Comparison of Alliin Recovery from Allium sativum L. Using Soxhlet Extraction and Subcritical Water Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials and Sample Preparation
2.3. Soxhlet Extraction
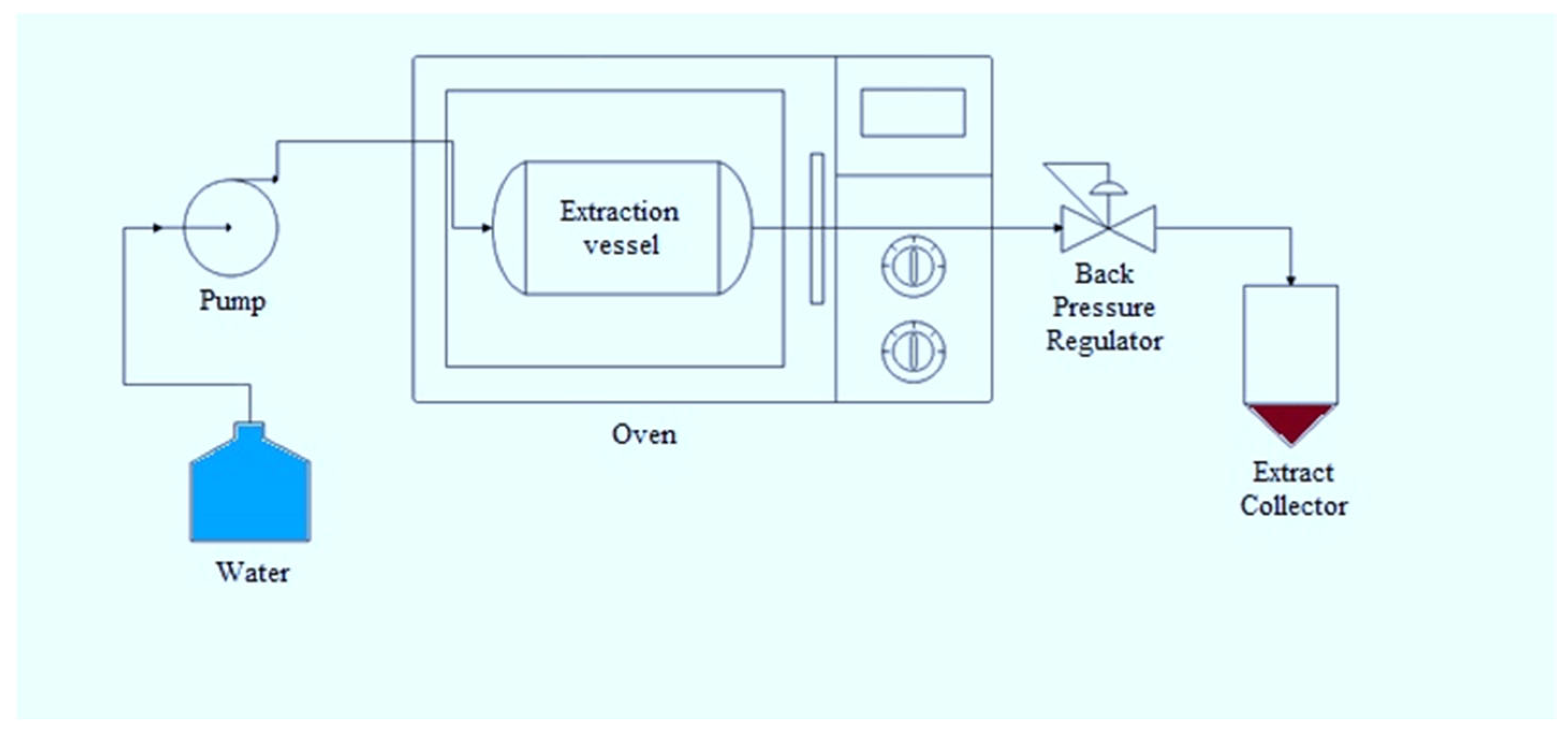
2.4. Subcritical Water Extraction
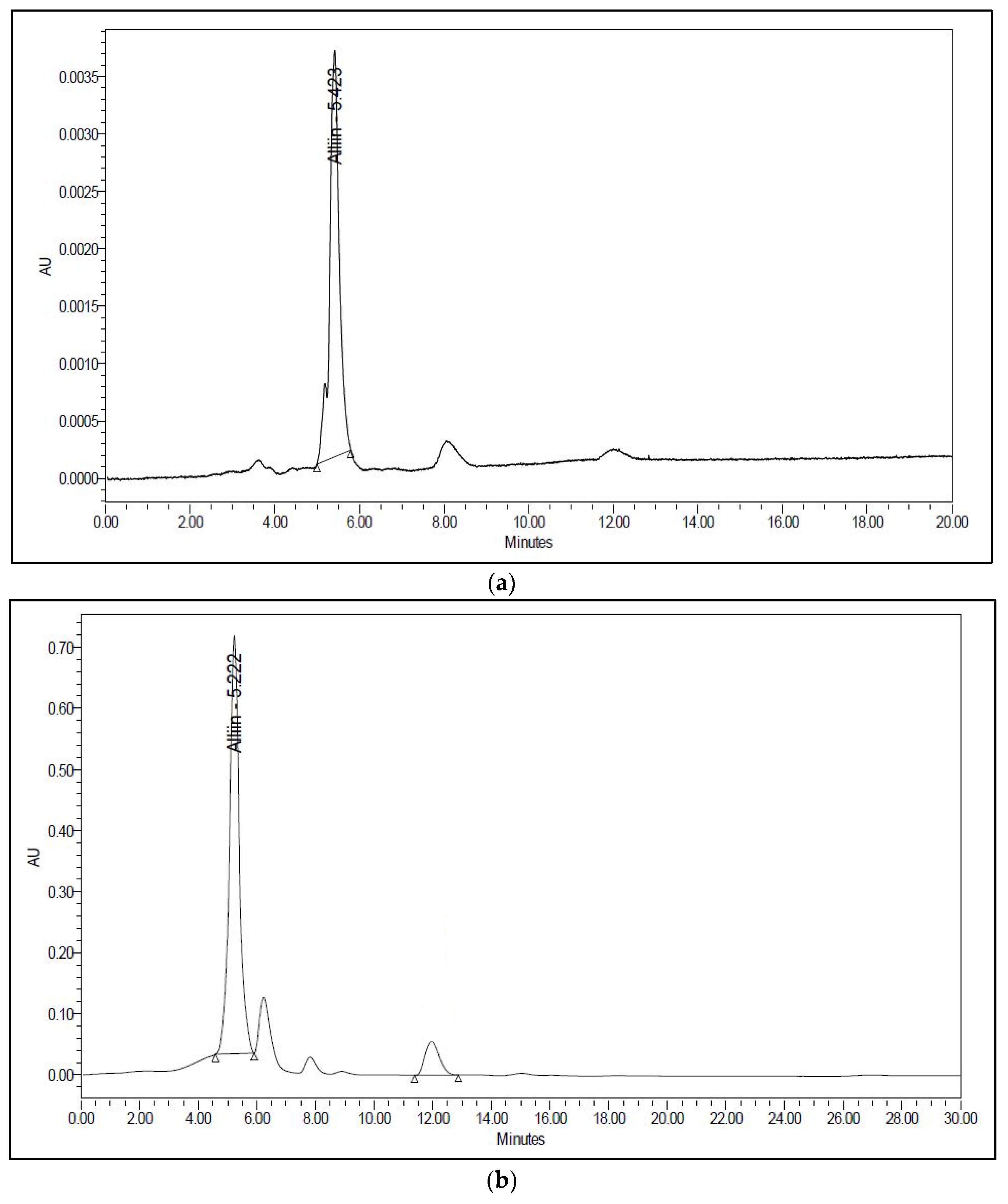
2.5. Analysis of Alliin by High-Performance Liquid Chromatography (HPLC)
2.6. Calculation of Extraction Yield
3. Results and Discussion
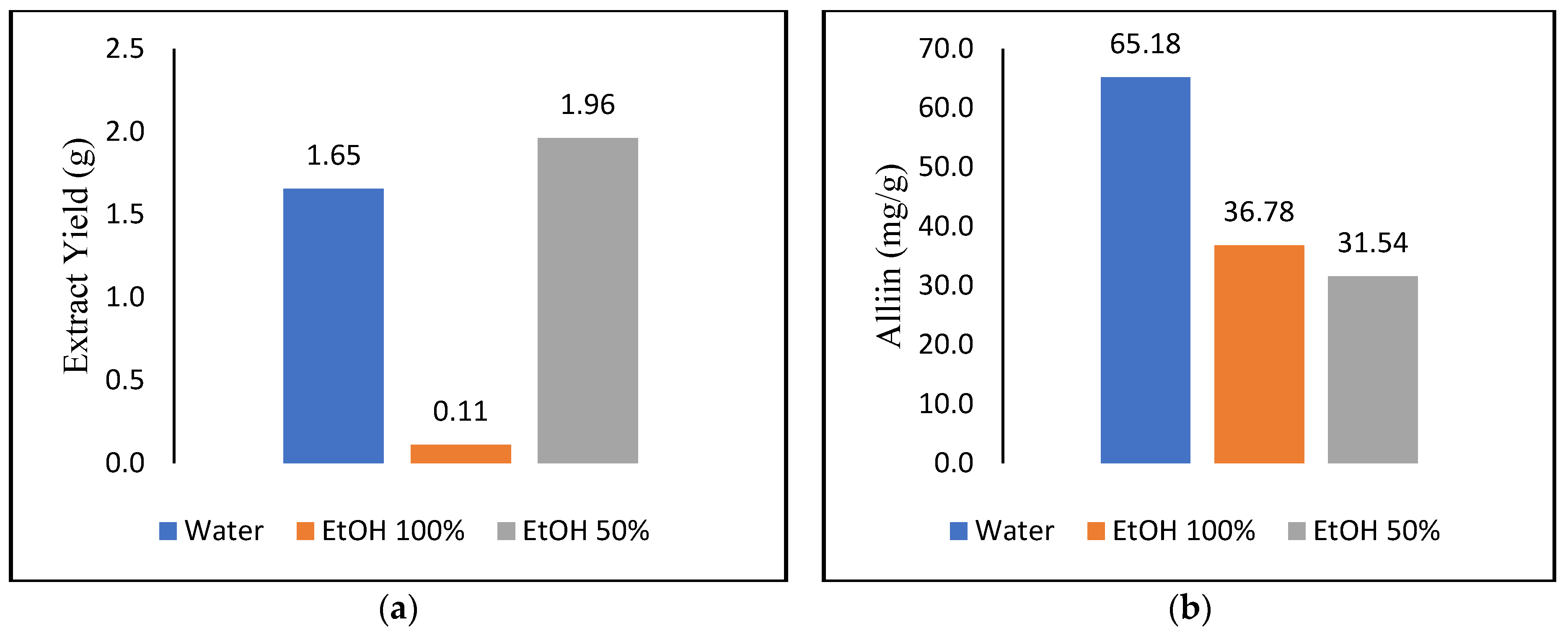
3.1. Soxhlet Extraction
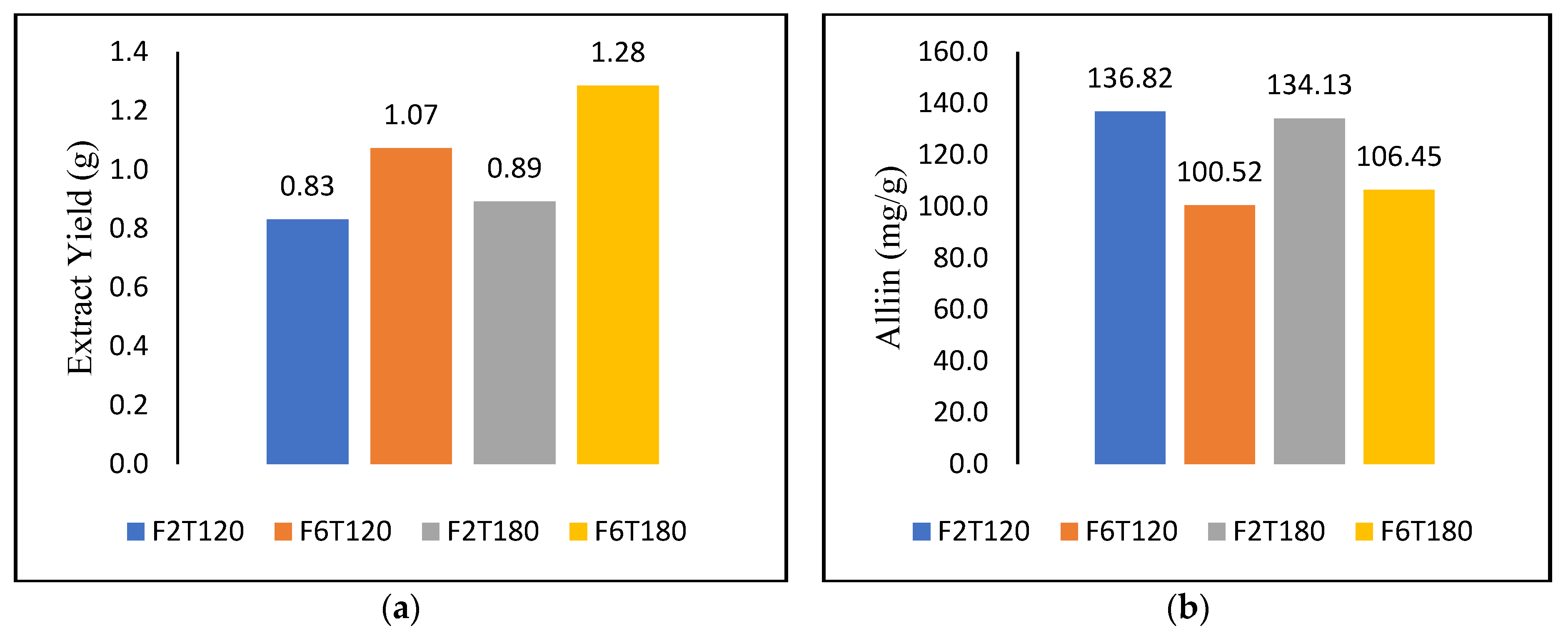
3.2. Subcritical Water Extraction
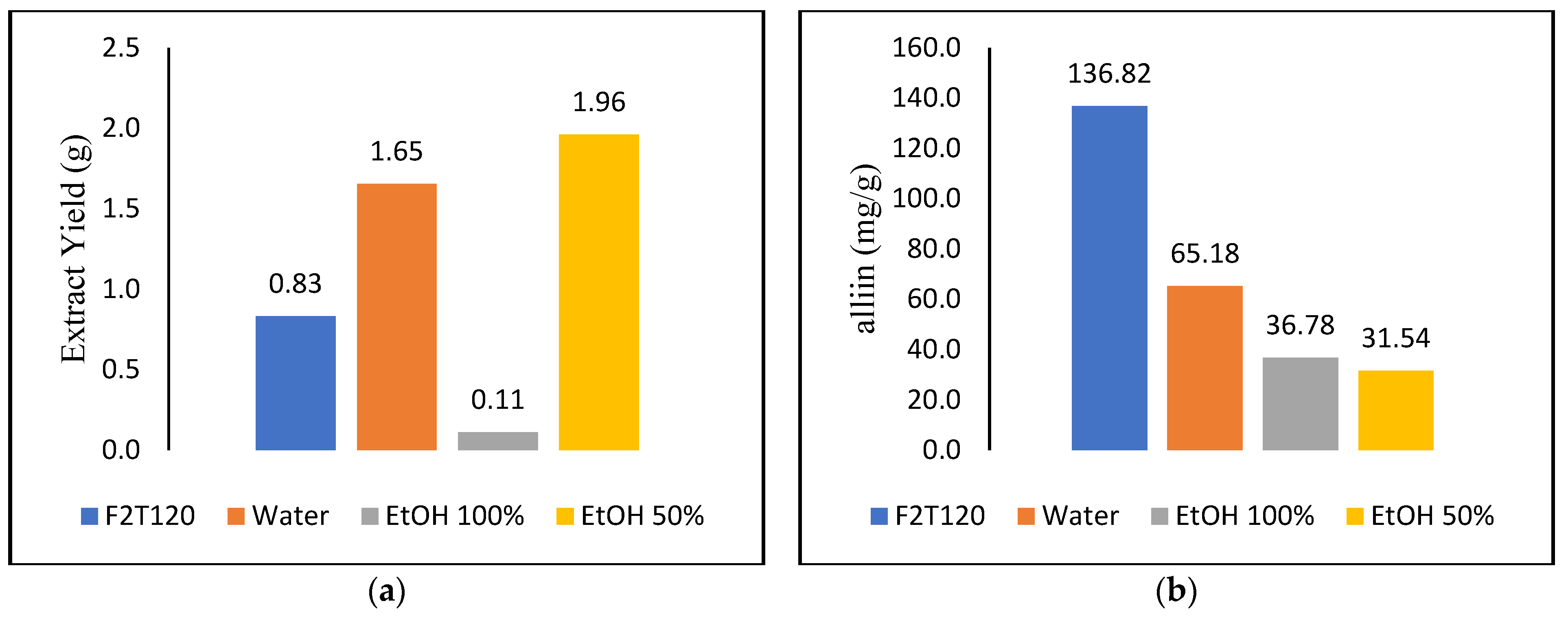
3.3. Comparisons of Subcritical Water Extraction and Soxhlet Extraction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fante, L.; Norena, C.P.Z. Quality of hot air dried and freeze-dried of garlic (Allium sativum L.). J. Food Sci. Technol. 2015, 52, 211–220. [Google Scholar] [CrossRef]
- Zaini, A.; Putra, N.; Idham, Z.; Norodin, N.M.; Rasidek, N.M.; Yunus, M.C. Mini review: Extraction of allicin from allium sativum using subcritical water extraction. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Marrakech, Morocco, 13–15 November 2020; p. 012023. [Google Scholar]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1. [Google