Abstract
Metal–organic frameworks (MOFs) and their derivatives have emerged as promising candidates for separator engineering in lithium–sulfur batteries (LSBs). This is attributed to their structural tunability, high porosity, and chemical versatility. Despite their potential, challenges such as lithium polysulfide (LiPS) shuttling, sluggish redox kinetics, and poor interfacial stability still hinder the practical deployment of LSBs. This review examines recent advances in MOF- and MOF derivative-based materials for separator modification, focusing on design strategies, functional mechanisms, and electrochemical performance. Pristine MOFs are classified into the following three key structural tuning strategies: control of the pore microenvironment, engineering of metal sites, and enhancement of electrical conductivity. Meanwhile, MOF derivatives are examined using compositional categories to highlight their distinct chemical characteristics and catalytic functionalities for LiPS regulation. Key findings demonstrate that these materials can effectively suppress polysulfide migration, accelerate LiPS redox reactions, and improve lithium-ion transport across the separator. The review also identifies remaining challenges and suggests future perspectives for bridging material-level innovations with system-level applications. Overall, MOF-based separator materials represent a versatile and impactful approach for advancing the electrochemical performance and stability of next-generation LSBs.
1. Introduction
The growing global demand for energy, combined with the overexploitation of fossil fuel resources and their negative environmental impacts (such as pollution and the greenhouse effect), has increased the need for sustainable energy solutions [1,2]. Renewable energy sources, including solar, wind, tidal, and geothermal energy, have emerged as viable alternatives to conventional fossil fuels, offering a more sustainable and environmentally friendly energy supply [3]. However, their intermittent nature requires the development of efficient energy storage technologies. Consequently, advancing next-generation energy storage systems has become a critical area of research to ensure stable and long-term energy conversion and utilization [4]. Lithium-ion batteries (LIBs) have been widely adopted in various applications, from portable electronics to electric vehicles, due to their high energy density, long cycle life, and relatively low self-discharge rates [5]. However, the energy density of conventional LIBs is fundamentally limited by the theoretical capacities of their electrode materials, making it increasingly challenging to meet the evolving energy storage demands of modern technologies, including high-end electronic device applications such as electric vehicles and large-scale energy storage systems [6]. This is particularly true in the electric vehicle industry, which seeks batteries with a greater energy density (exceeding 300 Wh kg−1), extended lifespan, and cost-effectiveness to provide longer driving distances and an improved performance [7]. Additionally, as consumer electronics continue to advance, there is a growing demand for batteries that are more compact and lightweight, while also enabling faster charging and enhanced durability to support increasingly sophisticated and power-intensive devices [8]. To overcome the potential drawbacks of LIBs mentioned above, researchers have been exploring alternative battery technologies that ensure energy density improvements while maintaining safety, stability, and affordability. Among them, lithium–sulfur batteries (LSBs) have gained significant attention as one of the promising next-generation energy storage solutions. The concept of LSBs was first introduced in 1962 by Ulam and Herbert, who proposed sulfur as a high-capacity cathode material for rechargeable batteries [9]. Since then, LSBs have been extensively studied due to their exceptionally high theoretical specific capacity of 1672 mAh g−1 and energy density of 2600 Wh kg−1, which far exceed those of conventional [10] LIBs by several-fold [11]. Moreover, the natural abundance, non-toxicity, and low cost of sulfur further enhance the commercial viability of LSBs, making them an attractive candidate for future-oriented applications requiring a high energy density and cost-effectiveness [12,13].
1.1. Mechanisms of LSBs
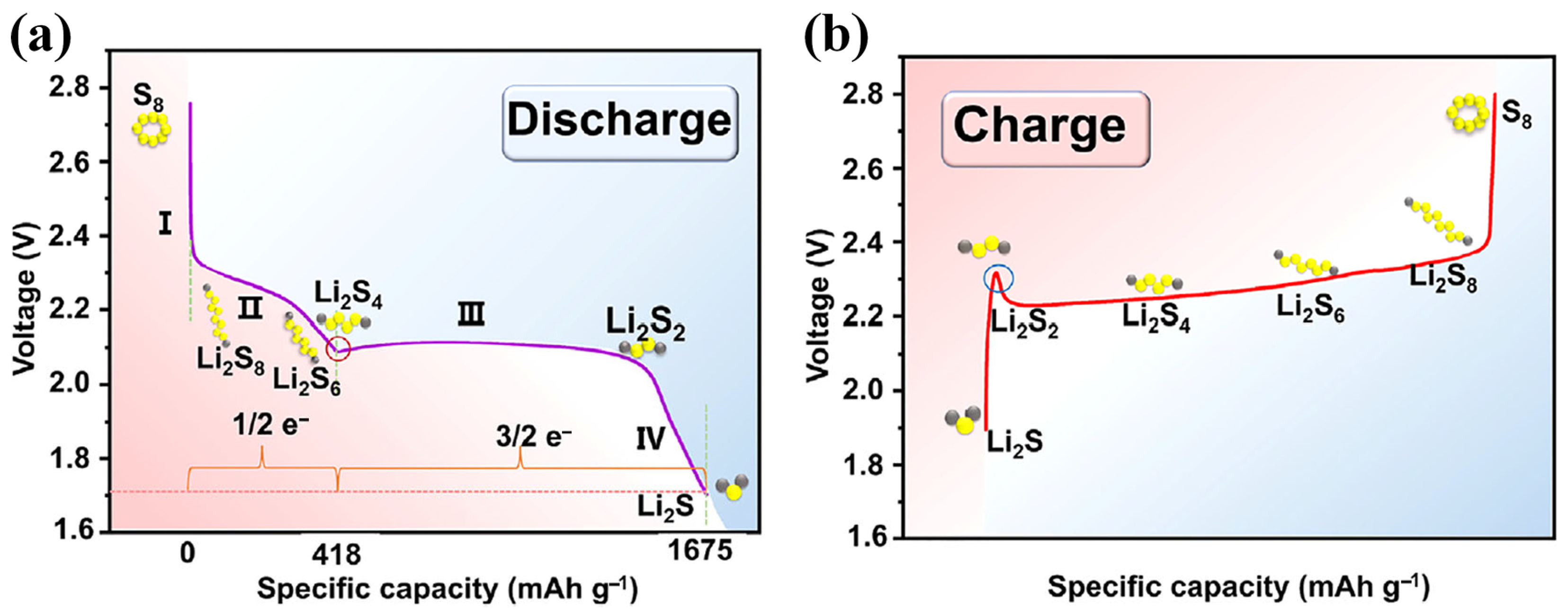
Unlike conventional LIBs that rely on the intercalation/deintercalation of Li ions, LSBs operate through a conversion-type electrochemical mechanism driven by a multi-electron and multi-phase sulfur redox reaction (SRR) [14]. This unique SRR mechanism involves a series of redox transitions among sulfur species (S8 to Li2S through a series of intermediate lithium polysulfides (LiPSs)), accompanied by solid–liquid, liquid–liquid, liquid–solid, and solid–solid phase transformations, as illustrated in Figure 1 [10,15,16]. During the discharging of LSBs (Figure 1a), elemental sulfur (S8) undergoes a stepwise reduction, as shown below.
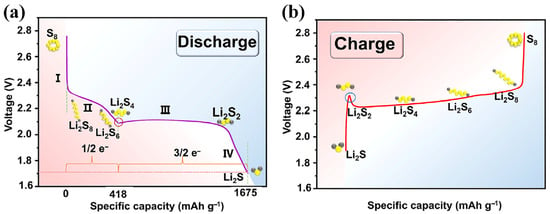
Figure 1.
Schematic diagram of SRR mechanism during (a) discharging and (b) charging of LSBs (reproduced with permission from Ref. [5], copyright 2024 Elsevier).
- (1)
- Stage I: Solid–Liquid Transition (S8 → Li2S8)
The discharge process begins with the reduction of solid S8 to soluble long-chain lithium polysulfides (Li2S8), which dissolve into the electrolyte. This solid–liquid conversion accounts for the initial voltage slope in the discharge profile.
- (2)
- Stage II: Liquid-Phase Redox Reaction (Li2S8 → Li2S6 → Li2S4)
The soluble high-order Li2S8 undergoes further reduction, transitioning into shorter-chain Li2S6. This reaction occurs primarily in the liquid phase and corresponds to the first discharge plateau at approximately 2.3 V (vs. Li+/Li). In the following step, the dissolved Li2S6 undergoes sequential reduction toward intermediate Li2S4, corresponding to the second voltage slope.
- (3)
- Stage III: Liquid–Solid Precipitation (Li2S4 → Li2S2)
Upon further reduction, Li2S4 precipitates into solid Li2S2, leading to a liquid–solid transition. This phase transformation is reflected in the second voltage plateau near 2.05 V (vs. Li+/Li).
- (4)
- Stage IV: Solid–Solid Phase Transformation (Li2S2 → Li2S)
The accumulated solid-phase Li2S2 on the cathode surface is continuously reduced to Li2S (the final discharged product of LSBs) until reaching the cutoff voltage. Owing to the sluggish solid–solid conversion process, this stage governs the overall rate of the SRR [17].
Stage I and II contribute approximately 25% of the theoretical discharge capacity (~418 mAh g−1), while the second discharge platform (Stage III and IV) accounts for about 75% of the theoretical capacity (~1257 mAh g−1) [18,19]. During the charging of LSBs (Figure 1b), the reactions proceed in reverse: Li2S is oxidized stepwise to regenerate intermediate polysulfides and, eventually, elemental sulfur. However, due to the low solubility and poor conductivity of Li2S (
), a remarkable overpotential is typically observed before the first charge plateau, indicating slow oxidation kinetics [20].
1.2. Challenges for LSBs
Despite over half a century of extensive research and development, the practical realization of LSBs remains hindered by several critical and interrelated challenges. First, the inherently insulating nature of sulfur and its discharged products (Li2S2/Li2S) severely limits both electron and ion transport, leading to sluggish electrochemical kinetics and poor active material utilization. This limitation not only slows sulfur conversion, but also promotes the accumulation of intermediate LiPSs [21,22]. Second, the dissolution and migration of LiPSs during cycling present a significant issue. High-order LiPSs (Li2Sx, 4 ≤ x ≤ 8) readily dissolve into the electrolyte and diffuse across the separator due to concentration gradients and slow transformation rates, triggering the well-known “shuttle effect” [23]. Beyond depleting active sulfur, this continuous polysulfide shuttling amplifies other problems by fueling parasitic reactions at the lithium anode, increasing interfacial resistance, and accelerating capacity fading and Coulombic efficiency (CE) loss [24,25]. Third, the substantial volume expansion of the sulfur cathode (up to 80% during lithiation) induces severe mechanical stress, which pulverizes the cathode, disrupts its structure, and promotes the detachment of active material [26]. This degradation not only lowers cycling stability and energy density, but also exposes more LiPSs to the electrolyte, creating a feedback loop where mechanical and electrochemical degradation intensifies the shuttle process and further worsens cell performance [27,28]. Fourth, the instability of the lithium metal anode further exacerbates these challenges in LSBs. Non-uniform Li+ deposition on the anode surface induces dendrite formation, dead lithium, and interfacial instability, allowing dendrites to penetrate the separator and cause internal short circuits [6,29]. Simultaneously, persistent side reactions between lithium and diffusing LiPSs continuously consume active lithium and increase impedance at the anode interface. This degradation not only worsens lithium anode instability, but also accelerates the shuttle effect by generating additional polysulfide species. Together, these intertwined processes establish a vicious cycle that further impairs charge transfer and severely limits the long-term cycling stability and safety of LSBs [30,31]. Taken together, these intertwined challenges mutually reinforce one another, creating a vicious cycle that must be broken through advanced material engineering and cell design before LSBs can reach commercial viability.
1.3. Separator Modification for High-Performance LSBs
To achieve high-performance LSBs, extensive research efforts have been dedicated to mitigating the LiPS shuttle effect, which remains one of the most persistent challenges in ensuring long-term cycling stability. The underlying mechanisms involve the dissolution of higher-order polysulfides into the electrolyte during discharge and their uncontrolled migration across the separator driven by concentration gradients and slow redox conversion kinetics [32,33,34]. Once these polysulfides reach the lithium anode, they trigger parasitic reactions that continuously deplete active material and exacerbate interfacial instability. Over repeated charge–discharge cycles, this process worsens, resulting in accelerated capacity fade, increased impedance, and declining CE. Thus, the shuttle effect not only limits the sulfur utilization at the cathode, but also shortens cell lifespan and raises serious safety concerns. A major line of investigation has focused on the rational design of sulfur cathodes, particularly through the incorporation of conductive carbon matrices. These porous carbon frameworks serve not only as efficient electron pathways, but also offer spatial confinement for sulfur and its intermediate LiPSs. More recently, hybrid systems combining carbon with polar materials (such as metal oxides, sulfides, nitrides, and carbides) have been developed to further enhance chemical affinity toward LiPSs [35]. These carbon/polar material composites have demonstrated an improved adsorption capacity and catalytic activity for the redox conversion between soluble LiPSs and insoluble Li2S2/Li2S, ultimately alleviating the shuttle effect and improving the utilization of active materials. Various fabrication approaches have been reported, including mechanical mixing (ex situ), in situ growth to enhance interfacial bonding, and derivation from organic precursors to produce integrated structures with uniform active site distribution [36,37]. However, these strategies often involve synthetic complexity and may incorporate a considerable amount of electrochemically inactive components, which could compromise the overall energy density of LSBs [38].
Despite these advancements in cathode engineering, it has become increasingly evident that suppressing LiPS shuttling cannot be fully achieved by cathode design alone. Conventional separators, generally composed of porous polymer membranes, possess relatively large macropore structures that fail to inhibit the diffusion of soluble LiPSs [39,40]. This uncontrolled transport allows LiPSs to shuttle freely between electrodes, which accelerates capacity fading and deteriorates battery stability during repeated cycling. These inherent limitations in separator architecture necessitated a shift toward more functionally engineered separator systems. This conceptual shift was notably pioneered by Manthiram and co-workers, who, in 2012, introduced a bifunctional microporous carbon interlayer between the cathode and separator [41]. Their approach demonstrated that the separator could be transformed from a passive ionic conduit into an electrochemically functional component capable of suppressing LiPSs migration and enhancing sulfur utilization. This work catalyzed a wave of research into separator modification as a viable and effective complement to cathode-focused strategies. Consequently, separator modification has emerged as a complementary and efficient strategy that targets LiPSs migration more directly and can be integrated into cell design with a minimal impact on energy density [42].
Interestingly, many of the functional materials originally developed for cathode engineering (such as porous carbon frameworks, polar inorganic compounds, and their hybrids) have also been extensively applied in separator modification [43]. Carbon-based materials, including carbon nanotubes, graphene oxide, and hollow carbon nanospheres, provide a high conductivity and physical confinement for LiPSs [44]. In parallel, polar inorganic materials such as metal oxides, sulfides, and nitrides offer not only chemical affinity and catalytic activity, but also strong chemical adsorption toward LiPSs, enabling the more effective immobilization of soluble intermediates [45,46]. When applied in separator modification, these materials serve as multifunctional components that combine physical obstruction, electrostatic repulsion, chemical adsorption, and catalytic conversion. This design synergy improves LiPS retention and redox conversion. It also helps to regulate lithium-ion flux and suppress dendritic growth [47,48]. As a result, the separator becomes a significant component that contributes directly to improving the electrochemical stability and safety of LSBs.
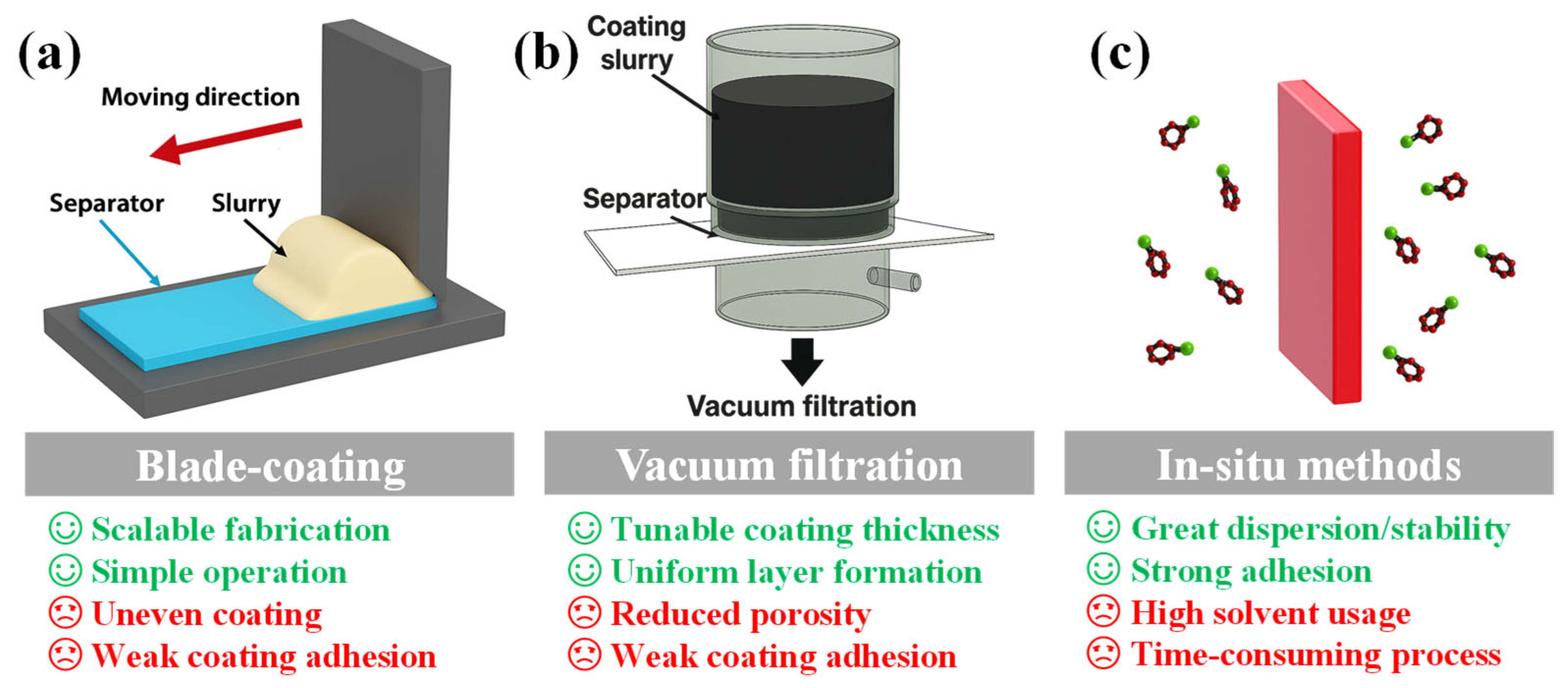
To translate material-level strategies into practical battery configurations, the method of implementing separator modification is equally critical. Broadly, fabrication strategies can be categorized into ex situ and in situ approaches [49]. In ex situ methods, functional materials are pre-synthesized and then applied to commercial separators through techniques such as blade coating, vacuum filtration, dip-coating, and spin coating. These methods are relatively simple and scalable, but may suffer from non-uniform dispersion, weak interfacial adhesion, and increased separator thickness, which could negatively impact ionic conductivity and mechanical integrity [50]. In contrast, in situ methods involve the direct growth or deposition of functional phases onto the separator surface, allowing for enhanced interfacial bonding, uniformity, and structural stability. However, these strategies often require complex procedures, extended reaction times, and strict environmental control, making them less suitable for large-scale implementation. Figure 2 presents typical ex situ and in situ separator modification processes, along with a comparative overview of their respective benefits and drawbacks [39,51].
