Lectin Activity in Commonly Consumed Plant-Based Foods: Calling for Method Harmonization and Risk Assessment
Abstract
:1. Introduction
1.1. Plant Lectins and Human Health
1.2. Analysis of Active Lectins
1.3. Current Risk Assesment
2. Materials and Methods
2.1. Samples
2.2. Preparation of Samples, Controls, and Commercially Available Lectins
2.3. Nitrogen Content Analysis
2.4. Lectin Analysis (Hemagglutination Assay)
2.4.1. Preparation of Erythrocyte Cell Suspension
2.4.2. Preparation of Trypsin Treated (TT) Erythrocytes
2.4.3. Extraction of Lectin Samples and Controls
2.4.4. Preparation of Dilution Series
2.4.5. Sample Application on Microtiter Plates
2.4.6. Reading and Interpretation of Results
2.4.7. Calculation of the Hemagglutination Activity Unit (HAU)
3. Results and Discussion
3.1. Determination of the Limit of Detection and Assay Uncertainty
3.2. Blood Pellet Shape
3.3. Non-Trypsin Treated (NTT) Hemagglutination Assay
3.4. Trypsin Treated (TT) Hemagglutination Assay
3.5. The Effect of Preparation
3.6. Evaluation of the Non-Trypsin Treated (NTT) and the Trypsin Treated (TT) Assays
3.7. Threshold Limits of Active Lectins in Plant-Based Products
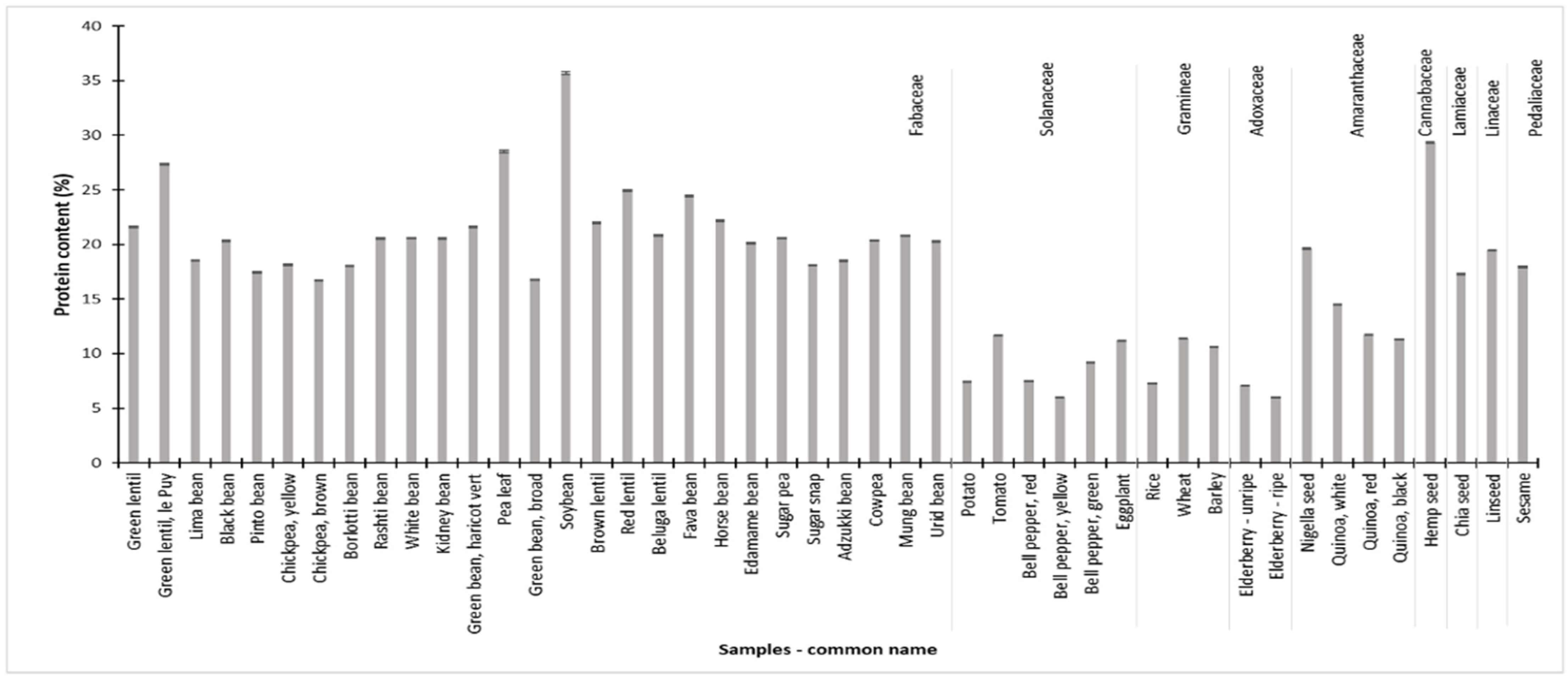
3.8. Nitrogen and Protein Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lannoo, N.; Van Damme, E.J.M. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nöthlings, U.; Schulze, M.B.; Weikert, C.; Boeing, H.; van der Schouw, Y.; Bamia, C.; Benetou, V.; Lagiou, P.; Krogh, V.; Beulens, J.W.J.; et al. Intake of Vegetables, Legumes, and Fruit, and Risk for All-Cause, Cardiovascular, and Cancer Mortality in a European Diabetic Population. J. Nutr. 2008, 138, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, A.; Weighell, W.; Guzman, R.P.; Zahradka, P.; Taylor, C.G. Feasibility and Tolerability of Daily Pulse Consumption in Individuals with Peripheral Artery Disease. Can. J. Diet. Pract. Res. 2017, 78, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The most important dietary predictor of survival in older people of different ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar]
- Figueira, N.; Curtain, F.; Beck, E.; Grafenauer, S. Consumer Understanding and Culinary Use of Legumes in Australia. Nutrients 2019, 11, 1575. [Google Scholar] [CrossRef] [Green Version]
- Pasqualone, A.; Costantini, M.; Coldea, T.E.; Summo, C. Use of Legumes in Extrusion Cooking: A Review. Foods 2020, 9, 958. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Peumans, W.; Van Damme, E. Prevalence, biological activity and genetic manipulation of lectins in foods. Trends Food Sci. Technol. 1996, 7, 132–138. [Google Scholar] [CrossRef]
