Hu, Y.; Zheng, K.; et al. Role of mTOR complex in IGF-1 induced neural differentiation of DPSCs. Histochem. J. 2019, 50, 273–283. [Google Scholar] [CrossRef]
- Shang, Y.C.; Chong, Z.Z.; Wang, S.; Maiese, K. Erythropoietin and Wnt1 govern pathways of mTOR, Apaf-1, and XIAP in inflammatory microglia. Curr. Neurovasc. Res. 2011, 8, 270–285. [Google Scholar] [CrossRef]
- Dai, C.; Tang, S.; Biao, X.; Xiao, X.; Chen, C.; Li, J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol. Biol. Rep. 2019, 46, 1963–1972. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Stress in the brain: Novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res. Rev. 2005, 49, 1–21. [Google Scholar] [CrossRef]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef]
- Crespo, M.C.; Tomé-Carneiro, J.; Pintado, C.; Dávalos, A.; Visioli, F.; Burgos-Ramos, E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. Biofactors 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Pal, P.B.; Sonowal, H.; Shukla, K.; Srivastava, S.K.; Ramana, K.V. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-α1/mTOR pathway. J. Mol. Endocrinol. 2019, 63, 11–25. [Google Scholar] [CrossRef]
- Malla, R.; Wang, Y.; Chan, W.K.; Tiwari, A.K.; Faridi, J.S. Genetic ablation of PRAS40 improves glucose homeostasis via linking the AKT and mTOR pathways. Biochem. Pharmacol. 2015, 96, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Alves, H.R.; Lomba, G.S.B.; Gonçalves-De-Albuquerque, C.F.; Burth, P. Irisin, Exercise, and COVID-19. Front. Endocrinol. 2022, 13, 879066. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, H.; Rong, Z.; Chen, L. Wnt family member 1 (Wnt1) overexpression-induced M2 polarization of microglia alleviates inflammation-sensitized neonatal brain injuries. Bioengineered 2022, 13, 12409–12420. [Google Scholar] [CrossRef]
- Du, L.-L.; Chai, D.-M.; Zhao, L.-N.; Li, X.-H.; Zhang, F.-C.; Zhang, H.-B.; Liu, L.-B.; Wu, K.; Liu, R.; Wang, J.-Z.; et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J. Alzheimer’s Dis. 2014, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Maiese, K.; Chong, Z.Z.; Shang, Y.C.; Wang, S. Novel directions for diabetes mellitus drug discovery. Expert Opin. Drug Discov. 2012, 8, 35–48. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.-C.; Wang, K.-F.; Chen, X.-Y. Aβ peptide secretion is reduced by Radix Polygalae-induced autophagy via activation of the AMPK/mTOR pathway. Mol. Med. Rep. 2015, 12, 2771–2776. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Wu, Q.-Y.; Zheng, R.; Chen, C.; Chen, Y.; Liu, Q.; Hoffmann, P.R.; Ni, J.-Z.; Song, G.-L. Selenomethionine Mitigates Cognitive Decline by Targeting Both Tau Hyperphosphorylation and Autophagic Clearance in an Alzheimer’s Disease Mouse Model. J. Neurosci. 2017, 37, 2449–2462. [Google Scholar] [CrossRef]
- Peixoto, C.A.; de Oliveira, W.H.; Araújo, S.M.D.R.; Nunes, A.K.S. AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp. Neurol. 2017, 298, 31–41. [Google Scholar] [CrossRef]
- Kim, S.H.; Yu, H.S.; Huh, S.; Kang, U.G.; Kim, Y.S. Electroconvulsive seizure inhibits the mTOR signaling pathway via AMPK in the rat frontal cortex. Psychopharmacology 2021, 239, 443–454. [Google Scholar] [CrossRef]
- Barcena, M.L.; Tonini, G.; Haritonow, N.; Breiter, P.; Milting, H.; Baczko, I.; Müller-Werdan, U.; Ladilov, Y.; Regitz-Zagrosek, V. Sex and age differences in AMPK phosphorylation, mitochondrial homeostasis, and inflammation in hearts from inflammatory cardiomyopathy patients. Aging Cell 2023, 00, e13894. [Google Scholar] [CrossRef]
- Zhong, S.; Chen, W.; Wang, B.; Gao, C.; Liu, X.; Song, Y.; Qi, H.; Liu, H.; Wu, T.; Wang, R.; et al. Energy stress modulation of AMPK/FoxO3 signaling inhibits mitochondria-associated ferroptosis. Redox Biol. 2023, 63, 102760. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Kumari, R.; Ilyas, A.; Tyagi, S.; Kumar, R.; Poddar, N.K. Crosstalk between epigenetics and mTOR as a gateway to new insights in pathophysiology and treatment of Alzheimer’s disease. Int. J. Biol. Macromol. 2021, 192, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Damstra-Oddy, J.L.; Warren, E.C.; Perry, C.J.; Desfougères, Y.; Fitzpatrick, J.K.; Schaf, J.; Costelloe, L.; Hind, W.; Downer, E.J.; Saiardi, A.; et al. Phytocannabinoid-dependent mTORC1 regulation is dependent upon inositol polyphosphate multikinase activity. Br. J. Pharmacol. 2021, 178, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Ciccotosto, G.D.; Cappai, R.; Wang, Y.; Tang, S.; Hoyer, D.; Schneider, E.K.; Velkov, T.; Xiao, X. Rapamycin Confers Neuroprotection against Colistin-Induced Oxidative Stress, Mitochondria Dysfunction, and Apoptosis through the Activation of Autophagy and mTOR/Akt/CREB Signaling Pathways. ACS Chem. Neurosci. 2017, 9, 824–837. [Google Scholar] [CrossRef]