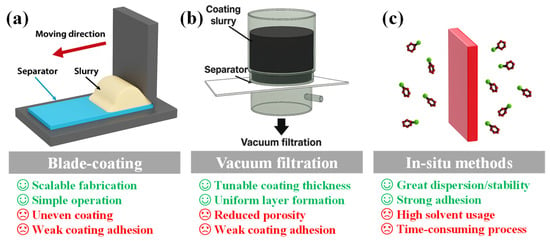
Figure 2.
Advantages and drawbacks of representative ex situ and in situ approaches for LSB separator modification. (a) Blade-coating, (b) Vacuum filtration, (c) In situ methodes.
1.4. Metal–Organic Frameworks (MOFs) for LSB Separator Modification
MOFs represent a unique class of crystalline porous materials, constructed through the coordination of metal ions or clusters with organic ligands [52,53]. Since their initial introduction in the mid-1990s [54], MOFs have undergone rapid development, giving rise to tens of thousands of structural variants with exceptional compositional and architectural diversity. Their highly tunable structures, high surface areas, and abundant pore volumes have positioned MOFs as ideal candidates in various energy applications, including LSBs.
In the context of LSBs, MOFs offer multifunctional roles when employed in separator modification. The dissolution and migration of LiPSs remains a major issue in LSBs, contributing to severe capacity fading and low CE. MOFs address this challenge through a combination of physical confinement, chemical interactions, and catalytic activity. In particular, the high porosity of MOFs plays a critical role in mitigating LiPS migration. The highly ordered pore structure and well-regulated pore size distribution allow these materials to serve as selective barriers that physically hinder LiPS diffusion without impeding lithium-ion transport [55,56]. Moreover, the large specific surface area associated with these porous frameworks provides abundant interaction sites to immobilize LiPSs at the separator interface, further suppressing shuttle effects [57]. This structural balance combining uniform micropores with extensive surface exposure ensures the effective spatial confinement of LiPSs, enhances interfacial contact, and promotes stable electrochemical cycling over extended operation. The polar metal sites and functional groups (Ex: –NH2, –SO3H) of MOFs enable strong chemisorption via Lewis acid–base interactions, where Lewis-acidic metal centers and proton-donating surface groups bind Lewis-basic polysulfide anions through coordinate bonding and electrostatic attractions [58]. This complementary interaction not only suppresses LiPS dissolution into the electrolyte, but also promotes more uniform Li+ flux across the separator. Furthermore, transition metals (such as Co and Ni) in the MOF framework act as catalytic centers that facilitate the reversible cleavage and formation of S–S bonds, thereby accelerating LiPS redox kinetics and improving overall electrochemical performance [59].
Another key advantage of MOFs lies in their synthetic flexibility. Through deliberate choices of metal nodes and linkers, researchers can tailor the pore size, charge distribution, and chemical functionality of MOFs for targeted interaction with LiPSs [60]. This tunability has led to the exploration of pristine MOFs, MOF composites, and MOF-derived materials in separator engineering. However, pristine MOFs often suffer from issues such as mechanical fragility and poor interfacial adhesion. To overcome this, hybrid approaches involving polymers, carbon matrices, and in situ transformation into derivatives have been explored to enhance the stability and processability of MOFs while preserving their functional advantages [61].
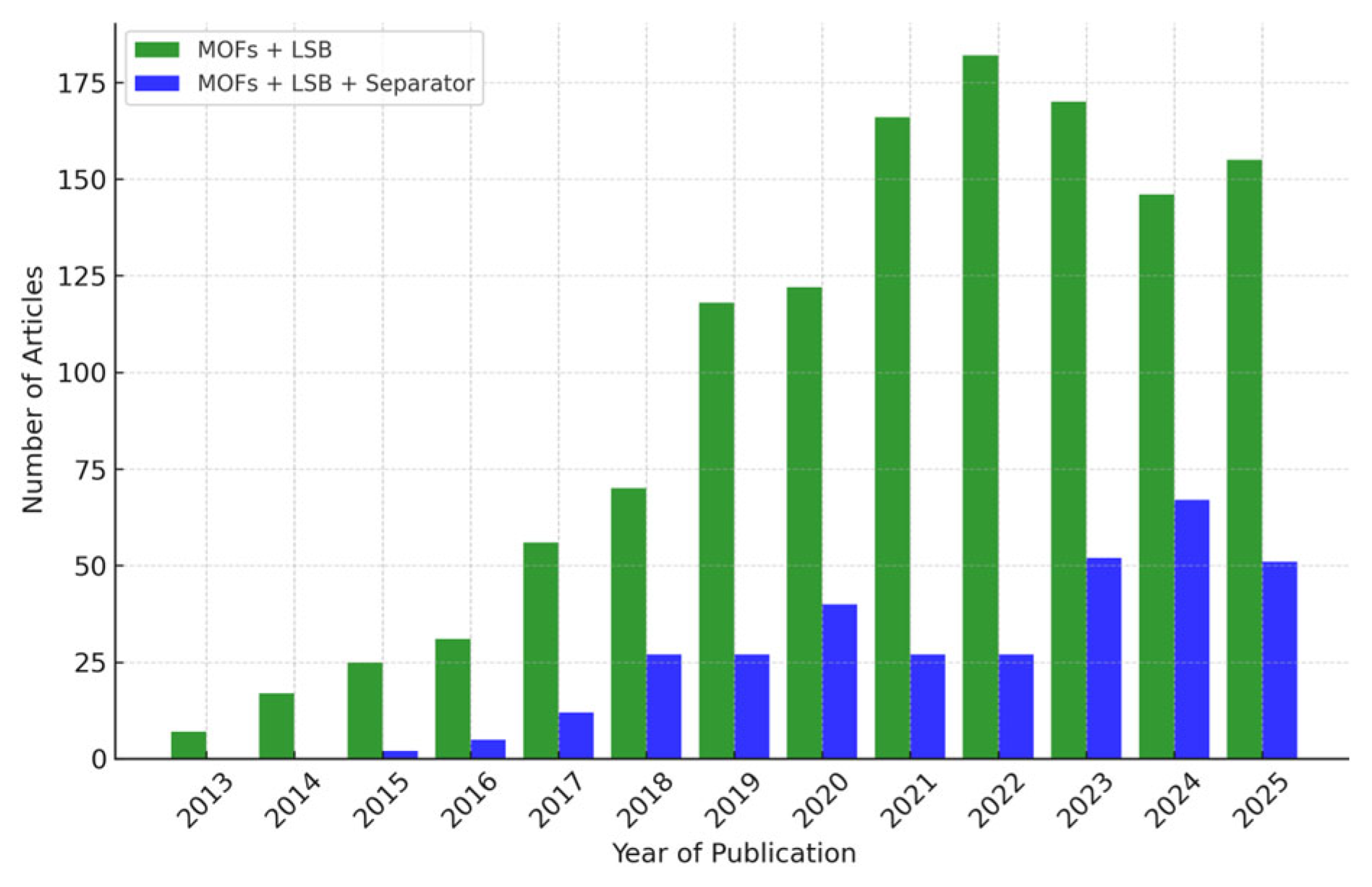
The increasing academic attention paid toward MOFs in the field of LSBs is well supported by publication trends over the past decade. As shown in Figure 3, the number of research articles utilizing MOFs in high-performance LSBs grew markedly from only 7 in 2013 to over 180 by 2021, indicating a sharp rise in interest. Within this expanding body of literature, studies specifically focusing on separator modification have also steadily increased, reflecting the growing recognition of this approach as a viable strategy for improving LSB performance.
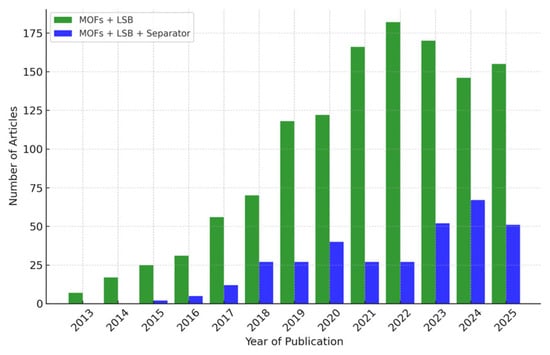
Figure 3.
The statistics of the number of scientific research papers published on (1) MOFs+LSB (Green) and (2) MOFs+LSB+separator (Blue) in the past decade (obtained from Web of Science).
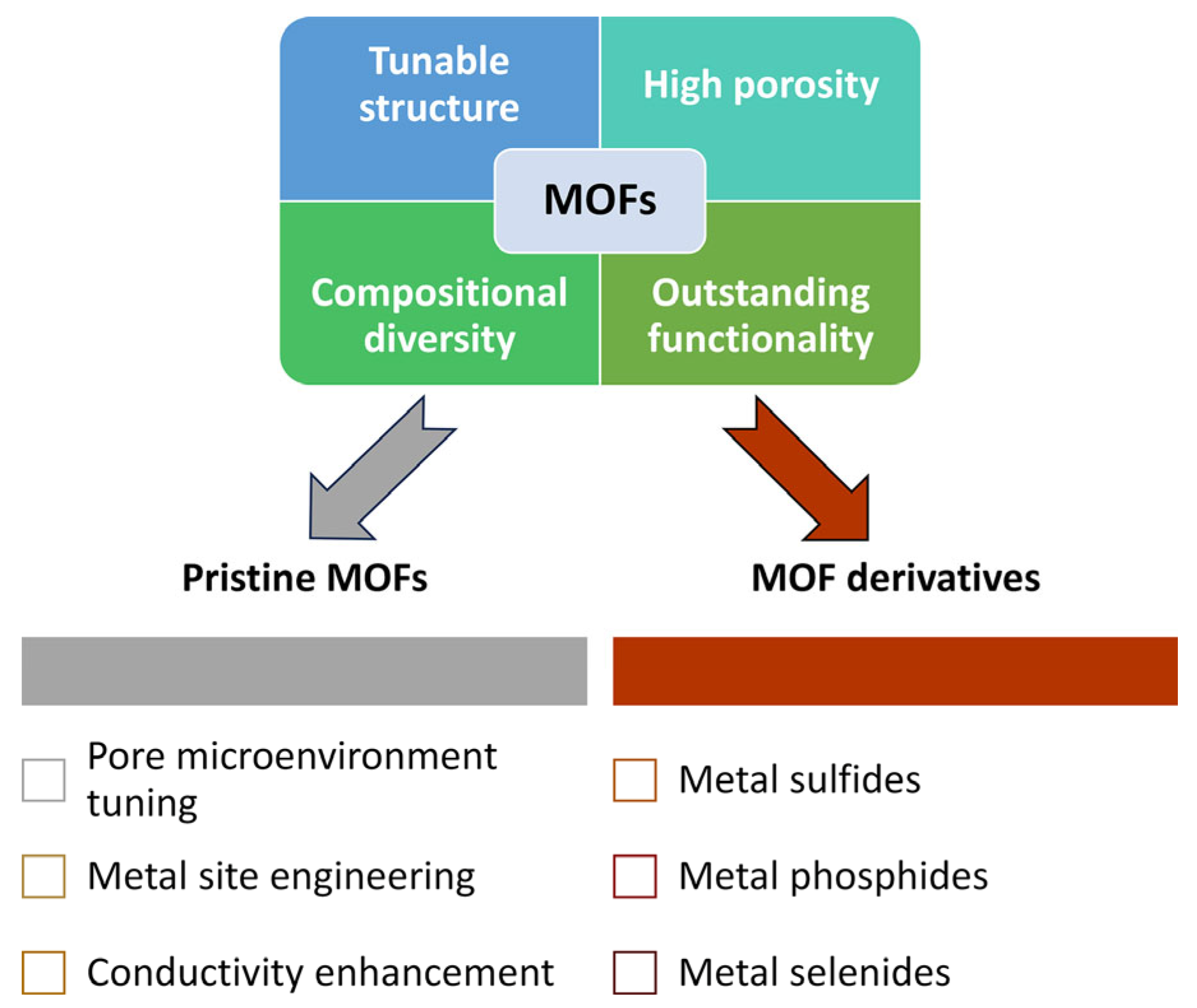
Based on the above features, this review summarizes the recent research progress in LSB separator modification using MOFs and their derivatives with a primary emphasis on cathode-side polysulfide regulation to enhance the performance of LSBs. To help readers navigate this discussion more clearly, pristine MOFs are organized by their structural tuning strategies (such as pore microenvironment control, metal site engineering, and conductivity enhancement), reflecting the primary directions for tailoring their separator functionality. In contrast, MOF derivatives are classified by their final composition (including metal sulfides, phosphides, and selenides) to highlight the distinct catalytic and adsorption properties that emerge from these different material types.
2. The MOFs and Their Derivatives for LSB Separator Fabrication
2.1. Pristine MOF-Based Separators
Among the various approaches to incorporating MOFs into LSB separators, the use of pristine MOFs without significant structural transformation has received considerable attention due to their well-defined porous architectures, tunable chemical environments, and structural regularity. By carefully designing the intrinsic properties of pristine MOFs, such as their pore chemistry, metal coordination centers, electrical conductivity, and morphological features, researchers have developed diverse strategies to suppress LiPSs migration and enhance separator functionality. This section categorizes and discusses representative MOF design strategies based on the following major aspects of pristine MOFs: pore microenvironment tuning, metal site engineering, and electrical conductivity enhancement. Each aspect is illustrated with representative studies that demonstrate their implementation in separator modification for LSBs.
2.1.1. Tailoring Pore Microenvironments in Pristine MOFs
The pore microenvironment of MOFs plays a decisive role in determining their functional performance as separator materials in LSBs. By precisely adjusting pore size, geometry, and surface chemistry, pristine MOFs can be tailored to selectively regulate ion transport while inhibiting the diffusion of LiPSs [62,63]. Strategies such as ligand functionalization, the incorporation of polar groups (Ex: –NH2, –SO3H), and the engineering of narrow microporous channels have been employed to enhance LiPSs confinement via both physical sieving and electrostatic interactions. In particular, the introduction of chemically interactive sites along the pore walls enables targeted interactions with soluble LiPS species, thereby minimizing their shuttling effect across the separator [64]. These modifications can be achieved without compromising the structural integrity of the MOF, allowing for precise control over separator selectivity at the molecular scale. A number of studies have demonstrated that rational tuning of the pore microenvironment leads to significant improvements in the cycling stability and CE of LSBs.
