Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms
Abstract
1. Introduction
2. Materials and Methods
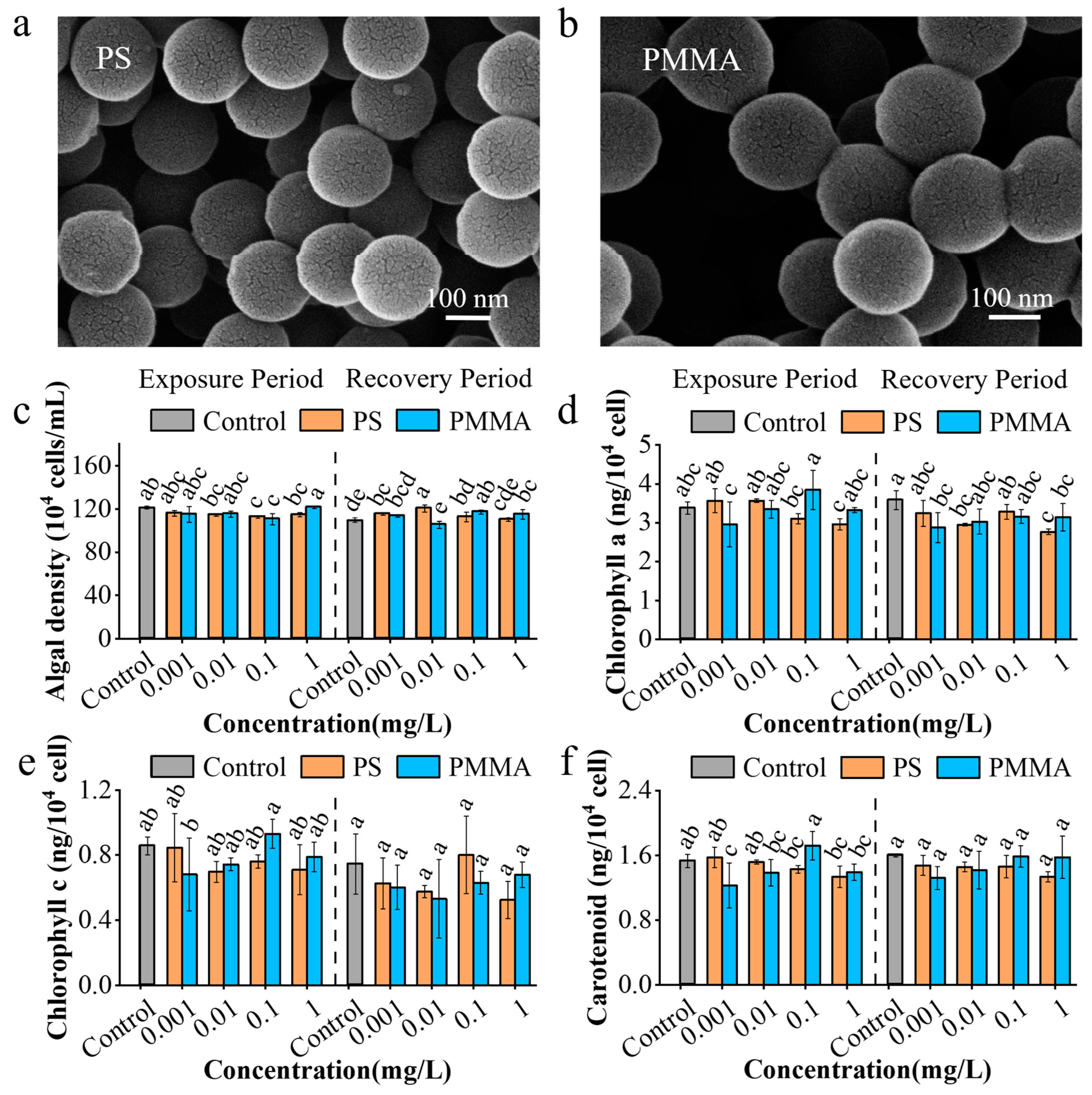
2.1. Characterization of MPs
2.2. Exposure and Recovery Experiments
2.3. Measurements of the Photosynthetic Pigment Contents
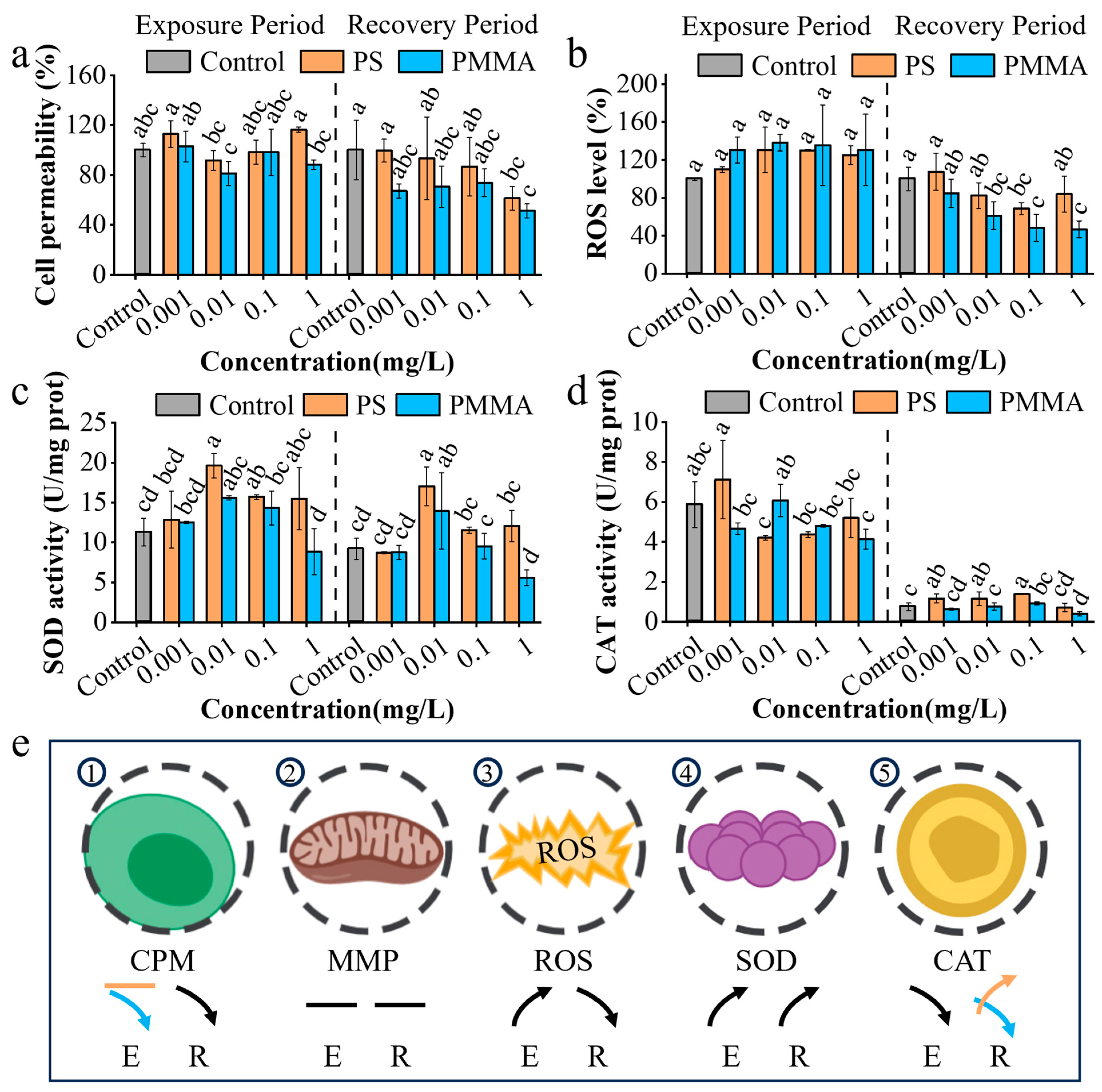
2.4. Oxidative Stress Responses to MP Exposure
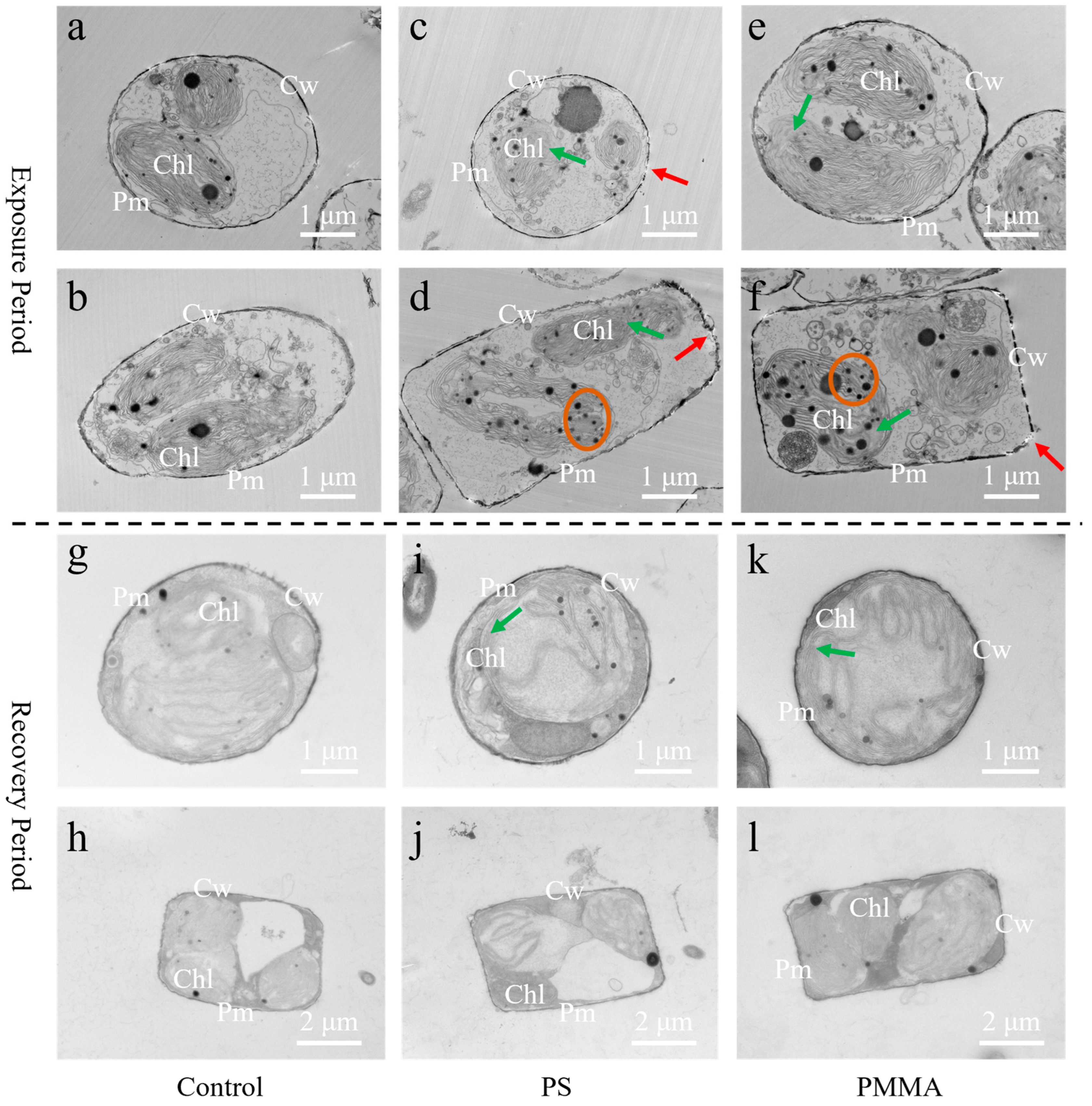
2.5. Cellular Ultrastructure Damage
2.6. Nitrogen Assimilation Measurements
2.7. Metabolomics Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Persistence and Recovery of Growth and Photosynthesis to MPs
3.2. Cellular Oxidative Stress Response to MPs
3.3. Damage of Cellular Ultrastructure
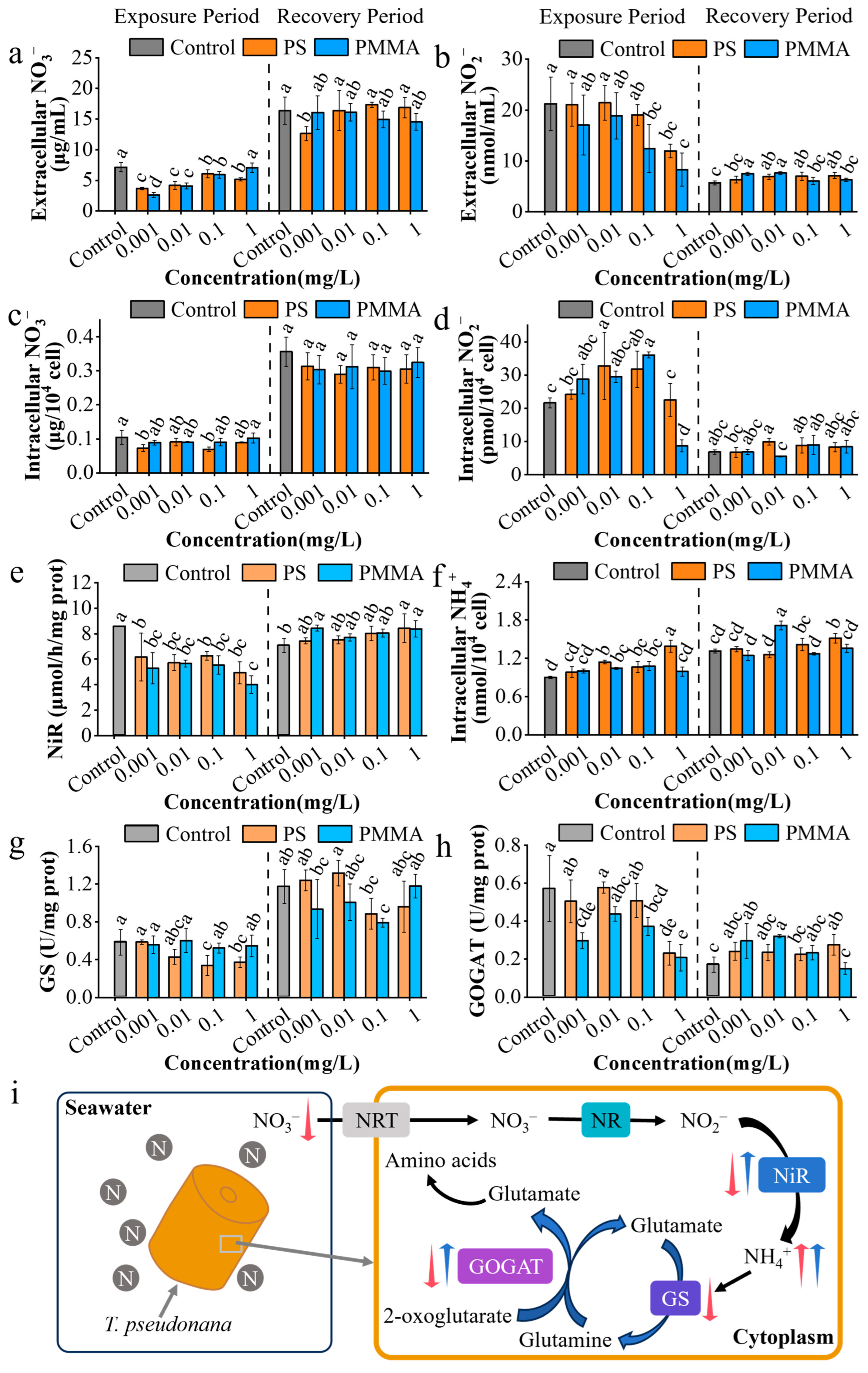
3.4. Effects of MPs on Nitrogen Assimilation
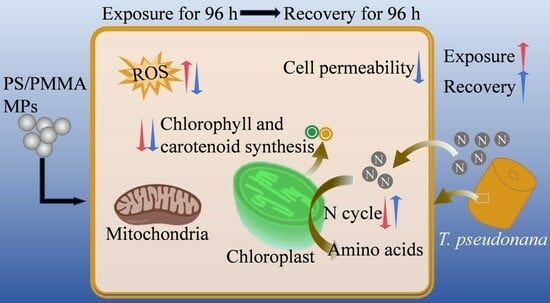
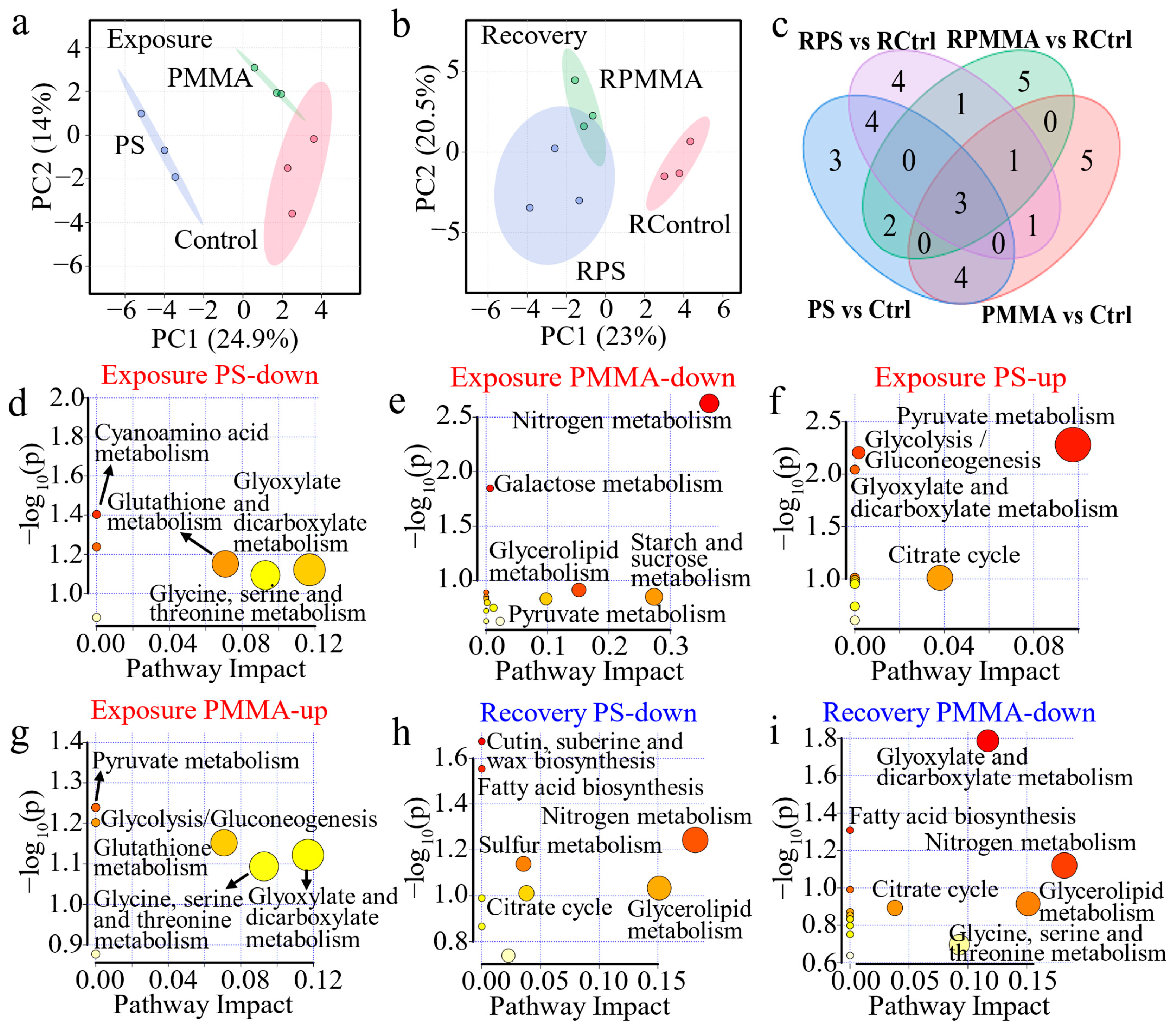
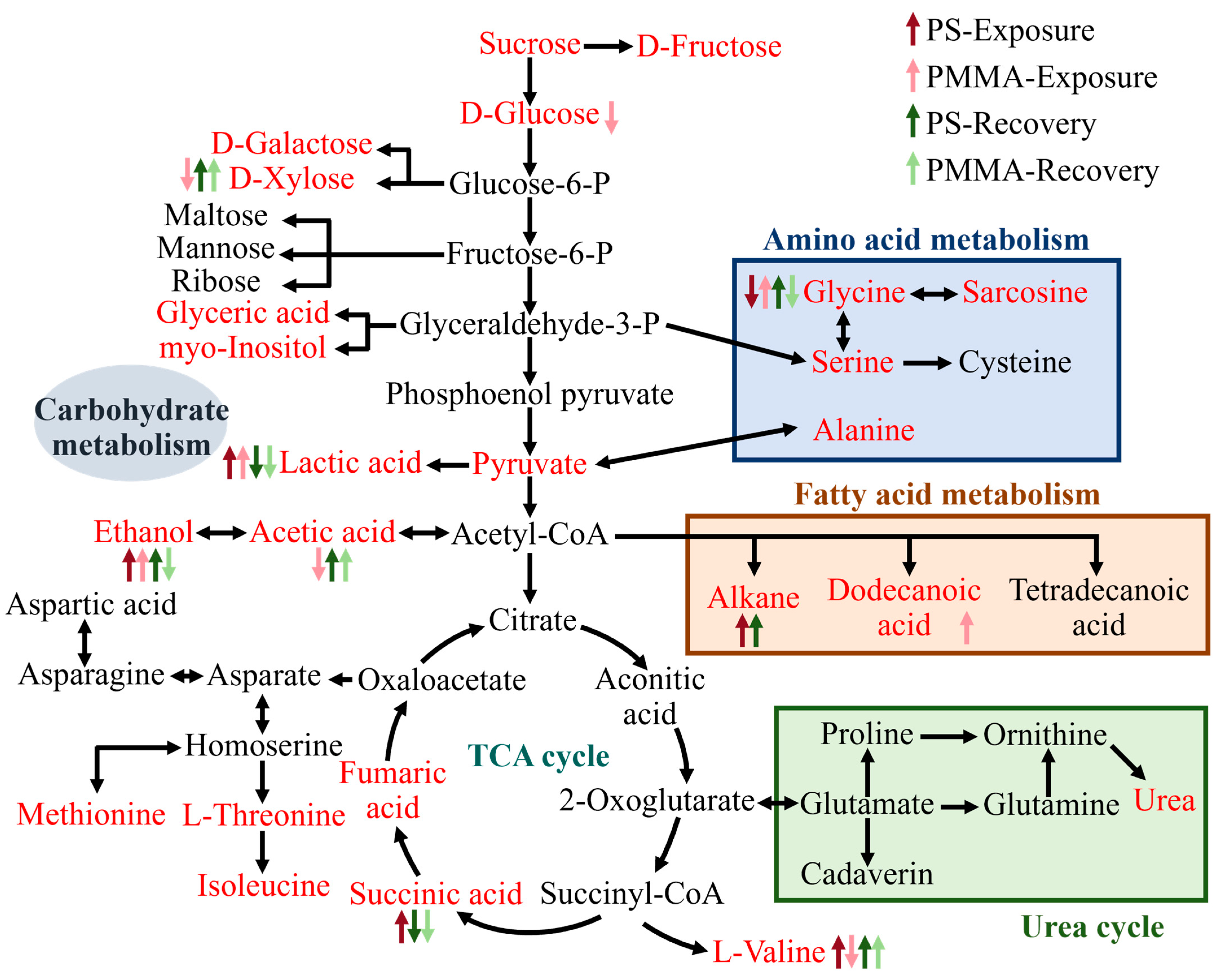
3.5. Mechanisms of Algal Toxicity and Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Vidal, F.; van der Marel, E.R.; Kerr, R.W.F.; McElroy, C.; Schroeder, N.; Mitchell, C.; Rosetto, G.; Chen, T.T.D.; Bailey, R.M.; Hepburn, C.; et al. Designing a circular carbon and plastics economy for a sustainable future. Nature 2024, 626, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Garside, M. Global Plastic Production 1950–2022. Statista. 2024. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 26 December 2024).
