Reactive Anti-Solvent Engineering via Kornblum Reaction for Controlled Crystallization in (FA0.83MA0.17Cs0.05)Pb(I0.85Br0.15)3 Perovskite Solar Cells
Abstract
1. Introduction
2. Results
2.1. The Selection of Chemical Reactions
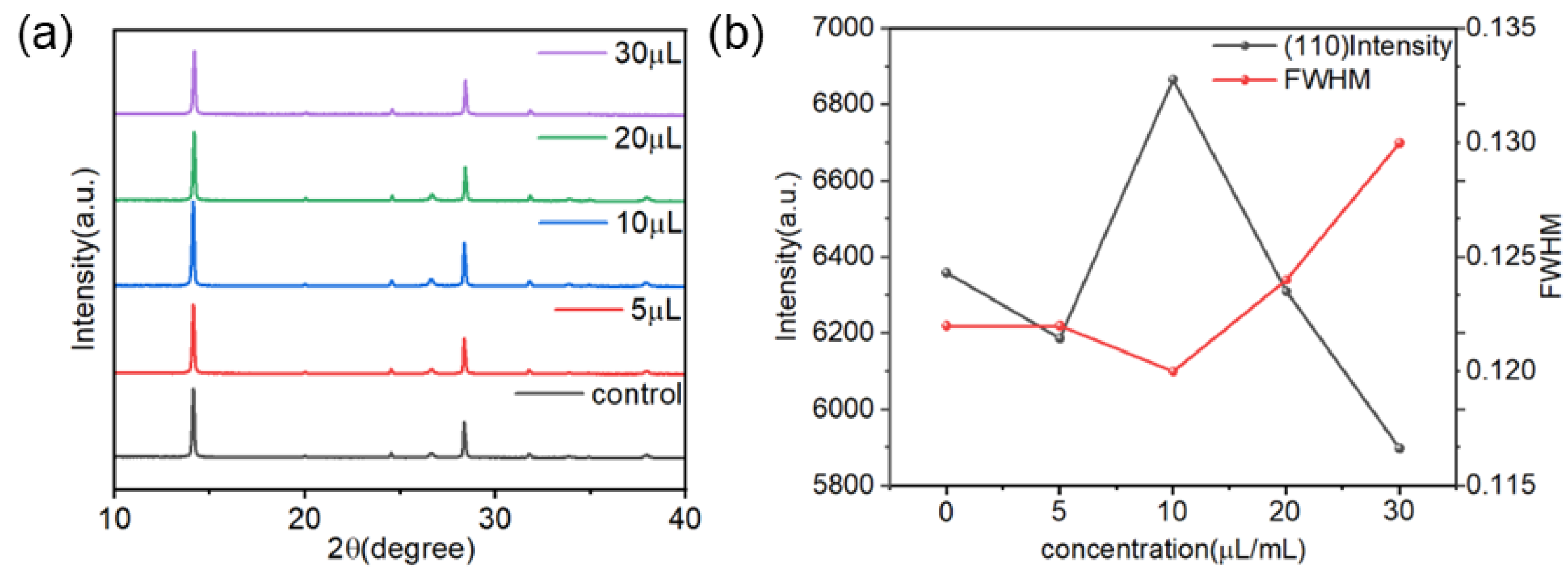
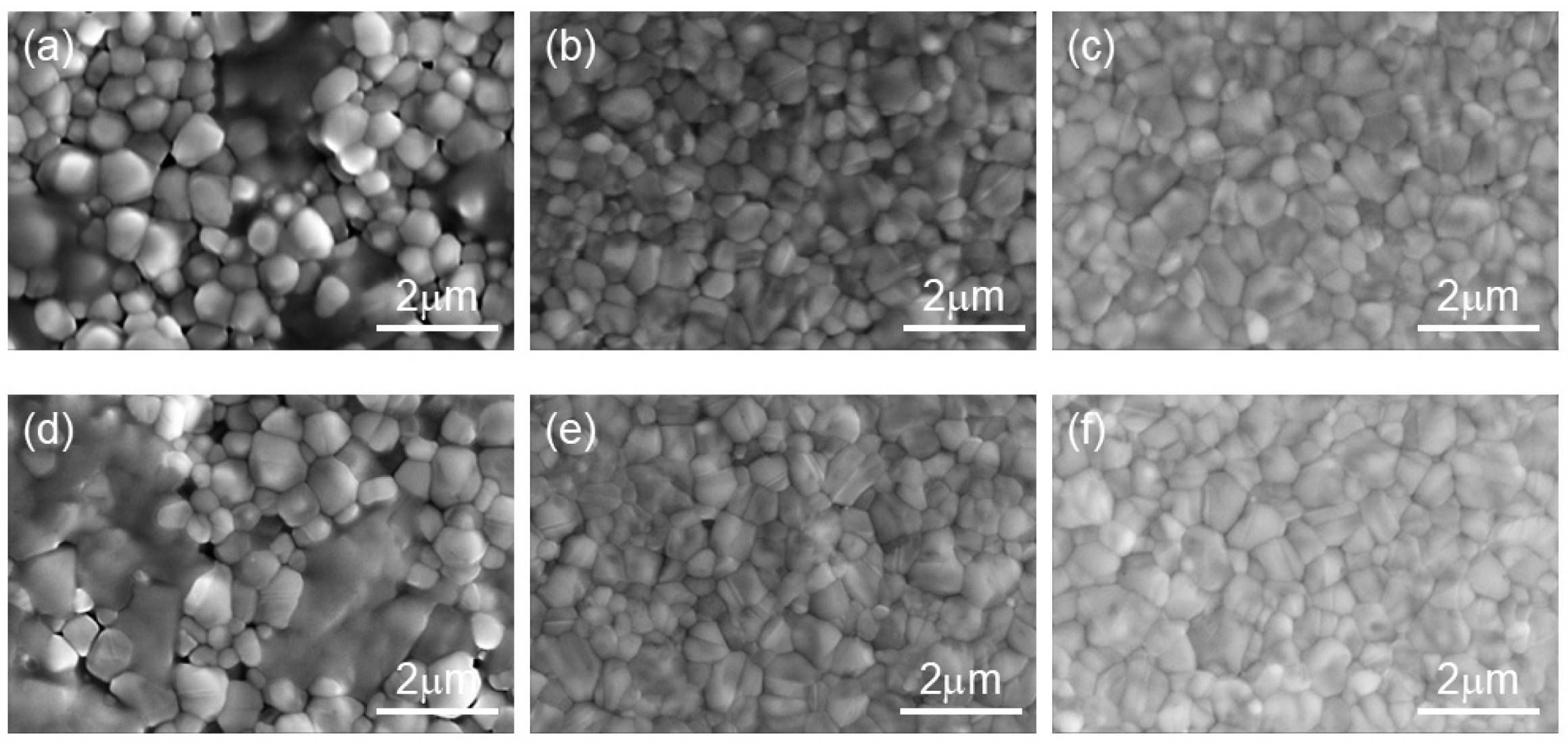
2.2. The Influence of TCC on the Morphology of Perovskite
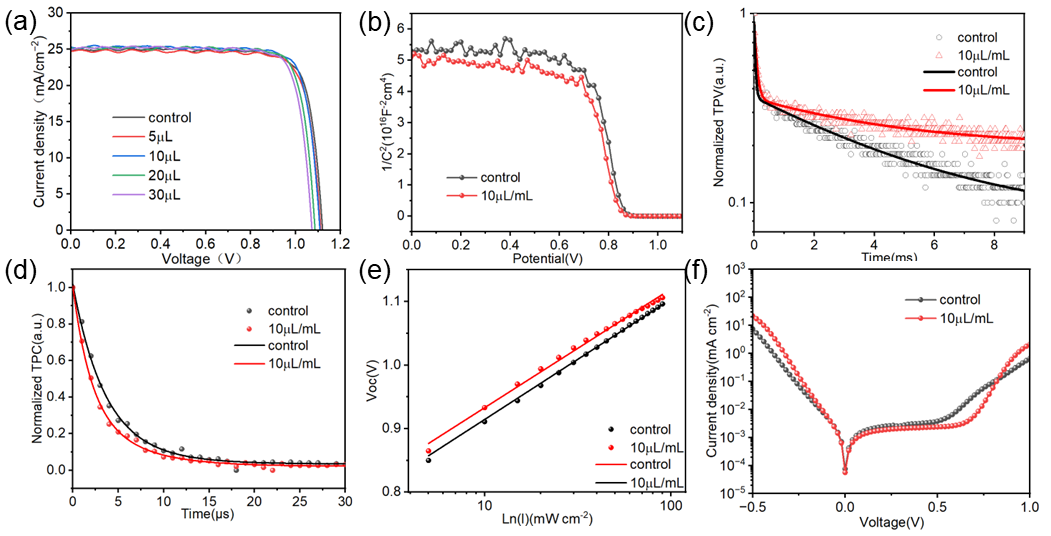
2.3. Effect of TCC on the Photovoltaic Performance of PSCs
