A Proposal to Perform High Contrast Imaging of Human Palatine Tonsil with Cross Polarized Optical Coherence Tomography
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
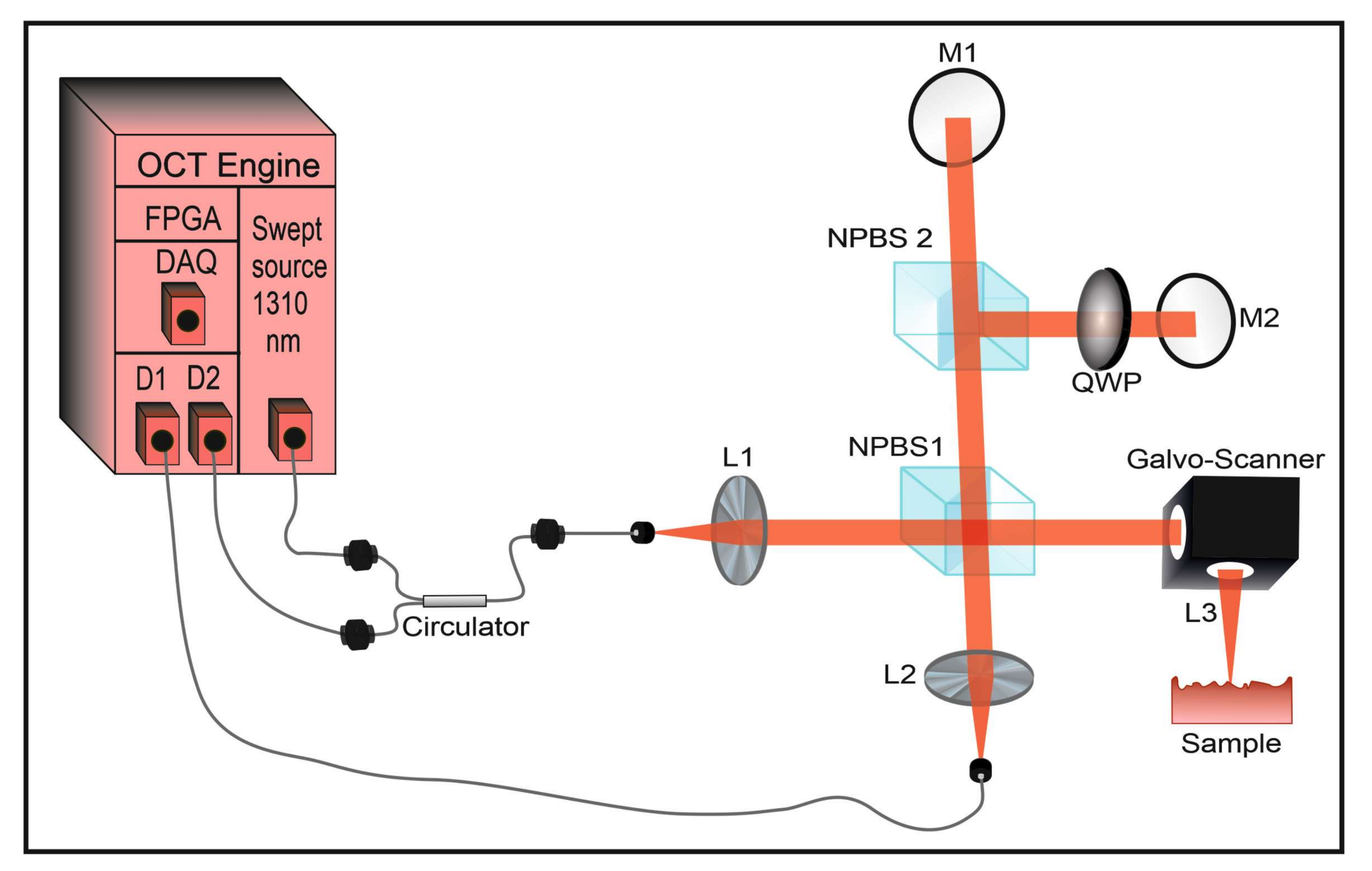
2.2. The OCT-Imaging
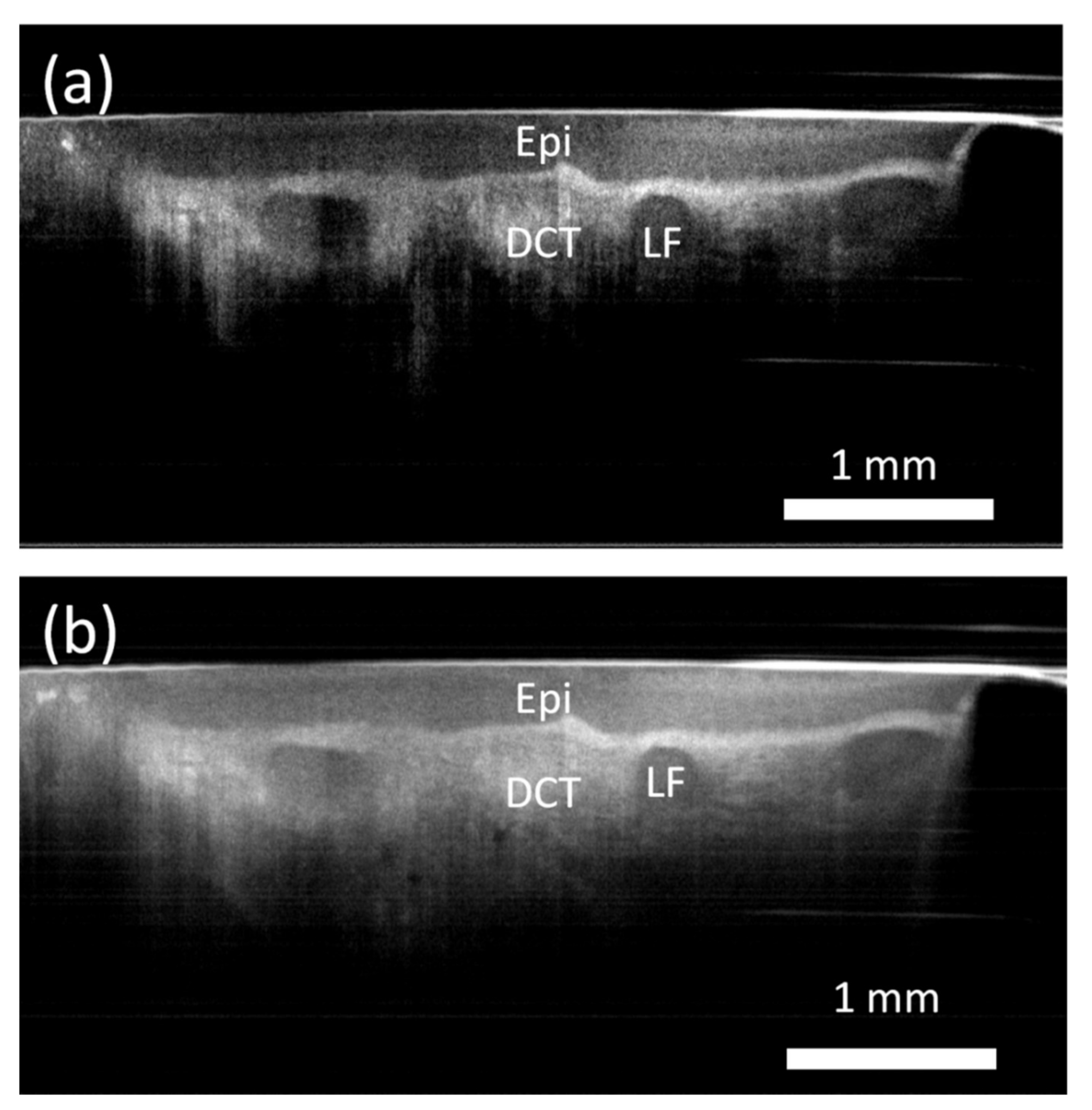
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrasco, A.; Sjölander, I.; Van Acker, A.; Dernstedt, A.; Fehrm, J.; Forsell, M.; Friberg, D.; Mjösberg, J.; Rao, A. The Tonsil Lymphocyte Landscape in Pediatric Tonsil Hyperplasia and Obstructive Sleep Apnea. Front. Immunol. 2021, 12, 674080. [Google Scholar] [CrossRef] [PubMed]
- Scadding, G.K. Immunology of the tonsil: A review. J. R. Soc. Med. 1990, 83, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Nave, H.; Gebert, A.; Pabst, R. Morphology and immunology of the human palatine tonsil. Anat. Embryol. 2001, 204, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, C.; Kanagalingam, J.; Zainal, A.; Ahmed, H.; Singh, A.; Patel, K.S. The association between periodontal disease and peritonsillar infection: A prospective study. Otolaryngol. Head Neck Surg. 2002, 126, 91–94. [Google Scholar] [CrossRef]
- Barone, A.; Chatelain, S.; Derchi, G.; Di Spirito, F.; Martuscelli, R.; Porzio, M.; Sbordone, L. Antibiotic’s effectiveness after erupted tooth extractions: A retrospective study. Oral Dis. 2020, 26, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, C.C.; Tolley, N.S.; Narula, P.A. Tonsillitis. BMJ Clin. Evid. 2014, 22, 0503. [Google Scholar]
- Burton, M.J.; Glasziou, P.P.; Chong, L.Y.; Venekamp, R.P. Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst. Rev. 2014, 2014, CD001802. [Google Scholar] [CrossRef]
- Munck, H.; Jørgensen, A.W.; Klug, T.E. Antibiotics for recurrent acute pharyngo-tonsillitis: Systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1221–1230. [Google Scholar] [CrossRef]
- Çelebi, İ.; Bozkurt, G.; Polat, N. Tonsillar Plasmacytoma: Clues on magnetic resonance imaging. BMC Med. Imaging 2018, 18, 19. [Google Scholar] [CrossRef]
- El Sherif, I.; Shembesh, F.M. A tonsillolith seen on MRI. Comput. Med. Imaging Graph. 1997, 21, 205–208. [Google Scholar] [CrossRef]
