Probiotics and the Gut–Brain Axis: Emerging Therapeutic Strategies for Epilepsy and Depression Comorbidity
Abstract
1. Introduction
2. Neuroinflammatory Pathways Linking Epilepsy and Depression: Different Mechanisms
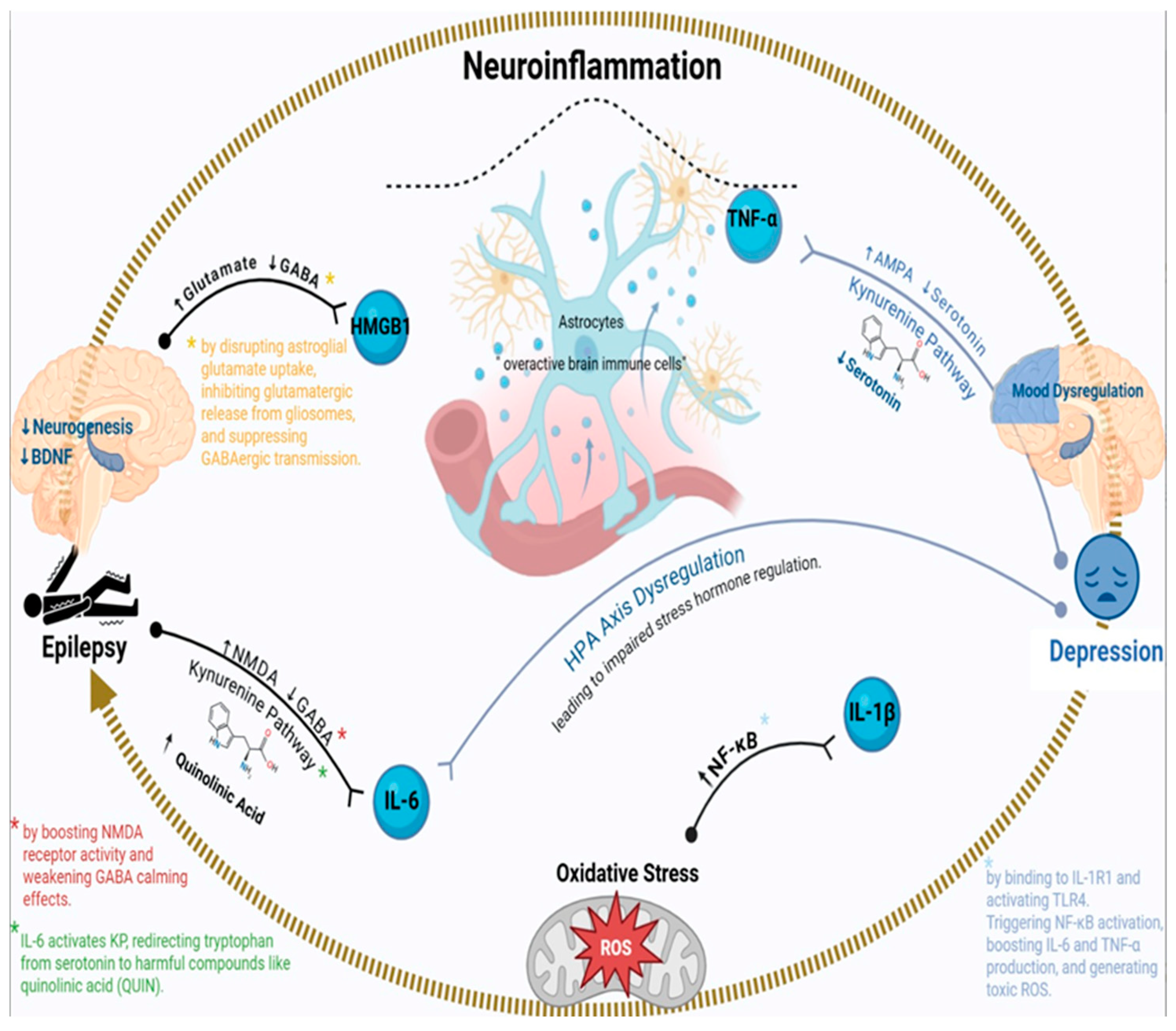
2.1. Introduction to Neuroinflammation in Epilepsy–Depression Comorbidity
2.2. Role of IL-6 in Epilepsy and Depression
2.3. TNF-α: A Dual Role in Neuronal Excitability and Mood Dysregulation
2.4. HMGB1-TLR4 Signaling: A Key Link Between Neuroinflammation and Hyperexcitability
2.5. Microglial and Astrocytic Dysfunction in Epilepsy–Depression Comorbidity
2.6. The Kynurenine Pathway: Bridging Inflammation to Serotonin Depletion
2.7. Oxidative Stress and Mitochondrial Dysfunction
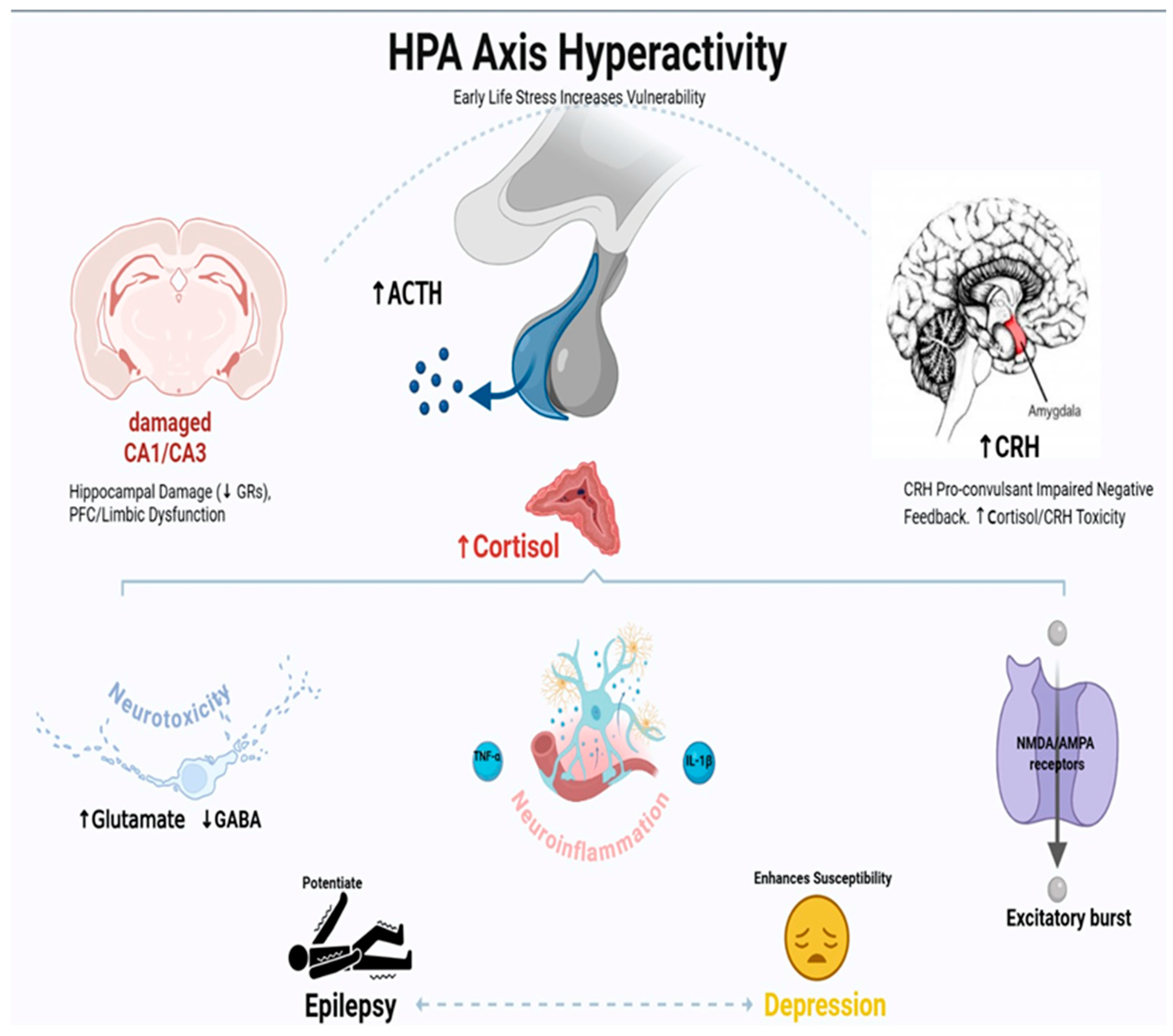
3. HPA Axis Dysregulation in Epilepsy–Depression Comorbidity: Mechanisms and Therapeutic Implications
3.1. Introduction to HPA Axis Dysfunction in Neurological Disorders
3.2. Neuroanatomical Basis of HPA Dysregulation in Epilepsy
3.3. Glucocorticoid-Mediated Neurotoxicity and Seizure Threshold
3.4. CRH and Its Role in Seizure Susceptibility and Mood Regulation
3.5. HPA–Immune System Interactions: The Neuroinflammatory Link
3.6. Developmental Aspects: Early Life Stress and Vulnerability
3.7. Clinical Evidence and Biomarker Correlations
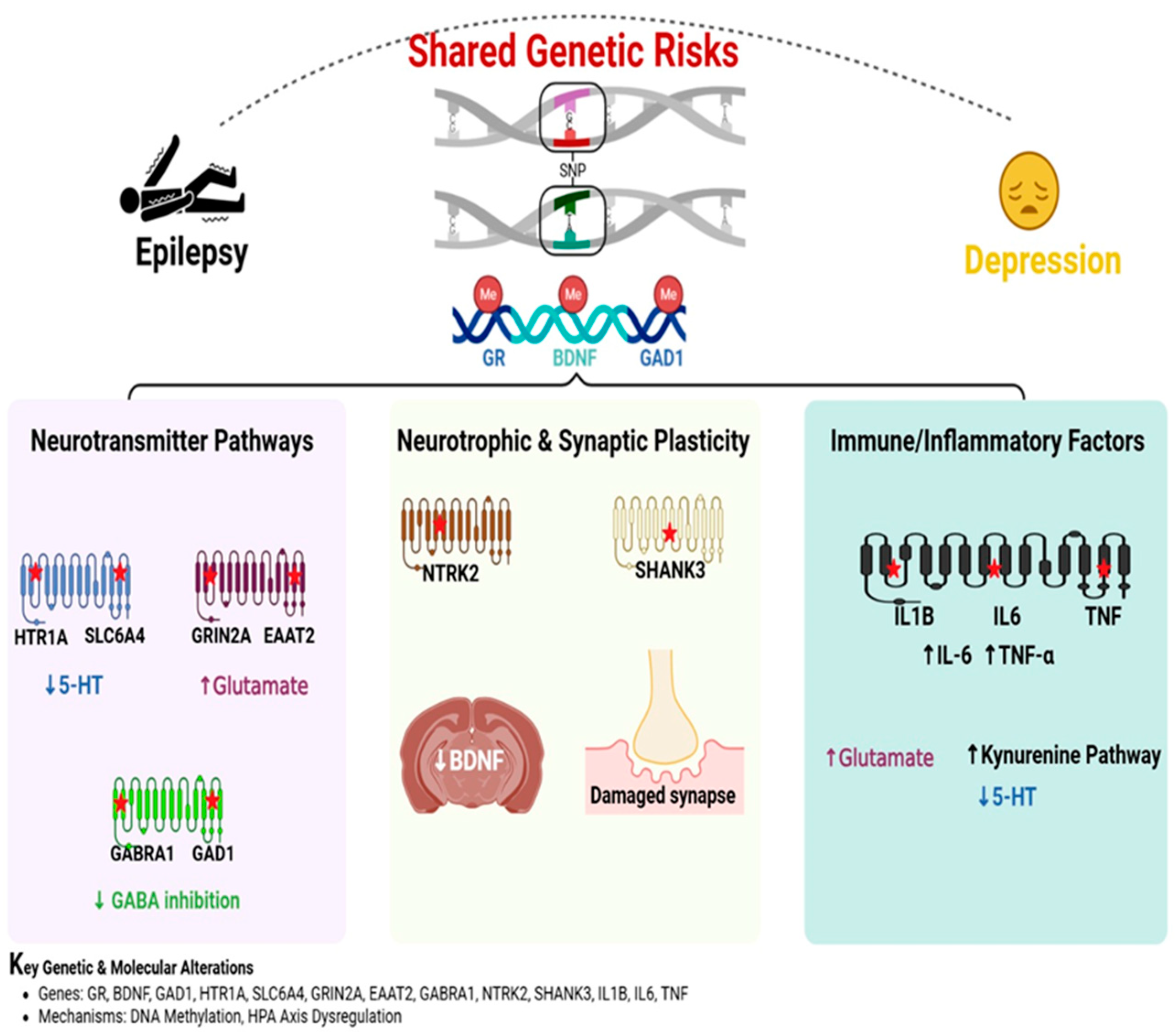
4. Genetic Predisposition as a Link Between Epilepsy and Depression: Shared Pathways and Mechanisms
4.1. Introduction to Genetic Overlap in Epilepsy and Depression
4.2. Neurotransmitter Pathways: Serotonin, Glutamate, and GABA
4.3. Neurotrophic and Synaptic Plasticity Pathways
4.4. Immune and Inflammatory Genetic Factors
4.5. Epigenetic Modifications and Gene-Environment Interactions
5. Genome-Wide Association Study (GWAS) Studies on Shared Genetic Risks in Epilepsy and Depression: Focus on BDNF and SLC6A4
5.1. Introduction to GWAS in Epilepsy–Depression Comorbidity
5.2. GWAS Methodology and Challenges in Studying Comorbidity
5.3. BDNF: A Neurotrophic Link Between Epilepsy and Depression
5.4. SLC6A4: Serotonin Transporter Dysfunction in Comorbidity
5.5. Other GWAS-Identified Shared Risk Loci
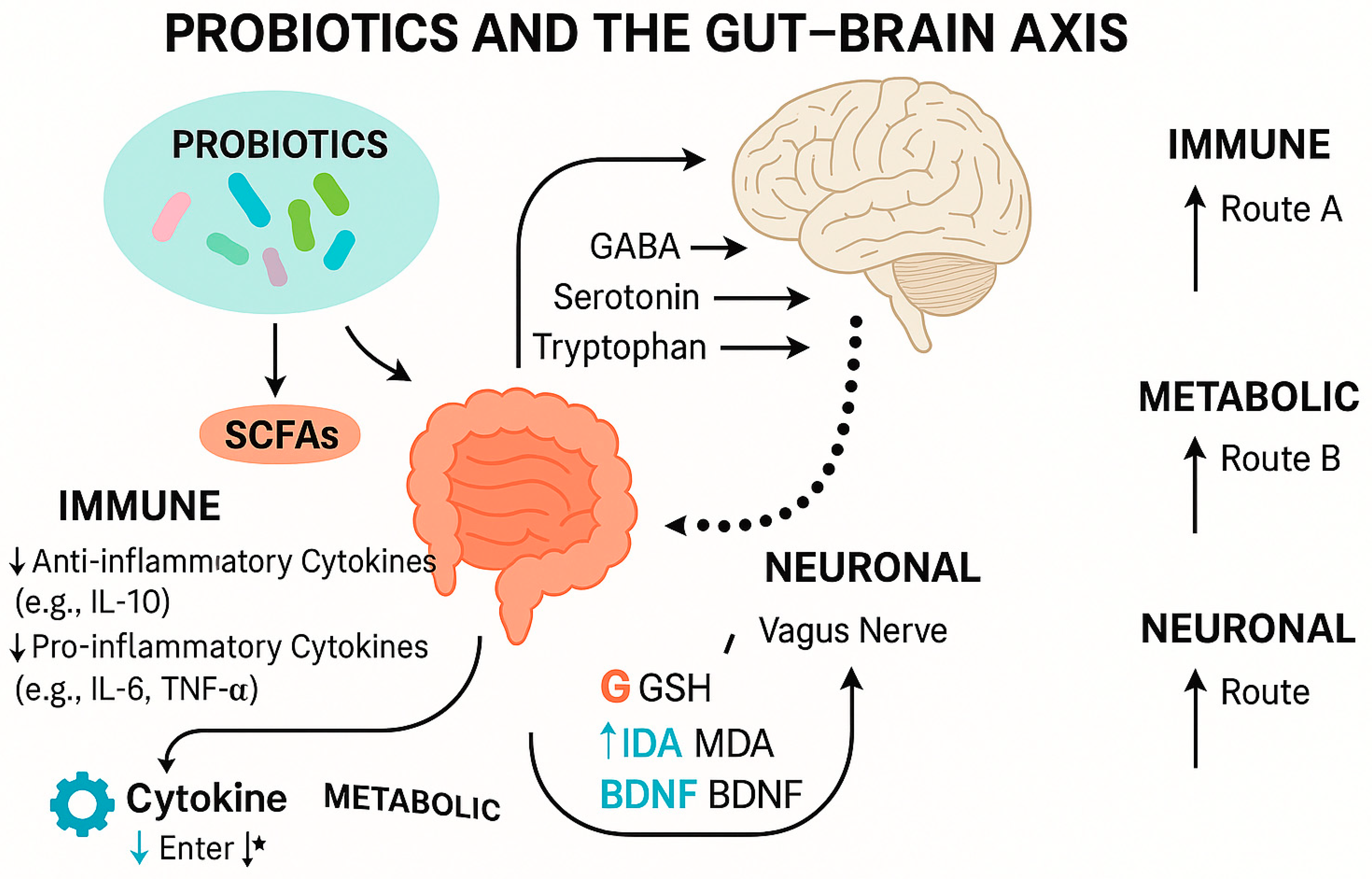
6. The Interplay Between Gut Microbiota and Gut–Brain Axis in Epilepsy and Depression
6.1. Possible Underlying Mechanisms of Gut Dysbiosis in Epilepsy and Depression
6.2. Therapeutic Basis and Mechanistic Evaluation of Probiotics in Gut–Brain Axis Modulation
6.3. Selection Criteria for Neuroactive Probiotic Strains
6.4. Mechanistic Insights and Biomarker Modulation
6.4.1. Inflammatory Pathways
6.4.2. Oxidative Stress Regulation
6.4.3. Neurotrophic Factor Modulation
6.4.4. Microbiota-Derived Metabolites: SCFAs and Tryptophan Metabolism
6.4.5. Immune Signaling
6.4.6. Neural Pathways: The Vagus Nerve and Other Neurotransmitters
6.5. Distinguishing Probiotics from Live Biotherapeutic Products (LBPs)
7. Different Therapeutic Mechanisms
7.1. Glutamatergic Modulation: Ketamine and Beyond
7.2. AMPA Receptor Potentiators (AMPAkines) for Epilepsy–Depression Comorbidity
7.3. Metabotropic Glutamate Receptor 5 (mGluR5) Antagonists for Epilepsy–Depression Comorbidity
7.4. Epigenetic Therapy
DNA Methyltransferase (DNMT) Inhibitors for Epilepsy–Depression Comorbidity: Insights from Preclinical Studies
7.5. Probiotic Interventions for Epilepsy–Depression Comorbidity: Insights from Preclinical Studies
7.6. Future Treatments
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3-HK | 3-hydroxykynurenine |
| 5-HT | 5-Hydroxytryptophan |
| 5-HTTLPR | Serotonin-transporter-linked promoter region |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AMPAkines | AMPA Receptor Potentiators |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| CRHR1 | Corticotropin-releasing hormone receptor 1 |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DNA | Deoxyribonucleic acid |
| DNMT | DNA methyltransferase |
| FST | Forced swim test |
| GABA | Gamma-aminobutyric acid |
| GAD | Glutamic acid decarboxylase |
| GRs | Glucocorticoid receptor |
| GWAS | Genome-wide association study |
| HMGB1 | High mobility group box 1 |
| HPA | Hypothalamic–pituitary–adrenal axis |
| IBS | Irritable bowel syndrome |
| IDO | Indoleamine 2,3-dioxygenase |
| IL-1R1 | Interleukin 1 receptor, type I |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin 6 |
| KD | Ketogenic diet |
| KMO | Kynurenine 3-monooxygenase |
| KP | Kynurenine pathway |
| KYNA | Kynurenic acid |
| mGluR5 | Metabotropic glutamate receptor 5 |
| MPEP | Methyl-phenyl-ethynyl pyridine |
| MRs | Mineralocorticoid receptors |
| MTEP | Methyl-thiazolyl-ethynyl-pyridine |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B |
| NMDA | N-methyl-D-aspartate |
| PTSD | Post-traumatic stress disorder |
| PVN | Paraventricular nucleus |
| RAGE | Advanced Glycation End Products |
| ROS | Reactive oxygen species |
| SNPs | Single-nucleotide polymorphisms |
| SSRIs | Selective serotonin reuptake inhibitors |
| TLE | Temporal lobe epilepsy |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TrkB | Tropomyosin receptor kinase B |
References
- Bølling-Ladegaard, E.; Dreier, J.W.; Kessing, L.V.; Budtz-Jørgensen, E.; Lolk, K.; Christensen, J. Directionality of the association between epilepsy and depression: A nationwide register-based cohort study. Neurology 2023, 100, e932–e942. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Stress-associated molecular and cellular hippocampal mechanisms common for epilepsy and comorbid depressive disorders. Biochemistry 2021, 86, 641–656. [Google Scholar] [CrossRef]
- Kanner, A.M. Major depression, anxiety disorder and suicidality in epilepsy: What should neurologists do? Epilepsy Behav. Rep. 2025, 30, 100758. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug resistance in epilepsy: Clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef] [PubMed]
- Peek, S.I.; Twele, F.; Meller, S.; Packer, R.M.A.; Volk, H.A. Epilepsy is more than a simple seizure disorder: Causal relationships between epilepsy and its comorbidities. Vet. J. 2024, 303, 106061. [Google Scholar] [CrossRef]
- Sălcudean, A.; Popovici, R.-A.; Pitic, D.E.; Sârbu, D.; Boroghina, A.; Jomaa, M.; Salehi, M.A.; Kher, A.A.M.; Lica, M.M.; Bodo, C.R. Unraveling the Complex Interplay Between Neuroinflammation and Depression: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 1645. [Google Scholar] [CrossRef] [PubMed]
- Elnady, R.E.; Abdon, M.S.; Shaheen, H.R.; Eladawy, R.M.; Azar, Y.O.; Al Raish, S.M. The Future of Alopecia Treatment: Plant Extracts, Nanocarriers, and 3D Bioprinting in Focus. Pharmaceutics 2025, 17, 584. [Google Scholar] [CrossRef]
- El-Ayouty, M.M.; Eltahawy, N.A.; Abd El-Sameaa, A.M.; Badawy, A.M.; Darwish, K.M.; Elhady, S.S.; Shokr, M.M.; Ahmed, S.A. In vivo determination of analgesic and anti-inflammatory activities of isolated compounds from Cleome amblyocarpa and molecular modelling for the top active investigated compounds. RSC Adv. 2024, 14, 24503–24515. [Google Scholar] [CrossRef]
- Pastorino, G.M.G.; Olivieri, M.; Viggiano, A.; Meccariello, R.; Roccella, M.; Parisi, L.; Cerulli Irelli, E.; Di Bonaventura, C.; Orsini, A.; Operto, F.F. Depressive symptoms in children and adolescents with epilepsy and primary headache: A cross-sectional observational study. Front. Neurol. 2024, 15, 1395003. [Google Scholar] [CrossRef]
- MuMunger Clary, H.M. Depression and epilepsy: The bidirectional relation Goes on and on…. Epilepsy Curr. 2023, 23, 222–224. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Almasri, R.S.; Bedir, A.S. Ancient Remedies, Modern Medicine: A Review of Antidiabetic, Cardioprotective, and Antimicrobial Activities of Date Palm (Phoenix dactylifera), Tomato (Solanum lycopersicum), Fenugreek (Trigonella foenum-graecum), and Ashwagandha (Withania somnifera). Biology 2025, 14, 695. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of inflammatory mechanisms in major depressive disorder: From etiology to potential pharmacological targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Mukhtar, I. Inflammatory and immune mechanisms underlying epileptogenesis and epilepsy: From pathogenesis to treatment target. Seizure 2020, 82, 65–79. [Google Scholar] [CrossRef]
- Yu, C.; Deng, X.; Xu, D. Microglia in epilepsy. Neurobiol. Dis. 2023, 185, 106249. [Google Scholar] [CrossRef]
- Kosanovic Rajacic, B.; Sagud, M.; Begic, D.; Nikolac Perkovic, M.; Kozmar, A.; Rogic, D.; Mihaljevic Peles, A.; Bozicevic, M.; Pivac, N. Increased Interleukin-6 Levels in Responders with Treatment-Resistant Depression After Bright Light Therapy. Biomolecules 2025, 15, 295. [Google Scholar] [CrossRef]
