Abstract
The coordination of rare-earth metal ions (Ln3+) to polyoxometalates (POM) is regarded as a way of modifying and controlling their properties, such as single-molecular magnetism or luminescent behavior. The half-sandwich complexes of Ln3+ with monolacunary Keggin POMs (Ln3+/POM = 1:1) are of particular interest, since the Ln3+ retains its ability to coordinate extra ligands. Thus, the knowledge of the exact structures of 1:1 Ln/POM complexes is important for the development of reliable synthetic protocols for hybrid complexes. In this work, we isolated three 1:1 Gd3+/POM complexes of the general formula Cat4Gd(PW11O39)·xH2O (Cat = K+ or Me4N+). Complex (Me4N)2K2[Gd(H2O)2PW11O39]·5H2O (1) is polymeric, revealing a layered structural motif via bridging Gd3+ and K+ ions. Complexes (Me4N)6K2[Gd(H2O)3PW11O39]2·20H2O (2) and (Me4N)7K[Gd(H2O)3PW11O39]2·12H2O (3) are classified as dimeric; the difference between them consists of the different crystal packing of the polyoxometalates, which is induced by a variation in the cationic composition. Isostructural complexes have also been characterized for praseodymium, europium, terbium and dysprosium. The coordination number of Ln3+ (8) persists in all the compounds, while the binding mode of the POM varies, giving rise to different architectures with two or three H2O co-ligands per Ln3+. However, whatever the particular structure and exact composition, the {Ln(PW11O39)} moieties are always involved in bonding with each other with the formation either of polymeric chains or dimeric units. In water, these aggregates can dissociate with the formation of [Ln(H2O)4PW11O39]4-. This behavior must be taken into account when choosing L for the design of hybrid {Ln(L)POM} complexes.
1. Introduction
The class of polyoxometalates (POMs) is characterized by great structural diversity with a small number of stable, well-studied structural types [1,2,3]. Heterometallic derivatives of POMs obtained for a large number of chemical elements with different electronic configurations exhibit various electronic [4,5], magnetic [6,7,8,9,10], catalytic [11,12,13,14], optical [15], or other useful properties [16,17]. The wide possibilities of modification determine POM applicability in such areas as biology and medicine, electrochemistry, materials and surface sciences, solar energy conversion, etc. [18,19,20,21]. It has been shown that almost all transition and non-transition metals can be included in the vacancy of lacunary Keggin-type POMs, which are the most common [22].
The combination of polyoxometalates with lanthanide (Ln) ions is also interesting from the point of view of unique functional properties, such as, for example, luminescence [23]. One of the principles for the formation of new functional materials is the binding of POM fragments through the Ln ions due to their large coordination numbers, resulting in solid-state oligomers [24,25] and large ring structures [26,27]. POMs can serve as connectors and transfer agents for different monolacunary POMs [28].
A lot of different lanthanide complexes with the monovacant Keggin-type anion, [PW11O39]7–, have been well studied to date. These Ln-POMs can be divided into several structurally determined types. First of all, there are the monomeric species containing only one polyoxometalate unit. A series of chromophore-Ln-POMs [N(CH3)4]3K2[Ln(C7H5O2)(H2O)2(PW11O39)]·11H2O (Ln = Eu, Tb, Tm, Lu) was prepared using LnCl3·6H2O and benzoic acid. Also, with the variation of the molar ratio of Eu:Tb:Tm ions, a series of multicenter-Ln analogues [N(CH3)4]3K2[EuxTbyTm1−x−y(C7H5O2)(H2O)2(PW11O39)]·11H2O were synthesized [29]. More organic–inorganic hybrid lanthanide-based POMs, [N(CH3)4]3K2[Ln(C7H5O2)(H2O)2(PW11O39)]·11H2O, were synthesized using the same conditions with HoCl3·6H2O [30], SmCl3·6H2O [31] and TbCl3·6H2O [32]. In all these cases, the dilacunary [P2W19O69(H2O)]14– was used as a source of the resulting Keggin anion. The interaction of phthalocyanine complexes of lanthanides, [Ln(Pc)(OAc)] (Ln = Y, Dy, Tb), with the [PW11O39]7– anion in a 1:1:1 mixture of CH3CN:MeOH:CH2Cl2 also yielded a row of monomeric complexes (N(nBu)4)4H2[Ln(Pc)(PW11O39)] [33].
The second group includes dimeric complexes connected through the organic bridging ligands. In 2009, the series of polyoxoanions, [{(PW11O39)Ln(H2O)(CH3COO)}2]10– (Ln = Sm, Eu, Gd, Tb, Ho, Er), constructed from two monolanthanide substituted {PW11-Ln} units bridged by two acetato ligands, was published [34]. The row was continued with Dy-, Y- and Lu-containing analogues in 2012 [35]. In 2013, Na2[Cu(en)2(H2O)]4[{(PW11O39)Sm(H2O)(CH3COO)}2]·10H2O [36] and K2[Cu(en)2(H2O)]4[{(PW11O39)Tb(H2O)(CH3COO)}2]·15H2O [37] had the same hybrid acetate-bridging dimeric structures modified by [Cu(en)2(H2O)]2+ (en = 1,2-ethylenediamine) fragments. In 2012, four new oxalate-bridging lanthanide-substituted phosphotungstates {[(PW11O39)Ln(H2O)]2(C2O4)}10− (Ln = Y, Dy, Ho, Er) were synthesized by the reaction of a Keggin-type anion with Ln cations and oxalate ligands in aqueous solution [38]. The double-tartaric bridged phosphotungstates formulated as [N(CH3)4]6K3H7[Ln(C4H2O6)(PW11O39)]2·27H2O (Ln = Dy, Er, Yb [39] and Tm [40]) have been obtained using the dilacunary [P2W19O69(H2O)]14– as a precursor. In 2012, the hydrothermal conditions were successfully used to prepare the three-dimensional architectures [Ln(HL)(L)(H2O)6{Ln(H2L)0.5(PW11O39H)Ln(H2O)4}]2·nH2O (Ln = La, Ce, Pr; H2L = 2,5-pyridinedicarboxylic acid) based on lanthanide-substituted POM units [41].
