Impact of Different Proportions of Purple Rice and Chanterelles Powder on Physicochemical, Sensory, and Starch Digestibility Properties in Wheat Bread
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Purple Rice and Chanterelle Mushroom Powder
2.2. Preparation of Bread
2.3. Bread Quality
2.3.1. Determination of Bread Composition
2.3.2. Specific Volume
2.3.3. Colorimetric Analysis
2.3.4. Texture Analysis
2.4. Total Phenolic and Total Flavonoid Contents
2.5. Determination of Antioxidant Capacity
2.6. In Vitro Starch Digestion Analysis
2.7. Calculation of Rapidly Digestible, Slowly Digestible, and Resistant Starch
2.8. Determination of Volatile Organic Compounds in Bread
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Analysis
3.2. Bread Quality Analysis
3.2.1. Specific Volume
| White | 1CB | 2CB | 5PB | 10PB | 1C5PB | 2C5PB | 1C10PB | 2C10PB | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Nutritional composition | Moisture (g/100 g) | 36.74 ± 0.79 d | 35.29 ± 0.57 a,b | 34.82 ± 0.76 a | 36.54 ± 0.61 c,d | 36.23 ± 0.44 c,d | 36.22 ± 0.65 b,c | 35.93 ± 0.38 a,b | 35.54 ± 0.55 a,b | 35.22 ± 0.36 a,b |
| Ash (g/100 g) | 2.69 ± 0.053 a | 2.74 ± 0.036 a,b | 2.77 ± 0.048 b | 2.81 ± 0.02 b,c | 2.92 ± 0.031 d | 2.87 ± 0.04 c,d | 2.90 ± 0.036 d | 3.01 ± 0.036 e | 3.05 ± 0.051 e | |
| Protein (g/100 g) | 14.67 ± 0.15 b,c | 15.60 ± 0.31 d | 15.95 ± 0.17 d | 14.61 ± 0.13 b | 14.23 ± 0.39 a | 14.83 ± 0.13 b,c | 15.04 ± 0.12 c | 14.81 ± 0.12 b,c | 15.02 ± 0.07 c | |
| Total fat (g/100 g) | 10.28 ± 0.25 c | 10.63 ± 0.24 d | 10.7 ± 30.19 d | 9.72 ± 0.20 b | 9.19 ± 0.21 a | 9.84 ± 0.04 b | 9.91 ± 0.32 b | 9.75 ± 0.09 b | 9.82 ± 0.08 b | |
| TDF (g/100 g) | 3.29 ± 0.19 a | 4.42 ± 0.11 b | 4.79 ± 0.06 d,e | 4.36 ± 0.21 b | 4.54 ± 0.06 b,c | 4.73 ± 0.05 c,d | 4.90 ± 0.07 d,e | 4.84 ± 0.08 d,e | 4.98 ± 0.06 e | |
| SDF (g/100 g) | 0.43 ± 0.05 a | 0.46 ± 0.05 a,b | 0.47 ± 0.04 a,b | 0.48 ± 0.03 a,b | 0.48 ± 0.05 a,b | 0.50 ± 0.04 a,b | 0.51 ± 0.03 a,b | 0.52 ± 0.03 b | 0.53 ± 0.04 b | |
| IDF (g/100 g) | 2.94 ± 0.09 a | 3.89 ± 0.08 b | 4.23 ± 0.09 c,d | 3.82 ± 0.07 b | 3.92 ± 0.04 b | 4.13 ± 0.13 c | 4.33 ± 0.11 d,e | 4.27 ± 0.14 d | 4.43 ± 0.06 e | |
| Carbohydrates (%) | 32.33 ± 0.81 b | 31.32 ± 1.01 a,b | 30.95 ± 0.70 a | 31.97 ± 0.77 a,b | 33.89 ± 0.71 c | 31.55 ± 0.74 a,b | 31.32 ± 0.48 a,b | 32.05 ± 0.58 a,b | 31.96 ± 0.34 a,b | |
