Structural Study of Mismatched Disila-Crown Ether Complexes
Abstract
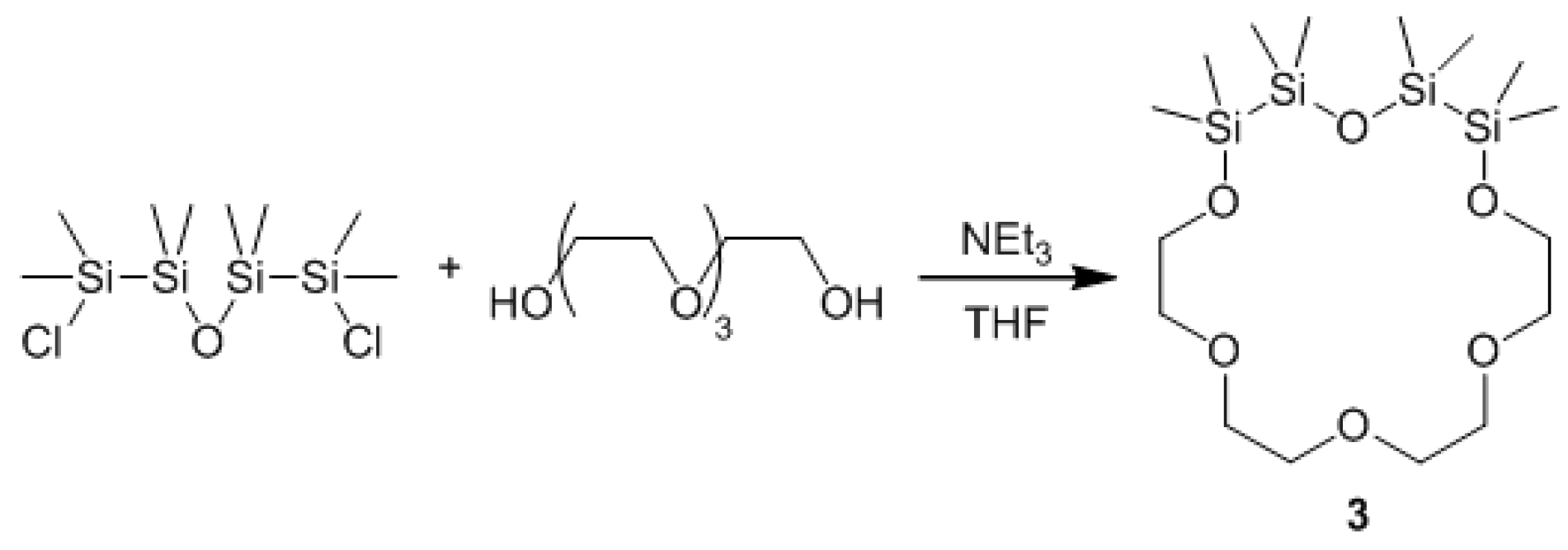
:1. Introduction
2. Results and Discussion
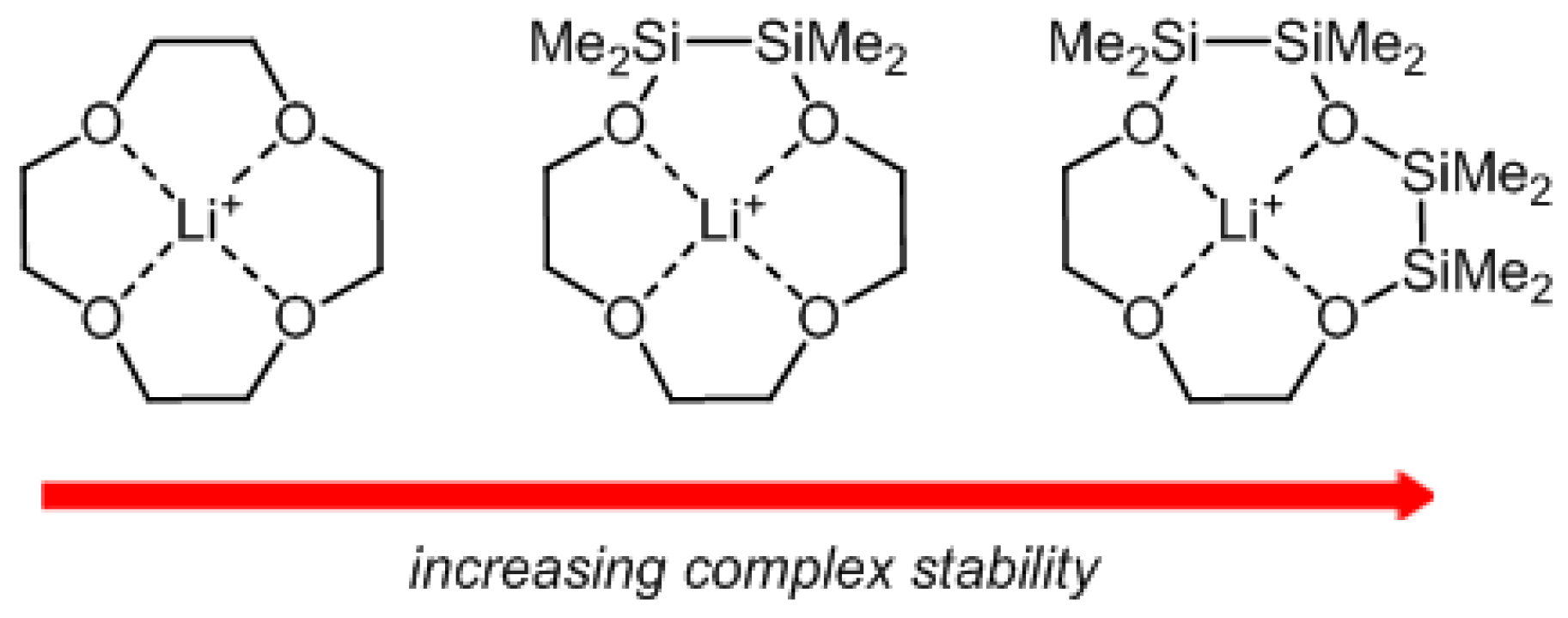
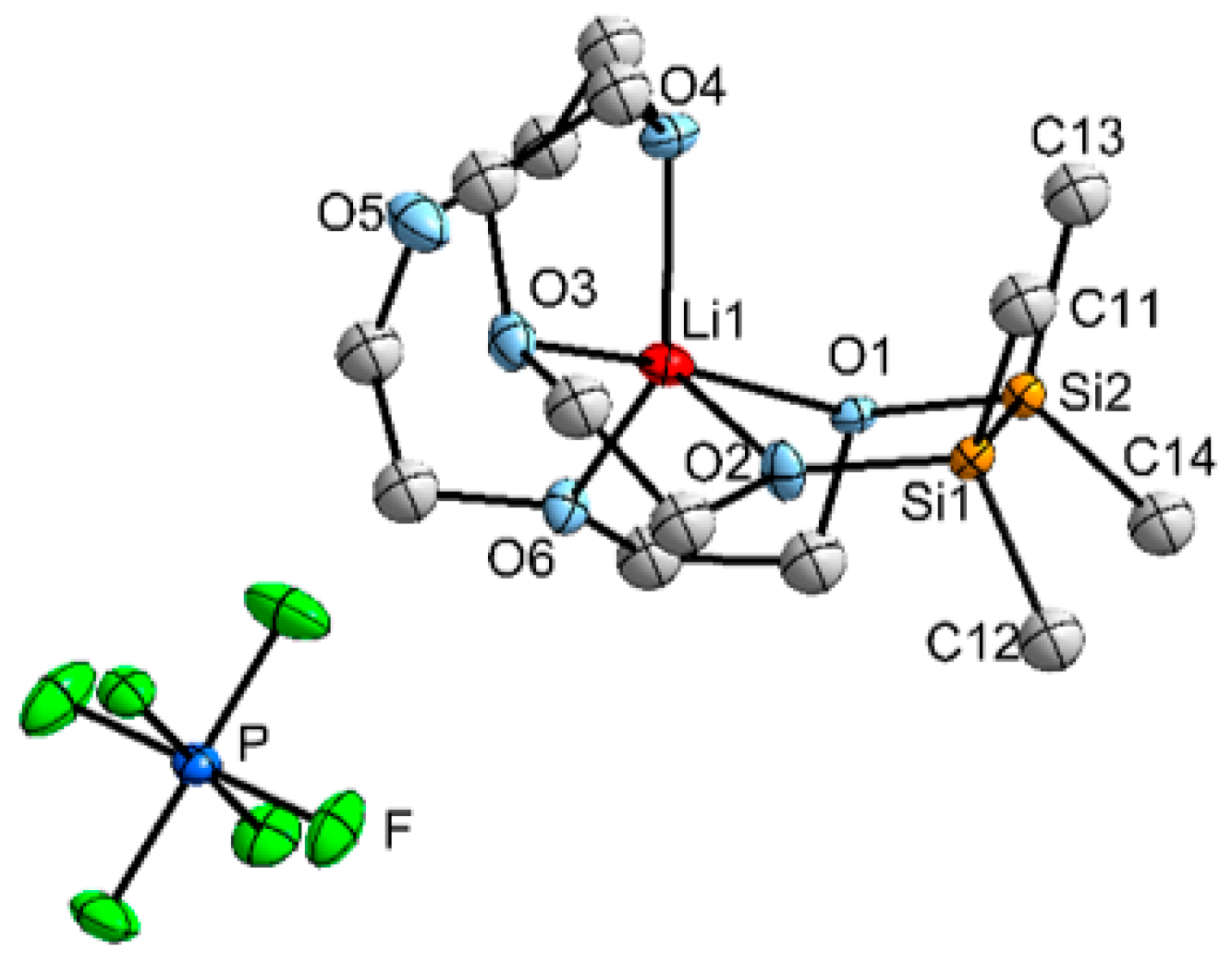
2.1. Mismatch Complexes Involving 1,2-Disila[18]crown-6 with Li+ and Na+
2.2. Determination of ΔEgeom in 1,2-Disila[18]crown-6 Complexes
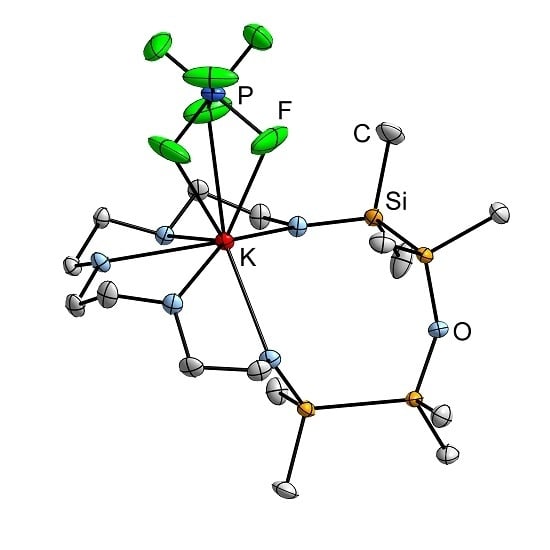
2.3. Mismatch Involving 1,2,4,5-Tetrasila[18]crown-6 and K+
2.4. The Inverse Case: 1,2-Disila[15]crown-5 and Ba2+
3. Materials and Methods
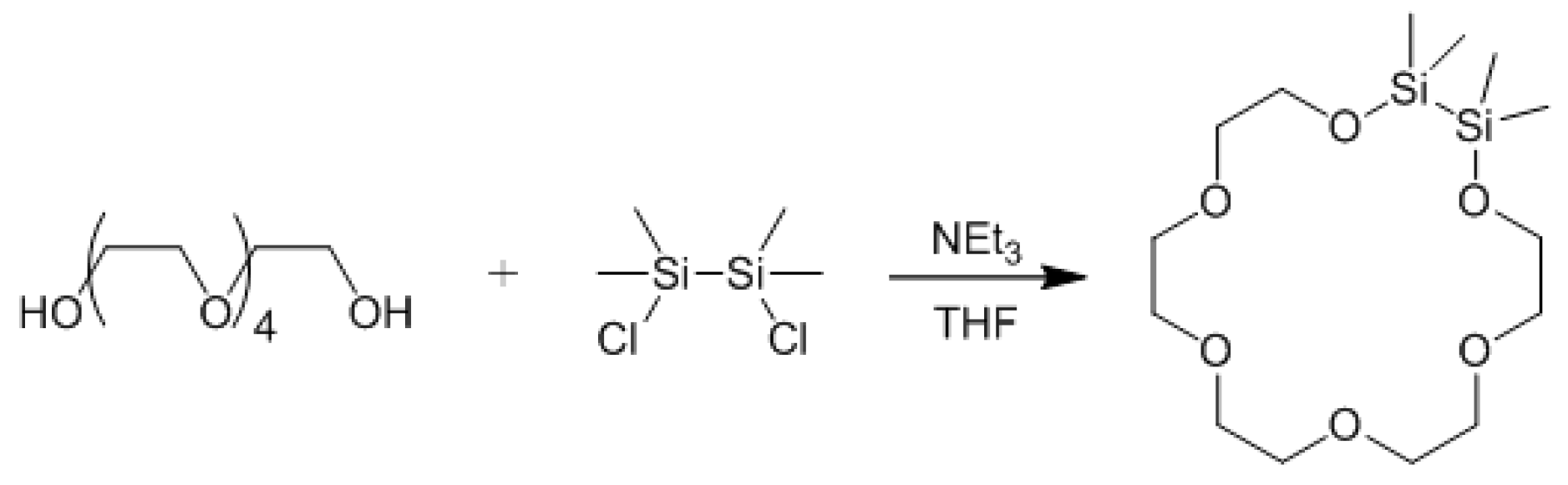
3.1. General Experimental Technique
3.2. Computational Details
3.3. Crystal Structures
3.4. Experimental Section
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liebau, F. Untersuchungen über die Grösse des Si–O–Si–Valenzwinkels. Acta Cryst. 1961, 14, 1103–1109. [Google Scholar] [CrossRef]
- Almenningen, A.; Bastiansen, O.; Ewing, V.; Hedberg, K.; Trætteberg, M. The Molecular Structure of Disiloxane, (SiH3)2O. Acta Chem. Scand. 1963, 17, 2455–2460. [Google Scholar] [CrossRef]
- Stone, F.G.A.; Seyferth, D. The Chemistry of Silicon Involving Probable Use of d-Type Orbitals. J. Inorg. Nucl. Chem. 1955, 1, 112–118. [Google Scholar] [CrossRef]
