Abstract
Activation of the dialkylpalladium complex (phen)Pd(CH3)2 (phen = 1,10-phenanthroline) with B(C6F5)3 affords a competent catalyst for the dimerization of vinyl silanes. All organic products of the catalytic dimerization of trialkoxyvinylsilanes were characterized by in situ NMR spectroscopy and GC–MS. The putative palladium cation was characterized by NMR spectroscopy. Upon activation, the palladium complex generated products in moderate yield (60–70%) and selectivity (~60:40, dimer:disproportionation products).
1. Introduction
Transformations of chemical feedstocks, small molecules such as ethylene and styrene, are of interest in the area of organometallic chemistry [1,2,3,4,5]. Of particular interest are the products of homocoupling reactions of unsaturated substances; such processes attract attention because they function as model systems for both oligomerization and polymerization reactions of alkenes [6,7]. A wide variety of homocoupling reactions of unsaturated substrates are known [8,9,10,11,12,13,14,15,16,17,18,19,20]; however, only a few exist that involve vinyl silanes [21,22,23,24,25,26,27,28,29].
Trialkoxyvinylsilanes are attractive substrates to target as they are used extensively in materials and surface science [30,31,32,33,34,35,36,37,38], nanotechnology [39,40] for various applications including modification of surfaces [31,37], formation of sol–gel [36,37], and organogelators [32,34,35], to obtain novel organic–inorganic hybrid materials [33], preparation of nanocomposites [39], and several hybrid [38] and supported catalysts. In most of these applications, the nature of the precursor trialkoxysilane has a profound effect on the properties of the final product [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Therefore, the availability of a diverse pool of trialkoxysilane precursors would enable incorporation of unprecedented properties to those end products [30,31,32,33,34,35,36,37,38,39,40,41]. In organic syntheses, trialkoxyvinylsilanes are reaction partners in numerous transformations including Heck reactions [42,43], Friedel–Crafts alkylations [44,45], Diels–Alder reactions [46,47], and 1,3-dipolar additions [48,49].
Similarly, bis(trialkoxysilanes) containing unsaturated double bonds would be even more important reagents in building molecular complexity via aforementioned transformations since they may contain two similar or dissimilar alkoxysilane groups [50]. Surprisingly, bis(trialkoxysilanes) containing unsaturated double bonds, such as 1,4-bis(trimethoxysilyl)butenes, are very rarely used in organic syntheses or materials science [50]. Synthetic methods for their preparation are scarce [51,52]. For example, Khvostenko et al. reported the formation of (E)-1,4-bis(trimethoxysilyl)-1-butene in 80% yield with 49% conversion of starting trimethoxy(vinyl)silane in the presence of a complex Ti(OBu)4:Ph3P:Et3Al (1:1:6) catalytic system [51]. Keller and Matusiak observed that, in the presence of alkylidenedinitrosylmolybdenum complexes (Mo(NO)2(CHMe)(OR)2(AlCl2)(EtAlCl2) (R = Et, O-i-Pr)), self-metathesis and dimerization of trimethyl(vinyl)silane takes place, resulting in formation of 1,2-bis(trimethylsilyl)ethene (yield = 20%) and 1,4-bis(trimethylsilyl)but-2-ene (yield = 30%) as major reaction products, respectively [52]. A number of transition-metal complexes are known to mediate homocoupling of (vinyl)silanes via disproportionation leading to 1,2- or 1,1-diylbis(trialkoxy/alkylsilyl)ethene derivatives (Scheme 1) [53]. We were intrigued by the recent report of Jun and coworkers that a simple iridium catalyst could affect vinyl silane dimerization in a regioselective manner (Scheme 1) [29].
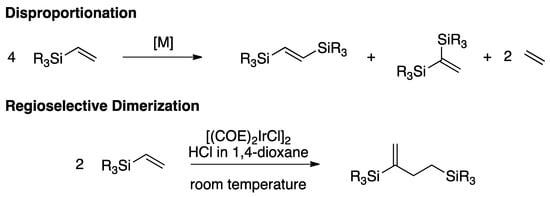
Scheme 1.