| Calories (kcal) | 287.09 ± 2.81 a | 292.21 ± 2.20 b | 293.70 ± 3.47 b | 282.48 ± 3.60 a | 284.29 ± 2.56 a | 283.41 ± 2.86 a | 284.42 ± 1.42 a | 284.9 ± 2.09 a | 286.17 ± 0.84 a | |
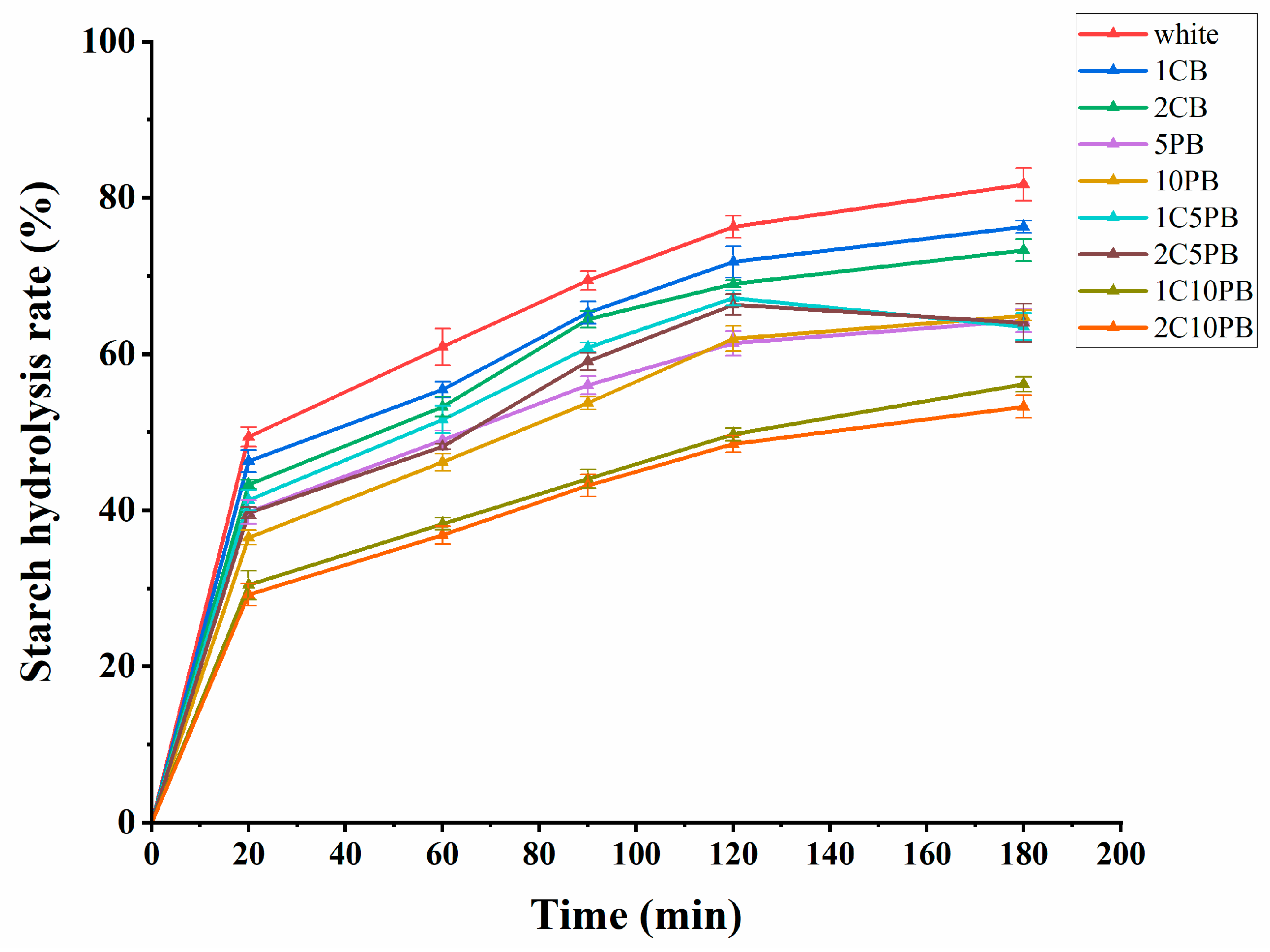
| Hydrolysis index | HI(%) | 95.50 ± 0.19 d | 90.15 ± 1.51 d | 89.03 ± 1.34 d | 81.19 ± 2.12 c | 74.48 ± 2.29 b | 89.66 ± 1.68 d | 88.66 ± 1.49 d | 56.82 ± 2.17 a | 57.33 ± 0.75 a |
| eGI (%) | 97.71 ± 0.64 d | 86.76 ± 2.51 d | 84.93 ± 1.15 d | 78.19 ± 1.82 c | 72.38 ± 1.97 b | 86.35 ± 1.44 d | 84.62 ± 1.28 d | 57.17 ± 1.87 a | 57.61 ± 0.64 a | |
| Starch content | RDS (%) | 48.69 ± 0.74 f | 46.27 ± 0.64 f | 43.74 ± 0.01 e | 39.03 ± 1.22 d | 35.23 ± 0.72 b | 37.17 ± 0.51 c | 35.93 ± 0.45 b,c | 32.86 ± 0.98 a | 32.03 ± 1.11 a |
| SDS (%) | 29.11 ± 1.05 d | 28.78 ± 0.89 c,d | 27.85 ± 0.96 b,c | 26.96 ± 0.99 b,c | 25.73 ± 0.78 a,b | 27.38 ± 2.04 b,c | 27.91 ± 1.07 b,c | 24.98 ± 1.17 a,b | 23.74 ± 1.97 a | |
| RS (%) | 22.49 ± 2.37 a | 24.05 ± 0.66 a,b | 26.05 ± 0.43 b | 31.15 ± 0.74 c | 34.71 ± 0.65 d | 33.05 ± 0.16 c,d | 34.29 ± 0.23 d | 40.73 ± 0.74 e | 41.58 ± 2.31 e |
3.2.2. Color
3.2.3. Textural Characteristics of Breads
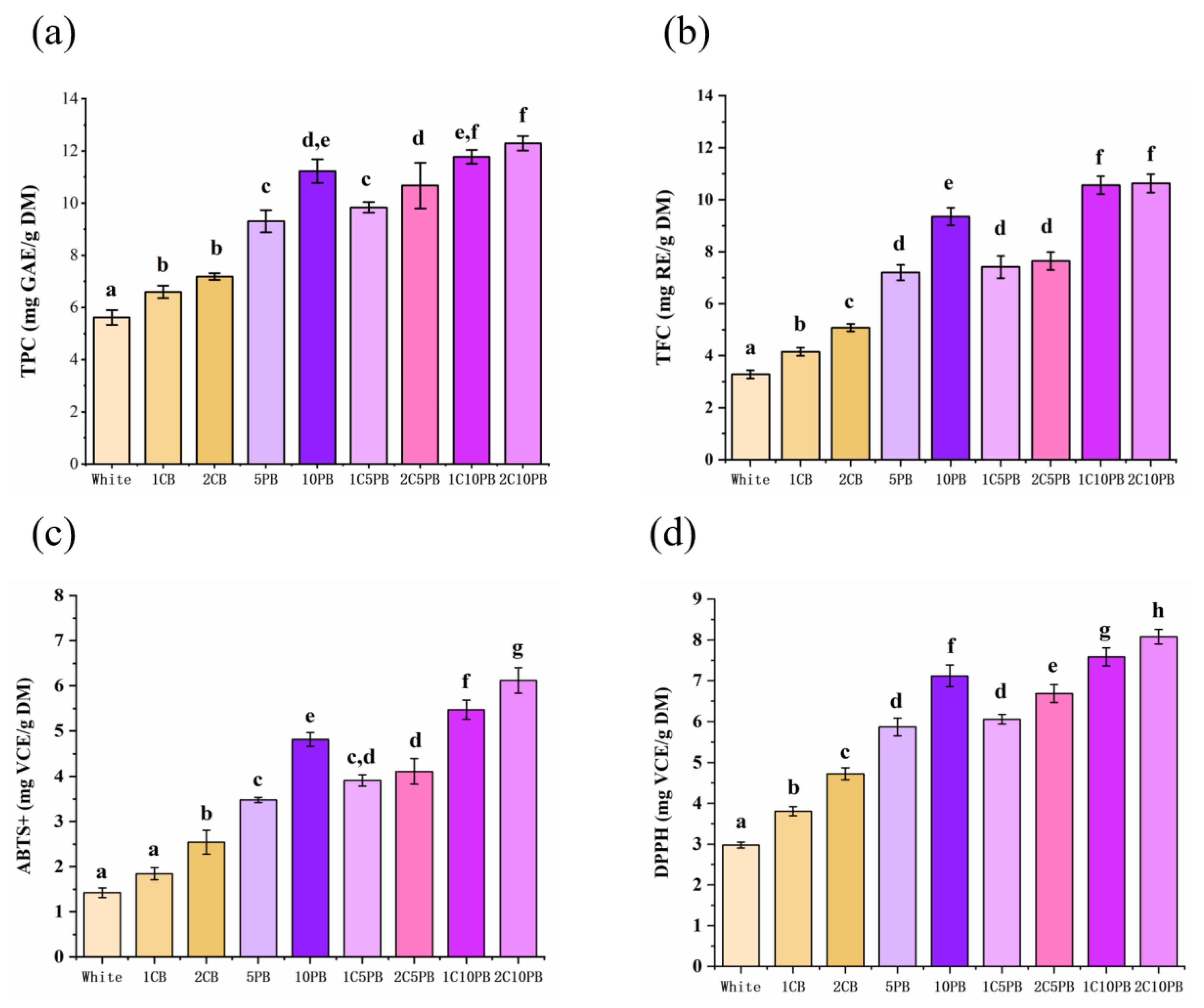
3.3. Total Phenolic Content and Total Flavonoid Content
3.4. Antioxidant Capacity Analysis
3.5. In Vitro Starch Digestibility Analysis
3.6. Volatile Compound Analysis in Bread
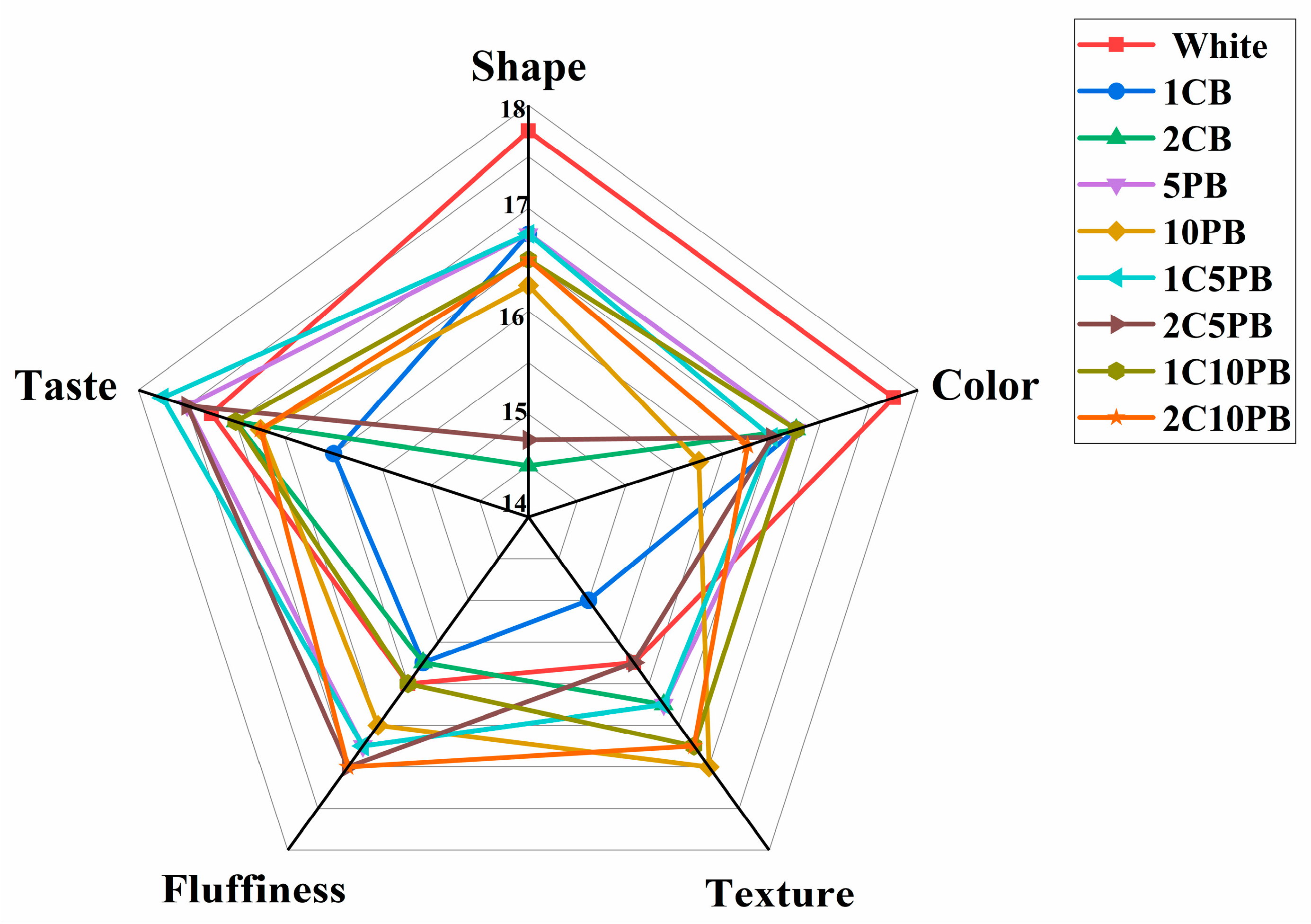
3.7. Sensory Attribute
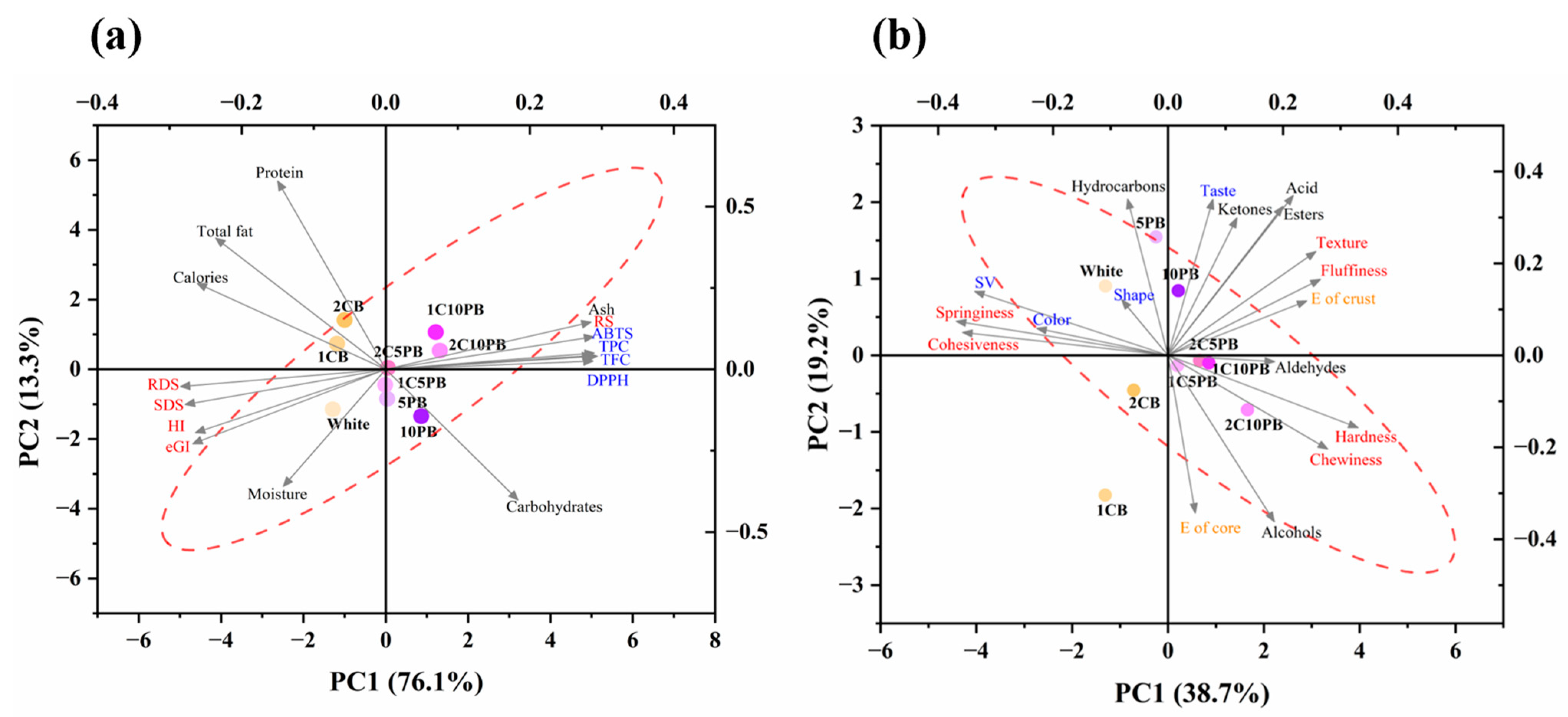
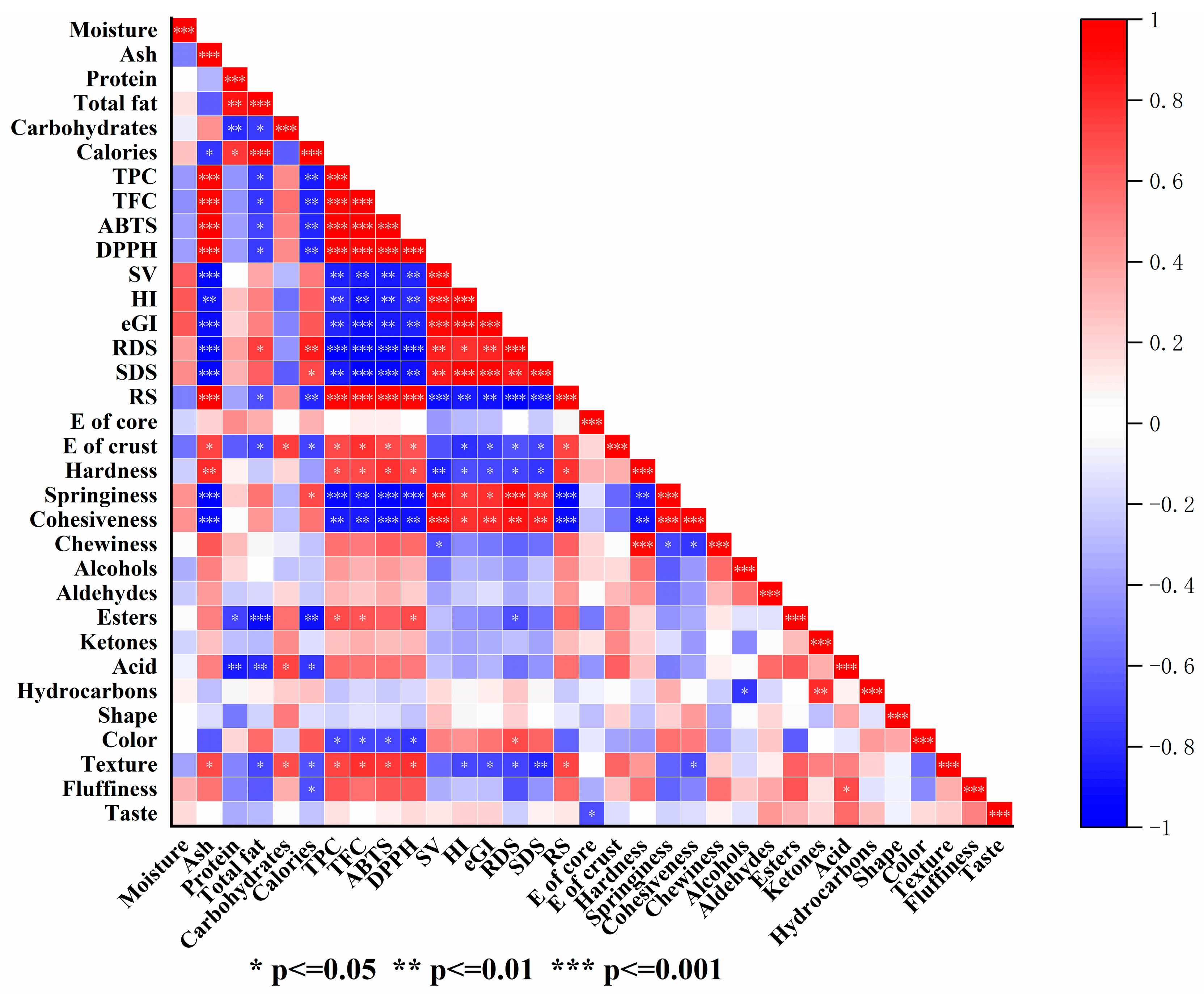
3.8. Principal Component Analysis (PCA) and Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowe, N.M. The global challenge of hidden hunger: Perspectives from the field. Proc. Nutr. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Padhani, Z.A. Alleviating hidden hunger: An infallible bridge to improved health and nutrition. Lancet Glob. Health 2022, 10, e1539–e1540. [Google Scholar] [CrossRef]
- Sharma, P.; Aggarwal, P.; Kaur, A. Biofortification: A new approach to eradicate hidden hunger. Food Rev. Int. 2017, 33, 1–21. [Google Scholar] [CrossRef]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019, 10, 8016–8025. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Chang, C.J.; Lin, C.S.; Lu, C.C.; Ko, Y.F.; Tseng, S.F.; Lai, H.C.; Young, J.D. Anti-obesogenic and antidiabetic effects of plants and mushrooms. Nat. Rev. Endocrinol. 2016, 13, 149–160. