Alternative Solvents for Pectin Extraction: Effects of Extraction Agents on Pectin Structural Characteristics and Functional Properties
Abstract
1. Introduction
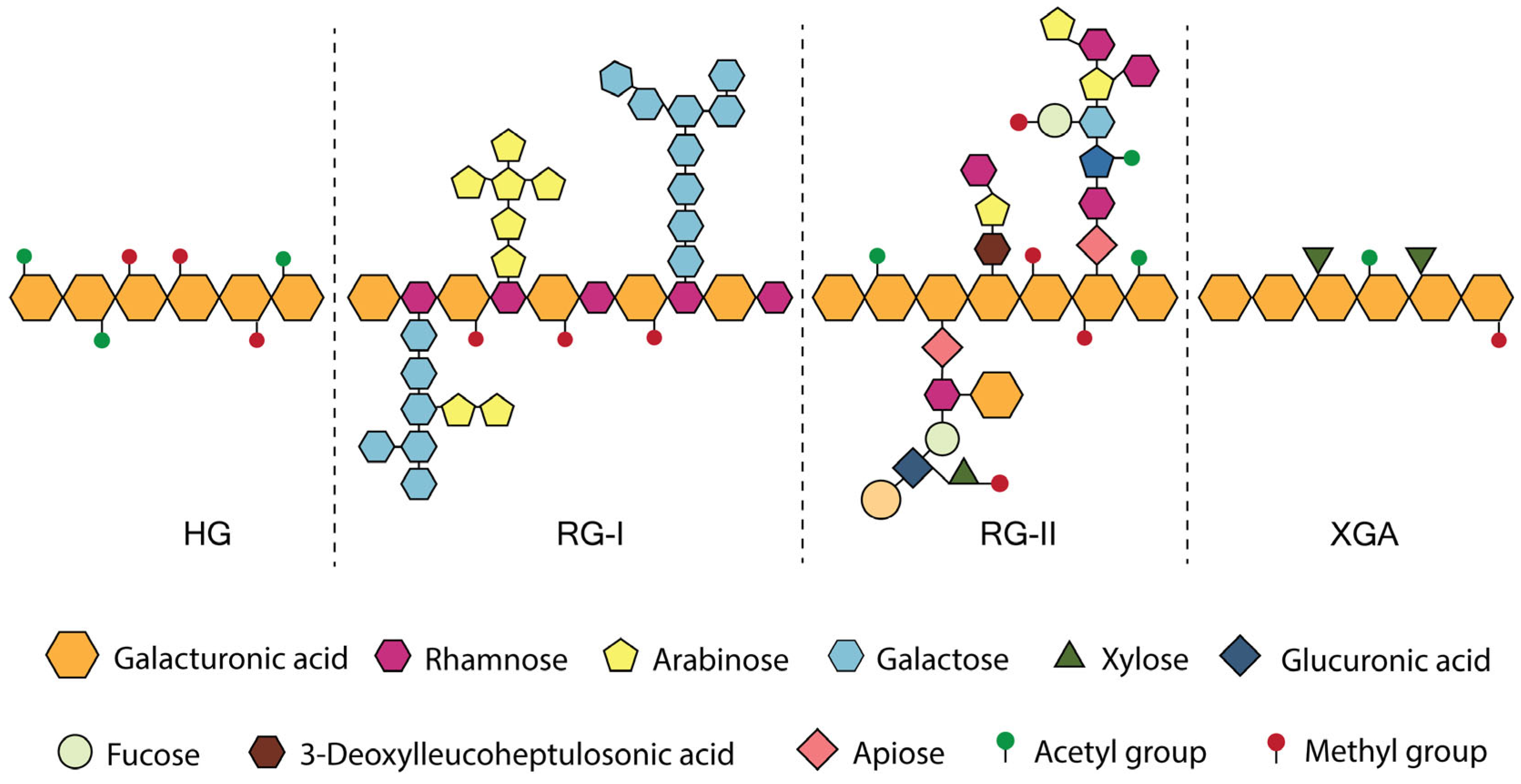
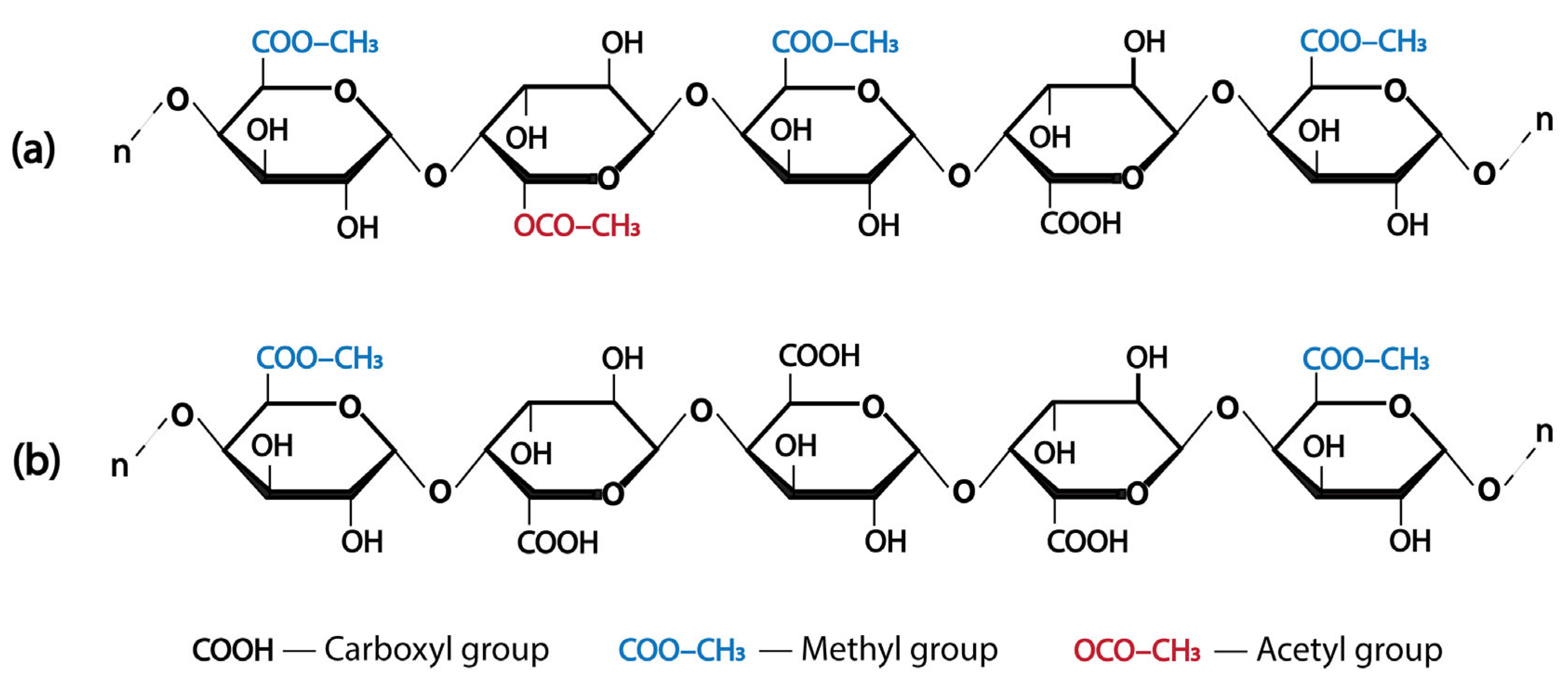
2. Pectin
3. Conventional Pectin Extraction
4. Alternative Solvents for Pectin Extraction
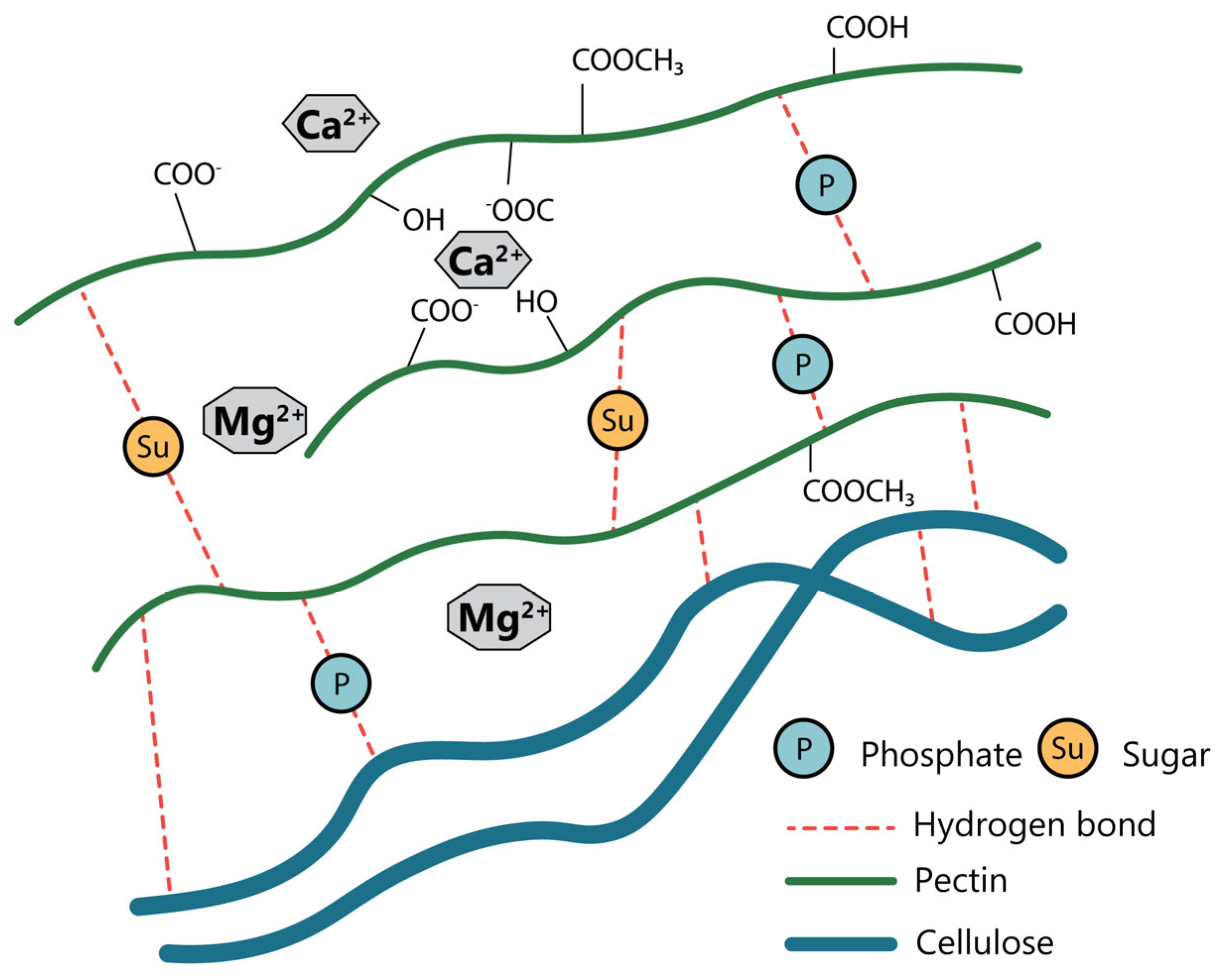
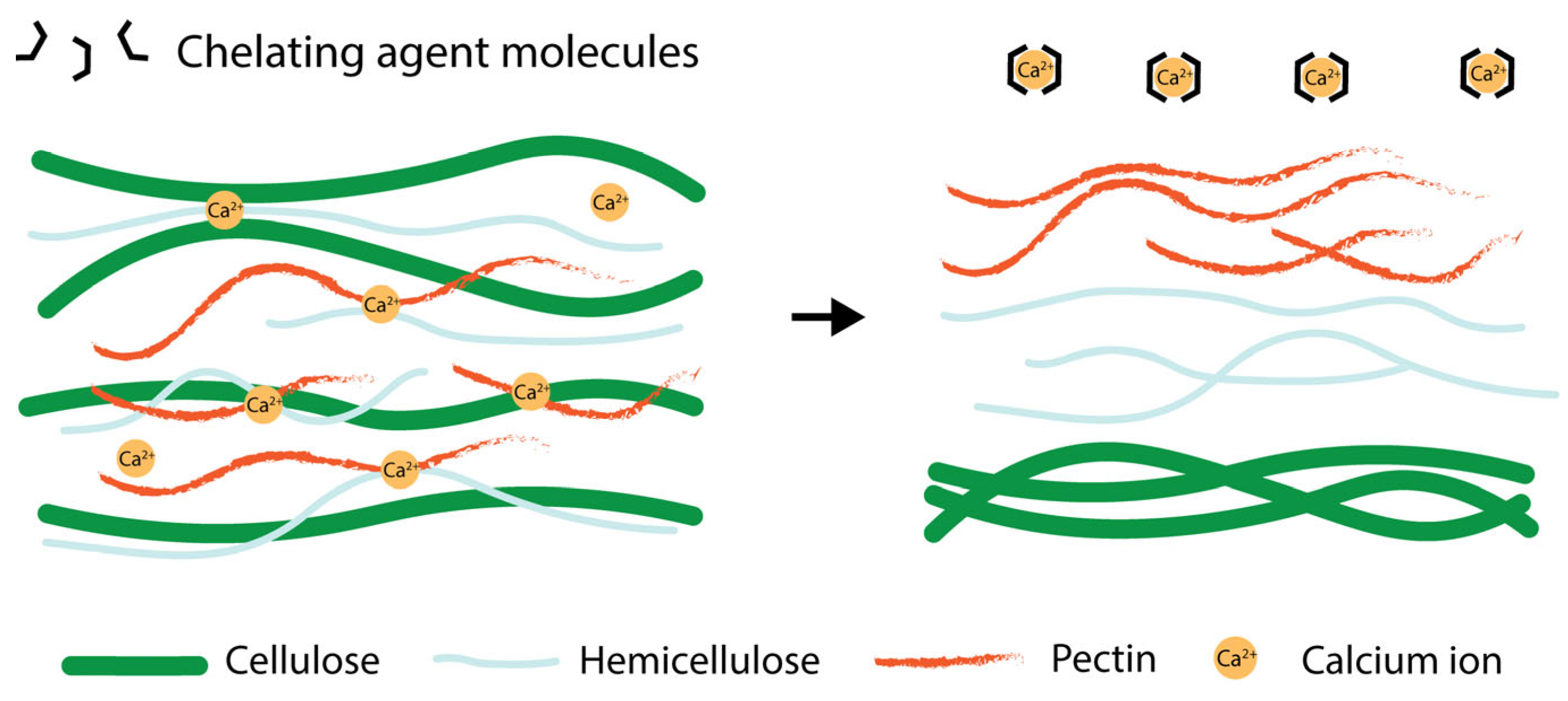
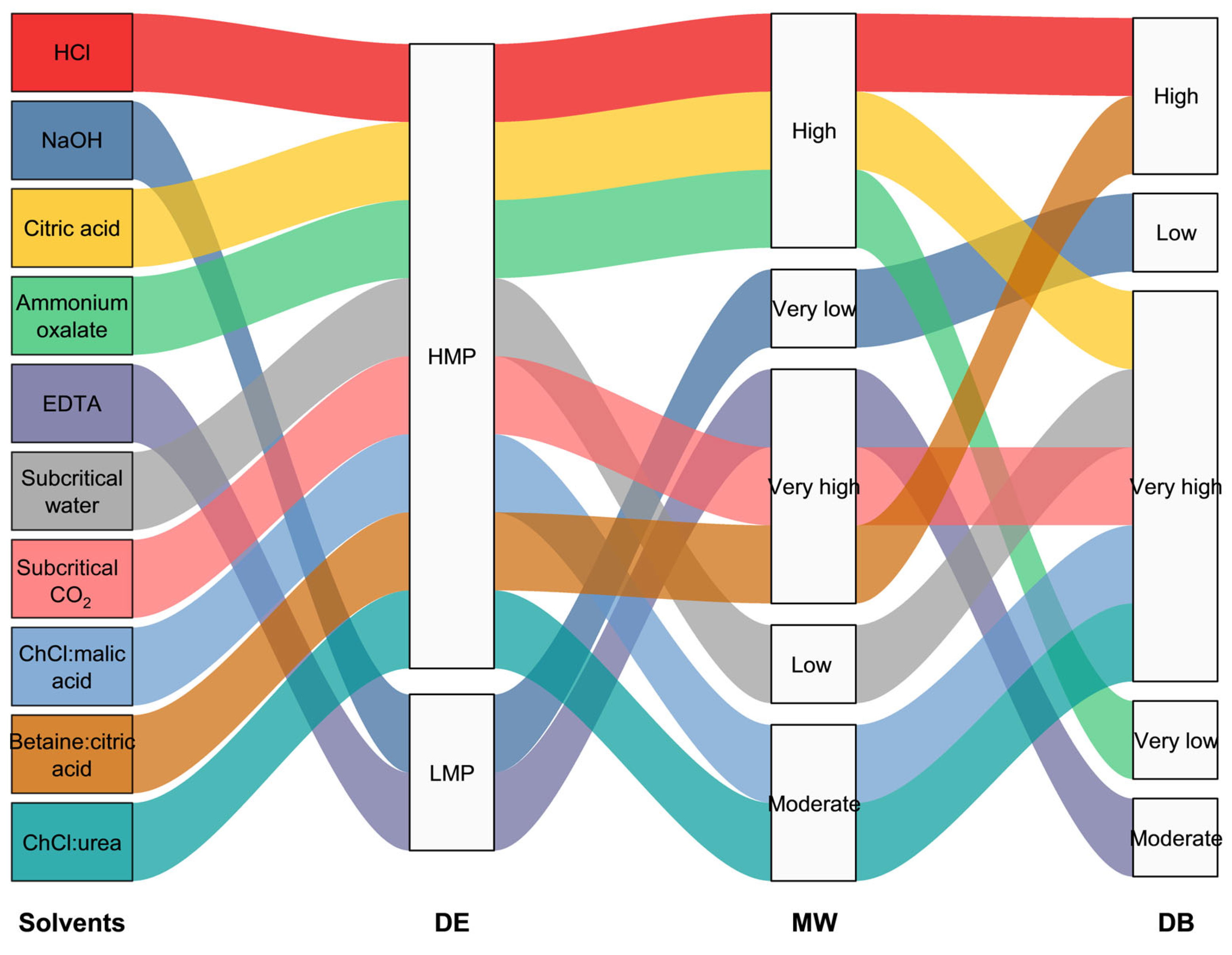
4.1. Chelating Agents
| Materials | Solvents | Extraction Conditions | Yield | Product Structures | Functionality | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| GalA (%) | DE (%) | MW (kDa) | Branching | ||||||
| Orange peel | * HCl, pH 2.5 | 90 °C for 90 min in water bath | 29.6 | - | - | 84.5 | - | - | [68] |
| Ammonium oxalate, 0.25% | 30.1 | 91.4 | |||||||
| EDTA, 0.5% | 27.7 | 102 | |||||||
| Sweet potato residue | * NaOH, pH 11 | SLR 1:25 for 90 min at 80 °C using conventional heating, 2 times | 3.16 | 38.3 | 0.14 | 44.5 | HG 20.5%, RG-I 75.4% | Highest anti-inflammation (1st) | [69] |
| * HCl, pH 3.0 | 2.14 | 54.1 | 14.3 | 69.8 | HG 43.1%, RG-I 46.9% | Anti-inflammation (4th) | |||
| Water | 2.32 | 41.5 | 47.5 | 58.4 | HG 37.1%, RG-I 63.2% | Anti-inflammation (2nd) | |||
| EDTA, 1% | 2.17 | 50.8 | 10.6 | 71.3 | HG 36.57% and RG-I 55.24% | Anti-inflammation (3rd) | |||
| Apple | CDTA, pH 6.5 | Extraction at 80 °C for 30 min | 31.9 | 49.2 | 74.0 | high MW | - | - | [21] |
| Sugar beet | 27.5 | 48.4 | 55.0 | low MW | - | - | |||
| Prickly pear peel | SHMP, 0.75% | Using ohmic heating process with field strength of 12.5 V/cm at 90 °C for 45 min | 3.32 | 72.6 | 21.2 | - | - | - | [71] |
| Mango peel | Citric acid, pH 2.5 | SLR 1:40, for 2 h at 80 °C using conventional heating | 17.9 | Sugar content 55.9% | 66.5 | up to 904 | Highly branched | - | [22] |
| Mango peel | Citric acid, pH 2.5 | SLR 1:40, for 15 min at 80 °C using conventional heating | 16.7 | 52.2 | 86.8 | 2858 | - | Emulsion | [72] |
| Citric acid, pH 2.5 | SLR 1:40, for 15 min at 80 °C using UAE | 17.2 | 53.4 | 86.8 | 2320 | - | |||
| Orange peel | Citric acid, pH 2.41 | Under thermal process at 86.36 °C for 64.85 min | 13.9 | AUA 77.3% | 48.2 | EW 2380 | - | - | [73] |
| Chicory root | * NaOH, pH 12 | SLR 1:20 for 1 h at 85 °C using conventional heating | 13.7 | 57.5 | 4 | 239 | DB 1.3 | Sensitive to calcium ion, rigid gel | [74] |
| Citric acid, pH 2 | 13.3 | 66.2 | 34 | 314 | DB 3.4 | Thickening property | |||
| Sodium citrate, 0.5% | 8.8 | 63.7 | 47.7 | 204 | DB 1.0 | Weak syneresis | |||
| Ammonium oxalate, 0.5% | 14.8 | 71.7 | 31 | 337 | DB 0.7 | Superior hardness, rigid gel | |||
| Sunflower head | Ammonium oxalate, 0.76% | Extraction time 1.34 h, SLR 1:15 | 7.36 | 76.2 | 39.2 | 316 | - | Antioxidant | [24] |
| Tomato waste | Ammonium oxalate/oxalic acid, pH 3.26 | Extraction time 15+15 min at 80 °C with 37 kHz UAE | 34.6 | AUA 57.16% | 89.0 | - | - | - | [75] |
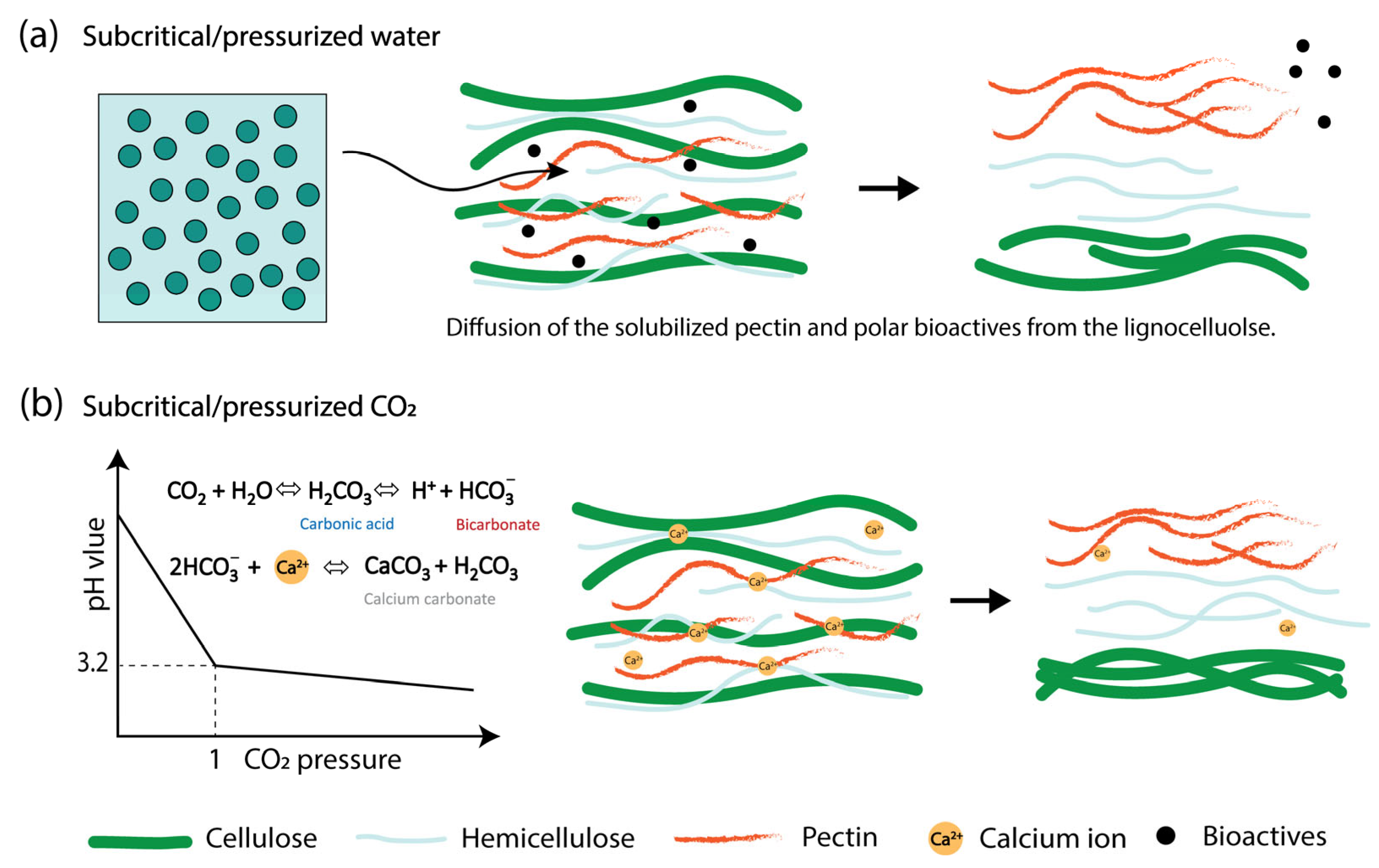
4.2. Subcritical Fluid Extraction
4.3. Supercritical Fluid Extraction
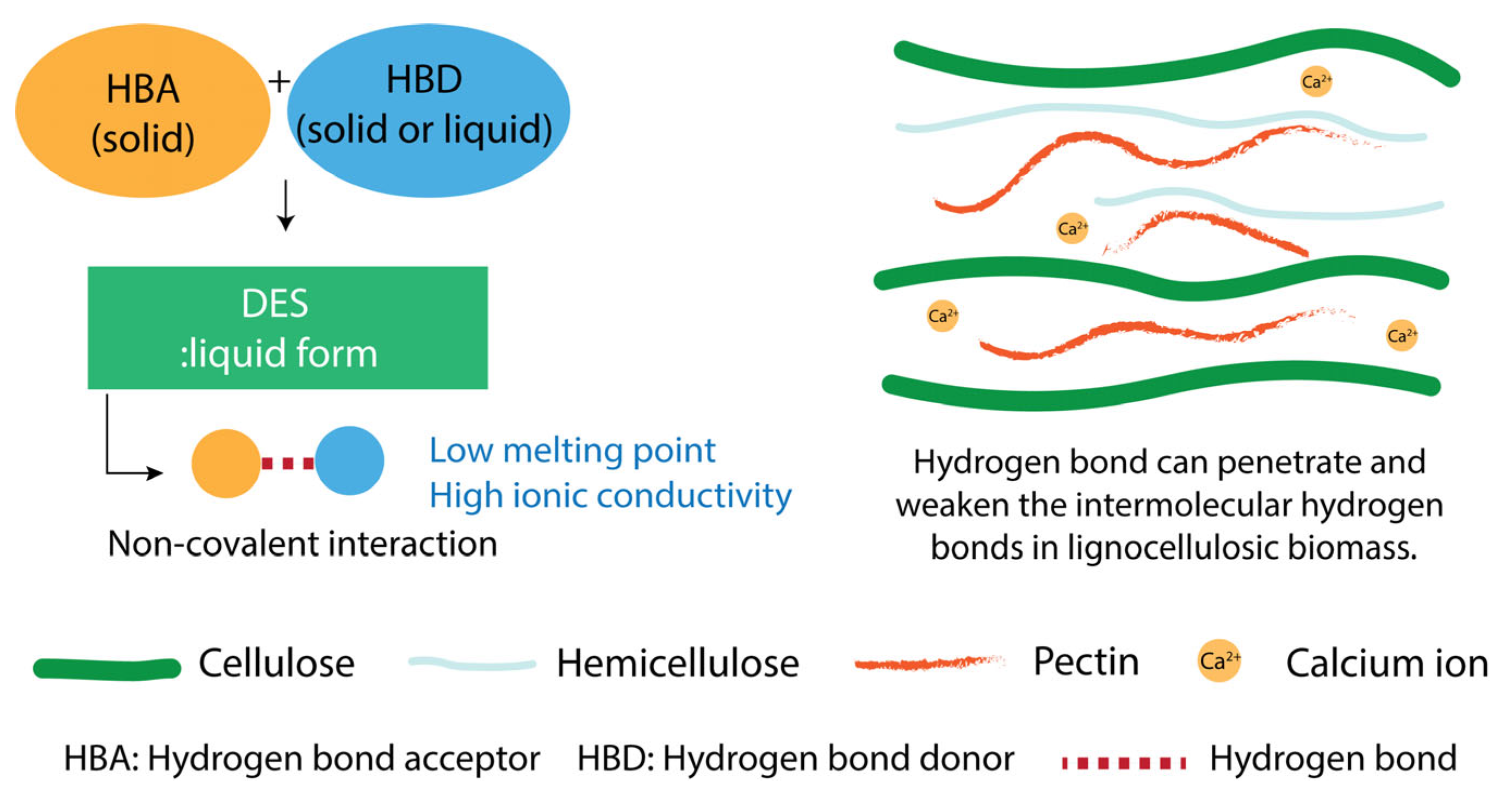
4.4. Deep Eutectic Solvents
5. Functional Properties Related to the Structural Characteristics of Pectin
5.1. Structural Influence on Physicochemical Properties
5.2. Structural Influence on Biological Properties
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUA | Anhydrouronic acid |
| CDTA | Cyclohexane diamine tetraacetic acid |
| DB | Degree of branching |
| DE | Degree of esterification |
| DES | Deep eutectic solvent |
| EAE | Enzyme-assisted extraction |
| EDTA | Ethylenediaminetetraacetic acid |
| EW | Equivalent weight |
| GalA | Galacturonic acid |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HG | Homogalacturonan |
| HMP | High-methoxyl pectin |
| LMP | Low-methoxyl pectin |
| MAE | Microwave-assisted extraction |
| MW | Molecular weight |
| SHMP | Sodium hexametaphosphate |
| SLR | Solid–liquid ratio |
| UAE | Ultrasound-assisted extraction |
| XGA | Xylogalacturonan |
References
- Voragen, A.G.J.; Coenen, G.-J.; Verhoef, R.P.; Schols, H.A. Pectin, a Versatile Polysaccharide Present in Plant Cell Walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Salazar Ripoll, C.S.; Hincapié-Llanos, G.A. Evaluation of Sources and Methods of Pectin Extraction from Fruit and Vegetable Wastes: A Systematic Literature Review (SLR). Food Biosci. 2023, 51, 102278. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, W.-Y.; Chen, T.-T.; Yan, J.-K. Effects of Structural and Conformational Characteristics of Citrus Pectin on Its Functional Properties. Food Chem. 2021, 339, 128064. [Google Scholar] [CrossRef] [PubMed]
