Abstract
A library of unsymmetrical SCN pincer palladacycles, [ClPd{2-pyr-6-(RSCH2)C6H3}], R = Et, Pr, Ph, p-MePh, and p-MeOPh, pyr = pyridine, has been synthesized via C–H bond activation, and used, along with PCN and N’CN unsymmetrical pincer palladacycles previously synthesized by the authors, to determine the extent to which the trans influence is exhibited in unsymmetrical pincer palladacycles. The trans influence is quantified by analysis of structural changes in the X-ray crystal and density functional theory (DFT) optimized structures and a topological analysis of the electron density using quantum theory of atoms in molecules (QTAIM) to determine the strength of the Pd-donor atom interaction. It is found that the trans influence is controlled by the nature of the donor atom and although the substituents on the donor-ligand affect the Pd-donor atom interaction through the varied electronic and steric constraints, they do not influence the bonding of the ligand trans to it. The data indicate that the strength of the trans influence is P > S > N. Furthermore, the synthetic route to the family of SCN pincer palladacycles presented demonstrates the potential of late stage derivitization for the effective synthesis of ligands towards unsymmetrical pincer palladacycles.
1. Introduction
Palladacycles have been extensively studied since their discovery in 1965 by Cope and Siekman [1]. They have been widely used as catalysts or pre-catalysts in organic reactions, such as in Heck and Suzuki–Miyaura cross-couplings [2,3,4,5]. Pincer palladacycles are an interesting type of palladacycle, of which there are two different types. The majority of pincer palladacycles studied have been of the symmetrical YCY type, such as NCN [6], SCS [7], PCP [8], and SeCSe [9,10]. There are limited numbers of reported unsymmetrical pincer palladacycles owing to their more difficult synthesis. For example, Szabó et al. synthesized unsymmetrical PCS pincer palladacycles from 1,3-bis(bromomethyl)benzene, albeit in low overall yield (38%) [11]. However, the unsymmetrical PCS pincer palladacycle reported showed enhanced catalytic activity when compared to related symmetrical pincer palladacycles [11,12]. Recently, we reported the synthesis of an unsymmetrical SCN pincer palladacycle by C–H bond activation [13], and novel unsymmetrical PCN and N’CN analogues [14] (Figure 1).
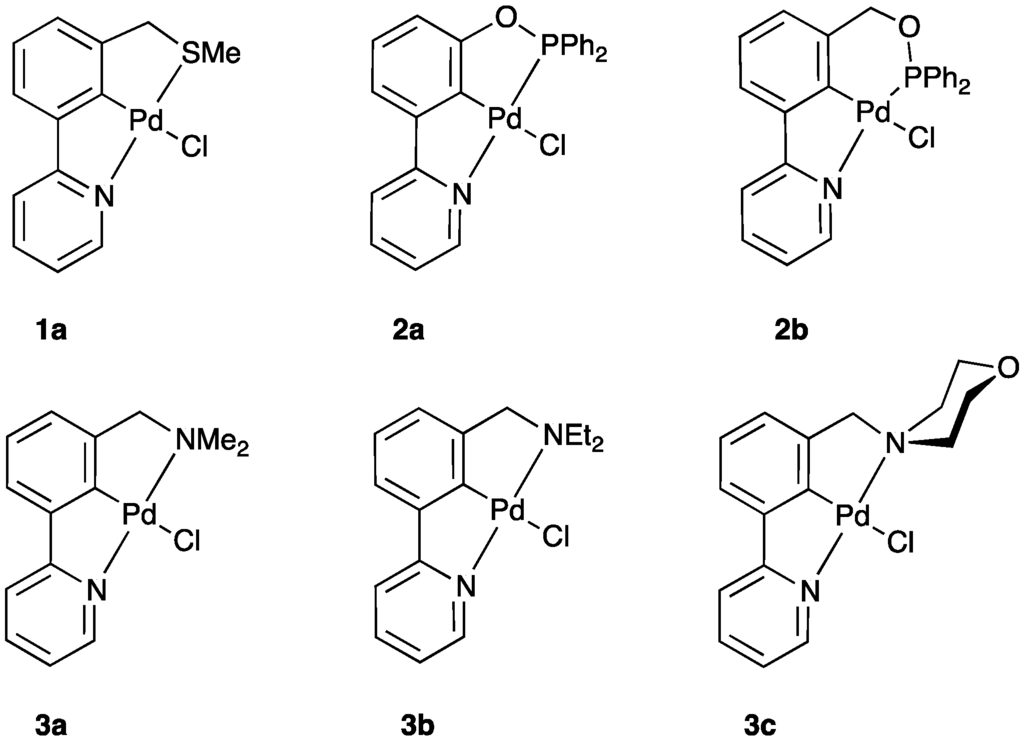
Figure 1.
Unsymmetrical pincer palladacylces, SCN (1a) [13], PCN (2a–2b), and N’CN (3a–3c) [14].
The pincer palladacycle structures are stabilized by an intramolecular coordination to the metal of the two donor atoms in the side arms. Their reactivity and other properties are influenced by the donor group around the metal [3]. The attractive feature of pincer palladacycles is the possibility for fine-tuning the catalytic activity by varying the two side arms to modify the palladium environment, by changing the donor atoms and their substituents, providing the opportunity to alter hard/soft acid base properties, or by changing the ring size, giving rise to varying steric hindrance [12]. These factors provide the potential for hemilabile coordination of the donor ligand arms with the metal center, an important consideration in the design of pincer palladacycles [15,16,17]. This can lead to different physical and chemical properties of the donor ligand arms, resulting in preferential decoordination of one of the ligand arms, providing the opportunity to fine tune the catalytic activity of unsymmetrical pincer palladacycles [18,19,20,21]. An excellent example by Wendt and co-workers reported the hemilabile nature of nitrogen and phosphorus donor atoms by reacting with a strong nucleophile (MeLi) [22]. The results showed that the nitrogen donor atom arm decoordinated from the Pd center, while the phosphorus donor atom arm remained coordinated to the Pd center (Scheme 1). It is clear that the different properties of the side arms result in hemilability due to the changing strength and/or nature of interaction between donor atoms and the Pd center.
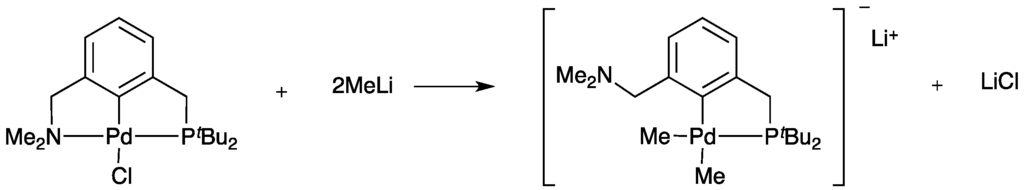
Scheme 1.
Reaction of unsymmetrical pincer palladacycles with MeLi showing the hemilability of the nitrogen donor atom arm by Wendt et al. [22].
Pincer palladacycles exist in a square planar configuration at the Pd(II) center, and a key factor determining the strength of the interaction between Pd and the donor atoms is the trans influence, potentially affecting hemilability of donor atom arms. The trans influence is defined by Pidcock [23] as “the tendency of a ligand to weaken the bond trans to itself”. The “trans influence” affects the structure in the ground, or thermodynamic state. Therefore, sometimes, it is called the thermodynamic trans effect, while the “trans effect” is related to the kinetic rate of reaction, depending on substitution of the bond trans to itself. The trans influence has been used to explain the stability of square planar complexes [24], while the trans effect has been used to study reaction pathways [25]. There are many experimental studies into the trans influence, generally using spectroscopic or X-ray crystallographic methods [26,27,28]. Additionally, density functional theory (DFT) structure optimization and molecular orbital analysis have been employed in the study of the trans influence and give a good explanation of the trans influence in organometallic complexes [29,30,31,32,33].