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Ni, C.; Jia, Q.; Ding, G.; Wu, X.; Yang, M. Low-glycemic index diets as an intervention in metabolic diseases: A systematic review and meta-analysis. Nutrients 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, S.R.; Moses, J.A.; Anandharamakrishnan, C. Prediction of in-vitro glycemic responses of biscuits in an engineered small intestine system. Food Res. Int. 2021, 147, 110459. [Google Scholar] [CrossRef] [PubMed]
- Yamuangmorn, S.; Prom-U-Thai, C. The potential of high-anthocyanin purple rice as a functional ingredient in human health. Antioxidants 2021, 10, 833. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef]
- Gu, W.; Peng, Y.; Wang, R.; Wang, R.; Wu, H.; Zhu, J.; Ni, X.; Xiong, Q. Comparison of metabolites and main nutritional components between uncooked and cooked purple rice. Metabolites 2023, 13, 1018. [Google Scholar] [CrossRef]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans. Food Funct. 2019, 10, 8230–8239. [Google Scholar] [CrossRef]
- Nowacka-Jechalke, N.; Nowak, R.; Juda, M.; Malm, A.; Lemieszek, M.; Rzeski, W.; Kaczyński, Z. New biological activity of the polysaccharide fraction from Cantharellus cibarius and its structural characterization. Food Chem. 2018, 268, 355–361. [Google Scholar] [CrossRef]
- Drewnowska, M.; Falandysz, J. Investigation on mineral composition and accumulation by popular edible mushroom common chanterelle (Cantharellus cibarius). Ecotoxicol. Environ. Saf. 2015, 113, 9–17. [Google Scholar] [CrossRef]
- Nyman, A.A.T.; Aachmann, F.L.; Rise, F.; Ballance, S.; Samuelsen, A.B.C. Structural characterization of a branched (1 → 6)-α-mannan and β-glucans isolated from the fruiting bodies of Cantharellus cibarius. Carbohydr. Polym. 2016, 146, 197–207. [Google Scholar] [CrossRef]
- Tian, R.; Zhang, Y.Z.; Cheng, X.; Xu, B.; Wu, H.; Liang, Z.Q.; Rahman, M.; Wang, Y.; Zeng, N.K. Structural characterization, and in vitro hypoglycemic activity of a polysaccharide from the mushroom Cantharellus yunnanensis. Int. J. Biol. Macromol. 2023, 253, 127200. [Google Scholar] [CrossRef]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Gromkowska-Kępka, K.; Naliwajko, S.K.; Moskwa, J.; Bielecka, J.; Grabia, M.; Borawska, M.; Socha, K. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother. 2021, 133, 111090. [Google Scholar] [CrossRef]