Homocoupling of vinyl silanes via disproportionation or dimerization.
Pd-complexes are not typically employed in catalyzing the disproportionation of (vinyl)silanes, although they do mediate other reactions, which include dehydrogenative silation, hydrosilation, and self- and co-polymerizations of various (vinyl)silyl compounds with olefins [54,55,56,57]. For example, cationic Pd(II) complexes, [(phen)Pd(CH3)L]+[BAr′4]− (phen = 1,10-phenanthroline; L = Et2O, Me3SiC≡CSiMe3; Ar′ = 3,5-(CF3)2C6H3) are known to catalyze dehydrogenative silation and hydrosilation of (vinyl)silanes [54]. Additionally, (α-Diimine)PdMe+ complexes (α-diimine = (2,6-iPr2–C6H3)N=C(Me)C(Me)=N(2,6-iPr2–C6H3)) copolymerize (vinylsilyl)ethers with olefins forming OSiR3-containing polyolefins [55]. Moreover, the aforementioned complexes are able to polymerize (vinyl)ethers [56]. The palladium complex [(α-diimine)Pd(μ-Cl)]2+ dimerizes (vinyl)ethers, including (vinylsilyl)ether, to the corresponding acetals [57]. In this brief communication, we report on the capability of the in situ generated cationic system (Phen)PdMe to dimerize (vinyl)silanes to 1,4-bis(trialkoxysilyl)butenes. Products were characterized using a combination of GC–MS and multi-nuclear NMR analysis.
2. Results and Discussion
The cationic system (Phen)PdMe is readily prepared by mixing the known dimethyl precursor, (Phen)PdMe2 [58], with one equivalent of B(C6F5)3 (Scheme 2). Upon mixing, the newly formed compound is readily soluble in methylene chloride. When carried out in CD2Cl2 at room temperature, formation of a new ionic complex is clear from NMR analysis; the 11B NMR of the reaction mixture shows a new sharp peak at −16 ppm, while, in the 1H NMR of the same mixture, a new singlet shows up at 0.2 ppm. These new NMR signals represent the tetra-coordinated anionic boron species [MeB(C6F5)3]− [59,60]. From these observations, the formation of the ionic species [(Phen)PdMe]+[MeB(C6F5)3]− is proposed.
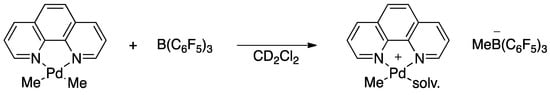
Scheme 2.
Synthesis of the putative Pd cation (phen)Pd(Me)(solv.).
Subsequently, we applied this complex as the catalyst (1 mol %) in the homodimerization of trimethoxy(vinyl)silane (1a) to furnish 1,4-bis(trimethoxysilyl)butene isomers (2aa and 3aa), 1,2-bis(triethoxy(silane))ethylene (4aa), and 1,1′-bis(trimethoxysilylmethyl)ethylene (5aa) (Scheme 3). Organic reaction products were identified based upon GC–MS and NMR analysis (see Supplementary Materials). There is moderate selectivity for homodimers 2aa and 3aa over disproportionation products 4aa and 5aa. Notably, control experiments that employed (Phen)PdMe2 or B(C6F5)3 alone, under otherwise identical conditions, did not affect the dimerization reaction. We believe this strongly implicates the cationic, coordinatively unsaturated [(Phen)PdMe]+ to be responsible for catalytic activity.
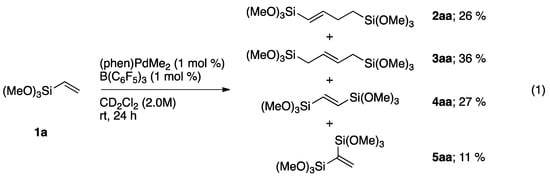
Scheme 3.
NMR-scale homodimerization studies of 1a catalyzed by (phen)Pd(Me)(solv.).
