1. Introduction
Flare gas is prevalent across numerous industrial sectors and has immense economic, environmental, and public health implications. The process discharges high levels of greenhouse gases (GHGs), namely carbon dioxide (CO
2) and methane (CH
4), which are key drivers of climate change. In addition to GHGs, flaring releases nitrogen oxides (NO
x) and sulfur oxides (SO
x), all of which have been linked to cardiovascular and respiratory disorders and have a detrimental effect on air quality [
1,
2,
3]. From an economic perspective, flaring constitutes a waste of valuable hydrocarbon resources that could otherwise be used for energy generation, chemical feedstock, or other industrial applications [
4,
5,
6].
Flare gas is generated by various processes, including safety devices such as pressure relief valves during accidental over-pressurization of plant equipment. Waste gas normally passes to flare stacks via piping systems and is burned at the stack tip. While flaring ensures safety in an emergency and safeguards against dangerous gas accumulation, frequent flaring aggravates environmental degradation and results in significant economic loss. The oil sector alone consumes approximately 140 billion cubic meters of natural gas annually, contributing to about 400 million tons of CO
2 emissions [
7]. If recovered, this gas could supply the annual electricity demand for the entire African continent, equivalent to 750 billion kWh [
4,
8].
In opposition to these challenges, flare gas treatment systems have been developed into key technologies for reducing environmental damage and increasing resource efficiency. Effective treatment starts with the efficient separation of surface gas. Initial phase separation processes are necessary to divide gas components and facilitate further treatment steps. The objective is to create dependable systems that minimize environmental effects and convert waste gas into economically feasible commodities.
Over the past decades, various technologies have been developed to treat and recover flare gas. Amine absorption is widely applied for removing acid gases, particularly hydrogen sulfide (H
2S) and CO
2. Tertiary amines such as methyl diethanolamine (MDEA) are especially valued for their high selectivity toward H
2S and relatively lower energy requirements for regeneration compared to primary or secondary amines [
9]. Dual-stage amine absorption systems have been shown to effectively reduce acid gas content while retaining high methane recovery [
10]. However, they offer limited selectivity for heavier hydrocarbons and require significant thermal energy for solvent regeneration. In contrast, supercritical carbon dioxide (sc-CO
2) extraction—although rarely applied to flare gas—has been widely used in other industries such as natural product extraction, polymer processing, and enhanced oil recovery. Above its critical point (31 °C, 7.38 MPa), CO
2 exhibits liquid-like density and gas-like diffusivity, resulting in strong selectivity for non-polar hydrocarbons. This makes sc-CO
2 particularly effective for recovering heavier hydrocarbons such as pentane and hexane. Nevertheless, it is less efficient at completely removing acid gases like H
2S, typically requiring an additional treatment step.
Other technologies for gas treatment have also been investigated, including membrane separation, cryogenic processing [
11], and physical solvent absorption [
12]. Membrane systems are compact and can be energy-efficient for CO
2 or H
2S removal [
13], but they are prone to fouling and performance degradation over time. Cryogenic methods achieve high recovery of heavier hydrocarbons by deep cooling yet require substantial refrigeration energy and complex equipment. Physical solvents such as Selexol and Rectisol can effectively remove bulk CO
2 without chemical reactions [
14], but their efficiency declines for low-concentration contaminants and often involves high solvent circulation rates. Each of these approaches presents trade-offs between selectivity, removal efficiency, and operational complexity, limiting their suitability as standalone solutions for flare gas recovery.
Supercritical CO
2 has also been explored in emerging applications such as the Brayton cycle for power generation from flare gases [
15], enhanced oil recovery, and foam-entrapped CO
2 for mobility control. These studies highlight sc-CO
2’s versatility and potential to contribute to both emission reduction and energy recovery. However, direct application of sc-CO
2 for flare gas treatment remains limited, with most research focusing on related but distinct uses.
While amine absorption and sc-CO2 extraction can each contribute to flare gas treatment, neither technology alone provides a complete solution. Amine systems are highly effective for acid gas removal but require significant regeneration energy and offer limited hydrocarbon selectivity. In contrast, sc-CO2 is well known in other industries for its ability to selectively recover hydrocarbons, yet its direct application to flare gas treatment has been rarely explored in the literature and remains underdeveloped. Moreover, hybrid configurations that combine amine absorption with sc-CO2 to exploit the strengths of both processes have not been systematically studied, leaving a clear gap in the field. This study addresses these gaps by systematically evaluating three two-stage flare gas treatment configurations—dual-stage amine absorption, dual-stage sc-CO2 absorption, and a hybrid process of sc-CO2 followed by amine absorption using Aspen HYSYS V12.1 simulations (Aspen Technology, Inc., Burlington, MA, USA), with recycling processes included. The performance of each configuration is analyzed in terms of acid gas removal, hydrocarbon recovery, and energy consumption, and the feasibility of integrating the most effective process with GTL technology is investigated.
2. Methodology
This study systematically compared and optimized flare gas treatment configurations using Aspen HYSYS V12.1 simulation software. The primary goal was to identify the efficiency, selectivity, and environmental impact of three treatment configurations:
Two-stage CO2 supercritical absorption.
Two-stage amine absorption.
Hybrid configuration of CO2 supercritical absorption and amine absorption.
The process involved simulating flare gas treatment under controlled conditions, testing different compression, absorption, and product recovery stages to identify the most effective system design.