- Khubber, S.; Kazemi, M.; Amiri Samani, S.; Lorenzo, J.M.; Simal-Gandara, J.; Barba, F.J. Structural-Functional Variability in Pectin and Effect of Innovative Extraction Methods: An Integrated Analysis for Tailored Applications. Food Rev. Int. 2023, 39, 2352–2377. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Gurak, P.D.; Cladera-Olivera, F.; Marczak, L.D.F.; Karwe, M. Combined Effect of High-Pressure and Conventional Heating on Pectin Extraction from Passion Fruit Peel. Food Bioprocess Technol. 2016, 9, 1021–1030. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Bagherian, H.; Zokaee Ashtiani, F.; Fouladitajar, A.; Mohtashamy, M. Comparisons between Conventional, Microwave- and Ultrasound-Assisted Methods for Extraction of Pectin from Grapefruit. Chem. Eng. Process. Process Intensif. 2011, 50, 1237–1243. [Google Scholar] [CrossRef]
- Şen, E.; Göktürk, E.; Hajiyev, V.; Uğuzdoğan, E. Comparisons of Pulsed Ultrasound-Assisted and Hot-acid Extraction Methods for Pectin Extraction under Dual Acid Mixtures from Onion (Allium cepa L.) Waste. Food Sci. Nutr. 2023, 11, 7320–7329. [Google Scholar] [CrossRef]
- Maneerat, N.; Tangsuphoom, N.; Nitithamyong, A. Effect of Extraction Condition on Properties of Pectin from Banana Peels and Its Function as Fat Replacer in Salad Cream. J. Food Sci. Technol. 2017, 54, 386–397. [Google Scholar] [CrossRef]
- Owusu, F.W.A.; Acquah, P.G.J.; Boakye-Gyasi, M.E.L.; Johnson, R.; Yeboah, G.N.; Archer, M.-A.; Antwi, M.B.; Asare, S.O. Pharmaceutical Assessment of the Impact of the Method of Extraction on the Suitability of Pectin from Plantain (Musa paradisiaca) Peels as a Suspending Agent in Oral Liquid Formulations. Sci. World J. 2023, 2023, 8898045. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M. Lemon Juice Improves the Extractability and Quality Characteristics of Pectin from Yellow Passion Fruit By-Product as Compared with Commercial Citric Acid Extractant. Bioresour. Technol. 2009, 100, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The Emulsifying and Emulsion-Stabilizing Properties of Pectin: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Öztürk, T.; Özbek, H.N.; Koçak Yanık, D. Environmentally Friendly Approach to Pectin Extraction from Grapefruit Peel: Microwave-Assisted High-Pressure CO2/H2O. Foods 2024, 13, 476. [Google Scholar] [CrossRef]
- Min, B.; Lim, J.; Ko, S.; Lee, K.-G.; Lee, S.H.; Lee, S. Environmentally Friendly Preparation of Pectins from Agricultural Byproducts and Their Structural/Rheological Characterization. Bioresour. Technol. 2011, 102, 3855–3860. [Google Scholar] [CrossRef]
- Rahmani, Z.; Khodaiyan, F.; Kazemi, M.; Sharifan, A. Optimization of Microwave-Assisted Extraction and Structural Characterization of Pectin from Sweet Lemon Peel. Int. J. Biol. Macromol. 2020, 147, 1107–1115. [Google Scholar] [CrossRef]
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave Assisted Extraction of Pectin from Waste Citrullus lanatus Fruit Rinds. Carbohydr. Polym. 2014, 101, 786–791. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing Green Methods for Pectin Extraction from Waste Orange Peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- Elgharbawy, A.; Hayyan, A.; Hayyan, M.; Mirghani, M.; Salleh, H.; Rashid, S.; Ngoh, G.; Liew, S.; Nor, M.; bin Mohd Yusoff, M.Y.Z.; et al. Natural Deep Eutectic Solvent-Assisted Pectin Extraction from Pomelo Peel Using Sonoreactor: Experimental Optimization Approach. Processes 2019, 7, 416. [Google Scholar] [CrossRef]
- Van Audenhove, J.; Bernaerts, T.; De Smet, V.; Delbaere, S.; Van Loey, A.M.; Hendrickx, M.E. The Structure and Composition of Extracted Pectin and Residual Cell Wall Material from Processing Tomato: The Role of a Stepwise Approach versus High-Pressure Homogenization-Facilitated Acid Extraction. Foods 2021, 10, 1064. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Thibault, J.-F. Structure and Properties of Apple and Sugar-Beet Pectins Extracted by Chelating Agents. Carbohydr. Res. 1993, 244, 99–114. [Google Scholar] [CrossRef]
- Jamsazzadeh Kermani, Z.; Shpigelman, A.; Kyomugasho, C.; Van Buggenhout, S.; Ramezani, M.; Van Loey, A.M.; Hendrickx, M.E. The Impact of Extraction with a Chelating Agent under Acidic Conditions on the Cell Wall Polymers of Mango Peel. Food Chem. 2014, 161, 199–207. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Lü, X. Pectin Extracted from Apple Pomace and Citrus Peel by Subcritical Water. Food Hydrocoll. 2014, 38, 129–137. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Jing, J.; Zhao, Q.; Ren, L.; Hu, Z. Optimization of Sunflower Head Pectin Extraction by Ammonium Oxalate and the Effect of Drying Conditions on Properties. Sci. Rep. 2021, 11, 10616. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lahaye, M. Natural Deep Eutectic Solvents Pretreatment as an Aid for Pectin Extraction from Apple Pomace. Food Hydrocoll. 2021, 115, 106601. [Google Scholar] [CrossRef]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Dang, G.; Li, J.; Yin, C.; Wang, W.; Zhang, K.; Zhong, R.; Chen, L.; Zhang, H.; Schroyen, M. Deciphering Pectin: A Comprehensive Overview of Its Origins, Processing, and Promising Utility. ACS Omega 2025, 10, 1–15. [Google Scholar] [CrossRef]