- Craig, D.P.; Maccoll, A.; Nyholm, R.S.; Orgel, L.E. Chemical bonds involving d-orbitals. Part I. J. Chem. Soc. 1954, 332–353. [Google Scholar] [CrossRef]
- Emeléus, H.J.; Onyszchuk, M. The Reaction of Methyldisoxanes and 1:1 Dimethyldisilthiane with Boron and Hydrogen Halides. J. Chem. Soc. 1958, 604–609. [Google Scholar] [CrossRef]
- Pitt, C.G. Hyperconjugation and its Role in Group IV Chemistry. J. Organomet. Chem. 1973, 61, 49–70. [Google Scholar] [CrossRef]
- Shambayati, S.; Schreiber, S.L.; Blake, J.F.; Wierschke, S.G.; Jorgenson, W.L. Structure and basicity of silyl ethers: A crystallographic and ab initio inquiry into the nature of silicon-oxygen interactions. J. Am. Chem. Soc. 1990, 112, 697–703. [Google Scholar] [CrossRef]
- Cypryk, M.; Apeloig, Y. Ab Initio Study of Silyloxonium Ions. Organometallics 1997, 16, 5938–5949. [Google Scholar] [CrossRef]
- Gillespie, R.J.; Johnson, S.A. Study of Bond Angles and Bond Lenghts in Disiloxane and Related Molecules in Terms of the Topology of the Electron Density and Its Laplacian. Inorg. Chem. 1997, 36, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Hesse, M.F.; Paulmann, C.; Luger, P.; Beckmann, J. How to Make the Ionic Si–O Bond More Covalent and the Si–O–Si Linkage a Better Acceptor for Hydrogen Bonding. Inorg. Chem. 