- Jiang, X.; Meng, W.; Li, L.; Meng, Z.; Wang, D. Adjuvant therapy with mushroom polysaccharides for diabetic complications. Front. Pharmacol. 2020, 11, 498943. [Google Scholar] [CrossRef]
- Cirlincione, F.; Venturella, G.; Gargano, M.L.; Ferraro, V.; Gaglio, R.; Francesca, N.; Rizzo, B.A.; Russo, G.; Moschetti, G.; Settanni, L.; et al. Functional bread supplemented with Pleurotus eryngii powder: A potential new food for human health. Int. J. Gastron. Food Sci. 2022, 27, 100449. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Dong, Y.N.; Karboune, S. A review of bread qualities and current strategies for bread bioprotection: Flavor, sensory, rheological, and textural attributes. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1937–1981. [Google Scholar] [CrossRef]
- Duguma, H.T.; Fehlberg, J.; Macke, P.; Matuana, L.M.; Cho, S.; Almenar, E. Impact of the replacement of polyethylene with waste from orange juicing on the physico-chemical, microbiological, and sensory quality of packaged bread. Food Packag. Shelf Life 2025, 47, 101417. [Google Scholar] [CrossRef]
- Jiménez, F.E.G.; Luna, H.E.R.; Avalos, S.R.; Antonio del Ángel Zumaya, J.; Vera, G.V.; Tapia, A.A.M.; Gerónimo, R.I.G.; Lovillo, A.P. Development of low-glycemic white bread using Oxalis tuberosa dietary fiber as a functional ingredient for nutraceutical benefits. Appl. Food Res. 2025, 5, 100649. [Google Scholar] [CrossRef]
- Turfani, V.; Narducci, V.; Durazzo, A.; Galli, V.; Carcea, M. Technological, nutritional and functional properties of wheat bread enriched with lentil or carob flours. LWT 2017, 78, 361–366. [Google Scholar] [CrossRef]
- Pejcz, E.; Lachowicz-Wiśniewska, S.; Nowicka, P.; Wojciechowicz-Budzisz, A.; Harasym, J. Enhancing antioxidant activity and modulating FODMAPs levels in inoculated sourdough rye bread. LWT 2024, 197, 115905. [Google Scholar] [CrossRef]
- Nudel, A.; Cohen, R.; Abbo, S.; Kerem, Z. Developing a nutrient-rich and functional wheat bread by incorporating Moringa oleifera leaf powder and gluten. LWT 2023, 187, 115343. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.; Jin, Z.; Jiao, A. Effects of highland barley protein and β-glucan addition on the flour structure, bread quality and starch digestibility of whole wheat bread. Int. J. Food Sci. Technol. 2023, 58, 6068–6080. [Google Scholar] [CrossRef]
- AOAC Official Method 925.09; Total Protein (Kjeldahl Method) (20th ed.). AOAC International: Gaithersburg, MD, USA, 2016.