The largest number of examples to date refers to the third type—dimeric complexes in which POM fragments are linked directly through lanthanide cations. In 1971, Peacock and Weakley isolated several dimeric compounds, formulated as K11[CeIII(PW11O39)2]·24H2O, K10[CeIII(PW11O39)2]·25H2O, K11[Pr(PW11O39)2]·22H2O K11[Nd(PW11O39)2]·26H2O [42]. In 2004, the structure of Cs11Eu(PW11O39)2]·28H2O was published [43]. The CeIV sandwiched between two lacunary Keggin-type [PW11O39]7– anions resulted in a lanthanide polyoxometalate (NH4)2[N(CH3)4]6Na2[Ce(PW11O39)2]·14H2O [44]. The central {CeIVO8} polyhedron reveals the square-antiprismatic geometry. The analogues dimeric compound was further prepared for praseodymium, [(CH3)4NH]4Na3[Pr(PW11O39)2]·12H2O [45]. The two isostructural anions, [CeIII(PW11O39)2]11– and [CeIV(PW11O39)2]10–, were isolated as dimethylammonium salts with the same square-antiprismatic coordination of the central {CeIII/IVO8} polyhedron [46]. Interestingly that for similar complexes, [N(CH3)4]10H[La(PW11O39)2]·21H2O and [N(CH3)4]10H[Ce(PW11O39)2]·19H2O, an approximate cubic {LnO8} geometry was found [47]. Potassium- or mixed potassium/caesium salts of [Ln(PW11O39)2]11− dimeric anions were obtained and characterized for ten lanthanids (Ln = Pr, Nd, Eu, Gd, Tb, Dy, Ho, Er, Tm and Yb) [48]. Three phosphors based on polyoxotungstates, K3Cs8[Eu(PW11O39)2]·11H2O, K3Cs8[Sm(PW11O39)2]·10H2O and K5Cs6[Dy(PW11O39)2]·15H2O, were prepared and characterized [49]. In 2017, a dysprosium-containing phosphotungstate compound, K2[N(CH3)4]5H4[Dy(PW11O39)2]·21H2O, was published [50].
Separately, a series of novel polyoxometalate trimers, H3[N(CH3)4]14[NaLn(PW11O39)3]·18H2O (Ln = Nd, Sm, Eu), can be noted. Herein, the dimeric unit {Ln(PW11O39)2} and Na-substituted POM monomer {NaPW11O39} linked to form an unprecedented linear structure [51].
Organic–inorganic hybrid enantiomeric compounds of K1.3Na3.2H6.5[L-Pr(PW11O39)2]·8.3L-proline·21.5H2O, K1.3Na3.2H6.5[D-Pr(PW11O39)2]·8.3D-proline·17H2O, K1.3Na3.2H6.5[L-Er(PW11O39)2]·8.3L-proline·22.5H2O [52] and KNa3[Hproline]7[Sm(PW11O39)2]·D-proline·18H2O [53] were obtained by using L- and D-proline as chiral auxiliary agents. The series was expanded by the isolation of [Ln(PW11O39)2]11– polyanions (Ln = La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Er, Tm, Yb and Y) using proline [54].
In 2008, compound [Cu(en)2]2H8[Gd(PW11O39)2]·(H2en)0.5·3H2O prepared under hydrothermal conditions was described [55]. In 2010, more inorganic–organic hybrid Ln-POM compounds with en-copper-complexes, H8[Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][La(PW11O39)2]}2·18H2O, H6[Na2(en)2(H2O)5][Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][Ce(PW11O39)2]}2·16H2O, H6[Na2(en)2(H2O)5][Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][Pr(PW11O39)2]}2·18H2O, H6[Na2(en)2(H2O)4][Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][Nd(PW11O39)2]}2·14H2O, H6[Na2(en)2(H2O)5][Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][Sm(PW11O39)2]}2·20H2O and H7[Cu(en)2]2[Sm(PW11O39)2]·10H2O, were published [56]. In 2011, complexes H14[Cu(en)2]4[Ce(PW11O39)2]2·2en·21H2O, H14[Cu(en)2]4[Pr(PW11O39)2]2·11H2O and H8[Cu(en)2H2O]4[Cu(en)2]{[Cu(en)2][Pr(PW11O39)2]}2·2en·12H2O were presented [57]. A series of 3d–4f heterometallic monovacant Keggin phosphotungstate derivatives, [Cu(dap)(H2O)2]0.5[Cu(dap)2]4H2[Pr(PW11O39)2]·3H2O (dap = 1,2-diaminopropane), [Cu(en)2(H2O)]2[Cu(en)2]1.5H4[Pr(PW11O39)2]·10H2O [58], [Cu(en)2]2H6[Ce(PW11O39)2]·8H2O, [Cu(dap)2(H2O)][Cu(dap)2]4.5[Dy(PW11O39)2]·4H2O [59], [Cu(dap)2]5.5[Y(PW11O39)2]·4H2O [60], Cu(dap)2(H2O)][Cu(dap)2]4.5[Sm(PW11O39)2]·5H2O, [Cu(dap)2(H2O)][Cu(dap)2]4.5[Er(PW11O39)2]·4H2O [61], [Cu(dap)2(H2O)][Cu(dap)2]4.5[La(PW11O39)2]·4H2O [62] and H[Cu(dap)2(H2O)][Cu(dap)2]4[Eu(PW11O39)2]·13H2O [63], was prepared in hydrothermal conditions.