In this study, we have investigated the trans influence in both model and experimentally-characterized unsymmetrical pincer palladacycles, using DFT calculations and quantum theory of atoms in molecules (QTAIM) analysis. Additionally, in order to determine the effect of varying the substituents on the donor atom, we have synthesized a library of unsymmetrical SCN pincer palladacycles, providing the opportunity to vary the steric and electronic properties on the sulfur atom. We have then used these palladacycles to further investigate the trans influence using DFT.
2. Results and Discussion
2.1. SCN Pincer Palladacycle Synthesis
Our previous work has demonstrated a novel synthetic route to an unsymmetrical SCN pincer palladacycle, via a key biaryl benzyl bromide intermediate 5 (Scheme 2) [13]. By changing the sulfur nucleophile in step c (Scheme 2), the ability to synthesize a library of SCN pincer ligands is possible. This provides the opportunity to vary the thioether substituent to tune the steric and electronic properties of the sulfur atom, which will be bound to the palladium atom in the resulting palladacycle. The SCN ligands then undergo C–H bond activation with in situ-generated Pd(MeCN)4(BF4) [13,34] synthesizing a library of SCN pincer palladacycles 1b–1f (Scheme 2, Table 1).
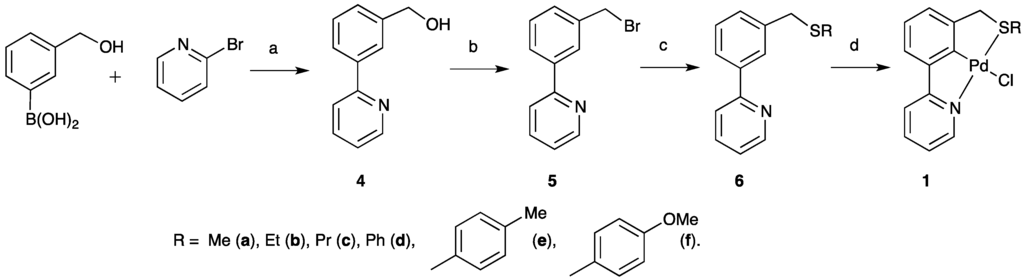
Scheme 2.
A synthesis of SCN pincer palladacycles 1b–1f via a key biaryl benzyl bromide intermediate 5, based on the previous synthesis of 1a. (Step a = Pd(PPh3)4, K3PO4, Tol/EtOH/H2O; b = 48% HBr in H2O; c = NaSMe, EtOH; d = (i) PdCl2, AgBF4, MeCN; and (ii) NaCl, H2O/MeCN).

Table 1.
SCN pincer palladacycle synthesis yields.
The SCN ligand syntheses presented in Table 1 reveal excellent to moderate yields, followed by C–H bond activation, also in moderate to excellent yield. Single crystals of palladacycles 1b–d and 1f were obtained by slow evaporation of dichloromethane (DCM) from a saturated solution, and were characterized by X-ray crystallography (Figure 2). The CCDC numbers for the structures are 1486787 for 1b, 1486788 for 1c, 1486789 for 1d and 1486790 for 1f. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
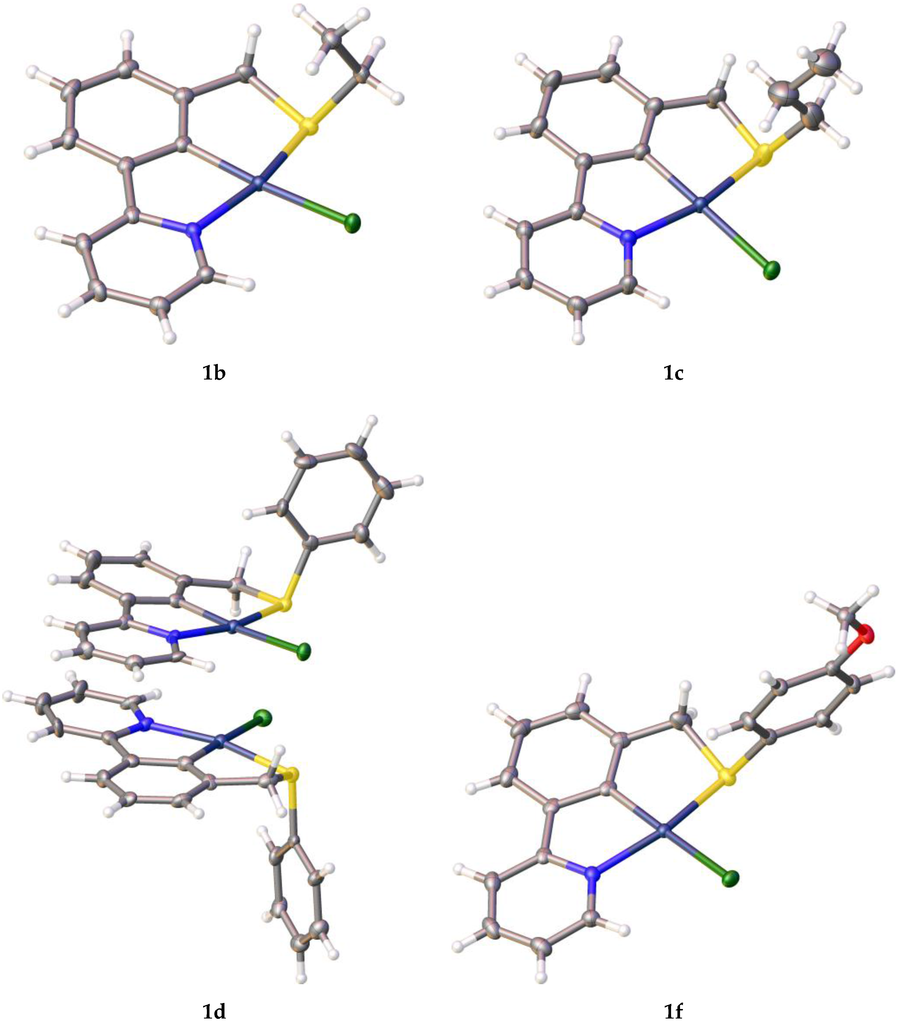
Figure 2.
X-ray crystallographic structures of 1b–d, 1f.
2.2. Investigaing the Trans Influence
To determine the accuracy of the Perdew–Burke–Ernzerhof exchange–correlation functional (PBE) for optimizing the YCY’ pincer complexes, we have analyzed the mean signed errors (MSE), which is the average of the deviation between calculation and experiment, and the mean unsigned errors (MUE), which is the average of the absolute deviation between calculated and experimental Pd–L bond lengths (L = Y, Y’, C and Cl). The MSE for 1a–1d, 1f is 0.001 Å, for 2a–2b is 0.012 Å and for 3a–3c is 0.001 Å. The MUE for 1a–1d, 1f is 0.017 Å, for 2a–2b is 0.012 Å and for 3a–3c is 0.001 Å.
A topological analysis of the electron density was performed using QTAIM. The bond path is a single line of locally-maximum density linking the nuclei of any two bonded atoms in a molecule [35]. The minimum along this path is defined as the bond critical point (BCP) and the magnitude of the electron density ρ(r) at this point can be used to determine the strength of the interaction.