Under these non-optimized reaction conditions triethoxy(vinyl)silane also dimerizes to form the corresponding dimers with 70% combined yield as a mixture of isomers (Scheme 4). Disproportionation of (vinyl)silanes was somewhat minimized in the reaction of the bulkier substrate 1b compared to 1a; 1,2- and 1,1-bis(trialkoxylsilyl)ethene isomers produced in combined yields of 38% (4aa and 5aa, Scheme 4) and 24% (4bb and 5bb, Scheme 4) with trimethoxy- and triethoxy(vinyl)silane, respectively.
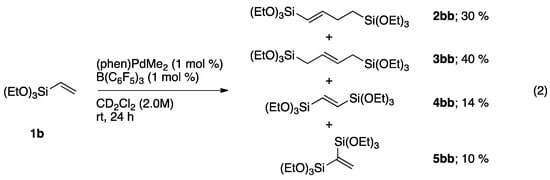
Scheme 4.
NMR-scale homodimerization studies of 1b catalyzed by (phen)Pd(Me)(solv.).
In competing reactions of 1a with styrene (1c), under similar reaction conditions, the cross-dimer product 6 was obtained as the major product after a 96-h reaction period (Scheme 5, top). At 96 h, the conversion of trimethoxy(vinyl)silane was only 76%. Homodimerization and disproportionation of trimethoxy(vinyl)silane also took place, forming corresponding products in trace amounts (as evidenced by GC–MS). In a similar competition experiment between trimethoxy(vinyl)silane and ethylene (1d), no cross-dimer product between alkene monomers or homodimerization product of trimethoxy(vinyl)silane was observed (Scheme 5, bottom). Taken together, our results lead us to propose the catalytic cycle shown in Scheme 6. Of particular significance, the work of Elsby and Johnson, in closely related C–H silylation chemistry employing a nickel catalyst, lends support to our proposed mechanism [61].
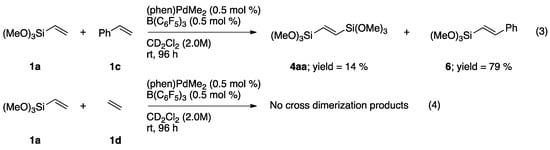
Scheme 5.
NMR-scale cross-dimerization studies of 1a with 1c (top) or 1d (bottom) catalyzed by (phen)Pd(Me)(solv.).
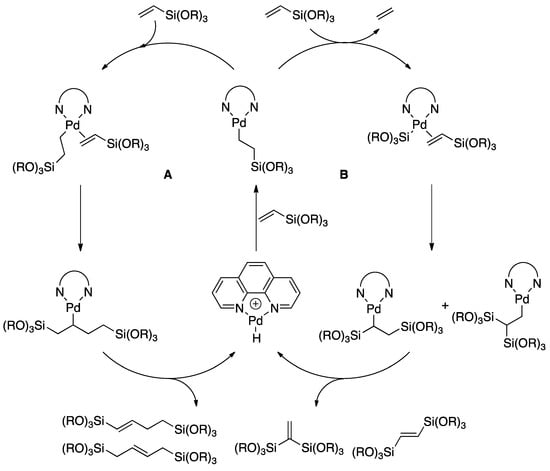
Scheme 6.
Proposed mechanism of dimerization and disproportionation to account for product distribution.
3. Conclusions
In summary, we disclosed a (vinyl)silane dimerization reaction leading to a mixture of 1,4-bis(trimethylsilyl)but-2-ene and 1,4-bis(trimethylsilyl)but-1-ene as major products. This reaction is believed to be mediated by the [(Phen)PdMe]+[MeB(C6F5)3]− complex generated in situ at a 1 mol % catalyst loading. The substrate scope of the reaction over various (vinyl)silanes and more detailed mechanistic studies are underway in our laboratories to elaborate on this preliminary communication.
Supplementary Materials
The following are available online at http://www.mdpi.com/2304-6740/6/4/102/s1: general remarks; typical procedure for catalytic dimerization reactions; NMR data of catalytic reactions. Reference [54] is cited in the supplementary materials.
Author Contributions
Conceptualization, S.P. and M.F.; Project Administration, M.F.; Performing experiments and characterization of compounds, S.P.; Writing (all), M.F.
