Clinical and Patient-Reported Outcomes for Intraoral (Palatal and Tuberosity) Soft Tissue Grafts in Root Coverage Procedures: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. PICO Question
2.2. Main Outcomes
2.3. Eligibility Criteria
2.4. Search Strategy
2.5. Paper Selection
2.6. Data Collection
2.7. Risk of Bias (Quality) Assessment
2.8. Statistical and Data Analysis
3. Results
3.1. Study Selection
3.2. Soft Tissue Grafts Harvested from the Tuberosity Area
3.2.1. Graft Dimension
3.2.2. Tissue Thickness
3.2.3. Post-Operative Pain/Discomfort
3.3. Soft Tissue Grafts Harvested from the Hard Palate
3.3.1. Graft Dimension
3.3.2. Tissue Thickness
3.3.3. Post-Operative Pain/Discomfort
3.4. Risk of Bias Assessment
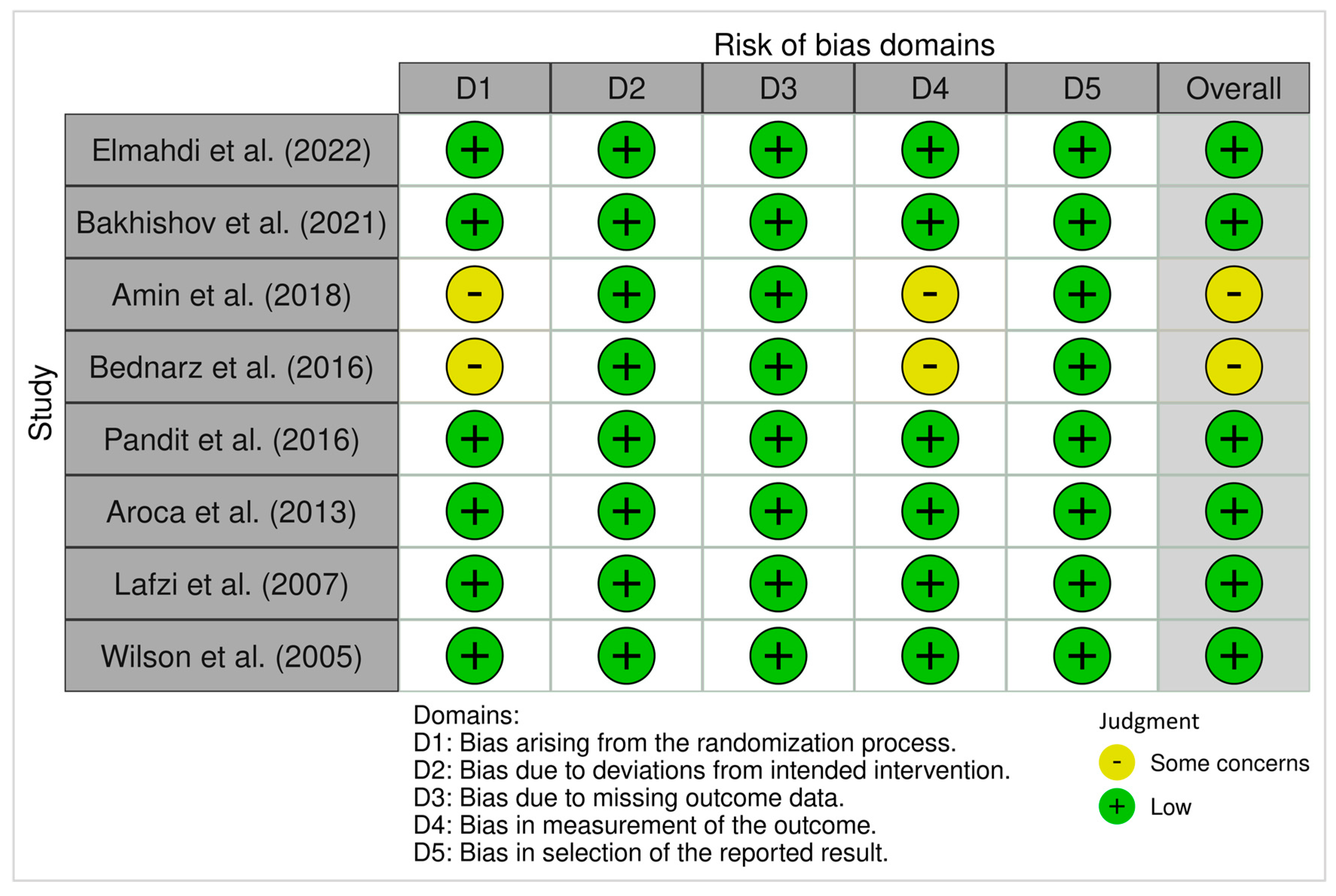
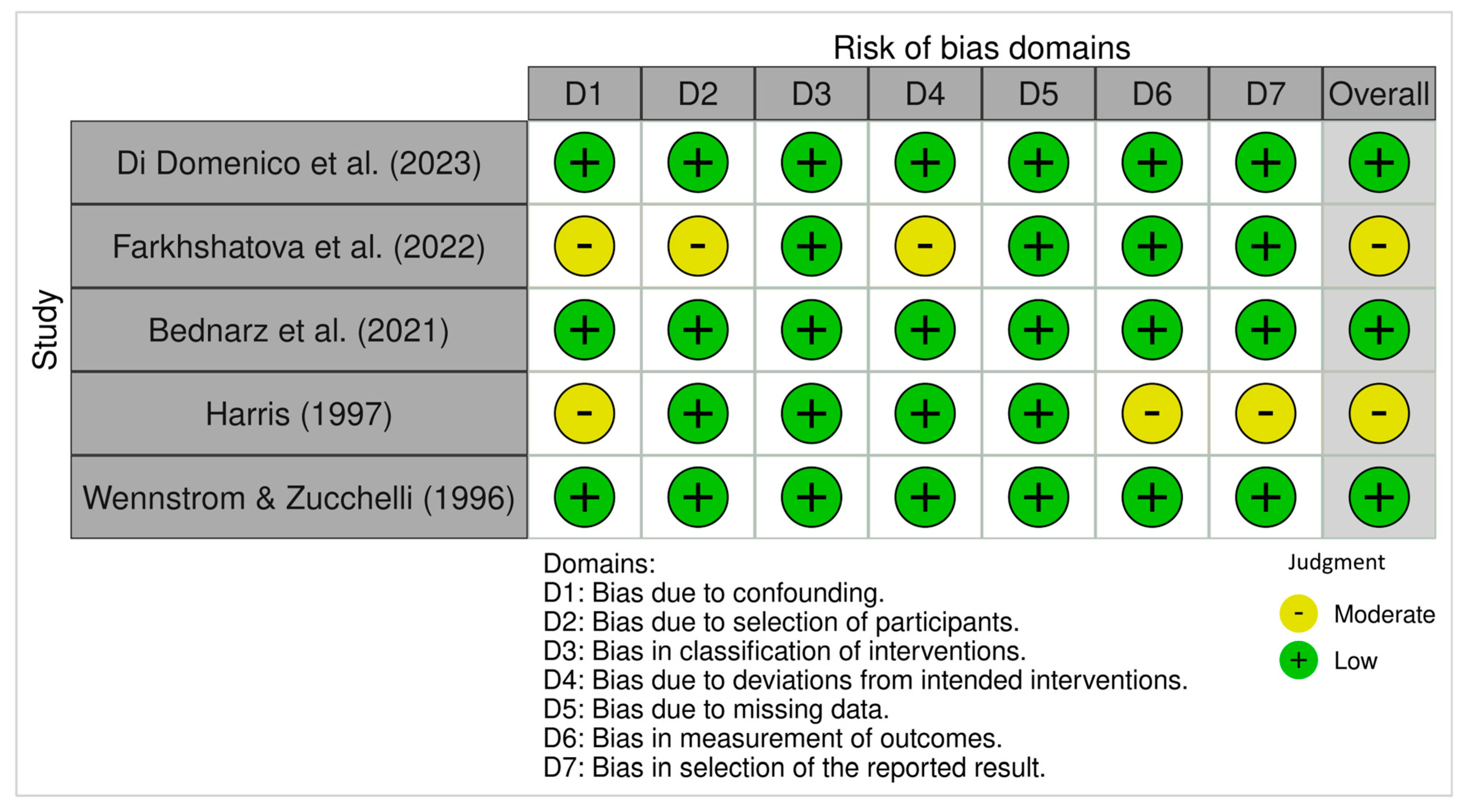
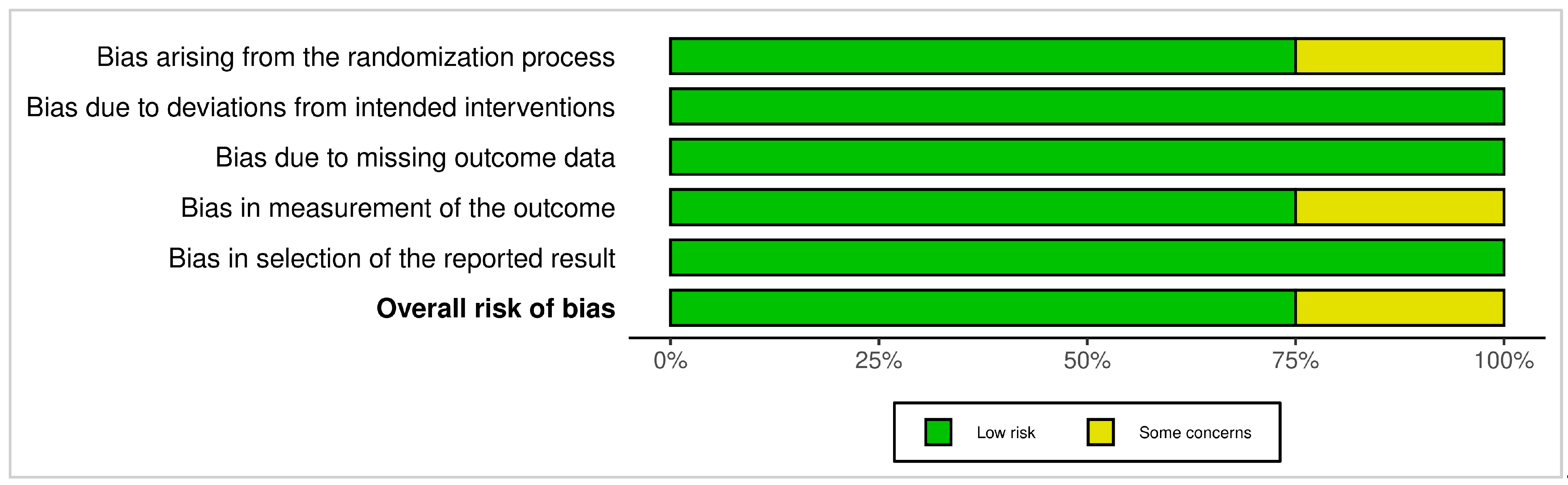
3.4.1. ROB 2 (Randomized Trials)
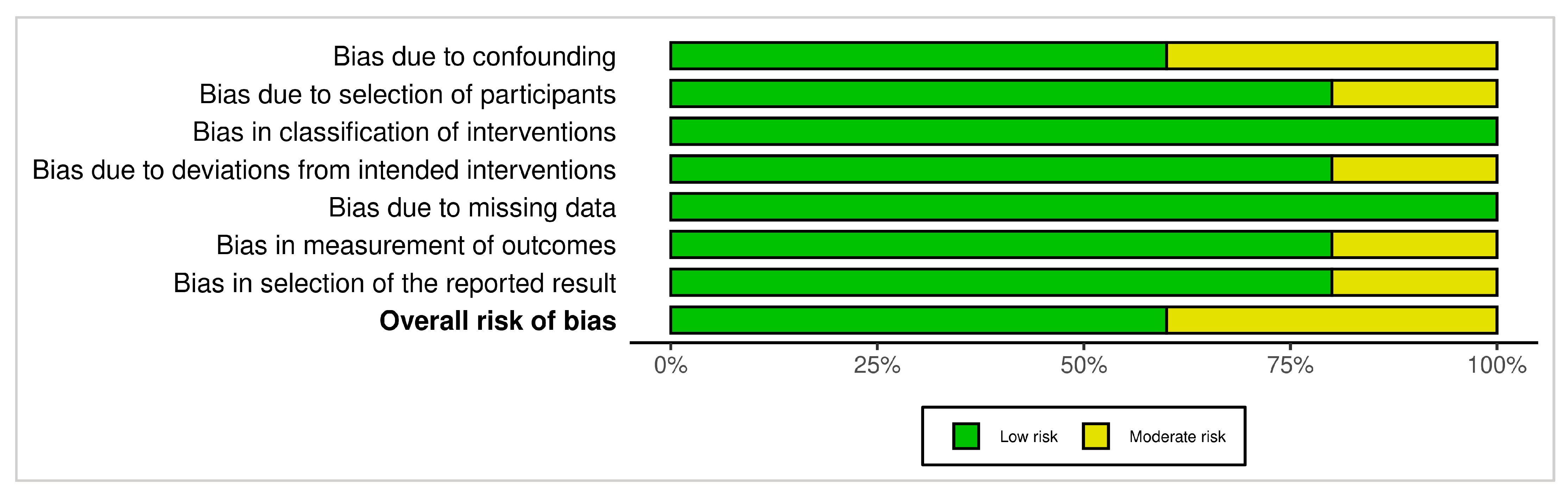
3.4.2. ROBINS-I (Non-Randomized Studies)
3.5. The Heterogeneity Across Studies by Each Major Outcome and Timepoint
- Gingival Thickness (GT)
- 2.
- Keratinized Tissue Width (KTW)
- 3.
- Patient-Reported Outcomes (Pain/Satisfaction—VAS or Questionnaire)
- 4.
- Clinical Outcomes (Recession Coverage, %RC, %CRC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chambrone, L.; Tatakis, D.N. Periodontal Soft Tissue Root Coverage Procedures: A Systematic Review from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S8–S51. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.N.; Bissada, N.F.; Ricchetti, P.A.; Silva, A.P.B.; Demko, C.A. Tuberosity versus palatal donor sites for soft tissue grafting: A split-mouth clinical study. Quintessence Int. 2018, 49, 589–598. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Greenwell, H.; Wang, H.L. Is a soft tissue graft harvested from the maxillary tuberosity the approach of choice in an isolated site? J. Periodontol. 2019, 90, 821–825. [Google Scholar] [CrossRef]
- Harris, R.J. The Connective Tissue With Partial Thickness Double Pedicle Graft: The Results of 100 Consecutively-Treated Defects. J. Periodontol. 1994, 65, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Zuhr, O.; Bäumer, D.; Hürzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41, S123–S142. [Google Scholar] [CrossRef]
- Langer, B.; Langer, L. Subepithelial CTG for Root Coverage. J. Periodontol. 1985, 56, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, H.C.; Atkins, J.H. Free autogenous gingival grafts, I. Principles of successful grafting. Periodontics 1968, 6, 121–129. [Google Scholar] [PubMed]
- Shahbazi, A.; Grimm, A.; Feigl, G.; Gerber, G.; Székely, A.D.; Molnár, B.; Windisch, P. Analysis of blood supply in the hard palate and maxillary tuberosity—Clinical implications for flap design and soft tissue graft harvesting (a human cadaver study). Clin. Oral. Investig. 2018, 23, 1153–1160. [Google Scholar] [CrossRef]
- Reiser, D.D.S.G.M.; Bruno, D.D.S.J.F.; Mahan, D.D.S.P.E.; Larkin, L.H. The Subepithelial Connective Tissue Graft Palatal Donor Site: Anatomic Considerations for Surgeons. Int. J. Periodontics Restor. Dent. 1996, 16, 130–137. [Google Scholar]
- Studer, S.P.; Allen, E.P.; Rees, T.C.; Kouba, A. The Thickness of Masticatory Mucosa in the Human Hard Palate and Tuberosity as Potential Donor Sites for Ridge Augmentation Procedures. J. Periodontol. 1997, 68, 145–151. [Google Scholar] [CrossRef]