- McPherson, L.L. The Effect of the Consumption of Red Kidney Beans (Phaseolus vulgaris) on the Growth of Rats and the Implications for Human Populations. J. R. Soc. Health 1990, 110, 222–226. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Huang, Y.; Li, M.; Lu, J.; Jin, N.; He, Y.; Fan, B. Phytohemagglutinin content in fresh kidney bean in China. Int. J. Food Prop. 2019, 22, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Bisen, P.S.; Bhagyawant, S.S. Chickpea Lectin Inhibits Human Breast Cancer Cell Proliferation and Induces Apoptosis through Cell Cycle Arrest. Protein Pept. Lett. 2018, 25, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Damme, E.J.M.V.; Peumans, W.J.; Barre, A.; Rougé, P. Plant lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Van Damme, E.J.; Roy, S.; Barre, A.; Rouge, P.; Van Leuven, F.; Peumans, W.J. The major elderberry (Sambucus nigra) fruit protein is a lectin derived from a truncated type 2 ribosome-inactivating protein. Plant J. 1997, 12, 1251–1260. [Google Scholar] [CrossRef]
- Tripathi, A.; Thakur, N.; Katoch, R. Studies on Lectins from Major Vigna species. Indian J. Agric. Biochem. 2018, 31, 93. [Google Scholar] [CrossRef]
- Pusztai, A.; Ewen, S.; Grant, G.; Peumans, W.; van Damme, E.; Rubio, L.; Bardocz, S. Relationship between Survival and Binding of Plant Lectins during Small Intestinal Passage and Their Effectiveness as Growth Factors. Digestion 1990, 46 (Suppl. 2), 308–316. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, A. Dietary lectins are metabolic signals for the gut and modulate immune and hormone functions. Eur. J. Clin. Nutr. 1993, 47, 691–699. [Google Scholar] [PubMed]
- Banwell, J.G.; Howard, R.; Kabir, I.; Costerton, J.W. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can. J. Microbiol. 1988, 34, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Pusztai, A.; Clarke, E.M.W. Immunocytochemical localization of ingested kidney bean (Phaseolus vulgaris) lectins in rat gut. J. Mol. Histol. 1980, 12, 201–208. [Google Scholar] [CrossRef]
- He, S.; Simpson, B.K.; Sun, H.; Ngadi, M.O.; Ma, Y.; Huang, T. Phaseolus vulgaris lectins: A systematic review of characteristics and health implications. Crit. Rev. Food Sci. Nutr. 2017, 58, 70–83. [Google Scholar] [CrossRef] [Green Version]
- Rodhouse, J.C.; Haugh, C.A.; Roberts, D.; Gilbert, R.J. Red kidney bean poisoning in the UK: An analysis of 50 suspected incidents between 1976 and 1989. Epidemiol. Infect. 1990, 105, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Vichova, P.; Jahodar, L. Plant poisonings in children in the Czech Republic, 1996−2001. Hum. Exp. Toxicol. 2003, 22, 467–472. [Google Scholar] [CrossRef]