- AOAC Official Method 978.04; Total Ash in Foods (Gravimetric Method) (20th ed.). AOAC International: Gaithersburg, MD, USA, 2016.
- AOAC Official Method 920.85; Moisture in Products (Oven Method) (20th ed.). AOAC International: Gaithersburg, MD, USA, 2016.
- AOAC Official Method 985.29; Total Dietary Fiber in Foods (Enzymatic-Gravimetric Method) (20th ed.). AOAC International: Gaithersburg, MD, USA, 2016.
- Nouska, C.; Irakli, M.; Palakas, P.; Lytou, A.E.; Bouloumpasi, E.; Biliaderis, C.G.; Lazaridou, A. Influence of sesame cake on physicochemical, antioxidant and sensorial characteristics of fortified wheat breads. Food Res. Int. 2024, 178, 113980. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Brennan, M.; Brennan, C.; Qin, Y.; Cheng, G.; Liu, Y. Physical, chemical, sensorial properties and in vitro digestibility of wheat bread enriched with yunnan commercial and wild edible mushrooms. LWT 2022, 169, 113923. [Google Scholar] [CrossRef]
- Waewkum, P.; Singthong, J. Functional properties and bioactive compounds of pigmented brown rice flour. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100289. [Google Scholar] [CrossRef]
- Chen, T.; He, S.; Zhang, J.; Wang, H.; Jia, Y.; Liu, Y.; Xie, M.; Cheng, G. Effects of ultra-high-pressure treatment on chemical composition and biological activities of free, esterified and bound phenolics from Phyllanthus emblica L. Fruits. Molecules 2024, 29, 3181. [Google Scholar] [CrossRef]
- Liu, X.; Lu, K.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Effect of purple yam flour substitution for wheat flour on in vitro starch digestibility of wheat bread. Food Chem. 2019, 284, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, S.; Durante, C.; Celli, S.; Mariani, M.; Marchetti, A.; Cocchi, M.; Strani, L. Optimization of an analytical method based on Spme-Arrow and chemometrics for the characterization of the aroma profile of commercial bread. Chemom. Intell. Lab. Syst. 2023, 241, 104940. [Google Scholar] [CrossRef]
- Adzqia, F.; Suwonsichon, S.; Thongngam, M. Effects of white sorghum flour levels on physicochemical and sensory characteristics of gluten-free bread. Foods 2023, 12, 4113. [Google Scholar] [CrossRef]
- ISO 13299; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile (2nd ed.). International Organization for Standardization: Geneva, Switzerland, 2016.
- Mazi, T.A.; Stanhope, K.L. Erythritol: An in-depth discussion of its potential to be a beneficial dietary component. Nutrients 2023, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Bordier, V.; Teysseire, F.; Schlotterbeck, G.; Senner, F.; Beglinger, C.; Meyer-Gerspach, A.C.; Wölnerhanssen, B.K. Effect of a chronic intake of the natural sweeteners xylitol and erythritol on glucose absorption in humans with obesity. Nutrients 2021, 13, 3950. [Google Scholar] [CrossRef]
- World Health Organization. Use of Non-Sugar Sweeteners: WHO Guideline; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Cappelli, A.; Oliva, N.; Cini, E. Stone milling versus roller milling: A systematic review of the effects on wheat flour quality, dough rheology, and bread characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- Koriyama, T.; Kurosu, Y.; Hosoya, T. Enhancing bread quality with steam-treated moringa (Moringa oleifera) powder. Foods 2025, 14, 927. [Google Scholar] [CrossRef]
- Ortiz de Erive, M.; He, F.; Wang, T.; Chen, G. Development of β-glucan enriched wheat bread using soluble oat fiber. J. Cereal Sci. 2020, 95, 103051. [Google Scholar] [CrossRef]
- Tao, X.; Su, Q.; Wu, H.; Zhan, L.; Huang, W.; Zheng, J.; Zhong, Q.; Chen, P. Structure and physicochemical properties of a new variety of purple rice (Oryza sativa L. indica) starch. Food Sci. Biotechnol. 2023, 32, 793–802. [Google Scholar] [CrossRef]
- Montes, L.; Santamaria, M.; Garzon, R.; Rosell, C.M.; Moreira, R. Effect of polyphenols from Ascophyllum nodosum seaweeds on the rheology and digestion of corn starch gels and gluten-free bread features. Heliyon 2024, 10, e27469. [Google Scholar] [CrossRef]