2009, 48, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Passmore, J.; Rautiainen, J.M. On The Lower Basicity of Siloxanes Compared to Ethers. Eur. J. Inorg. Chem. 2012, 6002–6010. [Google Scholar] [CrossRef]
- Cameron, T.S.; Decken, A.; Krossing, I.; Passmore, J.; Rautiainen, J.M.; Wang, X.; Zeng, X. Reactions of a Cyclodimethyldisiloxane (Me2SiO)6 with Silver Salts of Weakly Coordinating Anions; Crystal Structures of [Ag(Me2SiO)6][Al] ([Al] = FAl{OC(CF3)3}3], [Al{OC(CF3)3}4]) and Their Comparison with [Ag(18-Crown-6)]2[SbF6]2. Inorg. Chem. 2013, 52, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Decken, A.; Passmore, J.; Wang, W. Cyclic Dimethylsiloxanes as Pseudo Crown Ethers: Syntheses and Characterization of Li(Me2SiO)5[Al{OC(CF3)3}4], Li(Me2SiO)6[Al{OC(CF3)3}4] and Li(Me2SiO)6[Al{OC(CF3)2Ph}4]. Angew. Chem. Int. Ed. 2006, 45, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Ritch, J.S.; Chivers, T. Silicon Analogues of Crown Ethers and Cryptands: A New Chapter in Host–Guest Chemistry? Angew. Chem. Int. Ed. 2007, 46, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Von Hänisch, C.; Hampe, O.; Weigend, F.; Stahl, S. Stepwise Synthesis and Coordination Compound of an Inorganic Cryptand. Angew. Chem. Int. Ed. 2007, 46, 4775–4779. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Ouchi, M.; Hakushi, T. Molecular Design of Crown Ethers. 3. Extraction of Alkaline Earth and Heavy Metal Picrates with 14- to 17-Crown-5 and 17- to 22-Crown-6. Bull. Chem. Soc. Jpn. 1985, 58, 525–530. [Google Scholar] [CrossRef]
- Ouchi, M.; Inoue, Y.; Kanzaki, T.; Hakushi, T. Ring-contracted Crown Ethers: 14-Crown-5, 17-Crown-6, and Their Sila-analogues. Drastic Decrease in Cation-binding Ability. Bull. Chem. Soc. Jpn. 1984, 57, 887–888. [Google Scholar] [CrossRef]
- Reuter, K.; Buchner, M.R.; Thiele, G.; von Hänisch, C. Stable Alkali-Metal Complexes of Hybrid Disila-Crown Ethers. Inorg. Chem. 2016, 55, 4441–4447. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Thiele, G.; Hafner, T.; Uhlig, F.; von Hänisch, C. Synthesis and coordination ability of a partially silicon based crown ether. Chem. Commun. 2016, 52, 13265–13268. [Google Scholar] [CrossRef] [PubMed]
- Dalley, N.K.; Lamb, J.D.; Nazarenko, A.Y. Crystal Structure of the Complex of 1,4,7,10,13,16-Hexacyclooctadecane with Lithium Picrate Dihydrate. Supramol. Chem. 1997, 8, 345–350. [Google Scholar]
- Chadwick, S.; Ruhlandt-Senge, K. The Remarkable Structural Diversity of Alkali Metal Pyridine-2-thiolates with Mismatched Crown Ethers. Chem. Eur. J. 1998, 4, 1768–1780. [Google Scholar] [CrossRef]