Funding
This research received no external funding.
Acknowledgments
Start-up funds from Texas Tech University are gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- McLain, S.J.; Sancho, J.; Schrock, R.R. Selective dimerization of monosubstituted. α-olefins by tantalacyclopentane catalysts. J. Am. Chem. Soc. 1980, 102, 5610–5618. [Google Scholar] [CrossRef]
- Pillai, S.M.; Ravindranathan, M.; Sivaram, S. Dimerization of ethylene and propylene catalyzed by transition-metal complexes. Chem. Rev. 1986, 86, 353–399. [Google Scholar] [CrossRef]
- Skupińska, J. Oligomerization of α-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Piers, W.E.; Shapiro, P.J.; Bunel, E.E.; Bercaw, J.E. Coping with Extreme Lewis Acidity: Strategies for the Synthesis of Stable, Mononuclear Organometallic Derivatives of Scandium. Synlett 1990, 74–84. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Ohmiya, H.; Jamison, T.F. Alpha-olefins as alkenylmetal equivalents in catalytic conjugate addition reactions. Angew. Chem. Int. Ed. 2008, 47, 1893–1895. [Google Scholar] [CrossRef] [PubMed]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Kondo, T.; Takagi, D.; Tsujita, H.; Ura, Y.; Wada, K.; Mitsudo, T.-A. Highly Selective Dimerization of Styrenes and Linear Co-dimerization of Styrenes with Ethylene Catalyzed by a Ruthenium Complex. Angew. Chem. Int. Ed. 2007, 46, 5958–5961. [Google Scholar] [CrossRef] [PubMed]
- Tobisu, M.; Hyodo, I.; Onoe, M.; Chatani, N. Rhodium-catalysed anomalous dimerization of styrenes involving the cleavage of the ortho C–H bond. Chem. Commun. 2008, 45, 6013–6015. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.-Y.; He, L. Catalytic Intermolecular Tail-to-Tail Hydroalkenylation of Styrenes with α Olefins: Regioselective Migratory Insertion Controlled by a Nickel/N-Heterocyclic Carbene. Angew. Chem. Int. Ed. 2010, 49, 9182–9186. [Google Scholar] [CrossRef] [PubMed]
- Ez-Zoubir, M.; d’Herouville, F.L.B.; Brown, J.A.; Ratovelomanana-Vidal, V.; Michelet, V. Stereoselective Ir (III)-catalyzed dimerization reaction of enynes: An entry to functionalized polyunsaturated and cyclic systems. Chem. Commun. 2010, 46, 6332–6334. [Google Scholar] [CrossRef] [PubMed]
- Barlow, M.G.; Bryant, M.J.; Haszeldine, R.N.; Mackie, A.G.J. Organic reactions involving transition metals III. The palladium (II)-catalysed dimerization of olefinic compounds. Organomet. Chem. 1970, 21, 215–226. [Google Scholar]
- Dawans, F. Dimerisation stereospecifique du styrene en presence de bis(trifluoroacetate de nickel π-allyle). Tetrahedron Lett. 1971, 12, 1943–1946. [Google Scholar] [CrossRef]
- Sen, A.; Lai, T.-W. Oligomerization and isomerization of olefins by. eta. 3-allyl complexes of palladium. The role of the allyl group. Organometallics 1983, 2, 1059–1060. [Google Scholar] [CrossRef]
- Grenouillet, P.; Neibecker, D.; Tkatchenko, I. Cationic allylmetal complexes. 9. Dimerization of acrylates catalyzed by allylpalladium complexes. Role of the ligand and indirect evidence for the occurrence of hydridopalladium species. Organometallics 1984, 3, 1130–1132. [Google Scholar] [CrossRef]
- Wu, G.; Rheingold, L.A.; Heck, R.F. Cinnolinium salt synthesis from cyclopalladated azobenzene complexes and alkynes. Organometallics 1987, 6, 2386–2391. [Google Scholar] [CrossRef]