- Fuchs, J.; Rauber-Lüthy, C.; Kupferschmidt, H.; Kupper, J.; Kullak-Ublick, G.-A.; Ceschi, A. Acute plant poisoning: Analysis of clinical features and circumstances of exposure. Clin. Toxicol. 2011, 49, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, H.; Date, K. The “White Kidney Bean Incident” in Japan. Methods Mol. Biol. 2014, 1200, 39–45. [Google Scholar] [CrossRef]
- Pilegaard, K.; Olesen, P.T. Forespørgsel om Mulig Forgiftning Forårsaget af "Gourmetbønner"/Snitbønner. J. nr 13/01534; The Danish Technical University: Lyngby, Denmark, 2013. [Google Scholar]
- de la Barca, A.; Vázquez-Moreno, L.; Robles-Burgueño, M. Active soybean lectin in foods: Isolation and quantitation. Food Chem. 1991, 39, 321–327. [Google Scholar] [CrossRef]
- Rizzi, C.; Galeoto, L.; Zoccatelli, G.; Vincenzi, S.; Chignola, R.; Peruffo, A.D. Active soybean lectin in foods: Quantitative determination by ELISA using immobilised asialofetuin. Food Res. Int. 2003, 36, 815–821. [Google Scholar] [CrossRef]
- Hwang, K.M.; Murphree, S.A.; Sartorelli, A.C. A quantitative spectrophotometric method to measure plant lectin-induced cell agglutination. Cancer Res. 1974, 34, 3396–3402. [Google Scholar]
- Zubčević, N.; Fočak, M.; Suljević, D. Highly specific hemagglutination activity of plant lectins in specific species: Case of Fabaceae and Solanaceae. Bulg. J. Agric. Sci. 2018, 24, 391–397. [Google Scholar]
- Bhagyawant, S.S.; Gautam, A.; Chaturvedi, S.K.; Shrivastava, N. Hemagglutinating activity of chickpea extracts for lectin. Int. J. Pharm. Phytopharm. Res. 2015, 5, 1–6. [Google Scholar]
- Adamová, L.; Malinovská, L.; Wimmerová, M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/natural-toxins-in-food (accessed on 30 September 2021).
- FDA. Bad Bug Book. Handbook of Food Borne Pathogenic Microorganisms and Natural Toxins, 2nd edition; USA, 2012; Phytohaemagglutinin (Kidney Bean Lectin). Available online: https://www.fda.gov/media/83271/download.
- DVFA. Available online: https://www.foedevarestyrelsen.dk/Foedevarer/kend_kemien/Sider/Specifikke-foedevarer.aspx (accessed on 3 September 2021).
- ILDIS. International Legume Database & Information Service. Available online: http://www.ildis.org/LegumeWeb/ (accessed on 3 September 2021).
- Plants of de World Online. Available online: http://www.plantsoftheworldonline.org (accessed on 10 May 2020).
- LIS. Legume Information System. Available online: https://legumeinfo.org/organism/Phaseolus/coccineus (accessed on 3 September 2021).
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Protein; U.S. Department of Agriculture: Washington, DC, USA, 1941; p. 183.