- Yao, S.; Ni, N.; Li, X.; Wang, N.; Bian, Y.; Jiang, X.; Song, Y.; Bolan, N.S.; Zhang, Q.; Tsang, D.C.W. Interactions between white and black carbon in water: A case study of concurrent aging of microplastics and biochar. Water Res. 2023, 238, 120006. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent trends in degradation of microplastics in the environment: A state-of-the-art review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Rosso, B.; Scoto, F.; Hallanger, I.G.; Larose, C.; Gallet, J.C.; Spolaor, A.; Bravo, B.; Barbante, C.; Gambaro, A.; Corami, F. Characteristics and quantification of small microplastics (<100 µm) in seasonal svalbard snow on glaciers and lands. J. Hazard. Mater. 2024, 467, 133723. [Google Scholar] [CrossRef] [PubMed]
- Graham, P.M.; Pattinson, N.B.; Bakir, A.; McGoran, A.R.; Nel, H.A. Determination of microplastics in sediment, water, and fish across the Orange-Senqu River basin. Water Res. 2024, 266, 122394. [Google Scholar] [CrossRef]
- Yang, S.; Lu, X.; Wang, X. A perspective on the controversy over global emission fluxes of microplastics from ocean into the atmosphere. Environ. Sci. Technol. 2024, 58, 12304–12312. [Google Scholar] [CrossRef] [PubMed]
- Welsh, B.; Paterson, A.M.; Yao, H.; McConnell, C.; Aherne, J. The fate of microplastics in rural headwater lake catchments. Environ. Sci. Technol. 2024, 58, 16570–16577. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Zaheer, M.; Jin, W.; Yang, L. Biodegradation of macro- and micro-plastics in environment: A review on mechanism, toxicity, and future perspectives. Sci. Total Environ. 2023, 858, 160108. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Tayyab, M.; Pastorino, P.; Barcelò, D.; Yaseen, Z.M.; Grossart, H.