Preparation of Antarctic Krill Oil Emulsion and Its Stability under Catalase Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material, Chemicals and Reagents
2.2. Preparation of Antarctic Shrimp Oil Emulsion
2.3. CAT Affected the Status of Antarctic Shrimp Oil Emulsion
2.4. CAT Activity and Emulsion Stability
2.5. CAT Affected the Lipid Oxidation of Antarctic Shrimp Oil Emulsion
2.6. Measurement of the Turbidity and Stability
2.7. Measurement of the Particle Size and ζ-Potential
2.8. Transmission Electronic Microscopy (TEM) Observation
2.9. Measurement of the CAT Activity
2.10. Measurement of the TBARS
2.11. Measurement of the Hydroxyl Radicals
2.12. Statistical Analysis
3. Results
3.1. Optimization of the Antarctic Shrimp Oil Emulsion Preparation Conditions
3.1.1. Impacts of Different Emulsifiers on the Emulsion Properties
3.1.2. Impacts of Different Oil Concentrations on Emulsion Properties
3.1.3. Impacts of Different Ratios of Surfactant to Oil on the Emulsion Properties
3.1.4. Preparation of Emulsion at Optimal Conditions
3.2. Impacts of CAT on the Emulsion Status
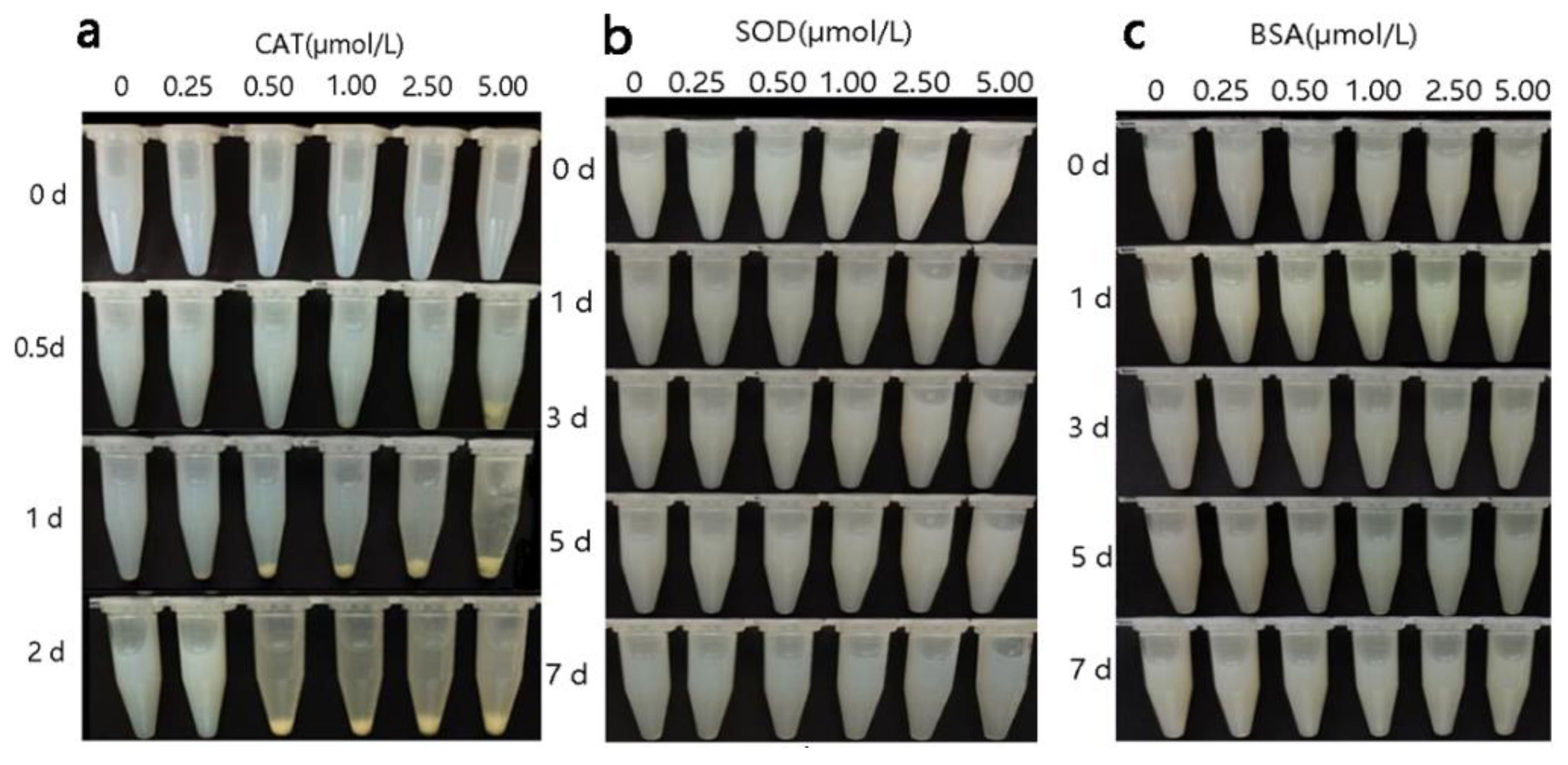
3.2.1. Visual Observation
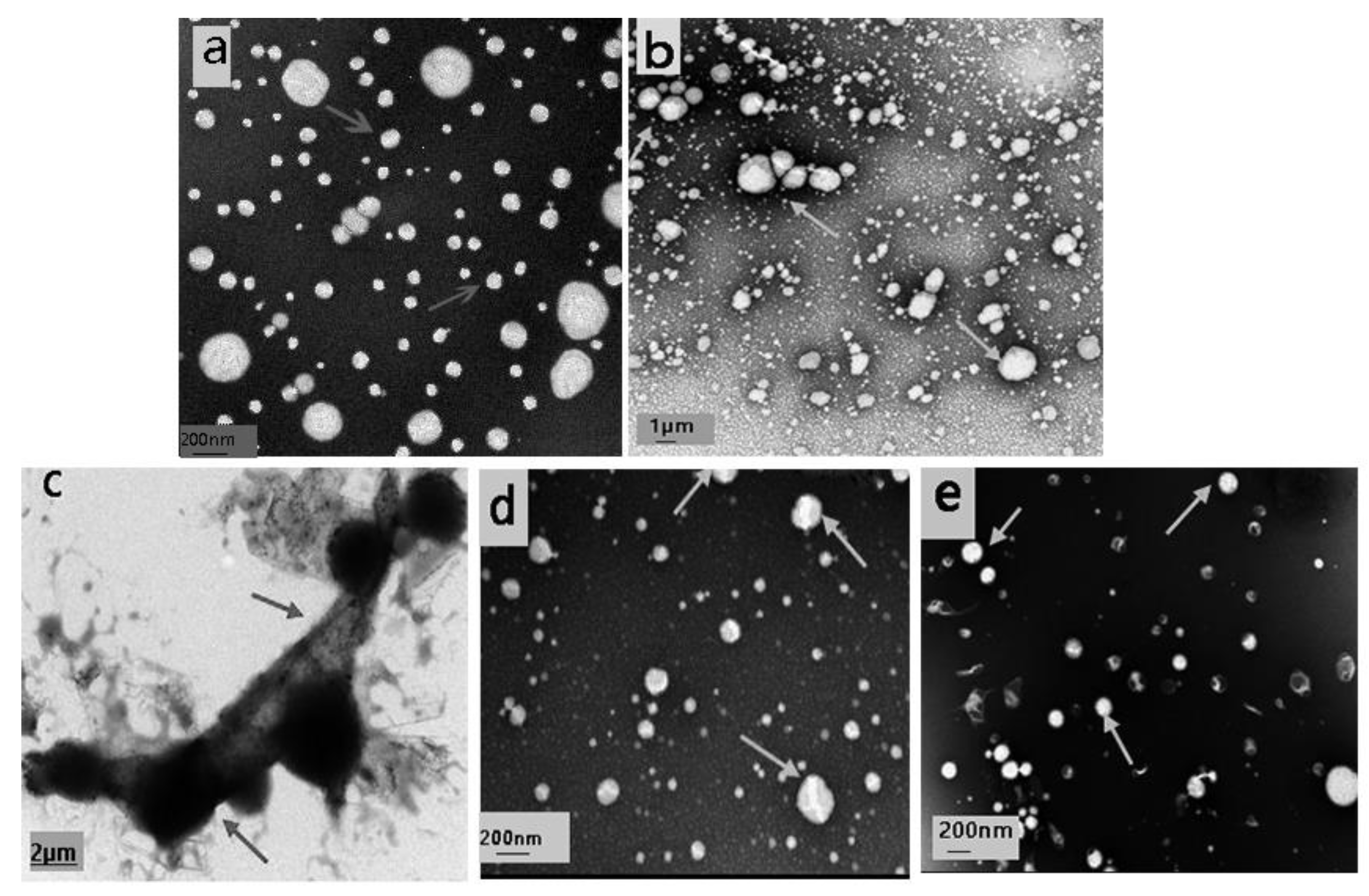
3.2.2. Micromorphology Observation
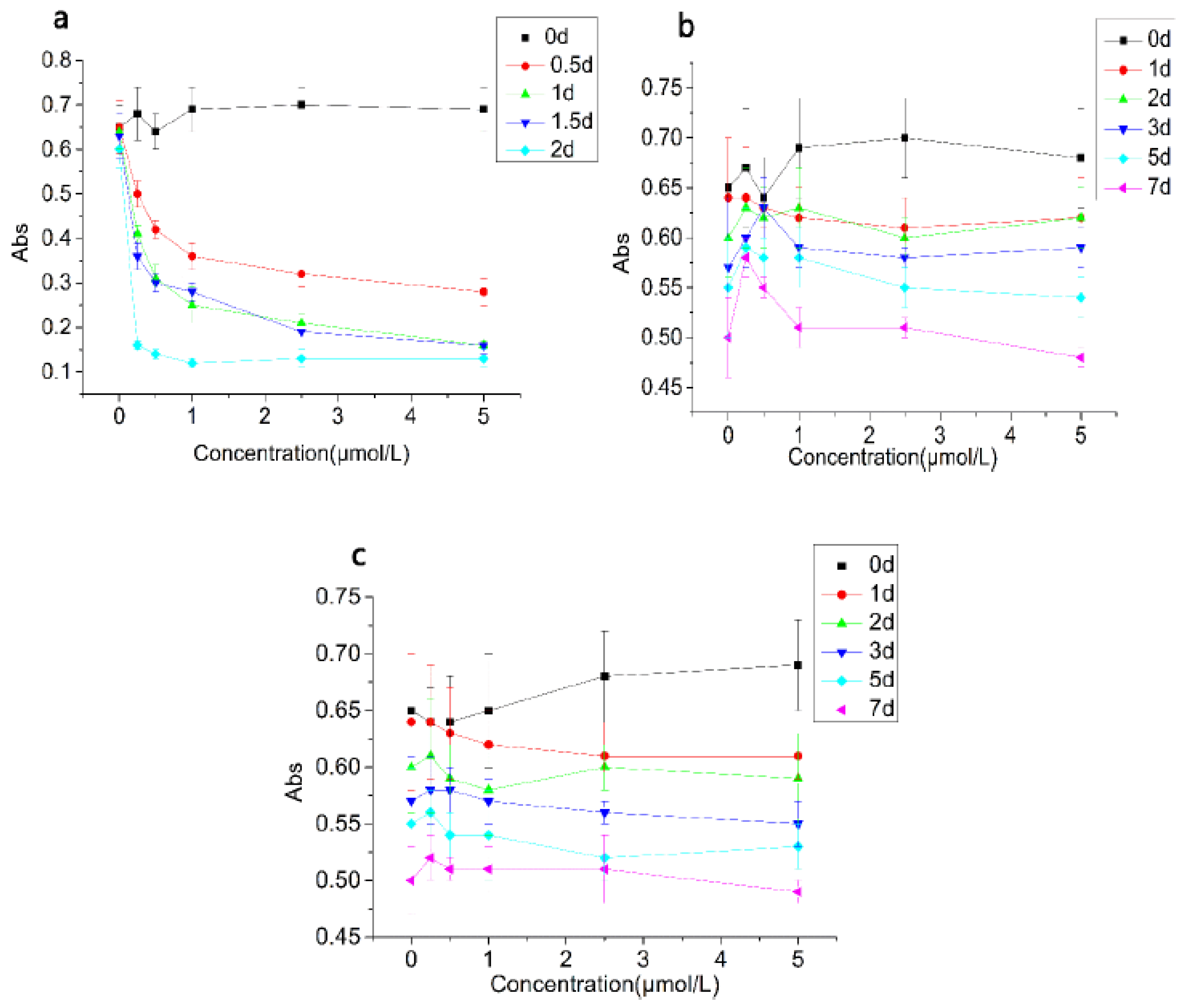
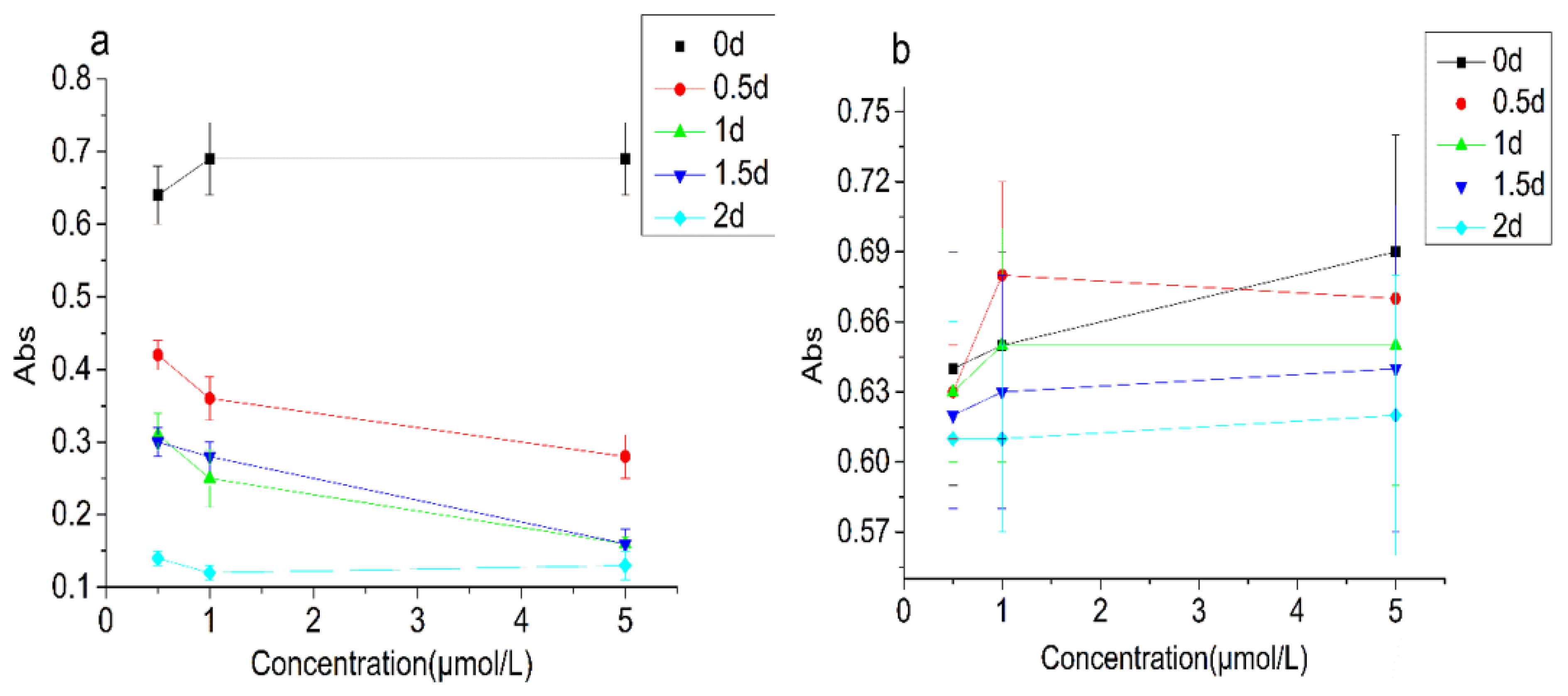
3.2.3. Turbidity and Stability Changes
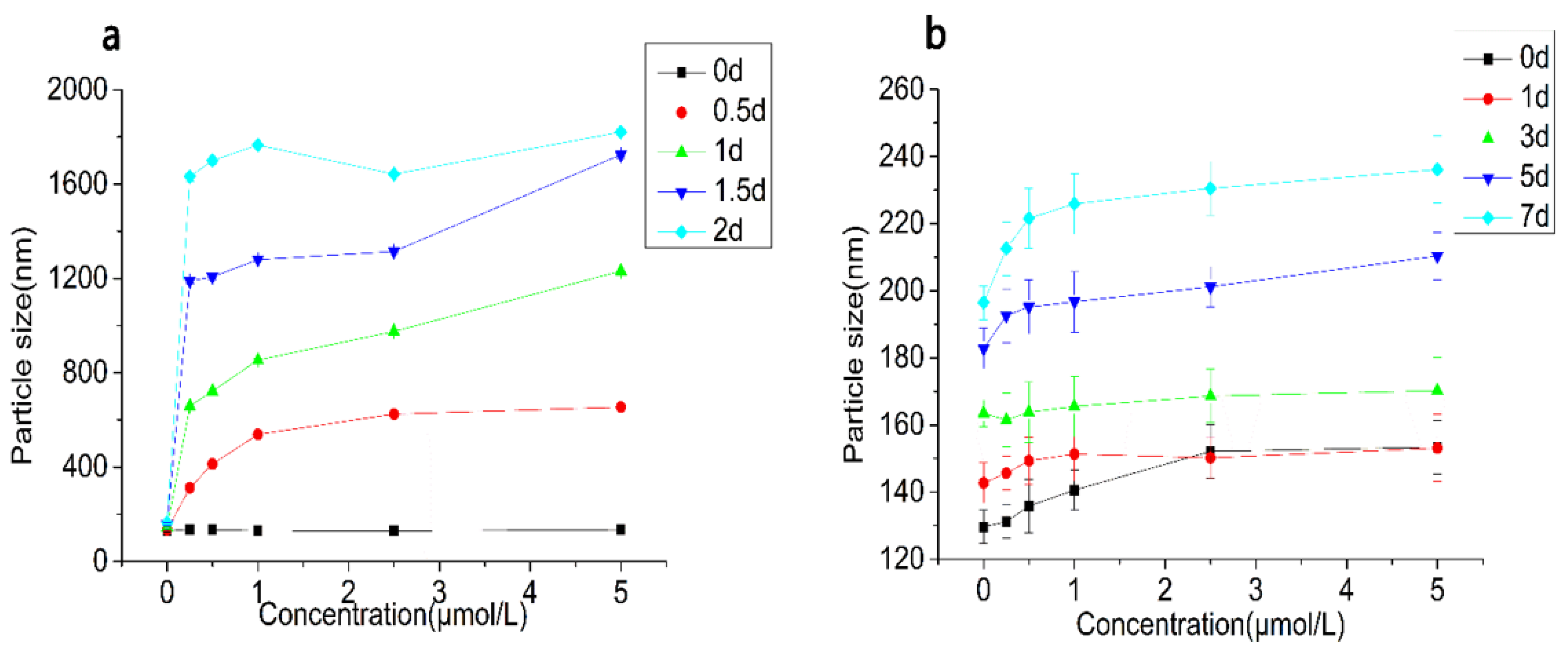
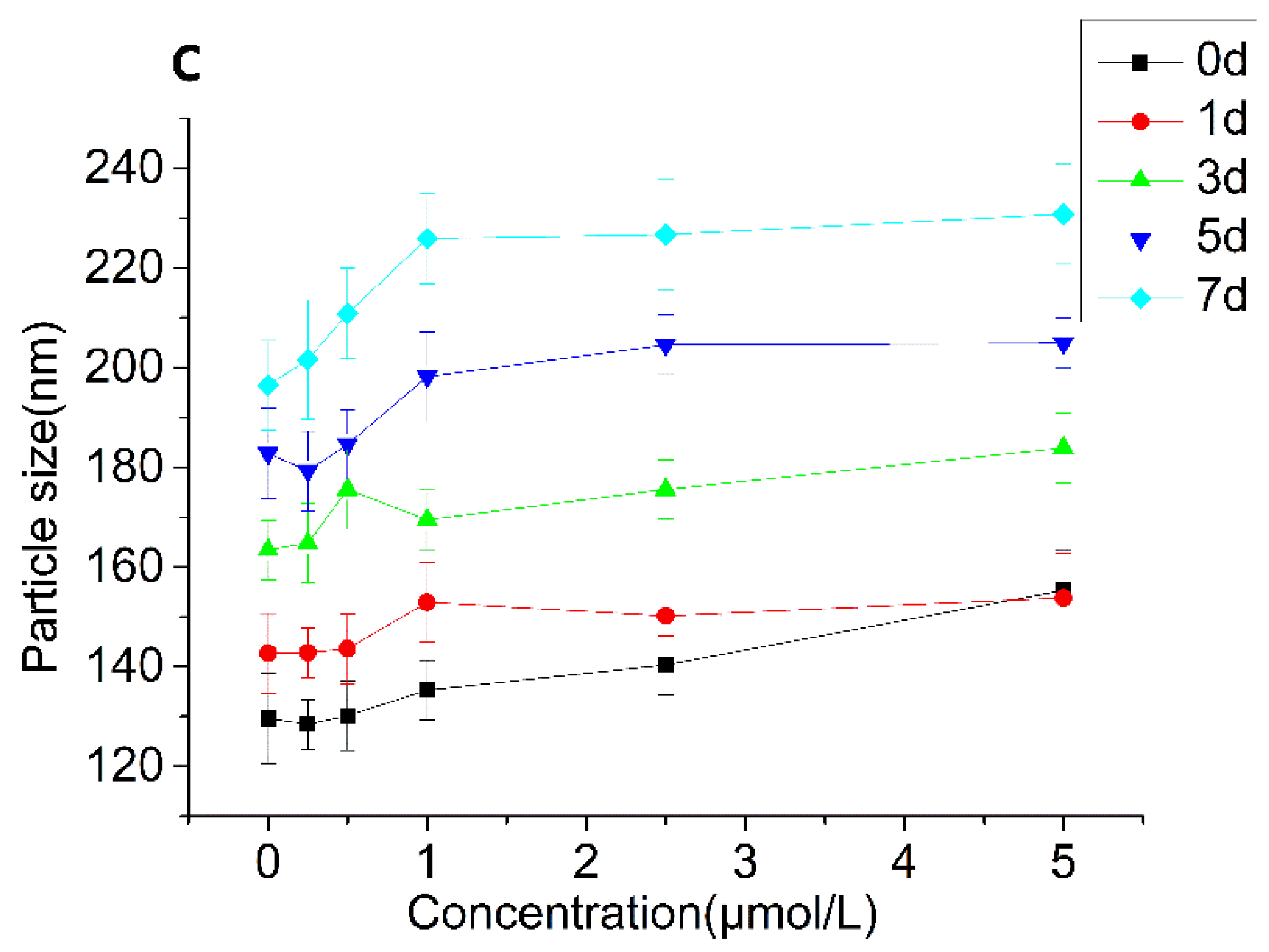
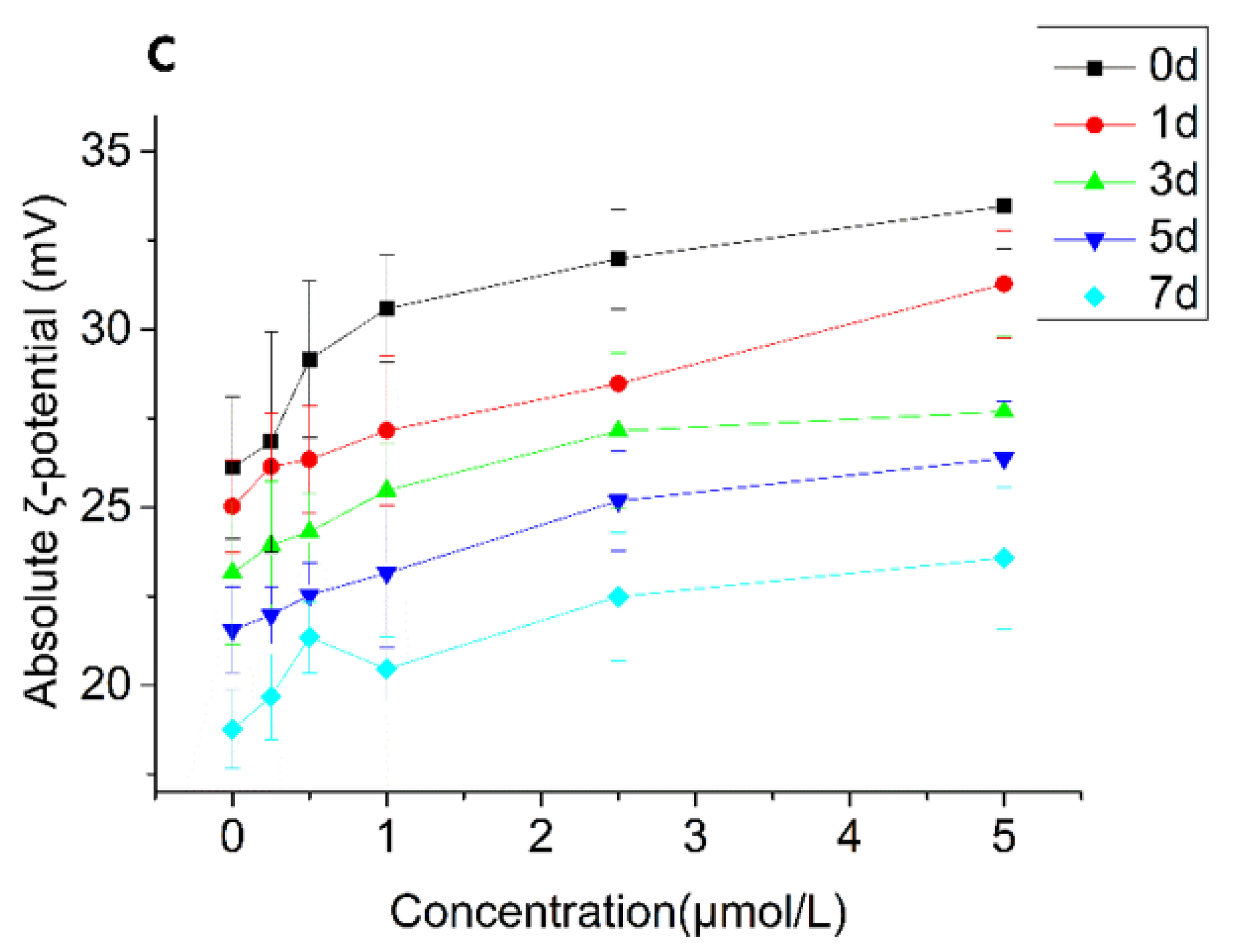
3.2.4. Particle Size and ζ-Potential Changes
3.3. Correlation Analysis between CAT Activity and Emulsion Stability and Lipid Oxidation
3.3.1. CAT Activity and Emulsion Stability
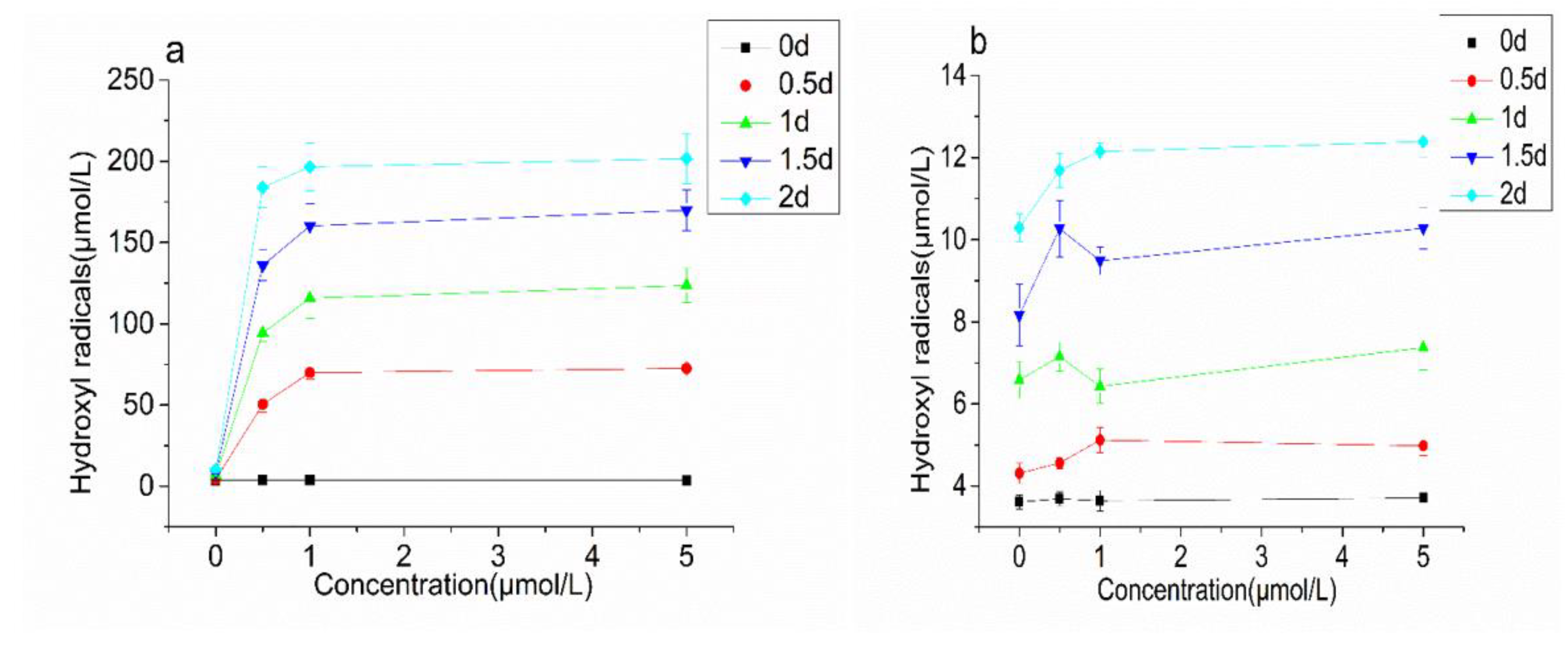
3.3.2. CAT Activity and Lipid Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Li, Y.; Dai, Y.; Han, L.; Zhu, Y.; Xue, C.; Wang, P.; Wang, J. Peptides from Antarctic Krill (Euphausia superba) Improve Osteoarthritis via Inhibiting HIF-2α-Mediated Death Receptor Apoptosis and Metabolism Regulation in Osteoarthritic Mice. J. Agric. Food Chem. 2019, 67, 3125–3133. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic Krill (Euphausia superba) Oil: A Comprehensive Review of Chemical Composition, Extraction Technologies, Health Benefits, and Current Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Guo, J.; Zhao, M.; Gong, Y.; Qiao, Y.B. Effect of Coagulation Bath Temperature on Mechanical, Morphological, and Thermal Properties of Cellulose/Antarctic Krill Protein Composite Fibers. Langmuir 2020, 36, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Song, L.; Guan, J.; Sun, C.; Zhou, D.; Zhu, B. Encapsulation of Antarctic krill oil in yeast cell microcarriers: Evaluation of oxidative stability and in vitro release. Food Chem. 2021, 338, 128089. [Google Scholar] [CrossRef]
- Xie, D.; Mu, H.; Tang, T.; Wang, X.; Wei, W.; Jin, J.; Wang, X.; Jin, Q. Production of three types of krill oils from krill meal by a three-step solvent extraction procedure. Food Chem. 2018, 248, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Ma, L.; Li, H.; Wang, Z.; Xu, J.; Xue, C. The oxidation mechanism of phospholipids in Antarctic krill oil promoted by metal ions. Food Chem. 2020, 333, 127448. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Li, Y.; Zhou, Z. Limonin Enhances the Antifungal Activity of Eugenol Nanoemulsion against Penicillium Italicum In Vitro and In Vivo Tests. Microorganisms 2021, 9, 969. [Google Scholar] [CrossRef] [PubMed]
- Hirvi, Y.; Griffiths, M. Milk Catalase Activity as an indicator of thermization treatments used in the manufacture of cheddar cheese. J. Dairy Sci. 1998, 81, 338–345. [Google Scholar] [CrossRef]
- Gamboa, J.; Montilla, A.; Ana, C.; Mar, V. Effects of conventional and ultrasound blanching on enzyme inactivation and carbohydrate content of carrots. Eur. Food Res. Technol. 2012, 234, 1071–1079. [Google Scholar] [CrossRef] [Green Version]
- Akbas, E.; Soyler, B.; Oztop, M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT 2018, 96, 266–273. [Google Scholar] [CrossRef]