Several AIM parameters determined at the Pd–Y and Pd–Y’ BCPs are provided in the Supplementary Information (electron density ρ(r), Laplacian of the density ∇2ρ(r), delocalization index, ellipticity, bond degree parameter, etc.) which can be used to determine the nature and strength of the interaction; the conclusions regarding the nature of the bonding are in complete agreement with our previous work on the nature of the bonding in symmetrical pincer palladacycles and, so, are not presented again here [36]. In the present work, the focus is on the trans influence and, so, the magnitude of the electron density ρ(r) at the BCP is used to determine the increase or decrease in the strength of the Pd–Y interaction when the ligand trans to it is varied.
2.2.1. Trans Influence in Model Palladacycles I–III
Normally, the trans influence has been studied in systems with four monodentate ligands coordinated to the metal center to form a square planar complex. Furthermore, a trans Pt–Cl bond length, situated trans to the donor atom arm, of a square planar complex is normally used to consider the trans influence [37,38,39,40]. From the unsymmetrical SCN pincer palladacycle structures, we do not have a Cl atom for monitoring the strength of the trans influence in this way. Therefore, first, simple model palladacycles I–III (Figure 3) have been studied using DFT to evaluate the trans influence in CY palladacycles before studying the unsymmetrical YCY’ pincer palladacycles. The models I–III contain a Cl ligand trans to a donor atom group (NMe2, SMe2, and PMe2, respectively) to monitor the strength of the trans influence. A topological analysis of the electron density was performed using QTAIM and the magnitude of the electron density ρ(r) at the bond critical point (BCP), the minimum along the bond path between interacting atoms, was used to determine the strength of the Pd–Cl interaction. A larger ρ(r) value corresponds to a stronger interaction between atoms [41] and, therefore, can be used to study the trans influence in palladacycles I–III. When ρ(r) at the BCP of Pd–Cl bond has a high value (strong interaction), it indicates that the donor atom trans to Cl has a weak trans influence, whereas a low ρ(r) value (weak interaction) indicates that the donor atom trans to the Pd–Cl bond has a strong trans influence. A relative change in bond length is a physical manifestation that indicates the strength of the trans influence. When the Pd–Cl bond is situated trans to a donor atom that exhibits a strong trans influence, the Pd–Cl bond lengthens compared to when the Pd–Cl bond is situated trans to a weak trans influence donor atom. The data provided in Table 2 show that the ρ(r) value of the Pd–Cl bond of III is smaller than that in II, which is smaller than in I, indicating that the trans influence of PMe2 is greater than that of SMe which is greater than NMe2. The ρ(r) data is supported by the bond lengths, with I having the shortest Pd–Cl bond length and III a significantly longer Pd–Cl bond length than in I and II, again demonstrating the stronger PMe2 trans influence. Based on this analysis the ordering of the trans influence series is PMe2 > SMe > NMe2.
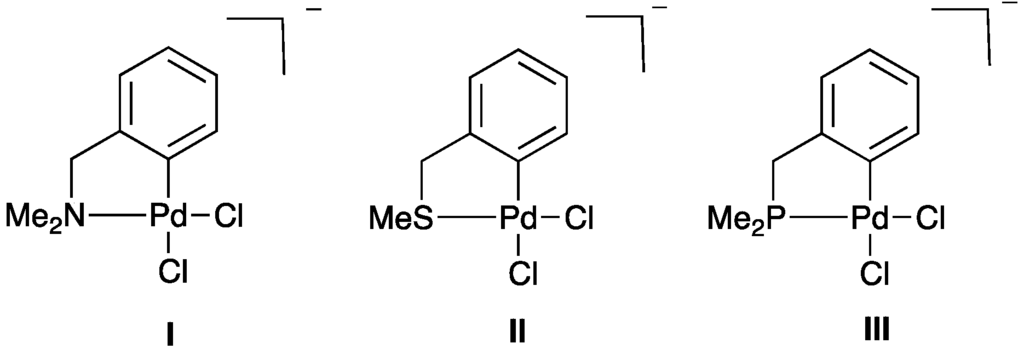
Figure 3.
Model palladacycles I–III studied to investigate the trans influence.

Table 2.
The electron density ρ(r) and Pd–Cl bond lengths.
2.2.2. Trans Influence in Model Unsymmetrical YCY’ Pincer Palladacycles
In order to extend our investigation of the trans influence to unsymmetrical pincer palladacycles, the palladacycles IV–VI have been studied using DFT and QTAIM, and their bond strengths and bond lengths compared to previous results found for symmetrical pincer palladacycles PdNCN, PdSCS and PdPCP [36] (Figure 4). Considering the ρ(r) value at the BCP of the Pd–Y bond in IV–VI, the ρ(r) value of the Pd–P bond of V (0.110 a.u) and VI (0.114 a.u.) are greater compared to the ρ(r) values for the Pd–P bond in the PdPCP (0.101 a.u.) [36]. This is due to the weaker trans influence of N and S, compared to P, leading to stronger Pd–P bonds in V and VI (Table 3). The ρ(r) value of the Pd–S bond of IV (0.097 a.u.) increases, whereas the ρ(r) value of the Pd–S bond of V (0.082 a.u.) decreases, compared to the ρ(r) value of the Pd–S bond in the PdSCS (0.091 a.u.), therefore showing that S has a moderate trans influence. Furthermore, the ρ(r) values of the Pd–N bond of IV (0.083 a.u.) and VI (0.075 a.u.) decrease compared to the ρ(r) value of the Pd–N bond in the PdNCN (0.086 a.u.) [36] indicating that P and S exhibit a stronger trans influence than N.
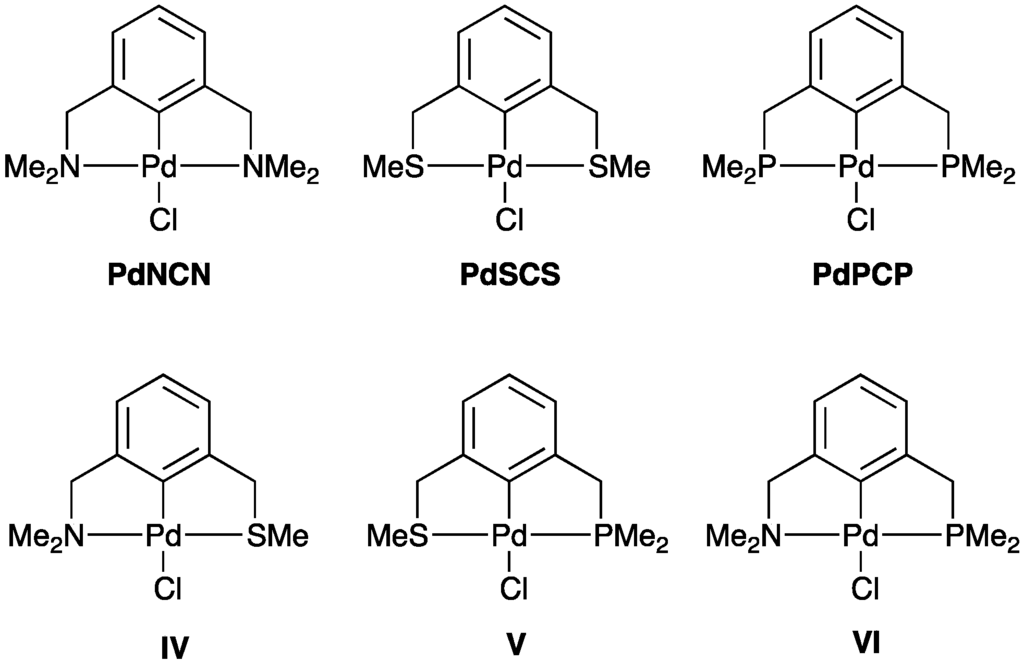
Figure 4.