- Sieghart, W.; Chiou, L.-C.; Ernst, M.; Fabjan, J.; Savić, M.M.; Lee, M.T. α6-Containing GABAA receptors: Functional roles and therapeutic potentials. Pharmacol. Rev. 2022, 74, 238–270. [Google Scholar] [CrossRef]
- Marsland, A.L.; Gianaros, P.J.; Abramowitch, S.M.; Manuck, S.B.; Hariri, A.R. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol. Psychiatry 2008, 64, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Eladawy, R.M.; Ahmed, L.A.; Salem, M.B.; Hammam, O.A.; Mohamed, A.F.; Salem, H.A.; El-Sayed, R.M. Impact of different gastric acid suppressants on chronic unpredictable mild stress-induced cognitive impairment in rats: A possible involvement of gut dysbiosis. Toxicol. Appl. Pharmacol. 2024, 492, 117126. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Caldito, N. Role of tumor necrosis factor-alpha in the central nervous system: A focus on autoimmune disorders. Front. Immunol. 2023, 14, 1213448. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-T.; Qian, F.; Liu, L.; Peng, X.-C.; Huang, J.-R.; Ren, B.-X.; Tang, F.-R. Astroglial connexins in epileptogenesis. Seizure 2021, 84, 122–128. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. BDNF unveiled: Exploring its role in major depression disorder serotonergic imbalance and associated stress conditions. Pharmaceutics 2023, 15, 2081. [Google Scholar] [CrossRef]
- Almasri, R.S.; Bedir, A.S.; Al Raish, S.M. Comprehensive Ethnopharmacological Analysis of Medicinal Plants in the UAE: Lawsonia inermis, Nigella sativa, Ziziphus spina-christi, Allium cepa, Allium sativum, Cymbopogon schoenanthus, Matricaria aurea, Phoenix dactylifera, Portulaca oleracea, Reichardia tingitana, Salvadora persica, Solanum lycopersicum, Trigonella foenum-graecum, Withania somnifera, and Ziziphus lotus. Nutrients 2025, 17, 411. [Google Scholar] [PubMed]
- Krügel, U.; Fischer, J.; Radicke, S.; Sack, U.; Himmerich, H. Antidepressant effects of TNF-α blockade in an animal model of depression. J. Psychiatr. Res. 2013, 47, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ziring, D.; Desai, S.; Kim, S.; Wong, M.; Korin, Y.; Braun, J.; Reed, E.; Gjertson, D.; Singh, R.R. TNFα blockade in human diseases: An overview of efficacy and safety. Clin. Immunol. 2008, 126, 13–30. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, F.; Zhai, F.; Liang, S. Role of HMGB1/TLR4 and IL-1β/IL-1R1 signaling pathways in epilepsy. Front. Neurol. 2022, 13, 904225. [Google Scholar] [CrossRef] [PubMed]
- Aljarba, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Shokr, M.M.; Papadakis, M.; Alexiou, A.; Alruwaili, M.; Alrouji, M.; Alshammari, M.S.; Batiha, G.E.-S. The possible therapeutic role of advanced glycation end-product inhibitors in ischemic stroke. Brain Res. Bull. 2025, 222, 111236. [Google Scholar] [CrossRef]
- Shokr, M.M.; Eladawy, R.M. HMGB1: Different secretion pathways with pivotal role in epilepsy and major depressive disorder. Neuroscience 2025, 570, 55–67. [Google Scholar] [CrossRef]
- Shokr, M.; Abdelaziz, A.; Nasser, M.; El-adaway, R. The Possible Pathophysiological Alterations of Epilepsy and its Relation with Other Neurological Disorders. Sinai Int. Sci. J. 2025, 1, 46–58. [Google Scholar] [CrossRef]
- Shokr, M.M.; Badawi, G.A.; Elshazly, S.M.; Zaki, H.F.; Mohamed, A.F. Sigma 1 Receptor and Its Pivotal Role in Neurological Disorders. ACS Pharmacol. Transl. Sci. 2024, 8, 47–65. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Bonanno, G.; Raiteri, L.; Milanese, M.; Zappettini, S.; Melloni, E.; Pedrazzi, M.; Passalacqua, M.; Tacchetti, C.; Usai, C.; Sparatore, B. The high-mobility group box 1 cytokine induces transporter-mediated release of glutamate from glial subcellular particles (gliosomes) prepared from in situ-matured astrocytes. Int. Rev. Neurobiol. 2007, 82, 73–93. [Google Scholar]
- Balosso, S.; Liu, J.; Bianchi, M.E.; Vezzani, A. Disulfide-containing high mobility group box-1 promotes N-methyl-D-aspartate receptor function and excitotoxicity by activating Toll-like receptor 4-dependent signaling in hippocampal neurons. Antioxid. Redox Signal. 2014, 21, 1726–1740. [Google Scholar] [CrossRef]
- Azar, Y.O.; Badawi, G.A.; Zaki, H.F.; Ibrahim, S.M. Agmatine-mediated inhibition of NMDA receptor expression and amelioration of dyskinesia via activation of Nrf2 and suppression of HMGB1/RAGE/TLR4/MYD88/NF-κB signaling cascade in rotenone lesioned rats. Life Sci. 2022, 311, 121049. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Tang, J.; Peng, S.; Cai, X.; Rong, X.; Yang, L. Serum concentration of high-mobility group box 1, Toll-like receptor 4 as biomarker in epileptic patients. Epilepsy Res. 2023, 192, 107138. [Google Scholar] [CrossRef] [PubMed]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef]
- Dahalia, M.; Gupta, S.; Majid, H.; Vohora, D. Nidhi Pirfenidone regulates seizures through the HMGB1/TLR4 axis to improve cognitive functions and modulate oxidative stress and neurotransmitters in PTZ-induced kindling in mice. Front. Pharmacol. 2025, 15, 1528032. [Google Scholar] [CrossRef]
- Thergarajan, P.; O’Brien, T.J.; Jones, N.C.; Ali, I. Ligand-receptor interactions: A key to understanding microglia and astrocyte roles in epilepsy. Epilepsy Behav. 2025, 163, 110219. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’connor, J.C. Neuroinflammation and the kynurenine pathway in CNS disease: Molecular mechanisms and therapeutic implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Fang, S.; Wu, Z.; Guo, Y.; Zhu, W.; Wan, C.; Yuan, N.; Chen, J.; Hao, W.; Mo, X.; Guo, X. Roles of microglia in adult hippocampal neurogenesis in depression and their therapeutics. Front. Immunol. 2023, 14, 1193053. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Abd El-Maksoud, E.M.; Shokr, M.M.; Alexiou, A.; Papadakis, M.; Batiha, G.E. Paracetamol: The potential therapeutic pathways defining its clinical use. Inflammopharmacology 2025, 35, 2907–2918. [Google Scholar] [CrossRef]
- Sanz, P.; Rubio, T.; Garcia-Gimeno, M.A. Neuroinflammation and epilepsy: From pathophysiology to therapies based on repurposing drugs. Int. J. Mol. Sci. 2024, 25, 4161. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-H.; Su, T.; Wu, L.; Wu, J.-F.; Liu, D.; Zhu, L.-Q.; Yuan, M. Deregulation of the glymphatic system in Alzheimer’s disease: Genetic and non-genetic factors. Aging Dis. 2023, 16, 283. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.B. Kynurenine pathway of tryptophan metabolism: Regulatory and functional aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Q.; Li, K.; Khan, M.A.S.; Zhang, A.; Sinha, B.; Li, S.; Chang, S.L.; Brody, D.L.; Grinstaff, M.W. Tryptophan metabolism in Alzheimer’s disease with the involvement of microglia and astrocyte crosstalk and gut-brain axis. Aging Dis. 2024, 15, 2168. [Google Scholar] [CrossRef]
- Kolodziej, L.R.; Paleolog, E.M.; Williams, R.O. Kynurenine metabolism in health and disease. Amino Acids 2011, 41, 1173–1183. [Google Scholar] [CrossRef]
- Pathak, S.; Nadar, R.; Kim, S.; Liu, K.; Govindarajulu, M.; Cook, P.; Watts Alexander, C.S.; Dhanasekaran, M.; Moore, T. The influence of kynurenine metabolites on neurodegenerative pathologies. Int. J. Mol. Sci. 2024, 25, 853. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Schwarcz, R. Kynurenic acid as an antagonist of α7 nicotinic acetylcholine receptors in the brain: Facts and challenges. Biochem. Pharmacol. 2013, 85, 1027–1032. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Schwarcz, R.; Erhardt, S. Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities. Pharmacol. Rev. 2024, 76, 978–1008. [Google Scholar] [CrossRef] [PubMed]
- Madireddy, S.; Madireddy, S. Therapeutic strategies to ameliorate neuronal damage in epilepsy by regulating oxidative stress, mitochondrial dysfunction, and neuroinflammation. Brain Sci. 2023, 13, 784. [Google Scholar] [CrossRef]
- Vancassel, S.; Capuron, L.; Castanon, N. Brain kynurenine and BH4 pathways: Relevance to the pathophysiology and treatment of inflammation-driven depressive symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H. Potential Inflammatory Biomarker in Patients with Attention Deficit Hyperactivity Disorder. Int. J. Mol. Sci. 2022, 23, 13054. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Ciwun, M.; Lewkowicz, J.; Pawlak, D. Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders. Cells 2024, 13, 1259. [Google Scholar] [CrossRef]
- Skorobogatov, K.; De Picker, L.; Verkerk, R.; Coppens, V.; Leboyer, M.; Mueller, N.; Morrens, M. Brain versus blood: A systematic review on the concordance between peripheral and central kynurenine pathway measures in psychiatric disorders. Front. Immunol. 2021, 12, 716980. [Google Scholar] [CrossRef]
