Abstract
This research provides a sustainable way to treat water by removing heavy metal hazards (mercury ion) and biological pollutants (several strains of bacteria and fungi) through the eco-friendly synthesis of silver nanoparticles using the ethanol extract of the Saudi Haloxylon ammodendron shrub, which is planted in the Qassim desert. Further, this work confirms that these nanoparticles could be used as anticancer materials. The optimization factors of the biosynthesis of silver nanoparticles were studied and obtained (volume ratio = 1:2, pH = 7.5, and temperature = 60 °C). The scanning electron microscope micrographs showed the spherical shape and the huge numbers of silver nanoparticles accumulated, while X-ray diffraction measurements gave the crystal size of these nanoparticles in the range of 10.64 nm. The application findings of these biofabricated silver nanoparticles demonstrated effective detection and removal of different concentrations of mercury ions (0–2500 ppm) from the polluted aqueous solutions. The work revealed that Haloxylon ammodendron extract enhanced the antibacterial and antifungal activities of silver nanoparticles against different strains of bacteria and fungi. As well, the anticancer activity examinations of these nanoparticles and the extract showed good and reasonable results.
1. Introduction
With the industrial and economic development in the Kingdom of Saudi Arabia and climatic changes, the percentage of environmental pollution in the country is increasing [1,2,3]. By the way, this is in line with the 2030 vision of the Kingdom of Saudi Arabia towards a clean environment free of pollutants [4]. Universities devote all of their scientific and research resources to achieving this vision by utilizing cutting-edge, inexpensive scientific methods [5,6]. We will talk here briefly about the most important pollutants and then the techniques used to remove them.
The detrimental effects of heavy metals and their numerous harms, such as cancer, are recognized as one of the most crucial topics that must be addressed when discussing environmental contaminants linked to industrial growth [7,8,9].
Mercury is among the heavy chemical elements and may be found in nature in a variety of forms and is employed in a variety of industries. Despite this, the need to reduce its uses and innovate methods to detect it, as well as ways to get rid of it, has become more of an interest to researchers in the field of the environment [10,11,12].
Research has shown that many traditional techniques can be used in the treatment and disposal of mercury, for example, membrane filtration, soil utilization, sedimentation, adsorption, the electrocoagulation process, etc., but most of these techniques are time-consuming and financially costly and therefore useless [13,14,15].
On the other hand, climate change and the growth of epidemics and diseases are also a result of biological pollution, which is another negative side effect of industrial development. This particularly occurs when drinking water is contaminated with different microorganisms [16,17,18].
Recently, nanotechnology has become one of the most common and important modern methods for dealing with different kinds of pollution, such as heavy metals or microbial contamination [19,20]. Researchers have become interested in this technology because of the special and superior characteristics of nanomaterials in the field of pollution treatment in terms of cost, simplicity of preparation, controllability, and other relevant aspects [21,22,23,24].
Recently, research articles have been concerned with one of the most interesting global issues: wastewater treatment using different techniques [25,26]. In this regard, many modern scientific studies have shown the high efficiency of silver nanoparticles in the simple colorimetric detection of mercury ions and explained the mechanism of removing this ion from drinking water in an easy way [27,28].
By the way, there are also many scientific articles that have proven the ability of silver nanoparticles to get rid of microbes that cause diseases and pollution, depending on the characteristics of these nanoparticles, such as the size and quality of the catalysts used in the preparation, the pH of the reaction medium, etc. [29,30].
The effectiveness of nanoparticles in treating microbial infections has been demonstrated by the large number of recent scientific studies that have concentrated on employing nanoparticles to target microbes instead of antibiotics. This prompted scientists to develop several preparation methods to improve the biological activity of these nanoparticles as anticancer materials. The extraordinary properties of silver nanoparticles in various applications have piqued researchers’ interest in these sorts of nanoparticles for their potential antimicrobial and anticancer properties [30,31,32,33].
This research work presented the bio-manufacturing of silver nanoparticles using an ethanolic extract of Haloxylon ammodendron shrub under optimum conditions such as the ratio of the reactants, pH, and the temperature of the preparation. Furthermore, this study demonstrated the promising results of the applications of these nanoparticles in detecting mercury ions to mitigate their harmful effects on the environment, in addition to their ability to act as an alternative to antimicrobial and anticancer drugs.
The Haloxylon ammodendron plant is a big sandy shrub in the Qassim desert in Saudi Arabia, and its scientific name is known as a C4 plant because it uses the C4 carbonization pathway to enhance photosynthetic rate by limiting or decreasing photorespiration [34,35]. The Haloxylon ammodendron (HM) shrub has a variety of uses, such as halting sand creep and lowering carbon emissions, pollution, soil contamination, etc. [36,37].
The common chemical ingredients in Haloxylon ammodendron shrub were discovered and reported [38,39,40]. Scheme 1 illustrates the main chemical compounds of HM shrub.
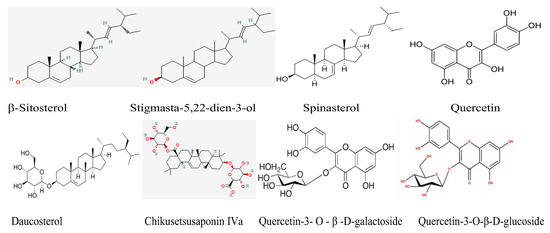
Scheme 1.
The main chemical compounds of the HM shrub.
2. Results and Discussion
2.1. Mechanism of Formation of Silver Nanoparticles
When talking about the mechanism of the formation of silver nanoparticles, the two correlated and important processes here are the oxidation and reduction between the silver nitrate chemical and the biological solution (HME) [41]. The proper conditions for the reaction, such as the pH, the volume ratio of the reactants, and the reaction temperature, must be provided for the synthesis of silver nanoparticles [42]. These factors affect the size and shape of the nanoparticles formed [43]. At the beginning of the reaction, the ions of the chemical silver nitrate dissociate into the positive silver ion and the negative nitrate ion as a first step [44]. Then, the process of reducing Ag+ to a neutral silver atom, Ag0, through the use of an ethanolic extract from the leaves [45].
In this step, the biologically active compounds (such as quercetin, which contains hydroxyl groups (-OH)) bond to the silver atom and form the nuclei [46]. Then, the particles accumulate on the nuclei due to the electrostatic attraction between them [47]. Temperature increases the reaction kinetics of the ions in the reaction medium and greatly accelerates the reduction process, resulting in the formation of Ag2O as a reaction product [48]. When the Ag+ group reacts with the negative ions OH, Ag-OH is formed, which is extremely unstable and easily oxidizes to Ag2O when dried at 50 °C.
At the end of the reaction, Ag-NPs and Ag2O-NPs may be formed in varying proportions, depending on the factors and conditions accompanying the reaction [49,50].