The first 2:2 type monolanthanide substituted POM dimers, [{(PW11O39H)Ln(H2O)3}2]6− (Ln = Nd, Gd), have been published in 2009. The binding of the [PW11O39Ln(H2O)3]4− anionic fragments in this structure was provided by two Ln-O-W bridges [34]. In 2017, a dysprosium-containing 2:2 type dimer, Na2[N(CH3)4]4H2[{Dy(PW11O39)(H2O)3}2]·28H2O, was obtained [50].
One more types of compounds include the chains built from Ln-POMs. Complex Al(H3O)[Eu(H2O)2PW11O39]·20H2O was obtained in 2004, and in the solid state, it was an infinite one-dimensional zigzag polymer. A Europium atom coordinated to the POM vacant site was served as the linking element and connected to neighboring {PW11O39} units via terminal oxygen atoms [43]. In 2011, the same 1-D zigzag chain was found for [N(CH3)4]4[Ce(H2O)2(PW11O39)]·2H2O with linking [Ce(H2O)2]3+ cations [64]. Another chain-like structure was found for [N(CH3)4]4[Tb(H2O)2(PW11O39)]·2H2O. In this case, the Tb3+ cation coordinated with the POM unit vacancy was connected to the terminal oxygen of only one adjacent {PW11O39} to form a linear chain [65].
Herein, we present a series of 1:1 Ln3+/POM complexes (Ln = Pr, Eu, Gd, Tb, Dy) of the general formula Cat4Ln(PW11O39)·xH2O (Cat = K+ or Me4N+). In the case of gadolinium compounds, we found three types of structures: complex (Me4N)2K2[Gd(H2O)2PW11O39]·5H2O (1Gd) is polymeric, revealing a layered structural motif, and complexes (Me4N)6K2[Gd(H2O)3PW11O39]2·20H2O (2Gd) and (Me4N)7K[Gd(H2O)3PW11O39]2·12H2O (3Gd) are classified as dimeric. For the sake of simplicity, we formulate the compounds with the K+ ions outside the brackets, even though they coordinate directly with the POMs. Other Ln-containing complexes are isostructural with the gadolinium analogues.
2. Results and Discussion
2.1. Synthesis
Several approaches were described in the literature for the preparation of Ln-POM complexes differing in the lacunary phosphotungstate precursors, such as [PW9O34]9− [43] or [P2W19O69(H2O)]14− [50]. In our work, we used a monolacunary [PW11O39]7− polyoxoanion to react with Ln cations, and the pH value was set at 5.4. As a result, we obtained crystals of three different kinds simultaneously. We were succeeded in structurally characterizing all three types of crystals for the gadolinium-containing compounds, and the unit cell parameters for the three types were also determined in the case of europium. For praseodymium, it was possible to determine the parameters of two types of crystals, and only one type was characterized in the case of dysprosium and terbium (Table 1). The resulting phases were additionally characterized by IR spectroscopy (Figure S1).

Table 1.
Unit cell parameters for selected crystals of the compounds: V (Å3), a (Å), b (Å), c (Å), α (°), β (°), and γ (°).
2.2. Structural Description
The structure of (Me4N)2K2[Gd(H2O)2PW11O39]·5H2O (1Gd) is presented in Figure 1a,b. The Gd is incorporated to the vacant site of the {PW11O39} POM, coordinating via four O atoms (Figure 1a). The Gd further coordinates two neighboring POMs, thus forming a chain with the {Gd2(POM)2} secondary building unit (Figure 1b), similarly to that in works [43,64]. The coordination sphere of Gd is supplemented by two H2O ligands, resulting in a {GdO8} polyhedron with the typical Gd–O distances. Potassium counterions join the chains into layers via K–O bonds with the POMs (Figure 2a,b). Me4N+, and disordered hydrate molecules are located between the layers.
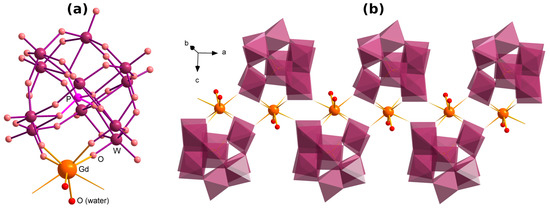
Figure 1.
(a) The structure of {Gd(H2O)2PW11O39} unit in compound 1Gd; (b) the corresponding {Gd(H2O)2PW11O39}n chain. The WO6 moieties are depicted as octahedra.
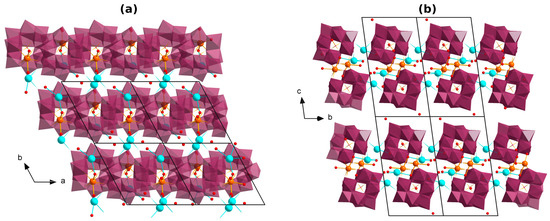
Figure 2.
(a) The structure of a layer composed from {Gd(H2O)2PW11O39}n chains bound with K+ cations in compound 1Gd; (b) the crystal packing of the layers. Me4N+ and hydrogens are not shown, the WO6 moieties are depicted as octahedra.
The structure of 2Gd and 3Gd somewhat resembles 1Gd in that they comprise a {Gd2(POM)2} secondary building unit. However, in 2Gd and 3Gd, it is not interlocked in a chain but is supplemented by two terminating H2O ligands, thus forming a centrosymmetric dimer structure (Figure 3). Analogous dimeric structures with different counterions (Na+/H+/Me4N+) have recently been reported [34,50]. The coordination polyhedron {GdO8} is similar in the compounds. Contrary to 1Gd, the structures of 2Gd and 3Gd reveal a disorder of K+ and Me4N+, i.e., the latter take partial occupancy, sharing similar positions with the hydrate molecules. The ratio of the cations is approximately similar within the series (tested for several crystals of 2 or 3). Due to the complicated disorder, positions of the counterions and the hydrate molecules are rather arbitrary. We speculate that the disordered K+ can both coordinate directly with the POM and bind via a hydrogen bond network. The number of the outer-sphere H2O and Me4N+ in 2Gd and 3Gd is larger than in 1Gd, so the POM species are more separated from each other in the former. The difference between 2Gd and 3Gd consists in different crystal packing of the POMs, which is induced by a variation in the outer-sphere composition (Figure 4). Specifically, in 2Gd, the POMs are located one above the other with the translation relation (P–1 space group), while in 3Gd, neighboring POMs are oriented by an angle of ca. 40° with respect to each other (relation by two-fold screw symmetry; P21/c space group).