Symmetrical NCN, SCS, and PCP pincer palladacycles (PdNCN, PdSCS and PdPCP) [36] and model unsymmetrical SCN (IV), PCS (V), and PCN (VI) pincer palladacycles.

Table 3.
Electron density ρ(r) in symmetrical and unsymmetrical pincer palladacycles (values are in atomic units). The donor atom is shown in bold for each side arm, Y and Y’.
Supporting the ρ(r) value results, the bond lengths of Pd–Y and Pd–Y’ are reported in Table 4. When the donor ligand Y has a trans influence the Pd–Y’ bond distance increases (and the ρ(r) value decreases) indicating a weakened interaction. By comparing with the symmetrical YCY pincer palladacycles it can be seen that the P donor ligand has a trans influence on the S donor ligand and the N donor ligand, and that the S donor ligand has a trans influence on the N donor ligand. For example, in VI, the PCN palladacycle, the P donor ligand has a strong influence on the N donor ligand trans to it, which manifests as an increased Pd–N (2.203 Å) bond distance compared to the Pd–N bond in PdNCN (2.140 Å), and a commensurate decrease in the Pd–P bond distance (2.222 Å) compared to the Pd–P bond length in PdPCP (2.287 Å) (Table 4). The results confirm the conclusion from the model systems with Cl as a reference, that P exhibits the greatest trans influence and N the least.

Table 4.
Calculated and experimental Pd–Y and Pd–Y’ bond distances in symmetrical and unsymmetrical pincer palladacycles (bond distances are in Å). The donor atom is shown in bold for each side arm, Y and Y’.
Based on the ρ(r) values and Pd–Y bond lengths, the ordering of the trans influence series is PMe2 > SMe > NMe2. This is in good agreement with that of Kapoor and Kakkar’s study [40] into the square planar Pt complexes using DFT calculations. Their results showed a trans influence series in order of P > S > N. Moreover, Sajith and Suresh [42] studied the correlation between ρ(r) and trans influence in a square planar Pd complex, showing good linear relation between ρ(r) and trans influence, with a trans influence series of PMe3 > SMe2 > NH3.
2.2.3. Trans Influence in Experimentally-Characterized Unsymmetrical YCY’ Pincer Palladacycles
In this section DFT and the QTAIM method is used to study the trans influence in 1a (PdSCN), 2a (PdPCN), and 3a (PdN’CN). By comparing the Pd–N bond length in the structures 1a, 2a, and 3a (optimized and experimental), the Pd–N bond is longest in 2a and shortest in 3a (Table 4). In addition, the smallest ρ(r) values for the Pd–N bond is in 2a (0.087 a.u.), while the largest is found in 3a (0.102 a.u.) with 1a (0.098 a.u.) intermediate (Table 3). The different Pd–N bond lengths and strengths demonstrate the difference in trans influence due to the nature of the donor atom of the Pd–Y bond. These results further confirm that the P donor ligand exhibits the strongest trans influence, while the N donor ligand has the weakest trans influence and that the trans influence series for the unsymmetrical pincer palladacycles considered is P > S > N.
The N donor ligand in the experimentally-characterized unsymmetrical SCN pincer palladacycle (Figure 1) is a pyridine rather than the amine considered in the previous section (IV). The change in electronic and steric effects when replacing NMe2 (IV) with pyridine (1a) in a SCN pincer palladacyle is reflected in the bond strength: ρ(r) value of the Pd–NMe2 bond is 0.083 a.u. in IV whereas the Pd–pyr is 0.098 a.u. in 1a, and the Pd–NMe2 bond length is 2.156 Å in IV and the Pd–pyr bond length is 2.074 Å in 1a, demonstrating the stronger Pd–pyridine bond (Table 3 and Table 4). However, this does not appear to effect the trans influence exerted on the SMe ligand when trans to these N donor ligands. The ρ(r) value of the Pd–S bond is 0.091 a.u. in PdSCS and increases to 0.097 a.u. in IV and 0.096 a.u. in 1a, and the bond length in PdSCS is 2.313 Å and shortens to 2.285 Å in IV and 2.288 Å in 1a. Thus, in both IV and 1a the Pd–S bond is strengthened relative to the symmetric PdSCS analog and, thus, can only be attributed to the effect of the N-donor ligand trans to it.
Furthermore, by comparing PdNCN where N = NMe2, to PdNCN’ (3a), where one of the amine ligands has been replaced by pyridine, we can assess the trans influence in an unsymmetrical pincer palladacycle where the donor atom is the same (N) for distinctly different donor ligands (NMe2 and pyr). In 3a the ρ(r) value of the Pd–NMe2 bond has not changed and the bond length has increased insignificantly (0.005 Å) from that in the symmetric PdNCN palladacycle. Therefore, we can conclude that, although the electronic and steric effects of the pyridine result in a considerably stronger bond to the Pd center, this stronger bond does not exert a trans influence on the amine donor ligand. Thus, it would appear the nature of the donor atom is the sole driver for the trans influence.
2.2.4. Trans Influence on Unsymmetrical Pincer Palladacycles: Donor Atom Substituent Effects
To determine whether the trans influence is induced when the substituents on the donor atom are varied, thereby introducing subtle electronic effects, the library of SCN pincer palladacycles synthesized in the present work (1b–1f), along with 1a, have been investigated computationally to determine the influence of the thioether group on the coordinated pyridine trans to it. The Pd–N bond distances (experimental and calculated) show very little change when the substituent on the S atom is changed (bond distance differences <0.005 Å, with the exception of the experimental Pd–N bond length for 1a) (Table 4). Similarly, the ρ(r) values at the Pd–N BCP in the SCN pincer palladacycles are unaffected by changing substitution on donor atom.
Furthermore, when the substituent is changed on the P (2a and 2b) (which both incorporate the phosphinite donor group) or the N (3a–3c), Figure 1, it does not alter the trans influence on the Pd–pyr interaction within the PCN or N’CN pincer palladacycles. The ρ(r) values for the Pd–pyr bond trans to the Pd–N’ bond is independent of the nature of the N’ ligand, and although the interactions (ρ(r) and bond length) due to the Pd–P ligands exhibit a slight difference (0.002 a.u. and 0.015 Å) they are extremely small.
3. Experimental Section
3.1. General Details
Solvents and chemicals were purchased from Sigma-Aldrich (Merck KGaA, Damstadt, Germany), VWR International (VWR, Radnor, PA, USA), Fisher Scientific (Fisher Scientific UK Ltd., Loughborough, UK) and Fluorochem (Fluorochem Ltd., Hadfield, UK) and used without further purification, with reactions taking place open to atmosphere and moisture.
3.2. Instrumentation
1H and 13C spectra were recorded on either a Varian 400 or 500 MHz spectrometer (Agilent Technologies, Yarnton, UK). High resolution mass spectrometry (HRMS) data were obtained on an electrospray ionization (ESI) mass spectrometer using a Bruker Daltonics Apex III (Brucker, Billerica, MA, USA), with source Apollo ESI, using methanol as the spray. Flash chromatography was performed on an automated ISCO RF75 (Teledyne ISCO Inc., Licoln, NE, USA). Gas chromatography (GC) measurements were obtained using a Perkin Elmer Autosystem XL Gas Chromatograph (PerkinElmer Inc., Waltham, MA, USA), utilizing a flame ionization detector, and a Supelco MDN-5S 30 m × 0.25 mm × 0.25 µm column, with a He mobile phase. Elemental analyses were run by the London Metropolitan University Elemental Analysis Service (ThermoFisher Scientific, Waltham, MA, USA). Crystal structures were obtained by the UK National Crystallography Service at the University of Southampton [43].