2.2. UV-Spectroscopy Analysis
In order to control the formation and stability of silver nanoparticles using the HME, the UV-Vis absorption spectra of the biosynthesized silver nanoparticles were recorded and analyzed. The UV-Vis spectra state the formation of silver nanoparticles as well as the effects of various factors (volume ratio, pH, and temperature) on this formation [51]. The formation of silver nanoparticles was observed by changing the solution color from light yellow to yellowish-brown to deep brown as the factors changed, which was caused by the vibrational excitation of surface plasmon in the silver nanoparticles [52]. A UV-Vis spectrometer detected the surface plasmon resonance (SPR) of Ag-NPs at 413–418 nm. For the biosynthesis of silver nanoparticles, this wavelength value of maximum absorption is consistent with the values measured in previous studies [53]. It depends on the concentrations of silver salt and the functional groups in the plant used in the reaction [54,55,56].
2.2.1. The Reactant Volume Ratio
When compared to the pure extract, Figure 1 shows the confirmation of the formation of silver nanoparticles. The formation of Ag-NPs was monitored in UV-Vis absorption spectra after different volumes of AgNO3, ranging from 20 mL to 80 mL, were added to a 20 mL HME. The absorbance peaks were shifted to a red wavelength and ranged from 414 nm to 418 nm. This indicates that the mean diameter of Ag-NPs has increased [57,58,59]. The sample V3, which had a volume ratio of 20 mL to 40 mL of reactants, had the highest absorption. This was the optimal volume ratio for the biosynthesis of Ag-NPs that was determined.
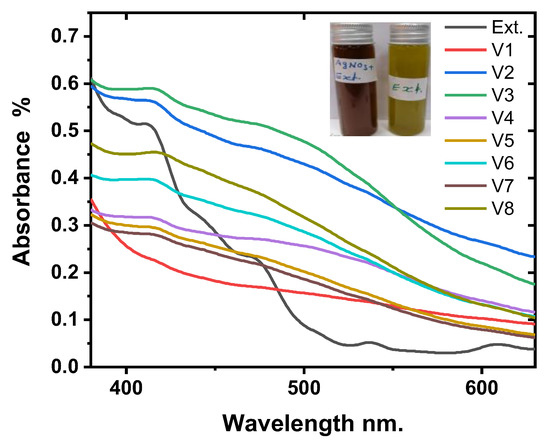
Figure 1.
The UV-Vis absorption spectra of Ag-NPs at different volume ratios.
2.2.2. The pH Factor
Figure 2 demonstrates the effect of pH on the formation of Ag-NPs. It is clear that when changing pH from 3 to 10, the UV-Vis absorbance peak increases and then decreases. The best absorption is for sample p3, pH = 7.5, and this means the probability of silver nanoparticle formation increases in the neutral reaction medium. As well as the absorption peak, which is related to the size and shape of the formed Ag-NPs, the role of the pH factor is to increase and alter the electrical charges of biomolecules, which in turn affect the capping, stability, and growth of Ag-NPs [56,60]. This study demonstrated that the prediction of Ag-NPs formation in the basic bio-reaction is greater than that in the acidic bio-reaction.
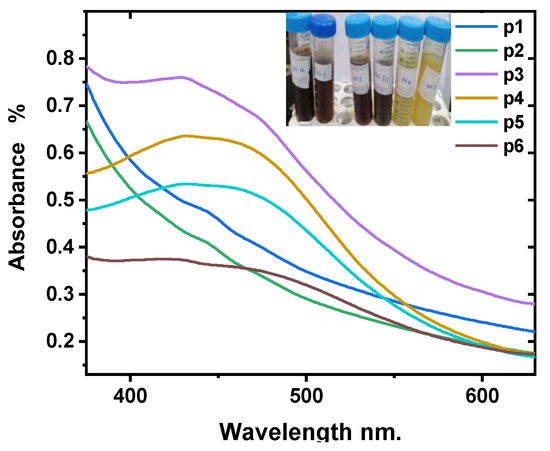
Figure 2.
UV-Vis absorption spectra of Ag-NPs at various pH levels.
2.2.3. The Reaction Temperature
From Figure 3, it is shown that the highest temperature has the highest absorbance. This set of experiments proposes that the high reaction temperature of Ag-NPs biosynthesis will accelerate the formation and growth of Ag-NPs more than at room temperature. This can be explained by the acceleration of the Ag+ reduction rate and then the regular nucleation of silver seed [32,61,62]. The maximum absorbance at high temperatures in sample T3 indicates that the Ag-NPs are smaller due to their formation at shorter wavelengths than at low temperatures.
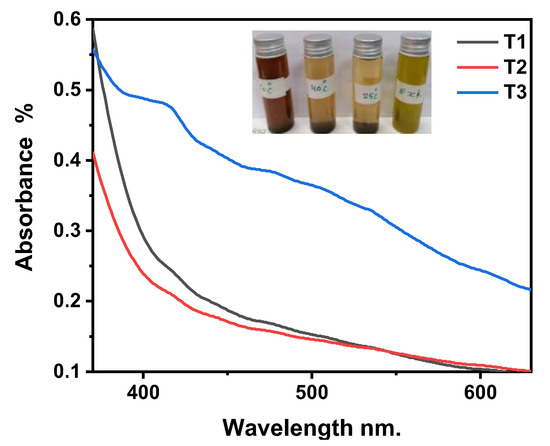
Figure 3.
The UV-Vis absorption spectra of Ag-NPs at different temperatures.
2.3. X-ray Diffraction Patters Analysis
X-ray diffraction is a good and effective technique for verifying the identity of the expected elements present in the prepared nanoparticle samples [63,64]. Figure 4 shows the XRD pattern of the prepared sample. The positions of the peaks indicate the arrangement of the elements’ atoms inside the samples [65]. By comparing these positions of peaks with standard XRD cards, it is shown that there are two phases of crystallinity, Ag and Ag2O, respectively. The peaks at 28.17°, 32.62°, 46.57°, 55.25°, and 57.76° correspond to the planes (100), (110), (200), (220), and (211) as standard data for Ag2O (JCPDS No. 76-1393). The other peaks at 38.53° and 77.27° correspond to the planes (111) and (311) as standard data for Ag (JCPDS No. 04-0783) [66,67,68]. The unit cell of the Ag2O nanoparticles, which are the dominant particles, is cubic (a = 0.476 nm).
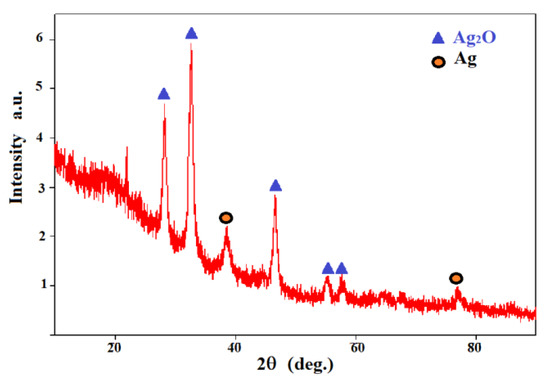
Figure 4.
XRD pattern of silver nanoparticles.
The crystal size of Ag2O nanoparticles was computed using the Scherrer formula [69]:
where k is a constant (~0.94), λ is the wavelength of the diffracted X-ray, β is the width of half of the maximum diffracted X-ray intensity, and θ is the angle of the diffracted X-ray. The crystal size of Ag2O nanoparticles at (110) was 10.64 nm.