Scholar] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Andreatta, A.; Foco, G.; Mabe, G.; Bottini, S. Extraction of garlic oil with quasi-critical solvents. In Proceedings of the 2nd Mercosur Congress on Chemical Engineering, Rio de Janeiro, Brazil, 14–18 August 2005; pp. 1–5. [Google Scholar]
- Kim, S.; Kim, D.-B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.-H. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Malik, M.Q.; Mujib, A.; Gulzar, B.; Zafar, N.; Syeed, R.; Mamgain, J.; Ejaz, B. Enrichment of alliin in different in vitro grown tissues of Allium sativum through CdCl2 elicitation as revealed by high performance thin layer chromatography (HPTLC). Ind. Crop. Prod. 2020, 158, 113007. [Google Scholar] [CrossRef]
- Zaini, A.S.; Aris, N.A.; Putra, N.R.; Hashib, S.A.; Kamaruddin, M.J.; Idham, Z.; Yunus, M.A.C. Comparison of charantin extract from Momordica charantia using modified supercritical carbon dioxide and soxhlet extraction method. Malays. J. Fundam. Appl. Sci. 2018, 14, 462–466. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Amiri, Z.N.; Najafpour, G.D.; Mohammadi, M.; Moghadamnia, A.A. Subcritical Water Extraction of Bioactive Compounds from Ginger (Zingiber officinale Roscoe). Int. J. Eng.-Iran. 2018, 31, 1991–2000. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Mayanga-Torres, P.C.; Marostica, M.R.; Rostagno, M.A.; Martinez, J.; Forster-Carneiro, T. Subcritical water extraction of flavanones from defatted orange peel. J. Supercrit. Fluid 2018, 138, 7–16. [Google Scholar] [CrossRef]
- Bose, S.; Laha, B.; Banerjee, S. Quantification of allicin by high performance liquid chromatography-ultraviolet analysis with effect of post-ultrasonic sound and microwave radiation on fresh garlic cloves. Pharmacogn. Mag. 2014, 10, S288. [Google Scholar] [CrossRef] [PubMed]
- Gunther, W.H. Garlic and other alliums—The lore and the science. J. Sulfur Chem. 2013, 34, 208. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Horita, C.N.; Pollonio, M.A.; Meireles, M.A.A. Allicin-rich extract obtained from garlic by pressurized liquid extraction: Quantitative determination of allicin in garlic samples. Food Public Health 2014, 4, 272–278. [Google Scholar]
- Idham, Z.; Putra, N.R.; Aziz, A.H.A.; Zaini, A.S.; Rasidek, N.A.M.; Mili, N.; Yunus, M.A.C. Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J. CO2 Util. 2022, 56, 101839. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Machmudah, S.; Shalleh, L.M.; Che Yunus, M.A. Recovery and solubility of flavonoid and phenolic contents from Arachis Hypogea in supercritical carbon dioxide assisted by ethanol as cosolvent. J. Food Process. Preserv. 