- Sanz-Martín, I.; Rojo, E.; Maldonado, E.; Stroppa, G.; Nart, J.; Sanz, M. Structural and histological differences between connective tissue grafts harvested from the lateral palatal mucosa or from the tuberosity area. Clin. Oral. Investig. 2018, 23, 957–964. [Google Scholar] [CrossRef]
- Jung, U.; Um, Y.; Choi, S. Histologic Observation of Soft Tissue Acquired From Maxillary Tuberosity Area for Root Coverage. J. Periodontol. 2008, 79, 934–940. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, G.L.; Di Martino, M.; Arrigoni, G.; Aroca, S.; de Sanctis, M. Multiple coronally advanced flap with a selective use of connective tissue graft: A 3-year prospective clinical and histological study. J. Periodontol. 2023, 94, 1200–1209. [Google Scholar] [CrossRef]
- Farkhshatova, R.; Gerasimova, L.; Usmanova, I.; Daurova, F.; Golub, A.; Aletdinova, S. Diagnosis and treatment of miller class i gingival recession using free connective tissue autograft from the hard palate. Clinical observation. Arch. Euromedica 2022, 12, e1. [Google Scholar] [CrossRef]
- Elmahdi, F.; Reda, A.; Hosny, M. Evaluation of Subepithelial Connective Tissue Graft Versus Acellular Dermal Matrix with Modified Coronally Advanced Tunnel Technique in Treatment of Multiple Gingival Recessions: A Randomized, Parallel-Design Clinical Trial. Int. J. Periodontics Restor. Dent. 2022, 42, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, W.; Majer, J.; Pakuszyńska-Błaszczyk, J.; Dominiak, M.; Gedrange, T.; Zielińska-Pałasz, A. Coronally advanced flap in the treatment of multiple adjacent gingival recessions along with a connective tissue graft harvested from augmented or nonaugmented palatal mucous membrane: A two-year comparative clinical evaluation. Appl. Sci. 2021, 11, 1081. [Google Scholar] [CrossRef]
- Bakhishov, H.; Isler, S.C.; Bozyel, B.; Yıldırım, B.; Tekindal, M.A.; Ozdemir, B. De-epithelialized gingival graft versus subepithelial connective tissue graft in the treatment of multiple adjacent gingival recessions using the tunnel technique: 1-year results of a randomized clinical trial. J. Clin. Periodontol. 2021, 48, 970–983. [Google Scholar] [CrossRef]
- Bednarz, W.; Zurek, J.; Gedrange, T.; Dominiak, M. A preliminary clinical comparison of the use of Fascia Lata allograft and autogenous connective tissue graft in multiple gingival recession coverage based on the tunnel technique. Adv. Clin. Exp. Med. 2016, 25, 587–598. [Google Scholar] [CrossRef]
- Pandit, N.; Khasa, M.; Gugnani, S.; Malik, R.; Bali, D. Comparison of two techniques of harvesting connective tissue and its effects on healing pattern at palate and recession coverage at recipient site. Contemp. Clin. Dent. 2016, 7, 3–10. [Google Scholar] [CrossRef]
- Aroca, S.; Molnár, B.; Windisch, P.; Gera, I.; Salvi, G.E.; Nikolidakis, D.; Sculean, A. Treatment of multiple adjacent Miller class i and II gingival recessions with a Modified Coronally Advanced Tunnel (MCAT) technique and a collagen matrix or palatal connective tissue graft: A randomized, controlled clinical trial. J. Clin. Periodontol. 2013, 40, 713–720. [Google Scholar] [CrossRef]