- Jiang, Z.; Sen, A. Tailored cationic palladium(II) compounds as catalysts for highly selective linear dimerization of styrene and linear polymerization of p-divinylbenzene. J. Am. Chem. Soc. 1990, 112, 9655–9657. [Google Scholar] [CrossRef]
- Tsuchimoto, T.; Kamiyama, S.; Negoro, R.; Shirakawa, E.; Kawakami, Y. Palladium-catalysed dimerization of vinylarenes using indium triflate as an effective co-catalyst. Chem. Commun. 2003, 40, 852–853. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Dong, G.; Venkataiah, B. Investigation of the behavior of arenediazonium salts with olefins in BmimPF6. Tetrahedron Lett. 2004, 45, 2775–2777. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Qiu, H.; Jiang, J.; Jiang, K.; Mao, J.; Lai, G. Dimerization of styrene to 1, 3-diphenyl-1-butene catalyzed by palladium-Lewis acid in ionic liquid. J. Mol. Catal. A Chem. 2006, 255, 16–18. [Google Scholar] [CrossRef]
- Yusupova, F.G.; Gailyunas, G.A.; Furley, I.I.; Panasenko, A.A.; Sheludyakov, V.D.; Tolstikov, G.A.; Yurjev, V.P. Linear co-oligomerization of vinylsilanes with butadiene as a method of synthesizing silicon-containing polyenes. J. Organomet. Chem. 1978, 155, 15–23. [Google Scholar] [CrossRef]
- Cros, P.; Triantaphylides, C.; Buono, G. Nickel-catalyzed codimerization of 1,3-cyclohexadiene and vinyltrimethylsilane. Effects of organophosphorus ligands. J. Org. Chem. 1988, 53, 185–187. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Troyanov, S.I.; Meetsma, A.; Hessen, B.; Teuben, J.H. Regioselective Homo-and Codimerization of α-Olefins Catalyzed by Bis (2,4,7-trimethylindenyl) yttrium Hydride. Organometallics 1998, 17, 284–286. [Google Scholar] [CrossRef]
- Ho, C.-Y.; He, L. Shuffle off the classic β-Si elimination by Ni–NHC cooperation: Implication for C–C forming reactions involving Ni-alkyl-β-silanes. Chem. Commun. 2012, 48, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yamamoto, H.; Yamane, M. Rhodium-Catalyzed Homocoupling of (1-Acyloxyvinyl) silanes: Synthesis of 1,3-Diene-2,3-diyl Diesters and Their Derivatives. Synlett 2009, 2831–2835. [Google Scholar] [CrossRef]
- Marciniec, B. Catalytic Coupling of sp2- and sp-Hybridized Carbon–Hydrogen Bonds with Vinylmetalloid Compounds. Acc. Chem. Res. 2007, 40, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, B.; Walczuk-Gusciora, E.; Pietraszuk, C. Activation of the Vinylic C–H Bond of Styrene by a Rhodium-Siloxide Complex: The Key Step in the Silylative Coupling of Styrene with Vinylsilanes. Organometallics 2001, 20, 3423–3428. [Google Scholar] [CrossRef]
- Marciniec, B.; Kownacki, I.; Kubicki, M. Synthesis, Structure, and Reactivity of [{Ir(cod)(μ-OSiMe3)}2] with Styrene and Vinylsilanes: Catalytic Activation of the Vinylic C–H Bond. Organometallics 2002, 21, 3263–3270. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, S.J.; Jun, C.-H. Ir(I)/HCl Catalyzed Head-to-Tail Homocoupling Reactions of Vinylsilanes. Org. Lett. 2012, 14, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, H.; Zhang, J.; Zhao, X. Synthesis and Structural Characterization of Multifunctional Silicone Co-Polymer. Adv. Mater. Res. 2012, 549, 147–151. [Google Scholar] [CrossRef]
