Construction and Application of Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acetamiprid in Traditional Chinese Medicine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Preparation of ACE Hapten and Antigen
2.3. Preparation of ACE Monoclonal Antibodies
2.4. Optimization of Working Parameters
2.5. Development of ic-ELISA
2.6. Specificity Evaluation of ic-ELISA
2.7. Application Evaluation of ic-ELISA
3. Results and Discussion
3.1. Characterization of Antigens and Screening of Antibodies
3.2. Optimization of ic-ELISA Conditions
3.3. Establishment of ic-ELISA Standard Curve
3.4. Evaluation of Specificity
3.5. Application in Actual Samples
3.6. Comparison with Other Immunoassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ospina, M.; Wong, L.-Y.; Baker, S.E.; Serafim, A.B.; Morales-Agudelo, P.; Calafat, A.M. Exposure to Neonicotinoid Insecticides in the U.S. General Population: Data from the 2015–2016 National Health and Nutrition Examination Survey. Environ. Res. 2019, 176, 108555. [Google Scholar] [CrossRef]
- Wrobel, S.A.; Bury, D.; Belov, V.N.; Klenk, J.M.; Hauer, B.; Hayen, H.; Martino-Andrade, A.J.; Koch, H.M.; Brüning, T.; Käfferlein, H.U. Rapid Quantification of Seven Major Neonicotinoids and Neonicotinoid-like Compounds and Their Key Metabolites in Human Urine. Anal. Chim. Acta 2023, 1239, 340680. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, Y.; Wang, Y.; Lu, Q.; Ruan, H.; Luo, J.; Yang, M. A Comprehensive Review on the Pretreatment and Detection Methods of Neonicotinoid Insecticides in Food and Environmental Samples. Food Chem. X 2022, 15, 100375. [Google Scholar] [CrossRef]
- Mao, J.; Han, Y.; Wang, Z.; Wang, S.; Wei, Z.; Cao, H.; Yuan, H.; Ye, T.; Xu, F. Fabrication and Detection of Molecularly Imprinted Fluorescence Sensor Based on Acetamiprid. J. Food Sci. Biotechnol. 2022, 41, 40–47. [Google Scholar] [CrossRef]
- Phogat, A.; Singh, J.; Kumar, V.; Malik, V. Toxicity of the Acetamiprid Insecticide for Mammals: A Review. Environ. Chem. Lett. 2022, 20, 1453–1478. [Google Scholar] [CrossRef]
- Jactel, H.; Verheggen, F.; Thiéry, D.; Escobar-Gutiérrez, A.J.; Gachet, E.; Desneux, N. Alternatives to Neonicotinoids. Environ. Int. 2019, 129, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Modification of the Existing Maximum Residue Levels for Acetamiprid in Various Crops—2021-EFSA Journal—Wiley Online Library. Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2021.6830 (accessed on 9 December 2024).
- GB 2763-2021; Standard for Maximum Residue Limits of Pesticides in Food. National Health Commission of the People’s Republic of China: Beijing, China, 2021. Available online: https://2763.foodvip.net/pesticides/limit/112.html (accessed on 30 May 2025).
- Ji, C.; Xiao, L.; Wang, X.; Hua, M.Z.; Wu, Y.; Wang, Y.; Wu, Z.; He, X.; Xu, D.; Zheng, W.; et al. Simultaneous Determination of 147 Pesticide Residues in Traditional Chinese Medicines by GC–MS/MS. ACS Omega 2023, 8, 28663–28673. [Google Scholar] [CrossRef]
- Piemontese, L. Plant Food Supplements with Antioxidant Properties for the Treatment of Chronic and Neurodegenerative Diseases: Benefits or Risks? J. Diet. Suppl. 2017, 14, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Wang, K.-H.; Lin, R.-D.; Hsu, F.-L.; Huang, Y.-H.; Chang, H.-C.; Huang, C.-Y.; Lee, M.-H. Cosmetic Applications of Selected Traditional Chinese Herbal Medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, Y.; Zhang, L.; Li, C.; Wang, Z.; Liu, Y.; Geng, Z.; Shen, M.; Sun, L.; Wei, F.; et al. Levels and Health Risk of Pesticide Residues in Chinese Herbal Medicines. Front. Pharmacol. 2022, 12, 818268. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, J.; Lu, Q.; Tian, J.; Ke, T.; Guo, M.; Luo, J.; Yang, M. Residue Detection and Correlation Analysis of Multiple Neonicotinoid Insecticides and Their Metabolites in Edible Herbs. Food Chem. X 2023, 17, 100603. [Google Scholar] [CrossRef]