- Jiang, L.; Qi, Y.; Ma, C.; Liu, B.; Wang, Z.; Li, Y. Formation and Stability of Fish Oil Enriched Biocompatible Nano-emulsion. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 2018, 49, 387–395. [Google Scholar]
- Pazos, M.; Andersen, M.L.; Skibsted, L.H. Heme-Mediated Production of Free Radicals via Preformed Lipid Hydroperoxide Fragmentation. J. Agric. Food Chem. 2008, 56, 11478–11484. [Google Scholar] [CrossRef] [PubMed]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of metal ions on lipid oxidation, colour and physi-cochemical properties of cuttlefish (Sepia pharaonis) subjected to multiple freeze–thaw cycles. Food Chem. 2006, 95, 591–599. [Google Scholar] [CrossRef]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A Review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Panchal, B.; Truong, T.; Prakash, S.; Bansal, N.; Bhandari, B. Influence of emulsifiers and dairy ingredients on manufacturing, microstructure, and physical properties of butter. Foods 2021, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Kozak, D.; Anderson, W.; Vogel, R.; Chen, S.; Antaw, F.; Trau, M. Simultaneous Size and ζ-Potential Measurements of Indi-vidual Nanoparticles in Dispersion Using Size-Tunable Pore Sensors. ACS Nano 2012, 6, 6990–6997. [Google Scholar] [CrossRef]
- Mcdonald, R.E.; Hultin, H.O. Some Characteristics of the Enzymic Lipid Peroxidation System in the Microsomal Fraction of Flounder Skeletal Muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Du, H.; Cao, Y.; Li, Z.; Li, L.; Xu, H. Formation and mechanisms of hydroxyl radicals during the oxygenation of sediments in Lake Poyang, China. Water Res. 2021, 202, 117442. [Google Scholar] [CrossRef] [PubMed]
- DA, B.; Jenny, E.R.; Aisen, P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J. Biol. Chem. 1984, 259, 13391–13394. [Google Scholar]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Grene, A.R.; Neval, E.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 372, 1331–1341. [Google Scholar]