- Dai, Z.; Wang, Z.; Wu, J.; Xu, J.; Zhao, N. Vacuum-Dried Robust Bridged Silsesquioxane Aerogels. Adv. Mater. 2013, 25, 4494–4497. [Google Scholar]
- Monkiewicz, J.; Roth, S.; Springer, C.; Standke, B.; Weissenbach, K. Polymer Compositions Based on Olefinically Functionalized Siloxane Oligomerss with Organic Polymers and Inorganic Solids. Patent DE 102011086869 A1, 23 May 2013. [Google Scholar]
- Luyt, A.S.; Sibeko, M.A. Preparation and characterisation of vinylsilane crosslinked low-density polyethylene composites filled with nano clays. Polym. Bull. 2014, 71, 637–657. [Google Scholar] [CrossRef]
- Nishisako, H. Epoxy Resin Powder Coatings with Good Electric Insulation and Electrolyte Resistance. Patent JP 2008081528 A, 10 April 2008. [Google Scholar]
- Kim, H.J.; Park, W.B.; Trung, T.Q. Method for Preparing Silicone-Epoxy-Vinyl Resins for Adhesives with Good Filler Wettability and Adhesion. Patent KR 2012081719 A, 20 July 2012. [Google Scholar]
- Chen, Z.; Hu, Y.; Qu, X.; Xie, H.; Zhu, H. Corrosion inhibition of flaky aluminium powders prepared through sol–gel process. Corros. Sci. 2011, 53, 481–486. [Google Scholar]
- Corobea, M.C.; Donescu, D.; Ghiurea, M.; Nistor, C.L.; Petcu, C.; Serban, S. Polymer-silica hybrids obtained by microemulsion polymerization. Colloid Polym. Sci. 2007, 285, 1455–1462. [Google Scholar]
- Cakmak, M.; Cao, Y.; Soucek, M.D. Antireflective coatings using organically modified silica and polyimide via solution casting method. Polym. Eng. Sci. 2013, 53, 2228–2241. [Google Scholar]
- Deng, H.; Hou, Y.; Li, H.; Zeng, X. Preparation and Characterization of Organosilicon-Acrylate/Montmorillonite Nanocomposite Emulsions. Huagong Xinxing Cailiao 2010, 38, 32–34. [Google Scholar]
- Bosnyak, C.P.; Chou, C.-J.; Garcia-Meitin, E.I.; Read, A.E. Polymer Nanocomposite. In Proceedings of the Annual Technical Conference-Society of Plastics Engineers, San Francisco, CA, USA, 5–9 May 2002; Volume 2, pp. 1452–1456. [Google Scholar]
- Pavithran, C.; Nair, B.P. Clay Nanocomposite Forming Microcapsule Useful for Guest Encapsulation and Process Thereof. Patent WO 2010131258 A1, 18 November 2010. [Google Scholar]
- Chen, W.; Lu, C.; Xu, D. Palladium-catalyzed double arylations of terminal olefins in acetic acid. Tetrahedron 2012, 68, 1466–1474. [Google Scholar]
- Fujita, T.; Hirai, K.; Mino, T.; Sakamoto, M.; Shibuya, M.; Suzuki, S. Palladium-catalyzed Mizoroki-Heck type reaction with aryl trialkoxysilanes using hydrazone ligands. Tetrahedron 2012, 68, 429–432. [Google Scholar]
- Darses, S.; Genet, J.-P.; Martinez, R.; Simon, M.-O. C–C Bond Formation via C–H Bond Activation under Protic Conditions: On the Role of Phosphane Ligand and Cosolvent. J. Org. Chem. 2010, 75, 208–210. [Google Scholar]
- Chatani, N.; Kakiuchi, F.; Kamatani, A.; Murai, S.; Sekine, S.; Sonoda, M.; Tanaka, Y. Efficient catalytic addition of aromatic carbon-hydrogen bonds to olefins. Nature 1993, 366, 529–531. [Google Scholar]
- Pidaparthi, R.R.; Junker, C.S.; Welker, M.E.; Day, C.S.; Wright, M.W. Preparation of 2-Silicon-Substituted 1,3-Dienes and Their Diels-Alder/Cross-Coupling Reactions. J. Org. Chem. 2009, 74, 8290–8297. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Jung, D.U.; Jung, Y.S.; Lim, W.C.; Yoo, B.R. Method for Preparing Cyclohexenylsilane by Diels-Alder Reaction. Patent KR 2008107911 A, 11 December 2008. [Google Scholar]