- Gao, M.; He, K.; Zhang, J.; Liu, X.; Ma, L. Simultaneous Determination of 15 Neonicotinoid Pesticide and Metabolite Residues in Lycium by QuEChERS-UPLC-MS/MS Method. Chin. J. Anal. Lab. 2025, 44, 36–41. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. General Requirements for the Verification of Medicinal Materials and the Prepared Slices of Chinese Crude Drugs 0212. In Pharmacopoeia of People’s Republic of China, 2025th ed.; People’s Medical Publishing House: Beijing, China, 2025; ISBN 978-7-5214-5207-5. [Google Scholar]
- Zhang, Y.; Zhang, J.; Wang, Y.; Luo, Z.; Li, X.; Wang, Y.; Luo, J.; Yang, M. Unveiling the Contamination Patterns of Neonicotinoid Insecticides: Detection, Distribution, and Risk Assessment in Panax Notoginseng across Plant Parts. J. Agric. Food Chem. 2024, 72, 17834–17846. [Google Scholar] [CrossRef]
- Vichapong, J.; Burakham, R.; Srijaranai, S. Alternative Liquid–Liquid Microextraction as Cleanup for Determination of Neonicotinoid Pesticides Prior HPLC Analysis. Chromatographia 2016, 79, 285–291. [Google Scholar] [CrossRef]
- Balayiannis, G.P.; Karasali, H. Determination of Azoxystrobin, Topramezone, Acetamiprid, Fluometuron and Folpet in Their Commercially Available Pesticide Formulations by Liquid Chromatography. J. Environ. Sci. Health Part B 2021, 56, 503–511. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, H.; Lan, L.; Miao, S.; Gu, Y.; Cao, J.; Yuan, M.; Mao, X.; Hu, Q.; Ji, S. An Enhanced Approach for Targeted Multi-Residue Screening of Pesticides in Complex Herbal Medicines by Ultra High-Performance Liquid Chromatography Tandem Ion Mobility/Quadrupole Time-of-Flight Mass Spectrometry. Arab. J. Chem. 2023, 16, 105007. [Google Scholar] [CrossRef]
- Uchigashima, M.; Watanabe, E.; Ito, S.; Iwasa, S.; Miyake, S. Development of Immunoassay Based on Monoclonal Antibody Reacted with the Neonicotinoid Insecticides Clothianidin and Dinotefuran. Sensors 2012, 12, 15858–15872. [Google Scholar] [CrossRef]
- Przybylska, A.; Chrustek, A.; Olszewska-Słonina, D.; Koba, M.; Kruszewski, S. Determination of Patulin in Products Containing Dried Fruits by Enzyme-Linked Immunosorbent Assay Technique Patulin in Dried Fruits. Food Sci. Nutr. 2021, 9, 4211–4220. [Google Scholar] [CrossRef] [PubMed]
- Hojo, E.; Matsuura, N.; Kamiya, K.; Yonekita, T.; Morishita, N.; Murakami, H.; Kawamura, O. Development of a Rapid and Versatile Method of Enzyme-Linked Immunoassay Combined with Immunoaffinity Column for Aflatoxin Analysis. J. Food Prot. 2019, 82, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lang, Y.; Guo, B.; Cao, Z.; Cheng, J.; Cai, D.; Shentu, X.; Yu, X. Indirect Competitive Enzyme-Linked Immunosorbent Assay Based on Broad-Spectrum Antibody for Simultaneous Determination of Thirteen Fluoroquinolone Antibiotics in Rana Catesbeianus. Foods 2023, 12, 2530. [Google Scholar] [CrossRef]
- Fang, Q.; Zu, Q.; Hua, X.; Lv, P.; Lin, W.; Zhou, D.; Xu, Z.; Fan, J.; Li, X.; Cao, H. Quantitative Determination of Acetamiprid in Pollen Based on a Sensitive Enzyme-Linked Immunosorbent Assay. Molecules 2019, 24, 1265. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Yang, Q.; Li, F.; Sun, X. The Effects of Different Antigen–Antibody Pairs on the Results of 20 Min ELISA and 8 Min Chromatographic Paper Test for Quantitative Detection of Acetamiprid in Vegetables. Biosensors 2022, 12, 730. [Google Scholar] [CrossRef]
