The authors wish to make the following corrections to this paper [1]: Update the Figure 21 and Figure 22 due to the mistake on the symbols. After the publication of this work, we noted the mistake and issued an erratum for correction. Figure 21 and Figure 22 have now been corrected in this erratum.
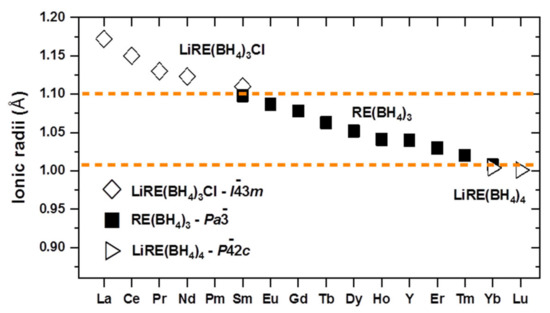
Figure 21.
Overview of borohydride phases obtained by mechanochemical reactions of RECl3 and LiBH4. The ionic radius of RE3+ cations in the solid-state are displayed in octahedral environment [388]. Reproduced with permission from Wegner, W.; Jaron, T.; Grochala, W. Polymorphism and hydrogen discharge from holmium borohydride, Ho(BH4)3, and KHo(BH4)4. International Journal of Hydrogen Energy, 2014, 39, pp. 20024–20030, Copyright (2014), with permission from Elsevier.
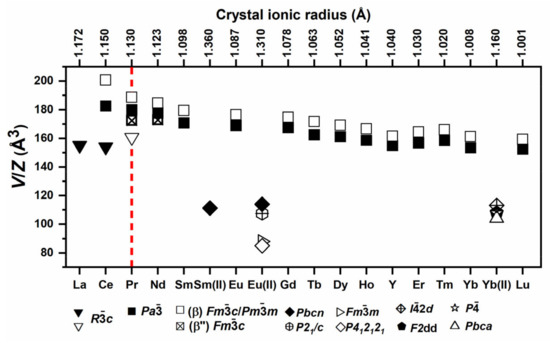
Figure 22.
Unit cell volumes (V) of the reported rare earth borohydrides divided by the number of formula units (Z) [391,392,395,396,399,400,402–407] are presented. The ionic crystal radius were taken from [388]. The high-temperature polymorphs are displayed with empty signs. The figure was adapted from [408] with permission from The Royal Society of Chemistry.
The authors and the Editorial Office would like to apologize for any inconvenience caused to the readers by these changes.
References
- Hadjixenophontos, E.; Dematteis, E.M.; Berti, N.; Wołczyk, A.R.; Huen, P.; Brighi, M.; Le, T.T.; Santoru, A.; Payandeh, S.; Peru, F.; et al. A Review of the MSCA ITN ECOSTORE—Novel Complex Metal Hydrides for Efficient and Compact Storage of Renewable Energy as Hydrogen and Electricity. Inorganics 2020, 8, 17. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).