- Abulaban, A.A.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Albuhadily, A.K.; Shokr, M.M.; Alexiou, A.; Papadakis, M.; Batiha, G.E. The janus face of astrocytes in multiple sclerosis: Balancing protection and pathology. Brain Res. Bull. 2025, 226, 111356. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Zsurka, G.; Kunz, W.S. Mitochondrial dysfunction and seizures: The neuronal energy crisis. Lancet Neurol. 2015, 14, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Tallarico, M.; Leo, A.; Guarnieri, L.; Zito, M.C.; De Caro, C.; Nicoletti, F.; Russo, E.; Constanti, A.; De Sarro, G.; Citraro, R. N-acetylcysteine aggravates seizures while improving depressive-like and cognitive impairment comorbidities in the WAG/Rij rat model of absence epilepsy. Mol. Neurobiol. 2022, 59, 2702–2714. [Google Scholar] [CrossRef]
- Bian, X.; Yang, W.; Lin, J.; Jiang, B.; Shao, X. Hypothalamic-Pituitary-Adrenal Axis and Epilepsy. J. Clin. Neurol. 2024, 20, 131. [Google Scholar] [CrossRef]
- Hooper, A.; Paracha, R.; Maguire, J. Seizure-induced activation of the HPA axis increases seizure frequency and comorbid depression-like behaviors. Epilepsy Behav. 2018, 78, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Eladawy, R.M.; El-Sayed, R.M.; Ahmed, L.A.; Mohamed, A.F.; Salem, H.A. Chronic Unpredictable Mild Stress Induced Cognitive Impairment: AMPK/mTOR Autophagic Signaling. Sinai Int. Sci. J. 2025, 1, 73–83. [Google Scholar] [CrossRef]
- Wulsin, A.C.; Solomon, M.B.; Privitera, M.D.; Danzer, S.C.; Herman, J.P. Hypothalamic-pituitary-adrenocortical axis dysfunction in epilepsy. Physiol. Behav. 2016, 166, 22–31. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology 2016, 41, 3–23. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2022, 8, 383–395. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 21, 403–416. [Google Scholar] [CrossRef]
- Maguire, J.; Salpekar, J.A. Stress, seizures, and hypothalamic–pituitary–adrenal axis targets for the treatment of epilepsy. Epilepsy Behav. 2013, 26, 352–362. [Google Scholar] [CrossRef]
- O’Toole, K.K.; Hooper, A.; Wakefield, S.; Maguire, J. Seizure-induced disinhibition of the HPA axis increases seizure susceptibility. Epilepsy Res. 2014, 108, 29–43. [Google Scholar] [CrossRef]
- Druzhkova, T.A.; Yakovlev, A.A.; Rider, F.K.; Zinchuk, M.S.; Guekht, A.B.; Gulyaeva, N.V. Elevated serum cortisol levels in patients with focal epilepsy, depression, and comorbid epilepsy and depression. Int. J. Mol. Sci. 2022, 23, 10414. [Google Scholar] [CrossRef]
- Sears, S.M.S.; Hewett, S.J. Influence of glutamate and GABA transport on brain excitatory/inhibitory balance. Exp. Biol. Med. 2021, 246, 1069–1083. [Google Scholar] [CrossRef]
- Azar, Y.O.; Ibrahim, S.M.; Zaki, H.F.; Elshazly, S.M.; Badawi, G.A. Targeting α-Klotho Protein by Agmatine and Pioglitazone Is a New Avenue against Diabetic Nephropathy. ACS Pharmacol. Transl. Sci. 2025, 8, 2493–2506. [Google Scholar] [CrossRef]
- Koning, A.-S.C.A.M.; Buurstede, J.C.; van Weert, L.T.C.M.; Meijer, O.C. Glucocorticoid and mineralocorticoid receptors in the brain: A transcriptional perspective. J. Endocr. Soc. 2019, 3, 1917–1930. [Google Scholar] [CrossRef]
- Perrelli, M.; Goparaju, P.; Postolache, T.T.; del Bosque-Plata, L.; Gragnoli, C. Stress and the CRH system, norepinephrine, depression, and type 2 diabetes. Biomedicines 2024, 12, 1187. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zhang, Y.; Sun, J.; Gao, L.; Ma, B.; Lu, J.; Ni, X. Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1. Endocrinology 2008, 149, 1389–1398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paretkar, T.; Dimitrov, E. The central amygdala corticotropin-releasing hormone (CRH) neurons modulation of anxiety-like behavior and hippocampus-dependent memory in mice. Neuroscience 2018, 390, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Li, X.-X.; Tu, X.-R.; Liu, R.; Xu, J.-W.; Lv, Y.-L.; Yao, Y.-Y. Hippocampal Crhr1 conditional gene knockout ameliorated the depression-like behavior and pathological damage in male offspring mice caused by chronic stress during pregnancy. Behav. Brain Res. 2024, 472, 115139. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry 1988, 21, 76–82. [Google Scholar] [CrossRef]
- Chen, X.; Gianferante, D.; Hanlin, L.; Fiksdal, A.; Breines, J.G.; Thoma, M.V.; Rohleder, N. HPA-axis and inflammatory reactivity to acute stress is related with basal HPA-axis activity. Psychoneuroendocrinology 2017, 78, 168–176. [Google Scholar] [CrossRef]
- Han, J.; Wang, Y.; Wei, P.; Lu, D.; Shan, Y. Unveiling the hidden connection: The blood-brain barrier’s role in epilepsy. Front. Neurol. 2024, 15, 1413023. [Google Scholar] [CrossRef] [PubMed]
- Waclawiková, B.; El Aidy, S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef]
- Rupasinghe, R.; Dezsi, G.; Ozturk, E.; Carron, S.; Hudson, M.R.; Casillas-Espinosa, P.M.; Jones, N.C. Early life adversity accelerates epileptogenesis and enhances depression-like behaviors in rats. Exp. Neurol. 2022, 354, 114088. [Google Scholar] [CrossRef] [PubMed]
- Babicola, L.; Ventura, R.; D’Addario, S.L.; Ielpo, D.; Andolina, D.; Di Segni, M. Long term effects of early life stress on HPA circuit in rodent models. Mol. Cell. Endocrinol. 2021, 521, 111125. [Google Scholar] [CrossRef] [PubMed]
- Golub, V.M.; Reddy, D.S. Post-traumatic epilepsy and comorbidities: Advanced models, molecular mechanisms, biomarkers, and novel therapeutic interventions. Pharmacol. Rev. 2022, 74, 387–438. [Google Scholar] [CrossRef]
- Jiang, S.; Postovit, L.; Cattaneo, A.; Binder, E.B.; Aitchison, K.J. Epigenetic modifications in stress response genes associated with childhood trauma. Front. Psychiatry 2019, 10, 808. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA axis in the pathomechanism of depression and schizophrenia: New therapeutic strategies based on its participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Sorge, S.T.; Hesdorffer, D.C.; Phelan, J.C.; Winawer, M.R.; Shostak, S.; Goldsmith, J.; Chung, W.K.; Ottman, R. Depression and genetic causal attribution of epilepsy in multiplex epilepsy families. Epilepsia 2016, 57, 1643–1650. [Google Scholar] [CrossRef][Green Version]
- Krishnan, V. Depression and anxiety in the epilepsies: From bench to bedside. Curr. Neurol. Neurosci. Rep. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.M.; Chauhan, L.; Bhardwaj, A.; Sharma, A.; Fayaz, F.; Kumar, B.; Alhashmi, M.; AlHajri, N.; Alam, M.S.; Pottoo, F.H. Brain-derived neurotropic factor in neurodegenerative disorders. Biomedicines 2022, 10, 1143. [Google Scholar] [CrossRef]
- Insel, B.J.; Ottman, R.; Heiman, G.A. Mood disorders in familial epilepsy: A test of shared etiology. Epilepsia 2018, 59, 431–439. [Google Scholar] [CrossRef]
- Thakran, S.; Guin, D.; Singh, P.; Singh, P.; Kukal, S.; Rawat, C.; Yadav, S.; Kushwaha, S.S.; Srivastava, A.K.; Hasija, Y. Genetic landscape of common epilepsies: Advancing towards precision in treatment. Int. J. Mol. Sci. 2020, 21, 7784. [Google Scholar] [CrossRef]
- Guiard, B.P.; Giovanni, G. Di Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: The missing link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef]
- Cardamone, L.; Salzberg, M.R.; O’brien, T.J.; Jones, N.C. Antidepressant therapy in epilepsy: Can treating the comorbidities affect the underlying disorder? Br. J. Pharmacol. 2013, 168, 1531–1554. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.-G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key factors in neurological and neurodegenerative disorders of the central nervous system. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.K.; Emdad, L.; Lee, S.-G.; Su, Z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P.B.; Sarkar, D. Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacol. Ther. 2011, 130, 1–8. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Kang, J.; Gallagher, M.J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010, 588, 1861–1869. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol. Sin. 2011, 32, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Badawi, G.A.; Shokr, M.M.; Elshazly, S.M.; Zaki, H.F.; Mohamed, A.F. Sigma-1 receptor modulation by clemastine highlights its repurposing as neuroprotective agent against seizures and cognitive deficits in PTZ-kindled rats. Eur. J. Pharmacol. 2024, 980, 176851. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, K.H.; Pilsner, J.R.; Lu, Q.; Wright, R.O.; Guilarte, T.R. Dysregulation of BDNF-TrkB signaling in developing hippocampal neurons by Pb2+: Implications for an environmental basis of neurodevelopmental disorders. Toxicol. Sci. 2012, 127, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Peterson, S.; Ford, K.; Singletary, K.; Peixoto, L. Critical periods and Autism Spectrum Disorders, a role for sleep. Neurobiol. Sleep Circadian Rhythm. 2023, 14, 100088. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Bufalino, C.; Hepgul, N.; Aguglia, E.; Pariante, C.M. The role of immune genes in the association between depression and inflammation: A review of recent clinical studies. Brain Behav. Immun. 2013, 31, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Landa-Solís, C.; Valle-Garcia, D.; Luna-Angulo, A.; Avilés-Arnaut, H.; Robles-Bañuelos, B.; Sánchez-Chapul, L.; Rangel-López, E. Kynurenines and Inflammation: A Remarkable Axis for Multiple Sclerosis Treatment. Pharmaceuticals 2024, 17, 983. [Google Scholar] [CrossRef]