- Lafzi, A.; Mostofi Zadeh Farahani, R.; Abolfazli, N.; Amid, R.; Safaiyan, A. Effect of connective tissue graft orientation on the root coverage outcomes of coronally advanced flap. Clin. Oral. Investig. 2007, 11, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G.; Mcguire, M.K.; Nunn, M.E. Evaluation of the Safety and Efficacy of Periodontal Applications of a Living Tissue-Engineered Human Fibroblast- Derived Dermal Substitute. II. Comparison to the Subepithelial Connective Tissue Graft: A Randomized Controlled Feasibility Study. J. Periodontol. 2005, 76, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. A Comparison of Two Tectiniques for Obtaining a Connective Tissue Graft From the Palate. Int. J. Periodontics Restor. Dent. 1997, 17, 260–271. [Google Scholar] [PubMed]
- Wennström, J.L.; Zucchelli, G. Increased gingival dimensions. A significant factor for successful outcome of root coverage procedures? A 2-year prospective clinical study. J. Clin. Periodontol. 1996, 23, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Wessel, J.R.; Tatakis, D.N. Patient Outcomes Following Subepithelial Connective Tissue Graft and Free Gingival Graft Procedures. J. Periodontol. 2008, 79, 425–430. [Google Scholar] [CrossRef]
- Dellavia, C.; Ricci, G.; Pettinari, L.; Allievi, C.; Grizzi, F.; Gagliano, N. Human Palatal and Tuberosity Mucosa as Donor Sites for Ridge Augmentation. Int. J. Periodontics Restor. Dent. 2014, 34, 179–186. [Google Scholar] [CrossRef]
- Azar, E.L.; Rojas, M.A.; Mandalunis, P.; Gualtieri, A.; Carranza, N. Histological evaluation of subepithelial connective tissue grafts harvested by two different techniques. Prelim. Study Hum. 2019, 32, 10–16. [Google Scholar]
- Ramos-Pilco, E.; Condori Salinas, Y.; Antonio Alarcón, M. Paladar o Tuberosidad, ¿Cuál es la Mejor Zona Donadora para un Injerto de Tejido Blando?: Una Revisión Sistemática Palate or Tuberosity, Which is the Best Donor Site for a Soft tissue graft? A Systematic Review. Int. J. Odontostomatol. 2020, 14, 602–609. [Google Scholar] [CrossRef]
- Konflanz, W.; Orth, C.C.; Celeste, R.K.; Muniz, F.W.M.G.; Haas, A.N. Influence of Donor Site and Harvesting Technique of Connective Tissue Graft on Root Coverage Outcomes of Single Gingival Recessions: Systematic Review and Meta-analyses. J. Int. Acad. Periodontol. 2021, 23, 79–98. [Google Scholar] [PubMed]

| Database | Keywords |
|---|---|
| PubMed | ((“soft tissue” OR “free gingival grafting” OR “gingival grafting” OR “connective tissue graft”) AND (“Tissue Donors” OR Tuberosity OR Palate) AND graft) |
| Web of Science | (TS = (“soft tissue” OR “free gingival grafting” OR “gingival grafting” OR “connective tissue graft”)) AND (TS = (“Tissue Donors” OR Tuberosity OR Palate)) AND TS = (Graft) |
| OVID Medline | (TS = (“soft tissue” OR “free gingival grafting” OR “gingival grafting” OR “connective tissue graft”)) AND (TS = (“Tissue Donors” OR Tuberosity OR Palate)) AND TS = (Graft) |
| Scopus | (TITLE-ABS-KEY (“soft tissue” OR “free gingival grafting” OR “gingival grafting” OR “connective tissue graft”)) AND (TITLE-ABS-KEY (“Tissue Donors” OR tuberosity OR palate)) AND (TITLE-ABS-KEY (Graft)) |
| Title | Author | Year | Study Type | Population Sample | Control Group | Test Group | Miller Class |
|---|---|---|---|---|---|---|---|
| Multiple Coronally Advanced Flap with a Selective Use of Connective Tissue Graft: A 3-Year Prospective Clinical and Histological Study | Giovanna Laura Di Domenico et al. [15] | 2023 | non-randomized prospective clinical trial + histological study | 23 patients, total of 93 recession (59 in maxilla, 34 mandible) | MCAF alone (39 sites) | MCAF + CTG (54 sites) | RT 1 |