- Zandleven, J.; Sørensen, S.O.; Harholt, J.; Beldman, G.; Schols, H.A.; Scheller, H.V.; Voragen, A.J. Xylogalacturonan Exists in Cell Walls from Various Tissues of Arabidopsis thaliana. Phytochemistry 2007, 68, 1219–1226. [Google Scholar] [CrossRef]
- Thibault, J.-F.; Renard, C.M.G.C.; Axelos, M.A.V.; Roger, P.; Crépeau, M.-J. Studies of the Length of Homogalacturonic Regions in Pectins by Acid Hydrolysis. Carbohydr. Res. 1993, 238, 271–286. [Google Scholar] [CrossRef]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of Pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The Primary, Secondary, and Structures of Higher Levels of Pectin Polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; McCartney, L.; Steele-King, C.G.; Marcus, S.E.; Mort, A.; Huisman, M.; van Alebeek, G.-J.; Schols, H.A.; Voragen, A.G.J.; Le Goff, A.; et al. A Xylogalacturonan Epitope Is Specifically Associated with Plant Cell Detachment. Planta 2004, 218, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Ghanbarzadeh, B.; Almasi, H. Biodegradable Polymers. In Biodegradation—Life of Science; InTech: London, UK, 2013. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K.; Rao, M.A. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Pinkaew, T.; Inthachat, W.; Khemthong, C.; Kemsawasd, V.; On-Nom, N.; Temviriyanukul, P. High Pectin Recovery from Cocoa Husks Using an Autoclave Approach: An Analysis of Its Physicochemical, Structural, and Genotoxicity Properties. Foods 2024, 13, 669. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Li, J.; Yan, L.; Li, S.; Ye, X.; Liu, D.; Ding, T.; Linhardt, R.J.; Orfila, C.; et al. Extraction and Characterization of RG-I Enriched Pectic Polysaccharides from Mandarin Citrus Peel. Food Hydrocoll. 2018, 79, 579–586. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Williams, M.A.K.; Tanhatan-Nasseri, A.; Ropartz, D.; Quéméner, B.; Bonnin, E. Innovative Enzymatic Approach to Resolve Homogalacturonans Based on Their Methylesterification Pattern. Biomacromolecules 2012, 13, 1615–1624. [Google Scholar] [CrossRef]
- May, C.D. Industrial Pectins: Sources, Production and Applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- Khamsucharit, P.; Laohaphatanalert, K.; Gavinlertvatana, P.; Sriroth, K.; Sangseethong, K. Characterization of Pectin Extracted from Banana Peels of Different Varieties. Food Sci. Biotechnol. 2018, 27, 623–629. [Google Scholar] [CrossRef]
- Gurev, A.; Cesko, T.; Dragancea, V.; Ghendov-Mosanu, A.; Pintea, A.; Sturza, R. Ultrasound- and Microwave-Assisted Extraction of Pectin from Apple Pomace and Its Effect on the Quality of Fruit Bars. Foods 2023, 12, 2773. [Google Scholar] [CrossRef]
- Picot-Allain, M.C.N.; Ramasawmy, B.; Emmambux, M.N. Extraction, Characterisation, and Application of Pectin from Tropical and Sub-Tropical Fruits: A Review. Food Rev. Int. 2022, 38, 282–312. [Google Scholar] [CrossRef]
- Yang, J.-S.; Mu, T.-H.; Ma, M.-M. Extraction, Structure, and Emulsifying Properties of Pectin from Potato Pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef]
- Chandel, V.; Biswas, D.; Roy, S.; Vaidya, D.; Verma, A.; Gupta, A. Current Advancements in Pectin: Extraction, Properties and Multifunctional Applications. Foods 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Pagán, J.; Ibarz, A.; Llorca, M.; Pagán, A.; Barbosa-Cánovas, G.V. Extraction and Characterization of Pectin from Stored Peach Pomace. Food Res. Int. 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Joslyn, M.A. The Chemistry of Protopectin: A Critical Review of Historical Data and Recent Developments. In Advances in Food Research; Elsevier: Amsterdam, The Netherlands, 1963; pp. 1–107. [Google Scholar]
- Kondratenko, V.V.; Kondratenko, T.Y. Enzyme Systems for Fragmentation of the Rhamnogalacturonan Sites Main Chains in Plant Tissue Protopectin Complex. Food Syst. 2023, 6, 188–201. [Google Scholar] [CrossRef]
- Sucharipa, R. Protopectin and Some Other Constituents of Lemon Peel. J. Am. Chem. Soc. 1924, 46, 145–156. [Google Scholar] [CrossRef]