- Wanatabe, S.; Ito, S.; Kamata, Y.; Omoda, N.; Yamazaki, T.; Munakata, H.; Kaneko, T.; Yuasa, Y. Development of Competitive Enzyme-Linked Immunosorbent Assays (ELISAs) Based on Monoclonal Antibodies for Chloronicotinoid Insecticides Imidacloprid and Acetamiprid. Anal. Chim. Acta 2001, 427, 211–219. [Google Scholar] [CrossRef]
- Sheng, W.; Zhang, B.; Zhao, Q.; Wang, S.; Zhang, Y. Preparation of a Broad-Spectrum Heterocyclic Aromatic Amines (HAAs) Antibody and Its Application in Detection of Eight HAAs in Heat Processed Meat. J. Agric. Food Chem. 2020, 68, 15501–15508. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Zhang, L.; Qin, J.; Dou, X.; Fu, Y.; Li, Q.; Zhao, X.; Yang, M. An Integrated Strategy for Rapid On-Site Screening and Determination of Prometryn Residues in Herbs. Anal. Bioanal. Chem. 2020, 412, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, J.; Zhang, Y.; Zhang, H.; Miao, K.; Luo, J.; Yang, M. Establishment of a Rapid and Sensitive Ic-ELISA for the Detection of Thiacloprid Residues in Honey and Medicinal Herbs Using a Novel Highly Specific Monoclonal Antibody. Ecotoxicol. Environ. Saf. 2024, 284, 116911. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Z.; Kong, L.; Huang, B.; Xu, Y.; Hou, R. AuNPs-Based Lateral Flow Immunoassay for Point-of-Needs Analysis of Four Neonicotinoids in Tea Samples: Effects of Grinding Degrees, Solvent Types and Contents on Extraction Efficiency. Food Chem. 2022, 397, 133790. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Yamasaki, T.; Hirakawa, Y.; Harada, A.; Iwasa, S.; Miyake, S. Organic Solvent-Free Immunoassay for Quantitative Detection of Neonicotinoid Acetamiprid Residues in Agricultural Products. Anal. Methods 2018, 10, 3162–3169. [Google Scholar] [CrossRef]
- Watanabe, E.; Miyake, S.; Baba, K.; Eun, H.; Endo, S. Immunoassay for Acetamiprid Detection: Application to Residue Analysis and Comparison with Liquid Chromatography. Anal. Bioanal. Chem. 2006, 386, 1441–1448. [Google Scholar] [CrossRef]
- Liu, B.; Zhai, R.; Abd El-Aty, A.M.; Zhang, J.; Liu, G.; Huang, X.; Lv, J.; Chen, J.; Liu, J.; Jin, M.; et al. Bimetallic Nanozyme-Assisted Immunoassay for the Detection of Acetamiprid in Vegetables. ACS Appl. Nano Mater. 2024, 7, 21833–21841. [Google Scholar] [CrossRef]
- Zhu, Z.; Shi, Q.; Wu, J.; He, K.; Feng, J.; Dong, S. Determination of Acetamiprid Residues in Vegetables by Indirect Competitive Chemiluminescence Enzyme Immunoassay. Foods 2022, 11, 2507. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, W.; Qin, Y.; Cui, P.; Song, G.; Hua, X.; Wang, L.; Wang, M. Inner Filter Effect-Based Immunoassay for the Detection of Acetamiprid Using Upconversion Nanoparticles and Gold Nanoparticles. Food Agric. Immunol. 2021, 32, 740–753. [Google Scholar] [CrossRef]
- Xu, R.; Xiang, Y.; Shen, Z.; Li, G.; Sun, J.; Lin, P.; Chen, X.; Huang, J.; Dong, H.; He, Z.; et al. Portable Multichannel Detection Instrument Based on Time-Resolved Fluorescence Immunochromatographic Test Strip for on-Site Detecting Pesticide Residues in Vegetables. Anal. Chim. Acta 2023, 1280, 341842. [Google Scholar] [CrossRef] [PubMed]





| Name | Structural Formula | IC50 (ng/mL) | Cross-Reactivity (%) |
|---|---|---|---|
| ACE |  | 13.61 | 100.0 |
| Imidacloprid |  | >1000 | <1.5 |
| Alatzin |  | >1000 | <1.5 |
| Dinotefuran |  | >1000 | <1.5 |
| Nitenpyram |  | >1000 | <1.5 |
| Carbendazim | 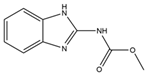 | >1000 | <1.5 |
| Chlopyrifos |  | >1000 | <1.5 |
| Omethoate |  | >1000 | <1.5 |
| Trichlorfon |  | >1000 | <1.5 |
| Thiacloprid |  | >1000 | <1.5 |
| TCM Sample | Spiked Level (μg/kg) | ic-ELISA | HPLC | ||||
|---|---|---|---|---|---|---|---|
| Mean (μg/kg) | Recovery (%) | RSD a (%) | Mean (μg/kg) | Recovery (%) | RSD (%) | ||