2020, 44, e14768. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Riaz, M.; Al-Tarouti, M.; Aloufi, F.; AlDarwish, A.; Alalaq, B.; Alhanfoush, B.; Khan, Z. Optimization of extraction and quantification technique for phenolics content of garlic (Allium sativum): An application for comparative phytochemical evaluation based on cultivar origin. Biomed. Chromatogr. 2020, 34, e4942. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Mandal, V.; Mandal, S.C. Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem. Eng. J. 2010, 50, 63–70. [Google Scholar] [CrossRef]
- Hao, G.X.; Cao, W.Q.; Li, T.; Chen, J.; Zhang, J.L.; Weng, W.Y.; Osako, K.; Ren, H.F. Effect of temperature on chemical properties and antioxidant activities of abalone viscera subcritical water extract. J. Supercrit. Fluid 2019, 147, 17–23. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, G.; Chen, S. Subcritical Water Extraction of Sesquiterpene Lactones from Inula racemose. ChemistrySelect 2020, 5, 488–494. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Veza, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L.; Yunus, M.A.C. Solubilization and Extraction of Valuable Compounds from Peanut skin in Subcritical Water. J. Food Process. Preserv. 2022, e17005. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Idham, Z.; Rizkiyah, D.N.; Jumakir, J.; Mastaza, M.H.A.; Yunus, M.A.C. Kinetic modelling and extraction of Orthosiphon stamineus stems and leaves by SC-CO2. Malays. J. Fundam. Appl. Sci. 2020, 16, 602–608. [Google Scholar]
- Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Machmudah, S.; Jumakir, J.; Waluyo, W.; Che Yunus, M.A. Procyanidin and proanthocyanidin extraction from Arachis hypogaea skins by using supercritical carbon dioxide: Optimization and modeling. J. Food Process. Preserv. 2021, 45, e15689. [Google Scholar] [CrossRef]
- Rizkiyah, D.N.; Jusoh, W.M.S.W.; Idham, Z.; Putra, N.R.; Che Yunus, M.A. Investigation of Phenolic, Flavonoid and Antioxidant Recovery and Solubility from Roselle Using Supercritical Carbon Dioxide: Experimental and Modelling. J. Food Process. Preserv. 2022, e16670. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Idham, Z.; Ruslan, M.S.H.; Yunus, M.A.C. Diffusivity optimization of supercritical carbon dioxide extraction with co-solvent-ethanol from peanut skin. Malays. J. Fundam. Appl. Sci. 2018, 14, 9–14. [Google Scholar]