- Sakai, T.; Ozaki, Y. Protopectin Solubilizing Enzyme That Does Not Catalyze the Degradation of Polygalacturonic Acid. Agric. Biol. Chem. 1988, 52, 1091–1093. [Google Scholar] [CrossRef]
- Belkheiri, A.; Forouhar, A.; Ursu, A.V.; Dubessay, P.; Pierre, G.; Delattre, C.; Djelveh, G.; Abdelkafi, S.; Hamdami, N.; Michaud, P. Extraction, Characterization, and Applications of Pectins from Plant By-Products. Appl. Sci. 2021, 11, 6596. [Google Scholar] [CrossRef]
- Ueno, H.; Tanaka, M.; Hosino, M.; Sasaki, M.; Goto, M. Extraction of Valuable Compounds from the Flavedo of Citrus junos Using Subcritical Water. Sep. Purif. Technol. 2008, 62, 513–516. [Google Scholar] [CrossRef]
- Mao, G.; Wu, D.; Wei, C.; Tao, W.; Ye, X.; Linhardt, R.J.; Orfila, C.; Chen, S. Reconsidering Conventional and Innovative Methods for Pectin Extraction from Fruit and Vegetable Waste: Targeting Rhamnogalacturonan I. Trends Food Sci. Technol. 2019, 94, 65–78. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Thibault, J.-F. Pectins in Mild Alkaline Conditions: β-Elimination and Kinetics of Demethylation. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 603–608. [Google Scholar]
- Nopiani, Y.; Hamzah, F.H.; Pato, U.; Zamzani, M. The Effect of Extraction Temperature on the Physicochemical Characteristics of Pectin from Banana Bunch. IOP Conf. Ser. Earth Environ. Sci. 2024, 1364, 012073. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and Structural Composition of Pomelo Peel Pectins Extracted under Acidic and Alkaline Conditions. Food Hydrocoll. 2019, 87, 237–244. [Google Scholar] [CrossRef]
- Cui, J.; Ren, W.; Zhao, C.; Gao, W.; Tian, G.; Bao, Y.; Lian, Y.; Zheng, J. The Structure–Property Relationships of Acid- and Alkali-Extracted Grapefruit Peel Pectins. Carbohydr. Polym. 2020, 229, 115524. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, D.; Zhang, Y.; Ma, M.; Shang, M.; Zhao, C.; Lu, X.; Zhao, C.; Zheng, J. Structural Characteristics and Gelling Properties of Citrus Pectins after Chemical and Enzymatic Modifications: Conformation Plays a Vital Role in Ca2+-Induced Gelation. Food Chem. 2024, 459, 140370. [Google Scholar] [CrossRef]
- Wang, T.; Tao, Y.; Lai, C.; Huang, C.; Ling, Z.; Yong, Q. Influence of Extraction Methods on Navel Orange Peel Pectin: Structural Characteristics, Antioxidant Activity and Cytoprotective Capacity. Int. J. Food Sci. Technol. 2023, 58, 1382–1393. [Google Scholar] [CrossRef]
- Peng, X.; Mu, T.; Zhang, M.; Sun, H.; Chen, J.; Yu, M. Optimisation of Production Yield by Ultrasound-/Microwave-Assisted Acid Method and Functional Property of Pectin from Sugar Beet Pulp. Int. J. Food Sci. Technol. 2015, 50, 758–765. [Google Scholar] [CrossRef]
- Forouhar, A.; Hamdami, N.; Djelveh, G.; Gardarin, C.; Pierre, G.; Ursu, A.V.; Michaud, P. The Effect of Ultrasound Pretreatment on Pectin Extraction from Watermelon Rind Using Microwave-Assisted Extraction. Appl. Sci. 2023, 13, 5558. [Google Scholar] [CrossRef]
- Adetunji, L.R.; Adekunle, A.; Orsat, V.; Raghavan, V. Advances in the Pectin Production Process Using Novel Extraction Techniques: A Review. Food Hydrocoll. 2017, 62, 239–250. [Google Scholar] [CrossRef]
- Wikiera, A.; Mika, M.; Grabacka, M. Multicatalytic Enzyme Preparations as Effective Alternative to Acid in Pectin Extraction. Food Hydrocoll. 2015, 44, 156–161. [Google Scholar] [CrossRef]
- Bethke, G.; Glazebrook, J. Cyclohexane Diamine Tetraacetic Acid (CDTA) Extraction of Plant Cell Wall Pectin. Bio Protoc. 2014, 4, e1357. [Google Scholar] [CrossRef]
- Habibi, Y.; Heyraud, A.; Mahrouz, M.; Vignon, M.R. Structural Features of Pectic Polysaccharides from the Skin of Opuntia ficus-indica Prickly Pear Fruits. Carbohydr. Res. 2004, 339, 1119–1127. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of Citrus Pectin Samples Extracted under Different Conditions: Influence of Acid Type and pH of Extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Ravn, H.C.; Meyer, A.S. Chelating Agents Improve Enzymatic Solubilization of Pectinaceous Co-Processing Streams. Process Biochem. 2014, 49, 250–257. [Google Scholar] [CrossRef]
- Kar, F.; Arslan, N. Effect of Temperature and Concentration on Viscosity of Orange Peel Pectin Solutions and Intrinsic Viscosity–Molecular Weight Relationship. Carbohydr. Polym. 1999, 40, 277–284. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, J.; Li, Q.; Liu, C.; Yue, R.; Zhang, Y.; Niu, F.; Zhu, H.; Ma, C.; Deng, S. Effects of Different Extraction Solvents on the Compositions, Primary Structures, and Anti-Inflammatory Activity of Pectin from Sweet Potato Processing By-Products. Carbohydr. Polym. 2025, 347, 122766. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, C. Microwave-Assisted Chelating Agent Extraction of Pectin from Hawthorn Wine Pomace. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 042003. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.A.; Contreras-Esquivel, J.C.; Benítez, B.J.L.; Morales-Oyervides, L.; Aguirre-Loredo, R.Y.; Montañez, J. Ohmic Heating Technology for the Extraction of Chelating Soluble Pectin from Red Prickly Pear (Opuntia lasiacantha P.) Peel Biomass. Food Bioprocess Technol. 2025, 18, 2648–2660. [Google Scholar] [CrossRef]