D = kλ/βcos θ
2.4. SEM Analysis
In Figure 5, the SEM micrograph illustrates the silver nanoparticles with their white color and small spherical particles. It was also clear from the SEM image that the capping agent provided stabilization for silver nanoparticles, caused them to spread out within the aggregates, and prevented them from gathering [70]. The size of these particles was calculated using ImageJ software, and their size distribution is shown in Figure 5b, with an average particle size of 10.97 nm. This value of average particle size is approximately similar to the value of crystal size of these silver nanoparticles from XRD analysis.
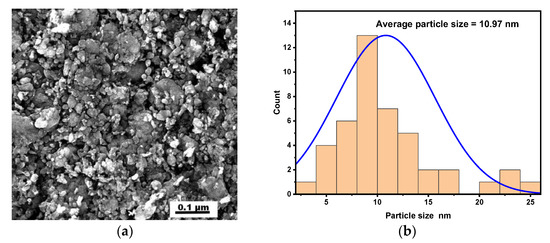
Figure 5.
(a,b) SEM image and size distribution of the silver nanoparticles.
2.5. FTIR Analysis
Infrared is a useful method for detecting the structural features of unknown substances by identifying the functional groups that absorb the IR energy. In Figure 6, the FT-IR spectra of the pure HME extract and the resultant silver nanoparticle solution are shown. It appears several bands, which represent various functional groups, exist in the chemical compounds available in the HME extract and the solution of silver nanoparticles. The main observation in the functional part of IR is that the broadband absorption of O-H at 3225 cm−1 in the IR spectra of HME extract became less peaking and broad and was shifted to 3280 cm−1 for the silver nanoparticles’ solution. This indicates a reduction in the intense intermolecular hydrogen bonds used in the reduction process to form silver nanoparticles. Furthermore, the difference in mass between the biological extract (HME) molecules and the resultant silver nanoparticles in the O-H absorption bands caused that shift in the wavenumber between them [71,72].
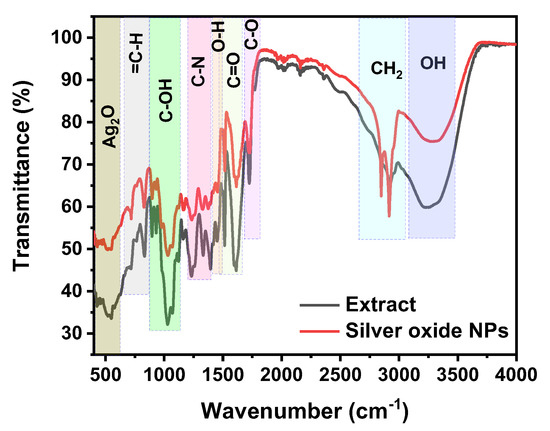
Figure 6.
FTIR measurements of HME and silver oxide nanoparticles.
On the other hand, the increased peak intensity in the IR spectrum of the resultant silver nanoparticles’ solution in the C-H absorption band is greater than that in the HME solution, which asserts that these functional groups also contributed to the reduction process [73].
In the fingerprint part of the IR spectrum, the peak assignment at 520 cm−1 is attributed to Ag-O stretch vibration groups, and this value is in good agreement with reported research [71,74]. The other absorption peaks that correspond to the functional groups that are available in the HME solution and contributed to the capping of the silver and silver oxide nanoparticles are shown in Table 1.

Table 1.
The functional groups’ absorption band identification for the HME extract and the silver/silver oxide nanoparticles’ solution.
2.6. Mercury Ions (Hg2+) Sensing Analysis
By looking at all the chemical compounds in the HME, it is clear that all the compounds are polyunsaturated rings with a few unsaturated bonds. These compounds do not contain fluorescent components, with the exception of quercetin compounds.
From Figure 7, the HME solution showed an emission spectrum with a peak at 493 nm and an absorption spectrum with peaks at 243 and 277 nm. These peaks are due to spin-allowed S0 → S2 electronic transitions. Two additional but lower intensity peaks are also observed at 329 nm and 416 nm for the absorption spectra, which indicate an S0 → S1 type electron transition that is spin-allowed [86]. A high Stokes shift between the absorption and emission spectra of about 216 nm is observed for the product solution (HME and Ag nanoparticles), which indicates a large increase in the dipole moment and an energy transfer through non-radiative processes, as shown in Figure 7. The spectra show that there are a variety of functional groups that can bind to metallic elements to create nanoparticles, as undertaken in this study [87,88,89,90].
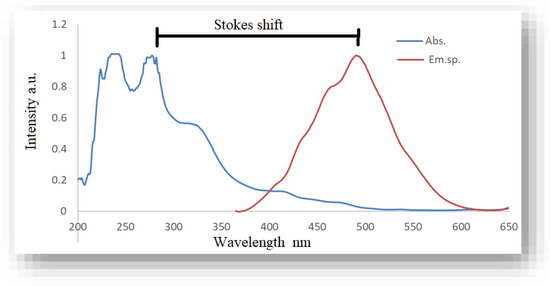
Figure 7.
The absorption and emission spectra of the HME.
It was found to be a 100% increase in the fluorescence intensity in the product solution (HME and Ag nanoparticles) compared to the pure HME solution, as in Figure 8a. Additionally, a hypochromic shift in the emission maximum spectra of the HME solution of about 20 nm is observed as a result of the presence of silver nanoparticles compared with the pure HgME solution, as in Figure 8b.
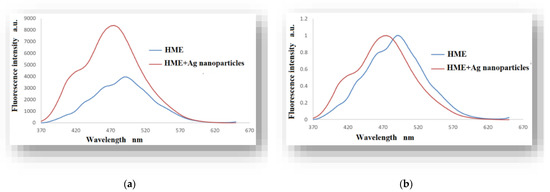
Figure 8.
(a) Fluorescence spectra of the HME and the product solution. (b) Normalized fluorescence spectra of the HME and the product solution, λex = 360 nm.
Figure 9 shows a major quenching in the fluorescence intensity in the presence of Hg2+ ions. By measuring the fluorescence intensity when mixing a fixed concentration of the nanoparticle solution (HME and Ag/Ag2O nanoparticles) with the Hg2+ solution, which is at various concentrations. The Hg2+ detection capability of the nanoparticle solution was assessed. Fluorescence intensity at 479 nm decreased consistently with increasing Hg2+ concentrations when compared to the fresh sample, which was free of Hg2+, indicating a clear inverse relationship between fluorescence intensity and Hg2+ quantities.
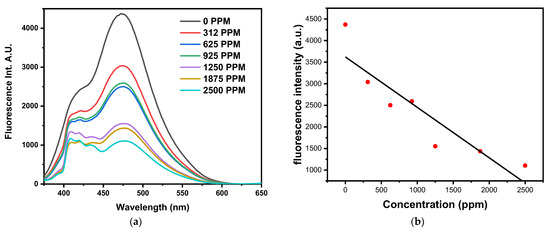
Figure 9.
(a,b): Fluorescence intensity of the product solution (HME and silver. nanoparticles) with different concentrations of Hg2+.