| Diagnosis and Treatment of Miller Class I Gingival Recession Using Free Connective Tissue Autograft from the Hard Palate. Clinical Observation. | Rushana Farkhshatova et al. [16] | 2022 | observational clinical study | 33 patients | No | patients with Miller class I, maximum no. of recessions 6 teeth per patient and minimum 1 tooth. | Class I = 24 recession zones |
| Evaluation of Subepithelial Connective Tissue Graft Versus Acellular Dermal Matrix with Modified Coronally Advanced Tunnel Technique in Treatment of Multiple Gingival Recessions: A Randomized, Parallel Design Clinical Trial | Fatema Elmahdi et al. [17] | 2022 | parallel group randomized controlled clinical trial | 24 patients | MCAT-SCTG modified coronally advanced tunnel with subepithelial connective tissue graft | MCAT-ADM acellular dermal matrix | RT1—facial maxillary or mandibular multiple adjacent gingival recessions |
| Coronally Advanced Flap in the Treatment of Multiple Adjacent Gingival Recessions Along with a Connective Tissue Graft Harvested from Augmented or Nonaugmented Palatal Mucous Membrane: A Two-Year Comparative Clinical Evaluation | Wojciech Bednarz et al. [18] | 2021 | prospective non- randomized comparative clinical trial | 35 patients with a total of 148 MAGRs | (NA-CTG + CAF) 71 gingival recessions treated with the nonaugmented connective tissue graft | (A-CTG + CAF) 77 recessions treated with augmented CTG with coronally advanced flap | Class I. 70 sites II. 60 III. 18 |
| De-Epithelialed Gingival Graft Versus Subepithelial Connective Tissue Graft in the Treatment of Multiple Adjacent Gingival Recessions Using the Tunnel Technique: 1-Year Results Of A Randomized Clinical Trial | Hikmat Bakhishov et al. [19] | 2021 | single-blinded, randomized clinical trial | 27 patients | SCTG + TUN subepithelial connective tissue graft with tunnel technique | DGG + TUN de-epithelialized gingival graft with the tunnel technique | class I and/or II (RT1) |
| Tuberosity Versus Palatal Donor Sites for Soft Tissue Grafting: A Split-Mouth Clinical Study | Peter Amin et al. [2] | 2018 | split-mouth, randomized clinical study | 20 participants, 10 for FGG, and 10 for CTG + CAF. Bilateral soft tissue grafting from tuberosity and palate carried in each patient, and served as their control | Tuberosity grafts (total 20 site): 10 FGG, 10 CTG | Palatal grafts (total 20 sites): 10 FGG, 10 CTG | Class I, II, or III, minimum of 2 mm recession |
| A Preliminary Clinical Comparison of the Use of Fascia Lata Allograft and Autogenous Connective Tissue Graft in Multiple Gingival Recession Coverage Based on the Tunnel Technique | Wojciech Bednarz et al. [20] | 2016 | comparative randomized clinical trial | 30 patients | (MCAT/CTG) modified coronally advanced tunnel technique with connective tissue graft (n = 40) | (MCAT/FL) Fascia Lata Allografts using a modified coronally advanced tunnel technique (n = 97) | Class I = 125 Class II = 12 |
| Comparison of Two Techniques of Harvesting Connective Tissue and its Effects on Healing Pattern at Palate and Recession Coverage at Recipient Site | Nymphea Pandit et al. [21] | 2016 | randomized clinical study | 16 patients with 30 sites | Group I: CTG harvested using Unigraft Knife | Group II, CTG harvested by the Langer and Langer technique | Class I and II (recession ≥ 2 mm) |