2.1. Simulation Framework and Process Design
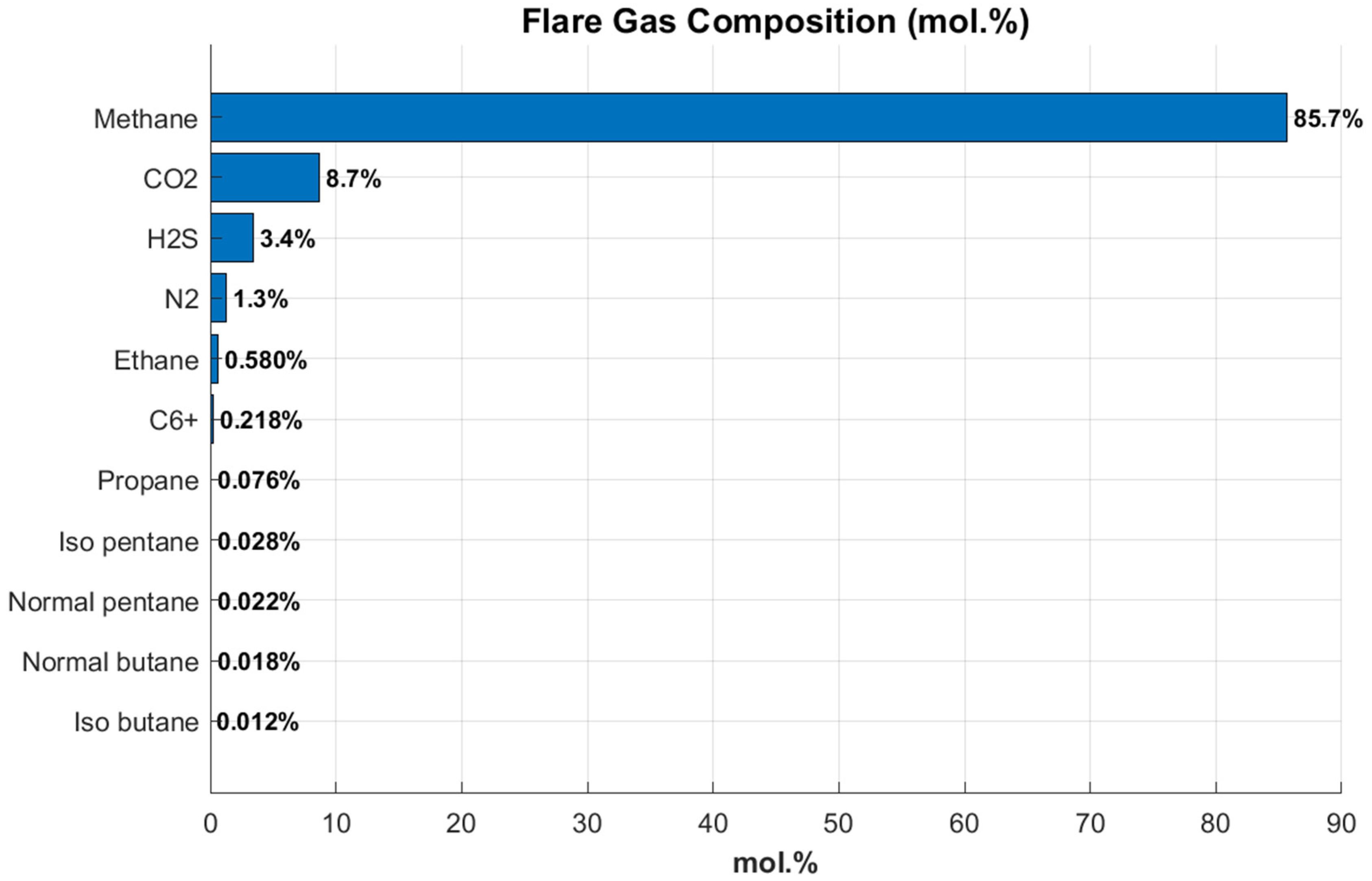
Initially, the flare gas was compressed to prepare it for downstream processing. This step was necessary to raise the pressure to levels suitable for the subsequent absorption stages. Each configuration was simulated using defined operating parameters to approximate real operating conditions. The flare gas feed was maintained at 30 °C, 0.4 bar, and a flow rate of 30 kmol/h, which are typical values for average gas compositions in oil and gas fields. It was subsequently compressed to reach the required column pressure. The detailed composition of the flare gas is presented in
Figure 1.
The compressed gas was processed through a series of amine absorption columns using 0.5 M methyl diethanolamine (MDEA) as the solvent in a two-stage absorption setup. The Acid Gas–Chemical Solvents fluid package was applied in the simulation. MDEA was chosen for its high selectivity toward acidic gases such as CO2 and H2S, as well as its relatively low regeneration energy. To ensure accurate process representation, chemical reactions of acidic gases were incorporated into the model.
In the dual-stage configuration for CO2 supercritical absorption, the gas was brought into contact with carbon dioxide under supercritical conditions. These conditions were achieved by maintaining temperature and pressure above 31 °C and 7.4 MPa. The high density and solvation capacity of supercritical CO2 enabled selective extraction of hydrocarbons, particularly the heavier fractions. The simulation also accounted for the phase behavior of CO2 under supercritical conditions, allowing precise modeling of hydrocarbon solubility.
The hybrid configuration combined the advantages of both methods. In this setup, the flare gas first underwent CO2 supercritical absorption to selectively recover hydrocarbons. The partially treated gas was then directed to an amine absorption unit to remove the remaining acidic gases. This configuration was designed to maximize hydrocarbon recovery while ensuring complete removal of H2S and CO2, thereby balancing operational efficiency with environmental compliance.
2.2. Gas-to-Liquids (GTL) Integration
In addition to the treatment simulations, this study explored the potential of applying Gas-to-Liquids (GTL) technology to convert flare gas into valuable liquid hydrocarbons [
16]. The GTL process was divided into three main stages:
- (a)
Synthesis Gas Production: The natural gas feedstock first underwent pre-treatment and heating, followed by autothermal reforming (ATR). Fischer–Tropsch (FT) synthesis requires synthesis gas with a hydrogen-to-carbon monoxide (H2/CO) ratio of approximately 2.3, which was optimized during the ATR step. The main reactions occurring in ATR included:
The load on the FT reactor was reduced by cooling the hot syngas, which also facilitated water removal.
- (b)
Fischer-Tropsch Synthesis: The syngas was introduced into an FT reactor operated isothermally at 210 °C [
17,
18]. Through this highly exothermic polymerization reaction, carbon monoxide and hydrogen were converted into hydrocarbons of varying chain lengths, as represented by the following general reaction:
Heat management features were integrated into the reactor design to maintain stable operation and ensure consistent hydrocarbon production.
- (c)
Product Upgrading: The FT products were further refined to enhance their economic value, yielding high-quality fuels such as diesel and synthetic crude oil [
19]. The FT synthesis generates a wide spectrum of hydrocarbons, primarily long-chain paraffins, along with smaller fractions of olefins and oxygenates. This unrefined product stream, commonly referred to as synthetic crude (syncrude), offers favorable properties such as zero sulfur content and a high cetane number. However, additional refining is required to meet commercial fuel specifications and enhance performance characteristics.
In this study, the product upgrading process is conceptually divided into the following main steps:
Heavy FT waxes (C20 and above) are cracked into lighter fractions, such as diesel, kerosene, and naphtha. Hydroisomerization improves cold-flow properties by introducing branching into paraffin chains. Both processes are typically carried out over bifunctional catalysts combining a metallic hydrogenation function (e.g., Pt, Ni) and an acidic cracking/isomerization function, under a hydrogen-rich atmosphere.
The hydroprocessed products are separated into desired fuel cuts through fractionation:
Naphtha (C5–C10) for petrochemical feedstock or gasoline blending, Kerosene (C10–C14) for jet fuel, and Diesel (C14–C20) for ultra-clean transportation fuel. Residual fractions for lubricant base oils or further cracking.
Trace oxygenates are removed, and olefins are saturated to improve stability and combustion quality. Hydrotreating also ensures compliance with stringent environmental regulations by delivering fuels with extremely low sulfur, nitrogen, and aromatic contents.
Final blending adjusts physical properties such as volatility, density, and cold-flow performance. FT-derived fuels can be blended with petroleum-derived fuels or used directly. In many cases, FT diesel and jet fuel can be used neat due to their superior combustion performance and environmental profile.
By integrating product upgrading into the GTL scheme, flare gas can be transformed not only into hydrocarbons but into premium, specification-grade fuels such as ultra-clean diesel and synthetic jet fuel. This step significantly increases the economic value of the process while supporting environmental sustainability by providing low-emission fuel alternatives to conventional petroleum products.