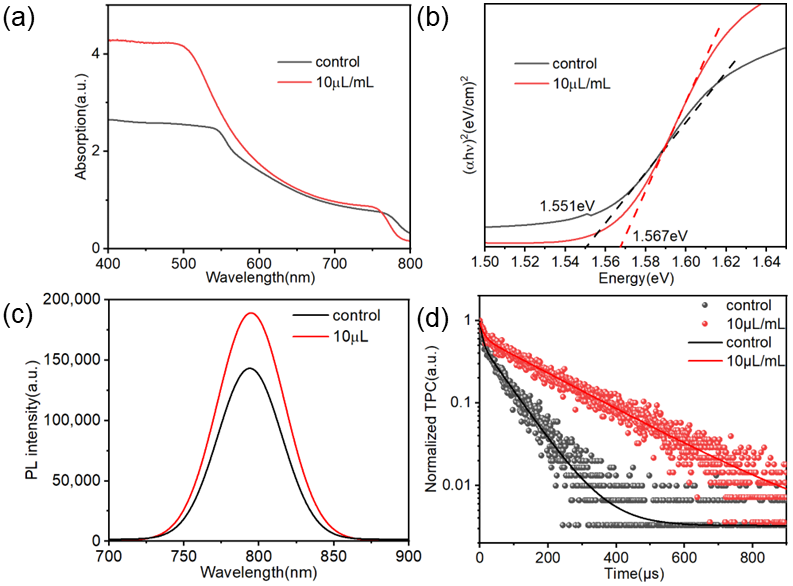
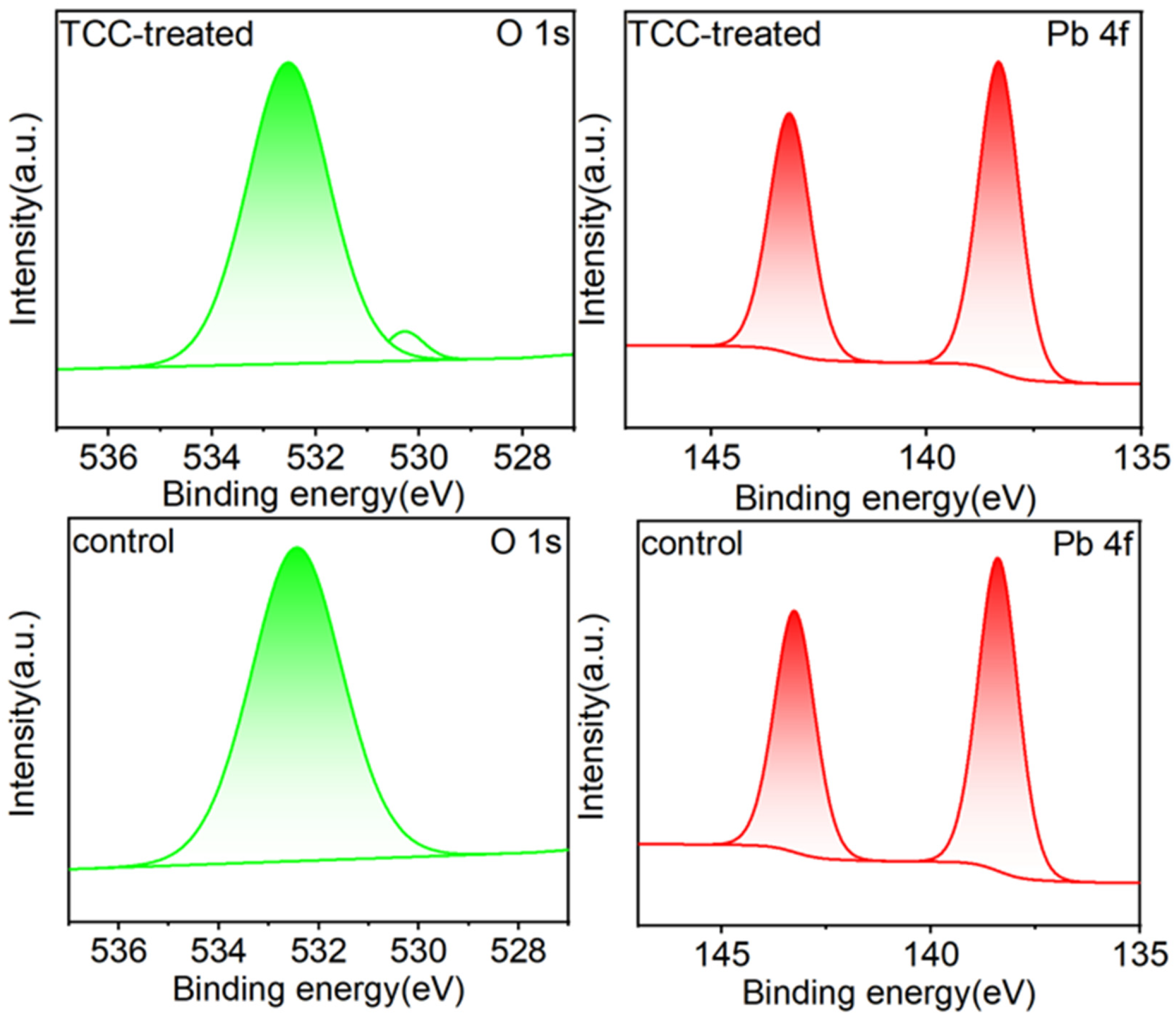
2.4. Mechanism of Action of TCC on Perovskite Films
2.5. The Impact of TCC on the Stability of PSCs

3. Materials and Methods
3.1. Materials
3.2. Preparation of the PSCs
3.3. Characterization and Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jeong, J.; Kim, B.; Seol, M.J.; Kim, S.Y. Light path management in devices containing halide perovskite materials with a high refractive index. Mater. Res. Bull. 2024, 175, 112777. [Google Scholar] [CrossRef]
- Dar, S.A.; Sengar, B.S. Breakthrough in sustainable photovoltaics: Achieving 30.86 % efficiency with innovative lead-free bilayer perovskite solar cells using SCAPS-1D and DFT framework. Sol. Energy Mater. Sol. Cells 2025, 282, 113352. [Google Scholar] [CrossRef]
- Li, Y. Research progress on preparation methods and photoelectric properties of perovskite solar cell[C/OL]//Mou, J.; Chen S. In Proceedings of the International Conference on Green Communication, Network, and Internet of Things (GCNIoT 2021), Kunming, China, 10–12 December 2021; Volume 47. Available online: https://www.spiedigitallibrary.org/conference-proceedings-of-spie/12085/2625439/Research-progress-on-preparation-methods-and-photoelectric-properties-of-perovskite/10.1117/12.2625439.full (accessed on 18 July 2025).