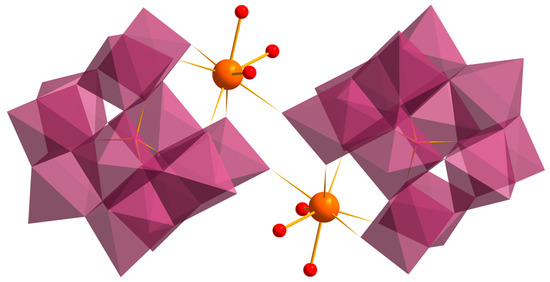
Figure 3.
The structure of a dimer {Gd(H2O)3PW11O39}2 in compounds 2Gd and 3Gd.
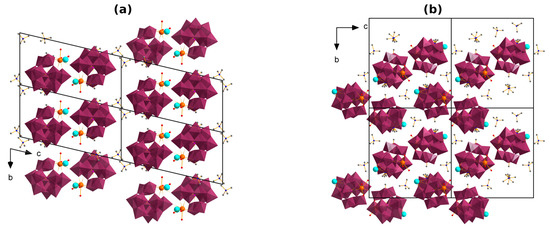
Figure 4.
Crystal packing of the dimers in compounds 2Gd (a) and 3Gd (b). The WO6 moieties are depicted as octahedra, hydrate molecules, hydrogens and the disorder are not shown.
Other lanthanide analogues with Pr, Eu, Tb and Dy also constitute three structural types 1–3, which are identified by unit cell parameters of single crystals (Table 1). One can note that the unit cell parameters generally correlate with the ionic radius of the lanthanides. At the same time, experiments within one lanthanide series reveal a quite large variation of the parameters above the instrument accuracy. Specifically, the unit cell volume for several crystals of 3Pr varies by 100 Å3, while the difference in parameter c can be up to 0.46 Å (Table 2). This is likely a consequence of the non-stoichiometric composition of disordered hydrate molecules in the compounds, which varies from crystal to crystal.

Table 2.
Unit cell parameters for several crystals of 3Pr (P21/c space group): V (Å3), a (Å), b (Å), c (Å), and β (°).
3. Experimental Section
3.1. Materials and Methods
The K7[PW11O39]·14H2O precursor was prepared according to the literature [66] and confirmed by IR spectra. Other chemicals were purchased commercially from Sigma Aldrich and used without further purification. Infrared spectra (4000–400 cm−1) were recorded on a Scimitar FTS 2000 spectrophotometer in KBr pressed pellets.
3.2. Synthesis
Synthesis of Cat4Gd(PW11O39)·xH2O: First, 1.00 g (0.31 mmol) of K7[PW11O39]·14H2O was dissolved in 15 mL of water, which was followed by the addition of 0.20 g (0.54 mmol) of GdCl3·6H2O in 15 mL of water dropwise, resulting in a pH = 4.7. The pH value was adjusted to 5.4 using 2M KOH solution under stirring. The reaction mixture was heated at 80 °C for 1 h after the solution was cooled to room temperature and filtered. Then, 0.20 g (1.30 mmol) of tetramethylammonium bromide was added under stirring. After 0.5 h, the resulting solution was filtered and left to evaporate at room temperature. A crystalline phase was obtained after one week. Yield: ca. 43% (based on K7[PW11O39]·14H2O).
Synthesis of Cat4Pr(PW11O39)·xH2O: The synthetic procedure was identical to that for Gd-POMs, but we used 0.2 g (0.54 mmol) of PrCl3·7H2O as the Ln reagent. A crystalline phase was obtained after one week. Yield: ca. 35% (based on K7[PW11O39]·14H2O).
Synthesis of Cat4Eu(PW11O39)·xH2O: The synthetic procedure was identical to that for Gd-POMs, but we used 0.2 g (0.55 mmol) of EuCl3·6H2O as the Ln reagent. A crystalline phase was obtained after one week. Yield: ca. 51% (based on K7[PW11O39]·14H2O).
Synthesis of Cat4Tb(PW11O39)·xH2O: The synthetic procedure was identical to that for Gd-POMs, but we used 0.2 g (0.54 mmol) of TbCl3·6H2O as the Ln reagent. A crystalline phase was obtained after one week. Yield: ca. 24% (based on K7[PW11O39]·14H2O).
Synthesis of Cat4Dy(PW11O39)·xH2O: The synthetic procedure was identical to that for Gd-POMs, but we used 0.2 g (0.53 mmol) of DyCl3·6H2O as the Ln reagent. A crystalline phase was obtained after one week. Yield: ca. 27% (based on K7[PW11O39]·14H2O).
3.3. X-Ray Diffraction on Single Crystals
Single-crystal XRD data for the compounds were collected at 150 K (Table S1) with a Bruker D8 Venture diffractometer with a CMOS PHOTON III detector (Bruker, Madison, Wisconsin, USA) and IμS 3.0 microfocus source (MoKα radiation (λ = 0.71073 Å), collimating Montel mirrors; Incoatec GmbH, Geesthacht, Germany). The crystal structures were solved using the SHELXT [67] and were refined using the SHELXL [68] programs with OLEX2 GUI [69]. Atomic displacement parameters for non-hydrogen atoms were refined in anisotropic approximation with the exception for the disordered hydrate molecules and Me4N+. For the latter, EADP constraints and SADI restraints were applied where needed. Hydrogen atoms were placed geometrically and refined in the riding model with the exception for those of the disordered hydrate molecules, which were not localized. The structures of 1–3Gd were deposited to the Cambridge Crystallographic Data Centre (CCDC) as a supplementary publication, No. 2270528-2270530.