From Figure 9, it is clear that the intensity of fluorescence decreases with the increase in the concentration of mercury ions, and this indicates the existence of an electrostatic interaction between mercury ions and silver nanoparticles, as mentioned in previous research. The potential reaction mechanism depends on the oxidation of mercury ions to the capping (hydroxyl) of the silver nanoparticles. This leads to the transformation of mercury ions into mercury particles (surrounded by hydroxyl). Next, a part of the mercury ions interacts with silver ions and turns into a silver–mercury molecule, and then gradually, the silver nanoparticles turn into normal silver particles, and the concentration of the fluorescence intensity of the product decreases [23,49,91].
Figure 10 shows the Stern–Volmer plot derived from the following equation of silver nanoparticle fluorescence quenching using Hg2+ as a quencher [92]:
where [Q] is the quencher concentration [Hg2+], I and I0 are the fluorescence intensities of studied quercetin Ag nanoparticles in the presence and absence of Hg2+, respectively, and KSV is the Stern–Volmer quenching constant.
I0/I = 1 + Ksv [Q]
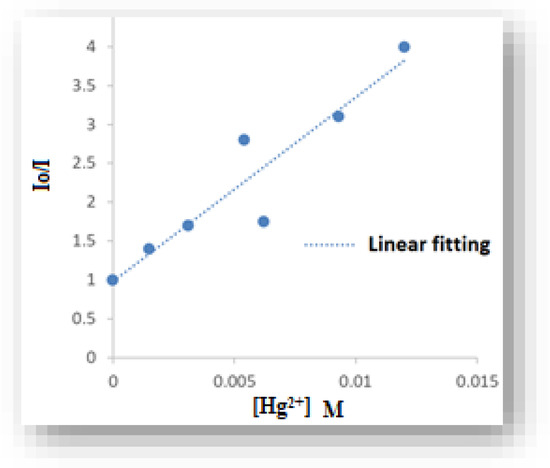
Figure 10.
The Stern–Volmer plot for fluorescence intensity vs. Hg2+ concentration.
In Figure 10, the system is said to follow the Stern–Volmer relationship. The value of the Stern–Volmer quenching constant was calculated from the slope of the linear fitting of plotting I0/I vs. [Hg2+] concentration and is equal to 2.3 × 1011 M−1, which is much higher than the diffusion control of HME, indicating that the mechanism of fluorescence quenching can be called a dynamic diffusion process [93].
2.7. Antibacterial and Antifungal Activities
The antibacterial and antifungal activities of the HME extract, AgNPs, ampicillin, and colitrimazole against Gram-positive bacteria, Gram-negative bacteria, and two common strains of fungi, respectively, were tested, and the results are listed in Table 2.

Table 2.
The antibacterial and antifungal activities of the HME extract, AgNPs, ampicillin, and colitrimazole.
The HME extract and AgNPs show a medium effect in inhibiting all kinds of bacteria and fungi, while the standard antibiotics show maximum inhibition for some kinds of microbes and noninhibition for other kinds (fungi). The second finding is that HME extract [94] enhances the antimicrobial activity of AgNPs [95], which explains why AgNPs’ antibacterial and antifungal activities are more effective than HME extract. Additionally, the outcomes demonstrated that silver nanoparticles are the most powerful inhibitors of all microbial life. This antibacterial activity of silver nanoparticles shows their potent capacity to halt the spread of numerous types of microbes and eradicate them. This antimicrobial activity was explained in numerous studies through different hypotheses, which have not been proven until now [29].
2.8. Anticancer Activity
Assays for cell proliferation are used to measure the number of cells over time, metabolic activity, and the frequency of cell division. Cytotoxicity, cell viability, or proliferation can all be assessed with the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay.
Generally, this MTT assay is an accepted paradigm for determining the complexes’ antitumor efficacy and also enables the assessment of metabolic cell activity [96]. The cytotoxicity of the HME extract and AgNPs against a human lung fibroblast line (WI38), hepatocellular carcinoma (HEPG-2), and breast cancer cell line of the mammary gland (MCF-7) was evaluated using different concentrations of compounds. The AgNPs exhibit potent anticancer properties and possess severe toxicity towards the tested cancer cell type. Table 3 provides the IC50 values for the HME extract and AgNPs.

Table 3.
The anticancer activity of the HME extract, AgNPs, and doxorubicin against WI38, HEPG-2, and MCF-7 cells.
The results revealed that the potency anticancer activity of HME extract was greater than that of silver nanoparticles or doxorubicin. In Table 3, the silver nanoparticles have more inhibitory activity in HEPG-2 cells.
The effect of reactive oxygen species (ROS) can be used to interpret the reaction mechanism of silver nanoparticles with cancer cells. The production of ROS is due to the biosynthesis of AgNPs using plants rich in OH groups. These silver nanoparticles react with the cell wall to form peroxide (H2O2) and change the function of the mitochondria by increasing the adenosine triphosphate (ATP) level, uncoupling the respiration process, ultimately causing cellular death [97,98,99].
3. Materials and Instruments
3.1. Materials
The Haloxylon ammodendron plant was collected from the Qassim desert in Saudi Arabia. Silver nitrate, nitric acid, ammonia, absolute ethanol, and mercury chloride were supplied by Sigma-Aldrich, deionized water from Qassim laboratory.
3.2. The Haloxylon ammodendron Ethanolic Extract Preparation
The Haloxylon ammodendron leaves were collected from the Qassim area, Saudi Arabia, during the springtime of 2023.
The Haloxylon ammodendron (HME) was washed several times with distilled water and once with deionized water. After drying out in the shade in the laboratory for five days, the Haloxylon ammodendron plant was ground into a fine powder. By soaking 100 g of plant powder in 300 mL of 100% ethanol for two days at room temperature using a magnetic stirrer, samples of the Haloxylon ammodendron plant were extracted. After that, the mixture was filtered. The ethanol extract of the Haloxylon ammodendron (HME) solution was cooled and stored in sterile vials until use.
3.3. The Biosynthesis of Ag Nanoparticles
In the preparation of the empirical tests, 0.001 mM of Ag(NO3) and HME were both used to achieve the best preparation of Ag nanoparticles, which was the result of several attempts. The effects of reactant volume ratio, pH, and reaction temperature on the biosynthesis of Ag nanoparticles were studied as follows:
3.3.1. The Reactant Volume Ratio
In this part, many experiments were conducted to determine the best volumetric ratio of the reactants by mixing a fixed amount of HME (20 mL) with different volumes of silver nitrate (20, 30, 40, 50, 60, 70, 80, and 90 mL). The samples were labeled with V1, V2, V3, V4, V5, V6, V7, and V8, respectively. The mixing process was carried out at room temperature with continuous stirring for 3 h. During each experiment, the mixture color changes from greenish-yellow to deep brown, and a precipitate forms, which indicates the formation of silver nanoparticles. This precipitate of silver nanoparticles was extracted using a centrifuge and then dried at 50 °C in an open oven for 8 h. Finally, after drying, the samples were prepared for X-ray diffraction and UV-vis absorption spectroscopy measurements.
3.3.2. The pH
In order to study the effect of pH on the biosynthesis of silver nanoparticles and determine the best reaction environment for the formation of these particles, several experiments were conducted. In these experiments, the volume ratio of the reactants (silver nitrate and HME) was fixed based on the best result of the experiments in the previous part. The pH of the mixture was adjusted by using drops of both nitric acid solution and ammonia solution. Samples were obtained at different pH values (3, 4, 7.5, 8, 9, and 10) while keeping the same previous preparation conditions, such as the temperature and speed of stirring the mixture and molar ratio. The samples were named p1, p2, p3, p4, p5, and p6, respectively. The same previous steps were followed in extracting the silver nanoparticle precipitate, and the same measurements were made.