2.3. Evaluation Metrics and Assumptions
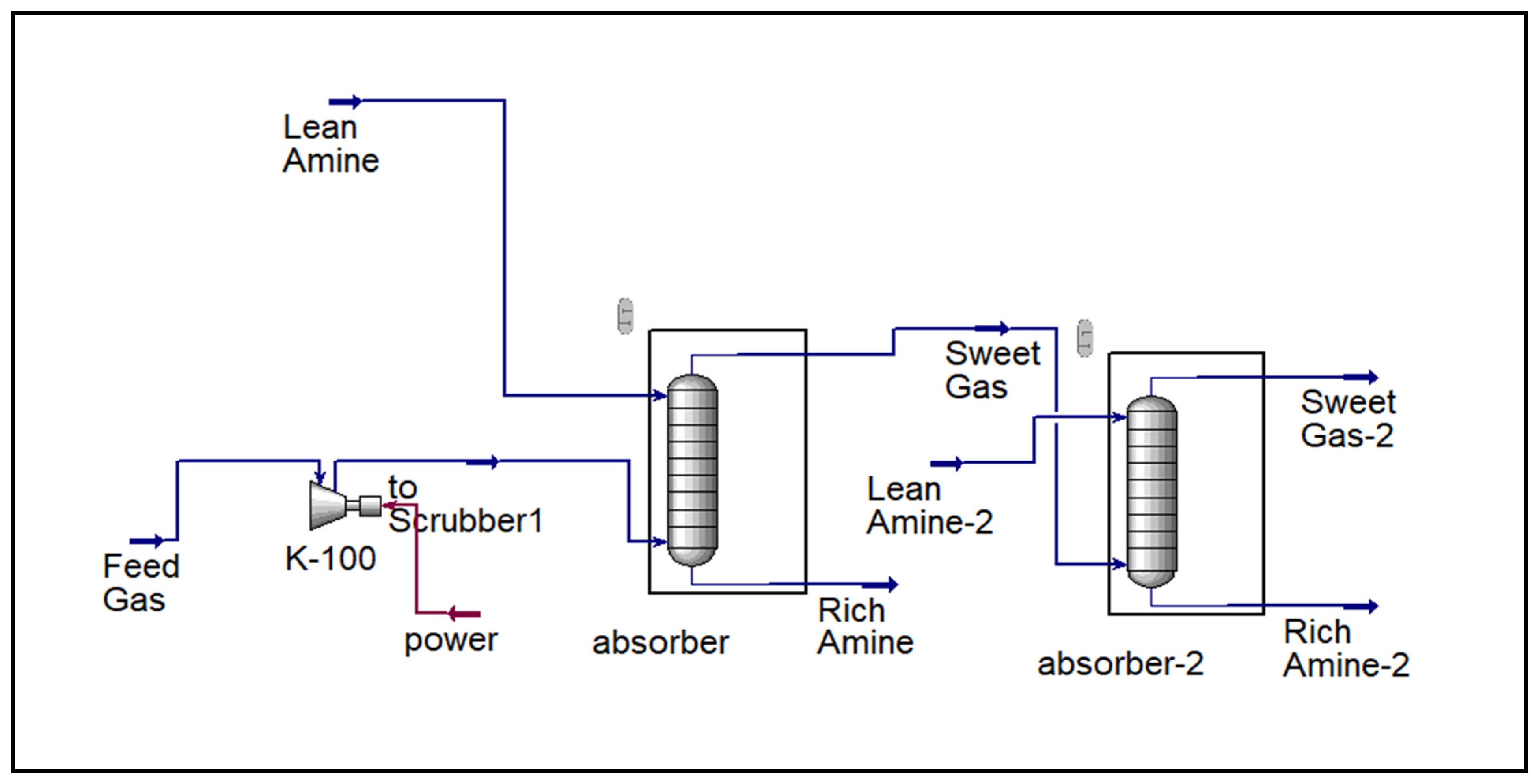
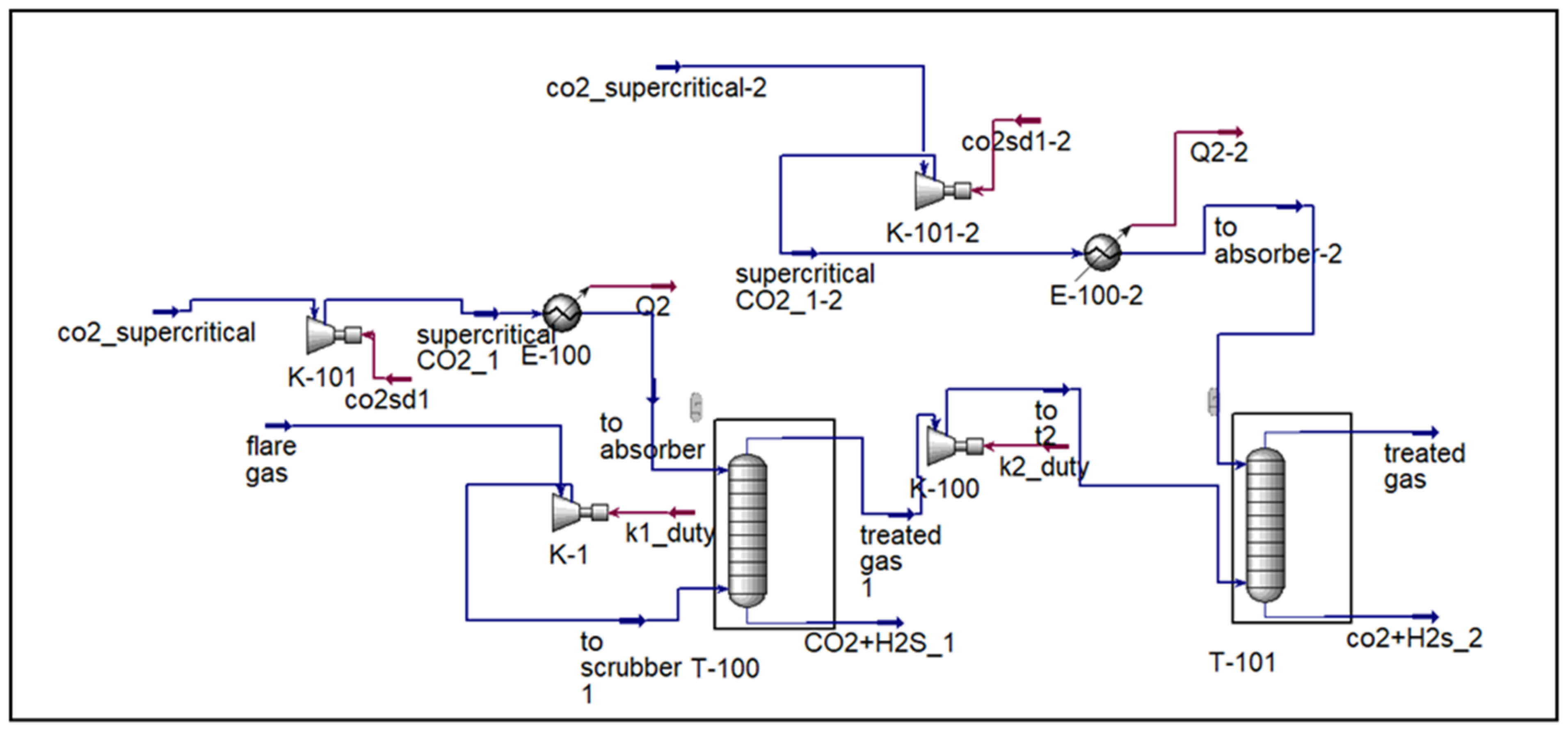
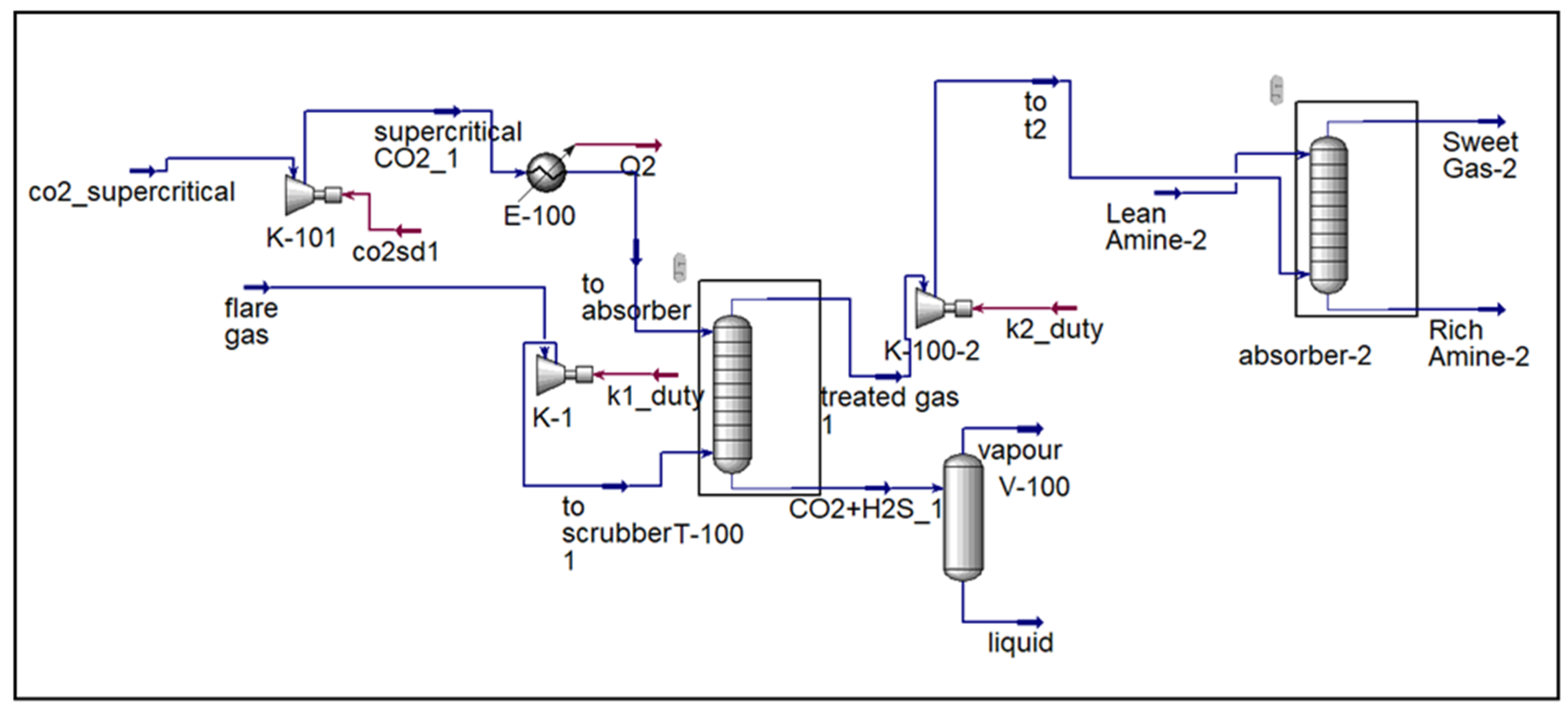
The overall process scheme is illustrated in
Figure 2,
Figure 3,
Figure 4 and
Figure 5, which present the design and operation of each configuration.
Figure 2,
Figure 3 and
Figure 4 illustrate the major differences among the three flare gas treatment configurations investigated in this study. The complete integrated process, including regeneration and recycling, is presented in
Figure 5.
The performance of the configurations was evaluated based on the following key metrics:
Acid Gas Removal Efficiency: The percentage of H2S removed from the gas stream.
Hydrocarbon Recovery: The quantity of hydrocarbons, particularly hexane, recovered during processing.
Energy Consumption: The total energy required for compression, cooling, and solvent regeneration.
The Acid Gas–Chemical Solvents thermodynamic package in Aspen HYSYS was employed to model chemical equilibria and mass transfer processes with high accuracy. The simulations assumed constant feed conditions and ideal mixing within the absorption units [
20].
The performance metrics were mathematically defined to enable a quantitative comparison among the evaluated flare gas treatment configurations:
Hydrocarbon Recovery Efficiency (η
hc):
where
and
are the molar flow rates of hydrocarbons at the inlet and outlet of the process, respectively.
H
2S Removal Efficiency (η
h2s):
where
and
are the molar flow rates of H
2S at the inlet and outlet of the process, respectively.
CO
2 Capture Efficiency (ηCO
2):
where
and
are the molar flow rates of CO
2 at the inlet and outlet of the process, respectively.
2.4. Practical Implementation in Malaysian Oilfields