- Ma, S.; Bao, Y.; Xu, M.; Yu, X.; Jiang, H. Effect of 3D printing and traditional molding on phenolic compounds and antioxidant activity in steamed bread. Food Chem. 2024, 454, 139699. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, A.B. The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds. Food Biosci. 2022, 47, 101706. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Zhang, L.; Li, J.; Fang, Z.; Li, C.; Chen, H.; Al-Asmari, F.; Sameeh, M.Y.; Wu, W.; et al. Feasibility analysis on the application of the Maillard reaction in developing lentinula edodes umami seasoning: Based on the umami substances, sensory quality, physicochemical properties of the products. Food Chem. X 2024, 24, 101943. [Google Scholar] [CrossRef]
- Begum, Y.A.; Deka, S.C. Effect of processing on structural, thermal, and physicochemical properties of dietary fiber of culinary banana bracts. J. Food Process. Preserv. 2019, 43, 14256. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Zamudio-Sosa, V.E.; Contreras-Angulo, L.A.; Leyva-López, N.; Heredia, J.B. Bioaccessibility of phenolic compounds from mistletoe infusions and effect of in vitro digestion on its antioxidant and pancreatic lipase inhibitory activity. Foods 2022, 11, 3319. [Google Scholar] [CrossRef]
- Kraithong, S.; Wang, S.; Junejo, S.A.; Fu, X.; Theppawong, A.; Zhang, B.; Huang, Q. Type 1 resistant starch: Nutritional properties and industry applications. Food Hydrocoll. 2022, 125, 107369. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Ma, Q.; Tian, B.; Nie, C.; Chen, Z.; Li, J. Health beneficial effects of resistant starch on diabetes and obesity via regulation of gut microbiota: A review. Food Funct. 2020, 11, 5749–5767. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Guan, W.; Zhang, J.; Yuan, L.; Brennan, C.S. Enhancing the nutritional properties of bread by incorporating mushroom bioactive compounds: The manipulation of the pre-dictive glycaemic response and the phenolic properties. Foods 2021, 10, 731. [Google Scholar] [CrossRef]
- Roman, L.; Martinez, M.M. Structural basis of resistant starch (RS) in bread: Natural and commercial alternatives. Foods 2019, 8, 267. [Google Scholar] [CrossRef]
- Bartkiene, E.; Rimsa, A.; Zokaityte, E.; Starkute, V.; Mockus, E.; Cernauskas, D.; Rocha, J.M.; Klupsaite, D. Changes in the physicochemical properties of chia (Salvia hispanica L.) seeds during solid-state and submerged fermentation and their influence on wheat bread quality and sensory profile. Foods 2023, 12, 2093. [Google Scholar] [CrossRef] [PubMed]
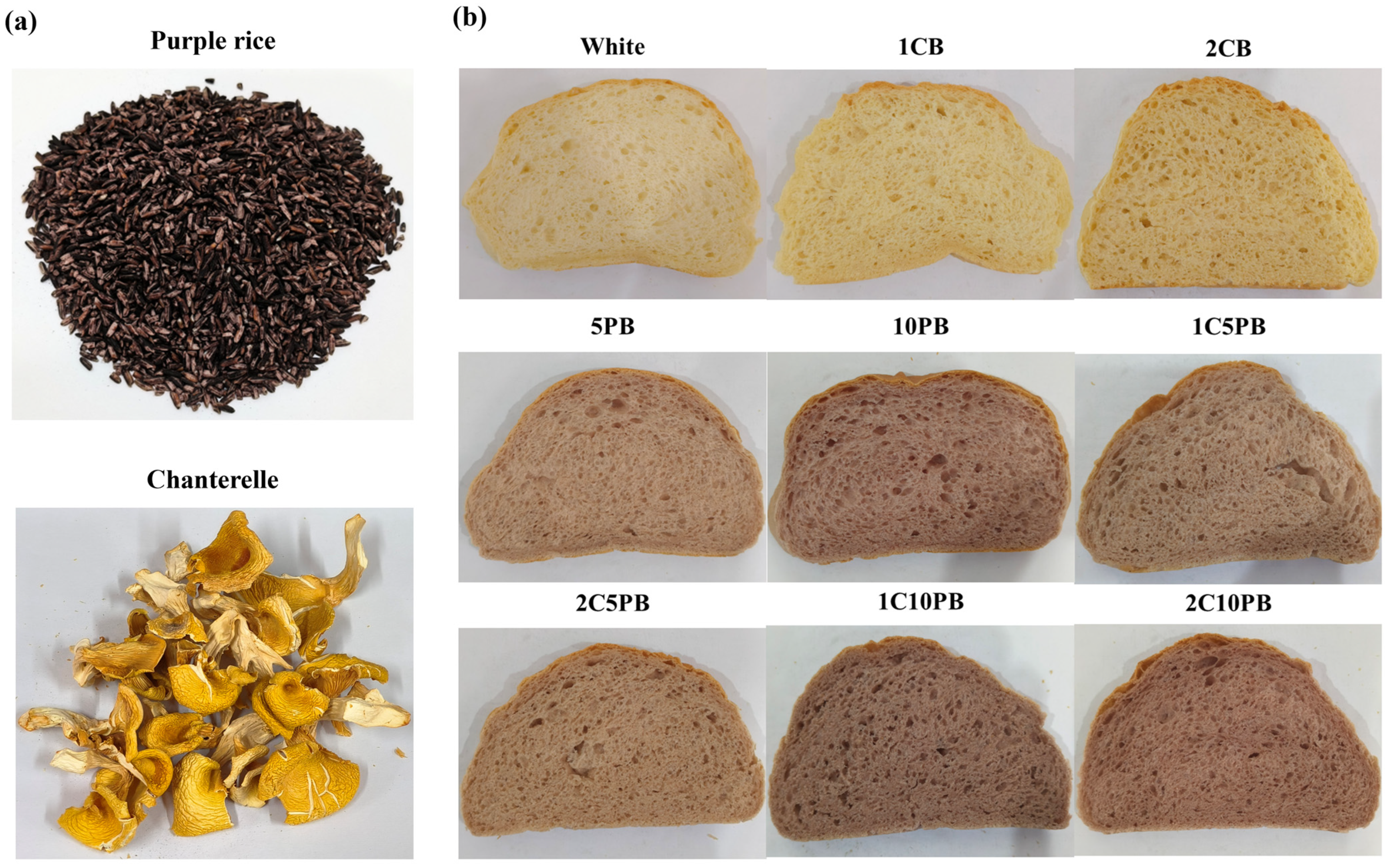
| Ingredients | CCP (g) | PRP (g) | Wheat Flour (g) | Salt (g) | Erythritol (g) | Milk (g) | Yeast (g) | Butter (g) |
|---|---|---|---|---|---|---|---|---|
| White | 0 | 0 | 250 | 2 | 15 | 175 | 5 | 15 |
| 1CB | 2.5 | 0 | 247.5 | 2 | 15 | 175 | 5 | 15 |
| 2CB | 5 | 0 | 245 | 2 | 15 | 175 | 5 | 15 |
| 5PB | 0 | 12.5 | 237.5 | 2 | 15 | 175 | 5 | 15 |
| 10PB | 0 | 25 | 225 | 2 | 15 | 175 | 5 | 15 |
| 1C5PB | 2.5 | 12.5 | 235 | 2 | 15 | 175 | 5 | 15 |
| 2C5PB | 5 | 12.5 | 232.5 | 2 | 15 | 175 | 5 | 15 |
| 1C10PB | 2.5 | 25 | 222.5 | 2 | 15 | 175 | 5 | 15 |
| 2C10PB | 5 | 25 | 220 | 2 | 15 | 175 | 5 | 15 |
| White | 1CB | 2CB | 5PB | 10PB | 1C5PB | 2C5PB | 1C10PB | 2C10PB | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Textural value | Hardness (N) | 3.20 ± 0.54 a | 4.49 ± 0.68 b | 6.33 ± 0.36 d | 5.84 ± 1.79 c,d | 4.80 ± 0.78 b,c | 6.98 ± 0.55 d,e | 8.16 ± 0.69 e | 8.07 ± 0.90 e | 13.79 ± 1.40 f |
| Springiness (N) | 0.91 ± 0.03 d | 0.89 ± 0.05 c,d | 0.87 ± 0.01 c,d | 0.86 ± 0.04 c | 0.82 ± 0.01 b | 0.80 ± 0.03 b | 0.79 ± 0.02 b | 0.75 ± 0.33 a | 0.73 ± 0.02 a | |
| Cohesiveness (N) | 0.66 ± 0.05 e | 0.66 ± 0.11 e | 0.55 ± 0.005 c,d | 0.59 ± 0.02 d | 0.56 ± 0.01 c,d | 0.56 ± 0.06 c,d | 0.52 ± 0.01 b,c | 0.48 ± 0.02 a,b | 0.44 ± 0.01 a | |
| Chewiness (N) | 1.88 ± 0.19 a | 2.65 ± 0.74 b,c | 3.02 ± 0.17 c,d | 2.95 ± 0.76 c,d | 2.19 ± 0.32 a,b | 3.19 ± 0.45 c,d | 3.35 ± 0.22 d | 2.89 ± 0.18 c,d | 4.29 ± 0.45 e | |
| Specific volume | SV (mL/g) | 3.59 ± 0.16 d | 3.29 ± 0.93 c,d | 3.11 ± 0.07 b,c | 3.24 ± 0.01 c,d | 3.02 ± 0.09 b,c | 3.20 ± 0.08 b,c | 2.89 ± 0.24 b | 2.38 ± 0.21 a | 2.33 ± 0.26 a |
| Color of bread core | L | 98.22 ± 0.87 f | 96.27 ± 2.12 f | 96.33 ± 1.60 f | 83.38 ± 2.03 e | 69.08 ± 1.74 b | 79.66 ± 1.55 d | 76.58 ± 1.57 c | 69.91 ± 1.44 b | 65.71 ± 1.26 a |