- Wang, M.; Huang, B.; Fan, C.; Zhao, K.; Hu, H.; Xu, X.; Pan, S.; Liu, F. Characterization and Functional Properties of Mango Peel Pectin Extracted by Ultrasound Assisted Citric Acid. Int. J. Biol. Macromol. 2016, 91, 794–803. [Google Scholar] [CrossRef]
- Iñiguez-Moreno, M.; Pizaña-Aranda, J.J.P.; Ramírez-Gamboa, D.; Ramírez-Herrera, C.A.; Araújo, R.G.; Flores-Contreras, E.A.; Iqbal, H.M.N.; Parra-Saldívar, R.; Melchor-Martínez, E.M. Enhancing Pectin Extraction from Orange Peel through Citric Acid-Assisted Optimization Based on a Dual Response. Int. J. Biol. Macromol. 2024, 263, 130230. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Pi, F.; Zhang, T.; Ai, C.; Yu, S. Rheological Characterization of RG-I Chicory Root Pectin Extracted by Hot Alkali and Chelators. Int. J. Biol. Macromol. 2020, 164, 759–770. [Google Scholar] [CrossRef]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound Assisted Extraction and Characterization of Pectin from Tomato Waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Wongthaweewatana, I.; Srinophakun, T.R.; Saramala, I.; Kasemwong, K. Production of Milk Analogues from Rice Bran Protein Hydrolysate Using the Subcritical Water Technique. Food Sci. Technol. 2021, 41, 722–729. [Google Scholar] [CrossRef]
- Tsuru, C.; Umada, A.; Noma, S.; Demura, M.; Hayashi, N. Extraction of Pectin from Satsuma Mandarin Orange Peels by Combining Pressurized Carbon Dioxide and Deionized Water: A Green Chemistry Method. Food Bioprocess Technol. 2021, 14, 1341–1348. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of Protein and Amino Acids from Deoiled Rice Bran by Subcritical Water Hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Ruthes, A.C.; Martínez-Abad, A.; Tan, H.-T.; Bulone, V.; Vilaplana, F. Sequential Fractionation of Feruloylated Hemicelluloses and Oligosaccharides from Wheat Bran Using Subcritical Water and Xylanolytic Enzymes. Green Chem. 2017, 19, 1919–1931. [Google Scholar] [CrossRef]
- Pattarapisitporn, A.; Noma, S.; Klangpetch, W.; Demura, M.; Hayashi, N. Extraction of Citrus Pectin Using Pressurized Carbon Dioxide and Production of Its Oligosaccharides. Food Biosci. 2024, 57, 103584. [Google Scholar] [CrossRef]
- Kumar, P.; Kermanshahi-pour, A.; Brar, S.K.; Brooks, M.S. Conversion of Lignocellulosic Biomass to Reducing Sugars in High Pressure and Supercritical Fluids: Greener Alternative for Biorefining of Renewables. Adv. Sustain. Syst. 2021, 5, 2000275. [Google Scholar] [CrossRef]
- Duan, X.; Zhu, Y.; Shu, C.; Gao, J.; Liu, F.; Pan, S. Extraction of Pectin from Satsuma Mandarin Peel: A Comparison of High Hydrostatic Pressure and Conventional Extractions in Different Acids. Molecules 2022, 27, 3747. [Google Scholar] [CrossRef]
- Anoraga, S.B.; Shamsudin, R.; Hamzah, M.H.; Sharif, S.; Saputro, A.D.; Basri, M.S.M. Optimization of Subcritical Water Extraction for Pectin Extraction from Cocoa Pod Husks Using the Response Surface Methodology. Food Chem. 2024, 459, 140355. [Google Scholar] [CrossRef]
- Jafarzadeh-Moghaddam, M.; Shaddel, R.; Peighambardoust, S.H. Sugar Beet Pectin Extracted by Ultrasound or Conventional Heating: A Comparison. J. Food Sci. Technol. 2021, 58, 2567–2578. [Google Scholar] [CrossRef]
- Pedraza-Guevara, S.; do Nascimento, R.F.; Canteri, M.H.G.; Muñoz-Almagro, N.; Villamiel, M.; Fernández-Ponce, M.T.; Cardoso, L.C.; Mantell, C.; Martinez de la Ossa, E.J.; Ibañez, E. Valorization of Unripe Papaya for Pectin Recovery by Conventional Extraction and Compressed Fluids. J. Supercrit. Fluids 2021, 171, 105133. [Google Scholar] [CrossRef]
- Pavlić, B.; Vidović, S.; Vladić, J.; Radosavljević, R.; Zeković, Z. Isolation of Coriander (Coriandrum sativum L.) Essential Oil by Green Extractions versus Traditional Techniques. J. Supercrit. Fluids 2015, 99, 23–28. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical Fluid Extraction: Recent Advances and Applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Dubey, K.K.; Marathe, S.J.; Singhal, R. Supercritical Fluid Extraction of Bioactives from Fruit Waste and Its Therapeutic Potential. Food Biosci. 2023, 52, 102418. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Hatakeyama, H.; Hatakeyama, T. Influence of Pectin Modification on Water Binding Properties. Food Hydrocoll. 2012, 27, 494–502. [Google Scholar] [CrossRef]