In Malaysian oilfields, where large volumes of gas are flared annually, the utilization of flare gas was the central focus of this study. The gas composition applied in the simulations was derived from established literature [
21,
22], ensuring accurate representation of flare gas properties. By integrating GTL technology, the study highlighted the potential to convert waste gas into economically valuable products, thereby reducing the environmental footprint while enhancing resource utilization
3. Results and Discussions
This research aimed to provide practical approaches for flare gas mitigation in Malaysian oilfields, where substantial volumes of gas are flared annually. By applying GTL technology, waste gas can be converted into valuable products while reducing both environmental impact and resource losses. The following discussion evaluates key performance indicators—including efficiency, product yield, and environmental benefits—to identify the most viable solutions for large-scale implementation.
3.1. Performance of Dual-Stage CO2 Supercritical Absorption
The dual-stage CO
2 supercritical absorption configuration demonstrated a high capacity for selective hydrocarbon recovery. Each absorber contained ten stages. Operating CO
2 in its supercritical state enhanced the solvation of heavier hydrocarbons, such as hexane, which are typically difficult to recover in conventional systems. Under these conditions, a hexane recovery rate of nearly 100% was achieved, making the process particularly suitable for applications that prioritize the extraction of specific hydrocarbons. Theoretically, this observation aligns with phase equilibrium principles, since nonpolar solutes such as hexane exhibit higher solubility in supercritical CO
2 due to favorable nonpolar–nonpolar molecular interactions [
23,
24]. These results validate both literature findings and thermodynamic expectations that supercritical CO
2 is particularly effective for heavy hydrocarbon recovery.