- Park, A.; Koh, H.C. NF-κB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch. Toxicol. 2019, 93, 1239–1253. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wang, Y.; Li, J.; Lu, G.; Liu, Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ. Health Prev. Med. 2019, 24, 4. [Google Scholar] [CrossRef]
- Javdan, N.; Ayatollahi, S.A.; Choudhary, M.I.; Al-Hasani, S.; Kobarfard, F.; Athar, A.; Pazoki-Toroudi, H. Capsaicin protects against testicular torsion injury through mTOR-dependent mechanism. Theriogenology 2018, 113, 247–252. [Google Scholar] [CrossRef]
- Han, K.; Jia, N.; Zhong, Y.; Shang, X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J. Cell. Biochem. 2017, 119, 3111–3117. [Google Scholar] [CrossRef]
- Dong, W.; Wang, R.; Ma, L.-N.; Xu, B.-L.; Zhang, J.-S.; Zhao, Z.-W.; Wang, Y.-L.; Zhang, X. Influence of age-related learning and memory capacity of mice: Different effects of a high and low caloric diet. Aging Clin. Exp. Res. 2015, 28, 303–311. [Google Scholar] [CrossRef]
- Hsieh, C.-F.; Liu, C.-K.; Lee, C.-T.; Yu, L.-E.; Wang, J.-Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef]
- Ka, M.; Smith, A.L.; Kim, W.-Y. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy 2017, 13, 1348–1363. [Google Scholar] [CrossRef]
- Martino, L.; Masini, M.; Novelli, M.; Beffy, P.; Bugliani, M.; Marselli, L.; Masiello, P.; Marchetti, P.; De Tata, V. Palmitate Activates Autophagy in INS-1E β-Cells and in Isolated Rat and Human Pancreatic Islets. PLoS ONE 2012, 7, e36188. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Uppal, K.; Liu, K.H.; Hu, X.; Orr, M.; Tran, V.; Go, Y.-M.; Jones, D.P. Antagonistic Interactions in Mitochondria ROS Signaling Responses to Manganese. Antioxidants 2023, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, M.; Rucci, N.; Falone, S. RNA methylation and cellular response to oxidative stress-promoting anticancer agents. Cell Cycle 2023, 22, 870–905. [Google Scholar] [CrossRef]
- Kim, K.-A.; Shin, Y.-J.; Akram, M.; Kim, E.-S.; Choi, K.-W.; Suh, H.; Lee, C.-H.; Bae, O.-N. High Glucose Condition Induces Autophagy in Endothelial Progenitor Cells Contributing to Angiogenic Impairment. Biol. Pharm. Bull. 2014, 37, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Du, J.; Jin, H.; Zhang, J.; Niu, M.; Qin, J. Recombinant Human Erythropoietin Protects Against Hippocampal Damage in Developing Rats with Seizures by Modulating Autophagy via the S6 Protein in a Time-Dependent Manner. Neurochem. Res. 2017, 43, 465–476. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, J.; Li, B.; Ding, Z.; Cheng, W.; Gao, F.; Zhang, Y.; Xu, Y.; Zhang, S. Cornin protects SH-SY5Y cells against oxygen and glucose deprivation-induced autophagy through the PI3K/Akt/mTOR pathway. Mol. Med. Rep. 2017, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-L.; Zhang, J.-B.; Guo, Y.-X.; Xia, T.-S.; Xu, L.-C.; Rahmand, K.; Wang, G.-P.; Li, X.-J.; Han, T.; Wang, N.-N.; et al. Xanthohumol ameliorates memory impairment and reduces the deposition of β-amyloid in APP/PS1 mice via regulating the mTOR/LC3II and Bax/Bcl-2 signalling pathways. J. Pharm. Pharmacol. 2021, 73, 1230–1239. [Google Scholar] [CrossRef]
- Lee, H.J.; Koh, S.-H.; Song, K.-M.; Seol, I.J.; Park, H.-K. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology 2016, 110, 93–100. [Google Scholar] [CrossRef]