| Treatment of Multiple Adjacent Miller Class I And II Gingival Recessions with a Modified Coronally Advanced Tunnel (MCAT) Technique and a Collagen Matrix or Palatal Connective Tissue Graft: A Randomized, Controlled Clinical Trial | Salvi Arocav et al. [22] | 2013 | randomized, controlled, split-mouth clinical trial | 22 patients, 156 recessions | 78 sites Modified Coronally Advanced Tunnel (MCAT) + (CTG) | 78 sites MCAT + bioresorbable collagen matrix (CM) | Class I and II |
| Effect of Connective Tissue Graft Orientation on the Root Coverage Outcomes of Coronally Advanced Flap | Ardeshir Lafzi et al. [23] | 2007 | randomized controlled clinical trial | 8 patients with a total of 16 bilateral recessions | P-teeth: periosteum contacting the tooth surface | P-flap: periosteum contacting the flap | Class I and II |
| Evaluation of the Safety and Efficacy of Periodontal Applications of a Living Tissue-Engineered Human Fibroblast derived Dermal Substitute. II. Comparison to the Subepithelial Connective Tissue Graft: A Randomized Controlled Feasibility Study | Thomas Wilson et al. [24] | 2005 | randomized controlled split-mouth study | 13 patients | CAF + SCTG | CAF + human fibroblast-derived dermal substitute (HF-DDS) either a single or double layer (8 sites, 5 sites, respectively) | Class I or II |
| A Comparison of Two Techniques for Obtaining a Connective Tissue Graft from the Palate | Randali J. Harris [25] | 1997 | non-randomized clinical trial | 26 patients | 13 patients with 19 defects treated with free gingival graft knife harvesting method (FGGK) | 13 patients with 15 defects treated with parallel incisions harvesting method (PI) | Class I or II |
| Increased Gingival Dimensions. A Significant Factor for Successful Outcome of Root Coverage Procedures? A 2-Year Prospective Clinical Study | Jan L. Wennstrom and Giovanni Zucchelli [26] | 1996 | non-randomized prospective clinical study | 67 patients, 103 recessions | CAF alone (n = 45) | CAF + CTG (n = 58) | Class I |
| Reference | Recipient Site | Donor Site | Graft Dimension | Rec. Dimension at Baseline | Rec Dimension at F.U | Other Findings | Patient Reported Outcome/Satisfaction | Follow Up Period |
|---|---|---|---|---|---|---|---|---|
| Di Domenico et al. (2023) [15] | CAF (split-full-split) with/out CTG | palate—no details | not mentioned | mean REC: -MCAF 1.97 ± 0.87 -MCAF + CTG 2.91 ± 1.01 (significantly lower at MCAF) | MCAF RecRed 1.69 ± 0.81 %RC 93 ± 21 CRC 1 yr 90% CRC 3 yr 86% MCAF + CTG RecRed 2.69 ± 1.08 %RC 93 ± 16 CRC 1 yr 90% CRC 3 yr 81% | mean MCAF KTW0 2.39 ± 1.02 KTW 3 yr 3.19 ± 1.21 ΔKTW 0.72 ± 1.11 ΔMTT 0.22 ± 0.89 mean MCAF + CTG KTW0 1.74 ± 0.89 KTW 3 yr 3.33 ± 1.2 ΔKTW 1.69 ± 1.59 ΔMTT 1.47 ± 0.77 | not mentioned | 1 and 3 years |
| Farkhshatova et al. (2022) [16] | Tunnel—two-layer technique | palate —CTG harvested according to the standard technique, one case as example was DGG | not mentioned | Rec height: 4.0 ± 0.2 mm | Rec height: at 14 days 0.8 ± 0.07 at 1 month 1.1 ± 0.04 In 3 patients, the height of residual recession 1 month after treatment was 1.5 ± 0.03 mm in the lower ant. area | Thickness of KG: At baseline 0.79 ± 0.09 at 14 days 2.8 ± 0.03 at 1 month 1.9 ± 0.08 KG increase: at 14 days 3.2 ± 0.13 | 4-point scale (0–3). Highest pain levels on the first day after surgery (−1.78 ± 0.29 points), decreasing significantly by days 3–7 (0.2 ± 0.11 points), and completely absent by days 7–14. | day 7, 14, then after 1 and 3 months |