The dual-stage sc-CO
2 process also demonstrated complete H
2S removal, as shown in
Table 1. A high concentration of CO
2 remains in the sweet gas stream, which is consistent with the physical absorption mechanism: since CO
2 is the solvent medium itself, it is not effectively separated from the mixture. This characteristic underscores the process’s suitability for selective hydrocarbon recovery, while indicating that complementary treatment steps may be required when deep CO
2 removal is a priority.
These findings suggest that while CO
2 supercritical absorption is highly effective for hydrocarbon recovery, it may need to be complemented with additional treatment steps to achieve full gas purification. The “Vapor Phase” mole fractions in
Table 1 represent the composition of the outlet gas stream only. Components such as pentane and hexane appear as zero in the vapor phase because, under the simulated operating conditions, they are almost completely absorbed into the dense supercritical CO
2 phase. Their contribution is therefore reflected in the overall recovery, even though they are absent from the gaseous outlet.
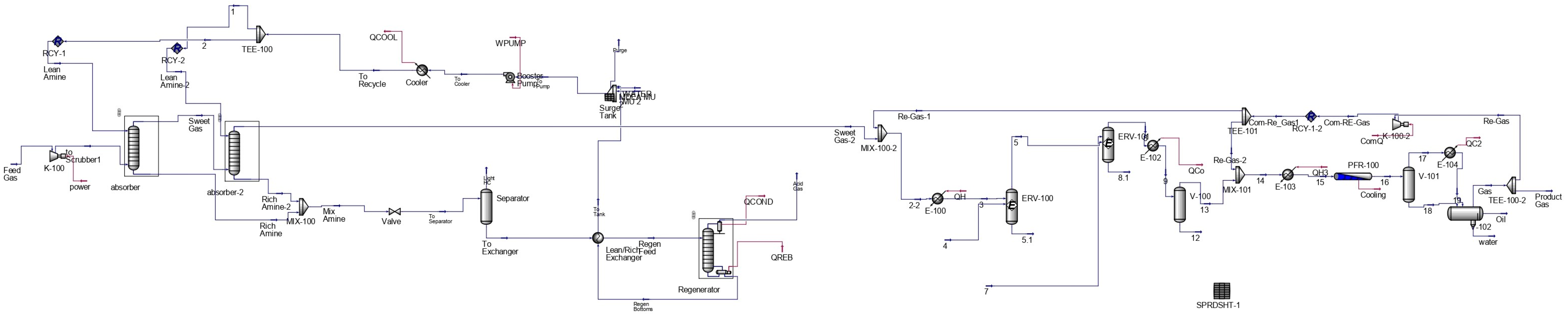
The effect of the flow ratio between supercritical CO
2 and the flare gas feed in the first absorber is illustrated in
Figure 6. Across the investigated range (0.5–2.5), the recovery efficiency remained consistently high, close to 100%. This behavior reflects the strong solvating power of dense supercritical CO
2 for non-polar hydrocarbons. Once the solvent-to-gas ratio is sufficient to maintain a stable supercritical phase, additional CO
2 provides little further benefit, since the heavier hydrocarbons are already almost completely extracted.
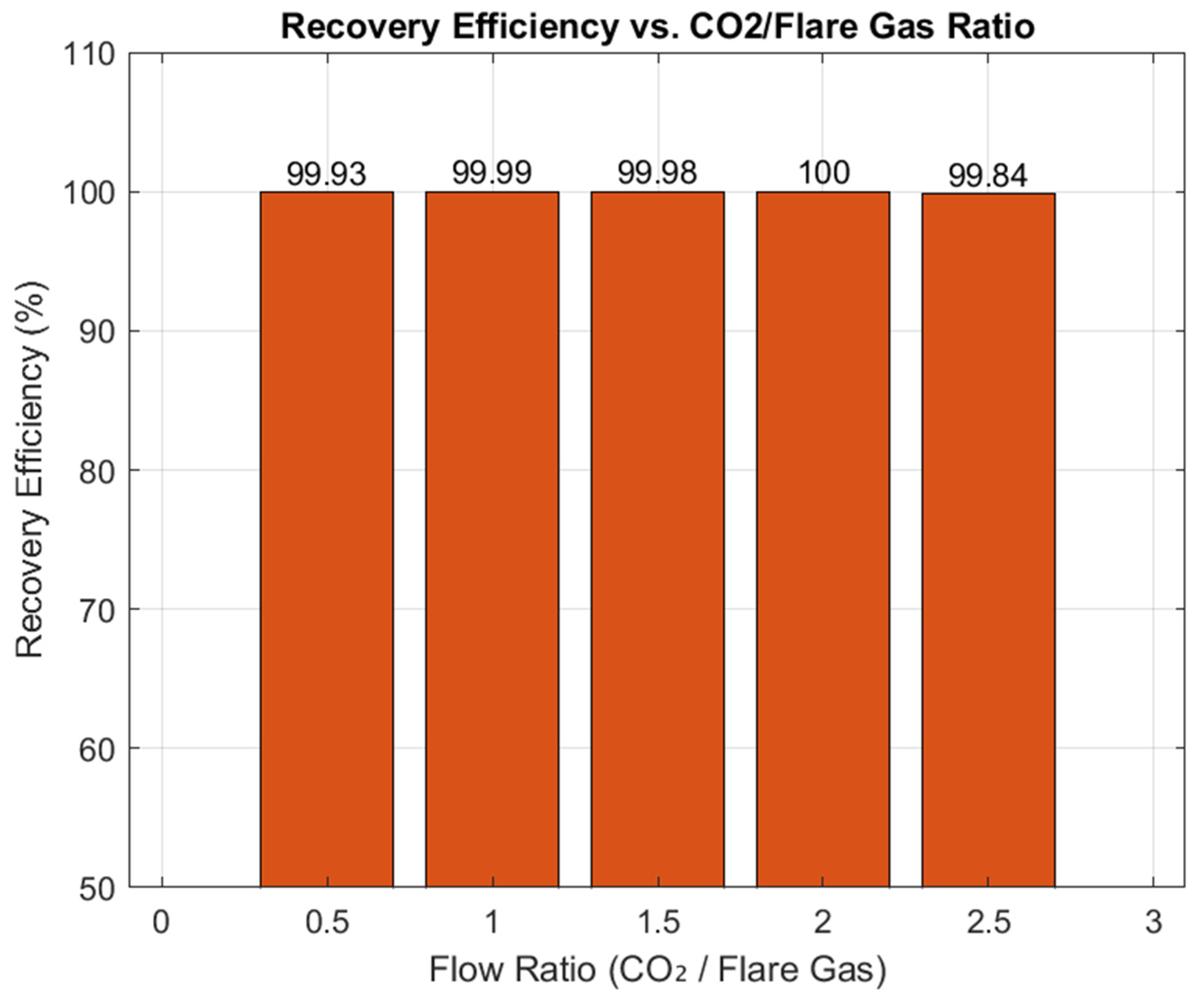
The effect of flare gas feed temperature (20–100 °C) on hexane recovery in the first absorber was evaluated while maintaining absorber pressure above the CO
2 critical point (7.38 MPa). As shown in
Figure 7, recovery remained essentially constant at ~100% across the entire range, confirming that recovery performance is pressure-dominated and only weakly influenced by feed temperature. This demonstrates the robustness of the process to upstream thermal variations.