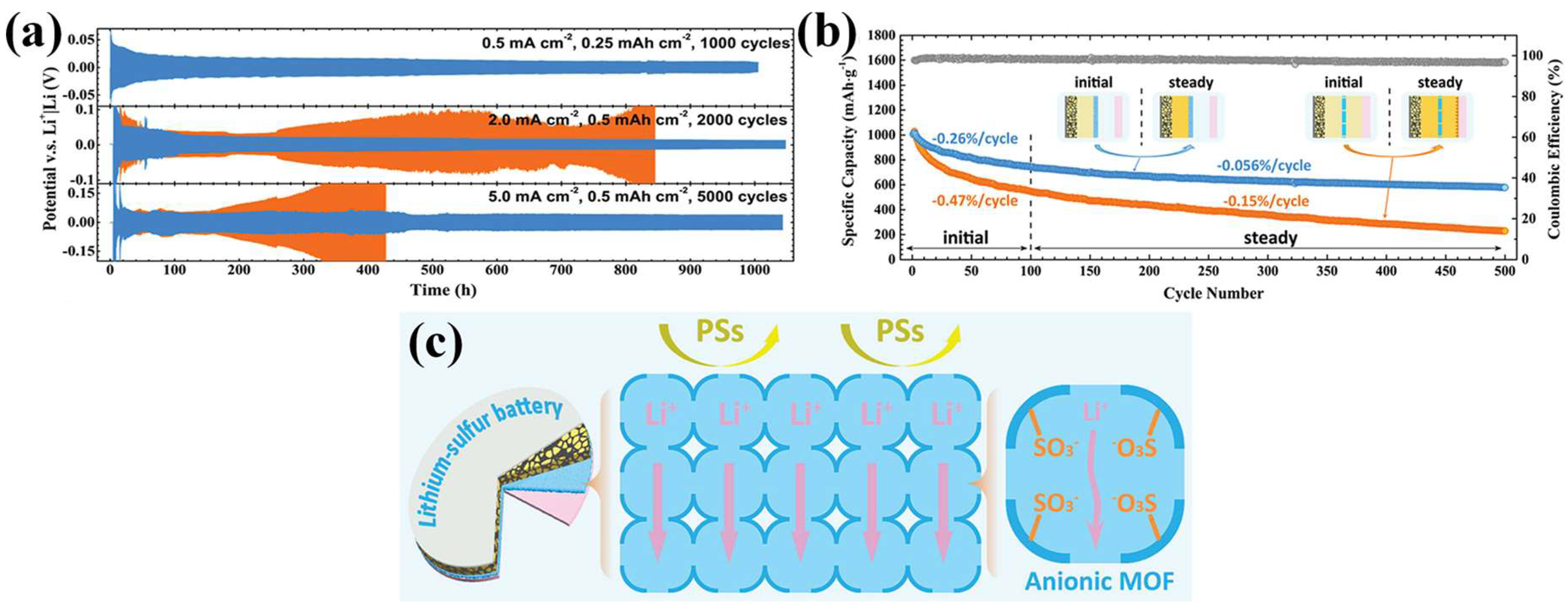
A representative study by Suriyakumar et al. demonstrated the use of UiO-66-NH2, a zirconium-based MOF functionalized with amine groups, for tuning the pore environment of separators in LSBs [65]. The introduction of amine functional groups into MOFs can enable hydrogen bonding with diffusing LiPSs, thereby impeding LiPS diffusion toward the anode. As a result, the LSB with an optimized separator presented a high initial discharge capacity of 1400 mAh g−1 at 0.1 C, representing about 83.5% of the theoretical capacity of sulfur. Even after 100 cycles, the cell retained approximately 600 mAh g−1, suggesting a stable cycling performance. A different approach was taken by Wang et al., who developed a bifunctional separator by incorporating UiO-66-SO3Li into a poly(vinylidene fluoride) (PVDF) matrix through a mixed-matrix membrane (MMM) approach [66]. The MOF was synthesized via cation exchange from UiO-66-SO3H, yielding a framework rich in sulfonate anions. These negatively charged functional groups provided electrostatic repulsion against LiPSs, while the interconnected channels facilitated directional Li+ transport across the separator. As a result, the Li//Li symmetric cell equipped with the MMM separator demonstrated an exceptional electrochemical performance, maintaining stable lithium plating and stripping over 1000 h at 5 mA cm−2 (Figure 4a). The galvanostatic voltage profile in this symmetric cell test is a representative electrochemical method to assess the long-term cycling stability of the separator and metal anode interface, as it clearly revealed dendrite formation, short-circuit events, and overpotential buildup [67]. Thus, this analysis provides direct insight into how effectively a modified separator can regulate lithium deposition and stripping without significant degradation or cell failure. Furthermore, the long-term cycling of the LSB at 0.5 C showed an excellent stability, with a capacity fading rate as low as 0.056% per cycle over 500 cycles (Figure 4b). As illustrated in Figure 4c, the following two key functions of the MMM separator are highlighted: the suppression of LiPS migration through electrostatic repulsion and the regulation of uniform Li+ deposition via directional ion transport across the membrane.
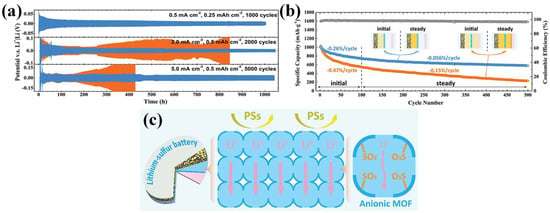
Figure 4.
(a) Lithium plating/stripping performance of Li|MMM|Li and Li|PP|Li symmetric cells under various current rates. (b) Long-term LSB cycling performance at 0.5 C. (c) Schematic illustration of the MMM separator for regulating Li plating and preventing LiPS shuttling in LSBs. (Reproduced with permission from Ref. [66], copyright 2020 Wiley-VCH).
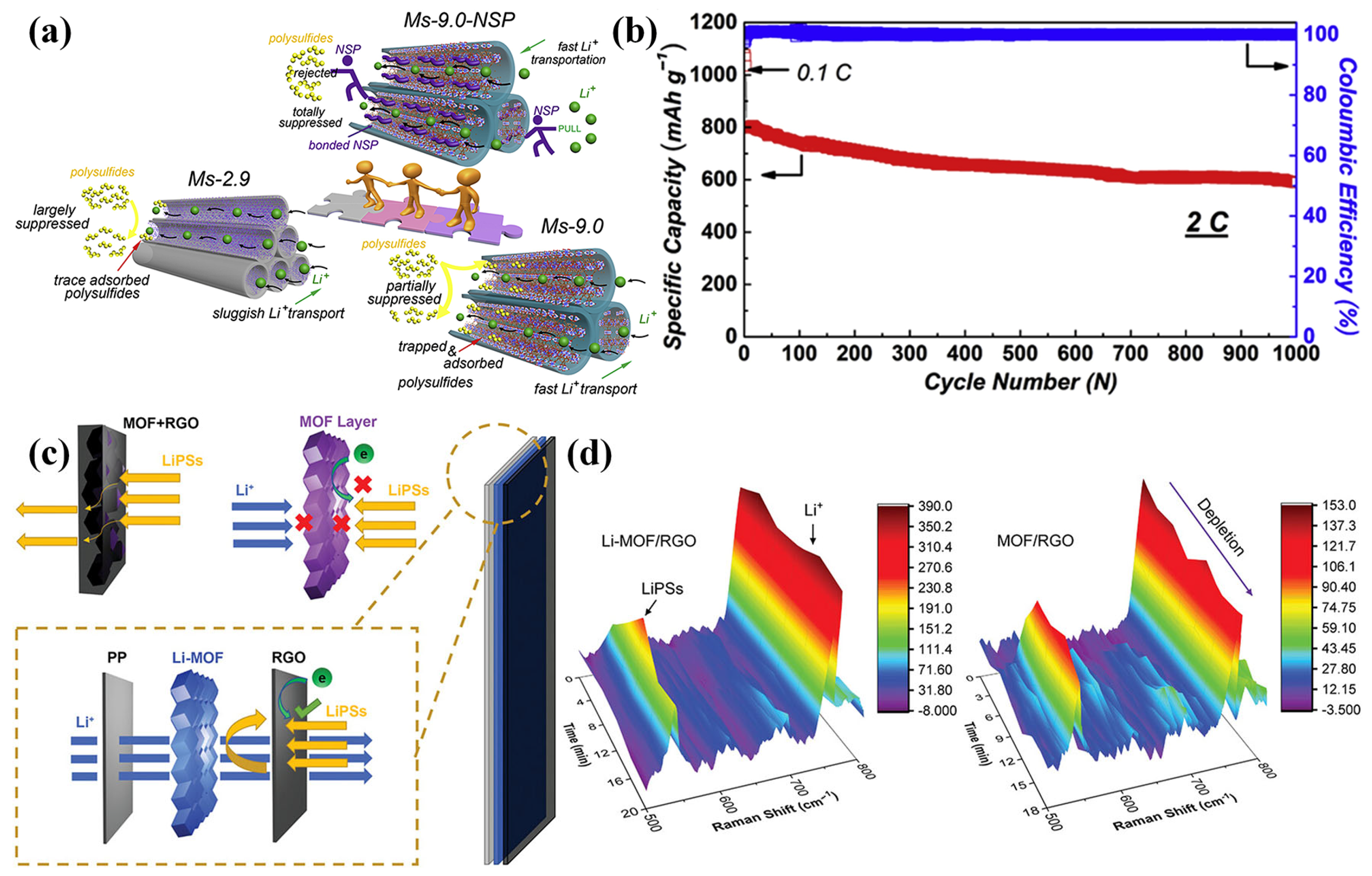
Building on previous efforts to address LiPS shuttling and dendritic Li growth, ion-sieving separators have been developed to simultaneously inhibit LiPS migration and facilitate fast, uniform Li+ transport and deposition [68]. In a study by Chang et al., a systematic comparison of three MOFs with different pore sizes was carried out to investigate their effects on LiPS trapping and Li+ transport behavior [69]. They observed that larger pore sizes, such as those in Ms-9.0, enhanced Li+ transport capacity and reduced polarization, but led to excessive polysulfide adsorption at copper sites, resulting in significant initial sulfur loss. To address this, they incorporated a negatively charged sulfonic polymer (NSP) into Ms-9.0, which effectively mitigated the strong interaction between the copper sites and LiPSs, enhancing the overall stability and performance of the separator. As described in Figure 5a, the incorporation of NSP into Ms-9.0 led to a reduction in pore size to 6.9 Å and altered the charge distribution within the channels. The resulting narrow, negatively charged nanochannels effectively repelled LiPSs while facilitating the attraction and transport of Li+ ions, thereby accelerating ionic mobility and alleviating voltage polarization. When cycled at 2 C (Figure 5b), LSBs using Ms-9.0-NSP exhibited an initial discharge capacity of 803 mAh g−1 and retained 589 mAh g−1 after 1000 cycles (corresponding to a capacity decay of 0.026% per cycle), showing the outstanding cycling stability of the Ms-9.0-NSP cell. Additionally, Cheng et al. suggested a pore-space-partitioned strategy to precisely control the pore structure of the MOF-based coating layer by tailoring the internal pore chemistry of the framework [70]. They developed an MOF structure, FJU-90, by introducing the nitrogen-rich ligand 2,4,6-tris(4-pyridyl)pyridine into the FJU-88 framework, which enabled precise tuning of the pore architecture and a reduction in pore size. The resulting material exhibited a balanced pore distribution, high surface area, and numerous catalytically active sites. These structural features contributed to the effective suppression of LiPS migration, improved Li+ conductivity, and facilitated the redox conversion of LiPSs, collectively leading to an enhanced cycling stability and rate capability under high-sulfur-loading conditions. A different strategy was reported by Zhou et al. [71], who modified ZIF-67 by introducing lithium bis(trifluoromethylsulfonyl)imide (LiTFSI), leading to the formation of a Li+-inserted MOF structure (Li-MOF, Figure 5c). To directly probe these processes, in situ Raman spectroscopy was conducted to monitor the dynamic behavior of LiPS diffusion and Li+ transport during battery discharge. A sealed quartz cuvette cell, consisting of a lithium anode, modified separator, and S/C cathode, is typically assembled to enable real-time observation of the polysulfide conversion process via in situ Raman spectroscopy. In situ Raman spectroscopy (Figure 5d) confirmed that the resulting Li-MOF functional layer effectively suppressed the diffusion of LiPSs, thereby improving sulfur utilization. The presence of Li+ ions within the MOF pores provided continuous pathways for ion transport, enhancing ion transfer kinetics and facilitating mass transport across the separator. When assembled into a Li-MOF/RGO separator, the battery demonstrated an excellent cycling stability, with a capacity fading rate of only 0.089% per cycle over 600 cycles at 1 C.
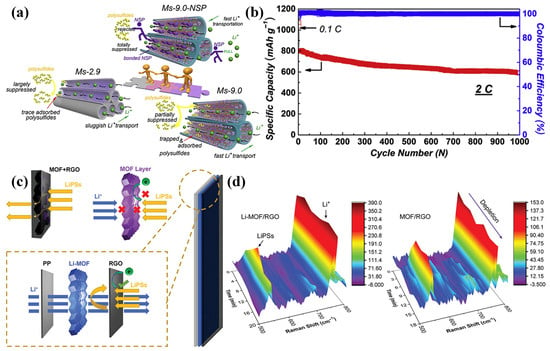
Figure 5.
(a) Schematics for Ms-2.9, Ms-9.0, and Ms-9.0-NSP diffusion of LiPSs and Li+. (b) Long-term cycling performance at 2 C. (Reproduced with permission from Ref. [69], copyright 2020 Elsevier.) (c) Schematic illustration for the mass and charge transfer mechanisms across various modified separators (MOF+RGO, MOF layer, and Li-MOF/RGO). (d) In situ Raman spectra with Li-MOF/RGO and MOF/RGO. (Reproduced with permission from Ref. [71], copyright 2021, Wiley-VCH.)
2.1.2. Engineering Metal Sites of MOFs
The arrangement of metal sites within MOF structures has emerged as an important design dimension for enhancing their functional roles in LSB separators [72]. Metal sites serve as catalytic centers that accelerate redox reactions, improve reaction kinetics, and provide chemically active sites for LiPS adsorption [73]. Recent advances have emphasized the role of defect engineering (such as the creation of metal vacancies or the incorporation of secondary metal species) in tuning the electronic environment and catalytic activity of MOFs [74,75]. These modifications strengthen chemical interactions with LiPSs and promote the more efficient utilization of catalytic sites, thereby facilitating faster polysulfide conversion across multiple stages of the redox process [76].
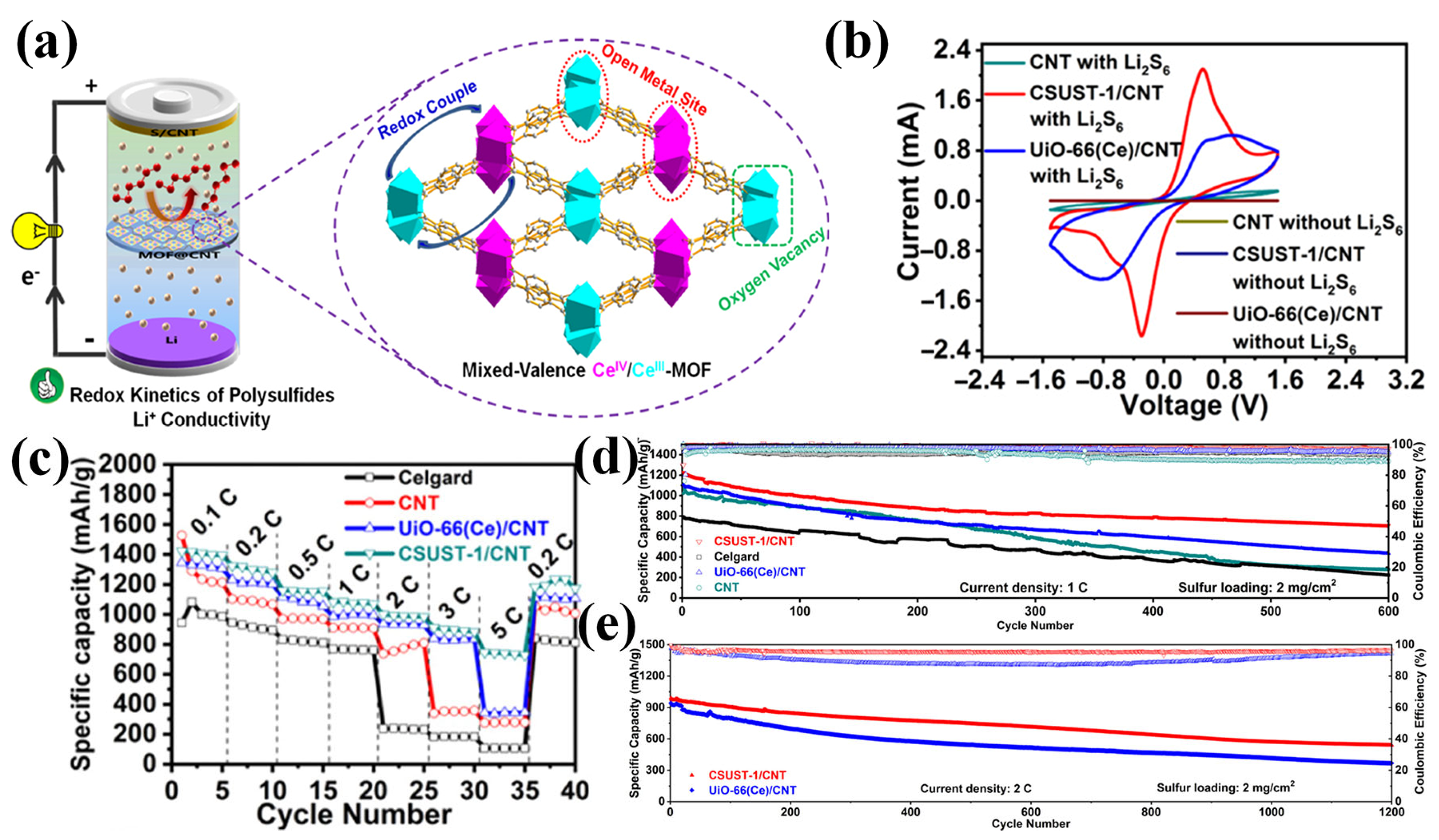
Jin et al. developed a mixed-valence cerium-based MOF (CSUST-1) containing spatially separated Ce(IV)/Ce(III) redox centers (Figure 6a) [77]. When combined with carbon nanotubes (CNTs), the resulting CSUST-1/CNT composite exhibited strong polysulfide adsorption, enhanced redox kinetics, and improved Li+ transport. These enhancements were attributed to the isolated multivalent Ce sites, the presence of oxygen vacancies, and the availability of exposed metal centers. Cyclic voltammetry (CV) measurements in symmetric cells with Li2S6 as the electrolyte (Figure 6b) confirmed that the improved redox activity originated from the catalytically active Ce sites within CSUST-1, as evidenced by a smaller voltage gap and larger CV curve area compared to the reference, indicating enhanced polysulfide conversion kinetics and reduced polarization. In rate performance tests of LSBs (Figure 6c), the CSUST-1/CNT-coated separator led to stable capacities even at high current densities. Compared to the sharp capacity drop observed in the UiO-66(Ce)/CNT cell when increasing the current from 3 C to 5 C, the CSUST-1/CNT cell exhibited a superior rate stability, attributed to its enhanced LiPSs redox kinetics. As expected, LSBs with CSUST-1/CNT delivered a superior long-term cycling performance at 1 C and 2 C, as shown in Figure 6d,e. This study clarified the working mechanism of mixed valence metal electrocatalysts for LiPS conversion. A bifunctional separator was also developed using a layer-by-layer assembly of ultra-thin MOF-Co nanosheets and bacterial cellulose [78]. The exposed Co and O sites on the nanosheet surfaces enabled a dual functionality: O atoms at the anode side promoted uniform Li+ deposition by regulating ion flux, while Co atoms at the cathode side suppressed the LiPS shuttle through Lewis acid–base interactions.