- Akutagawa, T.; Hasegawa, T.; Nakamura, T.; Takeda, S.; Inabe, T.; Sugiura, K.; Sakata, Y.; Underhill, A.E. Ionic Channel Structures in [(M+)x([18]crown-6)][Ni(dmit)2]2 Molecular Conductors. Chem. Eur. J. 2001, 7, 4902–4912. [Google Scholar] [CrossRef]
- Dankert, F.; Reuter, K.; Donsbach, C.; von Hänisch, C. A Structural Study of Alkaline Earth Metal Complexes with Hybrid Disila-Crown Ethers. Dalton Trans. 2017. [Google Scholar] [CrossRef] [PubMed]
- Olsher, U.; Izatt, R.M.; Bradshaw, J.S.; Dalley, N.K. Coordination chemistry of lithium ion: A crystal and molecular structure review. Chem. Rev. 1991, 91, 137–164. [Google Scholar] [CrossRef]
- Gingl, F.; Hiller, W.; Strähle, J. [Li(12-Krone-4)]Cl: Kristallstruktur und IR-Spektrum. Z. Anorg. Allg. Chem. 1991, 606, 91–96. [Google Scholar] [CrossRef]
- Liddle, S.T.; Clegg, W. A homologous series of crown-ether-complexed alkali metal amides as discrete ion-pair species: Synthesis and structures of [M(12-crown-4)2][PyNPh·PyN(H)Ph] (M = Li, Na and K). Polyhedron 2003, 22, 3507–3513. [Google Scholar] [CrossRef]
- Feldmann, C.; Okrut, A. Two Tricyclic Polychalcogenides in [Li(12-crown-4)2]2[Sb2Se12] and [Li(12-crown-4)2]4[Te12]·(12-crown-4)2. Z. Anorg. Allg. Chem. 2009, 635, 1807–1811. [Google Scholar] [CrossRef]