3.2. Performance of Dual-Stage Amine Absorption
Both absorbers in the system were designed with ten stages. The two-stage amine absorption process effectively removed acid gases, reducing H
2S concentrations to zero. This is consistent with literature reports [
25] and can be explained by the high reactivity of H
2S with MDEA. Theoretically, this efficiency is expected from acid–base equilibrium, where tertiary amines act as proton acceptors, shifting the equilibrium toward complete conversion of H
2S to its ionic species (RNH
3+, HS
−). The key reaction can be represented as:
In addition, the system achieved a methane recovery of 99.68%, making it a strong candidate for applications where methane retention is prioritized. The outlet gas composition is provided in
Table 2.
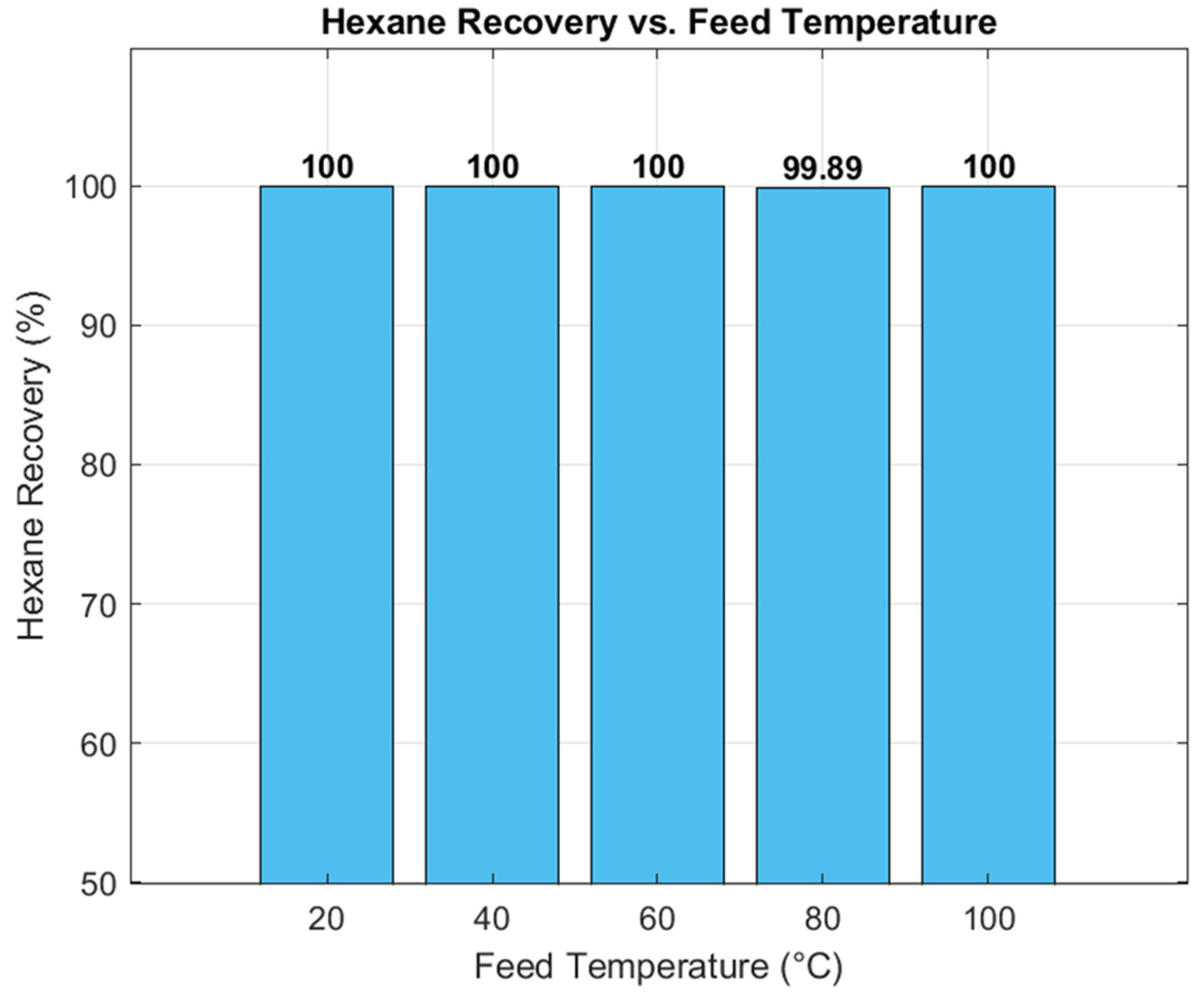
The effect of the lean amine–to–flare gas flow ratio on H
2S capture efficiency was evaluated (
Figure 8). Capture efficiency remained at 100% for ratios ≥ 1.25 but declined when the ratio fell below this threshold, reaching 95.44% at a ratio of 1.05. This indicates that maintaining a ratio above 1.25 is essential for complete H
2S removal. The reduction in efficiency at lower ratios is attributed to an insufficient supply of lean amine for optimal absorption. These results demonstrate that a flow ratio of 1.25 represents the optimal operating point, ensuring maximum H
2S removal with minimal amine consumption, thereby improving both process stability and efficiency in industrial applications.
3.3. Hybrid Configuration: CO2 Supercritical Absorption and Amine Absorption
The hybrid system, integrating supercritical CO
2 absorption with subsequent amine absorption, demonstrated strong separation performance. Each absorber was modeled with ten stages. The configuration achieved complete H
2S removal while maintaining selective hydrocarbon recovery. Hexane recovery reached 100%, and methane recovery was 91.8%, reflecting an optimized balance between selectivity and overall gas purification. A key advantage of the hybrid design is its ability to drastically reduce CO
2 in the sweet gas stream, overcoming the limitation of the standalone sc-CO
2 process where CO
2 remains at high concentrations. The outlet composition for the hybrid configuration is summarized in
Table 3.