3.3. Procedure
2-3-[(Ethylsulfanyl)methyl]phenylpyridine, 6b. Under an argon atmosphere, ethanethiol (2.42 mmol, 0.179 mL) and sodium hydride (2.41 mmol, 58 mg) were dissolved in dry DMF (dimethylformamide, 3 mL) and stirred at room temperature in a sealed microwave vial for 15 min. 2-[3-(Bromomethyl)phenyl]pyridine, 5 (1.61 mmol, 400 mg) in dry DMF (3 mL) was then added, and stirred under microwave irradiation (maximum power 300 W, dynamic heating) at 150 °C for 15 min. After cooling, the solvent was removed in vacuo and the crude mixture was diluted in H2O (25 mL) and DCM (25 mL). The product was extracted with DCM (2 × 25 mL), washed with H2O (5 × 25 mL) and brine (25 mL). The organic layers were dried over anhydrous MgSO4, filtered, and concentration in vacuo. The crude product was purified using flash column chromatography (7:3 DCM:EtOAc) yielding 263 mg of the expected product, 6c, as a yellow oil in 71% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 8.70 (d, J = 4.8 Hz, 1H), 7.96 (s, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.77–7.73 (m, 2H), 7.43 (dd, J = 7.5, 7.5 Hz, 1H), 7.39 (d, J = 7.5 Hz, 1H), 7.24 (ddd, 6.3, 4.8, 2.3 Hz, 1H), 3.81 (s, 2H), 2.48 (q, J = 7.5 Hz, 2H), 1.25 (t, J = 7.5 Hz, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 157.3, 149.7, 139.6, 139.2, 136.7, 129.4, 128.9, 127.4, 125.5, 122.1, 120.6, 36.0, 25.4, 14.4. HRMS (m/z). Calc. for [C14H15NS + H]+ 230.0998. Found 230.0998.
2-3-[(Propylsulfanyl)methyl]phenylpyridine, 6c. Same methodology as 6b, using propane-1-thiol (1.97 mmol, 0.178 mL), and reacting for 20 min in the microwave. After workup, 300 mg of the expected product, 6c was found, without purification in >99% yield as a yellow oil. 1H NMR (500 MHz), Chloroform-d δ (ppm): 8.70 (d, J = 4.8 Hz, 1H), 7.96 (s, 1H), 7.86 (d, J = 7.5 Hz, 1H), 7.78–7.73 (m, 2H), 7.43 (dd, J = 7.5, 7.5 Hz, 1H), 7.39 (d, J = 7.5 Hz, 1H), 7.24 (ddd, J = 6.5, 4.8, 2.1 Hz, 1H), 3.79 (s, 2H), 2.44 (t, J = 7.2 Hz, 2H), 1.64–1.57 (m, 2H), 0.96 (t, J = 7.3 Hz, 3H). ). 13C NMR (126 MHz), Chloroform-d δ (ppm): 157.2, 149.6, 139.6, 139.3, 136.7, 129.4, 128.8, 127.4, 125.5, 122.1, 120.6, 36.3, 33.6, 22.6, 13.5. HRMS (m/z). Calc. for [C15H17NS + H]+ 244.1154. Found 244.1155.
2-3-[(Phenylsulfanyl)methyl]phenylpyridine, 6d. Same methodology as 6b, using benzenethiol (1.86 mmol, 0.190 mL). After workup, 418 mg of the expected product, 6d as a yellow oil in 99% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 8.70 (d, J = 4.8 Hz, 1H), 7.93 (s, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.76–7.72 (m, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.39 (dd, J = 7.6, 7.6 Hz, 1H), 7.36–7.33 (m, 2H), 7.27–7.21 (m, 3H), 7.20–7.16 (m, 1H), 4.20 (s, 2H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 157.2, 149.6, 139.6, 138.0, 136.7, 130.0 (2C), 129.3 (2C), 128.9, 128.8 (2C), 127.4, 126.4, 125.8, 122.1, 39.2.
2-(3-[(4-Methylphenyl)sulfanyl]methylphenyl)pyridine, 6e. Under an argon atmosphere, 4-methylbenzenethiol (0.70 mmol, 87 mg) and trimethylamine (0.70 mmol, 0.099 mL) were dissolved in dry EtOH (2 mL) and stirred at room temperature in a sealed microwave vial for 15 min. 2-[3-(Bromomethyl)phenyl]pyridine, 5 (0.44 mmol, 110 mg) in dry EtOH (2 mL) was then added and the mixture was stirred under microwave irradiation (maximum power, 300 W, dynamic heating) at 150 °C for 20 min. After cooling, the solvent was removed in vacuo and the crude mixture diluted with H2O (25 mL) and EtOAc (25 mL). The product was extracted with EtOAc (2 × 25 mL), washed with H2O (2 × 25 mL) and brine (25 mL). The organic layers were dried over anhydrous MgSO4, filtered and concentrated in vacuo. The crude product was purified by flash chromatography (8:2 hexane:Et2O) yielding 65 mg of the expected product, 6e as a yellow oil in 51% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 8.70 (d, J = 4.8 Hz, 1H), 7.90 (s, 1H), 7.87 (d, J = 7.6 Hz, 1H), 7.72 (m, 1H), 7.65 (d, J = 8.0 Hz, 1H), 7.39 (dd, J = 7.6, 7.6 Hz, 1H), 7.32 (d, J = 7.6 Hz, 1H), 7.25 (d, J = 8.1 Hz, 2H), 7.21 (ddd, J = 7.4, 4.8, 1.2 Hz), 7.06 (d, J = 8.1 Hz, 2H), 4.15 (s, 2H), 2.30 (s, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 157.2, 149.6, 139.6, 138.3, 136.6, 132.4, 131.5, 130.9 (2C), 129.6 (2C), 129.3, 128.8, 127.4, 125.7, 122.1, 120.5, 39.9, 21.0. HRMS (m/z). Calc. for [C19H17NS + H]+ 292.1154. Found 292.1151.
2-(3-[(4-Methoxyphenyl)sulfanyl]methylphenyl)pyridine, 6f. Same method as 6b, using 4-methoxybenzenethiol (1.10 mmol, 0.136 mL). The crude product was purified using flash column chromatography (9:1 DCM:hexane) yielding 203 mg of the expected product, 6f as a yellow oil in 60% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 8.69 (ddd, J = 4.8, 1.8, 0.9 Hz, 1H), 7.86 (d, J = 7.7 Hz, 1H), 7.81 (s, 1H), 7.73 (ddd, J = 9.7, 7.9, 1.8 Hz, 1H), 7.65 (d, J = 7.7 Hz, 1H), 7.37 (dd, J = 7.7, 7.7 Hz, 1H), 7.28 (d, J = 8.8 Hz, 2H), 7.25–7.21 (m, 2H), 6.79 (d, J = 8.8 Hz, 2H), 4.07 (s, 2H), 3.76 (s, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 159.3, 157.2, 149.6, 139.5, 138.6, 136.6, 134.2 (2C), 129.4, 128.8, 127.5, 126.0 (2C), 125.6, 122.1, 120.6, 114.5, 55.3, 41.3. HRMS (m/z). Calc. for [C19H17NOS + H]+ 308.1104. Found 308.1109.