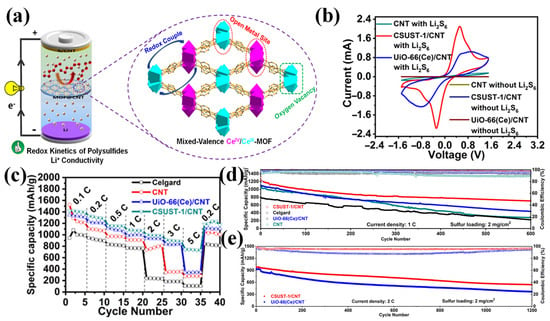
Figure 6.
(a) Schematics depicting the MOF framework structure of CSUST-1. (b) CV curves of the symmetric cells using various separators with/without Li2S6. (c) Rate performance and (d,e) long-term cycling performance (at 1 C and 2 C) of LSBs. (Reproduced with permission from Ref. [77], copyright 2021, American Chemical Society.)
Bimetallic MOFs have also attracted attention as separator-modifying materials for improving electrochemical performance. Zhou et al. developed a scalable method for synthesizing Zn/Co bimetallic ZIF nanosheets to fabricate modified separators for high-performance LSBs, wherein the Zn/Co ratio in the MOF framework could be precisely tuned by adjusting the precursor composition [79]. The Zn/Co-ZIF-coated separators demonstrated an excellent rate capability and cycling performance. Density functional theory (DFT) calculations demonstrated distinct Gibbs free energy profiles for each metal center toward various polysulfide species, with Zn sites favoring adsorption and Co sites enhancing catalytic conversion (Figure 7a). Structural characterizations confirmed the uniform distribution of Zn and Co within the nanosheet architecture, supporting the synergistic contribution of both metal centers to accelerated polysulfide conversion kinetics (Figure 7b). To address similar challenges, Leng et al. employed an electrospinning technique using polyacrylonitrile (PAN) to fabricate a porous Ni–Co MOF@PAN (NCMP) composite, which was applied as a functional separator in LSBs (Figure 7c) [80]. The resulting NCMP separator exhibited both an excellent rate capability and strong cycling stability. By an energy-dispersive X-ray spectroscopy analysis of the cycled Li metal anodes, the S peak signal obtained from the cell with the NCMP separator was weaker than that from the cell using a conventional Celgard separator, indicating that the NCMP structure effectively suppressed polysulfide shuttling during cycling (Figure 7d,e).
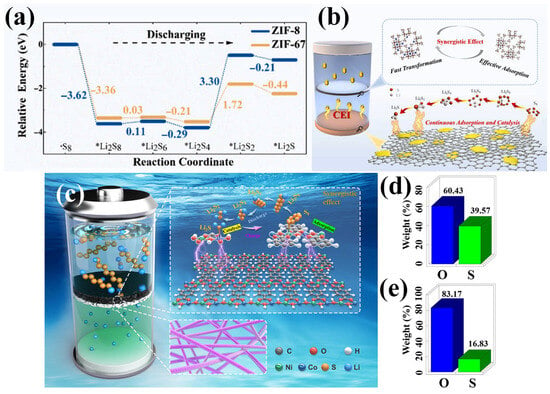
Figure 7.
(a) Energy profiles for the reduction of LiPSs on pure ZIF-8 (Zn) and ZIF-67 (Co) substrates. (b) Schematic of synergistic mechanism of Zn/Co-ZIF nanosheets on LiPSs. (Reproduced with permission from Ref. [79], copyright 2022, Elsevier.) (c) Schematic of NCMP separator multi-functions. Weight ratio of O/S obtained from energy-dispersive X-ray spectroscopy of the cycled Li metal anodes using (d) Celgard and (e) NCMP separator. (Reproduced with permission from Ref. [80], copyright 2023, Elsevier.)
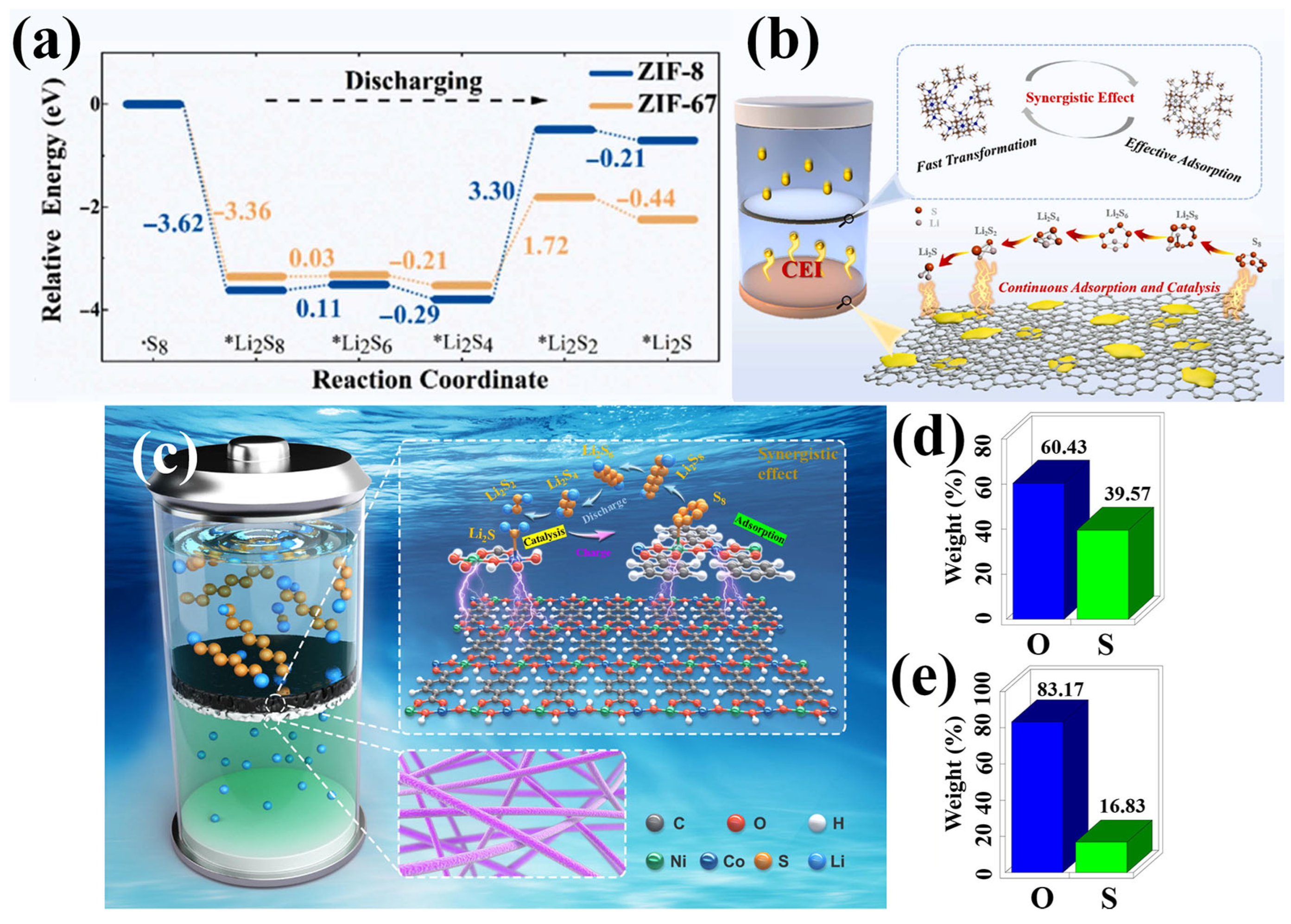
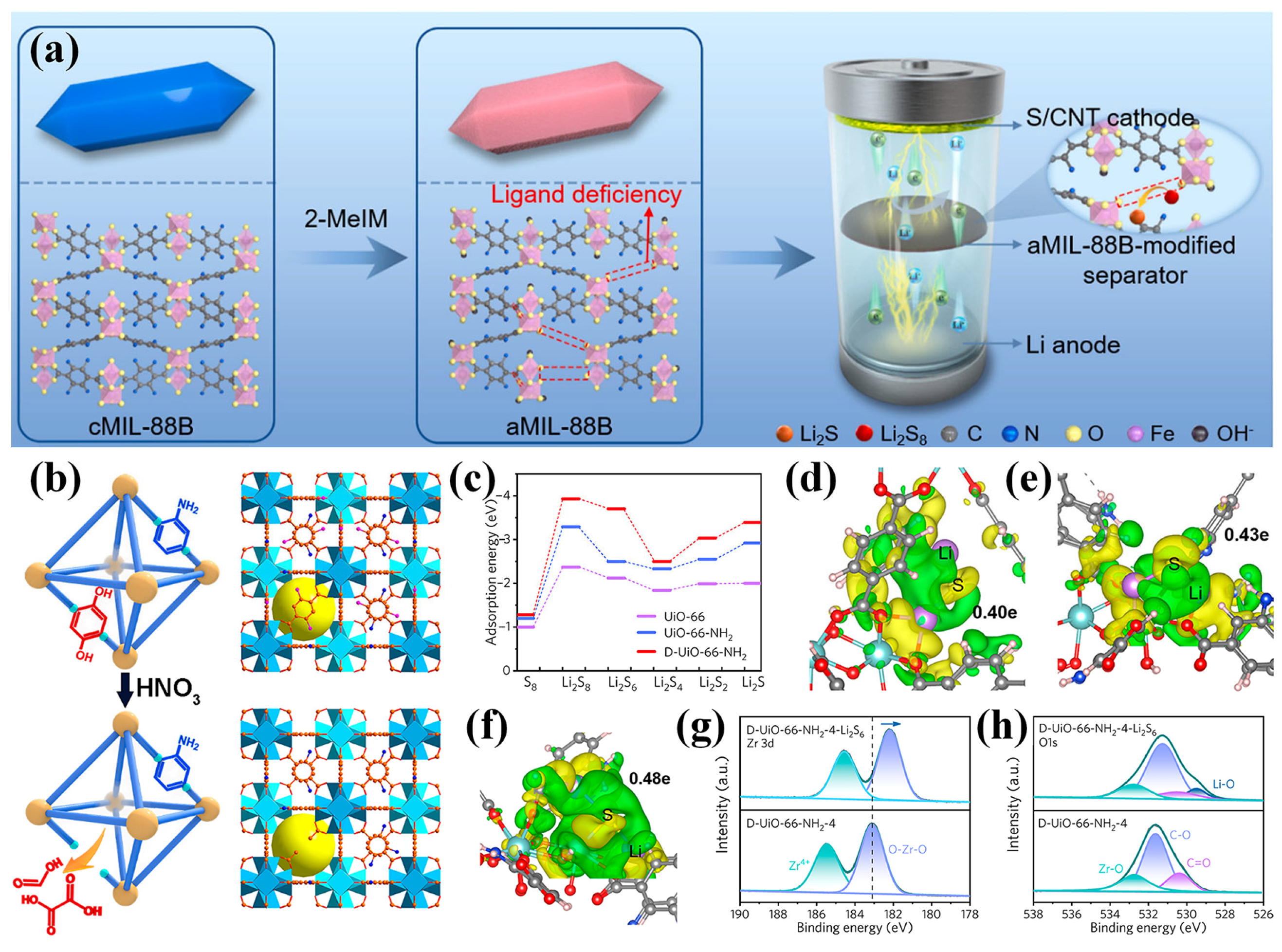
Structural defect engineering has emerged as an effective strategy to enhance the catalytic performance of MOFs for LiPS conversion in LSBs. Zhang et al. employed a ligand competition approach to synthesize an amorphous MOF (aMIL-88B), in which structural defects enhanced electrical conductivity, increased the exposure of active sites, and reinforced interactions with LiPSs (Figure 8a) [81]. As a result, cells with aMIL-88B-modified separators maintained a reversible capacity of 740 mAh g−1 over 500 cycles at 1 C. Li et al. introduced a defect-rich UiO-66-NH2 (D-UiO-66-NH2) via controlled nitrolysis, generating backbone vacancies that attenuated Li–S binding and improved charge transfer (Figure 8b) [82]. DFT calculations revealed that D-UiO-66-NH2 exhibited higher adsorption energies toward LiPSs compared to both UiO-66 and UiO-66-NH2 (Figure 8c), indicating a stronger immobilization capability due to the presence of abundant defects. Further analysis based on Bader charge and differential charge density distribution showed that D-UiO-66-NH2 enabled the highest charge transfer from Li2S (0.48 e) relative to the other structures (0.40 e for UiO-66 and 0.43 e for UiO-66-NH2), suggesting that the defect-rich framework effectively weakened the Li–S bond and lowered the decomposition energy barrier of Li2S (Figure 8d–f). XPS analysis further confirmed the chemical interaction between D-UiO-66-NH2 and LiPSs. After Li2S6 adsorption, both the Zr 3d and O 1s spectra exhibited notable shifts in binding energy (Figure 8g,h), indicating electron transfer and the formation of Li–O coordination, which supports the strong chemical affinity of the defect-rich MOF toward LiPSs. These findings collectively highlight the effectiveness of defect engineering in D-UiO-66-NH2 for enhancing LiPS confinement and redox catalysis in LSB separators. Expanding on this concept, Wang et al. developed a dual-defect UiO-66 structure incorporating both linker and cluster defects [83]. The linker defects exposed Zr active sites, facilitating the initial conversion of sulfur to intermediate LiPSs, while the cluster defects enhanced the interaction with short-chain species, promoting their further reduction to Li2S. The spatial proximity of these distinct defects enabled a synergistic catalytic effect that supported complete sulfur redox under high sulfur loading. As a result, cells employing this separator maintained an areal capacity of 10.4 mAh cm−2 after 45 cycles at a sulfur loading of 12.9 mg cm−2, demonstrating the effectiveness of defect-rich MOFs in improving LiPS conversion.
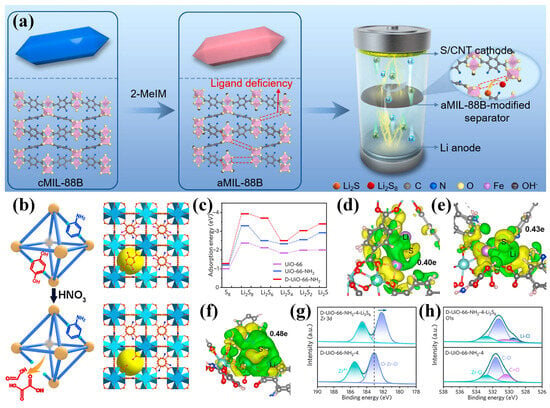
Figure 8.
(a) Schematic illustrating the introduction of aMIL-88B for modified separator fabrication. (Reproduced with permission from Ref. [81], copyright 2021, Elsevier.) (b) Schematic of D-UiO-66-NH2. (c) Calculated adsorption energies of S8 and Li2Sn (n = 1, 2, 4, 6, and 8) on UiO-66, UiO-66-NH2, and D-UiO-66-NH2. Differential charge density distribution for the Li2S molecule adsorbed on (d) UiO-66, (e) UiO-66-NH2, and (f) D-UiO-66-NH2. High-resolution XPS spectra of (g) Zr 3d and (h) O 1s of D-UiO-66-NH2 before/after Li2S6 adsorption. (Reproduced with permission from Ref. [82], copyright 2021, American Chemical Society.)
2.1.3. Enhancing Electrical Conductivity of MOFs
While MOFs have demonstrated considerable potential in suppressing LiPS shuttling and accelerating redox reactions through structural and chemical tailoring, their intrinsically low electrical conductivity remains a critical limitation [84]. This poor conductivity often leads to increased interfacial resistance between the separator and the cathode, ultimately hindering charge transfer efficiency. In response, recent advances have focused on developing electrically conductive MOFs as functional components for separator modification [85]. Incorporating such MOFs onto commercial separators not only provides physical barriers to block LiPS diffusion, but also significantly lowers interfacial resistance, thereby facilitating faster electrochemical conversion kinetics during battery operation [86].