3.3.3. The Reaction Temperature
To find out the effect of reaction temperature on the biosynthesis of silver nanoparticles and to obtain the suitable and optimal reaction temperature for the formation of these particles, experiments were conducted. In these experiments, the volume ratio of the reactants and pH were fixed based on the best results from the earlier experiments in the previous parts. The mixture was heated at different temperatures (25, 40, and 60 °C), and the resulting samples were labeled T1, T2, and T3, respectively. The formed silver nanoparticle precipitate was extracted using the same previous steps, and then XRD and UV-Vis absorption spectra were measured.
3.4. Characterization Instrumentation and Techniques
The pH values in all experiments were measured using a JENWAY 3510 pH meter from the UK.
An Agilent spectrometer (Cary 600 FTIR, USA) was used to analyze the Fourier-transform infrared (FTIR) spectra in the solid state. It was conducted in the 4000–400 cm−1 wavenumber range.
Using scanning electron microscopy-SEM (JOEL Jsm-5500), the size and shape of biosynthesized silver nanoparticles were examined. For the size investigation of diverse samples, a 30 kV accelerating voltage and various magnification techniques were used.
A Shimadzu spectrophotometer (UV-1650PC, Japan) with a 1 cm quartz cell and a wavelength range of 250–650 nm was applied to study the UV–Vis spectra of the HME and the AgNPs.
For fluorescence measurements, seven samples of Hg2+ ions were prepared at different concentrations (0, 312, 625, 925, 1250, 18750, and 2500 ppm) using DI water and HgCl2. A required amount of silver nanoparticles’ solution, which was biosynthesized using Haloxylon ammodendron, was added to the Hg2+ samples and monitored by the Jasco fluorescence spectrometer FP-8200.
A Rigaku XRD diffractometer (Ultima IV, USA) with Cu-K (λ = 0.15418 nm) as anode material was employed to study the nanostructure of the Ag nanoparticles. The XRD system was operated at 30 mA and 40 kV.
3.5. In Vitro Antimicrobial Studies
The biological activity of the extraction and its AgNps was investigated in vitro as an antimicrobial against some different types of bacteria and fungi. It was tested on four pathogenic bacterial strains (Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli) and two types of fungi (Candida albicans and Aspergillus flavus) using the agar-well diffusion method [31]. In this method, the microbial cells (bacteria or fungi) were inoculated on Petri dishes, and the test samples (HM extraction and AgNps) were implanted inside the holes, which were made in the middle of these microbial cell dishes. Afterward, these dishes were incubated for different hours depending on the microbe’s type (18–24 h at 37 °C), and thereafter, the diffusion of microbes was determined by measuring the zone of inhibition. The presence of antimicrobial activity is indicated by the absence of microbe growth near the test samples. The antimicrobial activity test process was duplicated three times using the same concentration. The antibiotics ampicillin and clotrimazole were used as standard antibiotics against bacteria and fungi, respectively.
3.6. Anticancer Activity
The viability assay was used to evaluate cytotoxicity, and the cancer cells (hepatocellular carcinoma (HEPG-2), human lung fibroblast (WI38), and mammary gland breast cancer (MCF-7) were used to study the anticancer activity of the samples. As well, doxorubicin was used as a standard anticancer drug for comparison [96].
4. Conclusions
This work demonstrated the viability of synthesizing silver nanoparticles from the Saudi Haloxylon ammodendron shrub and identified the ideal parameters for their manufacture (the reactant volume ratios, pH, and temperature). The preparation conditions helped form two types of silver nanoparticles due to the presence of oxygen gas during drying. These two types of nanoparticles are silver oxide nanoparticles and silver nanoparticles. Through X-ray diffraction analysis, it turned out that the formation percentage of silver oxide nanoparticles is greater than that of silver nanoparticles. The results of infrared and ultraviolet spectroscopy of the materials resulting from the interaction of silver nitrate salt with the HME extract confirmed the formation of silver nanoparticles (whether silver oxide or silver) due to the presence of oxidizing and active substances in the HME extract. The microscopic images obtained using the scanning electron microscopy technique showed the spherical shape of these particles and that the formed nanoparticles accumulate in large numbers, which explains the broadening of the ultraviolet spectrum curves of these nanoparticles. Experiments with fluorescence quenching, which was used in this research as a technique to get rid of mercury ions in aqueous solutions, showed the high ability of these nanoparticles to remove these heavy ionic pollutants.
The HME extract showed promising results as an antibacterial, antifungal, and anticancer material. The use of HME extract in the biosynthesis of silver nanoparticles enhanced the biological activity of these nanoparticles and made them more effective at inhibiting different microbes and cancer cells.
Author Contributions
Conceptualization, A.N.A.-H. and T.M.A.; methodology, A.N.A.-H. and R.A.A.; validation, A.N.A.-H., T.M.A. and R.A.A.; formal analysis, A.N.A.-H. and R.A.A.; investigation, R.A.A. and A.N.A.-H.; resources, A.N.A.-H.; data curation, A.N.A.-H.; writing—original draft preparation, A.N.A.-H. and R.A.A.; writing—review and editing, A.N.A.-H. and T.M.A.; visualization, A.N.A.-H.; supervision, A.N.A.-H.; project administration, A.N.A.-H.; funding acquisition, R.A.A. and A.N.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Qassim University”, represented by the deanship of scientific research, under the number (COS-2022-1-1-J-24993) during the academic year 1444 A H/2022 A D.
Acknowledgments
The authors gratefully acknowledge “Qassim University”, represented by the deanship of scientific research, on the financial support for this research under the number (COS-2022-1-1-J-24993) during the academic year 1444 A H/2022 A D.
Conflicts of Interest
The authors confirm that they do not have any conflict of interest related to the work in a manuscript.