- Wang, H.; Shuai, X.; Ye, S.; Zhang, R.; Wu, M.; Jiang, S.; Li, Y.; Wu, D.; He, J. Recent Advances in the Development of Bitter Gourd Seed Oil: From Chemical Composition to Potential Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 10678–10690. [Google Scholar] [CrossRef]
- Páramos, P.R.S.; Granjo, J.F.O.; Corazza, M.L.; Matos, H.A. Extraction of High Value Products from Avocado Waste Biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Casquete, R.; de Guía Córdoba, M.; Benito, M.J.; Hernández, A.; Ruiz-Moyano, S.; Martín, A. Functional Properties of Extracts and Residual Dietary Fibre from Pomegranate (Punica granatum L.) Peel Obtained with Different Supercritical Fluid Conditions. LWT 2021, 145, 111305. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Benvenutti, L.; Sanchez-Camargo, A.d.P.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as Potential Solvents for Anthocyanin and Pectin Extraction from Myrciaria cauliflora Fruit By-Product: In Silico and Experimental Approaches for Solvent Selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Kim, K.H.; Dutta, T.; Sun, J.; Simmons, B.; Singh, S. Biomass Pretreatment Using Deep Eutectic Solvents from Lignin Derived Phenols. Green Chem. 2018, 20, 809–815. [Google Scholar] [CrossRef]
- Liew, S.Q.; Ngoh, G.C.; Yusoff, R.; Teoh, W.H. Acid and Deep Eutectic Solvent (DES) Extraction of Pectin from Pomelo (Citrus grandis (L.) Osbeck) Peels. Biocatal. Agric. Biotechnol. 2018, 13, 1–11. [Google Scholar] [CrossRef]
- Haniman, A.; Elgharbawy, A.A.M.; Wae-Hayee, M.; Mohd Noor, N.A.; Jawahar, B.M.; Hussain, M.; Dewi, I.A. Clean Extraction of Pectin from Dragon Fruit Peels, Pomelo Peels, Okra, and Pineapple Peels Using Deep Eutectic Solvents and Ionic Liquids. Halalsphere 2024, 4, 23–33. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C.-Y. Deep Eutectic Solvents (DES) Mediated Extraction of Pectin from Averrhoa bilimbi: Optimization and Characterization Studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef]
- Turan, O.; Isci, A.; Yılmaz, M.S.; Tolun, A.; Sakiyan, O. Microwave-Assisted Extraction of Pectin from Orange Peel Using Deep Eutectic Solvents. Sustain. Chem. Pharm. 2024, 37, 101352. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, L.; Li, S.; Meng, T.; Wang, L.; Zhang, W. The Effect of Sonication-Synergistic Natural Deep Eutectic Solvents on Extraction Yield, Structural and Physicochemical Properties of Pectins Extracted from Mango Peels. Ultrason. Sonochem. 2022, 86, 106045. [Google Scholar] [CrossRef]
- Fu, M.; Sun, X.; Fei, C.; Li, D.; Zhang, D.; Tuo, X.; Gao, S.; Han, X.; Xiu, J.; Wang, J.; et al. Optimization and Characterization of Pectin Extracted from Hawthorn by Deep Eutectic Solvent. Int. J. Biol. Macromol. 2024, 256, 128688. [Google Scholar] [CrossRef]
- Liu, H.; Lin, J.; Hu, Y.; Lei, H.; Zhang, Q.; Tao, X.; Zhang, D.; Niu, H. Deep Eutectic Solvent (DES)-Assisted Extraction of Pectin from Ficus carica Linn. Peel: Optimization, Partial Structure Characterization, Functional and Antioxidant Activities. J. Sci. Food Agric. 2024, 104, 5149–5162. [Google Scholar] [CrossRef] [PubMed]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Subcritical Water Extraction (SWE) Modified by Deep Eutectic Solvent (DES) for Pectin Recovery from a Brazilian Berry By-Product. J. Supercrit. Fluids 2022, 189, 105729. [Google Scholar] [CrossRef]
- Vithanage, C.R.; Grimson, M.J.; Wills, P.R.; Harrison, P.; Smith, B.G. Rheological and Structural Properties of High-Methoxyl Esterified, Low-Methoxyl Esterified and Low-Methoxyl Amidated Pectin Gels. J. Texture Stud. 2010, 41, 899–927. [Google Scholar] [CrossRef]
- Guo, R.; Fan, R.; Hu, J.; Zhang, X.; Han, L.; Wang, M.; He, C. Valorization of Apple Pomace: Structural and Rheological Characterization of Low-Methoxyl Pectins Extracted with Green Agents of Citric Acid/Sodium Citrate. Food Chem. X 2024, 24, 102010. [Google Scholar] [CrossRef]
- Khubber, S.; Chaturvedi, K.; Thakur, N.; Sharma, N.; Yadav, S.K. Low-Methoxyl Pectin Stabilizes Low-Fat Set Yoghurt and Improves Their Physicochemical Properties, Rheology, Microstructure and Sensory Liking. Food Hydrocoll. 2021, 111, 106240. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, S. The Effect of Degree of Esterification of Pectin on the Interaction between Pectin and Wheat Gluten Protein. Food Hydrocoll. 2023, 136, 108272. [Google Scholar] [CrossRef]
- Oakenfull, D.; Scott, A. Hydrophobic Interaction in the Gelation of High Methoxyl Pectins. J. Food Sci. 1984, 49, 1093–1098. [Google Scholar] [CrossRef]