- Grezenko, H.; Ekhator, C.; Nwabugwu, N.U.; Ganga, H.; Affaf, M.; Abdelaziz, A.M.; Rehman, A.; Shehryar, A.; Abbasi, F.A.; Bellegarde, S.B. Epigenetics in neurological and psychiatric disorders: A comprehensive review of current understanding and future perspectives. Cureus 2023, 15, e43960. [Google Scholar] [CrossRef]
- Mitchell, C.; Schneper, L.M.; Notterman, D.A. DNA methylation, early life environment, and health outcomes. Pediatr. Res. 2016, 79, 212–219. [Google Scholar] [CrossRef]
- Slavich, G.M.; Roos, L.G.; Mengelkoch, S.; Webb, C.A.; Shattuck, E.C.; Moriarity, D.P.; Alley, J.C. Social Safety Theory: Conceptual foundation, underlying mechanisms, and future directions. Health Psychol. Rev. 2023, 17, 5–59. [Google Scholar] [CrossRef]
- Lee, R.S.; Sawa, A. Environmental stressors and epigenetic control of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology 2015, 100, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.; Giombelli, L.; Werlang, I.C.; Abujamra, A.L.; Secchi, T.L.; Brondani, R.; Bragatti, J.A.; Bizzi, J.W.J.; Leistner-Segal, S.; Bianchin, M.M. Methylation of BDNF and SLC6A4 gene promoters in Brazilian patients with temporal lobe epilepsy presenting or not psychiatric comorbidities. Front. Integr. Neurosci. 2021, 15, 764742. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Roy, B.; Korla, P.K. Genome-wide methylome-based molecular pathologies associated with depression and suicide. Neuropsychopharmacology 2025, 50, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.J. Genome wide association studies (GWAS) and common forms of human epilepsy. Epilepsy Behav. 2013, 28, S63–S65. [Google Scholar] [CrossRef]
- Zuberi, S.M.; Wirrell, E.; Yozawitz, E.; Wilmshurst, J.M.; Specchio, N.; Riney, K.; Pressler, R.; Auvin, S.; Samia, P.; Hirsch, E. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1349–1397. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.M.; Holst, K.K.; Adamsen, D.; Klein, A.B.; Frokjaer, V.G.; Jensen, P.S.; Svarer, C.; Gillings, N.; Baare, W.F.C.; Mikkelsen, J.D. BDNF Val66met and 5-HTTLPR polymorphisms predict a human in vivo marker for brain serotonin levels. Hum. Brain Mapp. 2015, 36, 313–323. [Google Scholar] [CrossRef]
- Boscutti, A.; Pigoni, A.; Delvecchio, G.; Lazzaretti, M.; Mandolini, G.M.; Girardi, P.; Ferro, A.; Sala, M.; Abbiati, V.; Cappucciati, M. The influence of 5-HTTLPR, BDNF Rs6265 and COMT Rs4680 polymorphisms on impulsivity in bipolar disorder: The role of gender. Genes 2022, 13, 482. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Hernández, L.M.; Taylor, M.K. The Val66Met variant of brain-derived neurotrophic factor is linked to reduced telomere length in a military population: A pilot study. Sci. Rep. 2024, 14, 27013. [Google Scholar] [CrossRef]
- Ferreira Fratelli, C.; Willatan Siqueira, J.; Rodrigues Gontijo, B.; de Lima Santos, M.; de Souza Silva, C.M.; Rodrigues da Silva, I.C. BDNF genetic variant and its genotypic fluctuation in major depressive disorder. Behav. Neurol. 2021, 2021, 7117613. [Google Scholar] [CrossRef]
- AlRuwaili, R.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Ali, N.H.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E.-S. The possible role of brain-derived neurotrophic factor in epilepsy. Neurochem. Res. 2024, 49, 533–547. [Google Scholar] [CrossRef]
- Harika-Germaneau, G.; Lafay-Chebassier, C.; Langbour, N.; Thirioux, B.; Wassouf, I.; Noël, X.; Jaafari, N.; Chatard, A. Preliminary evidence that the short allele of 5-HTTLPR moderates the association of psychiatric symptom severity on suicide attempt: The example in obsessive-compulsive disorder. Front. Psychiatry 2022, 13, 770414. [Google Scholar] [CrossRef]
- Miozzo, R.; Eaton, W.W.; Bienvenu, O.J., III; Samuels, J.; Nestadt, G. The serotonin transporter gene polymorphism (SLC6A4) and risk for psychiatric morbidity and comorbidity in the Baltimore ECA follow-up study. Compr. Psychiatry 2020, 102, 152199. [Google Scholar] [CrossRef] [PubMed]
- Risch, N.; Herrell, R.; Lehner, T.; Liang, K.-Y.; Eaves, L.; Hoh, J.; Griem, A.; Kovacs, M.; Ott, J.; Merikangas, K.R. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA 2009, 301, 2462–2471. [Google Scholar] [CrossRef]
- Lauerer, R.J.; Lerche, H. Voltage-gated calcium channels in genetic epilepsies. J. Neurochem. 2024, 168, 3853–3871. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zhang, H.; Li, J.; Huang, H.; Weng, J.; Tan, H.; Guo, T.; Wang, M.; Xie, J. The epigenetic modification of DNA methylation in neurological diseases. Front. Immunol. 2024, 15, 1401962. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, F.; Ericson, M.; Story, D.; Hulce, V.D.; Dunbar, G.L. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005, 7, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Mazarati, A.; Siddarth, P.; Baldwin, R.A.; Shin, D.; Caplan, R.; Sankar, R. Depression after status epilepticus: Behavioural and biochemical deficits and effects of fluoxetine. Brain 2008, 131, 2071–2083. [Google Scholar] [CrossRef]
- Mazarati, A.M.; Pineda, E.; Shin, D.; Tio, D.; Taylor, A.N.; Sankar, R. Comorbidity between epilepsy and depression: Role of hippocampal interleukin-1β. Neurobiol. Dis. 2010, 37, 461–467. [Google Scholar] [CrossRef]
- Mazarati, A.M.; Shin, D.; Kwon, Y.S.; Bragin, A.; Pineda, E.; Tio, D.; Taylor, A.N.; Sankar, R. Elevated plasma corticosterone level and depressive behavior in experimental temporal lobe epilepsy. Neurobiol. Dis. 2009, 34, 457–461. [Google Scholar] [CrossRef]
- Smolders, I.; Clinckers, R.; Meurs, A.; De Bundel, D.; Portelli, J.; Ebinger, G.; Michotte, Y. Direct enhancement of hippocampal dopamine or serotonin levels as a pharmacodynamic measure of combined antidepressant–anticonvulsant action. Neuropharmacology 2008, 54, 1017–1028. [Google Scholar] [CrossRef]
- Koh, S.; Magid, R.; Chung, H.; Stine, C.D.; Wilson, D.N. Depressive behavior and selective downregulation of serotonin receptor expression after early-life seizures: Reversal by environmental enrichment. Epilepsy Behav. 2007, 10, 26–31. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Pechlivanova, D.; Atanasova, T.; Markova, P.; Lozanov, V.; Stoynev, A. Diurnal variations in depression-like behavior of Wistar and spontaneously hypertensive rats in the kainate model of temporal lobe epilepsy. Epilepsy Behav. 2011, 20, 277–285. [Google Scholar] [CrossRef]
- Danober, L.; Deransart, C.; Depaulis, A.; Vergnes, M.; Marescaux, C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog. Neurobiol. 1998, 55, 27–57. [Google Scholar] [CrossRef]
- Jones, N.C.; Salzberg, M.R.; Kumar, G.; Couper, A.; Morris, M.J.; O’Brien, T.J. Elevated anxiety and depressive-like behavior in a rat model of genetic generalized epilepsy suggesting common causation. Exp. Neurol. 2008, 209, 254–260. [Google Scholar] [CrossRef]
- Jones, N.C.; Lee, H.E.; Yang, M.; Rees, S.M.; Morris, M.J.; O’Brien, T.J.; Salzberg, M.R. Repeatedly stressed rats have enhanced vulnerability to amygdala kindling epileptogenesis. Psychoneuroendocrinology 2013, 38, 263–270. [Google Scholar] [CrossRef]
- Rosenkranz, J.A.; Venheim, E.R.; Padival, M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry 2010, 67, 1128–1136. [Google Scholar] [CrossRef]
- Eskelund, A.; Budac, D.P.; Sanchez, C.; Elfving, B.; Wegener, G. Female Flinders Sensitive Line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 2016, 329, 337–348. [Google Scholar] [CrossRef]
- Sarkisova, K.; van Luijtelaar, G. The WAG/Rij strain: A genetic animal model of absence epilepsy with comorbidity of depressiony. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 854–876. [Google Scholar] [CrossRef] [PubMed]