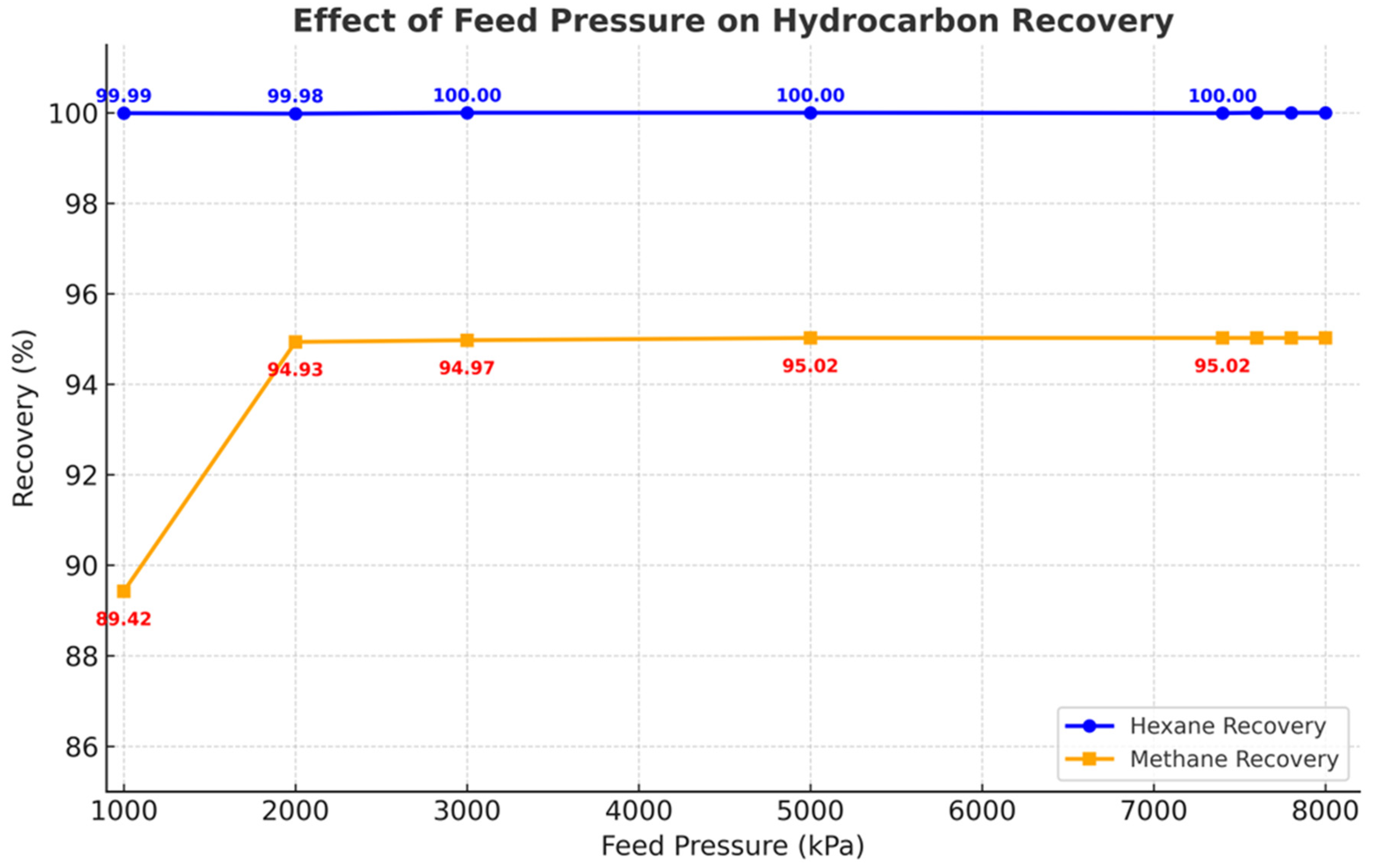
The influence of feed pressure on hydrocarbon recovery in the hybrid sc-CO
2/amine absorption system was evaluated in the range of 1000–8000 kPa (
Figure 9). This range was chosen for sensitivity analysis, even though the actual process operates under CO
2 supercritical conditions (≥7400 kPa).
Hexane recovery remained essentially constant at ~100% across the entire pressure range, confirming the strong affinity of supercritical CO2 for heavier hydrocarbons and its insensitivity to pressure variation once adequate solvent density is achieved.
Methane recovery exhibited stronger pressure dependence. At 1000 kPa, recovery was limited to 89.4%, but increased sharply as pressure rose, reaching ~95% by 2000 kPa. Beyond this point, methane recovery remained stable, with values between 94.9% and 95.0%. Notably, within the supercritical pressure region of CO2 (≥7400 kPa), methane recovery values were nearly identical (~95.02%), indicating that the hybrid process delivers consistent methane performance once supercritical conditions are reached.
3.4. Gas-to-Liquids (GTL) Integration
The GTL process demonstrates strong feasibility for converting flare gas into valuable liquid hydrocarbons. As shown in the process flow scheme (
Figure 5), the sequence begins with autothermal reforming (ATR), which generates high-purity synthesis gas with an optimal H
2/CO ratio of approximately 2.3, required for the subsequent Fischer–Tropsch (FT) synthesis step. In the FT reactor, this syngas is efficiently converted under isothermal conditions into long-chain hydrocarbons, yielding high-value products such as synthetic crude oil. Implementation of GTL technology not only reduces environmental impacts associated with gas flaring but also enhances the economic value of flare gas, improving profitability and sustainability of operations.
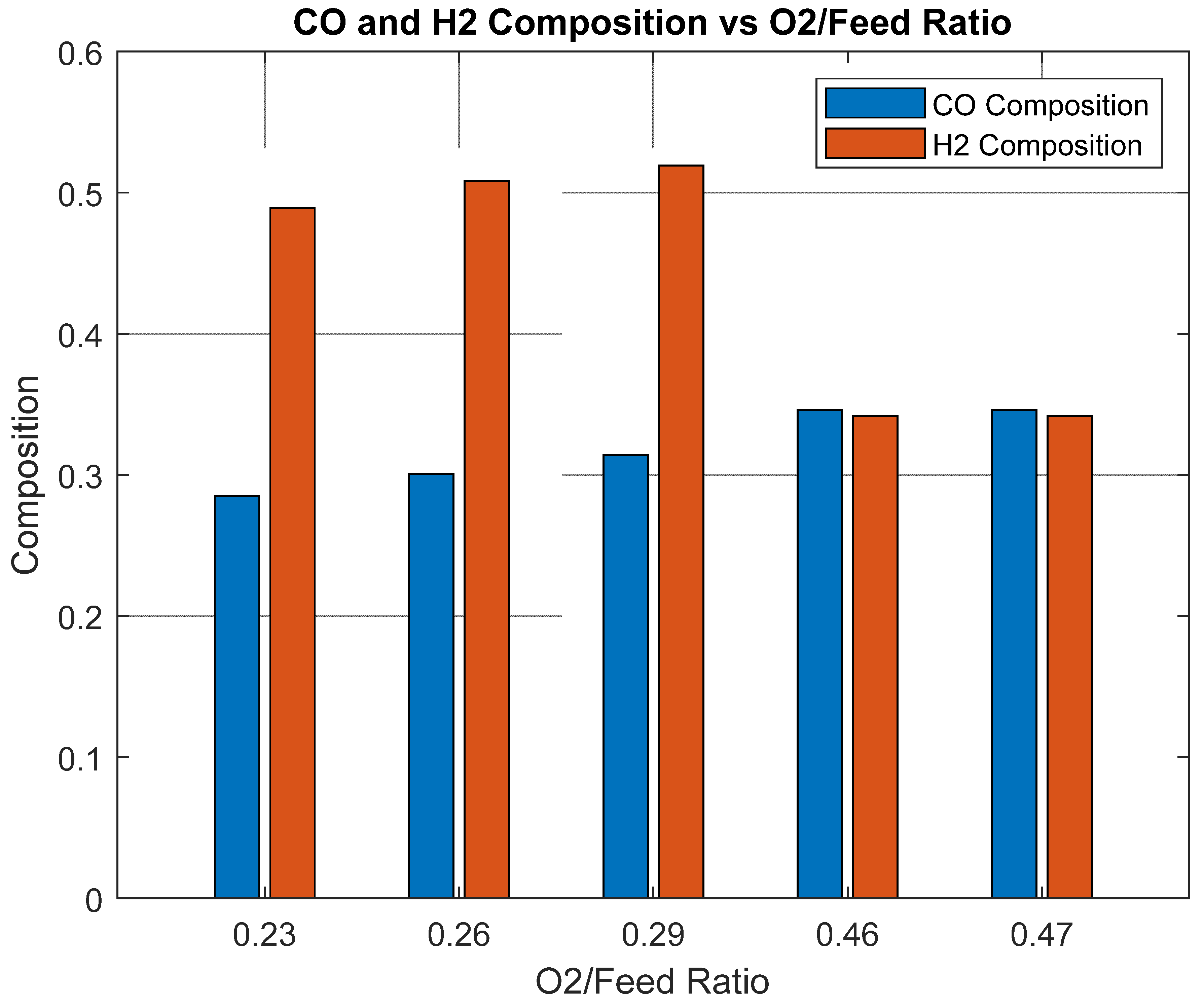
Figure 10 illustrates the influence of the O
2/feed ratio on CO and H
2 composition in the ATR step, a critical determinant of GTL process performance. As the O
2/feed ratio decreases from 0.47 to 0.23, the CO fraction declines from 0.3459 to 0.2849, while H
2 increases from 0.3417 to 0.4892. This trend reflects the shift from partial oxidation at higher O
2/feed ratios—favoring CO formation—to enhanced steam reforming at lower ratios, which promotes H
2 generation at the expense of CO. Consequently, the H
2/CO ratio is strongly affected. At an O
2/feed ratio of 0.47, the ratio approaches 1:1, which is suboptimal for FT synthesis. In contrast, an O
2/feed ratio of 0.23 yields an H
2/CO ratio of 1.72:1, closer to the desired value of ~2:1 for Fischer–Tropsch operation. Thus, an O
2/feed ratio of 0.23 is considered optimal for achieving the required syngas composition, balancing CO and H
2 production to meet process specifications for efficient GTL conversion.
While the preceding sections detail the technical performance of each configuration, a concise comparative overview is valuable for highlighting their practical implications.
Table 4 summarizes the three investigated flare gas treatment processes—dual-stage amine absorption, dual-stage supercritical CO
2 absorption, and the hybrid configuration—in terms of capital and operating expenditure (CAPEX and OPEX), acid gas removal efficiency, environmental risk, and greenhouse gas (GHG) mitigation potential. This integrated comparison not only underscores the technical trade-offs but also provides insights into economic and environmental considerations that are critical for real-world implementation.
3.5. Implications for GHG Emissions, Cost Reduction, and Environmental Benefits
This study evaluated the performance Beyond process performance, the broader impact of flare gas treatment lies in its ability to mitigate greenhouse gas (GHG) emissions, reduce operational costs, and deliver environmental benefits. Flaring of untreated gas contributes directly to CO2, CH4, and SOx emissions, which have severe climate and health consequences. The adoption of advanced flare gas recovery systems therefore offers a dual advantage: compliance with emission regulations and improved resource utilization.