- Turbomole, version 7.0; Turbomole Is a Development of University of Karlsruhe and Forschungszentrum Karlsruhe 1989–2007, Turbomole GmbH 2016; Turbomole GmbH: Karlsruhe, Germany, 2007.
- Weigend, F.; Ahlrichs, R. Balanced bass sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-fitting basis sers for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Dolg, M.; Stoll, H.; Savin, A.; Preuss, H. Energy-adjusted pseudopotentials for the rare earth elements. Theoret. Chim. Acta 1989, 75, 173–194. [Google Scholar] [CrossRef]
- Stoll, H.; Metz, B.; Dolg, M. Relativistic energy-consistent pseudopotentials—Recent developments. J. Comput. Chem. 2002, 23, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 2010, 132, 154104–154119. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Willcott, M.R. MestRe Nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin. Trans. 1993, 2, 799–805. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL14; Program for the Refinement of Crystal Structures; Universität Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Hildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Diamond—Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2012. [Google Scholar]
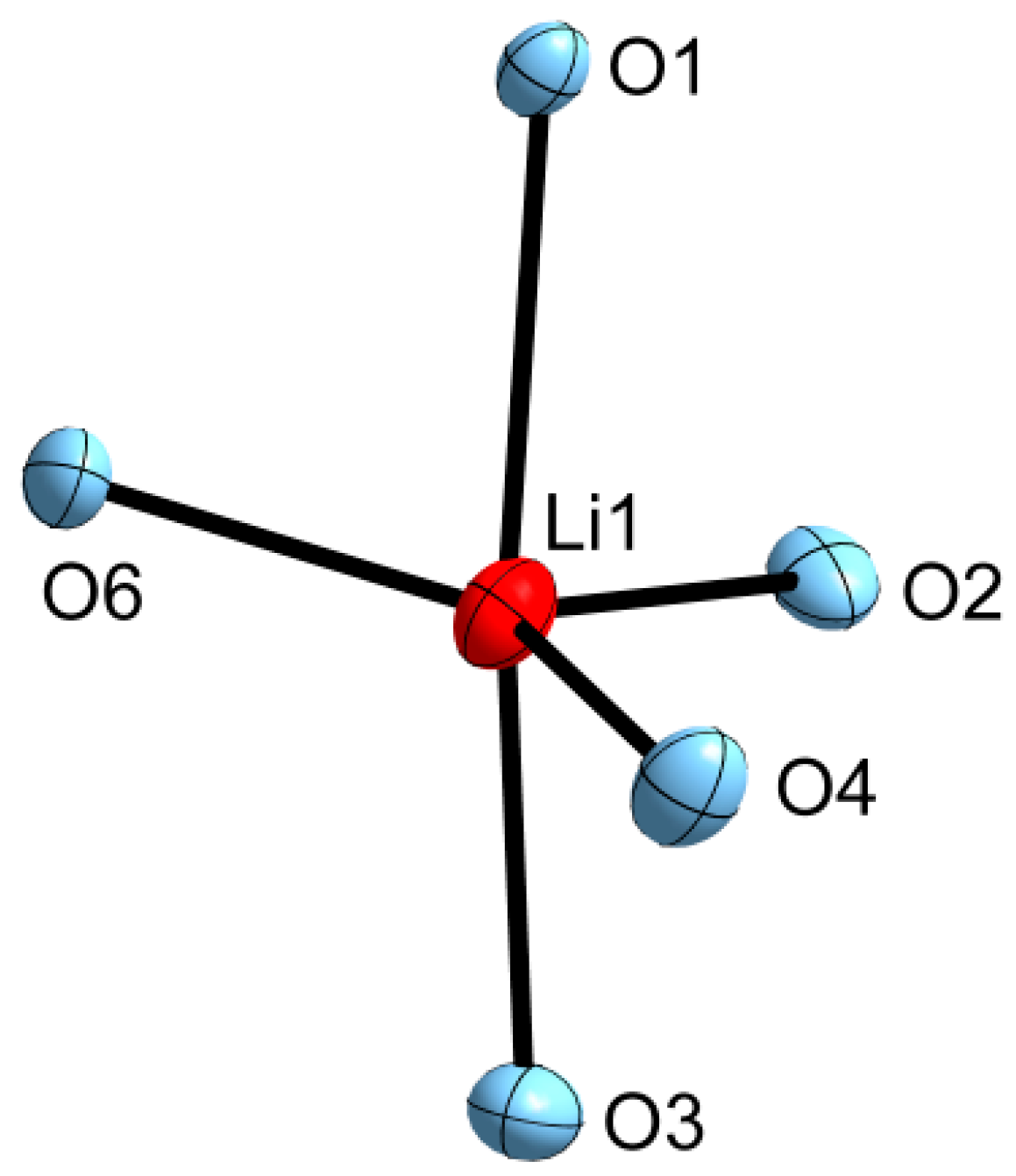

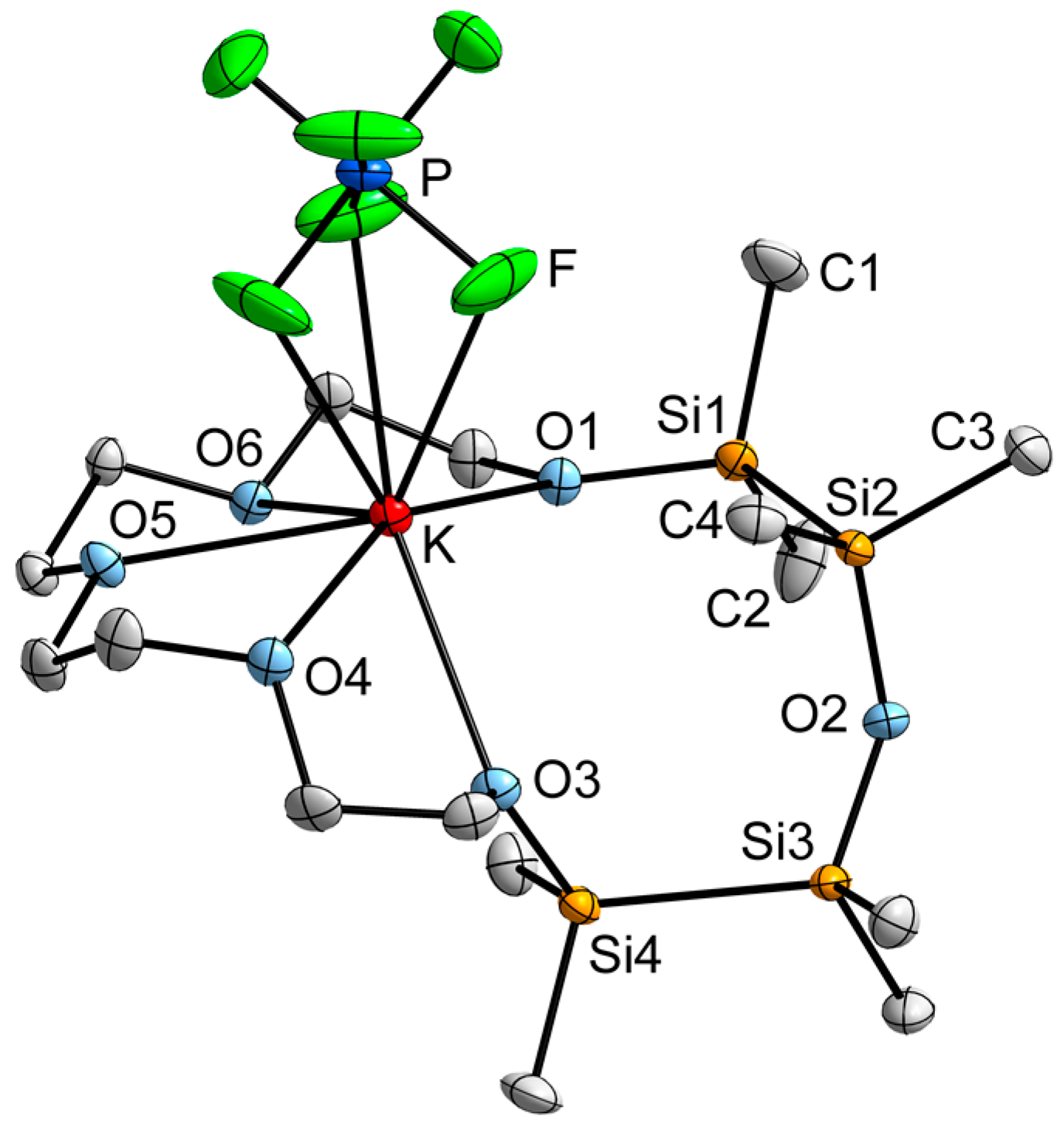
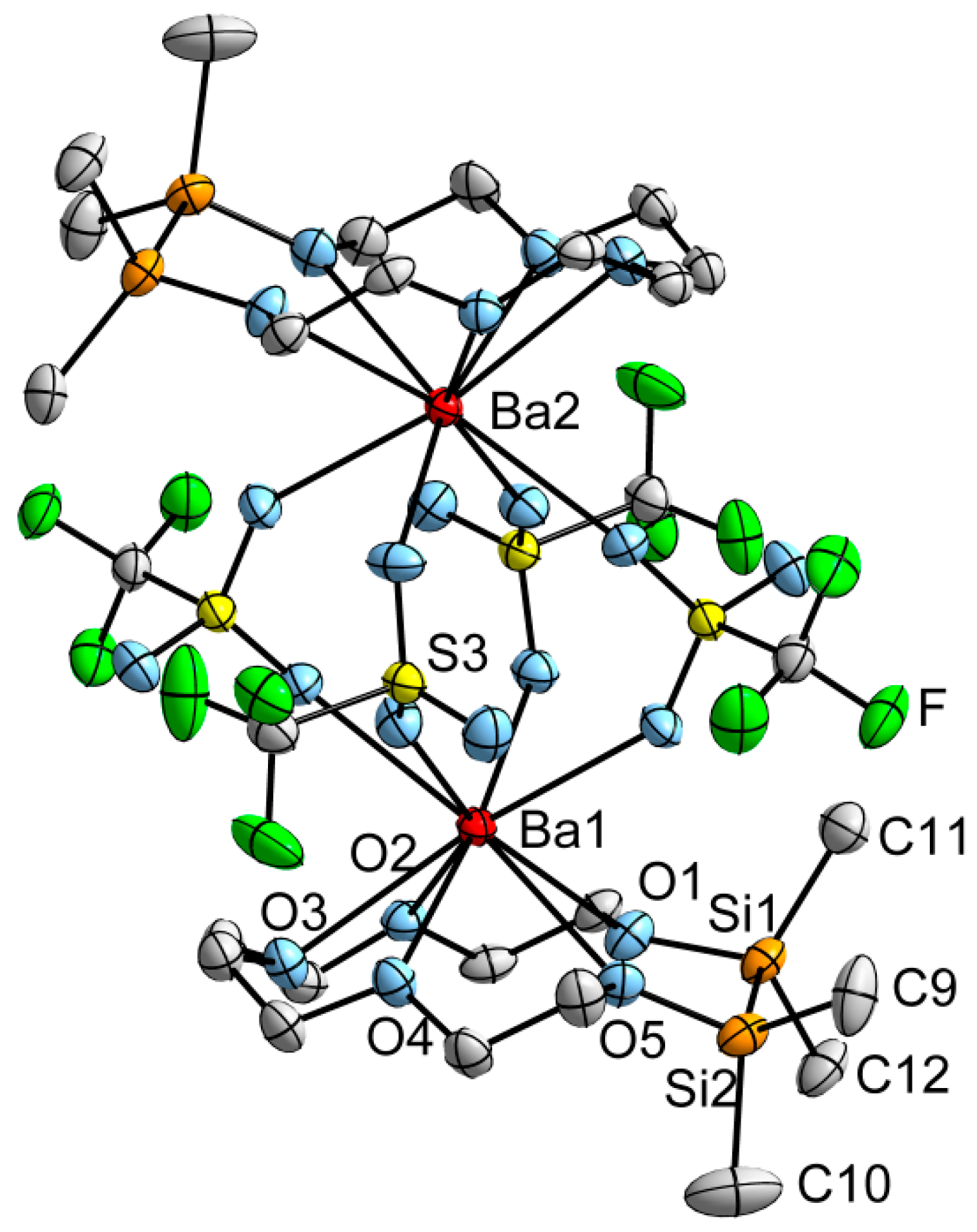
| Empirical Formula | C14H30Li1O6Si2F6P1 | C14H32F6Na1O6P1Si2 | C16H40F6K1O6Si4P1 | C28H55Ba2F12O22S4Si4 |
|---|---|---|---|---|
| Formula weight (g·mol−1) | 502.47 | 520.53 | 624.91 | 1487.00 |
| Crystal color, shape | colorless plank | colorless block | colorless plank | colorless block |
| Crystal size (mm) | 0.134 × 0.189 × 0.382 | 0.060 × 0.271 × 0.284 | 0.138 × 0.140 × 0.539 | 0.232 × 0.245 × 0.509 |
| Crystal system | monoclinic | triclinic | monoclinic | triclinic |
| Space group | P21/c | P | P21/n | P |
| Formula units | 4 | 2 | 4 | 2 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) |
| Unit cell dimensions | a = 9.189(1) b = 23.663(1) c = 10.608(1) β = 94.332(1) | a = 8.512(1) b = 11.468(1) c = 13.996(1) α = 105.64(1) β = 103.51(1) γ = 103.20(1) | a = 9.379(1) b = 22.608(2) c = 14.906(1) β = 104.62(1) | a = 11.269(2) b = 12.458(3) c = 20.817(4) α = 78.69(3) β = 83.62(3) γ = 78.63(3) |
| Cell volume (Å3) | 2300.0(2) | 1215.83(17) | 3058.4(4) | 2801.6(11) |
| Ρcalc (g/cm3) | 1.451 | 1.422 | 1.357 | 1.763 |
| μ (Mo Kα) (mm−1) | 0.298 | 0.301 | 0.446 | 1.739 |
| 2θ range | 2.384–25.299 | 2.578–25.237 | 2.289–25.319 | 1.695–26.373 |