- Byun, C.; Zheng, Y.; Pierce, A.; Wagner, W.L.; Scheller, H.V.; Mohnen, D.; Ackermann, M.; Mentzer, S.J. The Effect of Calcium on the Cohesive Strength and Flexural Properties of Low-Methoxyl Pectin Biopolymers. Molecules 2019, 25, 75. [Google Scholar] [CrossRef]
- Liang, R.; Wang, L.; Chen, J.; Liu, W.; Liu, C. Alkylated Pectin: Synthesis, Characterization, Viscosity and Emulsifying Properties. Food Hydrocoll. 2015, 50, 65–73. [Google Scholar] [CrossRef]
- Jiang, W.; Qi, J.-R.; Huang, Y.; Zhang, Y.; Yang, X.-Q. Emulsifying Properties of High Methoxyl Pectins in Binary Systems of Water-Ethanol. Carbohydr. Polym. 2020, 229, 115420. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, B.; Pan, Y.; Li, X.-M.; Cheng, J.-S.; Chen, H.-Q. Effects of Pectin with Different Molecular Weight on Gelatinization Behavior, Textural Properties, Retrogradation and In Vitro Digestibility of Corn Starch. Food Chem. 2018, 264, 58–63. [Google Scholar] [CrossRef]
- Katsuda, M.S.; McClements, D.J.; Miglioranza, L.H.S.; Decker, E.A. Physical and Oxidative Stability of Fish Oil-in-Water Emulsions Stabilized with β-Lactoglobulin and Pectin. J. Agric. Food Chem. 2008, 56, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, X.; Du, Y.; Tang, P.; Chen, L.; Chen, W.; Zheng, Z. Pectins Rich in RG-I Extracted from Watermelon Peel: Physicochemical, Structural, Emulsifying, and Antioxidant Properties. Foods 2024, 13, 2338. [Google Scholar] [CrossRef]
- Chen, T.-T.; Zhang, Z.-H.; Wang, Z.-W.; Chen, Z.-L.; Ma, H.; Yan, J.-K. Effects of Ultrasound Modification at Different Frequency Modes on Physicochemical, Structural, Functional, and Biological Properties of Citrus Pectin. Food Hydrocoll. 2021, 113, 106484. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H. Ultrasonic Degradation of Sweet Potato Pectin and Its Antioxidant Activity. Ultrason. Sonochem. 2017, 38, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, S.; Waterhouse, G.I.N.; Zhou, T.; Du, Y.; Sun-Waterhouse, D.; Wu, P. Yeast Fermentation of Apple and Grape Pomaces Affects Subsequent Aqueous Pectin Extraction: Composition, Structure, Functional and Antioxidant Properties of Pectins. Food Hydrocoll. 2022, 133, 107945. [Google Scholar] [CrossRef]
- Wu, D.; Zheng, X.; Hu, W.; Zhu, K.; Yu, C.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Anti-Inflammation Effects of Highly Purified Low-Mw RG-I Pectins on LPS-Activated Macrophages. Bioact. Carbohydr. Diet. Fibre 2021, 26, 100283. [Google Scholar] [CrossRef]
- Ogutu, F.O.; Mu, T.-H.; Sun, H.; Zhang, M. Ultrasonic Modified Sweet Potato Pectin Induces Apoptosis like Cell Death in Colon Cancer (HT-29) Cell Line. Nutr. Cancer 2018, 70, 136–145. [Google Scholar] [CrossRef]
- Hino, S.; Nishimura, N.; Morita, T. Hairy Region Concentrate of Pectin Strongly Stimulates Mucin Secretion in HT29-MTX Cells, but to a Lessor Degree in Rat Small Intestine. J. Nutr. Sci. Vitaminol. 2020, 66, 331–338. [Google Scholar] [CrossRef]
- Méndez-Albiñana, P.; Rodrigues-Díez, R.; Rodríguez-Rodríguez, P.; Moreno, R.; Muñoz-Valverde, D.; Casani, L.; Villamiel, M.; Blanco-Rivero, J. Structure and Properties of Citrus Pectin as Influencing Factors of Biomarkers of Metabolic Syndrome in Rats Fed with a High-Fat Diet. Curr. Res. Food Sci. 2025, 10, 101014. [Google Scholar] [CrossRef]
- Ren, T.; Fan, X.; Wu, Q.; Wu, Y.; Sun, X.; Tong, H. Structural Insights and Therapeutic Potential of Plant-Based Pectin as Novel Therapeutic for Type 2 Diabetes Mellitus: A Review. Int. J. Biol. Macromol. 2025, 307, 141876. [Google Scholar] [CrossRef]
- Brouns, F.; Theuwissen, E.; Adam, A.; Bell, M.; Berger, A.; Mensink, R.P. Cholesterol-Lowering Properties of Different Pectin Types in Mildly Hyper-Cholesterolemic Men and Women. Eur. J. Clin. Nutr. 2012, 66, 591–599. [Google Scholar] [CrossRef]
| Materials | Solvents | Extraction Conditions | Yield | Product Structures | Functionality | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| GalA (%) | DE (%) | MW (kDa) | Branching | ||||||
| Citrus peel | Subcritical water | SLR 1:30 at 120 °C for 5 min | 22.0 | 68.9 | 74.7 | 69.5 | Ara 3.10%, Gal 2.52% | Higher anti-tumor | [23] |
| Apple pomace | SLR 1:30 150 °C for 5 min | 16.7 | 40.1 | 86.0 | 53.4 | Ara 2.33%, Gal 4.58% | Higher antioxidant | ||
| Yuzu flavedo | Subcritical water | Hot compressed water at 160 °C with a pressure of 20 MPa | 0.95 | - | - | - | - | - | [51] |
| Subcritical water + 0.4% SHMP | 5.17 | - | - | - | - | ||||
| Subcritical water + 0.4% SHMP + 50 mM HCl | 15.6 | - | - | - | - | ||||