-P.; Khan, Z.H.; Li, G. Decoding the molecular concerto: Toxicotranscriptomic evaluation of microplastic and nanoplastic impacts on aquatic organisms. J. Hazard. Mater. 2024, 472, 134574. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Tagliabue, A. Feedbacks between phytoplankton and nutrient cycles in a warming ocean. Nat. Geosci. 2024, 17, 495–502. [Google Scholar] [CrossRef]
- Bolaños, L.M.; Karp-Boss, L.; Choi, C.J.; Worden, A.Z.; Graff, J.R.; Haëntjens, N.; Chase, A.P.; Della Penna, A.; Gaube, P.; Morison, F.; et al. Small phytoplankton dominate western North Atlantic biomass. ISME J. 2020, 14, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zheng, X.; Han, Z.; Li, Y.; He, H.; Lin, T.; Xu, H. Polystyrene microplastics enhanced the effect of PFOA on Chlorella sorokiniana: Perspective from the cellular and molecular levels. J. Hazard. Mater. 2024, 465, 133455. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, B.-Y.; Zhang, X.; Xu, Q.; Yang, L.; Chen, J.; Zhou, X.; Zhang, Y. The aging of microplastics exacerbates the damage to photosynthetic performance and bioenergy production in microalgae (Chlorella pyrenoidosa). Water Res. 2024, 259, 121841. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Dai, M.; Liu, P.; Chen, T.; Hu, L.; Luo, H.; Zhou, Q.; Du, M.; Pan, X. Phthalocyanine blue leaching and exposure effects on Microcystis aeruginosa (cyanobacteria) of photoaged microplastics. J. Hazard. Mater. 2024, 469, 133984. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, X.; Gao, P.; Zhao, X.; Ma, C.; Wang, L.; Sun, H.; Sun, L.; Liu, C. Combined effects of microplastics and excess boron on Microcystis aeruginosa. Sci. Total Environ. 2023, 891, 164298. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chu, Z.; Li, X.; Feng, C.; Zhang, Y.; Wang, C.; Zhang, J.; Xu, C.; Wang, J.; Tang, H. Proteomic insights into composition-dependent effects of microplastics on freshwater microalgae Chlamydomonas reinhardtii. Environ. Sci. Nano 2024, 11, 3440–3456. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.Y.; Jung, W.S.; Kim, H.S.; Oh, B.; Park, J.; Choi, Y.-E. Effects of micro-sized biodegradable plastics on Microcystis aeruginosa. Sci. Total Environ. 2024, 912, 169044. [Google Scholar] [CrossRef]
- Yan, B.; Liu, Q.; Li, J.; Wang, C.; Li, Y.; Zhang, C. Microplastic pollution in marine environment: Occurrence, fate, and effects (with a specific focus on biogeochemical carbon and nitrogen cycles). In Microplastic Pollution; Springer: Singapore, 2021; pp. 105–126. [Google Scholar] [CrossRef]
- Xue, Y.; Song, K.; Wang, Z.; Xia, Z.; Li, R.; Wang, Q.; Li, L. Nanoplastics occurrence, detection methods, and impact on the nitrogen cycle: A review. Environ. Chem. Lett. 2024, 22, 2241–2255. [Google Scholar] [CrossRef]
- Kang, W.; Sun, S.; Hu, X. Microplastics trigger the Matthew effect on nitrogen assimilation in marine diatoms at an environmentally relevant concentration. Water Res. 2023, 233, 119762. [Google Scholar] [CrossRef]
- Nik Mut, N.N.; Na, J.; Jung, J. A review on fate and ecotoxicity of biodegradable microplastics in aquatic system: Are biodegradable plastics truly safe for the environment? Environ. Pollut. 2024, 344, 123399. [Google Scholar] [CrossRef]