References
- Almosabbeh, I.A.; Almoree, M.A. The relationship between manufacturing production and economic growth in the Kingdom of Saudi Arabia. J. Econ. Stud. 2018, 45, 674–690. [Google Scholar] [CrossRef]
- DeNicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Climate change and water scarcity: The case of Saudi Arabia. Ann. Glob. Health 2015, 81, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, A.M.; Alamri, Y.A. The impact of the green Middle East initiative on sustainable development in the Kingdom of Saudi Arabia. J. Saudi Soc. Agric. Sci. 2023, 22, 35–46. [Google Scholar] [CrossRef]
- Almulhim, A.I. Understanding public awareness and attitudes toward renewable energy resources in Saudi Arabia. Renew. Energy 2022, 192, 572–582. [Google Scholar] [CrossRef]
- Gadhoum, Y. A Proposed Model of a Future University in the Era of the Artificial Intelligence Transformative Society: From Why to How. Creat. Educ. 2022, 13, 1098–1119. [Google Scholar] [CrossRef]
- Makki, A.A.; Alqahtani, A.Y. Modeling the Enablers to FinTech Innovation in Saudi Arabia: A Hybrid Approach Using ISM and ANP. Systems 2022, 10, 181. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Kumar, L.; Mohan, L.; Anand, S.; Bhardwaj, D.; Bharadvaja, N. Phyconanoremediation: A sustainable approach to deal with environmental pollutants heavy metals and dyes. Vegetos 2022, 1–16. [Google Scholar] [CrossRef]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef]
- Bhan, A.; Sarkar, N.N. Mercury in the environment: Effect on health and reproduction. Rev. Environ. Health 2005, 20, 39–56. [Google Scholar] [CrossRef]
- Kao, R.T.; Dault, S.; Pichay, T. Understanding the mercury reduction issue: The impact of mercury on the environment and human health. J. Calif. Dent. Assoc. 2004, 32, 574–579. [Google Scholar] [CrossRef]
- Teng, H.; Altaf, A.R. Elemental mercury (Hg0) emission, hazards, and control: A brief review. J. Hazard. Mater. Adv. 2022, 5, 100049. [Google Scholar] [CrossRef]
- Zolnikov, T.R.; Ortiz, D.R. A systematic review on the management and treatment of mercury in artisanal gold mining. Sci. Total Environ. 2018, 633, 816–824. [Google Scholar] [CrossRef]
- Kumari, S.; Jamwal, R.; Mishra, N.; Singh, D.K. Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef]
- Qadri, R.; Faiq, M.A. Freshwater pollution: Effects on aquatic life and human health. In Fresh Water Pollution Dynamics and Remediation; Qadri, H., Bhat, R., Mehmood, M., Dar, G., Eds.; Springer: Singapore, 2020; pp. 15–26. [Google Scholar] [CrossRef]
- Daud, M.K.; Nafees, M.; Ali, S.; Rizwan, M.; Bajwa, R.A.; Shakoor, M.B.; Arshad, M.U.; Chatha, S.A.S.; Deeba, F.; Murad, W. Drinking water quality status and contamination in Pakistan. BioMed Res. Int. 2017, 2017, 7908183. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Mallikarjunaiah, S.; Pattabhiramaiah, M.; Metikurki, B. Application of nanotechnology in the bioremediation of heavy metals and wastewater management. In Nanotechnology for Food, Agriculture, and Environment. Nanotechnology in the Life Sciences; Thangadurai, D., Sangeetha, J., Prasad, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 297–321. [Google Scholar] [CrossRef]
- Cheriyamundath, S.; Vavilala, S.L. Nanotechnology-based wastewater treatment. Water Environ. J. 2021, 35, 123–132. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Qiu, Y.; Liu, X.; Huang, W.; Yan, N.; Qu, Z. Utilization of Ag nanoparticles anchored in covalent organic frameworks for mercury removal from acidic waste water. J. Hazard. Mater. 2020, 389, 121824. [Google Scholar] [CrossRef]
- Pacheco, S.; Medina, M.; Valencia, F.; Tapia, J. Removal of inorganic mercury from polluted water using structured nanoparticles. J. Environ. Eng. 2006, 132, 342–349. [Google Scholar] [CrossRef]
- Sanjeevappa, H.K.; Nilogal, P.; Rayaraddy, R.; Martis, L.J.; Osman, S.M.; Badiadka, N.; Yallappa, S. Biosynthesized unmodified silver nanoparticles: A colorimetric optical sensor for detection of Hg2+ ions in aqueous solution. Results Chem. 2022, 4, 100507. [Google Scholar] [CrossRef]
- Parham, H.; Zargar, B.; Shiralipour, R. Fast and efficient removal of mercury from water samples using magnetic iron oxide nanoparticles modified with 2-mercaptobenzothiazole. J. Hazard. Mater. 2012, 205, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and application of Granular activated carbon from biomass waste materials for water treatment: A review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Vivas-Rodríguez, M.; Yánez-Jácome, G.; Fernández, L.; de las Nieves Piña, M. Colorimetric Detection and Adsorption of Mercury Using Silver Nanoparticles: A Bibliographic and Patent Review. Nanosci. Nanotechnol.-Asia 2021, 11, 4–23. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, A.; Mahto, A.; Raval, I.H.; Modi, K.M.; Haldar, S.; Jain, V.K. Oxacalix [4] arene templated silver nanoparticles as dual readout sensor: Developing portable kit for rapid detection of methylmercury and its speciation. Sens. Actuators B Chem. 2020, 317, 128180. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Dilshad, E.; Bibi, M.; Sheikh, N.A.; Tamrin, K.F.; Mansoor, Q.; Maqbool, Q.; Nawaz, M. Synthesis of functional silver nanoparticles and microparticles with modifiers and evaluation of their antimicrobial, anticancer, and antioxidant activity. J. Funct. Biomater. 2020, 11, 76. [Google Scholar] [CrossRef]
- Abd El-Halim, H.F.; Mohamed, G.G.; Anwar, M.N. Antimicrobial and anticancer activities of Schiff base ligand and its transition metal mixed ligand complexes with heterocyclic base. Appl. Organomet. Chem. 2018, 32, e3899. [Google Scholar] [CrossRef]
- Osman, M.S.; Al-qubati, M.; Saeed, M.; Abdulqawi, N.; Algradee, M.A.; Alwan, A.; Sultan, A.M. Effective inhibition of waterborne and fungal pathogens using ZnO nanoparticles prepared from an aqueous extract of propolis: Optimum biosynthesis, characterization, and antimicrobial activity. Appl. Nanosci. 2023, 13, 4515–4526. [Google Scholar] [CrossRef]
- Al-Fakeh, M.S.; Osman, S.O.M.; Gassoumi, M.; Rabhi, M.; Omer, M. Characterization, antimicrobial and anticancer properties of palladium nanoparticles biosynthesized optimally using Saudi propolis. Nanomaterials 2021, 11, 2666. [Google Scholar] [CrossRef]
- Lovio-Fragoso, J.P.; De Jesús-Campos, D.; Razo-Mendivil, F.G.; García-Coronado, H.; Domínguez-Rosas, E.; Trillo-Hernández, E.A.; Hayano-Kanashiro, C.; Hernández-Oñate, M.Á. Desert plant transcriptomics and adaptation to abiotic stress. In Transcriptome Profiling; Elsevier: Amsterdam, The Netherlands, 2023; pp. 199–256. [Google Scholar]
- Sage, R.F.; Sultmanis, S. Why are there no C4 forests? J. Plant Physiol. 2016, 203, 55–68. [Google Scholar] [CrossRef]
- Squires, V.R.; Qi, L. Sustainable Land Management in Greater Central Asia: An Integrated and Regional Perspective; Routledge: London, UK, 2017; ISBN 1317394054. [Google Scholar]
- Öztürk, M.; Altay, V.; Nazish, M.; Ahmad, M.; Zafar, M. Halophyte Plant Diversity and Public Health; Springer Nature: Berlin, Germany, 2023; ISBN 3031219449. [Google Scholar]
- Kang, W.; Song, Y. Chemical Constituents of Haloxylon ammodendron. Chem. Nat. Compd. 2015, 51, 557–558. [Google Scholar] [CrossRef]