- Aspestrand, F.; Kolbenstvedt, A.; Boysen, M. Staging of carcinoma of the palatine tonsils by computed tomography. J. Comput. Assist. Tomogr. 1988, 12, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kito, S.; Tanaka, T.; Nishida, I.; Awano, S.; Fujita, Y.; Saeki, K.; Matsumoto-Takeda, S.; Wakasugi-Sato, N.; Habu, M.; et al. Prevalence and imaging characteristics of detectable tonsilloliths on 482 pairs of consecutive CT and panoramic radiographs. BMC Oral Health 2013, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Kay-Rivest, E.; Saint-Martin, C.; Daniel, S.J. High-frequency ultrasound: A novel diagnostic tool to measure pediatric tonsils in 3 dimensions. Otolaryngol. Head Neck Surg. 2019, 161, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, T.; Yamada, Y.; Tanami, Y.; Hattori, S.; Sato, Y.; Hosokawa, M.; Oguma, E. Evaluation of the normal tonsils in pediatric patients with ultrasonography. J. Ultrasound Med. 2017, 36, 1029–1036. [Google Scholar] [CrossRef]
- Herrmann, K.H.; Hoffmann, F.; Ernst, G.; Pertzborn, D.; Pelzel, D.; Geißler, K.; Guntinas-Lichius, O.; Reichenbach, J.R.; von Eggeling, F. High-resolution MRI of the human palatine tonsil and its schematic anatomic 3D reconstruction. J. Anat. 2022, 240, 166–171. [Google Scholar] [CrossRef]
- Pahlevaninezhad, H.; Lee, A.M.; Rosin, M.; Sun, I.; Zhang, L.; Hakimi, M.; MacAulay, C.; Lane, P.M. Optical coherence tomography and autofluorescence imaging of human tonsil. PLoS ONE 2014, 9, e115889. [Google Scholar] [CrossRef]
- Monici, M. Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 2005, 11, 227–256. [Google Scholar] [CrossRef]
- Banerjee, B.; Miedema, B.E.; Chandrasekhar, H.R. Role of basement membrane collagen and elastin in the autofluorescence spectra of the colon. J. Investig. Med. 1999, 47, 326–332. [Google Scholar]
- Sharma, S.; Hartl, G.; Naveed, S.K.; Blessing, K.; Sharma, G.; Singh, K. Input polarization-independent polarization-sensitive optical coherence tomography using a depolarizer. Rev. Sci. Instrum. 2020, 91, 043706. [Google Scholar] [CrossRef]