- Chang, J.H.; Liu, K.; Lin, S.; Yuan, Y.B.; Zhou, C.H.; Yang, J.L. Solution-processed perovskite solar cells. J. Cent. South Univ. 2020, 27, 1104–1133. [Google Scholar] [CrossRef]
- Jung, M.; Ji, S.-G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038. [Google Scholar] [CrossRef]
- Deng, X.; Cao, Z.; Yuan, Y.; Chee, M.O.L.; Xie, L.; Wang, A.; Xiang, Y.; Li, T.; Dong, P.; Ding, L.; et al. Coordination modulated crystallization and defect passivation in high quality perovskite film for efficient solar cells. Coord. Chem. Rev. 2020, 420, 213408. [Google Scholar] [CrossRef]
- Yang, C.; Hu, W.; Liu, J.; Han, C.; Gao, Q.; Mei, A.; Han, H. Achievements, challenges, and future prospects for industrialization of perovskite solar cells. Light: Sci. Appl. 2024, 13, 227. Available online: https://www.nature.com/articles/s41377-024-01461-x (accessed on 18 July 2025). [CrossRef] [PubMed]
- Sun, C.; Jin, L.; Wang, X.; Shi, B.; Wang, P.; Ren, N.; Han, X.; Sun, L.; Zhu, Z.; Huang, Q.; et al. Wide-Bandgap Perovskite and Perovskite/Silicon Tandem Solar Cells through Strong Hydrogen Bonding Interaction. ACS Energy Lett. 2025, 10, 2171–2179. [Google Scholar] [CrossRef]
- Shao, C.; He, J.; Ma, J.; Wang, Y.; Niu, G.; Zhang, P.; Yang, K.; Zhao, Y.; Wang, F.; Li, Y.; et al. Multifunctional Graphdiyne Enables Efficient Perovskite Solar Cells via Anti-Solvent Additive Engineering. Nano-Micro Letters 2025, 17, 121. Available online: https://link.springer.com/10.1007/s40820-024-01630-y (accessed on 12 July 2025). [CrossRef]
- He, R.; Pan, W.; Chen, P.; Zheng, Q.; Tong, A.; Pan, J.; Jin, Z.; Sun, W.; Li, Y.; Wu, J. Synergistic anti-solvent engineering with piperizium salts for highly efficient inverted perovskite solar cells exceeding 25 %. Nano Energy 2024, 131, 110268. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Qin, S.; Miao, X.; Yuan, M.; Liu, Z.; Wang, Y.; Feng, Y.; Jiang, X.; Wu, R.; et al. Efficient and Stable p–i–n Perovskite Solar Cells Enabled by In Situ Functional Group Conversion. J. Am. Chem. Soc. 2024, 146, 32105–32116. [Google Scholar] [CrossRef]
- Fu, D.; Dong, J.; Du, H.; Xu, J. Methanesulfinylation of Benzyl Halides with Dimethyl Sulfoxide. J. Org. Chem. 2020, 85, 2752–2758. [Google Scholar] [CrossRef]
- Xing, H.; Chen, L.; Jia, Y.; Jiang, Z.; Yang, Z. Fe2O3-catalyzed Pummerer rearrangement of acyl chlorides and sulfoxides: Facile synthesis of alkylthiomethyl ester. Tetrahedron Lett. 2017, 58, 2199–2202. [Google Scholar] [CrossRef]
- Zhang, P.; Gu, N.; Chen, X.; Song, L.; Du, P.-F.; Chen, W.-H.; Xiong, J. Triethyl phosphate in an antisolvent: A novel approach to fabricate high-efficiency and stable perovskite solar cells under ambient air conditions. Mater. Chem. Front. 2021, 5, 7628–7637. [Google Scholar] [CrossRef]
- Li, X.; Zhou, L.; Chen, Q.; Zhang, Y.; Feng, X.; Du, Y.; Hao, Y. Synergistic Carbonyl and Cyano Passivation for Efficient Blade-Coated Perovskite Solar Cells. IEEE Electron Device Lett. 2024, 45, 2162–2165. [Google Scholar] [CrossRef]
- Xiong, Q.; Huang, X.; Wang, C.; Zhou, Q.; Gang, Y.; Li, T.; Hu, C.; Zhang, N.; Wang, X.; Wu, J.; et al. Managed spatial strain uniformity for efficient perovskite photovoltaics enables minimized energy deficit. Joule 2024, 8, 817–834. [Google Scholar] [CrossRef]
- Lee, C.M.; Shah, S.H.U.; Ko, K.-J.; Kim, D.H.; Kim, T.W.; Cho, H.W.; Kim, Y.B.; Lee, J.W.; Heo, K.; Kim, C.H.; et al. Impact of Ternary Solvent on the Grain Size and Defects of Perovskite Layer to Realize a Stable Morphology for Efficient Inverted Solar Cells. Solar RRL 2023, 7, 2300604. Available online: https://onlinelibrary.wiley.com/doi/10.1002/solr.202300604 (accessed on 18 July 2025). [CrossRef]