- Haida, S.; Kribii, A. Chemical composition, phenolic content and antioxidant capacity of Haloxylon scoparium extracts. South Afr. J. Bot. 2020, 131, 151–160. [Google Scholar] [CrossRef]
- Lachkar, N.; Lamchouri, F.; Bouabid, K.; Boulfia, M.; Senhaji, S.; Stitou, M.; Toufik, H. Mineral composition, phenolic content, and in vitro antidiabetic and antioxidant properties of aqueous and organic extracts of Haloxylon scoparium aerial parts. Evid.-Based Complement. Altern. Med. 2021, 2021, 9011168. [Google Scholar] [CrossRef]
- Litvin, V.A.; Galagan, R.L.; Minaev, B.F. Kinetic and mechanism formation of silver nanoparticles coated by synthetic humic substances. Colloids Surfaces A Physicochem. Eng. Asp. 2012, 414, 234–243. [Google Scholar] [CrossRef]
- Hebeish, A.A.; El-Rafie, M.H.; Abdel-Mohdy, F.A.; Abdel-Halim, E.S.; Emam, H.E. Carboxymethyl cellulose for green synthesis and stabilization of silver nanoparticles. Carbohydr. Polym. 2010, 82, 933–941. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Groenaga Infante, H.; Stokes, E.; Shaw, A.M. Silver nanoparticle enhanced silver ion stress response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef]
- Nayeri, F.D.; Mafakheri, S.; Mirhosseini, M. Phyto-mediated silver nanoparticles via Mellisa officinalis aqueous and methanolic extracts: Synthesis, characterization and biological properties. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.A.; Kabary, H.; Khedr, M.; Emam, A.N. Antibiofilm, antimicrobial and cytotoxic activity of extracellular green-synthesized silver nanoparticles by two marine-derived actinomycete. RSC Adv. 2020, 10, 10361–10367. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, O.A.D.; Moiraghi, R.; Macchione, M.A.; Godoy, J.A.; Pérez, M.A.; Coronado, E.A.; Macagno, V.A. Silver oxide particles/silver nanoparticles interconversion: Susceptibility of forward/backward reactions to the chemical environment at room temperature. RSC Adv. 2012, 2, 2923–2929. [Google Scholar] [CrossRef]
- Ismail, M.; Khan, M.I.; Akhtar, K.; Seo, J.; Khan, M.A.; Asiri, A.M.; Khan, S.B. Phytosynthesis of silver nanoparticles; naked eye cellulose filter paper dual mechanism sensor for mercury ions and ammonia in aqueous solution. J. Mater. Sci. Mater. Electron. 2019, 30, 7367–7383. [Google Scholar] [CrossRef]
- Hosseinpour-Mashkani, S.M.; Ramezani, M. Silver and silver oxide nanoparticles: Synthesis and characterization by thermal decomposition. Mater. Lett. 2014, 130, 259–262. [Google Scholar] [CrossRef]
- Ghaemi, M.; Gholamipoor, S. Controllable synthesis and characterization of silver nanoparticles using Sargassum angostifolium. Iran. J. Chem. Chem. Eng. 2017, 1, 1–10. [Google Scholar]
- Zhao, X.; Yan, L.; Xu, X.; Zhao, H.; Lu, Y.; Wang, Y.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J. Synthesis of silver nanoparticles and its contribution to the capability of Bacillus subtilis to deal with polluted waters. Appl. Microbiol. Biotechnol. 2019, 103, 6319–6332. [Google Scholar] [CrossRef]
- Gradess, R.; Abargues, R.; Habbou, A.; Canet-Ferrer, J.; Pedrueza, E.; Russell, A.; Valdés, J.L.; Martínez-Pastor, J.P. Localized surface plasmon resonance sensor based on Ag-PVA nanocomposite thin films. J. Mater. Chem. 2009, 19, 9233–9240. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Mohamed, S.O.; El-Naggar, K.; Khalil, M.M.H. Green Synthesis of Silver Nanoparticles using Egyptian Propolis Extract and its Antimicrobial Activity. Egypt. J. Chem. 2022, 65, 5–6. [Google Scholar]
- Han, Y.; Lupitskyy, R.; Chou, T.-M.; Stafford, C.M.; Du, H.; Sukhishvili, S. Effect of oxidation on surface-enhanced Raman scattering activity of silver nanoparticles: A quantitative correlation. Anal. Chem. 2011, 83, 5873–5880. [Google Scholar] [CrossRef]
- Abbasi, B.A.; Iqbal, J.; Nasir, J.A.; Zahra, S.A.; Shahbaz, A.; Uddin, S.; Hameed, S.; Gul, F.; Kanwal, S.; Mahmood, T. Environmentally friendly green approach for the fabrication of silver oxide nanoparticles: Characterization and diverse biomedical applications. Microsc. Res. Tech. 2020, 83, 1308–1320. [Google Scholar] [CrossRef]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Anigol, L.B.; Charantimath, J.S.; Gurubasavaraj, P.M. Effect of concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Org. Med. Chem. Int. J. 2017, 3, 1–5. [Google Scholar]
- Yang, Z.; Qian, H.; Chen, H.; Anker, J.N. One-pot hydrothermal synthesis of silver nanowires via citrate reduction. J. Colloid Interface Sci. 2010, 352, 285–291. [Google Scholar] [CrossRef]
- Nishimura, S.; Mott, D.; Takagaki, A.; Maenosono, S.; Ebitani, K. Role of base in the formation of silver nanoparticles synthesized using sodium acrylate as a dual reducing and encapsulating agent. Phys. Chem. Chem. Phys. 2011, 13, 9335–9343. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Basak, M.; Rahman, M.L.; Ahmed, M.F.; Biswas, B.; Sharmin, N. The use of X-ray diffraction peak profile analysis to determine the structural parameters of cobalt ferrite nanoparticles using Debye-Scherrer, Williamson-Hall, Halder-Wagner and Size-strain plot: Different precipitating agent approach. J. Alloys Compd. 2022, 895, 162694. [Google Scholar] [CrossRef]
- Ameh, E.S. A review of basic crystallography and X-ray diffraction applications. Int. J. Adv. Manuf. Technol. 2019, 105, 3289–3302. [Google Scholar] [CrossRef]
- Yang, H.; Ren, Y.; Wang, T.; Wang, C. Preparation and antibacterial activities of Ag/Ag+/Ag3+ nanoparticle composites made by pomegranate (Punica granatum) rind extract. Results Phys. 2016, 6, 299–304. [Google Scholar] [CrossRef]
- Gauri, B.; Vidya, K.; Sharada, D.; Shobha, W. Synthesis and characterization of Ag/AgO nanoparticles as alcohol sensor. Res. J. Chem. Environ. 2016, 20, 1–5. [Google Scholar]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus mediated synthesis of silver oxide nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef]
- Scherrer, P. Nachr Ges wiss goettingen. Math. Phys. 1918, 2, 98–100. [Google Scholar]
- Priya, M.M.; Selvi, B.K.; Paul, J.A. Green synthesis of silver nanoparticles from the leaf extracts of Euphorbia hirta and Nerium indicum. Dig. J. Nanomater. Biostruct. 2011, 6, 869–877. [Google Scholar]
- Wan Mat Khalir, W.K.A.; Shameli, K.; Jazayeri, S.D.; Othman, N.A.; Che Jusoh, N.W.; Hassan, N.M. Biosynthesized silver nanoparticles by aqueous stem extract of Entada spiralis and screening of their biomedical activity. Front. Chem. 2020, 8, 620. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy, Brooks; Cole Cengage Learn: Boston, MA, USA, 2009; pp. 381–417. [Google Scholar]
- Yu, L.; He, R.; Zhang, Y.; Gao, J. Effect of surface treatment on flexural and tribological properties of poly (p-phenylene benzobisoxazole)/polyimide composites under normal and elevated temperature. Materials 2018, 11, 2131. [Google Scholar] [CrossRef]
- Yong, N.L.; Ahmad, A.; Mohammad, A.W. Synthesis and characterization of silver oxide nanoparticles by a novel method. Int. J. Sci. Eng. Res 2013, 4. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=89e46bd619abcbf4e233925c6a26ffa47b755e6c (accessed on 5 June 2023).