4. Conclusions
A series of 1:1 Ln3+/POM (Ln = Pr, Eu, Gd, Tb, Dy) complexes of the general formula Cat4Ln(PW11O39)·xH2O (Cat = K+ or Me4N+) was synthesized in an aqueous solution. It was shown that the {Ln(PW11O39)} fragments are always involved in binding with each other to form either polymer chains or dimeric units, and the Ln3+ coordination number is the same in all cases and equals 8. It was also found that for the isostructural compounds, the unit cell parameters mainly correlate with the corresponding ionic radius of the lanthanides. The description of these compounds is important for the study of other monovacant POMs of the Keggin type in an aqueous solution and should be taken into account when developing {Ln-POM} hybrid complexes.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics11080327/s1, Figure S1: IR spectra of Cat4Ln(PW11O39)·xH2O complexes (Ln from the top to the bottom): Gd, Eu, Pr, Tb and Dy; Table S1. Crystal data and structure refinement for 1–3Gd.
Author Contributions
Conceptualization, M.N.S.; methodology, V.S.K. and M.N.S.; analysis, V.S.K. and T.S.S.; investigation, V.S.K. and T.S.S.; writing—original draft preparation, V.S.K. and T.S.S.; writing—review and editing, V.S.K. and M.N.S.; visualization, V.S.K. and T.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation, № 121031700313-8 (The Nikolaev Institute of Inorganic Chemistry SB RAS).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the NIIC SB RAS team for the access to IR and SCXRD facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Pope, M.T.; Müller, A. Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar] [CrossRef]
- Yamase, T.; Pope, M.T. Polyoxometalates Chemistry for Nano-Composite Design; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaita-Arino, A.; Coronado, E.; Loss, D. Quantum computing with molecular spin systems. J. Mater. Chem. 2009, 19, 1672–1677. [Google Scholar] [CrossRef]
- Charron, G.; Giusti, A.; Mazerat, S.; Mialane, P.; Gloter, A.; Miserque, F.; Keita, B.; Nadjo, L.; Filoramo, A.; Rivière, E.; et al. Assembly of a magnetic polyoxometalate on SWNTs. Nanoscale 2010, 2, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Newton, G.N.; Yamashita, S.; Hasumi, K.; Matsuno, J.; Yoshida, N.; Nihei, M.; Shiga, T.; Nakano, M.; Nojiri, H.; Wernsdorfer, W.; et al. Redox-Controlled Magnetic {Mn13} Keggin Systems. Angew. Chem. Int. Ed. 2011, 50, 5715–5720. [Google Scholar] [CrossRef]
- AlDamen, M.A.; Cardona-Serra, S.; Clemente-Juan, J.; Coronado, E.; Gaita-Arino, A.; Marti-Gastaldo, C.; Luis, F.; Montero, O. Mononuclear Lanthanide Single Molecule Magnets Based on the Polyoxometalates [Ln(W5O18)2]9− and [Ln(β2-SiW11O39)2]13− (LnIII = Tb, Dy, Ho, Er, Tm, and Yb). Inorg. Chem. 2009, 48, 3467–3479. [Google Scholar] [CrossRef]
- Ritchie, C.; Ferguson, A.; Nojiri, H.; Miras, H.N.; Song, Y.-F.; Long, D.-L.; Burkholder, E.; Murrie, M.; Kögerler, P.; Brechin, E.K.; et al. Polyoxometalate-Mediated Self-Assembly of Single-Molecule Magnets: {[XW9O34]2[MnIII4MnII2O4(H2O)4]}12−. Angew. Chem. Int. Ed. 2008, 47, 5609–5612. [Google Scholar] [CrossRef]
- AlDamen, M.A.; Clemente-Juan, J.M.; Coronado, E.; Marti-Gastaldo, C.; Gaita-Ariño, A. Mononuclear Lanthanide Single-Molecule Magnets Based on Polyoxometalates. J. Am. Chem. Soc. 2008, 130, 8874–8875. [Google Scholar] [CrossRef]
- Compain, J.D.; Mialane, P.; Dolbecq, A.; Mbomekallé, I.M.; Marrot, J.; Sécheresse, F.; Rivière, E.; Rogez, G.; Wernsdorfer, W. Iron Polyoxometalate Single-Molecule Magnets. Angew. Chem. Int. Ed. 2009, 48, 3077–3081. [Google Scholar] [CrossRef]
- Weiner, H.; Finke, R.G. An All-Inorganic, Polyoxometalate-Based Catechol Dioxygenase That Exhibits >100 000 Catalytic Turnovers. J. Am. Chem. Soc. 1999, 121, 9831–9842. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Timofeeva, M.N.; Melgunov, M.S.; Shmakov, A.N.; Chesalov, Y.A.; Dybtsev, D.N.; Fedin, V.P.; Kholdeeva, O.A. Heterogeneous selective oxidation catalysts based on coordination polymer MIL-101 and transition metal-substituted polyoxometalates. J. Catal. 2008, 257, 315–323. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Yamaguchi, K.; Mizuno, N. Zinc(II) Containing γ-Keggin Sandwich-Type Silicotungstate: Synthesis in Organic Media and Oxidation Catalysis. Angew. Chem. Int. Ed. 2010, 49, 6096–6100. [Google Scholar] [CrossRef]
- Okuhara, T.; Mizuno, N.; Misono, M. Catalysis by heteropoly compounds—Recent developments. Appl. Catal. A 2001, 222, 63–77. [Google Scholar] [CrossRef]