| Satsuma mandarin peel | * HCl, pH 2.5 | SLR 1:25, at 90 °C for 90 min | 10.4 | 63 | 75 | 65 | High HG region | - | [77] |
| Pressurized CO2 | SLR 1:25 under 1 MPa at 90 °C for 90 min | 3.8 | 61 | >90 | 85 | Rich in Ara and Gal | - | ||
| Satsuma mandarin peel | * HCl, pH 1.4 | Extraction in water bath at 85 °C for 70 min | 16 | 76.6 | 71.4 | 1871 | - | Emulsion | [83] |
| Citric acid, pH 1.4 | 18 | 84.1 | 69.2 | 1674 | - | ||||
| High hydrostatic pressure | SLR 1:50 in citric acid solution pH 1.4, treated at 500 MPa for 10 min | 19 | 77.0 | 67.7 | 1201 | - | |||
| Cocoa pod husk | Citric acid, pH 3 | Extraction at 95 °C for 10 min | 3.20 | AUA 17.5% | 29.8 | EW 1429.5 | - | - | [84] |
| Subcritical water | SLR 1:15 extracted at 120 °C for 10 min | 6.58 | AUA 66.5% | 37.9 | EW 486.3 | - | |||
| Sugar beet pulp | * Acid extraction pH 1 | Extraction at 90 °C for 4 h | 20.8 | 68.2 | 57.0 | 102 | - | - | [85] |
| Subcritical water | SLR 1:30 130 °C for 20 min | 20.7 | 73.0 | 84.2 | 23.5 | - | |||
| Unripe papaya | * Nitric acid | SLR 1:75 extracted at 80 °C 30 min | 14.9 | 38.0 | - | - | HG 27.7%, RG-I 55.9% | - | [86] |
| Citric acid | 5.90 | 31.2 | - | 127 | HG 22.2%, RG-I 56.1% | ||||
| Pressurized H2O + citric acid | SLR 1:75 40 MPa, 80 °C 30 min | 4.60 | 41.1 | - | 68 | HG 25.5%, RG-I 63.6% | |||
| Pressurized CO2 + H2O+ citric acid | 16.8 | 51.2 | - | 149 | HG 37.4%, RG-I 64.0% | ||||
| Materials | Solvents | Extraction Conditions | Yield | Products Structure | Functionality | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| GalA (%) | DE (%) | MW (kDa) | Branching | ||||||
| Averrhoa bilimbi (starfruit) | ChCl/citric acid, (1:1) | DES 3.74% at 80 °C for 2.5 h | 14.4 | 54 | - | highly branched sugar | Antioxidant | [100] | |
| Orange peel | 0.05 M sulfuric acid, pH 1.14 | Incubated at 80 °C for 60 min | 12.6 | 47.7 | 72.9 | - | - | - | [101] |
| ChCl/formic acid, (1:2) | 8% DES, maceration at 90 °C, 60 min | 46 | 22.3 | 85.6 | - | - | - | ||
| 8% DES, 360 W for 15 min using microwave | 40 | 29.5 | 74.4 | - | - | - | |||
| Mango peel | * HCl, pH 2.5 | SLR 1:40 at 90 °C for 2 h | 13.2 | 61.5 | 65.6 | 706 | HG 59.4%, RG-I 18.2% | - | [102] |
| ChCl/malic acid | SLR 1:30 at 90 °C for 2 h | 30.0 | 65.9 | 87.1 | 641 | HG 63.56%, RG-I 19.25% | - | ||
| Betaine:citric acid | 27.6 | 68.1 | 83.4 | 782 | HG 66.3%, RG-I 18.6% | ||||
| Hawthorn | * HCl, pH 1.5 | SLR 1:20 at 90 °C for 90 min | 3.07 | 78.1 | 63.5 | 275 | HG 94.0%, RG-I 5.98% | - | [103] |
| SLR 1:15 UAE 70 °C for 40 min | 3.32 | 83.7 | 65.5 | 309 | HG 94.73%, RG-I 5.27% | - | |||
| ChCl/urea, (1:3) | SLR 1:30 at 80 °C for 60 min | 4.33 | 88.9 | 60.9 | 157 | HG 93.8%, RG-I 6.25% | - | ||
| Extraction Methods | Advantages | Disadvantages |
|---|---|---|
| Acid extraction | - High yield - Simple and low-cost | - Harsh conditions can degrade neutral sugars - High environmental burden from acid waste |
| Alkali extraction | - Effective in extracting pectin from hard tissues - Low cost | - Leads to de-esterification and degradation - Alters functional properties - Generates alkaline waste |
| Chelating agents | - Preserves side chains and DE - Gentle on pectin structure | - High ash content - Need additional purification steps |
| Subcritical fluids | - Environmentally friendly - Retain bioactivity | - Requires high-pressure equipment - Limited scalability |
| Supercritical fluids | - Selective extraction of bioactive compounds - Solvent-free | - High equipment and operation cost - Low pectin yield |
| DESs | - Tunable solvent properties - Preserve bioactivity | - Need optimization of HBA/HBD ratio - Residual solvent may remain in the product |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattarapisitporn, A.; Noma, S. Alternative Solvents for Pectin Extraction: Effects of Extraction Agents on Pectin Structural Characteristics and Functional Properties. Foods 2025, 14, 2644. https://doi.org/10.3390/foods14152644
Pattarapisitporn A, Noma S. Alternative Solvents for Pectin Extraction: Effects of Extraction Agents on Pectin Structural Characteristics and Functional Properties. Foods. 2025; 14(15):2644. https://doi.org/10.3390/foods14152644
Chicago/Turabian StylePattarapisitporn, Alisa, and Seiji Noma. 2025. "Alternative Solvents for Pectin Extraction: Effects of Extraction Agents on Pectin Structural Characteristics and Functional Properties" Foods 14, no. 15: 2644. https://doi.org/10.3390/foods14152644
APA StylePattarapisitporn, A., & Noma, S. (2025). Alternative Solvents for Pectin Extraction: Effects of Extraction Agents on Pectin Structural Characteristics and Functional Properties. Foods, 14(15), 2644. https://doi.org/10.3390/foods14152644