2-3-[(Ethylsulfanyl)methyl]phenylpyridine chloro-palladacycle, 1b. Under an argon atmosphere, PdCl2 (1.17 mmol, 208 mg) was dissolved in dry MeCN (10 mL) and heated under reflux until a red solution had formed. AgBF4 (2.36 mmol, 460 mg) in dry MeCN (5 mL) was added to the PdCl2 solution and heated under reflux for 2 h, forming a white precipitate. The precipitate was filtered off, and 6b (1.13 mmol, 260 mg) dissolved in dry MeCN (10 mL), was added to the filtrate and heated under reflux for 4 h. The solution was cooled to room temperature, filtered over celite, and the solvent removed in vacuo. The crude solid was dissolved in MeCN (5 mL), and NaCl (26.0 mmol, 1.52 g) dissolved in H2O (5 mL) was added, and stirred at room temperature for 3 h. The solvent was removed in vacuo, and the crude mixture dissolved in DCM (25 mL) and H2O (25 mL). The crude product was extracted with DCM (2 × 25 mL), washed with H2O (2 × 25 mL) and brine (25 mL), and dried over anhydrous Na2SO4. The mixture was filtered over celite, and the solvent removed in vacuo, yielding 347 mg of the expected product, 1b as a yellow solid in 83% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 9.15 (d, J = 5.5 Hz, 1H), 7.84 (ddd, J = 7.8, 7.8 Hz, 1H), 7.64 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 7.7 Hz, 1H), 7.26–7.23 (m, 1H), 7.08 (dd, J = 7.7, 7.7 Hz, 1H), 7.03 (d, J = 7.7 Hz, 1H), 4.25 (bs, 2H), 3.20 (q, J = 7.4 Hz, 2H), 1.57 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 165.5, 165.3, 150.5, 148.1, 144.4, 139.0, 125.0, 124.7, 122.9, 122.2, 118.7, 45.8, 33.8, 14.8. HRMS (m/z). Calc. for [C14H14NPdS]+ 333.9876. Found 333.9878. Elemental Analysis. Calc. (%) for C14H14NPdSCl: C 45.42, H 3.81, N 3.78; found C 45.50, H 3.75, N 3.83.
2-3-[(Propylsulfanyl)methyl]phenylpyridine chloro-palladacycle, 1c. Same method as 1b using 6c (0.55 mmol, 113 mg), yielding 179 mg of the expected product, 1c as a yellow solid in 85% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 9.11 (d, J = 5.5 Hz, 1H), 7.82 (ddd, J = 7.8, 7.8, 1.7 Hz, 1H), 7.62 (d, J = 7.7 Hz, 1H), 7.30 (d, J = 7.8 Hz, 1H), 7.22 (ddd, J = 7.5, 5.5, 1.3 Hz, 1H), 7.05 (dd, 7.7, 7.7 Hz, 1H), 7.00 (d, J = 7.7 Hz, 1H), 4.27 (bs, 2H), 3.15 (t, J = 7.8 Hz, 2H), 1.96 (m, 2H), 1.07 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 165.5, 165.3, 150.5, 148.1, 144.4, 139.0, 125.0, 124.6, 122.9, 122.1, 118.7, 46.6, 41.4, 23.3, 13.3. HRMS (m/z). Calc. for [C15H16NPdS]+ 348.0033. Found 348.0032. Elemental Analysis. Calc. (%) for C15H16NPdSCl: C 46.89, H 4.20, N 3.65; found: C 47.02, H 4.08, N 3.56.
2-3-[(Phenylsulfanyl)methyl]phenylpyridine chloro-palladacycle, 1d. Same method as 1b using 6d (1.51 mmol, 418 mg). The crude product was purified using flash column chromatography (100% consisting of 98:2 DCM:MeOH) yielding 446 mg of the expected product 1d as a yellow solid in 71% yield. 1H NMR (500 MHz), Chloroform-d δ (ppm): 9.14 (d, J = 5.5 Hz, 1H), 7.91–7.89 (m, 2H), 7.83 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H), 7.62 (d, J = 7.7 Hz, 1H), 7.36–7.33 (m, 3H), 7.29 (d, J = 7.7 Hz, 1H), 7.20 (ddd, J = 7.7, 5.5, 1.2 Hz, 1H), 7.06 (dd, J = 7.7, 7.7 Hz, 1H), 7.00 (d, J = 7.7 Hz, 1H), 4.63 (s, 2H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 166.0, 165.5, 150.8, 147.8, 144.6, 139.1, 132.8, 131.9 (2C), 129.9, 129.6 (2C), 124.9, 124.8, 122.9, 122.3, 118.8, 53.1. HRMS (m/z). Calc. for [C18H14NPdS]+ 381.9876. Found 381.9876. Elemental Analysis. Calc. (%) for C18H14NPdSCl: C 51.69, H 3.37, N 3.35; found C 51.50, H 3.28, N 3.41.
2-(3-[(4-Methylphenyl)sulfanyl]methylphenyl)pyridine, 1e. Same method as 1b, using 6e (0.54 mmol, 158 mg). 1H NMR (500 MHz), Chloroform-d δ (ppm): 9.19 (d, J = 5.5 Hz, 1H), 7.84 (dd, J = 7.6, 7.6 Hz, 1H), 7.79 (d, J = 8.1 Hz, 2H), 7.64 (d, J = 7.6 Hz, 1H), 7.33 (d, J = 7.6 Hz, 1H), 7.23–7.21 (m, 1H), 7.16 (d, J = 8.1 Hz, 2H), 7.08 (dd, J = 7.6, 7.6 Hz, 1H), 7.00 (d, J = 7.6 z, 1H), 4.60 (bs, 2H), 2.32 (s, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 166.0, 165.5, 150.7, 147.9, 144.5, 140.4, 139.1, 132.0 (2C), 130.3 (2C), 129.4, 124.8, 122.9, 122.2, 118.8, 53.5, 21.2. HRMS (m/z). Calc. for [C19H16NPdS]+ 396.0033. Found 396.0050. Elemental Analysis. Calc. (%) for C19H16NPdSCl: C 52.63, H 3.84, N 3.29; found C 52.79, H 3.73, N 3.24.
2-(3-[(4-Methoxyphenyl)sulfanyl]methylphenyl)pyridine, 1f. Same method as 1b, using 1e (0.62 mmol, 190 mg). 1H NMR (500 MHz), Chloroform-d δ (ppm): 9.18 (d, J = 5.5 Hz, 1H), 7.86–7.83 (m, 3H), 7.65 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 7.6 Hz, 1H), 7.23 (ddd, J = 7.6, 5.5, 1.4 Hz, 1H), 7.08 (dd, J = 7.6, 7.6 Hz, 1H), 6.99 (d, J = 7.6 Hz, 1H), 6.87 (d, J = 8.9 Hz, 1H), 4.58 (s, 2H), 3.77 (s, 3H). 13C NMR (126 MHz), Chloroform-d δ (ppm): 165.9, 165.5, 161.2, 150.8, 147.8, 144.5, 139.1, 134.0 (2C), 124.8, 123.5, 122.9, 122.2, 118.8, 115.1 (2C), 55.5, 54.4. HRMS (m/z). Calc. for [C19H16NOPdS]+ 411.9982. Found 411.9991. Elemental Analysis. Calc. (%) for C19H16NOPdSCl: C 50.91, H 3.60, N 3.12; found C 50.80, H 3.47, N 3.19.