- Sarkisova, K.Y.; Midzianovskaia, I.S.; Kulikov, M.A. Depressive-like behavioral alterations and c-fos expression in the dopaminergic brain regions in WAG/Rij rats with genetic absence epilepsy. Behav. Brain Res. 2003, 144, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.A.; Hensler, J.G.; Sankar, R.; Shin, D.; Burke, T.F.; Mazarati, A.M. Plasticity of presynaptic and postsynaptic serotonin 1A receptors in an animal model of epilepsy-associated depression. Neuropsychopharmacology 2011, 36, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Tai, C.; Westenbroek, R.E.; Yu, F.H.; Cheah, C.S.; Potter, G.B.; Rubenstein, J.L.; Scheuer, T.; De La Iglesia, H.O.; Catterall, W.A. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature 2012, 489, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Bahceci, D.; Anderson, L.L.; Brown, C.V.O.H.; Zhou, C.; Arnold, J.C. Adolescent behavioral abnormalities in a Scn1a+/− mouse model of Dravet syndrome. Epilepsy Behav. 2020, 103, 106842. [Google Scholar] [CrossRef]
- Coleman, E.M.; White, M.; Antonoudiou, P.; Weiss, G.L.; Scarpa, G.; Stone, B.; Maguire, J. Early life stress influences epilepsy outcomes in mice. Epilepsy Behav. 2025, 163, 110217. [Google Scholar] [CrossRef]
- Wulsin, A.C.; Franco-Villanueva, A.; Romancheck, C.; Morano, R.L.; Smith, B.L.; Packard, B.A.; Danzer, S.C.; Herman, J.P. Functional disruption of stress modulatory circuits in a model of temporal lobe epilepsy. PLoS ONE 2018, 13, e0197955. [Google Scholar] [CrossRef]
- Lévesque, M.; Herrington, R.; Leclerc, L.; Rogawski, M.A.; Avoli, M. Allopregnanolone decreases interictal spiking and fast ripples in an animal model of mesial temporal lobe epilepsy. Neuropharmacology 2017, 121, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, J.; Sun, J.; Liang, Q.; Zhan, Y.; Yang, Z.; Zhang, Y.; Jin, L.; Hu, C.; Zhao, Y.-T. Ketogenic diet attenuates neuroinflammation and restores hippocampal neurogenesis to improve CUMS induced depression-like behavior in mice. Food Funct. 2025, 16, 3408–3422. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Tian, S.; Zhang, Y.; Che, L.; Li, Q.; Qu, Z.; Wang, W. A ketogenic diet may improve cognitive function in rats with temporal lobe epilepsy by regulating endoplasmic reticulum stress and synaptic plasticity. Mol. Neurobiol. 2024, 61, 2249–2264. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.A.; Hensler, J.G.; Sankar, R.; Shin, D.; Burke, T.F.; Mazarati, A.M. Interleukin-1beta causes fluoxetine resistance in an animal model of epilepsy-associated depression. Neurotherapeutics 2012, 9, 477–485. [Google Scholar] [CrossRef]
- Krahl, S.E.; Senanayake, S.S.; Pekary, A.E.; Sattin, A. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J. Psychiatr. Res. 2004, 38, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Filatov, G.; Smith, S.J.; Gammaitoni, A.R.; Lothe, A.; Reeder, T. Fenfluramine increases survival and reduces markers of neurodegeneration in a mouse model of Dravet syndrome. Epilepsia Open 2024, 9, 300–313. [Google Scholar] [CrossRef]
- Forthoffer, N.; Tarrada, A.; Brissart, H.; Maillard, L.; Hingray, C. Anxiety and depression in newly diagnosed epilepsy: A matter of psychological history? Front. Neurol. 2021, 12, 744377. [Google Scholar] [CrossRef]
- Sarikaya, I. PET studies in epilepsy. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 416. [Google Scholar] [PubMed]
- Kanner, A.M. Depression in epilepsy: A neurobiologic perspective. Epilepsy Curr. 2005, 5, 21–27. [Google Scholar] [CrossRef]
- Lee, S.K. Epilepsy in the elderly: Treatment and consideration of comorbid diseases. J. Epilepsy Res. 2019, 9, 27. [Google Scholar] [CrossRef]
- Swailem, S.K.; Bamogaddam, F.A.; Al-Attas, A.A. The prevalence of depression in patients with epilepsy in the Kingdom of Saudi Arabia. Cureus 2024, 16, e55570. [Google Scholar] [CrossRef]
- Guo, W.; Li, Y.; Zhang, Y.; Lv, X.; Wang, S.; Zhang, S.; Wang, E.; Chen, X.; Li, Y. Risk analysis of depression among adult patients with epilepsy of different sex: A retrospective single-center study from China. Front. Psychiatry 2023, 14, 1283983. [Google Scholar] [CrossRef]
- Briellmann, R.S.; Hopwood, M.J.; Jackson, G.D. Major depression in temporal lobe epilepsy with hippocampal sclerosis: Clinical and imaging correlates. J. Neurol. Neurosurg. Psychiatry 2007, 78, 1226–1230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Operto, F.F.; Pastorino, G.M.G.; Pippa, F.; Padovano, C.; Vivenzio, V.; Scuoppo, C.; Pistola, I.; Coppola, G. Psychiatric symptoms and parental stress in children and adolescents with epilepsy. Front. Neurol. 2021, 12, 778410. [Google Scholar] [CrossRef] [PubMed]
- Altman, K.; Shavit-Stein, E.; Maggio, N. Post stroke seizures and epilepsy: From proteases to maladaptive plasticity. Front. Cell. Neurosci. 2019, 13, 397. [Google Scholar] [CrossRef]
- Hu, M.; Qin, B.; Li, T.; Wei, C.; Su, D.; Tan, Z. Efficacy of rTMS for poststroke epilepsy and its effects on patients’ cognitive function and depressive status. BMC Neurol. 2024, 24, 25. [Google Scholar] [CrossRef]
- Soncin, L.-D.; Mcgonigal, A.; Kotwas, I.; Belquaid, S.; Giusiano, B.; Faure, S.; Bartolomei, F. Post-traumatic stress disorder (PTSD) in patients with epilepsy. Epilepsy Behav. 2021, 121, 108083. [Google Scholar] [CrossRef]
- Bakvis, P.; Spinhoven, P.; Giltay, E.J.; Kuyk, J.; Edelbroek, P.M.; Zitman, F.G.; Roelofs, K. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia 2010, 51, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. Most antidepressant drugs are safe for patients with epilepsy at therapeutic doses: A review of the evidence. Epilepsy Behav. 2016, 61, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M.; Kozak, A.M.; Frey, M. The use of sertraline in patients with epilepsy: Is it safe? Epilepsy Behav. 2000, 1, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Navidhamidi, M.; Mehranfard, N. Vagal nerve stimulation for refractory epilepsy: A brief. Neuropsychiatry 2016, 6, 149–160. [Google Scholar]
- Kim, J.S.; Kim, D.Y.; Jo, H.J.; Hwang, Y.H.; Song, J.Y.; Yang, K.I.; Hong, S.B. Effect of long-term treatment with vagus nerve stimulation on mood and quality of life in Korean patients with drug-resistant epilepsy. J. Clin. Neurol. 2021, 17, 385. [Google Scholar] [CrossRef]
- Shin, H.-R.; Kim, M.; Park, K.-I. Electroconvulsive therapy and seizure: A double-edged sword? Encephalitis 2023, 3, 103. [Google Scholar] [CrossRef]
- Lex, H.; Nevers, S.W.; Jensen, E.L.; Ginsburg, Y.; Maixner, D.F.; Mickey, B.J. Long-term quality of life in treatment-resistant depression after electroconvulsive therapy. J. Affect. Disord. 2021, 291, 135–139. [Google Scholar] [CrossRef]
- Miller, I.O.; Sotero de Menezes, M.A. SCN1A Seizure Disorders. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1318/ (accessed on 17 August 2025).
- Scheffer, I.E.; Nabbout, R. SCN1A-related phenotypes: Epilepsy and beyond. Epilepsia 2019, 60, S17–S24. [Google Scholar] [CrossRef]
- D’Andrea Meira, I.; Romão, T.T.; Pires do Prado, H.J.; Krüger, L.T.; Pires, M.E.P.; da Conceição, P.O. Ketogenic diet and epilepsy: What we know so far. Front. Neurosci. 2019, 13, 5. [Google Scholar] [CrossRef]
- Guerreiro, D.; Almeida, A.; Ramalho, R. Ketogenic Diet and Neuroinflammation: Implications for Neuroimmunometabolism and Therapeutic Approaches to Refractory Epilepsy. Nutrients 2024, 16, 3994. [Google Scholar] [CrossRef]
- de Araujo Filho, G.M.; Mazetto, L.; Gomes, F.L.; Marinho, M.M.; Tavares, I.M.; Caboclo, L.O.S.F.; Centeno, R.S.; Yacubian, E.M.T. Pre-surgical predictors for psychiatric disorders following epilepsy surgery in patients with refractory temporal lobe epilepsy and mesial temporal sclerosis. Epilepsy Res. 2012, 102, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Nowacki, A.; Jehi, L.; Busch, R. Predicting Depression Following Temporal Lobe Epilepsy Surgery in Adults (1651). Neurology 2020, 94, 1651. [Google Scholar] [CrossRef]
- Guille, C.; Spencer, S.; Cavus, I.; Epperson, C.N. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: Implications for diagnosis and treatment. Epilepsy Behav. 2008, 13, 12–24. [Google Scholar] [CrossRef]
- Herzog, A.G. Catamenial epilepsy: Definition, prevalence pathophysiology and treatment. Seizure 2008, 17, 151–159. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of gut microbiota from the perspective of the gut–brain axis: Role in the provocation of neurological disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Appleton, J. The gut-brain axis: Influence of microbiota on mood and mental health. Integr. Med. A Clin. J. 2018, 17, 28. [Google Scholar]