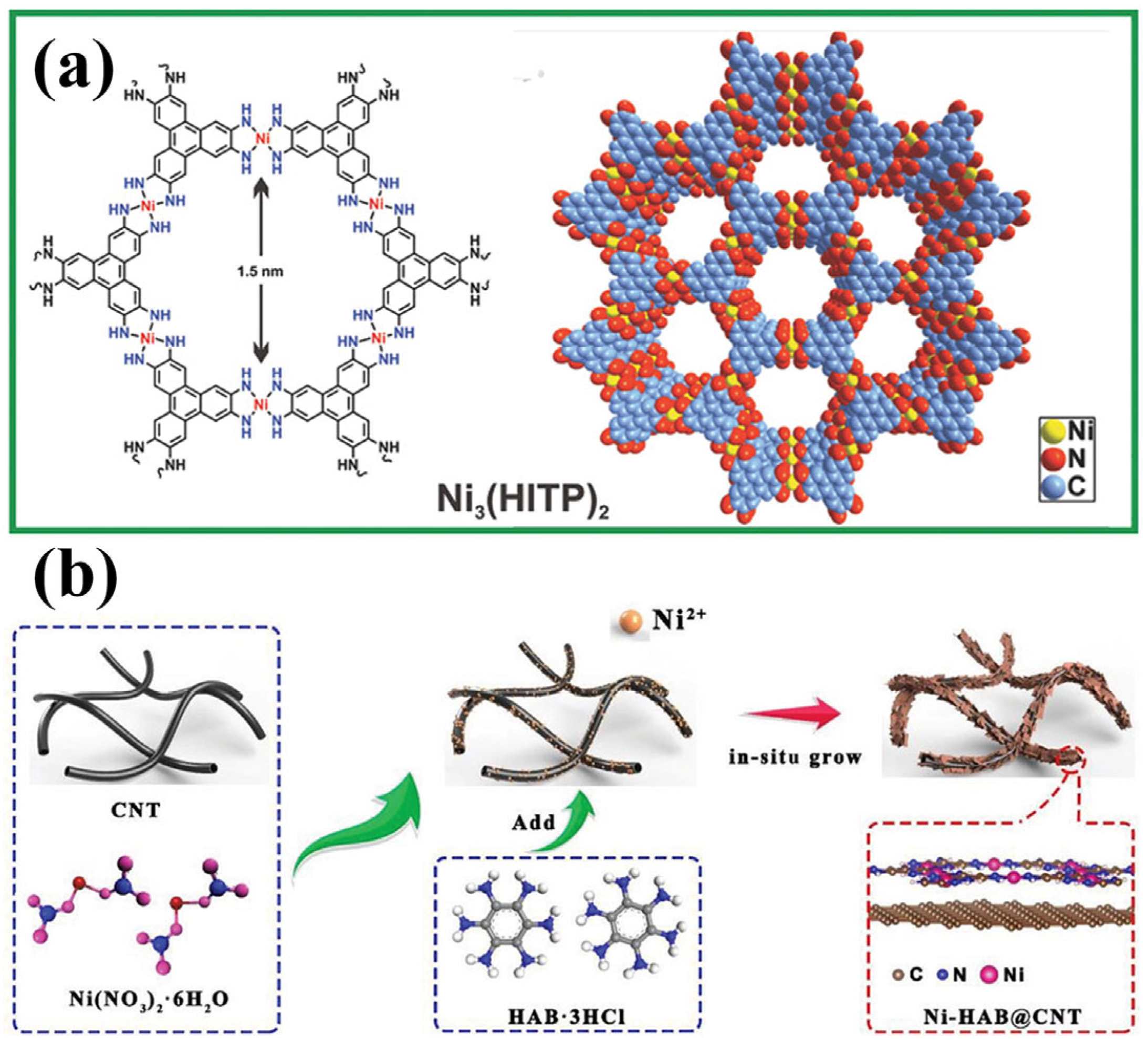
A representative example of a conductive MOF applied to separator modification is Ni3(HITP)2, which was utilized by Zang et al. due to its high electrical conductivity, ordered microporous structure, and large specific surface area (Figure 9a) [87]. This 2D layered framework, constructed from π-conjugated ligands, exhibited an exceptional conductivity of up to 4000 S m−1, surpassing that of activated carbon and porous graphite. Using interface-induced growth, the researchers directly coated Ni3(HITP)2 onto commercial separators, forming an ultra-thin and uniform conductive layer with a minimal mass loading of 0.066 mg cm−2. When introduced into LSBs, the Ni3(HITP)2/PP separator delivered a high initial discharge capacity of 1244 mAh g−1 and retained 1139 mAh g−1 after 100 cycles at 0.2 C, corresponding to 92% capacity retention and an average decay rate of only 0.08% per cycle. In another study, Guo et al. synthesized a highly crystalline 2D conductive MOF (Figure 9b), Ni-HAB, by coordinating Ni2+ with hexaaminobenzene (HAB), the smallest π-conjugated ligand, via in situ dsp2 hybridization on the surface of carbon nanotubes (CNTs) [88]. This π–d conjugated framework of Ni-HAB@CNT featured an excellent electrical conductivity, minimal steric hindrance, and a high density of delocalized electrons, all of which contributed to significantly accelerated polysulfide redox kinetics. Electrochemical characterization (including Tafel profiles and in situ Raman spectra) validated the rapid reaction kinetics enabled by the Ni-HAB@CNT-modified separator. Even under demanding conditions, with a low electrolyte-to-sulfur (E/S) ratio of 5 μL mg−1 and a high sulfur loading of 6.5 mg cm−2, the LSBs achieved an areal capacity of 6.29 mAh cm−2, underscoring the effectiveness of Ni-HAB in conductive separator designs.
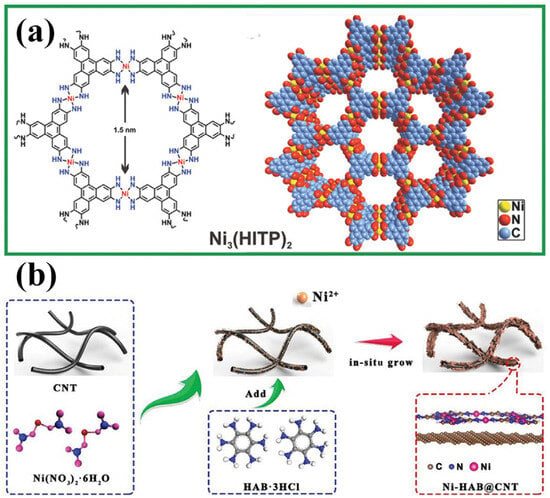
Figure 9.
(a) Schematic illustration of 2D layered structure of Ni3(HITP)2. (Reproduced with permission from Ref. [87], copyright 2018, Wiley-VCH.) (b) Schematic diagrams of synthetic route of Ni-HAB@CNT. (Reproduced with permission from Ref. [88], copyright 2023, Wiley-VCH.)
2.2. MOF-Derivative-Based Separators
MOFs possess ordered structures, tunable porosity, and well-defined compositions, making them excellent templates for deriving various functional materials such as porous carbons, metal oxides, metal sulfides, and heterostructures [89,90]. When thermochemically or chemically converted, these MOF derivatives inherit the high surface area and morphology of their parent structures, while acquiring an enhanced physicochemical stability, conductivity, and catalytic activity [91]. In this context, the term “MOF derivatives” broadly refers to materials derived from MOFs via post-synthetic treatments that produce inorganic or carbon–inorganic hybrid structures, a usage that is common in the literature. Such hybrid attributes offer significant advantages when these materials are utilized to engineer LSB separators. The advantages of MOF derivatives for separator modification in LSBs include the following:
- Structural versatility and inherited porosity: MOF derivatives often preserve the inherent porous architectures of their parent frameworks, resulting in abundant ion-diffusion channels and extensive active interfaces for LiPS interaction. This structural advantage enables effective LiPS confinement and Li+ transport simultaneously.
- Improved chemical and electrochemical stability: Compared to pristine MOFs, which may suffer from instability in electrolyte environments, MOF derivatives (especially carbonized or oxidized forms) exhibit a superior chemical robustness, ensuring prolonged durability during long-term cycling.
- High electronic conductivity from carbon-interconnected frameworks: MOF derivatives synthesized via thermal treatment frequently form conductive carbon skeletons. These frameworks not only suppress shuttle effects, but also act as secondary sulfur hosts within the separator, enabling sulfur species to participate reversibly in redox reactions. This contributes to higher sulfur utilization and an improved long-term cycling stability.
- Strong polarity and tunable surface chemistry: Most MOF derivatives exhibit a high polarity and surface activity. These features enhance the chemical anchoring of LiPSs and facilitate redox kinetics. Depending on their specific compositions, certain derivatives may provide stronger LiPS adsorption while others function more effectively as catalytic centers.
- Compatibility with scalable processing methods: MOF derivatives, available in powder, nanosheet, or hybrid composite forms, can be readily integrated into separators using industrially scalable techniques such as slurry casting, vacuum filtration, and spray coating. This enables practical implementation in commercial LSB configurations.
Taken together, these properties position MOF derivatives as promising multifunctional components for separator design in LSBs. Through rational composition control and hierarchical structuring, these derivatives not only physically suppress polysulfide migration, but also contribute to sustained redox catalysis and stable electrochemical performance across a wide range of operational conditions.
2.2.1. Metal Sulfides
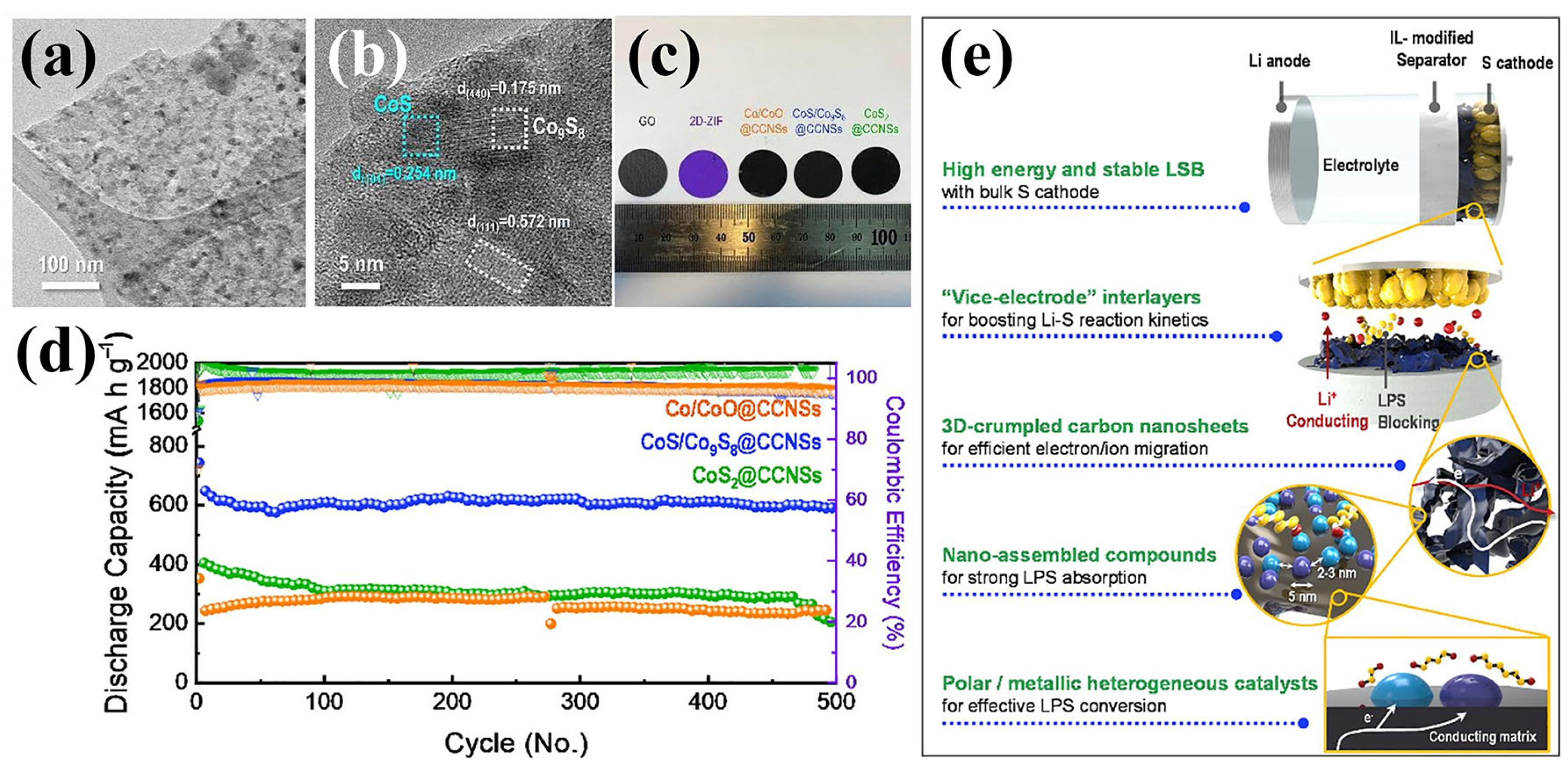
A representative study by Seo et al. demonstrated a 3D crumpled carbon nanosheet (CCNS)-based interlayer embedded with polar cobalt sulfide heterostructures (CoS/Co9S8), derived from 2D ZIF-67 templates [92]. These MOF derivatives were synthesized through a one-step carbonization/sulfidation strategy and subsequently deposited onto a commercial separator via vacuum filtration, forming a highly conductive and uniformly coated functional layer. As confirmed by the TEM images in Figure 10a,b, the CoS/Co9S8 nanoparticles were uniformly dispersed within the carbon matrix, preserving the nanosheet morphology and offering a high surface accessibility. The digital photograph (Figure 10c) shows the uniform macroscopic appearance and color contrast of the different modified separators, including CoS/Co9S8@CCNS, Co/CoO@CCNS, and CoS2@CCNS. Among them, the CoS/Co9S8@CCNS-modified separator exhibited the most favorable electrochemical behavior. As displayed in Figure 10d, the CoS/Co9S8@CCNS separator led to a significantly enhanced performance: the LSB delivered a discharge capacity of 911 mAh g−1 at 0.2 C after 100 cycles and maintained over 600 mAh g−1 for 500 cycles at 1 C, corresponding to a low-capacity fading rate of 0.018% per cycle. These improvements were attributed to the synergistic effects of (i) the polar CoS/Co9S8 domains acting as polysulfide adsorbents and catalytic centers and (ii) the 3D crumpled conductive carbon framework facilitating electron/ion transport. (Figure 10e) Of particular importance is that these findings highlight how carefully designed 3D CCNSs, decorated with diverse polar nanostructures, can significantly enhance LSB performance. A SO42−-assisted, surfactant-free synthesis yielded uniform 2D ZIF nanosheets that, upon precise annealing, transformed into tailored Co-based derivatives on N-doped carbon nanosheets. Among them, CoS/Co9S8@CCNSs exhibited optimal LiPS adsorption and conversion kinetics due to their heterogeneous nanostructure and abundant nanoscopic voids. This hierarchical architecture not only improved ionic and electronic pathways, but also enabled more effective LiPS immobilization and catalysis, demonstrating that the structural and compositional design of these heterostructured catalysts was critical to the interlayer’s overall functionality and cycling stability. As another representative example of ZIF-67-derived separators, Zhang et al. synthesized a hollow-structured MoS2/Co9S8/C composite via a sulfidation process [93]. The outer MoS2 shell offered abundant active sites that facilitated LiPS adsorption and ion transport, while the inner Co9S8 phase embedded in the amorphous carbon matrix enhanced electrical conductivity and strengthened chemical affinity toward LiPSs. These integrated structural and compositional features effectively suppressed the shuttle effect and promoted polysulfide conversion, leading to an improved electrochemical performance in LSBs. Building upon similar design principles, Qian et al. developed a ZIF-67-derived hollow Co9S8 coating as a separator modifier for LSBs [94]. The hollow architecture provided physical confinement for polysulfides, while the intrinsic polarity and high electrical conductivity of Co9S8 enabled strong chemical interaction and fast electron transfer, thus accelerating the redox kinetics of LiPSs and enhancing battery performance.
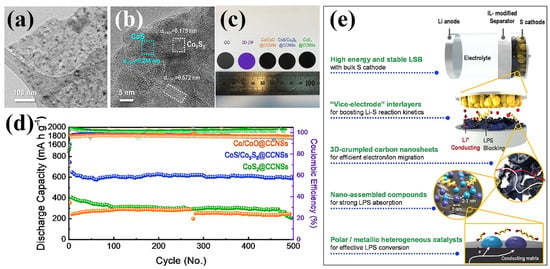
Figure 10.
(a,b) TEM images of CoS/Co9S8@CCNSs. (c) Digital image of various modified separators. (d) Long-term cycle performance of LSBs using prepared separators at 1 C. (e) Schematic representation of synergetic enhancement mechanism of CoS/Co9S8@CCNSs. (Reproduced with permission from Ref. [92], copyright 2020, Elsevier.)
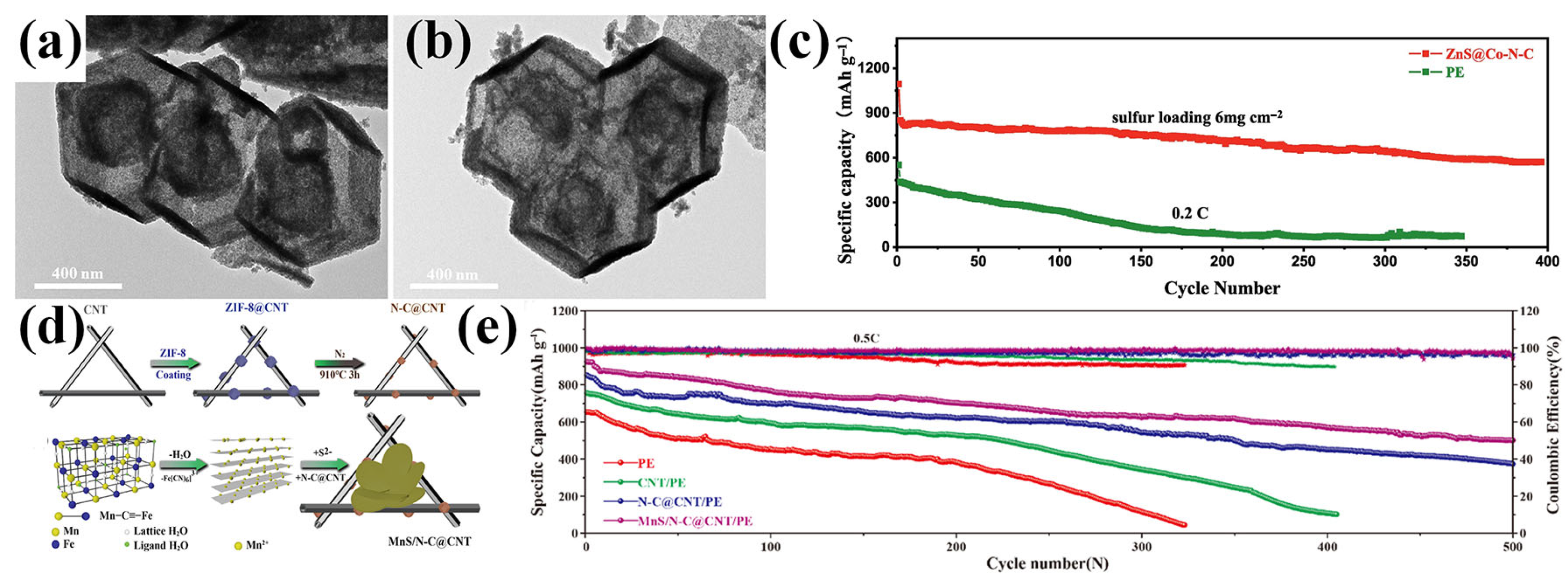
Zinc sulfide (ZnS) has been widely recognized for its catalytic activity in polysulfide conversion, while Co–N-doped carbon (Co–N–C) structures are known for their strong chemical affinity toward LiPSs [95]. Leveraging these complementary features, Jin et al. designed a core–shell-structured ZnS@Co–N–C composite through room-temperature solution synthesis using ZIF-8/ZIF-67 as bimetallic precursors, followed by vulcanization and calcination treatments (Figure 11a,b) [96]. In this hierarchical structure, the ZnS core provided catalytic activity to accelerate LiPS conversion reactions, while the surrounding Co–N–C nanocage shell offered abundant adsorption sites and conductive pathways for efficient charge transport. The hollow double-shelled architecture not only enhanced the physical confinement of LiPSs, but also improved electrolyte affinity through surface oxygen-containing groups on the carbon matrix. This design suppressed the shuttle effect and promoted uniform lithium-ion diffusion across the separator interface, effectively reducing polarization during cycling. As a result, the ZnS@Co–N–C-modified separator significantly improved both the discharge capacity and cycling stability of LSBs (Figure 11c).