| a | −3.58 ± 0.67 a | −3.24 ± 0.31 a | −2.92 ± 0.35 a | 0.18 ± 0.28 b | 4.47 ± 0.39 d,e | 1.92 ± 0.29 c | 3.12 ± 0.49 c,d | 4.29 ± 0.37 d,e | 5.21 ± 0.26 e | |
| b | 26.90 ± 1.12 e | 29.77 ± 0.80 f | 31.02 ± 1.22 f | 17.25 ± 0.63 b,c | 13.81 ± 0.25 a | 18.63 ± 0.34 c | 21.93 ± 3.68 d | 15.17 ± 0.17 a,b | 15.39 ± 0.34 a,b | |
| E | 24.34 ± 1.07 c | 26.85 ± 1.42 d | 27.84 ± 0.92 d,e | 15.86 ± 0.91 a | 24.95 ± 1.45 c,d | 19.11 ± 1.21 b | 23.72 ± 3.85 c | 24.76 ± 1.24 c,d | 29.72 ± 2.07 e | |
| Color of bread crust | L | 86.39 ± 0.15 c | 87.32 ± 1.21 c | 68.07 ± 4.29 a | 74.57 ± 1.81 b | 64.46 ± 1.61 a | 75.54 ± 0.60 b | 74.53 ± 1.09 b | 64.44 ± 0.06 a | 64.54 ± 1.04 a |
| a | 3.98 ± 0.42 b | 1.25 ± 0.19 a | 8.69 ± 1.55 e | 5.74 ± 1.24 b,c | 8.15 ± 1.49 d,e | 4.56 ± 0.27 b,c | 3.58 ± 1.67 a,b | 8.67 ± 0.32 e | 6.51 ± 0.71 c,d | |
| b | 33.98 ± 1.49 d | 31.71 ± 1.30 c,d | 16.76 ± 4.04 a | 28.04 ± 1.74 b,c | 27.43 ± 1.63 b,c | 25.49 ± 0.91 b | 30.75 ± 1.46 c,d | 29.03 ± 0.29 b,c | 25.74 ± 2.62 b | |
| E | 30.98 ± 1.42 b,c | 28.31 ± 1.47 a,b | 25.26 ± 3.53 a | 30.19 ± 2.67 b | 36.87 ± 2.57 d | 27.36 ± 0.43 a,b | 32.08 ± 2.02 b,c | 38.05 ± 0.23 d | 35.42 ± 0.99 c,d |
| Chemical Types | Volatile Compounds | Odor | White | 1CB | 2CB | 5PB | 10PB | 1C5PB | 2C5PB | 1C10PB | 2C10PB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | Ethyl alcohol | Alcohol, spicy | 8.17 ± 0.32 | 12.76 ± 0.48 | 12.23 ± 1.45 | 13.06 ± 0.19 | 11.35 ± 0.41 | 3.34 ± 0.69 | 8.12 ± 1.15 | 13.26 ± 0.87 | 12.32 ± 0.74 |
| 3-Methyl-1-Butanol | Malty, alcohol | 7.29 ± 0.88 | 3.51 ± 0.88 | 10.73 ± 1.68 | 9.53 ± 0.31 | 10.13 ± 0.28 | 9.92 ± 0.42 | 10.41 ± 0.51 | 11.96 ± 0.12 | 13.26 ± 0.86 | |
| Phenylethyl alcohol | Rose, honey | 3.13 ± 0.56 | 5.47 ± 0.45 | 6.31 ± 0.33 | 3.23 ± 0.83 | 2.59 ± 0.37 | 4.70 ± 0.16 | 5.43 ± 0.42 | 3.92 ± 0.29 | 3.10 ± 0.45 | |
| Hexanol | Green, flower, sweet | 0.79 ± 0.16 | 0.49 ± 0.12 | 0.56 ± 0.10 | 0.69 ± 0.06 | 0.93 ± 0.05 | 0.51 ± 0.10 | 0.49 ± 0.04 | 1.06 ± 0.11 | 1.09 ± 0.14 | |
| 2,3-Butanediol | Neutral, soft | 0.72 ± 0.42 | nd | nd | 0.29 ± 0.09 | 0.54 ± 0.06 | 0.12 ± 0.04 | 0.58 ± 0.09 | 0.70 ± 0.06 | 0.86 ± 0.04 | |
| 1-Heptanol | Grassy, vegetable | nd | 9.91 ± 0.21 | nd | 0.64 ± 0.16 | 0.40 ± 0.18 | 0.41 ± 0.09 | 0.81 ± 0.09 | 0.35 ± 0.11 | 0.37 ± 0.13 | |
| 3,7-Dimethyl-1-Octanol | NF | 0.39 ± 0.06 | 0.20 ± 0.07 | 0.31 ± 0.09 | nd | nd | nd | nd | nd | nd | |
| 3-None-1-ol | Flower, fruity | 0.33 ± 0.06 | 0.53 ± 0.14 | 0.94 ± 0.27 | 0.28 ± 0.09 | 0.43 ± 0.05 | 0.51 ± 0.09 | 0.55 ± 0.08 | 0.64 ± 0.13 | 0.68 ± 0.15 | |
| 1-Octen-3-ol | Mushroom | nd | 0.16 ± 0.04 | 0.24 ± 0.04 | nd | nd | 0.11 ± 0.07 | 0.19 ± 0.04 | 0.16 ± 0.05 | 0.21 ± 0.06 | |
| Aldehydes | Nonanal | Rose, almondy | 1.64 ± 0.15 | 1.24 ± 0.12 | 1.66 ± 0.18 | 1.35 ± 0.12 | 1.55 ± 0.13 | 1.19 ± 0.19 | 1.04 ± 0.14 | 1.59 ± 0.06 | 1.47 ± 0.23 |
| Hexanal | Balsamic, grassy | 1.47 ± 0.14 | 1.63 ± 0.17 | 1.16 ± 0.11 | 0.40 ± 0.19 | 0.55 ± 0.14 | 0.85 ± 0.19 | 1.14 ± 0.24 | 0.67 ± 0.15 | 1.54 ± 0.27 | |
| Benzaldehyde | Almondy, caramel | 1.13 ± 0.11 | 1.37 ± 0.21 | 2.45 ± 0.28 | 0.65 ± 0.17 | 0.79 ± 0.14 | 0.92 ± 0.16 | 1.33 ± 0.13 | 1.46 ± 0.09 | 1.64 ± 0.13 | |
| Decanal | Sweet flower | 0.52 ± 0.14 | 0.69 ± 0.20 | 0.54 ± 0.14 | 0.41 ± 0.07 | 0.49 ± 0.07 | 0.36 ± 0.04 | 0.31 ± 0.04 | 0.41 ± 0.09 | 0.47 ± 0.08 | |
| 2-Nonenal | Grassy, citrusy | 0.51 ± 0.16 | 0.45 ± 0.15 | 0.53 ± 0.16 | 0.46 ± 0.09 | 0.59 ± 0.19 | 0.44 ± 0.12 | 0.54 ± 0.11 | 0.47 ± 0.07 | 0.50 ± 0.12 | |
| 2-Octenal | Nut, bake | 0.41 ± 0.16 | 0.39 ± 0.24 | 0.52 ± 0.16 | nd | nd | 0.25 ± 0.08 | 0.36 ± 0.04 | 0.22 ± 0.06 | 0.25 ± 0.04 | |
| Phenylacetaldehyde | Rose | 0.180.04 | 0.37 ± 0.07 | 0.81 ± 0.14 | 0.18 ± 0.04 | 0.21 ± 0.04 | 0.56 ± 0.09 | 0.62 ± 0.07 | 0.45 ± 0.10 | 0.61 ± 0.11 | |
| 2-Heptenaldehyde | Citrusy | 0.14 ± 0.05 | nd | nd | 0.17 ± 0.07 | 0.24 ± 0.05 | 0.26 ± 0.07 | 0.36 ± 0.06 | 0.46 ± 0.08 | 0.31 ± 0.07 | |
| Esters | Ethyl octanoate | Fruity | 2.42 ± 0.11 | 2.30 ± 0.23 | 3.02 ± 0.27 | 2.36 ± 0.23 | 3.03 ± 0.25 | 1.89 ± 0.19 | 2.20 ± 0.22 | 2.70 ± 0.21 | 1.97 ± 0.22 |
| Ethyl decanoate | Fruity, brandy | 0.35 ± 0.05 | 0.49 ± 0.05 | 0.67 ± 0.06 | 0.50 ± 0.07 | 0.79 ± 0.10 | 0.49 ± 0.07 | 0.49 ± 0.11 | 0.63 ± 0.04 | 0.53 ± 0.06 | |