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Grant, G.; More, L.J.; McKenzie, N.H.; Stewart, J.C.; Pusztai, A. A survey of the nutritional and haemagglutination properties of legume seeds generally available in the UK. Br. J. Nutr. 1983, 50, 207–214. [Google Scholar] [CrossRef]
- Nachbar, M.S.; Oppenheim, J. Lectins in the United States diet: A survey of lectins in commonly consumed foods and a review of the literature. Am. J. Clin. Nutr. 1980, 33, 2338–2345. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin Digestibility and Stability of Elderberry Antioxidants to Heat Treatment In Vitro. Molecules 2017, 22, 95. [Google Scholar] [CrossRef] [Green Version]
- Pompeu, D.G.; Mattioli, M.A.; Ribeiro, R.; Gonçalves, D.B.; Magalhães, J.; Marangoni, S.; Da Silva, J.A.; Granjeiro, P.A. Purification, partial characterization and antimicrobial activity of Lectin from Chenopodium Quinoa seeds. Food Sci. Technol. 2015, 35, 696–703. [Google Scholar] [CrossRef] [Green Version]
- Ayyagari, R.; Rao, B.N.; Roy, D. Lectins, trypsin inhibitors, BOAA and tannins in legumes and cereals and the effects of processing. Food Chem. 1989, 34, 229–238. [Google Scholar] [CrossRef]
- Contreras, S.; Tagle, M.A. Toxic factors in Chilean legumes III. Hemagglutinating activity. Arch. Latinoam. Nutr. 1974, 24, 191–199. [Google Scholar]
- Roy, F.; Boye, J.; Simpson, B. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- De Mejía, E.G.; Prisecaru, V.I. Lectins as Bioactive Plant Proteins: A Potential in Cancer Treatment. Crit. Rev. Food Sci. Nutr. 2005, 45, 425–445. [Google Scholar] [CrossRef]
- Ebere, U.; Godswill, A.C. Effect of some processing methods on hemagglutinin activity of lectin extracts from selected grains (cereals and legumes). J. Adv. Acad. Res. 2016, 2, 24–59. [Google Scholar]
- Bender, A.E.; Reaidi, G.B. Toxicity of Kidney Beans (Phaseolus vulgaris) With Particular Reference to Lectins. J. Plant Foods 1982, 4, 15–22. [Google Scholar] [CrossRef]
- Norberg, S. The Food Safety Facts on Slow Cooking. Available online: https://www.safefood.net/Blog/January-2017/The-food-safety-facts-on-slow-cooking (accessed on 3 September 2021).
- Pusztai, A.; Palmer, R. Nutritional evaluation of kidney beans (Phaseolus vulgaris): The toxic principle. J. Sci. Food Agric. 1977, 28, 620–623. [Google Scholar] [CrossRef]

| Species and Common Plant Name | Sugar Specificity † | References |
|---|---|---|
| Chenopodium quinoa Willd. (quinoa—black, red, white) | (GlcNAc)n | [12] |
| Cicer arietinum L. (chickpea) | Complex | [12] |
| Glycine max L. (soybean) | GalNAc > Gal | [12] |
| Hordeum vulgare L. (barley) | GlcNAc | [12] |
| Lens culinaris L. (beluga lentil, brown lentil, green lentil, green lentil le Puy, red lentil) | Man/Glc | [12] |
| Oryza sativa L. (rice) | GlcNAc | [12] |
| Phaseolus lunatus L. (lima bean) | GalNAc | [8,12] |
| Phaseolus vulgaris L. (black bean, borlotti bean, green bean—haricot vert, kidney bean, rashti bean, white bean) | Complex | [12] |
| Pisum sativum L. (pea) | Man/Glc | [12] |
| Sambucus nigra L.—fruit (elderberry) | Gal/GalNAc | [12] |
| Solanum lycopersicum L. (tomato) | (GlcNAc)n | [12] |
| Solanum tuberosum L. (potato) | GalNAc > Gal | [12,13] |
| Triticum aestivum L. (wheat) | GlcNAc | [12] |
| Vicia faba L. (fava bean, horse bean) | Man/Glc | [12] |