- De Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography—A review [Invited]. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, H.-C.; Ahsen, O.O.; Lee, B.; Choi, W.; Potsaid, B.; Liu, J.; Jayaraman, V.; Cable, A.; Kraus, M.F. Depth-encoded all-fiber swept source polarization sensitive OCT. Biomed. Opt. Express 2014, 5, 2931–2949. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Xiang, S. Cross-polarized backscatter in optical coherence tomography of biological tissue. Opt. Lett. 1998, 23, 1060–1062. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Blessing, K.; Hartl, G.; Waldner, M.; Singh, K. Swept source cross-polarized optical coherence tomography for any input polarized light. J. Opt. 2020, 22, 045301. [Google Scholar] [CrossRef]
- Hartl, G.R.; Parmar, A.; Sharma, G.; Singh, K. Cross-Polarized Optical Coherence Tomography System with Unpolarized Light. Photonics 2022, 9, 76. [Google Scholar] [CrossRef]
- Gelikonov, V.; Gelikonov, G. New approach to cross-polarized optical coherence tomography based on orthogonal arbitrarily polarized modes. Laser Phys. Lett. 2006, 3, 445. [Google Scholar] [CrossRef]
- Kuranov, R.V.; Sapozhnikova, V.; Turchin, I.; Zagainova, E.; Gelikonov, V.; Kamensky, V.; Snopova, L.; Prodanetz, N. Complementary use of cross-polarization and standard OCT for differential diagnosis of pathological tissues. Opt. Express 2002, 10, 707–713. [Google Scholar] [CrossRef]
- Yao, X.; Gan, Y.; Ling, Y.; Marboe, C.C.; Hendon, C.P. Multicontrast endomyocardial imaging by single-channel high-resolution cross-polarization optical coherence tomography. J. Biophotonics 2018, 11, e201700204. [Google Scholar] [CrossRef]
- Alghilan, M.A.; Lippert, F.; Platt, J.A.; Eckert, G.J.; González-Cabezas, C.; Fried, D.; Hara, A.T. In vitro longitudinal evaluation of enamel wear by cross-polarization optical coherence tomography. Dent. Mater. 2019, 35, 1464–1470. [Google Scholar] [CrossRef]
- Gladkova, N.; Kiseleva, E.; Robakidze, N.; Balalaeva, I.; Karabut, M.; Gubarkova, E.; Feldchtein, F. Evaluation of oral mucosa collagen condition with cross-polarization optical coherence tomography. J. Biophotonics 2013, 6, 321–329. [Google Scholar] [CrossRef]