- Li, X.; Luo, J.; Zeng, H.; Zhu, L.; Lu, X. Microplastics decrease the toxicity of sulfamethoxazole to marine algae (Skeletonema costatum) at the cellular and molecular levels. Sci. Total Environ. 2022, 824, 153855. [Google Scholar] [CrossRef]
- Leblanc, K.; Quéguiner, B.; Diaz, F.; Cornet, V.; Michel-Rodriguez, M.; de Madron, X.D.; Bowler, C.; Malviya, S.; Thyssen, M.; Grégori, G.; et al. Nanoplanktonic diatoms are globally overlooked but play a role in spring blooms and carbon export. Nat. Commun. 2018, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Irion, S.; Christaki, U.; Berthelot, H.; L’Helguen, S.; Jardillier, L. Small phytoplankton contribute greatly to CO2-fixation after the diatom bloom in the Southern Ocean. ISME J. 2021, 15, 2509–2522. [Google Scholar] [CrossRef]
- Dedman, C.J.; Christie-Oleza, J.A.; Fernández-Juárez, V.; Echeveste, P. Cell size matters: Nano- and micro-plastics preferentially drive declines of large marine phytoplankton due to co-aggregation. J. Hazard. Mater. 2022, 424, 127488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.H.; Wang, Z.Q.; Li, D.R.; Li, L.Y.; Zhao, Y.R.; Tang, X.X.; Zhao, Y. Reactive oxygen species mediated extracellular polymeric substances production assisting the recovery of Thalassiosira pseudonana from polystyrene micro and nanoplastics exposure. Environ. Pollut. 2024, 348, 123850. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tang, X.; Li, L.; Zhang, B.; Wang, Z.; Liu, Z.; Zhao, Y. UV-B radiation aging changed the environmental behavior of polystyrene micro-/nanoplastics-adsorption kinetics of BDE-47, plankton toxicities and joint toxicities with BDE-47. J. Hazard. Mater. 2024, 480, 136198. [Google Scholar] [CrossRef]
- Li, D.R.; Tang, X.X.; Xu, X.Z.; Zhao, Y.R.; Li, L.Y.; Zhang, B.H.; Zhao, Y. UV-B radiation alleviated detrimental effects of polymethyl methacrylate microplastics on marine diatom. Sci. Total Environ. 2023, 892, 164388. [Google Scholar] [CrossRef]
- Fernández Severini, M.D.; Villagran, D.M.; Buzzi, N.S.; Sartor, G.C. Microplastics in oysters (Crassostrea gigas) and water at the Bahía Blanca Estuary (Southwestern Atlantic): An emerging issue of global concern. Reg. Stud. Mar. Sci. 2019, 32, 100829. [Google Scholar] [CrossRef]
- Chouchene, K.; da Costa, J.P.; Chamkha, M.; Ksibi, M.; Sayadi, S. Effects of microplastics’ physical and chemical properties on aquatic organisms: State-of-the-art and future research trends. TrAC Trends Anal. Chem. 2023, 166, 117192. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Podbielska, M.; Szpyrka, E. Microplastics-an emerging contaminants for algae. critical review and perspectives. Sci. Total Environ. 2023, 885, 163842. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, X.; Kang, W.; Yao, M. Combined effects of microplastics and warming enhance algal carbon and nitrogen storage. Water Res. 2023, 233, 119815. [Google Scholar] [CrossRef] [PubMed]
- ISO 10253; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. ISO: Geneva, Switzerland, 2024.