- Arumugham, T.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Rinklebe, J.; Banat, F. Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications—A review. Chemosphere 2021, 271, 129525. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical water extraction of phenolic compounds from onion skin wastes (Allium cepa cv. Horcal): Effect of temperature and solvent properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. In Proceedings of the MATEC Web of Conferences, Semarang, Indonesia, 14 March 2018; p. 02005. [Google Scholar]
- Ramirez, D.A.; Altamirano, J.C.; Camargo, A.B. Multi-phytochemical determination of polar and non-polar garlic bioactive compounds in different food and nutraceutical preparations. Food Chem. 2021, 337, 127648. [Google Scholar] [CrossRef] [PubMed]
- Arsad, N.H.; Yunus, M.A.C.; Zaini, M.A.A.; Rahman, Z.A.; Idham, Z. Effect of Operating Conditions of Supercritical Carbon Dioxide on Piper Betle Leave Oil Yield and Antioxidant Activity. Int. J. Appl. Chem. 2016, 12, 741–751. [Google Scholar]
- Essien, S.O.; Young, B.; Baroutian, S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. [Google Scholar] [CrossRef]
- Rodrigues, L.G.G.; Mazzutti, S.; Siddique, I.; da Silva, M.; Vitali, L.; Ferreira, S.R.S. Subcritical water extraction and microwave-assisted extraction applied for the recovery of bioactive components from Chaya (Cnidoscolus aconitifolius Mill.). J. Supercrit. Fluids 2020, 165, 104976. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaini, A.S.; Putra, N.R.; Idham, Z.; Mohd Faizal, A.N.; Che Yunus, M.A.; Mamat, H.; Abdul Aziz, A.H. Comparison of Alliin Recovery from Allium sativum L. Using Soxhlet Extraction and Subcritical Water Extraction. ChemEngineering 2022, 6, 73. https://doi.org/10.3390/chemengineering6050073
Zaini AS, Putra NR, Idham Z, Mohd Faizal AN, Che Yunus MA, Mamat H, Abdul Aziz AH. Comparison of Alliin Recovery from Allium sativum L. Using Soxhlet Extraction and Subcritical Water Extraction. ChemEngineering. 2022; 6(5):73. https://doi.org/10.3390/chemengineering6050073
Chicago/Turabian StyleZaini, Ahmad Syahmi, Nicky Rahmana Putra, Zuhaili Idham, Azrul Nurfaiz Mohd Faizal, Mohd Azizi Che Yunus, Hasmadi Mamat, and Ahmad Hazim Abdul Aziz. 2022. "Comparison of Alliin Recovery from Allium sativum L. Using Soxhlet Extraction and Subcritical Water Extraction" ChemEngineering 6, no. 5: 73. https://doi.org/10.3390/chemengineering6050073
APA StyleZaini, A. S., Putra, N. R., Idham, Z., Mohd Faizal, A. N., Che Yunus, M. A., Mamat, H., & Abdul Aziz, A. H. (2022). Comparison of Alliin Recovery from Allium sativum L. Using Soxhlet Extraction and Subcritical Water Extraction. ChemEngineering, 6(5), 73. https://doi.org/10.3390/chemengineering6050073