- Zhang, T.R.; Liu, S.Q.; Kurth, D.G.; Faul, C.F.J. Organized Nanostructured Complexes of Polyoxometalates and Surfactants that Exhibit Photoluminescence and Electrochromism. Adv. Funct. Mater. 2009, 19, 642–652. [Google Scholar] [CrossRef]
- Cooper, G.J.T.; Cronin, L. Real-Time Direction Control of Self Fabricating Polyoxometalate-Based Microtubes. J. Am. Chem. Soc. 2009, 131, 8368–8369. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Wang, H.; Nakanishi, R.; Hamanaka, S.; Kitaura, R.; Shinohara, H.; Yokoyama, T.; Yoshikawa, H.; Awaga, K. Nanohybridization of Polyoxometalate Clusters and Single-Wall Carbon Nanotubes: Applications in Molecular Cluster Batteries. Angew. Chem. Int. Ed. 2011, 50, 3471–3474. [Google Scholar] [CrossRef] [PubMed]
- Geisberger, G.; Paulus, S.; Carraro, M.; Bonchio, M.; Patzke, G.R. Synthesis, Characterisation and Cytotoxicity of Polyoxometalate/Carboxymethyl Chitosan Nanocomposites. Chem.–Eur. J. 2011, 17, 4619–4625. [Google Scholar] [CrossRef]
- Wall, M.J.; Wigmore, G.; Lopatar, J.; Frenguelli, B.G.; Dale, N. The novel NTPDase inhibitor sodium polyoxotungstate (POM-1) inhibits ATP breakdown but also blocks central synaptic transmission, an action independent of NTPDase inhibition. Neuropharmacology 2008, 55, 1251–1258. [Google Scholar] [CrossRef]
- Musumeci, C.; Luzio, A.; Pradeep, C.P.; Miras, H.N.; Rosnes, M.H.; Song, Y.F.; Long, D.L.; Cronin, L.; Pignataro, B. Programmable Surface Architectures Derived from Hybrid Polyoxometalate-Based Clusters. J. Phys. Chem. C 2011, 115, 4446–4455. [Google Scholar] [CrossRef]
- Grzhegorzhevskii, K.V.; Shevtsev, N.S.; Abushaeva, A.R.; Chezganov, D.S.; Ostroushko, A.A. Prerequisites and prospects for the development of novel systems based on the Keplerate type polyoxomolybdates for the controlled release of drugs and fluorescent molecules. Russ. Chem. Bull. 2020, 69, 804–814. [Google Scholar] [CrossRef]
- Cavaleiro, A.M.V.; Pedrosa de Jesus, J.D.; Noguera, H.I.S. Metal Clusters in Chemistry; Wiley-VCH: London, UK, 1999. [Google Scholar] [CrossRef]
- Yamase, T. Photo- and Electrochromism of Polyoxometalates and Related Materials. Chem. Rev. 1998, 98, 307–326. [Google Scholar] [CrossRef]
- Sadakane, M.; Dickman, M.H.; Pope, M.T. Controlled Assembly of Polyoxometalate Chains from Lacunary Building Blocks and Lanthanide-Cation Linkers. Angew. Chem. Int. Ed. 2000, 39, 2914–2916. [Google Scholar] [CrossRef]
- Mialane, P.; Lisnard, L.; Mallard, A.; Marrot, J.; Antic-Fidancev, E.; Aschehoug, P.; Vivien, D.; Secheresse, F. Solid-State and Solution Studies of {Lnn(SiW11O39)} Polyoxoanions: An Example of Building Block Condensation Dependent on the Nature of the Rare Earth. Inorg. Chem. 2003, 42, 2102–2108. [Google Scholar] [CrossRef] [PubMed]
- Muller, A.; Peters, F.; Pope, M.T.; Gatteschi, D. Polyoxometalates: Very Large Clusters—Nanoscale Magnets. Chem. Rev. 1998, 98, 239–271. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, K.; Dickman, M.H.; Pope, M.T. Self-Assembly of Supramolecular Polyoxometalates: The Compact, Water-Soluble Heteropolytungstate Anion [AsIII12CeIII16(H2O)36W148O524]76−. Angew. Chem. Int. Ed. Engl. 1997, 36, 1445–1448. [Google Scholar] [CrossRef]
- Belai, N.; Sadakane, M.; Pope, M.T. Formation of Unsymmetrical Polyoxotungstates via Transfer of Polyoxometalate Building Blocks. NMR Evidence Supports the Kinetic Stability of the Pentatungstate Anion, [W5O18]6–, in Aqueous Solution. J. Am. Chem. Soc. 2001, 123, 2087–2088. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhi, M.; Chen, H.; Singh, V.; Ma, P.; Wang, J.; Niu, J. Well-tuned white-light-emitting behaviours in multicenter-Ln polyoxometalate derivatives: A photoluminescence property and energy transfer pathway study. Spectrochim. Acta—A Mol. Biomol. Spectrosc. 2019, 223, 117294. [Google Scholar] [CrossRef]
- Wang, K.; Feng, S.; Ma, P. Synthesis, characterization and photoluminescence properties of a benzoic modified lanthanide-containing polyoxometalate. Inorg. Chem. Commun. 2019, 108, 107511. [Google Scholar] [CrossRef]
- Wu, H.; Yan, B.; Liang, R.; Singh, V.; Ma, P.; Wang, J.; Niu, J. An organic chromophore—Modified samarium-containing polyoxometalate: Excitation-dependent color tunable behavior from the organic chromophores to the lanthanide ion. Dalton Trans. 2020, 49, 388–394. [Google Scholar] [CrossRef]
- Wang, K.; Feng, S.; Ma, P. Synthesis, characterization and photoluminescence properties of an organic–inorganic hybrid monolacunary Keggin-type polyoxotungstate. Inorg. Chem. Commun. 2021, 129, 108621. [Google Scholar] [CrossRef]