| Vigna angularis L. (adzukki bean) | Gal | [13] |
| Vigna mungo L. (urid bean) | Gal | [13] |
| Vigna radiata L. (mung bean) | Gal | [14] |
| Vigna unguiculata L. (cowpea) | Gal | [14] |
| Family Common Name | Species Name | Geographic Origin | Sample Preparation | |
|---|---|---|---|---|
| Soaking | Boiling | |||
| Adoxaceae | ||||
| Elderberry—ripe | Sambucus nigra L. | Denmark | - | 15 min |
| Elderberry—unripe | Sambucus nigra L. | Denmark | - | 15 min |
| Amaranthaceae | ||||
| Nigella seed | Nigella sativa L. | India/Egypt | - | 5 min |
| Quinoa, black | Chenopodium quinoa Willd. | Peru, Bolivia | - | 15 min |
| Quinoa, white | Chenopodium quinoa Willd. | Unknown | - | 20 min |
| Quinoa, red | Chenopodium quinoa Willd. | Peru, Bolivia | - | 15 min |
| Cannabaceae | ||||
| Hemp seed | Cannabis sativa L. | China | - | 5 min |
| Fabaceae | ||||
| Adzukki bean | Vigna angularis L. | Unknown | 12 h | 45 min |
| Beluga lentil | Lens culinaris L. | Turkey | - | 20 min |
| Black bean | Phaseolus vulgaris L. | China | 12 h | 30 min |
| Borlotti bean | Phaseolus vulgaris L. | China | 12 h | 45 min |
| Brown lentil | Lens culinaris L. | Canada | - | 20 min |
| Chickpea, brown | Cicer arietinum L. | India | 12 h | 1 h |
| Chickpea, yellow | Cicer arietinum L. | EU | 12 h | 1 h |
| Cowpea | Vigna unguiculata L. | Argentina | 12 h | 30 min |
| Edamame bean | Glycine max L. | Denmark | - | 5 min |
| Fava bean | Vicia faba L. | Unknown | 12 h | 1 h |
| Green bean, broad | Phaseolus coccineus L. | Spain | - | 10 min |
| Green bean, haricot vert | Phaseolus vulgaris L. | Nederland | - | 10 min |
| Green lentil | Lens culinaris L. | Turkey | - | 20 min |
| Green lentil, le Puy | Lens culinaris L. | Turkey | - | 20 min |
| Horse bean | Vicia faba L. | Denmark | - | 5 min |
| Kidney bean | Phaseolus vulgaris L. | Unknown | 12 h | 30 min |
| Lima bean | Phaseolus lunatus L. | Unknown | 12 h | 30 min |
| Mung bean | Vigna radiata L. | Uzbekistan | 12 h | 20 min |
| Pea leaves | Pisum sativum L. | Denmark | - | - |
| Pinto bean | Phaseolus vulgaris L. | Unknown | 12 h | 45 min |
| Rashti bean | Phaseolus vulgaris L. | Unknown | 12 h | 45 min |
| Red lentil | Lens culinaris L. | Turkey | - | 10 min |
| Soybean | Glycine max L. | Canada | 12 h | 45 min |
| Sugar pea | Pisum sativum L. | Denmark | 5 min | |
| Sugar snap | Pisum sativum L. | Denmark | - | 5 min |
| Urid bean | Vigna mungo L. | Myanmar | 12 h | 20 min |
| White bean | Phaseolus vulgaris L. | China | 12 h | 45 min |
| Gramineae | ||||
| Barley | Hordeum vulgare L. | Denmark | - | 15 min |
| Rice | Oryza sativa L. | Unknown | - | 15 min |
| Wheat | Triticum aestivum L. | Denmark | - | 15 min |
| Lamiaceae | ||||
| Chia seed | Salvia hispanica L. | Paraguay | 20 min | - |
| Linaceae | ||||
| Linseed | Linum usitatissimum L. | India | - | 5 min |
| Pedaliaceae | ||||
| Sesame | Sesamum indicum L. | Uganda/India/Pakistan | - | 5 min |
| Solanaceae | ||||
| Bell pepper, green | Capsicum annuum L. | Nederland | - | 5 min |
| Bell pepper, red | Capsicum annuum L. | Nederland | - | 5 min |
| Bell pepper, yellow | Capsicum annuum L. | Nederland | - | 5 min |
| Eggplant | Solanum melongena L. | Nederland | - | 10 min |
| Potato | Solanum tuberosum L. | Denmark | - | 20 min |