| Elmahdi et al. (2022) [17] | modified coronally advanced tunnel (MCAT) technique + CTG | Palate, single incision method | -CTG thickness 1 to 1.5 mm -ADM height of 6 to 7 mm apico-coronal from CEJ | ADM GRD0 2.87 ± 0.31 GRW0 3.28 ± 1.44 CTG GRD0 2.76 ± 0.89 GRW0 4 ± 1.48 | 9 M ADM RD 0.76 ± 0.1 RW 0.87 ± 0.70 RD reduction 2.10 ± 0.64 RW reduction 2.41 ± 1.19 RC% 72.72 ± 23.36 9 M CTG RD 0.53 ± 0.48 RW 1.59 ± 1.96 RD reduction 2.23 ± 0.68 RW reduction 2.41 ± 1.94 RC% 82.62 ± 16.30 | ADM KTW0 3.03 ± 0.72 KTW9 3.12 ± 0.69 KTWΔ 0.21 ± 0.84 GT0 1.10 ± 0.20 GT9 1.65 ± 0.39 GTΔ 0.53 ± 0.41 CTG KTW0 2.65 ± 0.92 KTW9 3.82 ± 1.3 KTWΔ 1.15 ± 1.16 GT0 1.33 ± 0.54 GT9 2.26 ± 0.63 GTΔ 0.94 ± 0.52 | Patient esthetic satisfaction and postoperative pain recorded using VAS. CTG group significantly higher pain levels during the first four postsurgical days. Patient satisfaction at 9 months: no difference btw grp (ADM 8.24 ± 0.43, CTG 8.24 ± 0.65) | daily till day 14, then at 9 months |
| Bednarz et al. (2021) [18] | CAF (trapizoidal flap) + CTG | palate—trap door | about 1 mm in thickness | Control (NA-CTG + CAF) GRD 3.08 ± 1.14 GRW 3.64 ± 1.25 Test (A-CTG + CAF) GRD 2.72 ± 1.00 GRW 3.47 ± 0.92 | Control (NA-CTG + CAF)) 6 M GRD 0.30 ± 0.49 GRW 0.85 ± 1.24 ARC% 91.47 ± 15.50 CRC% 66.20 12 M GRD 0.34 ± 0.49 GRW 0.95 ± 1.16 ARC% 90.48 ± 12.66 CRC% 57.75 24 M GRD 0.37 ± 0.55 GRW 0.98 ± 1.21 ARC% 89.90 ± 13.36 CRC% 57.75 Test (A-CTG + CAF) 6 M GRD 0.21 ± 0.37 GRW 0.69 ± 1.14 ARC% 93.22 ± 12.24 CRC% 71.43 12 M GRD 0.22 ± 0.36 GRW 0.73 ± 1.13 ARC% 92.64 ± 12.29 CRC% 68.83 24 M GRD 0.27 ± 0.44 GRW 0.80 ± 1.21 ARC% 91.76 ± 13.29 CRC% 67.53 | Attached Gingiva AG Control (NA-CTG + CAF) Baseline 1.51 ± 1.78 6 M 3.93 ± 1.33 12 M 3.91 ± 1.29 24 M 3.87 ± 1.33 Test (A-CTG + CAF) Baseline 1.33 ± 1.41 6 M 3.73 ± 1.00 12 M 3.70 ± 0.94 24 M 3.69 ± 0.98 | Not mentioned | 6, 12, and 24 months |
| Bakhishov et al. (2021) [19] | modified Tunnel + CTG | -palate -DGG with intra-oral de-epithelialization -SCTG single-incision SI | DGG thickness 1.40 ± 0.11 width 20.42 ± 3.53 height 4.67 ± 0.49 SCTG thickness 1.37 ± 0.11 width 17.57 ± 2.38 height 4.64 ± 0.50 | DGG RD 2.63 ± 0.89 RW 2.95 ± 0.87 SCTG RD 3.0 ± 1.19 RW 3.45 ± 0.68 | DGG RD 6 M 0.52 ± 0.73 12 M 0.27 ± 0.54 RW 6 M 0.80 ± 0.99 12 M 0.77 ± 1.16 MRC% 6 M 84.72 ± 19.72 12 M 91.72 ± 16.59 CRC% 6 M 70.0 (21/30) 12 M 76.7 (23/30) SCTG RD 6 M 0.76 ± 0.91 12 M 0.68 ± 0.99 RW 6 M 1.19 ± 1.42 12 M 1.06 ± 1.41 MRC% 6 M 79.62 ± 22.51 12 M 83.16 ± 23.32 CRC% 6 M 48.4 (15/31) 12 M 61.3 (19/31) | DGG KTH baseline 2.70 ± 1.29 6 M 3.97 ± 1.03 12 M 4.20 ± 1.03 GT baseline 0.73 ± 0.19 6 M 1.54 ± 0.44 12 M 1.58 ± 0.54 SCTG KTH baseline 2.42 ± 1.18 6 M 3.48 ± 0.93 12 M 4.13 ± 0.96 GT baseline 0.74 ± 0.20 6 M 1.51 ± 0.38 12 M 1.57 ± 0.29 Histological results: Cellularity higher in SCTG, vascularity no difference, DGG showed partly epithelial remnants in superficial portions | Using VAS; No significant differences between groups in respect to postoperative pain, patients’ discomfort, sensitivity and the amount of systemic analgesic consumption | -Patient reported outcome 1, 2, 3, 7, 14 and 28 days. -Clinical outcome 6 and 12 months. |