| LJF | 5.0 | 4.69 | 93.87 | 7.46 | 4.45 | 88.93 | 2.02 |
| 50.0 | 50.56 | 101.13 | 8.25 | 51.05 | 102.10 | 1.80 | |
| 500.0 | 478.53 | 95.71 | 8.28 | 486.23 | 97.25 | 5.83 | |
| JSF | 5.0 | 4.90 | 98.00 | 3.33 | 5.05 | 100.93 | 3.95 |
| 50.0 | 49.27 | 98.53 | 5.89 | 49.93 | 99.87 | 2.02 | |
| 500.0 | 478.07 | 95.61 | 9.13 | 509.61 | 101.92 | 3.23 | |
| LF | 5.0 | 4.34 | 86.87 | 8.93 | 4.23 | 84.53 | 6.28 |
| 50.0 | 43.84 | 87.69 | 12.05 | 43.73 | 87.47 | 7.40 | |
| 500.0 | 444.03 | 88.81 | 9.64 | 430.43 | 86.09 | 6.42 | |
| CRP | 5.0 | 4.67 | 93.33 | 8.81 | 4.58 | 91.53 | 6.06 |
| 50.0 | 52.40 | 104.80 | 7.56 | 50.50 | 101.00 | 5.44 | |
| 500.0 | 507.50 | 101.5 | 7.01 | 481.80 | 96.36 | 5.81 | |
| BL | 5.0 | 4.81 | 96.20 | 6.71 | 4.49 | 89.80 | 1.55 |
| 50.0 | 49.80 | 99.60 | 7.56 | 50.27 | 100.53 | 5.60 | |
| 500.0 | 465.87 | 93.17 | 9.82 | 505.47 | 97.26 | 2.71 | |
| Method | Sensitivity | Detection Range | Cross-Reactivity | Matrix | Sample Preparation | Reference |
|---|---|---|---|---|---|---|
| AuNPs-LFIA | Cut-off value 10.0 ng/mL | — a | Thiacloprid(30%), Imidaclothiz(30%), Imidacloprid(3%) | Tea | Extracted with 100% methanol and diluted with PBST | [31] |
| dc-ELISA | IC50 0.16 ng/mL | 0.043–0.6 ng/mL | Thiacloprid(43.8%), IM-2-1(2.1%) | Chinese chive | Extracted with water and diluted with water | [32] |
| ELISA | I50 0.6 ng/g | 0.18–3 ng/g | Thiacloprid(40%), Imidacloprid(0.62%) | Peach, apple, strawberry, cucumber, eggplant, and tomato | Extracted with 100% methanol and diluted with water | [33] |
| Au@Pt-assisted ic-ELISA | IC50 25.58 μg/L | 1.85–327.19 μg/L | — a | Chinese cabbage, cucumber, and zucchini | Extracted with 100% methanol and diluted with PBS | [34] |
| ic-CLEIA | IC50 10.24 ng/mL | 0.70–96.31 ng/mL | Clothianidin(8.63%), Thiacloprid(4.79%), Nitenpyram(1.96%) | Chinese cabbage and cucumber | Extracted with 99.5% acetone and diluted with sub-boiling water | [35] |
| IFE-IA | SC50 0.04 μg/L | 0.002–0.58 μg/L | Thiacloprid(36.4%) | Soil, pear, wheat, and cucumber | Extracted with borate saline buffer (0.14 mol/L) containing 20% methanol and diluted with buffer | [36] |
| TRFIS | LOD 0.056–0.074 mg/kg | 0.25–1.75 mg/kg | — a | Celery cabbage, cauliflower, and baby cabbage | Extracted with 100% methanol and diluted with PBST | [37] |
| ic-ELISA b | IC50 13.61 ng/mL | 1.00–150.99 ng/mL | Thiacloprid(<1.5%), Imidacloprid(<1.5%) | LJF, LF, BL, CRP, and JSF | Extracted with 10% ethanol | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, B.; Xie, X.; Liu, Y.; Li, H.; Jin, H.; Lin, Y.; Wei, F.; Wang, Y. Construction and Application of Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acetamiprid in Traditional Chinese Medicine. Toxics 2025, 13, 982. https://doi.org/10.3390/toxics13110982
Zhou T, Zhang B, Xie X, Liu Y, Li H, Jin H, Lin Y, Wei F, Wang Y. Construction and Application of Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acetamiprid in Traditional Chinese Medicine. Toxics. 2025; 13(11):982. https://doi.org/10.3390/toxics13110982
Chicago/Turabian StyleZhou, Tingting, Biao Zhang, Xuan Xie, Yuanxi Liu, Hailiang Li, Hongyu Jin, Yongqiang Lin, Feng Wei, and Ying Wang. 2025. "Construction and Application of Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acetamiprid in Traditional Chinese Medicine" Toxics 13, no. 11: 982. https://doi.org/10.3390/toxics13110982
APA StyleZhou, T., Zhang, B., Xie, X., Liu, Y., Li, H., Jin, H., Lin, Y., Wei, F., & Wang, Y. (2025). Construction and Application of Indirect Competitive Enzyme-Linked Immunosorbent Assay for Acetamiprid in Traditional Chinese Medicine. Toxics, 13(11), 982. https://doi.org/10.3390/toxics13110982