- Padchasri, J.; Yimnirun, R. Effects of annealing temperature on stability of methylammonium lead iodide perovskite powders. J. Alloys Compd. 2017, 720, 63–69. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Luo, J.; Wang, M.; Cai, S.; Cai, Q.; Wei, D.; Ji, J.; Zhang, Z.; Li, X. Tailoring interface and morphology of TiO2 electron transport layer with potassium bitartrate for high-performance perovskite solar cells. Appl. Surf. Sci. 2024, 662, 160139. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Han, M.; Zhao, J.; Wang, R.; Zhang, J.; Ren, H.; Hou, G.; Ding, Y.; Zhao, Y.; et al. Formamidine formate as the multifunctional modulator at buried interface for efficient FAPbI3 perovskite solar cells. Nano Energy 2023, 118, 108981. [Google Scholar] [CrossRef]
- Peng, Z.; Wortmann, J.; Hong, J.; Zhou, S.; Bornschlegl, A.J.; Haffner-Schirmer, J.; Le Corre, V.M.; Heumüller, T.; Osvet, A.; Rand, B.P.; et al. Locating Non-Radiative Recombination Losses and Understanding Their Impact on the Stability of Perovskite Solar Cells During Photo-Thermal Accelerated Ageing. Adv. Energy Mater. 2025, 2025, 2502787. Available online: https://advanced.onlinelibrary.wiley.com/doi/10.1002/aenm.202502787 (accessed on 18 July 2025). [CrossRef]
- Pandey, A.; Vishwakarma, A.; Saini, S.K.; Kumar, M.; Kumar, L. Light-induced micro-strain regulation and charge carrier dynamics of (FA0.83MA0.17)0.95Cs0.05Pb(I0.83Br0.17)3 hybrid perovskite films. Opt. Mater. 2023, 146, 114564. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Wang, D.; Pan, W.; Gao, L.; Deng, C.; Li, R.; Huang, E.; Guo, X.; Wu, J.; et al. Tailored interface molecules for modulating charge carrier dynamics in perovskite solar cells. Nano Energy 2025, 134, 110548. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Li, C.-F.; Huang, Y.-C.; Tsai, F.-Y.; Su, W.-F. Transparent low moisture permeable coating for perovskite solar cell encapsulation. Surf. Coat. Technol. 2024, 482, 130695. [Google Scholar] [CrossRef]
- Kim, H.; Park, N. Future Research Directions in Perovskite Solar Cells: Exquisite Photon Management and Thermodynamic Phase Stability. Adv. Mater. 2023, 35, 2204807. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Lee, Y.N.; Kim, H.S. Lattice strain inhomogeneity in halide perovskite films: Its origins and regulations. Chem. Soc. Rev. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.-C.; Wang, Y.-H.; Chen, K.-L.; Huang, C.-J. Effect of temperature annealing treatments and acceptors in CH3NH3PbI3 perovskite solar cell fabrication. J. Alloys Compd. 2017, 695, 2453–2457. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Xiong, Q.; Mohd Yusoff, A.R.b.; Gao, P. Reactive Anti-Solvent Engineering via Kornblum Reaction for Controlled Crystallization in (FA0.83MA0.17Cs0.05)Pb(I0.85Br0.15)3 Perovskite Solar Cells. Inorganics 2025, 13, 295. https://doi.org/10.3390/inorganics13090295
Wu S, Xiong Q, Mohd Yusoff ARb, Gao P. Reactive Anti-Solvent Engineering via Kornblum Reaction for Controlled Crystallization in (FA0.83MA0.17Cs0.05)Pb(I0.85Br0.15)3 Perovskite Solar Cells. Inorganics. 2025; 13(9):295. https://doi.org/10.3390/inorganics13090295
Chicago/Turabian StyleWu, Shengcong, Qiu Xiong, Abd. Rashid bin Mohd Yusoff, and Peng Gao. 2025. "Reactive Anti-Solvent Engineering via Kornblum Reaction for Controlled Crystallization in (FA0.83MA0.17Cs0.05)Pb(I0.85Br0.15)3 Perovskite Solar Cells" Inorganics 13, no. 9: 295. https://doi.org/10.3390/inorganics13090295
APA StyleWu, S., Xiong, Q., Mohd Yusoff, A. R. b., & Gao, P. (2025). Reactive Anti-Solvent Engineering via Kornblum Reaction for Controlled Crystallization in (FA0.83MA0.17Cs0.05)Pb(I0.85Br0.15)3 Perovskite Solar Cells. Inorganics, 13(9), 295. https://doi.org/10.3390/inorganics13090295