The dual-stage amine system ensures complete removal of H2S and CO2, preventing their direct release into the atmosphere. However, its limited selectivity for heavier hydrocarbons results in partial flaring of higher-value components. In contrast, the sc-CO2 system maximizes recovery of C6+ hydrocarbons, reducing methane slip but leaving residual H2S, which upon combustion forms SO2. The hybrid system resolves both issues by fully eliminating acid gases and recovering significant fractions of methane (97%) and heavier hydrocarbons (96.3%), thereby achieving the greatest net reduction in GHG and acid gas emissions.
The economic benefits of flare gas recovery stem from two main factors: (i) reduced energy penalties from flaring and (ii) monetization of recovered hydrocarbons. Amine absorption systems incur high OPEX due to solvent regeneration requirements, while sc-CO2 systems demand high CAPEX for compression but operate at lower OPEX. The hybrid configuration strikes a balance by lowering regeneration energy demand relative to pure amine systems while still enabling recovery of higher hydrocarbons that can be sold or fed into downstream Gas-to-Liquids (GTL) processes. This not only offsets operational costs but can also provide additional revenue streams.
The integration of flare gas treatment with GTL conversion provides a sustainable pathway for transforming waste gas into ultra-clean liquid fuels such as diesel and jet fuel. These fuels have near-zero sulfur content and favorable combustion characteristics, supporting stricter environmental regulations and reducing local air pollution. Among the configurations, the hybrid process offers the most comprehensive environmental advantage: it ensures regulatory compliance (acid gas removal), reduces CO2-equivalent emissions, and contributes to circular resource utilization through hydrocarbon recovery and conversion.
Overall, the hybrid configuration demonstrates the highest potential for simultaneously reducing greenhouse gas emissions, lowering operational costs, and enhancing environmental sustainability, making it the most attractive option for large-scale deployment in oilfield operations.
4. Conclusions
This study evaluated the performance of three flare gas treatment configurations: dual-stage amine absorption, dual-stage CO2 supercritical absorption, and a hybrid system combining CO2 supercritical absorption followed by amine absorption. Aspen HYSYS V12.1 simulations were used to assess efficiency, selectivity, and environmental impact. The results demonstrate clear trade-offs among the configurations, underscoring the importance of selecting a process suited to specific operational objectives.
The dual-stage amine absorption system achieved the most effective removal of acid gases, with complete elimination of CO2 and H2S and high methane recovery (99.7%). However, it exhibited limited selectivity for heavier hydrocarbons such as hexane. In contrast, the dual-stage supercritical CO2 configuration provided nearly complete recovery of hydrocarbons, including 100% hexane, and full H2S removal, but retained CO2 at elevated concentrations in the sweet gas stream. The hybrid configuration proved the most promising, combining the strengths of both methods. It achieved complete H2S removal, reduced CO2 to very low levels (0.0012 mole fraction), maintained full hexane recovery, and secured methane recovery of 95.02%. These results demonstrate that integrating sc-CO2 with amine absorption resolves the trade-off between acid gas removal and hydrocarbon selectivity, establishing a technically viable pathway for flare gas treatment and resource utilization.
Beyond treatment simulations, the study also examined the integration of Gas-to-Liquids (GTL) technology as a sustainable approach to flare gas utilization. The autothermal reformer (ATR) enabled optimal syngas generation with an H2/CO ratio of 2.3, while the Fischer–Tropsch (FT) reactor converted this syngas into high-value long-chain hydrocarbons. This demonstrates that GTL technology not only mitigates environmental impacts of flaring but also enhances the economic value of flare gas, offering a feasible and profitable solution for regions facing severe flaring challenges.