| Reflections measured | 47204 | 13274 | 86701 | 24025 |
| Independent Reflections | 4181 [Rint = 0.0402] | 4422 [Rint = 0.0882] | 5566 [Rint = 0.0290] | 11417 [Rint = 0.0848] |
| R1 (I > 2σ(I)) | 0.0455 | 0.0535 | 0.0191 | 0.0435 |
| wR2 (all data) | 0.1103 | 0.1504 | 0.0360 | 0.1091 |
| GooF | 1.023 | 1.021 | 0.800 | 0.926 |
| Largest diff. peak and hole (e·Å−3) | 1.02/−0.65 | 0.60/−0.56 | 0.60/−0.61 | 1.61/−2.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reuter, K.; Dankert, F.; Donsbach, C.; Von Hänisch, C. Structural Study of Mismatched Disila-Crown Ether Complexes. Inorganics 2017, 5, 11. https://doi.org/10.3390/inorganics5010011
Reuter K, Dankert F, Donsbach C, Von Hänisch C. Structural Study of Mismatched Disila-Crown Ether Complexes. Inorganics. 2017; 5(1):11. https://doi.org/10.3390/inorganics5010011
Chicago/Turabian StyleReuter, Kirsten, Fabian Dankert, Carsten Donsbach, and Carsten Von Hänisch. 2017. "Structural Study of Mismatched Disila-Crown Ether Complexes" Inorganics 5, no. 1: 11. https://doi.org/10.3390/inorganics5010011
APA StyleReuter, K., Dankert, F., Donsbach, C., & Von Hänisch, C. (2017). Structural Study of Mismatched Disila-Crown Ether Complexes. Inorganics, 5(1), 11. https://doi.org/10.3390/inorganics5010011