- Sarwar, S.; Sanz, S.; van Leusen, J.; Nichol, G.S.; Brechin, E.K.; Kögerler, P. Phthalocyanine-polyoxotungstate lanthanide double deckers. Dalton Trans. 2020, 49, 16638–16642. [Google Scholar] [CrossRef]
- Niu, J.; Wang, K.; Chen, H.; Zhao, J.; Ma, P.; Wang, J.; Li, M.; Bai, Y.; Dang, D. Assembly Chemistry between Lanthanide Cations and Monovacant Keggin Polyoxotungstates: Two Types of Lanthanide Substituted Phosphotungstates [{(α-PW11O39H)Ln(H2O)3}2]6− and [{(α-PW11O39)Ln(H2O)(η2,μ-1,1)-CH3COO}2]10−. Cryst. Growth Des. 2009, 9, 4362–4372. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, C.; Chen, H.; Ma, P.; Wang, J.; Niu, J. Syntheses, structures and properties of dimeric rare earth derivatives based on monovacant Keggin-type polyoxotungstates. Inorg. Chim. Acta 2012, 391, 218–223. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Zhao, J.-W.; Yang, B.-F.; He, H.; Yang, G.-Y. Organic–inorganic hybrids based on monovacant Keggin-type polyoxotungstates and 3d–4f heterometals. CrystEngComm 2013, 15, 8186–8194. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Zhao, J.-W.; Yang, B.-F.; Wei, Q.; Yang, G.-Y. Two Organic–Inorganic Hybrids Assembled by Carboxylate-Bridging Lanthanide-Substituted Polyoxometalate Dimers with Copper–ethylendiamine Cations. J. Clust. Sci. 2014, 25, 667–680. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zhao, J.; Ma, P.; Wang, J.; Niu, J. Two types of oxalate-bridging rare-earth-substituted Keggin-type phosphotungstates {[(α-PW11O39)RE(H2O)]2(C2O4)}10− and {(α-x-PW10O38)RE2(C2O4)(H2O)2}3−. Dalton Trans. 2012, 41, 3764–3772. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Hu, F.; Wan, R.; Huo, Y.; Zhang, D.; Niu, J.; Wang, J. Magnetic double-tartaric bridging mono-lanthanide substituted phosphotungstates with photochromic and switchable luminescence properties. J. Mater. Chem. C 2016, 4, 5424–5433. [Google Scholar] [CrossRef]
- Wu, H.; Zhi, M.; Singh, V.; Li, H.; Ma, P.; Niu, J.; Wang, J. Elucidating white light emissions in Tm3+/Dy3+ codoped polyoxometalates: A color tuning and energy transfer mechanism study. Dalton Trans. 2018, 47, 13949–13956. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, D.; Ma, J.; Ma, P.; Niu, J.; Wang, J. Three-dimensional lanthanide polyoxometalate organic complexes: Correlation of structure with properties. CrystEngComm 2012, 14, 3205–3212. [Google Scholar] [CrossRef]
- Peacock, R.D.; Weakley, T.J.R. Heteropolytungstate complexes of the lanthanide elements. Part I. Preparation and reactions. J. Chem. Soc. A 1971, 1836–1839. [Google Scholar] [CrossRef]
- Zhang, C.; Howell, R.C.; Scotland, K.B.; Perez, F.G.; Todaro, L.; Francesconi, L.C. Aqueous Speciation Studies of Europium(III) Phosphotungstate. Inorg. Chem. 2004, 43, 7691–7701. [Google Scholar] [CrossRef]
- Fan, L.; Xu, L.; Gao, G.; Li, F.; Li, Z.; Qiu, Y. A novel polyoxometalate chain constructed from sandwich lanthanide-containing polyanion [Ce(PW11O39)2]10− and sodium ion linker. Inorg. Chem. Commun. 2006, 9, 1308–1311. [Google Scholar] [CrossRef]
- Fan, L.-H.; Xu, L.; Zhang, C.-H.; Li, F.-Y.; Li, Z.-K.; Liu, X.-Z. A novel polyoxometalate chain constructed from sandwich lanthanide-containing polyanions [Pr(PW11O39)2]11− and sodium cation linkers. Struct. Chem. 2007, 18, 917–921. [Google Scholar] [CrossRef]
- Iijima, J.; Ishikawa, E.; Nakamura, Y.; Naruke, H. Synthesis and structural investigation of sandwich polyoxotungstates containing cerium (III/IV) and mono-lacunary Keggin tungstophosphate units. Inorg. Chim. Acta 2010, 363, 1500–1506. [Google Scholar] [CrossRef]
- Iijima, J.; Naruke, H. Structural characterization of Keggin sandwich-type [LnIII(α-PW11O39)2]11− (Ln = La and Ce) anion containing a pseudo-cubic LnIIIO8 center. Inorg. Chim. Acta 2011, 379, 95–99. [Google Scholar] [CrossRef]
- Gupta, R.; Saini, M.K.; Doungmene, F.; de Oliveira, P.; Hussain, F. Lanthanoid containing phosphotungstates: The syntheses, crystal structure, electrochemistry, photoluminescence and magnetic properties. Dalton Trans. 2014, 43, 8290–8299. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.-R.; Lu, X.-N.; Liao, J.-S.; Zhang, C.-W.; You, H.-Y.; Liu, C.-M. Preparation and luminescence properties of phosphors of rare earth complexes based on polyoxotungstates. Mater. Res. Bull. 2015, 68, 16–21. [Google Scholar] [CrossRef]