- Zhang, X.; Hu, X.; Wu, H.; Mu, L. Persistence and recovery of ZIF-8 and ZIF-67 phytotoxicity. Environ. Sci. Technol. 2021, 55, 15301–15312. [Google Scholar] [CrossRef] [PubMed]
- Pabortsava, K.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef]
- Garcés-Ordóñez, O.; Mejía-Esquivia, K.A.; Sierra-Labastidas, T.; Patiño, A.; Blandón, L.M.; Espinosa Díaz, L.F. Prevalence of microplastic contamination in the digestive tract of fishes from mangrove ecosystem in Cispata, Colombian Caribbean. Mar. Pollut. Bull. 2020, 154, 111085. [Google Scholar] [CrossRef]
- Wu, D.; Deng, L.; Wang, T.; Du, W.; Yin, Y.; Guo, H. Aging process does not necessarily enhance the toxicity of polystyrene microplastics to Microcystis aeruginosa. Sci. Total Environ. 2023, 882, 163608. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Tan, H.; Wang, H.; Wang, J.; Song, K.; Xu, D.; Zhang, B.; Liu, Z.; Liu, X.; et al. Responses of different species of marine microalgae and their community to gear-derived microplastics. Water Res. 2025, 281, 123528. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972; p. 310. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. Assay of superoxide dismutase activity in a plate assay using WST-1. Free. Radic. Biol. Med. 2017, 103, 188–191. [Google Scholar] [CrossRef]
- Hadwan, M.H.; Abed, H.N. Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 2016, 6, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.Q.; Mu, L.; Gao, Z.W.; Hu, X.A. Persistence of algal toxicity induced by polystyrene nanoplastics at environmentally relevant concentrations. Sci. Total Environ. 2023, 876, 162853. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Guo, S.; Hu, X. Identifying the phytotoxicity and defense mechanisms associated with graphene-based nanomaterials by integrating multiomics and regular analysis. Environ. Sci. Technol. 2021, 55, 9938–9948. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Thomas, K.V.; Luo, Z.S.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef]
- Kang, W.L.; Li, X.K.; Sun, A.Q.; Yu, F.B.; Hu, X.G. Study of the persistence of the phytotoxicity induced by graphene oxide quantum dots and of the specific molecular mechanisms by integrating omics and regular analyses. Environ. Sci. Technol. 2019, 53, 3791–3801. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Srivastava, P. Photosynthetic pigments in diatoms. In Insights into the World of Diatoms, from Essentials to Applications; Springer Nature: Singapore, 2023; pp. 1–20. [Google Scholar] [CrossRef]
- Kuczyńska, P.; Jemioła-Rzemińska, M.; Strzałka, K. Pigments in diatoms. In Diatom Photosynthesis; Wiley: Hoboken, NJ, USA, 2024; pp. 137–189. [Google Scholar] [CrossRef]
- Kang, W.; Yu, F.; Wang, S.; Hu, X. Marine colloids promote the adaptation of diatoms to nitrate contamination by directional electron transfer. Environ. Sci. Technol. 2022, 56, 5694–5705. [Google Scholar] [CrossRef]
- Hou, X.; Mu, L.; Hu, X.A.; Guo, S.Q. Warming and microplastic pollution shape the carbon and nitrogen cycles of algae. J. Hazard. Mater. 2023, 447, 130775. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, A.; Romeo, D.; Wick, P. Understanding nanomaterial biotransformation: An unmet challenge to achieving predictive nanotoxicology. Small 2020, 16, 1907650. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, M.; Ouyang, D.; Tong, H.; Wu, M.; Su, L. Research progress on the protective effect of brown algae-derived polysaccharides on metabolic diseases and intestinal barrier injury. Int. J. Mol. Sci. 2022, 23, 10784. [Google Scholar] [CrossRef]
- Wang, D.J.; Yuan, X.R.; Jia, J.B.; He, S.; Zhou, X.X.; Yan, B. Al3+ reduces PM2.5-induced cytotoxicity in human bronchial epithelial cells via reducing ROS production. Air Qual. Atmos. Health 2021, 14, 903–909. [Google Scholar] [CrossRef]
- Wang, L.; Yang, T.; Pan, Y.; Shi, L.; Jin, Y.; Huang, X. The metabolism of reactive oxygen species and their effects on lipid biosynthesis of microalgae. Int. J. Mol. Sci. 2023, 24, 11041. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, X.; Zhang, L.; Zhang, S.; Li, Y.; Zhang, W.; Li, Q.; Zhao, Y.; Chen, X.; et al. Effect and mechanism of microplastics exposure against microalgae: Photosynthesis and oxidative stress. Sci. Total Environ. 2023, 905, 167017. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Zhou, Y.; Tan, C. Toxicity effects of polystyrene nanoplastics with different sizes on freshwater microalgae Chlorella vulgaris. Molecules 2023, 28, 3958. [Google Scholar] [CrossRef] [PubMed]