- Wang, G.-B.; Ni, Y.-L.; Zhou, X.-P.; Zhang, W.-F. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol. Cell. Biochem. 2013, 385, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Le, Y.; He, W.-Y.; He, J.; Wang, Y.-H.; Zhang, L.; Xiong, Q.-M.; Zheng, X.-Q.; Liu, K.-X.; Wang, H.-B. Abnormal Insulin-like Growth Factor 1 Signaling Regulates Neuropathic Pain by Mediating the Mechanistic Target of Rapamycin-Related Autophagy and Neuroinflammation in Mice. ACS Chem. Neurosci. 2021, 12, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- Temiz-Resitoglu, M.M.; Guden, D.S.; Senol, S.P.M.; Vezir, O.M.; Sucu, N.M.; Kibar, D.M.; Yılmaz, S.N.; Tunctan, B.; Malik, K.U.; Sahan-Firat, S. Pharmacological Inhibition of Mammalian Target of Rapamycin Attenuates Deoxycorticosterone Acetate Salt–Induced Hypertension and Related Pathophysiology: Regulation of Oxidative Stress, Inflammation, and Cardiovascular Hypertrophy in Male Rats. J. Cardiovasc. Pharmacol. 2021, 79, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Samadi, N.; Shahnazi, V.; Mihanfar, A.; Fattahi, A.; Latifi, Z.; Bahrami-Asl, Z.; Roshangar, L.; Nouri, M. Nicotinamide and its metabolite N1-Methylnicotinamide alleviate endocrine and metabolic abnormalities in adipose and ovarian tissues in rat model of Polycystic Ovary Syndrome. Chem. Interact. 2020, 324, 109093. [Google Scholar] [CrossRef] [PubMed]
- Kalender, A.; Selvaraj, A.; Kim, S.Y.; Gulati, P.; Brûlé, S.; Viollet, B.; Kemp, B.E.; Bardeesy, N.; Dennis, P.; Schlager, J.J.; et al. Metformin, Independent of AMPK, Inhibits mTORC1 in a Rag GTPase-Dependent Manner. Cell Metab. 2010, 11, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Bramante, C.; Ingraham, N.; Murray, T.; Marmor, S.; Hoversten, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Ong, A.N.; Hospital, C.C.C.H.; Tan, C.C.; Cañete, M.T.; Lim, B.A.; Robles, J. Association between Metformin Use and Mortality Among Patients with Type 2 Diabetes Mellitus Hospitalized for COVID-19 Infection. J. ASEAN Fed. Endocr. Soc. 2021, 36, 133–141. [Google Scholar] [CrossRef]
- Guo, W.; Qian, L.; Zhang, J.; Zhang, W.; Morrison, A.; Hayes, P.; Wilson, S.; Chen, T.; Zhao, J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J. Neurosci. Res. 2011, 89, 1723–1736. [Google Scholar] [CrossRef]
- Ou, X.; Lee, M.R.; Huang, X.; Messina-Graham, S.; Broxmeyer, H.E. SIRT1 Positively Regulates Autophagy and Mitochondria Function in Embryonic Stem Cells Under Oxidative Stress. Stem Cells 2014, 32, 1183–1194. [Google Scholar] [CrossRef]
- Pan, Y.-R.; Song, J.-Y.; Fan, B.; Wang, Y.; Che, L.; Zhang, S.-M.; Chang, Y.-X.; He, C.; Li, G.-Y. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Commun. Signal. 2020, 18, 27. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Pang, X.; Zhao, Z.; Yu, H.; Zhou, H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol. Cell. Biochem. 2018, 455, 127–134. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Chen, S.; Li, Z.; Ma, L.; Jia, X.; Wang, K.; Bao, J.; Liang, Y.; Chen, M.; et al. Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci. Rep. 2017, 7, srep41082. [Google Scholar] [CrossRef] [PubMed]
- van Vuren, E.J.; Steyn, S.F.; Brink, C.B.; Möller, M.; Viljoen, F.P.; Harvey, B.H. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed. Pharmacother. 2021, 135, 111200. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. From causes of aging to death from COVID-19. Aging 2020, 12, 10004–10021. [Google Scholar] [CrossRef]
- do Nascimento, I.J.B.; Cacic, N.; Abdulazeem, H.M.; Von Groote, T.C.; Jayarajah, U.; Weerasekara, I.; Esfahani, M.A.; Civile, V.T.; Marusic, A.; Jerončić, A.; et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J. Clin. Med. 2020, 9, 941. [Google Scholar] [CrossRef]
- Kurki, S.N.; Kantonen, J.; Kaivola, K.; Hokkanen, L.; MäyränpäÄ, M.I.; Puttonen, H.; Martola, J.; Pöyhönen, M.; Kero, M.; Tuimala, J.; et al. APOE ε4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: A Finnish biobank, autopsy and clinical study. Acta Neuropathol. Commun. 2021, 9, 199. [Google Scholar] [CrossRef]
- Maiese, K. Picking a bone with WISP1 (CCN4): New strategies against degenerative joint disease. J. Transl. Sci. 2016, 1, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-L.; Zhou, J.-Y.; Liang, S.-J.; Wang, X.-Q. Impaired intestinal stem cell activity in ETEC infection: Enterotoxins, cyclic nucleotides, and Wnt signaling. Arch. Toxicol. 2022, 96, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).