- Ali, A.; Sharma, R. A comparative study on the role of Lysine and BSA in Glycation-induced damage to DNA. Biosci. Bioeng. Commun. 2015, 1, 38–43. [Google Scholar]
- Johnson, D.G.; Niemczyk, M.P.; Minsek, D.W.; Wiederrecht, G.P.; Svec, W.A.; Gaines, G.L., III; Wasielewski, M.R. Photochemical electron transfer in chlorophyll-porphyrin-quinone triads: The role of the porphyrin-bridging molecule. J. Am. Chem. Soc. 1993, 115, 5692–5701. [Google Scholar] [CrossRef]
- Naruta, Y.; Maruyama, K. High oxygen-evolving activity of rigidly linked manganese(III) porphyrin dimers. A functional model of manganese catalase. J. Am. Chem. Soc. 1991, 113, 3595–3596. [Google Scholar] [CrossRef]
- Rebelo, S.L.; Neves, C.M.; de Almeida, M.P.; Pereira, E.; Simões, M.M.; Neves, M.G.P.; de Castro, B.; Medforth, C.J. Binary ionic iron(III) porphyrin nanostructured materials with catalase-like activity. Appl. Mater. Today 2020, 21, 100830. [Google Scholar] [CrossRef]
- Ferrón, J.; Alonso, E.; Baragiola, R.; Oliva-Florio, A. Ion-electron emission: The effect of oxidation. Surf. Sci. 1982, 120, 427–434. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Zhu, K.; Dai, Z. Preparation of Antarctic Krill Oil Emulsion and Its Stability under Catalase Treatment. Foods 2021, 10, 2797. https://doi.org/10.3390/foods10112797
Zheng Z, Zhu K, Dai Z. Preparation of Antarctic Krill Oil Emulsion and Its Stability under Catalase Treatment. Foods. 2021; 10(11):2797. https://doi.org/10.3390/foods10112797
Chicago/Turabian StyleZheng, Zhenxiao, Kai Zhu, and Zhiyuan Dai. 2021. "Preparation of Antarctic Krill Oil Emulsion and Its Stability under Catalase Treatment" Foods 10, no. 11: 2797. https://doi.org/10.3390/foods10112797
APA StyleZheng, Z., Zhu, K., & Dai, Z. (2021). Preparation of Antarctic Krill Oil Emulsion and Its Stability under Catalase Treatment. Foods, 10(11), 2797. https://doi.org/10.3390/foods10112797