- Yesilay, G.; Hazeem, L.; Bououdina, M.; Cetin, D.; Suludere, Z.; Barras, A.; Boukherroub, R. Influence of graphene oxide on the toxicity of polystyrene nanoplastics to the marine microalgae Picochlorum sp. Environ. Sci. Pollut. Res. 2022, 29, 75870–75882. [Google Scholar] [CrossRef]
- Li, X.; Qiu, H.; Zhang, P.; Song, L.; Romero-Freire, A.; He, E. Role of heteroaggregation and internalization in the toxicity of differently sized and charged plastic nanoparticles to freshwater microalgae. Environ. Pollut. 2023, 316, 120517. [Google Scholar] [CrossRef]
- Asadishad, B.; Chahal, S.; Akbari, A.; Cianciarelli, V.; Azodi, M.; Ghoshal, S.; Tufenkji, N. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition. Environ. Sci. Technol. 2018, 52, 1908–1918. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef]
- Tong, Y.C.; Feng, A.Q.; Hou, X.; Zhou, Q.X.; Hu, X.G. Nanoholes regulate the phytotoxicity of single-layer molybdenum disulfide. Environ. Sci. Technol. 2019, 53, 13938–13948. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; He, H.; Liu, K.; Li, Z.; Xi, Y.; Liao, Z.; Dao, G.; Huang, B.; Pan, X. Toxic mechanisms of the antiviral drug arbidol on microalgae in algal bloom water at transcriptomic level. J. Hazard. Mater. 2024, 473, 134678. [Google Scholar] [CrossRef]
- Dey, P.; Malik, A.; Singh, D.K.; Haange, S.-B.; von Bergen, M.; Jehmlich, N. Unveiling fungal strategies: Mycoremediation in multi-metal pesticide environment using proteomics. Sci. Rep. 2024, 14, 23171. [Google Scholar] [CrossRef]
- McNabney, D.W.G.; Mangal, V.; Kirkwood, A.E.; Simmons, D.D.B. Phytoplankton metabolite profiles from two Lake Ontario Areas of Concern reveal differences associated with taxonomic community composition. Sci. Total Environ. 2023, 871, 162042. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.A.; Bernier, M.; Mitchell, S.J.; Di Germanio, C.; Mattison, J.A.; Ehrlich, M.R.; Colman, R.J.; Anderson, R.M.; de Cabo, R. Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell Metab. 2020, 32, 100–116. [Google Scholar] [CrossRef]
- Jani, J.; Toor, G.S. Composition, sources, and bioavailability of nitrogen in a longitudinal gradient from freshwater to estuarine waters. Water Res. 2018, 137, 344–354. [Google Scholar] [CrossRef]
- Zehr, J.P.; Capone, D.G. Changing perspectives in marine nitrogen fixation. Science 2020, 368, 9514. [Google Scholar] [CrossRef] [PubMed]
- Treves, H.; Küken, A.; Arrivault, S.; Ishihara, H.; Hoppe, I.; Erban, A.; Höhne, M.; Moraes, T.A.; Kopka, J.; Szymanski, J.; et al. Carbon flux through photosynthesis and central carbon metabolism show distinct patterns between algae, C3 and C4 plants. Nat. Plants 2022, 8, 78–91. [Google Scholar] [CrossRef]
- Shan, S.; Manyakhin, A.Y.; Wang, C.; Ge, B.; Han, J.; Zhang, X.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. Mixotrophy, a more promising culture mode: Multi-faceted elaboration of carbon and energy metabolism mechanisms to optimize microalgae culture. Bioresour. Technol. 2023, 386, 129512. [Google Scholar] [CrossRef]
- Li, A.; Yan, Y.; Qiu, J.; Yan, G.; Zhao, P.; Li, M.; Ji, Y.; Wang, G.; Meng, F.; Li, Y.; et al. Putative biosynthesis mechanism of the neurotoxin β-N-methylamino-L-alanine in marine diatoms based on a transcriptomics approach. J. Hazard. Mater. 2023, 441, 129953. [Google Scholar] [CrossRef]
- Ming, H.; Yan, G.; Zhang, X.; Pei, X.; Fu, L.; Zhou, D. Harsh temperature induces Microcystis aeruginosa growth enhancement and water deterioration during vernalization. Water Res. 2022, 223, 118956. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhou, Q.; Yuan, P.; Gao, Y.; Sun, J.; Zou, W.; Hu, X. Natural nanocolloids regulate the fate and phytotoxicity of hematite particles in water. Water Res. 2023, 232, 119678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, L.; Hu, X. Integrating proteomics, metabolomics and typical analysis to investigate the uptake and oxidative stress of graphene oxide and polycyclic aromatic hydrocarbons. Environ. Sci. Nano 2018, 5, 115–129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, C.; Yang, H.; Du, Y.; Li, X. Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms. Toxics 2025, 13, 376. https://doi.org/10.3390/toxics13050376
Meng C, Yang H, Du Y, Li X. Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms. Toxics. 2025; 13(5):376. https://doi.org/10.3390/toxics13050376
Chicago/Turabian StyleMeng, Chongchong, Huijie Yang, Yuan Du, and Xiaokang Li. 2025. "Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms" Toxics 13, no. 5: 376. https://doi.org/10.3390/toxics13050376
APA StyleMeng, C., Yang, H., Du, Y., & Li, X. (2025). Persistence and Recovery of Polystyrene and Polymethyl Methacrylate Microplastic Toxicity on Diatoms. Toxics, 13(5), 376. https://doi.org/10.3390/toxics13050376