4. Computational Section
Geometry optimization calculations were performed using Gaussian09 [44], in the gas-phase. The minimized structures were confirmed by the absence of any imaginary modes of vibration using frequency analysis. All structures were optimized using the generalized gradient approximation (GGA) PBE density functional [45,46]. The SDD ECP basis set was used for Pd, and the 6-31+G(d,p) basis set was used for all other atoms (PBE/6-31+G(d,p)[SDD]). This methodology has been validated in our previous study into the structures of symmetrical pincer palladacycles [36]. The topological analysis using quantum theory of atoms in molecules (QTAIM) was performed using the Multiwfn program [47]. The ωB97XD[48]/6-311+G(2df,2p)[DGDZVP] model chemistry was used for these calculations. The all-electron relativistic DGDZVP basis set was used to treat Pd [49] as the bond path cannot be traced when treated using ECP.
5. Conclusions
It has been shown that the trans influence plays a key role in the stability of unsymmetrical pincer palladacycles, with the bond strength, and the bond length of the Pd-donor atom interaction affected significantly when trans to a ligand exhibiting a strong trans influence. The topological analysis of the electron density at the bond critical point, and the structure determination, show that the strength of the trans influence is in the order P > S > N. This is in agreement with previous work [40,42].
A library of SCN pincer palladacycles were synthesized via C–H bond activation and characterized using X-ray crystallography, demonstrating the utility of late stage derivitization. These SCN palladacycles, along with PCN and N’CN previously synthesized by the authors, were used to investigate the driving force for the trans influence. It was shown, by investigating the electron density at the bond critical point and changes in the Pd-donor ligand bond length, that it is the donor atom that is responsible for the trans influence. The electronic and steric factors of the ligand do not influence significantly the bond strength of the ligand trans to it. This demonstrates the important role of unsymmetrical pincer palladacycles, with different donor atoms, in the search for harnessing and exploiting hemilability in the design of effective new palladacycle catalysts.
Supplementary Materials
The following are available online at www.mdpi.com/2304-6740/4/3/25/s1, Section S1: QTAIM analysis data (electron density ρ(r), Laplacian of the electron density ∇2ρ(r), total energy H(r), bond degree parameter |H(r)/ρ(r)|, ellipticity ε, delocalization index δ, at the BCP of Pd–Cl (Table S1) and at the BCPs of Pd–Y and Pd–Y’ (Table S2)); Section S2: Cartesian coordinates and Section S3: Experimental spectral data.
Acknowledgments
We would like to thank the University of Sussex for a studentship (GWR), Royal Thai Government for scholarship (SB) and Christopher Dadswell for the support and use of the GC equipment, and Johnson Matthey for the loan of palladium salts. We would also like to thank Alaa Abdul-Sada at the University of Sussex and the EPSRC Mass Spectroscopy Service (University of Swansea) for mass spectrometry services.
Author Contributions
Sarote Boonseng performed the calculations, interpreted the data and wrote the theoretical section. Gavin W. Roffe performed the experiments, interpreted their data and wrote the experimental section. Rhiannon N. Jones synthesized 1d. Simon J. Coles and Graham J. Tizzard performed X-ray crystal structure analysis. Hazel Cox and John Spencer provided the idea, supervised the project and revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Pyr | Pyridine |
| DFT | Density Functional Theory |
| QTAIM | Quantum Theory of Atoms in Molecules |
| Tol | Toluene |
| HRMS | High Resolution Mass Spectrometry |
| ESI | Electrospray Ionization |
| GC | Gas Chromatography |
| DMF | Dimethylformamide |
| DCM | Dichloromethane |
| PBE | Perdew Burke Ernzerhof Exchange–Correlation Functional |
| MSE | Mean Signed Error |
| MUE | Mean Unsigned Error |
| BCP | Bond Critical Point |
| GGA | Generalized Gradient Approximation |
| SDD | Stuttgart-Dresden |
| ECP | Effective Core Potentials |
References
- Cope, A.C.; Siekman, R.W. Formation of covalent bonds from platinum or palladium to carbon by direct substitution. J. Am. Chem. Soc. 1965, 87, 3272–3273. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The potential of palladacycles: More than just precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-L.; Hao, X.-Q.; Gong, J.-F.; Song, M.-P. Symmetrical and unsymmetrical pincer complexes with group 10 metals: Synthesis via aryl C–H activation and some catalytic applications. Dalton Trans. 2011, 40, 5135–5150. [Google Scholar] [CrossRef] [PubMed]
- Selander, N.; Szabó, K.J. Catalysis by palladium pincer complexes. Chem. Rev. 2011, 111, 2048–2076. [Google Scholar] [CrossRef] [PubMed]
- Morales-Morales, D. Pincer complexes. Applications in catalysis. Rev. Soc. Quim. Mex. 2004, 48, 338–346. [Google Scholar] [CrossRef]
- Liu, B.-B.; Wang, X.-R.; Guo, Z.-F.; Lu, Z.-L. Mononuclear versus dinuclear palladacycles derived from 1,3-bis(N,N-dimethylaminomethyl)benzene: Structures and catalytic activity. Inorg. Chem. Commun. 2010, 13, 814–817. [Google Scholar] [CrossRef]
- Kruithof, C.A.; Dijkstra, H.P.; Lutz, M.; Spek, A.L.; Gebbink, R.J.M.K.; van Koten, G. X-Ray and NMR study of the structural features of SCS-pincer metal complexes of the group 10 triad. Organometallics 2008, 27, 4928–4937. [Google Scholar] [CrossRef]
- Kjellgren, J.; Aydin, J.; Wallner, O.A.; Saltanova, I.V.; Szabó, K.J. Palladium pincer complex catalyzed cross-coupling of vinyl epoxides and aziridines with organoboronic acids. Chem. Eur. J. 2005, 11, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Sheets, M. A SeCSe−Pd(II) pincer complex as a highly efficient catalyst for allylation of aldehydes with allyltributyltin. J. Org. Chem. 2006, 71, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Aydin, J.; Selander, N.; Szabó, K.J. Strategies for fine-tuning the catalytic activity of pincer-complexes. Tetrahedron Lett. 2006, 47, 8999–9001. [Google Scholar] [CrossRef]
- Gagliardo, M.; Selander, N.; Mehendale, N.C.; Van Koten, G.; Klein Gebbink, R.J.M.; Szabó, K.J. Catalytic performance of symmetrical and unsymmetrical sulfur-containing pincer complexes: Synthesis and tandem catalytic activity of the first PCS-pincer palladium complex. Chem. Eur. J. 2008, 14, 4800–4809. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; SanMartin, R.; Ines, B.; Herrero, M.T.; Domínguez, E. Recent advances in the use of unsymmetrical palladium pincer complexes. Curr. Org. Chem. 2009, 13, 878–895. [Google Scholar]