| Ethyl lactate | fruity | 0.15 ± 0.02 | nd | nd | nd | nd | nd | nd | nd | nd | |
| Octyl formate | NF | nd | 0.22 ± 0.06 | nd | 0.24 ± 0.04 | 0.61 ± 0.03 | nd | nd | 0.71 ± 0.03 | nd | |
| Propyl nonyl lactone | Almondy, peach | nd | nd | 0.17 ± 0.04 | 0.20 ± 0.04 | 0.27 ± 0.04 | 0.20 ± 0.04 | 0.18 ± 0.04 | nd | 0.20 ± 0.05 | |
| Phenylethyl acetate | Rose, honey | 0.19 ± 0.04 | 0.37 ± 0.07 | 0.77 ± 0.08 | 0.18 ± 0.04 | 0.22 ± 0.05 | 0.57 ± 0.09 | 0.60 ± 0.11 | 0.46 ± 0.12 | 0.60 ± 0.09 | |
| Decalactone | Fruity, peach | nd | nd | nd | 0.22 ± 0.04 | 0.30 ± 0.08 | 0.21 ± 0.06 | 0.23 ± 0.05 | 0.25 ± 0.05 | 0.27 ± 0.06 | |
| Ketones | 2-Nonanone | fruity | 0.42 ± 0.07 | nd | 0.44 ± 0.12 | 0.43 ± 0.13 | 0.46 ± 0.07 | nd | 0.33 ± 0.07 | 0.56 ± 0.09 | 0.40 ± 0.08 |
| Acid | 2-Methylbutyric acid | Cheese | 0.35 ± 0.04 | 0.54 ± 0.11 | 0.19 ± 0.04 | 0.50 ± 0.09 | 0.30 ± 0.07 | 0.40 ± 0.10 | 0.16 ± 0.07 | 0.33 ± 0.06 | 0.39 ± 0.09 |
| 3-Methylbutyric acid | Citrusy | 0.29 ± 0.054 | nd | nd | 0.22 ± 0.08 | 0.82 ± 0.19 | 0.29 ± 0.11 | 0.17 ± 0.05 | 0.37 ± 0.12 | 0.33 ± 0.12 | |
| Hydrocarbons | Dodecane | Insipid | 3.39 ± 0.49 | 4.64 ± 0.69 | 6.13 ± 0.56 | 4.49 ± 0.48 | 5.56 ± 0.24 | 3.95 ± 0.40 | 3.73 ± 0.55 | 4.77 ± 0.48 | 4.42 ± 0.45 |
| Undecane | Insipid | 2.59 ± 0.21 | 2.95 ± 0.26 | 3.53 ± 0.28 | 3.00 ± 0.18 | 3.52 ± 0.17 | 2.97 ± 0.21 | 2.78 ± 0.48 | 3.71 ± 0.45 | 2.62 ± 0.32 | |
| Decane | Insipid | 2.33 ± 0.44 | 1.70 ± 0.72 | 1.97 ± 0.63 | 1.74 ± 0.57 | 1.95 ± 0.51 | 1.67 ± 0.48 | 1.38 ± 0.51 | 1.90 ± 0.32 | nd | |
| 2,6-Dimethyloctane | Insipid | 0.55 ± 0.11 | nd | 0.18 ± 0.04 | 0.20 ± 0.07 | 0.43 ± 0.06 | nd | nd | 0.27 ± 0.07 | 0.34 ± 0.06 | |
| Tetradecane | Insipid | 0.29 ± 0.04 | 0.45 ± 0.06 | 0.62 ± 0.04 | 0.44 ± 0.12 | 0.67 ± 0.09 | 0.33 ± 0.13 | 0.52 ± 0.17 | 0.51 ± 0.16 | 0.54 ± 0.12 | |
| 2,6,6-Trimethyl Octane | Insipid | 0.25 ± 0.04 | nd | 0.34 ± 0.09 | 0.29 ± 0.04 | nd | nd | 0.30 ± 0.08 | nd | 0.36 ± 0.14 | |
| 4-Methyldodecane | Insipid | 0.29 ± 0.12 | 0.26 ± 0.09 | 0.33 ± 0.11 | 0.17 ± 0.05 | 0.32 ± 0.04 | 0.25 ± 0.08 | 0.32 ± 0.13 | 0.39 ± 0.10 | 0.45 ± 0.07 | |
| 2-Methyltetradecane | Insipid | 0.22 ± 0.04 | 0.28 ± 0.08 | 0.47 ± 0.09 | 0.32 ± 0.14 | 0.42 ± 0.09 | 0.28 ± 0.06 | 0.33 ± 0.11 | 0.36 ± 0.08 | 0.35 ± 0.10 | |
| Limonene | Flower, fruity, orange | 0.51 ± 0.03 | 0.95 ± 0.11 | 1.01 ± 0.13 | 0.59 ± 0.06 | 1.33 ± 0.14 | 0.74 ± 0.13 | 0.84 ± 0.14 | 1.34 ± 0.11 | 0.65 ± 0.11 | |
| Others | 2-Amylfuran | Flower, nut | 0.55 ± 0.11 | 0.43 ± 0.13 | 0.56 ± 0.09 | nd | nd | 0.11 ± 0.03 | 0.35 ± 0.07 | 0.20 ± 0.06 | 0.50 ± 0.11 |
| Indole | Flower, nut | nd | 0.28 ± 0.05 | 0.41 ± 0.07 | nd | nd | nd | nd | nd | nd | |
| Anisole | Fennel | 0.11 ± 0.02 | 0.18 ± 0.04 | 0.26 ± 0.08 | 0.25 ± 0.07 | 0.30 ± 0.10 | 0.19 ± 0.05 | 0.27 ± 0.07 | 0.22 ± 0.08 | 0.29 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Jia, Y.; Li, Z.; Wang, Z.; Wei, T.; Bi, F.; Wang, Y.; Qin, Y.; Khan, A.; Liu, Y.; et al. Impact of Different Proportions of Purple Rice and Chanterelles Powder on Physicochemical, Sensory, and Starch Digestibility Properties in Wheat Bread. Foods 2025, 14, 1343. https://doi.org/10.3390/foods14081343
Hu Y, Jia Y, Li Z, Wang Z, Wei T, Bi F, Wang Y, Qin Y, Khan A, Liu Y, et al. Impact of Different Proportions of Purple Rice and Chanterelles Powder on Physicochemical, Sensory, and Starch Digestibility Properties in Wheat Bread. Foods. 2025; 14(8):1343. https://doi.org/10.3390/foods14081343
Chicago/Turabian StyleHu, Yingrui, Yiqing Jia, Zhilin Li, Zhishuang Wang, Ting Wei, Feifei Bi, Yurou Wang, Yuyue Qin, Afsar Khan, Yaping Liu, and et al. 2025. "Impact of Different Proportions of Purple Rice and Chanterelles Powder on Physicochemical, Sensory, and Starch Digestibility Properties in Wheat Bread" Foods 14, no. 8: 1343. https://doi.org/10.3390/foods14081343
APA StyleHu, Y., Jia, Y., Li, Z., Wang, Z., Wei, T., Bi, F., Wang, Y., Qin, Y., Khan, A., Liu, Y., & Cheng, G. (2025). Impact of Different Proportions of Purple Rice and Chanterelles Powder on Physicochemical, Sensory, and Starch Digestibility Properties in Wheat Bread. Foods, 14(8), 1343. https://doi.org/10.3390/foods14081343