- Fock, E.; Parnova, R. Mechanisms of blood–brain barrier protection by microbiota-derived short-chain fatty acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chu, J.; Feng, S.; Guo, C.; Xue, B.; He, K.; Li, L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: A review. Biomed. Pharmacother. 2023, 164, 114985. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: From pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef]
- You, M.; Chen, N.; Yang, Y.; Cheng, L.; He, H.; Cai, Y.; Liu, Y.; Liu, H.; Hong, G. The gut microbiota–brain axis in neurological disorders. MedComm 2024, 5, e656. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, C.-L.; Kao, C.-H. Irritable bowel syndrome increases the risk of epilepsy: A population-based study. Medicine 2015, 94, e1497. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, B.; Verne, N.G. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef]
- Gecse, K.; Róka, R.; Séra, T.; Rosztóczy, A.; Annaházi, A.; Izbéki, F.; Nagy, F.; Molnár, T.; Szepes, Z.; Pávics, L. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion 2012, 85, 40–46. [Google Scholar] [CrossRef]
- Dlugosz, A.; Nowak, P.; D’amato, M.; Mohammadian Kermani, G.; Nyström, J.; Abdurahman, S.; Lindberg, G. Increased serum levels of lipopolysaccharide and antiflagellin antibodies in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol. Motil. 2015, 27, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Zong, G.; Wang, Z.; Shi, J.; Du, H.; Hu, J. Expression of inducible nitric oxide synthase in mast cells contributes to the regulation of inflammatory cytokines in irritable bowel syndrome with diarrhea. Neurogastroenterol. Motil. 2016, 28, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Khedr, L.H.; Eladawy, R.M.; Nassar, N.N.; Saad, M.A.E. Canagliflozin attenuates chronic unpredictable mild stress induced neuroinflammation via modulating AMPK/mTOR autophagic signaling. Neuropharmacology 2023, 223, 109293. [Google Scholar] [CrossRef]
- Lindefeldt, M.; Eng, A.; Darban, H.; Bjerkner, A.; Zetterström, C.K.; Allander, T.; Andersson, B.; Borenstein, E.; Dahlin, M.; Prast-Nielsen, S. The ketogenic diet influences taxonomic and functional composition of the gut microbiota in children with severe epilepsy. Npj Biofilms Microbiomes 2019, 5, 5. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Jin, F. Gut-brain psychology: Rethinking psychology from the microbiota–gut–brain axis. Front. Integr. Neurosci. 2018, 12, 33. [Google Scholar] [CrossRef]
- Wall, R.; Cryan, J.F.; Ross, R.P.; Fitzgerald, G.F.; Dinan, T.G.; Stanton, C. Bacterial neuroactive compounds produced by psychobiotics. Microb. Endocrinol. Microbiota-Gut-Brain Axis Health Dis. 2014, 817, 221–239. [Google Scholar]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Microb. Endocrinol. Microbiota-Gut-Brain Axis Health Dis. 2014, 817, 195–219. [Google Scholar]
- Margolis, K.G.; Stevanovic, K.; Li, Z.; Yang, Q.M.; Oravecz, T.; Zambrowicz, B.; Jhaver, K.G.; Diacou, A.; Gershon, M.D. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014, 63, 928–937. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The neuro-endocrinological role of microbial glutamate and GABA signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Reiter, A.; Bengesser, S.A.; Hauschild, A.-C.; Birkl-Töglhofer, A.-M.; Fellendorf, F.T.; Platzer, M.; Färber, T.; Seidl, M.; Mendel, L.-M.; Unterweger, R. Interleukin-6 gene expression changes after a 4-week intake of a multispecies probiotic in major depressive disorder—Preliminary results of the PROVIT study. Nutrients 2020, 12, 2575. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, R.; Deng, H.; Cui, P.; Li, C.; Yang, F.; Leong Bin Abdullah, M.F.I. Research protocol of the efficacy of probiotics for the treatment of alcohol use disorder among adult males: A comparison with placebo and acceptance and commitment therapy in a randomized controlled trial. PLoS ONE 2023, 18, e0294768. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Huang, Y.-Y.; Tsai, S.-Y.; Kuo, Y.-W.; Lin, J.-H.; Ho, H.-H.; Chen, J.-F.; Hsia, K.-C.; Sun, Y. Efficacy of probiotic supplements on brain-derived neurotrophic factor, inflammatory biomarkers, oxidative stress and cognitive function in patients with Alzheimer’s dementia: A 12-week randomized, double-blind active-controlled study. Nutrients 2023, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Shin, Y.-J.; Park, H.-S.; Jeong, J.-W.; Kim, J.Y.; Shim, J.-J.; Lee, J.-L.; Kim, D.-H. Lactobacillus casei and its supplement alleviate stress-induced depression and anxiety in mice by the regulation of BDNF expression and NF-κB activation. Nutrients 2023, 15, 2488. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, L.; Li, Y.; Chen, Q.; Wang, L.; Farag, M.A.; Liu, L.; Zhan, S.; Wu, Z.; Liu, L. Soy protein isolate-citrus pectin composite hydrogels induced by TGase and ultrasonic treatment: Potential targeted delivery system for probiotics. Food Hydrocoll. 2023, 143, 108901. [Google Scholar] [CrossRef]
- Wang, X.; Ma, R.; Liu, X.; Zhang, Y. Effects of long-term supplementation of probiotics on cognitive function and emotion in temporal lobe epilepsy. Front. Neurol. 2022, 13, 948599. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef] [PubMed]
- Rob, M.; Yousef, M.; Lakshmanan, A.P.; Mahboob, A.; Terranegra, A.; Chaari, A. Microbial signatures and therapeutic strategies in neurodegenerative diseases. Biomed. Pharmacother. 2025, 184, 117905. [Google Scholar] [CrossRef]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan metabolites along the microbiota-gut-brain axis: An interkingdom communication system influencing the gut in health and disease. Int. J. Tryptophan Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Phillips, C. Brain-derived neurotrophic factor, depression, and physical activity: Making the neuroplastic connection. Neural Plast. 2017, 2017, 7260130. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: A systematic review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain–gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 298797. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, S.; Majoie, H.J.M.; Van der Heijden, M.; Rijkers, K.; Leenen, L.; Aldenkamp, A.P. Vagus nerve stimulation has a positive effect on mood in patients with refractory epilepsy. Clin. Neurol. Neurosurg. 2012, 114, 336–340. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus nerve and underlying impact on the gut microbiota-brain axis in behavior and neurodegenerative diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Tan, C.; Yan, Q.; Ma, Y.; Fang, J.; Yang, Y. Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front. Neurol. 2022, 13, 1015175. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef]
- Kim, S.; Park, S.; Choi, T.G.; Kim, S.S. Role of short chain fatty acids in epilepsy and potential benefits of probiotics and prebiotics: Targeting “health” of epileptic patients. Nutrients 2022, 14, 2982. [Google Scholar] [CrossRef]
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- Chiappini, S.; d’Andrea, G.; De Filippis, S.; Di Nicola, M.; Andriola, I.; Bassetti, R.; Barlati, S.; Pettorruso, M.; Sensi, S.; Clerici, M. Esketamine in treatment-resistant depression patients comorbid with substance-use disorder: A viewpoint on its safety and effectiveness in a subsample of patients from the REAL-ESK study. Eur. Neuropsychopharmacol. 2023, 74, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Rafało-Ulińska, A.; Pałucha-Poniewiera, A. The effectiveness of (R)-ketamine and its mechanism of action differ from those of (S)-ketamine in a chronic unpredictable mild stress model of depression in C57BL/6J mice. Behav. Brain Res. 2022, 418, 113633. [Google Scholar] [CrossRef]
- Kawczak, P.; Feszak, I.; Bączek, T. Ketamine, Esketamine, and Arketamine: Their Mechanisms of Action and Applications in the Treatment of Depression and Alleviation of Depressive Symptoms. Biomedicines 2024, 12, 2283. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, X.; Wei, L.; Chen, Q.; Xu, Y.; Ni, P.; Deng, W.; Guo, W.; Hu, X.; Qi, X. LT-102, an AMPA receptor potentiator, alleviates depression-like behavior and synaptic plasticity impairments in prefrontal cortex induced by sleep deprivation. J. Affect. Disord. 2024, 367, 18–30. [Google Scholar] [CrossRef]
- Scharf, S.S.; Gasparini, F.; Spooren, W.; Lindemann, L. Drug Discovery for Targeted Pharmacotherapy of Fragile X Syndrome. In Fragile X Syndrome; Elsevier: Amsterdam, The Netherlands, 2017; pp. 363–399. [Google Scholar]
- Dyomina, A.V.; Kovalenko, A.A.; Zakharova, M.V.; Postnikova, T.Y.; Griflyuk, A.V.; Smolensky, I.V.; Antonova, I.V.; Zaitsev, A.V. MTEP, a selective mGluR5 antagonist, had a neuroprotective effect but did not prevent the development of spontaneous recurrent seizures and behavioral comorbidities in the rat lithium–pilocarpine model of epilepsy. Int. J. Mol. Sci. 2022, 23, 497. [Google Scholar] [CrossRef]