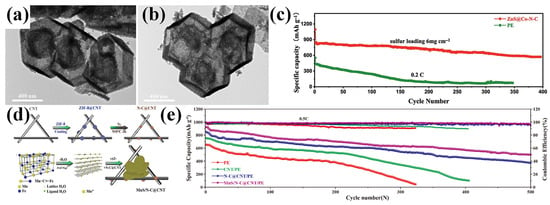
Figure 11.
(a,b) TEM images of ZnS@Co-N-C. (c) Cycle performance of LSBs with PE and ZnS@Co-N-C-modified separator at high sulfur loading mass of 6 mg cm−2. (Reproduced with permission from Ref. [96], copyright 2023, Elsevier.) (d) Schematic illustration of MnS/N-C@CNT preparation. (e) Cycling properties of LSBs with various separators at 0.5 C. (Reproduced with permission from Ref. [97], copyright 2021, American Chemical Society.)
Manganese sulfide (MnS), known for its strong chemical interaction with polysulfides, was employed by Li et al. to construct a multifunctional separator coating [97]. They synthesized MnS/N-C@CNT by embedding MnS nanoparticles into nitrogen-doped carbon-coated carbon nanotubes (CNTs) derived from ZIF-8 (Figure 11d). The CNT framework provided an excellent electronic and ionic conductivity, while its microporous structure, coupled with nitrogen doping, enhanced the adsorption of LiPSs. Meanwhile, the MnS nanocubes acted as catalytic centers to promote LiPS redox conversion. When applied as a separator interlayer, MnS/N-C@CNT delivered an initial capacity of 923.9 mAh g−1 at 0.5 C and retained 500.8 mAh g−1 over 500 cycles with a low-capacity decay rate of 0.08% per cycle, demonstrating an outstanding long-term electrochemical stability (Figure 11e).
2.2.2. Metal Phosphides
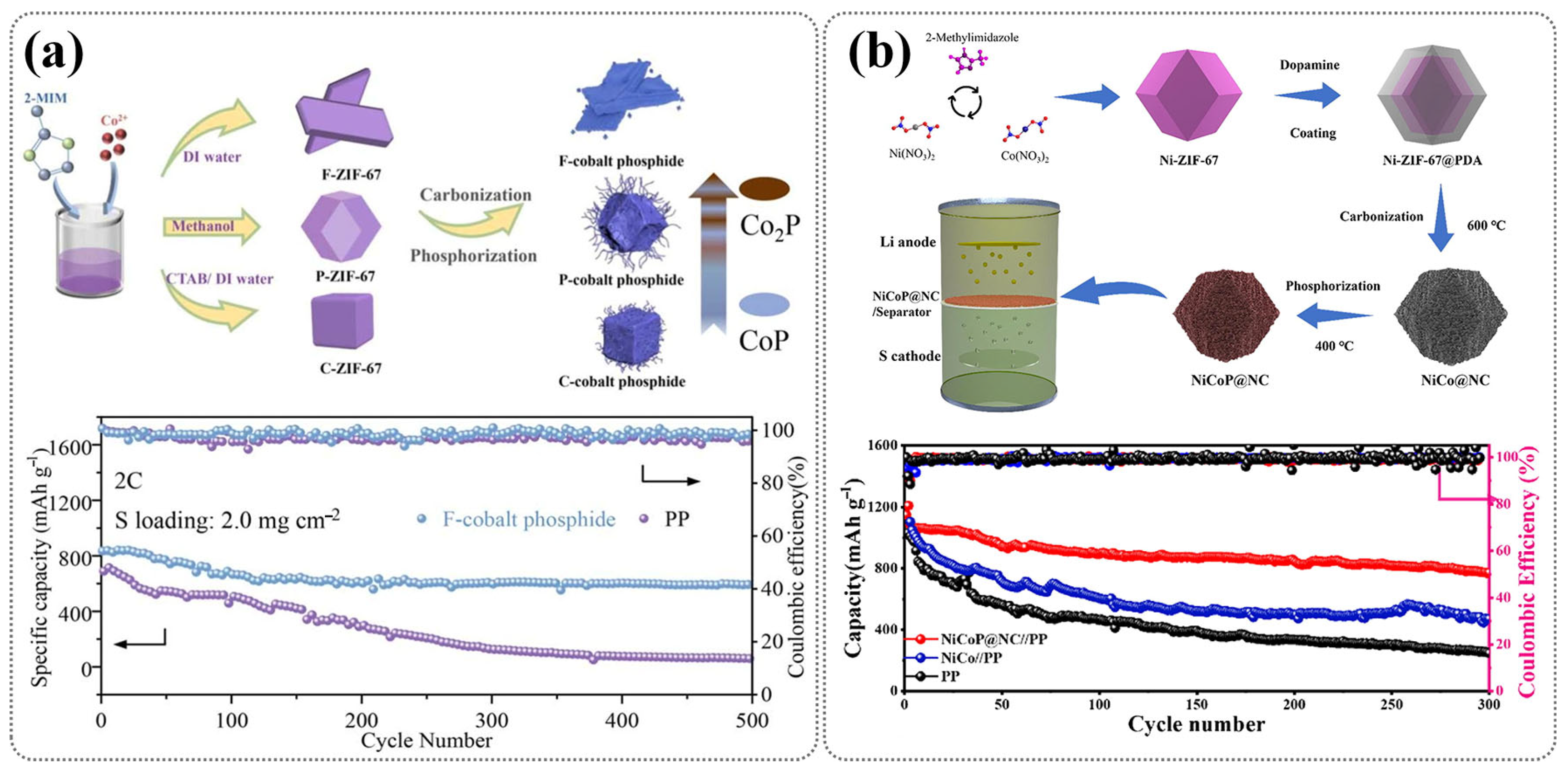
In addition to sulfide-based derivatives, ZIF-derived materials have also been widely employed to fabricate metal phosphides for LSB separator modification. Wang et al. synthesized cobalt phosphides with flake, polyhedral, and cubic morphologies from ZIF-67 precursors, followed by phosphorization [98]. As shown in Figure 12a, the flake-type CoP/Co2P heterostructure exhibited superior catalytic activity and polysulfide confinement, resulting in a markedly improved cycling performance compared to unmodified PP separators. This highlights the crucial role of morphology control in optimizing MOF-derived phosphide separators. Zhu et al. synthesized a nitrogen-doped dual-carbon network embedding Ni/Co bimetallic phosphides (NiCoP@NC) via the carbon–phosphide treatment of a Ni-ZIF-67@PDA precursor [99]. The resulting separator coating not only formed a physical barrier to mitigate polysulfide shuttling, but also provided abundant active sites to catalyze LiPS conversion, enabling an excellent cycling stability and rate performance (Figure 12b). These studies collectively highlight the versatility of ZIF-derived phosphides as multifunctional separator modifiers, offering both a strong chemical affinity toward LiPSs and efficient catalytic pathways that enhance the electrochemical stability of LSBs.
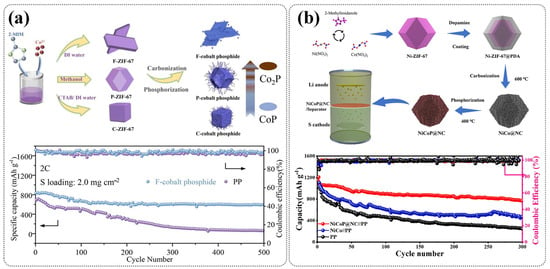
Figure 12.
(a) The synthesis process of ZIF-derived cobalt phosphides and corresponding cycling performance of LSBs at 2 C. (Reproduced with permission from Ref. [98], copyright 2023, Elsevier.) (b) Schematic illustration for preparation process of the NiCoP@NC-modified separator and long-term cyclic performance at 0.5 C using various separators. (Reproduced with permission from Ref. [99], copyright 2023, Elsevier.)
2.2.3. Metal Selenides
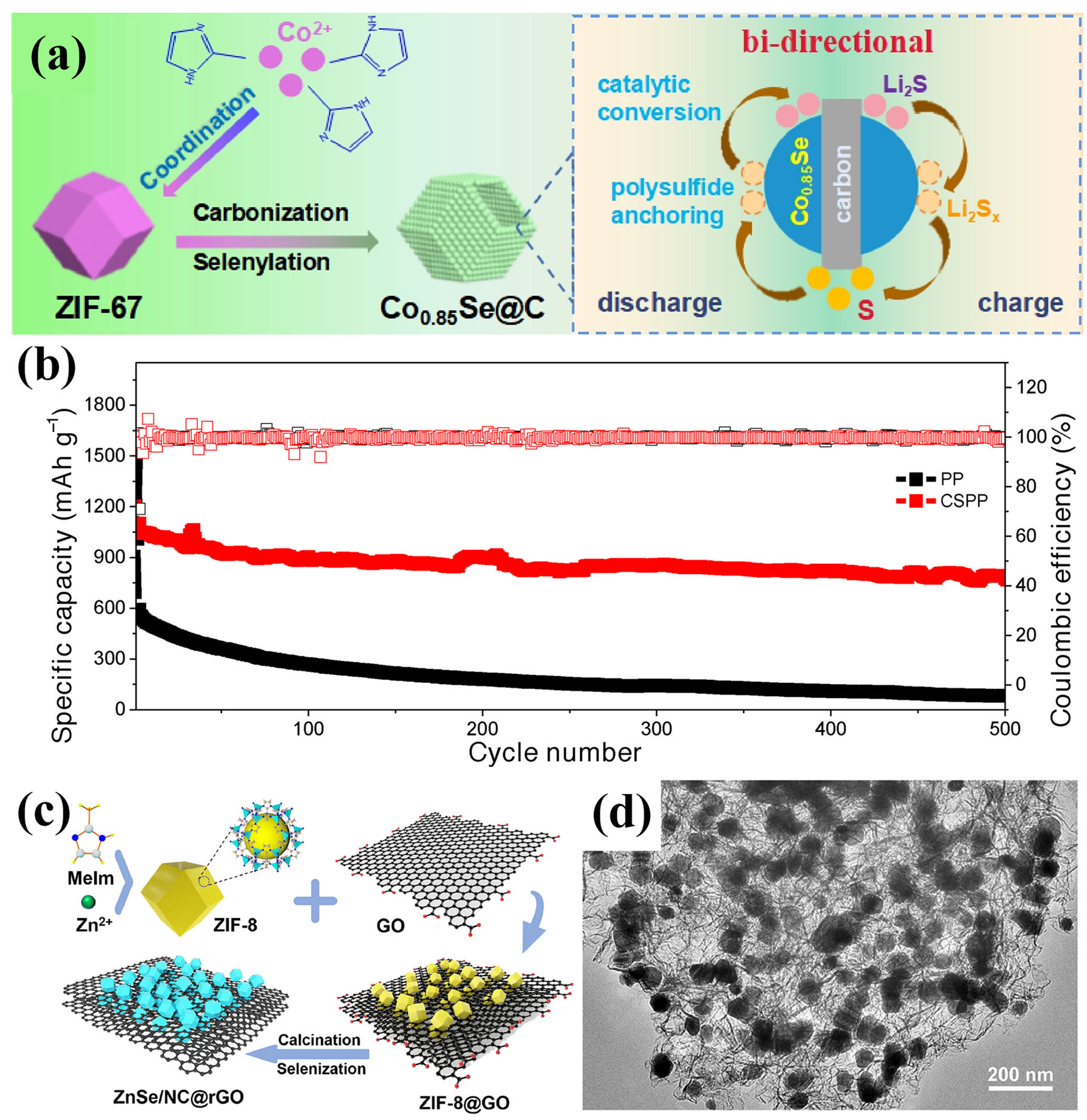
Zhang et al. developed a hollow-structured Co0.85Se/C composite, designed to function as an interlayer modifier on the separator, synthesized via the high-temperature selenization and carbonization of ZIF-67 (Figure 13a) [100]. During synthesis, cobalt ions within the MOF reacted with Se powder to form catalytically active Co0.85Se nanoparticles, while the organic ligands were transformed into a mesoporous carbon shell. This hollow Co0.85Se/C architecture offered a strong chemical adsorption of LiPSs and dual-direction electrocatalytic activity. Moreover, its porous structure provided abundant active sites and facilitated fast lithium-ion transport. When applied for the fabrication of a modified separator (CSPP), the LSB exhibited an outstanding long-term cycling performance at 1 C, retaining a high capacity of 791 mAh g−1 after 500 cycles with a minimal fading rate of 0.056% per cycle and a CE exceeding 99% (Figure 13b).
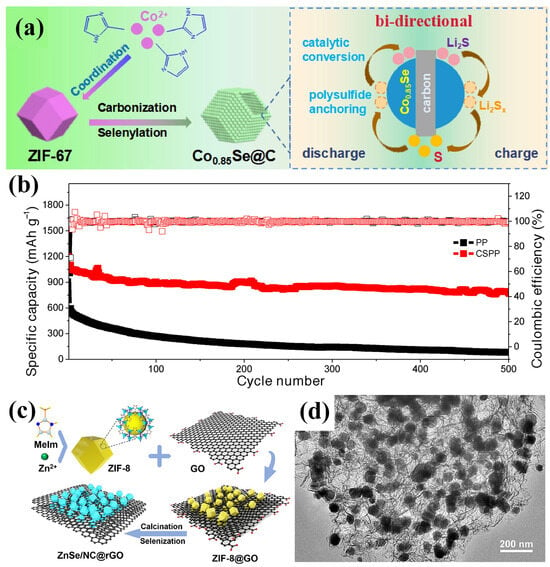
Figure 13.
(a) Schematic illustration of the synthesis of Co0.85Se/C and its role in accelerating catalytic LiPS redox reactions. (b) Cycling performance with commercial PP and CSPP separators at 1 C. (Reproduced with permission from Ref. [100], copyright 2022, Wiley-VCH.) (c) Schematic of the synthesis procedure for ZnSe/NC@rGO. (d) TEM image of ZnSe/NC@rGO. (Reproduced with permission from Ref. [101], copyright 2023, Elsevier.)
Sun et al. developed a ZnSe/NC@rGO composite by annealing ZIF-8@GO precursors. The resulting structure featured ZnSe nanoparticles encapsulated in N-doped carbon shells, uniformly embedded within a reduced graphene oxide (GO) matrix (Figure 13c,d) [101]. When applied as a separator coating in LSBs, this composite exhibited an enhanced chemisorption of LiPSs due to the polar ZnSe component, while the N-doped carbon and rGO network improved electrical conductivity and provided additional active sites. The ZnSe/NC@rGO-modified separator enabled stable Li deposition and efficient LiPS conversion, delivering 1057 mAh g−1 at 0.2 C after 100 cycles and maintaining 685 mAh g−1 at 3 C. Over 900 cycles at 1 C, it demonstrated an excellent cycling stability with only 0.043% capacity loss per cycle. Similarly, Wang et al. synthesized ZnSe/NC nanosheets by embedding ZnSe into nitrogen-doped carbon sheets derived from 2D ZIF-8 and employed them as a functional separator modifier in LSBs [102]. These nanosheets exhibited a high electrical conductivity and abundant polar sites, effectively suppressing LiPS migration and accelerating redox kinetics. As a result, the modified batteries achieved over 1500 cycles at 1 C with only 0.046% capacity decay per cycle and maintained a high areal capacity of 4.28 mAh cm−2 at a sulfur loading of 4.71 mg cm−2 after 50 cycles.
3. Conclusions and Future Perspectives
In this review, we systematically summarized recent advances in the application of MOFs and their derivatives as functional materials for separator modification in LSBs. MOFs, with their tunable structure, high porosity, and compositional diversity, have demonstrated significant potential as physical barriers, chemical adsorbents, and redox catalysts for mitigating the shuttle effect and improving Li+ transport across separators. Pristine MOFs were categorized based on the following three design strategies: pore microenvironment tuning, metal site engineering, and conductivity enhancement. In parallel, representative MOF derivatives (such as metal sulfides, phosphides, selenides, and composite carbons) were introduced as multifunctional platforms that exhibit an enhanced chemical stability and catalytic activity. The schematic summary is illustrated in Figure 14 to visually highlight the overall classification and key advantages of pristine MOFs and their derivatives. Table 1, summarizing the electrochemical performance of MOF-based separators versus other advanced separator materials, is also provided to illustrate their relative effectiveness in LSBs.