- Ma, P.; Hu, F.; Huo, Y.; Zhang, D.; Zhang, C.; Niu, J.; Wang, J. Magnetoluminescent Bifunctional Dysprosium-Based Phosphotungstates with Synthesis and Correlations between Structures and Properties. Cryst. Growth Des. 2017, 17, 1947–1956. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.-D.; Zhao, D.; Sun, Y.-Q.; Zheng, S.-T. A Series of Unprecedented Linear Mixed-Metal-Substituted Polyoxometalate Trimers: Syntheses, Structures, Luminescence, and Proton Conductivity Properties. Eur. J. Inorg. Chem. 2019, 3–4, 437–441. [Google Scholar] [CrossRef]
- Naruke, H.; Iijima, J.; Sanji, T. Enantioselective Resolutions and Circular Dichroism Studies of Lanthanide-Containing Keggin-Type [Ln(PW11O39)2]11– Polyoxometalates. Inorg. Chem. 2011, 50, 7535–7539. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Zhang, J.; Zhao, J.; Chen, L. Synthesis, structure, spectroscopic and ferroelectric properties of an acentric polyoxotungstate containing 1:2-type [Sm(α-PW11O39)2]11− fragment and d-proline components. Spectrochim. Acta—A Mol. Biomol. Spectrosc. 2015, 134, 101–108. [Google Scholar] [CrossRef]
- Iijima, J.; Naruke, H.; Sanji, T. On chirality induction in the crystalline solid-containing sandwich-type [Ln(α-PW11O39)2]11− polyoxotungstate and proline. RSC Adv. 2016, 6, 91494–91507. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Guo, G.-L.; Wang, J.-P. Hydrothermal synthesis and crystal structure of a heteropolyoxotungstate formed by sandwich-type heteropolyanion [Gd(PW11O39)2]11− and reduced [Cu(en)2]+ cations. J. Coord. Chem. 2008, 61, 2428–2436. [Google Scholar] [CrossRef]
- Du, D.-Y.; Qin, J.-S.; Li, S.-L.; Wang, X.-L.; Yang, G.-S.; Li, Y.-G.; Shao, K.-Z.; Su, Z.-M. A series of inorganic–organic hybrid compounds constructed from bis(undecatungstophosphate) lanthanates and copper-organic units. Inorg. Chim. Acta 2010, 363, 3823–3831. [Google Scholar] [CrossRef]
- Du, D.-Y.; Qin, J.-S.; Yuan, G.; Lan, Y.-Q.; Wang, X.-L.; Shao, K.-Z.; Su, Z.-M. Building block approach to a series of substituted Keggin-type inorganic–organic hybrids. Solid State Sci. 2011, 13, 1115–1121. [Google Scholar] [CrossRef]
- Chen, L.; Shi, D.; Wang, Y.; Cheng, H.; Geng, Z.; Zhao, J.; Ma, P.; Niu, J. Two 3d–4f heterometallic monovacant Keggin phosphotungstate derivatives. J. Coord. Chem. 2011, 64, 400–412. [Google Scholar] [CrossRef]
- Shi, D.; Chen, L.; Zhao, J.; Wang, Y.; Ma, P.; Niu, J. Two novel 2D organic–inorganic hybrid lacunary Keggin phosphotungstate 3d–4f heterometallic derivatives: [Cu(en)2]2H6[Ce(α-PW11O39)2]·8H2O and [Cu(dap)2(H2O)][Cu(dap)2]4.5[Dy(α-PW11O39)2]·4H2O. Inorg. Chem. Commun. 2011, 14, 324–329. [Google Scholar] [CrossRef]
- Shi, D.; Wang, Z.; Xing, J.; Li, Y.; Luo, J.; Chen, L.; Zhao, J. A 2-D Organic–Inorganic Hybrid Copper-Yttrium Heterometallic Monovacant Keggin Phosphotungstate Derivative: [Cu(dap)2]5.5[Y(α-PW11O39)2]·4H2O. Synth. React. Inorg. Metalorg. Nanometal. Chem. 2012, 42, 30–36. [Google Scholar] [CrossRef]
- Li, Y.; Tian, S.; Li, Y.-Z.; Zhao, J.; Ma, P.; Chen, L. Two 2D Cu–Ln heterometallic polyoxometalate aggregates constructed from bis(undecatungstophosphate)lanthanate units and copper-complex bridges. Inorg. Chim. Acta 2013, 405, 105–110. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Shi, D.; Zhao, J.; Chen, L. Synthesis, Structure, and Properties of a 2-D Organic–Inorganic Hybrid Phosphotungstate-Based CuII–LaIII Heterometallic Derivative. Synth. React. Inorg. Metalorg. Nanometal. Chem. 2014, 44, 171–176. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Yang, B.-F.; Yang, G.-Y. Two new 2D organic–inorganic hybrids assembled by lanthanide-substituted polyoxotungstate dimers and copper–complex linkers. Inorg. Chem. Commun. 2017, 84, 212–216. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, P.; Chen, H.; Wang, J.; Niu, J. Synthesis, structure, and properties of a 1-D cerium based on monovacant Keggin-type polyoxotungstate. J. Coord. Chem. 2011, 64, 2178–2185. [Google Scholar] [CrossRef]
- Ma, P.; Si, Y.; Wan, R.; Zhang, S.; Wang, J.; Niu, J. Synthesis, crystal structure, and properties of a 1-D terbium-substituted monolacunary Keggin-type polyoxotungstate . Spectrochim. Acta—A Mol. Biomol. Spectrosc. 2015, 138, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Contant, R. Relations entre les tungstophosphates apparentés à l’anion PW12O403−. Synthèse et propriétés d’un nouveau polyoxotungstophosphate lacunaire K10P2W20O70·24H2O. Can. J. Chem. 1987, 65, 568–573. [Google Scholar] [CrossRef]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).