- Lea, P.M., IV; Movsesyan, V.A.; Faden, A.I. Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors. Br. J. Pharmacol. 2005, 145, 527–534. [Google Scholar] [CrossRef]
- Du, Y.; Gao, F.; Sun, H.; Wu, C.; Zhu, G.; Zhu, M. Novel substituted 4-(Arylethynyl)-Pyrrolo [2, 3-d] pyrimidines negative allosteric modulators (NAMs) of the metabotropic glutamate receptor subtype 5 (mGlu5) treat depressive disorder in mice. Eur. J. Med. Chem. 2023, 261, 115855. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef]
- Witkin, J.M.; Pandey, K.P.; Smith, J.L. Clinical investigations of compounds targeting metabotropic glutamate receptors. Pharmacol. Biochem. Behav. 2022, 219, 173446. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Guo, J.; Chen, B.; Fang, J. Psychosis of Epilepsy: An Update on Clinical Classification and Mechanism. Biomolecules 2025, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Aumer, T.; Däther, M.; Bergmayr, L.; Kartika, S.; Zeng, T.; Ge, Q.; Giorgio, G.; Hess, A.J.; Michalakis, S.; Traube, F.R. The type of DNA damage response after decitabine treatment depends on the level of DNMT activity. Life Sci. Alliance 2024, 7, 9. [Google Scholar] [CrossRef]
- Parrish, R.R.; Buckingham, S.C.; Mascia, K.L.; Johnson, J.J.; Matyjasik, M.M.; Lockhart, R.M.; Lubin, F.D. Methionine increases BDNF DNA methylation and improves memory in epilepsy. Ann. Clin. Transl. Neurol. 2015, 2, 401–416. [Google Scholar] [CrossRef]
- Yan, L.; Geng, Q.; Cao, Z.; Liu, B.; Li, L.; Lu, P.; Lin, L.; Wei, L.; Tan, Y.; He, X. Insights into DNMT1 and programmed cell death in diseases. Biomed. Pharmacother. 2023, 168, 115753. [Google Scholar] [CrossRef]
- Kim, D.J. The Role of the DNA Methyltransferase Family and the Therapeutic Potential of DNMT Inhibitors in Tumor Treatment. Curr. Oncol. 2025, 32, 88. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, W.; Liu, S.; Qiao, X.; Xing, Y.; Zhou, Q.; Zhang, Z. Epigenetic regulation of neuroinflammation in Alzheimer’s Disease. Cells 2023, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Ansori, A.N.M.; Antonius, Y.; Susilo, R.J.K.; Hayaza, S.; Kharisma, V.D.; Parikesit, A.A.; Zainul, R.; Jakhmola, V.; Saklani, T.; Rebezov, M. Application of CRISPR-Cas9 genome editing technology in various fields: A review. Narra J. 2023, 3, e184. [Google Scholar] [CrossRef] [PubMed]
- Monsey, M.S.; Ota, K.T.; Akingbade, I.F.; Hong, E.S.; Schafe, G.E. Epigenetic alterations are critical for fear memory consolidation and synaptic plasticity in the lateral amygdala. PLoS ONE 2011, 6, e19958. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Gartlehner, G.; Hansen, R.A.; Carey, T.S.; Lohr, K.N.; Gaynes, B.N.; Randolph, L.C. Discontinuation rates for selective serotonin reuptake inhibitors and other second-generation antidepressants in outpatients with major depressive disorder: A systematic review and meta-analysis. Int. Clin. Psychopharmacol. 2005, 20, 59–69. [Google Scholar] [CrossRef]
- Eladawy, R.M.; Ahmed, L.A.; Salem, M.B.; El-Sayed, R.M.; Salem, H.A.; Mohamed, A.F. Probiotics reverse gut dysbiosis and memory impairment associated with esomeprazole use in chronically stressed rats: A significant neuroprotective role for cholecystokinin. Int. Immunopharmacol. 2025, 150, 114227. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Transforming life: A broad view of the developmental origins of health and disease concept from an ecological justice perspective. Int. J. Environ. Res. Public Health 2016, 13, 1075. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R. The effects of probiotics on depressive symptoms in humans: A systematic review. Ann. Gen. Psychiatry 2017, 16, 14. [Google Scholar] [CrossRef]
- Kumar, A.; Sivamaruthi, B.S.; Dey, S.; Kumar, Y.; Malviya, R.; Prajapati, B.G.; Chaiyasut, C. Probiotics as modulators of gut-brain axis for cognitive development. Front. Pharmacol. 2024, 15, 1348297. [Google Scholar] [CrossRef]
- Randeni, N.; Xu, B. Critical review of the cross-links between dietary components, the gut microbiome, and depression. Int. J. Mol. Sci. 2025, 26, 614. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Dyomina, A.V.; Kovalenko, A.A.; Roginskaya, A.I.; Melik-Kasumov, T.B.; Korneeva, M.A.; Chuprina, A.V.; Zhabinskaya, A.A.; Kolyhan, S.A.; Zakharova, M.V. Beneficial effects of probiotic Bifidobacterium longum in a Lithium–pilocarpine model of temporal lobe epilepsy in rats. Int. J. Mol. Sci. 2023, 24, 8451. [Google Scholar] [CrossRef]
- Yong, S.J.; Tong, T.; Chew, J.; Lim, W.L. Antidepressive mechanisms of probiotics and their therapeutic potential. Front. Neurosci. 2020, 13, 1361. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Dasriya, V.L.; Samtiya, M.; Ranveer, S.; Dhillon, H.S.; Devi, N.; Sharma, V.; Nikam, P.; Puniya, M.; Chaudhary, P.; Chaudhary, V. Modulation of gut-microbiota through probiotics and dietary interventions to improve host health. J. Sci. Food Agric. 2024, 104, 6359–6375. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.U.; Tareen, F.K.; Rehman, Z.; Saghir, K.A.; Ashraf, W.; Anjum, S.M.M.; Ahmad, T.; Alqahtani, F.; Imran, I. Probiotics by Modulating Gut–Brain Axis Together With Brivaracetam Mitigate Seizure Progression, Behavioral Incongruities, and Prevented Neurodegeneration in Pentylenetetrazole-Kindled Mice. CNS Neurosci. Ther. 2024, 30, e70078. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.D.M.; Santos, S.S.; Cruz, A.B.O.; de Jesus, M.V.A.C.; Lauria, P.S.S.; Lins, M.P.; Villarreal, C.F. Probiotics as Antioxidant Strategy for Managing Diabetes Mellitus and Its Complications. Antioxidants 2025, 14, 767. [Google Scholar] [CrossRef] [PubMed]
- Aboulgheit, A.; Karbasiafshar, C.; Zhang, Z.; Sabra, M.; Shi, G.; Tucker, A.; Sodha, N.; Abid, M.R.; Sellke, F.W. Lactobacillus plantarum probiotic induces Nrf2-mediated antioxidant signaling and eNOS expression resulting in improvement of myocardial diastolic function. Am. J. Physiol. Circ. Physiol. 2021, 321, H839–H849. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331. [Google Scholar] [PubMed]
- Ciltas, A.C.; Toy, C.E.; Güneş, H.; Yaprak, M. Effects of probiotics on GABA/glutamate and oxidative stress in PTZ-induced acute seizure model in rats. Epilepsy Res. 2023, 195, 107190. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, L.; Cheng, Y.; Lei, W.; Wang, Y.; Liu, X.; Zheng, N.; Shao, L.; Chen, X.; Sun, Y. Probiotics for the treatment of depression and its comorbidities: A systemic review. Front. Cell. Infect. Microbiol. 2023, 13, 1167116. [Google Scholar] [CrossRef]
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: Profound implications for diet and disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Khanna, H.N.; Roy, S.; Shaikh, A.; Bandi, V. Emerging role and place of probiotics in the management of pediatric neurodevelopmental disorders. Euroasian J. Hepato-Gastroenterol. 2022, 12, 102. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Yang, H.-C.; He, C.; Wang, Y.-H.; Zheng, J.; Wang, X.-Y.; Hao, F.-Y.; Feng, C.-W.; Ma, L.; Zhang, Y.-H. Inflammatory Links between Epilepsy and Depression: A Review of Mechanisms and Therapeutic Strategies. Front. Neurosci. 2025, 19, 1614297. [Google Scholar] [CrossRef]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A comprehensive review on the role of the gut microbiome in human neurological disorders. Clin. Microbiol. Rev. 2022, 35, e00338-20. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Shi, J.; Xie, J.; Li, Z.; He, X.; Wei, P.; Sander, J.W.; Zhao, G. The role of neuroinflammation and network anomalies in drug-resistant epilepsy. Neurosci. Bull. 2025, 41, 881–905. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharmacol. Sci. 2023, 44, 442–456. [Google Scholar] [CrossRef] [PubMed]
| Preclinical Studies on Epilepsy–Depression Comorbidity | ||||
|---|---|---|---|---|
| Experimental Model | Key Effects | Proposed Mechanisms | References | Limitations |
| Pentylenetetrazol-kindled rats | ↑ Depressive-like behavior in forced swim test (FST), ↓ hippocampal BDNF | GABAergic dysfunction, neuroinflammation (↑ IL-6, TNF-α) | [126] | Limited to animal models, specific tests, and human translation is difficult. |
| Pilocarpine-induced TLE (rats) | ↑ Anxiety/depression in (elevated plus maze, FST), hippocampal atrophy | Limbic hyperexcitability, HPA axis dysregulation (↑ cortisol) | [127,128,129,130] | Limited to animal models, specific tests, and direct human translation is difficult. |