- Hatamifard, A.; Nasrollahzadeh, M.; Sajadi, S.M. Biosynthesis, characterization and catalytic activity of an Ag/zeolite nanocomposite for base-and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of a variety of dyes at room temperature. New J. Chem. 2016, 40, 2501–2513. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour. Technol. 2009, 100, 501–504. [Google Scholar] [CrossRef]
- Sujatha, V.; Kaviyasri, G.; Venkatesan, A.; Thirunavukkarasu, C.; Acharya, S.; Dayel, S.B.; Al-Ghamdi, S.; Abdelzaher, M.H.; Shahid, M.; Ramesh, T. Biomimetic formation of silver oxide nanoparticles through Diospyros montana bark extract: Its application in dye degradation, antibacterial and anticancer effect in human hepatocellular carcinoma cells. J. King Saud Univ. 2023, 35, 102563. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Jeeva, K.; Thiyagarajan, M.; Elangovan, V.; Geetha, N.; Venkatachalam, P. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind. Crops Prod. 2014, 52, 714–720. [Google Scholar] [CrossRef]
- Mustafa Nadhim Owaid, G.A.N.; Rasim Farraj Muslim, R.S.O. Synthesis, characterization and antitumor efficacy of silver nanoparticle from Agaricus bisporus pileus, Basidiomycota. Walailak J. Sci. Technol. (WJST) 2020, 17, 75–87. [Google Scholar] [CrossRef]
- Hemmati, S.; Rashtiani, A.; Zangeneh, M.M.; Mohammadi, P.; Zangeneh, A.; Veisi, H. Green synthesis and characterization of silver nanoparticles using Fritillaria flower extract and their antibacterial activity against some human pathogens. Polyhedron 2019, 158, 8–14. [Google Scholar] [CrossRef]
- Jemal, K.; Sandeep, B.V.; Pola, S. Synthesis, characterization, and evaluation of the antibacterial activity of Allophylus serratus leaf and leaf derived callus extracts mediated silver nanoparticles. J. Nanomater. 2017, 2017, 4213275. [Google Scholar] [CrossRef]
- Yang, N.; Li, W.-H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind. Crops Prod. 2013, 48, 81–88. [Google Scholar] [CrossRef]
- Leng, Z.; Wu, D.; Yang, Q.; Zeng, S.; Xia, W. Facile and one-step liquid phase synthesis of uniform silver nanoparticles reduction by ethylene glycol. Optik 2018, 154, 33–40. [Google Scholar] [CrossRef]
- Rajput, J.K. Bio-polyphenols promoted green synthesis of silver nanoparticles for facile and ultra-sensitive colorimetric detection of melamine in milk. Biosens. Bioelectron. 2018, 120, 153–159. [Google Scholar]
- Al-Hazmy, S.M.; Zouaghi, M.O.; Al-Johani, J.N.; Arfaoui, Y.; Al-Ashwal, R.; Hammami, B.; Alhagri, I.A.; Alhemiary, N.A.; Hamdi, N. Chemosensing Properties of Coumarin Derivatives: Promising Agents with Diverse Pharmacological Properties, Docking and DFT Investigation. Molecules 2022, 27, 5921. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590. [Google Scholar] [CrossRef]
- Pettit, G.R.; Meng, Y.; Herald, D.L.; Graham, K.A.N.; Pettit, R.K.; Doubek, D.L. Isolation and Structure of Ruprechstyril from Ruprechtia tangarana. J. Nat. Prod. 2003, 66, 1065–1069. [Google Scholar] [CrossRef]
- Meneses-Sagrero, S.E.; Navarro-Navarro, M.; Ruiz-Bustos, E.; Del-Toro-Sánchez, C.L.; Jiménez-Estrada, M.; Robles-Zepeda, R.E. Antiproliferative activity of spinasterol isolated of Stegnosperma halimifolium (Benth, 1844). Saudi Pharm. J. 2017, 25, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Cornard, J.P.; Merlin, J.C. Spectroscopic and structural study of complexes of quercetin with Al (III). J. Inorg. Biochem. 2002, 92, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Schiesaro, I.; Burratti, L.; Meneghini, C.; Fratoddi, I.; Prosposito, P.; Lim, J.; Scheu, C.; Venditti, I.; Iucci, G.; Battocchio, C. Hydrophilic silver nanoparticles for Hg (II) detection in water: Direct evidence for mercury–silver interaction. J. Phys. Chem. C 2020, 124, 25975–25983. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- El-Daly, S.A.; Rahman, M.M.; Alamry, K.A.; Asiri, A.M. Fluorescence quenching of perylene DBPI dye by colloidal low-dimensional gold nanoparticles. J. Fluoresc. 2015, 25, 973–978. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Pan, Y.; Ninomiya, K.; Yuan, D.; Jia, X.; Yoshikawa, M.; Nakamura, S.; Matsuda, H.; Muraoka, O. A review of biologically active natural products from a desert plant Cistanche tubulosa. Chem. Pharm. Bull. 2019, 67, 675–689. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Park, T.-J.; Rohit, J.V.; Koduru, J.R. Antimicrobial activity of silver nanoparticles. In Nanoparticles in Pharmacotherapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 461–484. [Google Scholar]
- Hamida, R.S.; Ali, M.A.; Alfassam, H.E.; Momenah, M.A.; Alkhateeb, M.A.; Bin-Meferij, M.M. One-Step Phytofabrication Method of Silver and Gold Nanoparticles Using Haloxylon Salicornicum for Anticancer, Antimicrobial, and Antioxidant Activities. Pharmaceutics 2023, 15, 529. [Google Scholar] [CrossRef]
- Ullah, I.; Khalil, A.T.; Ali, M.; Iqbal, J.; Ali, W.; Alarifi, S.; Shinwari, Z.K. Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid. Med. Cell. Longev. 2020, 2020, 1215395. [Google Scholar] [CrossRef]
- Hailan, W.A.; Al-Anazi, K.M.; Farah, M.A.; Ali, M.A.; Al-Kawmani, A.A.; Abou-Tarboush, F.M. Reactive oxygen species-mediated cytotoxicity in liver carcinoma cells induced by silver nanoparticles biosynthesized using Schinus molle extract. Nanomaterials 2022, 12, 161. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).