| Amin et al. (2018) [2] | -CAF + CTG group (20 sites): split thickness flap. -FGG group (20 site): split-thickness flap with butt joint horizontal incision | palate and tuberosity -both harvested as epithelialized grafts via double blade scalpel handle -CTG de-epithailize extraorally flap | -Thickness 1.5 mm for all grafts harvested —For CTG cases, thickness of 1.0 mm was maintained after de-epithelialization. | not mentioned | mean RC% at 8 W (for 10 CAF and CTG pt): -Tuberosity 67 ± 12% -Palate 62 ± 13% | Mean GT (8 W): -Tuberosity CAF + CTG 2.9 ± 0.5 mm FGG 2.7 ± 0.7 mm -Palate CAF + CTG 2.3 ± 0.6 mm FGG 2.1 ± 0.7 mm Mean gingival thickness of the healed tuberosity grafts was greater than of the palatal grafts | mean pain score (10-point scale) at 2 W: -Tuberosity 2.6 ± 2.2 -Palate 5.9 ± 2.7 | 2, 4, and 8 weeks |
| Bednarz et al. (2016) [20] | MCAT modified coronally advanced tunnel | palate—SI harvesting technique | not mentioned | mm CTG RD0 2.50 ± 1.01 RW0 3.20 ± 0.99 mm FL RD0 2.19 ± 0.15 RW0 3.23 ± 1.09 | CTG RD3 0.25 ± 0.44 RD6 0.13 ± 0.33 RW3 0.40 ± 0.81 RW6 0.28 ± 0.75 %ARC3 94.28 ± 0.11 %ARC6 95.77 ± 0.11 %CRC3 90.00 ± 0.18 %CRC6 94.87 ± 0.14 FL RD3 0.06 ± 0.24 RD6 0.13 ± 0.47 RW3 0.21 ± 0.92 RW6 0.35 ± 1.18 %ARC3 97.99 ± 0.09 %ARC6 94.21 ± 0.20 %CRC3 97.34 ± 0.11 %CRC6 94.24 ± 0.20 | mm CTG HKT0 2.13 ± 1.47 HKT3 3.06 ± 1.83 HKT6 3.09 ± 0.95 mm FL HKT0 2.61 ± 1.36 HKT3 3.09 ± 1.03 HKT6 2.86 ± 1.60 | Not mentioned | 3 and 6 months |
| Pandit et al. (2016) [21] | full mucoperiosteal flap using horizontal and vertical incisions following Langer and Langer technique | palate—trap door technique: -group I Unigraft knife method. -group II Langer and Langer) | Thickness: -Unigraft knife 1.5 mm -Langer and Langer was not specified | mean Rec0: grp I 3.2 ± 1.42 grp II 3.6 ± 1.12 | mean grp I Rec 3 1.6 ± 1.18 Rec 6 1.53 ± 1.14 mean grp II Rec 3 1.43 ± 1.4 Rec 6 1.33 ± 1.38 | mean grp I KT0 2.8 ± 1.66 KT3 4.2 ± 1.74 KT6 4.27 ± 1.68 mean grp II KT0 2.4 ± 0.91 KT3 4.53 ± 1.25 KT6 4.67 ± 1.18 | Using Visual analog scale (VAS) at 1-week: both groups exhibited with no significant difference Using Verbal rating scale (5-points VRS) at 1-week: group I had moderate intensity pain; group had II dull pain | 1, 4, 12 weeks, 3, 6 months |
| Aroca et al. (2013) [22] | MCAT + CTG or CM | Palate —either modified distal wedge or SI | -CTG thickness 1–1.5 mm -CM width 5 mm | mean control GRD 1.8 ± 0.5 GRW 3.8 ± 0.9 mean test GRD 1.9 ± 0.6 GRW 3.8 ± 0.8 | mean control GRD12 0.2 ± 0.3 GRW12 0.5 ± 1.0 %RC12 90 ± 18 mean test GRD12 0.6 ± 0.5 GRW12 1.4 ± 1.2 %RC12 71 ± 21 | mean control KTW0 2.0 ± 0.7 KTW12 2.7 ± 0.8 GT0 0.8 ± 0.3 GT12 1.3 ± 0.4 mean test KTW0 2.1 ± 0.9 KTW12 2.4 ± 0.7 GT0 0.8 ± 0.2 GT12 1.0 ± 0.3 | using VAS at 12 M—patient complaint: control 12.8 ± 7.5 test 7.3 ± 3.4 -patient satisfaction: control 90.6 ± 7.9 test 92.9 ± 8.4 -The number of 100% satisfaction was higher in the test group, but not statistically significant | 6 and 12 months |
| Lafzi et al. (2007) [23] | CAF + CTG | palate—horizontal incision with vertical incision mesially | Thickness 0.9 mm | mean P-teeth RD 4.46 ± 0.48 RW 3.00 ± 0.65 mean P-flap RD 4.56 ± 0.56 RW 3.06 ± 0.49 | mean P-teeth RD 0.78 ± 0.99 RW 0.31 ± 0.37 CRC 50% of the cases %RC 81.77 mean P-flap RD 1.31 ± 1.16 RW 0.43 ± 0.41 CRC 37.5% of the cases %RC 72.39 | P-teeth LKT0 1.37 ± 0.53 LKT3 2.62 ± 0.64 P-flap LKT0 1.25 ± 0.49 LKT3 2.56 ± 0.56 | using patient questioning: esthetic concerns were met in PRC above 70%, gradual reduction in PRC declined to 60%, sudden dissatisfaction in PRCs below 60% | 1, 3 months |