| Kainic acid-induced status epilepticus (rats) | Persistent depressive phenotypes, synaptic loss in the prefrontal cortex | Glutamate excitotoxicity, ↓ neurogenesis | [131,132] | Limited to animal models, specific findings, and human translation is difficult. |
| Genetic epilepsy (genetic absence epilepsy rats) | Comorbid absence seizures + depression-like behavior | Thalamocortical dysrhythmia (T-type Ca2+ channels), serotonin deficiency | [133,134] | Limited to genetic animal model, specific findings, and human translation. |
| Chronic stress + TLE (mice) | Exacerbated seizure frequency + anhedonia (sucrose preference test) | Neurosteroid withdrawal (↓ allopregnanolone), ↑ CRH in the amygdala | [135,136] | Limited to animal model, specific findings, human translation difficult. |
| Flinders Sensitive Line (rats) | Spontaneous seizures + despair behavior (FST) | Serotonin transporter (SLC6A4) dysfunction, ↑ KP activity | [137] | Limited to animal models, specific findings, and human translation is difficult. |
| WAG/Rij rats (absence epilepsy) | Spike-wave discharges + depressive-like immobility | T-type Ca2+ channel hyperactivity, ↓ noradrenaline in locus coeruleus | [138,139] | Limited to animal models, specific findings, and human translation is difficult. |
| Corticosterone-treated mice | ↑ Seizure susceptibility + despair (FST) | GR receptor resistance, ↓ hippocampal neurogenesis | [129] | Limited to animal models, specific findings, and human translation is difficult. |
| Post-status epilepticus depression model (rats) | Spontaneous seizures + social withdrawal | Neuroinflammation (microglial activation, ↑ IL-1β), ↓ mammalian target of rapamycin signaling | [127,140] | Limited to the rat model of post-status epilepticus, specific behavioral and mechanistic findings, direct translation to human comorbidity is difficult. |
| Dravet syndrome (Scn1a+/− mice) | Severe seizures + anxiety/depression | Nav1.1 channel dysfunction, 5-HT depletion | [141,142] | Limited to the genetic animal model of Dravet syndrome, specific findings, and direct human translation are challenging. |
| Early-life stress + TLE (rats) | ↑ Epileptogenesis + adult depressive behavior | Epigenetic modifications (BDNF methylation), HPA axis hyperactivity | [143,144,145] | Limited to animal models of early-life stress and TLE, specific findings, and direct human translation are challenging. |
| Ketogenic diet (TLE mice) | ↓ Seizures + improved mood (FST) | ↑ GABA/glutamate ratio, ↓ neuroinflammation (IL-6, HMGB1) | [146,147] | Small sample size, short duration, unclear long-term effects, and species-specific responses. |
| IL-1β-infused epilepsy (rats) | Resistant to selective serotonin reuptake inhibitors (SSRIs), persistent depression | KP shift (↑ QUIN), ↓ serotonin synthesis | [128,148] | Single model, acute IL-1β effects, lacks human translation, limited mechanistic depth. |
| Vagus nerve stimulation (TLE rats) | Reduced seizures + antidepressant effects | ↑ Noradrenaline/serotonin in limbic regions, BDNF restoration | [149] | Species-specific, invasive, unclear long-term effects, and lacks human clinical correlation. |
| Fenfluramine-treated (Down syndrome mice) | ↓ Seizures + improved social interaction | Dual 5-HT/sigma-1 receptor modulation, ↑ GABAergic inhibition | [150] | Mouse model only, short-term effects, lacks human data, potential side effects. |
| Clinical Studies on Epilepsy–Depression Comorbidity | ||||
|---|---|---|---|---|
| Study Population | Key Findings | Proposed Mechanisms | References | Limitations |
| Newly diagnosed epilepsy patients | A history of depression is 7× more frequent before epilepsy onset | Shared neurobiological vulnerability (e.g., serotonin dysfunction, HPA axis dysregulation) | [151] | Limited scope, new patients, pre-existing depression focus. |
| Adults with TLE (positron emission tomography study) | Reduced 5-HT1A receptor binding in ipsilateral hippocampus/raphe | Serotonergic deficits in limbic networks | [152] | Limited to TLE adults, specific receptor. |
| Elderly epilepsy patients (≥55 yrs) | Depression is 3.7× more likely to precede epilepsy | Chronic stress-induced hyperexcitability and hippocampal atrophy | [153,154] | Limited to elderly patients, a specific temporal link. |
| Patients with drug-resistant epilepsy | A 55% prevalence of depression correlates with seizure frequency | Neuroinflammation (↑ IL-6, TNF-α), BDNF downregulation | [155,156] | Limited to drug-resistant epilepsy, correlational, specific biomarkers. |
| TLE patients with hippocampal sclerosis | Higher depression rates vs. non-TLE epilepsy | Limbic circuit disruption (amygdala-hippocampus-prefrontal connectivity loss) | [157] | Specific to TLE-HS, focused on limbic circuit disruption. |
| Pediatric epilepsy cohort | 13% depression prevalence; linked to low intelligence quotient/seizure severity | Early-life stress impacts neurodevelopment (e.g., GABA/glutamate imbalance) | [158] | Limited to pediatric cohort, correlational, specific factors, and early stress. |
| Post-stroke epilepsy patients | No correlation between lesion laterality and depression | Network dysfunction beyond structural damage | [159,160] | Limited to post-stroke, no lesion correlation, network dysfunction focus. |
| Post-traumatic stress disorder (PTSD) patients with epilepsy | 78% reported trauma exposure; 26% met PTSD criteria | CRH overactivation and HPA axis hyperactivity | [161,162] | Limited to PTSD-epilepsy, self-reported trauma, and HPA axis focus. |
| SSRI-treated epilepsy patients | Sertraline reduced seizure frequency in 60% of cases | Enhanced serotonergic transmission lowers seizure threshold | [163,164] | Limited to SSRI-treated, partial efficacy, and complex mechanisms. |
| Vagus nerve stimulation-treated refractory epilepsy | Improved mood scores alongside seizure reduction | Noradrenergic/serotonergic modulation via locus coeruleus activation | [165,166] | Limited to VNS-treated, correlational, specific mechanisms. |
| Epilepsy patients post- electroconvulsive therapy | Rapid antidepressant effects, but memory side effects | Seizure-induced neuroplasticity (BDNF upregulation, glutamate normalization) | [167,168] | |
| Genetic epilepsy (SCN1A mutations) | Higher depression scores in mutation carriers | GABAergic interneuron impairment | [169,170] | Limited to SCN1A genetic epilepsy, correlational, and specific mechanisms. |
| Ketogenic diet responders | 40% improvement in mood and seizure control | Anti-inflammatory effects (↓ IL-6), enhanced GABAergic tone | [171,172] | Limited to diet responders, specific mechanisms, not all patients. |
| Epilepsy surgery candidates | Pre-surgical depression predicted worse post-surgical outcomes | Limbic network instability persists despite seizure freedom | [173,174] | Limited to surgery candidates, predictive, network instability focus. |
| Women with catamenial epilepsy | Higher depression rates linked to hormonal fluctuations | Progesterone withdrawal → reduced allopregnanolone | [175,176] | Limited to catamenial epilepsy, hormonal link, and specific mechanisms. |
| Probiotic Strain | Mechanism of Action | Neurobiological/ Clinical Effect |
|---|---|---|
| Lactobacillus rhamnosus JB-1 | Modulates GABA receptors, vagus nerve signaling. | Anxiolytic, antidepressant effects [199]. |
| Bifidobacterium longum | Reduces IL-6, increases BDNF. | Cognitive enhancement, anti-inflammatory [200]. |
| Lactobacillus plantarum | Modulates tryptophan–serotonin pathway. | Mood regulation, synaptic plasticity [201]. |
| Multi-strain mix | ↑ SOD, ↓ MDA, ↓ IL-1β. | Improved oxidative balance and neuroprotection [198,202]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shokr, M.M.; Eladawy, R.M.; Azar, Y.O.; Al Raish, S.M. Probiotics and the Gut–Brain Axis: Emerging Therapeutic Strategies for Epilepsy and Depression Comorbidity. Foods 2025, 14, 2926. https://doi.org/10.3390/foods14172926
Shokr MM, Eladawy RM, Azar YO, Al Raish SM. Probiotics and the Gut–Brain Axis: Emerging Therapeutic Strategies for Epilepsy and Depression Comorbidity. Foods. 2025; 14(17):2926. https://doi.org/10.3390/foods14172926
Chicago/Turabian StyleShokr, Mustafa M., Reem M. Eladawy, Yasmena O. Azar, and Seham M. Al Raish. 2025. "Probiotics and the Gut–Brain Axis: Emerging Therapeutic Strategies for Epilepsy and Depression Comorbidity" Foods 14, no. 17: 2926. https://doi.org/10.3390/foods14172926
APA StyleShokr, M. M., Eladawy, R. M., Azar, Y. O., & Al Raish, S. M. (2025). Probiotics and the Gut–Brain Axis: Emerging Therapeutic Strategies for Epilepsy and Depression Comorbidity. Foods, 14(17), 2926. https://doi.org/10.3390/foods14172926