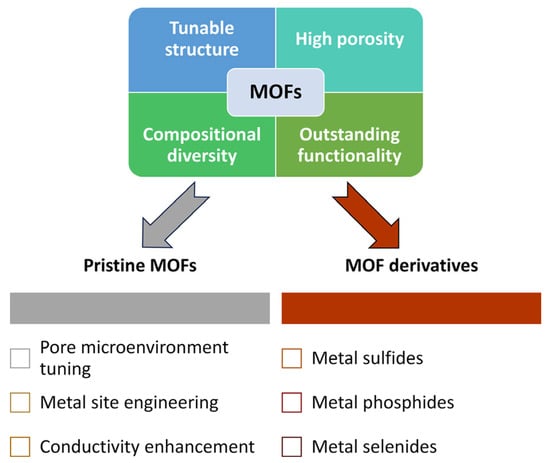
Figure 14.
Schematic summary of the key advantages of MOFs and their classification into pristine MOFs and MOF derivatives for LSB separators.

Table 1.
Electrochemical performance comparison of MOFs, MOF derivatives, and other advanced materials for comparison of LSB electrochemical performance employing separators modified with MOFs, MOF derivatives, and other recently reported advanced materials.
Despite promising performance improvements, several scientific and practical challenges remain before MOF-based separators can be widely adopted in commercial LSB systems. First, the intrinsic electronic insulation and mechanical fragility of many MOFs restrict their long-term operability under high-loading and lean-electrolyte conditions. Second, while MOF derivatives generally offer an improved conductivity and structural robustness, their synthesis often requires high-temperature pyrolysis or multistep processing, raising concerns about cost-effectiveness, scalability, and environmental sustainability. Furthermore, achieving a uniform dispersion of MOF or MOF-derived particles on commercial separator substrates without significantly increasing thickness or reducing porosity remains a technical barrier.
Going forward, engineering the interfaces between MOF-derived separators and other battery components will be especially critical for ensuring stable LSB operation, particularly at practical current densities. Targeted research into these interfacial interactions will be essential for improving ion transport, suppressing polysulfide migration, and enhancing overall cycling stability. Although recent reports have demonstrated a promising cell-level performance in pouch-type configurations, the translation of these advances to commercial-scale batteries is still in its early stages. Future efforts must, therefore, focus on rational design principles, cost-effective fabrication routes, and deeper mechanistic insights so that MOF-based separators can ultimately meet the stringent requirements for real-world LSB applications.
To address these limitations, future research efforts should focus on several key directions. First, the development of novel MOF compositions or post-synthetic functionalization techniques (such as ligand engineering, defect generation, and hybridization with conductive polymers or 2D materials) could further enhance conductivity, polarity, and structural integrity.
Second, integrating MOFs into hierarchical composite structures or 3D networks may provide synergistic effects on LiPS adsorption, redox catalysis, and Li+ diffusion. In particular, dual-function separators that combine polar sites for chemical trapping and conductive frameworks for electron/ion transport are expected to play a central role in next-generation high-performance LSBs.
Third, efforts should be made to design scalable, solution-processable coating strategies that ensure interfacial compatibility, mechanical stability, and long-term cycling durability. Vacuum filtration, blade coating, and in situ growth methods have shown promise, but their industrial viability must be validated through pilot-scale demonstrations. Additionally, advanced characterization tools, such as in situ/operando spectroscopy, X-ray imaging, and synchrotron-based techniques, are needed to unravel the dynamic interactions between LiPSs and MOF-based interlayers during battery operation.
Lastly, bridging fundamental material design with system-level integration will be essential. Future studies should consider how MOF-based separator modifications interact with other battery components, including high-loading sulfur cathodes, modified electrolytes, and lithium metal anodes. The compatibility of these materials in pouch cells and under practical conditions (such as elevated temperature, mechanical stress, and prolonged cycling) should be explored.
In summary, MOFs and their derivatives offer a unique platform for rational separator engineering in LSBs. With continued innovation in materials chemistry, processing strategies, and device integration, MOF-based separator technologies are expected to play a transformative role in overcoming long-standing bottlenecks and accelerating the commercialization of high-energy-density, long-life LSB systems.
Author Contributions
C.C. developed the concept; C.C. and M.K. developed the framework of the paper. C.C., T.-S.K., and M.K. conducted the data analysis and collected the various research papers; C.C. and T.-S.K. conducted writing—review and editing of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Sungshin Women’s University Research Grant of H20240022. All authors have given approval to the final version of the manuscript. This work was supported by Kyungpook National University Research Fund, 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Raza, H.; Bai, S.; Cheng, J.; Majumder, S.; Zhu, H.; Liu, Q.; Zheng, G.; Li, X.; Chen, G. Li-S Batteries: Challenges, Achievements and Opportunities. Electrochem. Energy Rev. 2023, 6, 29. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, L.; Cheng, H.M. Carbon-Based Fibers for Advanced Electrochemical Energy Storage Devices. Chem. Rev. 2020, 120, 2811–2878. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Yuan, H.; Yu, Y.; Tan, Y. Recent Progress and Challenge in Metal–Organic Frameworks for Lithium–Sulfur Battery Separators. Adv. Funct. Mater. 2024, 34, 2405890. [Google Scholar] [CrossRef]
- Lu, J.; Luo, S.; Qi, Z.; Chen, T.; Li, X.; Yuan, T.; Pang, Y.; Zheng, S. Recent advances in transition metal chalcogenide derivatives from metal-organic frameworks for lithium-sulfur batteries. Cell Rep. Phys. Sci. 2024, 5, 102028. [Google Scholar] [CrossRef]
- Min, B.C.; Park, J.B.; Choi, C.; Kim, D.-W. Dynamic construction of a composite solid electrolyte interphase for dendrite-free lithium metal batteries via lithium-antimony self-alloying. Adv. Compos. Hybrid Mater. 2024, 8, 4. [Google Scholar] [CrossRef]
- Gao, G.; Yang, X.; Bi, J.; Guan, W.; Du, Z.; Ai, W. Advanced engineering strategies for Li2S cathodes in lithium–sulfur batteries. J. Mater. Chem. A 2023, 11, 26318–26339. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Liu, H.; Li, D.; Zeng, Z.; Li, Y.; Ji, F.; Guo, Y.; Wei, Y.; Zhang, S.; et al. Prelithiation: A Critical Strategy Towards Practical Application of High-Energy-Density Batteries. Adv. Energy Mater. 2023, 13, 2300466. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium-Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, e2003666. [Google Scholar] [CrossRef]
- Qi, F.; Sun, Z.; Fan, X.; Wang, Z.; Shi, Y.; Hu, G.; Li, F. Tunable Interaction between Metal-Organic Frameworks and Electroactive Components in Lithium–Sulfur Batteries: Status and Perspectives. Adv. Energy Mater. 2021, 11, 2100387. [Google Scholar] [CrossRef]
- Seo, S.-D.; Choi, C.; Kim, D.-W. Fabrication of sulfur-impregnated porous carbon nanostructured electrodes via dual-mode activation for lithium–sulfur batteries. Mater. Lett. 2016, 172, 116–119. [Google Scholar] [CrossRef]
- Lee, D.; Choi, C.; Park, J.B.; Jung, S.W.; Kim, D.-W. Ingenious separator architecture: Revealing the versatile 3D heterostructured MXene-hydrogen titanate electrocatalysts for advanced lithium-sulfur battery. Energy Storage Mater. 2024, 70, 103529. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O2 and Li-S batteries with high energy storage. Nat. Mater. 2011, 11, 19–29. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, J.; Liang, X.; Li, Y.; Cui, J.; Chen, M. Tunable MOFs derivatives for stable and fast sulfur electrodes in Li-S batteries. Chem. Eng. J. 2022, 450, 138287. [Google Scholar] [CrossRef]
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.J.; Capiglia, C.; Deng, W.; Zhou, X.; Liu, Z.; Feng, Z.; Proietti Zaccaria, R. A Comprehensive Understanding of Lithium–Sulfur Battery Technology. Adv. Funct. Mater. 2019, 29, 1901730. [Google Scholar] [CrossRef]
- Pan, Z.; Brett, D.J.L.; He, G.; Parkin, I.P. Progress and Perspectives of Organosulfur for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103483. [Google Scholar] [CrossRef]
- Song, Z.; Jiang, W.; Li, B.; Qu, Y.; Mao, R.; Jian, X.; Hu, F. Advanced Polymers in Cathodes and Electrolytes for Lithium-Sulfur Batteries: Progress and Prospects. Small 2024, 20, e2308550. [Google Scholar] [CrossRef]
- Kolosnitsyn, V.S.; Kuzmina, E.V.; Karaseva, E.V. On the reasons for low sulphur utilization in the lithium–sulphur batteries. J. Power Sources 2015, 274, 203–210. [Google Scholar] [CrossRef]
- Jin, Z.; Lin, T.; Jia, H.; Liu, B.; Zhang, Q.; Li, L.; Zhang, L.; Su, Z.M.; Wang, C. Expediting the Conversion of Li(2)S(2) to Li(2)S Enables High-Performance Li-S Batteries. ACS Nano 2021, 15, 7318–7327. [Google Scholar] [CrossRef]
- Hu, A.; Zhou, M.; Lei, T.; Hu, Y.; Du, X.; Gong, C.; Shu, C.; Long, J.; Zhu, J.; Chen, W.; et al. Optimizing Redox Reactions in Aprotic Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2002180. [Google Scholar] [CrossRef]
- Moon, S.; Jung, Y.H.; Jung, W.K.; Jung, D.S.; Choi, J.W.; Kim, D.K. Encapsulated monoclinic sulfur for stable cycling of li-s rechargeable batteries. Adv. Mater. 2013, 25, 6547–6553. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, S.; Park, J.B.; Park, D.; Lee, S.; Choi, C.; Lee, H.; Jang, G.; Park, Y.S.; Yun, J.; et al. Electrochemically Active MoO3/TiN Sulfur Host Inducing Dynamically Reinforced Built-in Electric Field for Advanced Lithium-Sulfur Batteries. Small 2024, 20, e2406018. [Google Scholar] [CrossRef] [PubMed]
- Miao, K.; Chen, F.; Ma, C.; Zhou, J. Progresses and outlooks of all-solid-state lithium-sulfur batteries for practical development. Chem. Eng. J. 2025, 512, 162173. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, L.; Zhang, C.; Liu, L.; Li, Y.; Qiao, Z.; Lin, J.; Wei, Q.; Wang, L.; Xie, Q.; et al. Recent Advances and Strategies toward Polysulfides Shuttle Inhibition for High-Performance Li-S Batteries. Adv. Sci. 2022, 9, e2106004. [Google Scholar] [CrossRef]
- Seo, S.-D.; Choi, C.; Kim, B.-K.; Kim, D.-W. Fabrication of highly porous carbon as sulfur hosts using waste green tea bag powder for lithium–sulfur batteries. Ceram. Int. 2017, 43, 2836–2841. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, W.; Zhou, H.; Mao, D. A Review of Lithium–Sulfur Batteries Based on Metal–Organic Frameworks: Progress and Prospects. Batteries 2025, 11, 89. [Google Scholar] [CrossRef]
- Kim, J.S.; Hwang, T.H.; Kim, B.G.; Min, J.; Choi, J.W. A Lithium-Sulfur Battery with a High Areal Energy Density. Adv. Funct. Mater. 2014, 24, 5359–5367. [Google Scholar] [CrossRef]
- Choi, C.; Lee, D.-Y.; Park, J.B.; Kim, D.-W. Separators Modified Using MoO2@Carbon Nanotube Nanocomposites as Dual-Mode Li-Polysulfide Anchoring Materials for High-Performance Anti-Self-Discharge Lithium–Sulfur Batteries. ACS Sustain. Chem. Eng. 2020, 8, 15134–15148. [Google Scholar] [CrossRef]
- Jang, K.; Song, H.J.; Park, J.B.; Jung, S.W.; Kim, D.-W. Magnesium fluoride-engineered UiO-66 artificial protection layers for dendrite-free lithium metal batteries. Energy Environ. Sci. 2024, 17, 4622–4633. [Google Scholar] [CrossRef]
- Shi, P.; Cheng, X.B.; Li, T.; Zhang, R.; Liu, H.; Yan, C.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. Electrochemical Diagram of an Ultrathin Lithium Metal Anode in Pouch Cells. Adv. Mater. 2019, 31, e1902785. [Google Scholar] [CrossRef]
- Park, J.B.; Choi, C.; Yu, S.; Chung, K.Y.; Kim, D.W. Porous Lithiophilic Li–Si Alloy-Type Interfacial Framework via Self-Discharge Mechanism for Stable Lithium Metal Anode with Superior Rate. Adv. Energy Mater. 2021, 11, 2101544. [Google Scholar] [CrossRef]
- Xia, S.; Xu, X.; Wu, W.; Chen, Y.; Liu, L.; Wang, G.; Fu, L.; Zhang, Q.; Wang, T.; He, J.; et al. Advancements in functionalized high-performance separators for lithium-sulfur batteries. Mater. Sci. Eng. R Rep. 2025, 163, 100924. [Google Scholar] [CrossRef]
- Liu, M.; Hu, L.J.; Guan, Z.K.; Chen, T.L.; Zhang, X.Y.; Sun, S.; Shi, R.; Jing, P.; Wang, P.F. Tailoring Cathode-Electrolyte Interface for High-Power and Stable Lithium-Sulfur Batteries. Nano Micro Lett. 2024, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Gu, H.; Lee, C.; Sung, J.; Kim, D.H.; Han, J.; Oh, Y.S.; Ahn, S.; Jeon, I.; Park, J.W. Recent Advances in Achieving High Energy/Power Density of Lithium–Sulfur Batteries for Current and Near-Future Applications. Battery Energy 2025, e20240051. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Cheng, H.M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Cheng, Z.; Fransaer, J.; Luo, J.; Wübbenhorst, M. Cobalt-embedded 3D conductive honeycomb architecture to enable high-sulphur-loading Li-S batteries under lean electrolyte conditions. Nano Res. 2022, 15, 8091–8100. [Google Scholar] [CrossRef]
- Jin, M.; Sun, G.; Wang, Y.; Yuan, J.; Zhao, H.; Wang, G.; Zhou, J.; Xie, E.; Pan, X. Boosting Charge Transport and Catalytic Performance in MoS2 by Zn2+ Intercalation Engineering for Lithium-Sulfur Batteries. ACS Nano 2024, 18, 2017–2029. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Ji, J.; Cai, C.; Fu, Y. Temperature-dependent viscoelastic liquid MOFs based cellulose gel electrolyte for advanced lithium-sulfur batteries over an extensive temperature range. Energy Storage Mater. 2024, 64, 103065. [Google Scholar] [CrossRef]
- Kim, A.; Oh, S.H.; Adhikari, A.; Sathe, B.R.; Kumar, S.; Patel, R. Recent advances in modified commercial separators for lithium–sulfur batteries. J. Mater. Chem. A 2023, 11, 7833–7866. [Google Scholar] [CrossRef]
- Chen, M.; Shao, M.; Jin, J.; Cui, L.; Tu, H.; Fu, X. Configurational and structural design of separators toward shuttling-free and dendrite-free lithium-sulfur batteries: A review. Energy Storage Mater. 2022, 47, 629–648. [Google Scholar] [CrossRef]
- Su, Y.S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef]
- Jeong, Y.C.; Kim, J.H.; Nam, S.; Park, C.R.; Yang, S.J. Rational Design of Nanostructured Functional Interlayer/Separator for Advanced Li–S Batteries. Adv. Funct. Mater. 2018, 28, 1707411. [Google Scholar] [CrossRef]
- He, J.; Chen, Y.; Manthiram, A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li–S batteries. Energy Environ. Sci. 2018, 11, 2560–2568. [Google Scholar] [CrossRef]
- Choi, C.; Kim, D.-W. Silica-templated hierarchically porous carbon modified separators for lithium–sulfur batteries with superior cycling stabilities. J. Power Sources 2020, 448, 227462. [Google Scholar] [CrossRef]
- Chen, P.; Wang, T.; He, D.; Shi, T.; Chen, M.; Fang, K.; Lin, H.; Wang, J.; Wang, C.; Pang, H. Delocalized Isoelectronic Heterostructured FeCoOx Sy Catalysts with Tunable Electron Density for Accelerated Sulfur Redox Kinetics in Li-S batteries. Angew. Chem. Int. Ed. 2023, 62, e202311693. [Google Scholar] [CrossRef] [PubMed]
- Phung, J.; Zhang, X.; Deng, W.; Li, G. An overview of MOF-based separators for lithium-sulfur batteries. Sustain. Mater. Technol. 2022, 31, e00374. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Miao, L.; Xiang, J.; Wang, T.; Yuan, K.; Chen, J.; Huang, Y. A separator-based lithium polysulfide recirculator for high-loading and high-performance Li–S batteries. J. Mater. Chem. A 2018, 6, 5862–5869. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, Z.; Hua, W.; Liu, D.; Tao, T.; Rahman, M.M.; Lei, W.; Huang, S.; Chen, Y. Functionalized Boron Nitride Nanosheets/Graphene Interlayer for Fast and Long-Life Lithium–Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1602380. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- He, Y.; Qiao, Y.; Chang, Z.; Zhou, H. The potential of electrolyte filled MOF membranes as ionic sieves in rechargeable batteries. Energy Environ. Sci. 2019, 12, 2327–2344. [Google Scholar] [CrossRef]