- Gladkova, N.; Streltsova, O.; Zagaynova, E.; Kiseleva, E.; Gelikonov, V.; Gelikonov, G.; Karabut, M.; Yunusova, K.; Evdokimova, O. Cross-polarization optical coherence tomography for early bladder-cancer detection: Statistical study. J. Biophotonics 2011, 4, 519–532. [Google Scholar] [CrossRef]
- Podoleanu, A.G. Unbalanced versus balanced operation in an optical coherence tomography system. Appl. Opt. 2000, 39, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Blessing, K.; Schirmer, J.; Parmar, A.; Singh, K. Depth encoded input polarisation independent swept source cross-polarised optical coherence tomography probe. J. Phys. D Appl. Phys. 2021, 54, 305401. [Google Scholar] [CrossRef]
- Blessing, K.; Schirmer, J.; Sharma, G.; Singh, K. Novel input polarisation independent endoscopic cross-polarised optical coherence tomography probe. J. Biophotonics 2020, 13, e202000134. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, G.; Parmar, A.; Hoffmann, F.; Geißler, K.; von Eggeling, F.; Guntinas-Lichius, O.; Singh, K. A Proposal to Perform High Contrast Imaging of Human Palatine Tonsil with Cross Polarized Optical Coherence Tomography. Photonics 2022, 9, 259. https://doi.org/10.3390/photonics9040259
Sharma G, Parmar A, Hoffmann F, Geißler K, von Eggeling F, Guntinas-Lichius O, Singh K. A Proposal to Perform High Contrast Imaging of Human Palatine Tonsil with Cross Polarized Optical Coherence Tomography. Photonics. 2022; 9(4):259. https://doi.org/10.3390/photonics9040259
Chicago/Turabian StyleSharma, Gargi, Asha Parmar, Franziska Hoffmann, Katharina Geißler, Ferdinand von Eggeling, Orlando Guntinas-Lichius, and Kanwarpal Singh. 2022. "A Proposal to Perform High Contrast Imaging of Human Palatine Tonsil with Cross Polarized Optical Coherence Tomography" Photonics 9, no. 4: 259. https://doi.org/10.3390/photonics9040259
APA StyleSharma, G., Parmar, A., Hoffmann, F., Geißler, K., von Eggeling, F., Guntinas-Lichius, O., & Singh, K. (2022). A Proposal to Perform High Contrast Imaging of Human Palatine Tonsil with Cross Polarized Optical Coherence Tomography. Photonics, 9(4), 259. https://doi.org/10.3390/photonics9040259