| Wilson et al. (2005) [24] | CAF + CTG or HF-DDS | palate—no details | not mentioned | mean control RD 3.9 ± 0.88 RW 3.9 ± 0.88 mean test RD 3.7 ± 0.82 RW 4.2 ± 0.92 | mean control test RD1 0.7 ± 0.68 1.4 ± 0.70 RD3 1.4 ± 0.97 1.9 ± 0.57 RD6 1.4 ± 1.30 1.6 ± 0.92 RW6 2.4 ± 2.07 3.4 ± 1.77 %RC1 83.7 ± 15.3 62.8 ± 17.6 %RC3 64.2 ± 23.8 47.0 ± 20.3 %RC6 64.4 ± 31.9 56.7 ± 27.8 | mean control KT0 1.9 ± 0.88 KT6 2.1 ± 0.84 mean test KT0 1.9 ± 0.88 KT6 2.1 ± 0.64 | questionnaire was used for discomfort and satisfaction. Patient satisfaction in regard to tissue contours and color match was similar regardless of the graft material used. | 1 week after surgery, and at months 1, 3, and 6 |
| Harris (1997) [25] | partial thickness double pedicle graft technique | palate—free gingival graft knife method or parallel incisions method | size mm2—FGGK 85.9 ± 23.6—PI 86.9 ± 23.1 | mean FGGK RD 3.5 ± 0.8 RW 3.0 ± 0.6 mean PI RD 3.2 ± 0.9 RW 4.3 ± 2.0 | mean12 W FGGK PI RD 0.1 ± 0.2 0.1 ± 0.3 RW 0.2 ± 0.5 0.2 ± 0.8 RC% 98.3 ± 4.2 98.7 ± 5 CRC% 84.2 93.3 | mean FGGK KTW0 1.5 ± 1.4 KTW12 5.2 ± 1.6 mean PI KTW0 1.4 ± 1.2 KTW12 5.0 ± 1.2 | -Patients were asked to rate discomfort level, and grouped as: no pain or minimal pain, or greater than minimal pain. -PI produced less postoperative discomfort compared to the FGGK method. | 1, 2, 4, 8, 12 weeks postoperative |
| Wennstrom and Zucchelli (1996) [26] | CAF with/out CTG | palate—trap door technique | Thickness 1.5 to 2 mm | mean RD: control 4.1 ± 0.9 test 4.0 ± 1.0 | mean control test RD 6 M 0.2 ± 0.4 0.2 ± 0.3 RD 12 M 0.1. ± 0.3 0.1 ± 0.2 RD 24 M 0.2 ± 0.3 0.1 ± 0.2 %RC 6 M 96.4 ± 6.5 96.1 ± 6.7 %RC 12 M 97.7 ± 5.2 98.7 ± 3.3 %RC2 4 M 97.1 ± 6.2 98.9 ± 3.1 %CRC 6 M 74 72 %CRC 12 M 83 86 %CRC 24 M 80 88 | mean GH control baseline 1.1 ± 0.5 6 M 1.5 ± 0.5 12 M 2.3 ± 0.6 24 M 2.2 ± 0.6 mean GH test baseline 0.9 ± 0.5 6 M 3.5 ± 0.6 12 M 3.7 ± 0.7 24 M 3.7 ± 0.6 | not mentioned | 6, 12, 24 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyawar, S.; Al Zahra, F.; Aljoghaiman, E.; Aljofi, F.E.; Alagl, A.S.; Madi, M. Clinical and Patient-Reported Outcomes for Intraoral (Palatal and Tuberosity) Soft Tissue Grafts in Root Coverage Procedures: A Systematic Review. Dent. J. 2025, 13, 563. https://doi.org/10.3390/dj13120563
Alyawar S, Al Zahra F, Aljoghaiman E, Aljofi FE, Alagl AS, Madi M. Clinical and Patient-Reported Outcomes for Intraoral (Palatal and Tuberosity) Soft Tissue Grafts in Root Coverage Procedures: A Systematic Review. Dentistry Journal. 2025; 13(12):563. https://doi.org/10.3390/dj13120563
Chicago/Turabian StyleAlyawar, Suha, Fatima Al Zahra, Eman Aljoghaiman, Faisal E. Aljofi, Adel S. Alagl, and Marwa Madi. 2025. "Clinical and Patient-Reported Outcomes for Intraoral (Palatal and Tuberosity) Soft Tissue Grafts in Root Coverage Procedures: A Systematic Review" Dentistry Journal 13, no. 12: 563. https://doi.org/10.3390/dj13120563
APA StyleAlyawar, S., Al Zahra, F., Aljoghaiman, E., Aljofi, F. E., Alagl, A. S., & Madi, M. (2025). Clinical and Patient-Reported Outcomes for Intraoral (Palatal and Tuberosity) Soft Tissue Grafts in Root Coverage Procedures: A Systematic Review. Dentistry Journal, 13(12), 563. https://doi.org/10.3390/dj13120563