- Zhou, C.; He, Q.; Li, Z.; Meng, J.; Hong, X.; Li, Y.; Zhao, Y.; Xu, X.; Mai, L. A robust electrospun separator modified with in situ grown metal-organic frameworks for lithium-sulfur batteries. Chem. Eng. J. 2020, 395, 124979. [Google Scholar] [CrossRef]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dinca, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhu, C.; Gao, G. Recent Progress of Advanced Conductive Metal-Organic Frameworks: Precise Synthesis, Electrochemical Energy Storage Applications, and Future Challenges. Small 2022, 18, e2203140. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, H. Hydrothermal Synthesis of a Metal-Organic Framework Containing Large Rectangular Channels. J. Am. Chem. Soc. 2002, 117, 10401–10402. [Google Scholar] [CrossRef]
- Cheng, Z.; Fang, Y.; Dai, W.; Zhang, J.; Xiang, S.; Zhang, Z. Structural engineering of metal–organic layers toward stable Li–CO2 batteries. J. Mater. Chem. A 2023, 11, 1180–1187. [Google Scholar] [CrossRef]
- Kang, X.; He, T.; Zou, R.; Niu, S.; Ma, Y.; Zhu, F.; Ran, F. Size Effect for Inhibiting Polysulfides Shuttle in Lithium-Sulfur Batteries. Small 2024, 20, e2306503. [Google Scholar] [CrossRef]
- Li, J.; Xie, F.; Pang, W.; Liang, Q.; Yang, X.; Zhang, L. Regulate transportation of ions and polysulfides in all-solid-state Li-S batteries using ordered-MOF composite solid electrolyte. Sci. Adv. 2024, 10, eadl3925. [Google Scholar] [CrossRef]
- Cheng, Z.; Lian, J.; Zhang, J.; Xiang, S.; Chen, B.; Zhang, Z. Pristine MOF Materials for Separator Application in Lithium-Sulfur Battery. Adv. Sci. 2024, 11, e2404834. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Peng, R.; Liang, Z.; Zhou, X.; Luo, X.; Chen, R.; Li, P.; Yu, D. Metal Organic Frameworks as Polysulfide Reaction Modulators for Lithium Sulfur Batteries: Advances and Perspectives. Chemphyschem 2024, 25, e202400239. [Google Scholar] [CrossRef]
- Yuan, N.; Sun, W.; Yang, J.; Gong, X.; Liu, R. Multifunctional MOF-Based Separator Materials for Advanced Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2021, 8, 2001941. [Google Scholar] [CrossRef]
- Zhao, T.; Xiao, P.; Nie, S.; Luo, M.; Zou, M.; Chen, Y. Recent progress of metal-organic frameworks based high performance batteries separators: A review. Coord. Chem. Rev. 2024, 502, 215592. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.O.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.C.; Wu, Y.; Shu, Y.; Gong, X.; Ke, F.S.; Deng, H. Metal-Organic Frameworks for High Charge-Discharge Rates in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2018, 57, 3916–3921. [Google Scholar] [CrossRef] [PubMed]
- Suriyakumar, S.; Stephan, A.M.; Angulakshmi, N.; Hassan, M.H.; Alkordi, M.H. Metal–organic framework@SiO2 as permselective separator for lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 14623–14632. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, W.; Hua, J.; Wang, Y.; Yi, H.; Zhao, W.; Zhao, Q.; Jia, H.; Fei, B.; Pan, F. An Anionic-MOF-Based Bifunctional Separator for Regulating Lithium Deposition and Suppressing Polysulfides Shuttle in Li–S Batteries. Small Methods 2020, 4, 2000082. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, C.; Park, J.B.; Yu, S.; Kim, D.W. Fortifying Zinc Metal Anodes against Uncontrollable Side-Reactions and Dendrite Growth for Practical Aqueous Zinc Ion Batteries: A Novel Composition of Anti-Corrosive and Zn2+ Regulating Artificial Protective Layer. Adv. Energy Mater. 2023, 14, 2302493. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Chang, Z.; Qiao, Y.; Wang, J.; Deng, H.; He, P.; Zhou, H. Fabricating better metal-organic frameworks separators for Li–S batteries: Pore sizes effects inspired channel modification strategy. Energy Storage Mater. 2020, 25, 164–171. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Pan, H.; Zhang, J.; Xiang, S.; Cheng, Z.; Zhang, Z. Pore-space-partitioned MOF separator promotes high-sulfur-loading Li–S batteries with intensified rate capability and cycling life. J. Mater. Chem. A 2021, 9, 26929–26938. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.; Lei, T.; Chen, W.; Rao, G.; Xue, L.; Hu, A.; Fan, Y.; Huang, J.; Hu, Y.; et al. Ion-Inserted Metal-Organic Frameworks Accelerate the Mass Transfer Kinetics in Lithium-Sulfur Batteries. Small 2021, 17, e2104367. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.J.; Song, C.L.; Yang, Y.; Tan, H.C.; Li, G.H.; Cai, Y.P.; Wang, H. Cerium Based Metal-Organic Frameworks as an Efficient Separator Coating Catalyzing the Conversion of Polysulfides for High Performance Lithium-Sulfur Batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Ren, L.; Sun, K.; Wang, Y.; Kumar, A.; Liu, J.; Lu, X.; Zhao, Y.; Zhu, Q.; Liu, W.; Xu, H.; et al. Tandem Catalysis inside Double-Shelled Nanocages with Separated and Tunable Atomic Catalyst Sites for High Performance Lithium-Sulfur Batteries. Adv. Mater. 2024, 36, e2310547. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zeng, Q.; Xu, L.; Xiao, Y.; Xie, L.; Yang, J.; Rong, J.; Weng, J.; Zheng, C.; Zhang, Q.; et al. Multimodal Engineering of Catalytic Interfaces Confers Multi-Site Metal-Organic Framework for Internal Preconcentration and Accelerating Redox Kinetics in Lithium-Sulfur Batteries. Angew. Chem. Int. Ed. 2024, 63, e202318859. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Deng, N.; Li, Y.; Wang, H.; Zhang, M.; Kang, W.; Cheng, B. Advanced preparation and application of bimetallic materials in lithium-sulfur batteries: A review. J. Energy Chem. 2024, 88, 469–512. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Wang, J.; Zhang, D.; Zhu, Y.; Ling, S.; Huang, K.W.; Belmabkhout, Y.; Adil, K.; Zhang, Y.; et al. Imaging defects and their evolution in a metal-organic framework at sub-unit-cell resolution. Nat. Chem. 2019, 11, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Wang, M.; Wen, J.X.; Han, S.H.; Hong, X.J.; Cai, Y.P.; Li, G.; Fan, J.; Chao, Z.S. Oxygen Vacancy-Rich Mixed-Valence Cerium MOF: An Efficient Separator Coating to High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2021, 13, 3899–3910. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Wang, D.; Gao, T.; Song, J.; Zhou, P.; Xu, Z.; Yang, Z.; Xiao, N.; Guo, S. Single Atom Array Mimic on Ultrathin MOF Nanosheets Boosts the Safety and Life of Lithium-Sulfur Batteries. Adv. Mater. 2020, 32, e1906722. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, M.; Dong, C.; Wang, H.; Shen, C.; Wu, X.; An, Q.; Chang, G.; Xu, X.; Mai, L. The continuous efficient conversion and directional deposition of lithium (poly)sulfides enabled by bimetallic site regulation. Nano Energy 2022, 98, 107332. [Google Scholar] [CrossRef]
- Leng, X.; Zeng, J.; Yang, M.; Li, C.; Vattikuti, S.V.P.; Chen, J.; Li, S.; Shim, J.; Guo, T.; Ko, T.J. Bimetallic Ni–Co MOF@PAN modified electrospun separator enhances high-performance lithium-sulfur batteries. J. Energy Chem. 2023, 82, 484–496. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Zhang, Y.; Luo, D.; Yu, A.; Wang, X.; Chen, Z. Amorphizing metal-organic framework towards multifunctional polysulfide barrier for high-performance lithium-sulfur batteries. Nano Energy 2021, 86, 106094. [Google Scholar] [CrossRef]
- Li, S.; Lin, J.; Ding, Y.; Xu, P.; Guo, X.; Xiong, W.; Wu, D.Y.; Dong, Q.; Chen, J.; Zhang, L. Defects Engineering of Lightweight Metal-Organic Frameworks-Based Electrocatalytic Membrane for High-Loading Lithium-Sulfur Batteries. ACS Nano 2021, 15, 13803–13813. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Zhao, Y.; Luo, D.; Shui, L.; Li, Y.; Ma, G.; Zhu, Y.; Zhang, Y.; Zhou, G.; et al. Accelerated Multi-step Sulfur Redox Reactions in Lithium-Sulfur Batteries Enabled by Dual Defects in Metal-Organic Framework-based Catalysts. Angew. Chem. Int. Ed. 2023, 62, e202306901. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dinca, M. Electrically Conductive Porous Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef] [PubMed]
- Day, R.W.; Bediako, D.K.; Rezaee, M.; Parent, L.R.; Skorupskii, G.; Arguilla, M.Q.; Hendon, C.H.; Stassen, I.; Gianneschi, N.C.; Kim, P.; et al. Single Crystals of Electrically Conductive Two-Dimensional Metal-Organic Frameworks: Structural and Electrical Transport Properties. ACS Cent. Sci. 2019, 5, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Cong, C.; Ma, H. Advances of Electroactive Metal-Organic Frameworks. Small 2023, 19, e2207547. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Pei, F.; Huang, J.; Fu, Z.; Xu, G.; Fang, X. Large-Area Preparation of Crack-Free Crystalline Microporous Conductive Membrane to Upgrade High Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1802052. [Google Scholar] [CrossRef]
- Guo, T.; Ding, Y.; Xu, C.; Bai, W.; Pan, S.; Liu, M.; Bi, M.; Sun, J.; Ouyang, X.; Wang, X.; et al. High Crystallinity 2D pi-d Conjugated Conductive Metal-Organic Framework for Boosting Polysulfide Conversion in Lithium-Sulfur Batteries. Adv. Sci. 2023, 10, e2302518. [Google Scholar] [CrossRef]
- Seo, S.D.; Park, D.; Park, S.; Kim, D.W. “Brain-Coral-Like” Mesoporous Hollow CoS2@N-Doped Graphitic Carbon Nanoshells as Efficient Sulfur Reservoirs for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2019, 29, 1903712. [Google Scholar] [CrossRef]
- Cai, D.; Liu, B.; Zhu, D.; Chen, D.; Lu, M.; Cao, J.; Wang, Y.; Huang, W.; Shao, Y.; Tu, H.; et al. Ultrafine Co3Se4 Nanoparticles in Nitrogen-Doped 3D Carbon Matrix for High-Stable and Long-Cycle-Life Lithium Sulfur Batteries. Adv. Energy Mater. 2020, 10, 1904273. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Xue, J.; He, J.; Zhao, R.; Chen, Z.; Sun, Z.; Liu, H.K.; Dou, S.X. ZIF-67/ZIF-8 and its Derivatives for Lithium Sulfur Batteries. Adv. Funct. Mater. 2024, 35, 2414671. [Google Scholar] [CrossRef]
- Seo, S.-D.; Choi, C.; Park, D.; Lee, D.-Y.; Park, S.; Kim, D.-W. Metal-organic-framework-derived 3D crumpled carbon nanosheets with self-assembled CoxSy nanocatalysts as an interlayer for lithium-sulfur batteries. Chem. Eng. J. 2020, 400, 125959. [Google Scholar] [CrossRef]
- Jin, L.; Huang, B.; Qian, X.; Chen, J.; Cheng, J.; Hao, Q.; Zhang, K. Hollow bimetallic sulfide composite coated separator for hindering the shuttle of polysulfides in Li-S batteries. J. Alloys Compd. 2022, 926, 166515. [Google Scholar] [CrossRef]
- Jin, L.; Huang, B.; Qian, X. ZIF-67 Derived Hollow Co9S8 as an Efficient Polysulfides Prohibitor for High Performance Lithium-Sulfur Batteries. ChemElectroChem 2022, 9, e202101337. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.; Fan, H.; Cheng, F.; Su, D.; Wang, G. Promoting lithium polysulfide/sulfide redox kinetics by the catalyzing of zinc sulfide for high performance lithium-sulfur battery. Nano Energy 2018, 51, 73–82. [Google Scholar] [CrossRef]
- Jin, L.; Chen, J.; Fu, Z.; Qian, X.; Cheng, J.; Hao, Q.; Zhang, K. ZIF-8/ZIF-67 derived ZnS@Co-N-C hollow core-shell composite and its application in lithium-sulfur battery. Sustain. Mater. Technol. 2023, 35, e00571. [Google Scholar] [CrossRef]
- Li, F.; Qian, X.; Jin, L. MOF-Derived MnS/N–C@CNT Composites as Separator Coating Materials for Long-Cycling Li–S Batteries. ACS Sustain. Chem. Eng. 2021, 9, 15469–15477. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Wang, Q.; Li, Y.; Guo, S. Regulating the phase and catalytic activity of cobalt phosphide based on the morphological engineering of ZIF-67 to enhance the redox kinetics of polysulfides for high-performance lithium-sulfur batteries. J. Alloys Compd. 2023, 967, 171730. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, S.; Xiong, J.; Wan, P.; Jin, X.; Lu, S.; Zhang, Y.; Fan, H. MOF derived cobalt-nickel bimetallic phosphide (CoNiP) modified separator to enhance the polysulfide adsorption-catalysis for superior lithium-sulfur batteries. J. Colloid Interface Sci. 2023, 641, 942–949. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, H.; Shen, C.; Wei, B.; Wang, J.G. Catalytic Boosting Bidirectional Polysulfide Redox using Co0.85Se/C Hollow Structure for High-Performance Lithium-Sulfur Batteries. ChemElectroChem 2022, 9, e202101557. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Y.; Zhou, H.; Ma, C.; Zhang, Y.; Wang, J.; Qiao, W.; Ling, L. Boosting polysulfide confinement and redox kinetics via ZnSe/NC@rGO as separator modifier for high-performance lithium-sulfur batteries. Electrochim. Acta 2023, 445, 142026. [Google Scholar] [CrossRef]
- Wang, B.; Sun, D.; Ren, Y.; Zhou, X.; Ma, Y.; Tang, S.; Meng, X. MOFs derived ZnSe/N-doped carbon nanosheets as multifunctional interlayers for ultralong-Life lithium-sulfur batteries. J. Mater. Sci. Technol. 2022, 125, 97–104. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, G.; Liang, G.; Lan, D.; Zhang, S.; Wang, Y.; Li, D.; Wu, G. PEG-VN modified PP separator for high-stability and high-efficiency lithium-sulfur batteries. Acta Phys.-Chim. Sin. 2025, 41, 100082. [Google Scholar] [CrossRef]
- Lin, C.; Feng, P.; Wang, D.; Chen, X.; Fang, Y.; Gao, Y.; Zheng, Y.; Yan, Y.; Liu, M. Safe, Facile, and Straightforward Fabrication of Poly(N-vinyl imidazole)/Polyacrylonitrile Nanofiber Modified Separator as Efficient Polysulfide Barrier Toward Durable Lithium–Sulfur Batteries. Adv. Funct. Mater. 2024, 35, 2411872. [Google Scholar] [CrossRef]
- Wang, R.; Ma, C.; Li, C.; Zhang, W.; Zhang, C.; Wang, J.; Xiao, L.; Lv, B.; Guo, S.; Yao, S. Keggin-type potassium phosphotungstate modified functionalization of carbon nanotubes hybrid materials for capture and boost conversion of polysulfides in lithium sulfur batteries. J. Energy Storage 2025, 109, 115168. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Li, J.; Cui, H.; Liu, K.; Liu, Y.; Yang, Y.; Wang, M. Ion selective rapid transport enabled by functionalized CeO2/LiX zeolite modified separator in high performance lithium sulfur batteries. Chem. Eng. J. 2025, 503, 158218. [Google Scholar] [CrossRef]
- Li, Z.; Cong, Y.; Zhao, J.; Su, H.; Shang, Y.; Liu, H. Polyaniline-Coated Cobalt–Iron Oxide Composites Modified Separator for High Performance Lithium–Sulfur Batteries. ACS Appl. Electron. Mater. 2024, 6, 3780–3789. [Google Scholar] [CrossRef]
- Wang, T.; Shi, Z.; Wang, F.; Cui, S.; Zhang, Z.; Liu, W.; Jin, Y. Nanorod In2O3@C Modified Separator with Improved Adsorption and Catalytic Conversion of Soluble Polysulfides for High-Performance Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2024, 16, 18937–18948. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Ni, D.; Zhou, Y.; Yousaf, M.; Guo, Z.; Zhou, J.; Zhou, P.; Wang, Q.; Guo, S. Two Birds with One Stone: Interfacial Engineering of Multifunctional Janus Separator for Lithium-Sulfur Batteries. Adv. Mater. 2022, 34, e2107638. [Google Scholar] [CrossRef]
- Qi, X.; Huang, L.; Luo, Y.; Chen, Q.; Chen, Y. Ni3Sn2/nitrogen-doped graphene composite with chemisorption and electrocatalysis as advanced separator modifying material for lithium sulfur batteries. J. Colloid Interface Sci. 2022, 628, 896–910. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Jin, B.; Sheng, Q.; Li, Q.; Cui, M.; Li, Y.; Lang, X.; Jiang, Q. Functional Separator Modified with Reduced Graphene Oxide and Fe3S4 for High-Performance Lithium–Sulfur Batteries. ACS Appl. Nano Mater. 2023, 6, 1161–1170. [Google Scholar] [CrossRef]
- Yan, M.; Zhao, C.; Li, X. Preparation of Bacterial Cellulose/Ketjen Black-TiO2 Composite Separator and Its Application in Lithium-Sulfur Batteries. Polymers 2022, 14, 5559. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).