- Roffe, G.W.; Boonseng, S.; Baltus, C.B.; Coles, S.J.; Day, I.J.; Jones, R.N.; Press, N.J.; Ruiz, M.; Tizzard, G.J.; Cox, H.; et al. A synthetic, catalytic and theoretical investigation of an unsymmetrical SCN pincer palladacycle. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Roffe, G.W.; Tizzard, G.J.; Coles, S.J.; Cox, H.; Spencer, J. Synthesis of unsymmetrical N’CN and PCN pincer palladacycles and their catalytic evaluation compared with a related SCN pincer palladacycle. Org. Chem. Front. 2016, 3, 957–965. [Google Scholar] [CrossRef]
- Braunstein, P.; Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew. Chem. Int. Ed. 2001, 40, 680–699. [Google Scholar] [CrossRef]
- Khusnutdinova, J.R.; Milstein, D. Metal-ligand cooperation. Angew. Chem. Int. Ed. 2015, 54, 12236–12273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Chien, S.W.; Hor, T.S.A. Recent advances in metal catalysts with hybrid ligands. Coord. Chem. Rev. 2011, 255, 1991–2024. [Google Scholar] [CrossRef]
- Ramírez-Rave, S.; Estudiante-Negrete, F.; Toscano, R.A.; Hernández-Ortega, S.; Morales-Morales, D.; Grévy, J.M. Synthesis and characterization of new Pd(II) non-symmetrical Pincer complexes derived from thioether functionalized iminophosphoranes. Evaluation of their catalytic activity in the Suzuki–Miyaura couplings. J. Organomet. Chem. 2014, 749, 287–295. [Google Scholar] [CrossRef]
- Saha, D.; Verma, R.; Kumar, D.; Pathak, S.; Bhunya, S.; Sarkar, A. A “hemilabile” palladium–carbon bond: Characterization and its implication in catalysis. Organometallics 2014, 33, 3243–3246. [Google Scholar] [CrossRef]
- Poverenov, E.; Gandelman, M.; Shimon, L.J.W.; Rozenberg, H.; Ben-David, Y.; Milstein, D. Pincer “hemilabile” effect. PCN platinum(II) complexes with different amine “arm length”. Organometallics 2005, 24, 1082–1090. [Google Scholar]
- Gargir, M.; Ben-David, Y.; Leitus, G.; Diskin-Posner, Y.; Shimon, L.J.W.; Milstein, D. PNS-Type ruthenium pincer complexes. Organometallics 2012, 31, 6207–6214. [Google Scholar] [CrossRef]
- Fleckhaus, A.; Mousa, A.H.; Lawal, N.S.; Kazemifar, N.K.; Wendt, O.F. Aromatic PCN palladium pincer complexes. Probing the hemilability through reactions with nucleophiles. Organometallics 2015, 34, 1627–1634. [Google Scholar] [CrossRef]
- Pidcock, A.; Richards, R.E.; Venanzi, L.M. 195Pt–31P nuclear spin coupling constants and the nature of the trans-effect in platinum complexes. J. Chem. Soc. A Inorg. Phys. Theor. 1966, 1707–1710. [Google Scholar] [CrossRef]
- Appleton, T.G.; Clark, H.C.; Manzer, L.E. The trans-influence: Its measurement and significance. Coord. Chem. Rev. 1973, 10, 335–422. [Google Scholar] [CrossRef]
- Quagliano, J.V.; Schubert, L. The trans effect in complex inorganic compounds. Chem. Rev. 1952, 50, 201–260. [Google Scholar] [CrossRef]
- Rigamonti, L.; Rusconi, M.; Manassero, C.; Manassero, M.; Pasini, A. Quantification of cis and trans influences in [PtX(PPh3)3]+ complexes. A 31P NMR study. Inorg. Chim. Acta 2010, 363, 3498–3505. [Google Scholar] [CrossRef]
- Randaccio, L.; Bresciani-Pahor, N.; Toscano, P.J.; Marzilli, L.G. Bonding mode and trans influence of the nitromethyl ligand. Structure of trans-bis(dimethylglyoximato)(nitromethyl)(pyridine)cobalt(III). Inorg. Chem. 1981, 20, 2722–2724. [Google Scholar] [CrossRef]
- Otto, S.; Johansson, M.H. Quantifying the trans influence of triphenylarsine. Crystal and molecular structures of cis-[PtCl2(SMe2)(AsPh3)] and cis-[PtCl2(AsPh3)2]·CHCl3. Inorg. Chim. Acta 2002, 329, 135–140. [Google Scholar] [CrossRef]
- Kaltsoyannis, N.; Mountford, P. Theoretical study of the geometric and electronic structures of pseudo-octahedral d0 imido compounds of titanium: The trans influence in mer-[Ti(NR)Cl2(NH3)3] (R = But, C6H5 or C6H4NO2-4). Dalt. Trans. 1999, 781–790. [Google Scholar] [CrossRef]
- Lyne, P.D.; P. Mingos, D.M. The effects of back-bonding to phosphines on the trans influence in [Mo(NH)Cl3(PR3)2]0,±1 (R = H, Me and F). J. Organomet. Chem. 1994, 478, 141–151. [Google Scholar] [CrossRef]
- Jacobsen, H.; Berke, H. Tuning of the transition metal hydrogen bond: How do trans ligands influence bond strength and hydridicity? Dalt. Trans. 2002, 3117–3122. [Google Scholar] [CrossRef]
- Deeth, R.J. The trans influence in [RH(Ph3)3Cl]: A density functional theory study. Dalt. Trans. 1993, 3711–3713. [Google Scholar] [CrossRef]
- Burdett, J.K.; Albright, T.A. Trans influence and mutual influence of ligands coordinated to a central atom. Inorg. Chem. 1979, 18, 2112–2120. [Google Scholar] [CrossRef]
- Loeb, S.J.; Shimizu, G.K.H.; Wisner, J.A. Mono- versus dipalladation of the durene-based tetrathioether Ligand 1,2,4,5-(tBuSCH2)4C6H2. Structures of [PdCl((tBuSCH2)4C6H)] and [Pd2((tBuSCH2)4C6)(MeCN)2][BF4]2. Organometallics 1998, 17, 2324–2327. [Google Scholar] [CrossRef]
- Bader, R.F.W. A bond path: A universal indicator of bonded interactions. J. Phys. Chem. A 1998, 5639, 7314–7323. [Google Scholar] [CrossRef]
- Boonseng, S.; Roffe, G.W.; Spencer, J.; Cox, H. The nature of the bonding in symmetrical pincer palladacycles. Dalt. Trans. 2015, 44, 7570–7577. [Google Scholar] [CrossRef] [PubMed]
- Hartley, F.R. The cis- and trans-effects of ligands. Chem. Soc. Rev. 1973, 2. [Google Scholar] [CrossRef]
- Sajith, P.K.; Suresh, C.H. Bond dissociation energies of ligands in square planar Pd(II) and Pt(II) complexes: An assessment using trans influence. J. Organomet. Chem. 2011, 696, 2086–2092. [Google Scholar] [CrossRef]
- Manojlovic-Muir, L.J.; Muir, K.W. The trans-influence of ligands in platinum(II) complexes. The significance of the bond length data. Inorg. Chim. Acta 1974, 10, 47–49. [Google Scholar] [CrossRef]
- Kapoor, P.N.; Kakkar, R. Trans and cis influence in square planar Pt(II) complexes: A density functional study of [PtClX(dms)2] and related complexes. J. Mol. Struct. Theochem. 2004, 679, 149–156. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Sajith, P.K.; Suresh, C.H. Quantification of mutual trans influence of ligands in Pd(II) complexes: A combined approach using isodesmic reactions and AIM analysis. Dalton Trans. 2010, 39, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Coles, S.J.; Gale, P.A. Changing and challenging times for service crystallography. Chem. Sci. 2012, 3, 683–689. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision B.01. Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple . Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple [Phys. Rev. Lett. 77, 3865 (1996)]. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-Range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Sajith, P.K.; Suresh, C.H. Mechanisms of reductive eliminations in square planar Pd(II) complexes: Nature of eliminated bonds and role of trans influence. Inorg. Chem. 2011, 50, 8085–8093. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).