- Berthon-Gelloz, G.; Marchant, M.; Marko, I.E.; Straub, B.F. Palladium-Catalyzed Cyclopropanation of Alkenyl Silanes by Diazoalkanes: Evidence for a Pd0 Mechanism. Chem.-Eur. J. 2009, 15, 2923–2931. [Google Scholar] [CrossRef] [PubMed]
- Lorberth, J.; Nasim, M.; Petrosyan, V.S.; Zaitseva, G.S. Synthesis of silatranyl- and 3,7,10-trimethylsilatranyl-cyclopropanes. J. Organomet. Chem. 1992, 430, 269–272. [Google Scholar]
- Marciniec, B.; Pawluć, P.; Prukała, W. Silylative Coupling of Olefins with Vinylsilanes in the Synthesis of π-Conjugated Double Bond Systems. Eur. J. Org. Chem. 2010, 219–229. [Google Scholar] [CrossRef]
- Gailiunas, G.; Khvostenko, V.I.; Monakhova, E.S.; Nurtdinova, G.V.; Yusupova, F.G. Oligomerization transformations of vinylsilanes with acceptor substituents. Dokl. Akad. Nauk SSSR 1979, 249, 114–116. [Google Scholar]
- Keller, A.; Matusiak, R. Reaction of alkylidenedinitrosylmolybdenum complexes with vinyl trisubstituted silanes and substituted acetylenes. J. Mol. Catal. A Chem. 1996, 104, 213–219. [Google Scholar] [CrossRef]
- Bogdan, M. Catalysis by transition metal complexes of alkene silylation—recent progress and mechanistic implications. Coord. Chem. Rev. 2005, 249, 2374–2390. [Google Scholar]
- Brookhart, M.; Francis, C.R.; LaPointe, A.M. Mechanistic studies of palladium (II)-catalyzed hydrosilation and dehydrogenative silation reactions. J. Am. Chem. Soc. 1997, 119, 906–917. [Google Scholar]
- Jordan, R.F.; Luo, S. Copolymerization of silyl vinyl ethers with olefins by (α-diimine)PdR+. J. Am. Chem. Soc. 2006, 128, 12072–12073. [Google Scholar]
- Chen, C.; Jordan, R.F.J.; Luo, S. Cationic polymerization and insertion chemistry in the reactions of vinyl ethers with (α-diimine) PdMe+ species. J. Am. Chem. Soc. 2010, 132, 5273–5284. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jordan, R.F. Palladium-catalyzed dimerization of vinyl ethers to acetals. J. Am. Chem. Soc. 2010, 132, 10254–10255. [Google Scholar] [CrossRef] [PubMed]
- Aye, K.-T.; Canty, A.J.; Crespo, M.; Puddephatt, R.J.; Scott, J.D.; Watson, A.A. Alkyl halide transfer from palladium (IV) to platinum (II) and a study of reactivity, selectivity, and mechanism in this and related reactions. Organometallics 1989, 8, 1518–1522. [Google Scholar]
- Bibal, C.; Bourbigou, H.-O.; Chauvin, Y.; Santini, C.C.; Vallée, C. A selective synthesis of hydroxyborate anions as novel anchors for zirconocene catalysts. Dalton Trans. 2008, 2866–2870. [Google Scholar] [CrossRef] [PubMed]
- Bellachioma, G.; Cardaci, G.; Foresti, E.; Macchioni, A.; Sabatino, P.; Zuccaccia, C. Reactions of alkyl-iron(II) and -ruthenium(II) complexes with B(C6F5)3 and its water adducts. X-ray structure of a cyclometallated-iron(II) carbine. Inorg. Chim. Acta 2003, 353, 245–252. [Google Scholar] [CrossRef]
- Elsby, M.R.; Johnson, S.A. Nickel-Catalyzed C–H Silylation of Arenes with Vinylsilanes: Rapid and Reversible β-Si Elimination. J. Am. Chem. Soc. 2017, 139, 9401–9407. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).