| Tomato | Solanum lycopersicum L. | Denmark | - | 5 min |
| Family | Raw Material (HAU/g) | Processed Material (HAU/g) † | ||
|---|---|---|---|---|
| Common Name | NTT ± SD | TT ± SD | NTT ± SD | TT ± SD |
| Adoxaceae | ||||
| Elderberry—ripe | 52 ± 11 | - | ND | - |
| Elderberry—unripe | 104 ± 22 | - | 26 ± 6 | - |
| Amaranthaceae | ||||
| Nigella seed | 104 ± 22 | - | 26 ± 6 | - |
| Quinoa, black | 26 ± 6 | - | ND | - |
| Quinoa, white | 26 ± 6 | - | ND | - |
| Quinoa, red | 26 ± 6 | - | ND | - |
| Cannabaceae | ||||
| Hemp seed | ND | ND | ND | ND |
| Fabaceae | ||||
| Adzukki bean | ND | ND | ND | - |
| Beluga lentil | 1664 ± 353 | - | ND | - |
| Black bean | 26,429 ± 5603 | - | ND | - |
| Borlotti bean | 13,312 ± 2822 | - | ND | - |
| Brown lentil | 3328 ± 706 | - | ND | - |
| Chickpea, brown | ND | 13,312 ± 2822 | ND | 6656 ± 1411 |
| Chickpea, yellow | ND | 13,312 ± 2822 | ND | 13,312 ± 2822 |
| Cowpea | ND | ND | ND | - |
| Edamame bean | 416 ± 88 | - | ND | - |
| Fava bean | 1658 ± 351 | - | ND | - |
| Green bean, broad | 3328 ± 706 | - | 26 ± 6 | - |
| Green bean, haricot vert | 6656 ± 1411 | - | ND | - |
| Green lentil | ND | 3,407,872 ± 722,469 | ND | ND |
| Green lentil, le Puy | ND | 1,703,936 ± 361,234 | ND | ND |
| Horse bean | 1658 ± 351 | - | ND | - |
| Kidney bean | 13,214 ± 2828 | - | ND | - |
| Lima bean | 26,526 ± 5624 | - | ND | - |
| Mung bean | ND | ND | ND | - |
| Pea leaf | 4901 ± 1039 | - | - | - |
| Pinto bean | 13,563 ± 2875 | - | ND | - |
| Rashti bean | 13,312 ± 2822 | - | ND | - |
| Red lentil | 3328 ± 706 | - | ND | - |
| Soybean | 3328 ± 706 | - | ND | - |
| Sugar pea | 414 ± 88 | - | ND | - |
| Sugar snap | 208 ± 44 | - | ND | - |
| Urid bean | ND | ND | ND | - |
| White bean | 13,263 ± 2812 | - | ND | - |
| Gramineae | ||||
| Barley | ND | ND | ND | - |
| Rice | - | 208 ± 44 | ND | ND |
| Wheat | ND | ND | ND | - |
| Lamiaceae | ||||
| Chia seed | ND | ND | ND | ND |
| Linaceae | ||||
| Linseed | ND | ND | ND | ND |
| Pedaliaceae | ||||
| Sesame | ND | ND | ND | ND |
| Solanaceae | ||||
| Bell pepper, green | ND | ND | ND | ND |
| Bell pepper, red | 26 ± 6 | - | ND | - |
| Bell pepper, yellow | 26 ± 6 | - | ND | - |
| Eggplant | ND | ND | ND | ND |
| Potato | 826 ± 175 | - | ND | - |
| Tomato | 256 ± 54 | - | 104 ± 22 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamcová, A.; Laursen, K.H.; Ballin, N.Z. Lectin Activity in Commonly Consumed Plant-Based Foods: Calling for Method Harmonization and Risk Assessment. Foods 2021, 10, 2796. https://doi.org/10.3390/foods10112796
Adamcová A, Laursen KH, Ballin NZ. Lectin Activity in Commonly Consumed Plant-Based Foods: Calling for Method Harmonization and Risk Assessment. Foods. 2021; 10(11):2796. https://doi.org/10.3390/foods10112796
Chicago/Turabian StyleAdamcová, Anežka, Kristian Holst Laursen, and Nicolai Zederkopff Ballin. 2021. "Lectin Activity in Commonly Consumed Plant-Based Foods: Calling for Method Harmonization and Risk Assessment" Foods 10, no. 11: 2796. https://doi.org/10.3390/foods10112796
APA StyleAdamcová, A., Laursen, K. H., & Ballin, N. Z. (2021). Lectin Activity in Commonly Consumed Plant-Based Foods: Calling for Method Harmonization and Risk Assessment. Foods, 10(11), 2796. https://doi.org/10.3390/foods10112796







