Abstract
In this study, we evaluated the nitrous oxide production potential of denitrifying bacterial strains isolated from sediments of the urban wetland Santa María del Lago under anaerobic and aerobic conditions to determine their potential role in mitigating anthropogenic N2O emissions, which have increased by approximately 40% since 1980, and if these emissions could be related to the absence of the nitrous oxide reductase gene (nosZ). The results demonstrated that denitrifying bacteria belonging to the genus Bacillus were able to generate nitrous oxide in high concentrations under both aerobic (up to 83 nM/h) and anaerobic (up to 3865.5 nM/h) conditions in cultures with optimal concentrations of nitrate and carbon. The amplification of the nosZ gene as marker of denitrifying microorganisms showed that only 50% of strains possess this gene, and its presence did not correlate with nitrous oxide reduction under anoxic conditions. Interestingly, one strain was able to reduce nitrous oxide in the presence of air, which is promising for its potential use in aerobic bioremediation systems that require microorganisms with a high affinity for this greenhouse gas to reduce emissions into the atmosphere.
1. Introduction
Nitrous oxide is a greenhouse gas (GHG) with a concentration of 337.6 ppbV in the troposphere [1], a warming potential 310 times greater than that of CO2 on a 100-year scale, and a contribution to global warming of 5 to 7% [2]. A total of 40% of this GHG is derived from anthropogenic processes (agricultural, industrial, combustion, and human waste disposal) and 65% from biogenic processes in terrestrial, marine, and freshwater environments. In the latter, it has been estimated that inland waters with nitrogen addition (including lakes and wetlands) contribute up to 0.4 Tg N/y to the total emission of this GHG [3]. However, these calculations may be underestimated, as it has recently been reported that denitrification can generate N2O under anoxic, suboxic, microaerophilic, and even aerobic conditions through partial denitrification [4,5,6], which broadens the spectrum of the impact that global climate change can have on the microbial communities that generate N2O in aquatic ecosystems prone to eutrophication. Furthermore, denitrifying bacteria that can reduce nitrous oxide in the presence of oxygen have recently been isolated from natural environments, which raises great interest for their application in ecosystem bioremediation processes [7] in line with the global sustainable development goals of climate action, clean water, and sustainable cities.
In Colombia, N2O production has been reported in a few aquatic ecosystems such as Lake Sonso [8], the Prado reservoir [9], and the urban wetland Santa María del Lago-SML [10]. The SML wetland is part of a network of urban wetlands in Bogotá, Colombia, included in the Ramsar List of key areas for the conservation of biological diversity. In this area it has been reported that nitrification and denitrification contributed to the N2O production, depending on oxygen conditions, nutrients, pH, C/N ratio, and available organic matter [10], corroborating previous information on wetlands globally [11]. Microbial ecology studies conducted in the SML wetland in Bogotá demonstrate that denitrification contributes between 20 and 40% to the net production of this GHG [10] and that this process can be carried out by heterotrophic bacteria abundant in sediments such as Proteobacteria, Acidobacteria, and Aminicenantes which, along with bacteria possessing functional genes for nitrous oxide reduction like Candidatus competibacter denitrificans [12], may be involved in the cycling of this GHG. Additionally, spatial and temporal variation in trophic indices, C/N ratio, nutrient levels, and oxygen in the water column has been reported in this wetland [10,11,12,13,14]. Based on these precedents, the objectives of this research were to determine if there is production of this GHG by denitrification in the presence and absence of oxygen by native bacteria isolated from the wetland that demonstrate their metabolic versatility and detect the presence of functional denitrification genes in wetland sediments through shotgun metagenomic analysis.
2. Materials and Methods
In this study, controlled laboratory experiments were performed with denitrifying bacteria previously isolated from sediment samples, to quantify their capacity to produce nitrous oxide under both oxic and anoxic conditions, as well as in the presence of acetylene, and to assess their ability to reduce nitrate to nitrite or ammonium. Moreover, the presence of the nosZ gene, which encodes nitrous oxide reductase, was assessed in these isolates using polymerase chain reaction. Additionally, microorganisms involved in denitrification and nitrification processes were identified through functional gene annotation based on shotgun metagenomic sequencing coupled with bioinformatics analyses.
2.1. Reactivation of Strains and Selection
The denitrifying bacterial strains isolated from sediments of SML wetland collected in 2023 at two stations are as follows: S1 (4.69226 N–74.09464 W) and S2 (4.41226 N–74.05464 W) were reactivated on nutrient agar at 35 °C for 24 h for their microscopic characterization and subsequent experiments. The denitrifying activity of the isolated strains was verified by the presence of blue halos around the colony on culture media with bromothymol blue [4]. In the first set of experiments, five strains were used to estimate N2O production by taking samples directly from the culture on nutrient agar. In the second set of experiments, five strains were selected and adjusted to a McFarland standard of 0.5 (ʎ = 600 nm), and their optimal growth was monitored for 24 h in nitrate broth composed of (g/L): peptone 5.0; meat extract 3.0 and KNO3 1.0 for subsequent use.
2.2. N2O Production Experiments
A first set of experiments was conducted by adding inoculum of the strains growing on agar to amber flasks in triplicate, each one containing 50 mL of nitrate broth composed of (g/L): KNO3 0.5; CaCO3 5.0; glucose 5.5; saline solution 50 mL, and pH 7–7.3. The flasks were then sealed with butyl stoppers and metal caps. To each flask, 45 mL of ultrapure helium was added to create anoxic conditions, and they were incubated at 300 rpm for 24 h at 28 °C. Samples of 15 mL from the gas phase of each flask were taken at 6, 24, and 48 h using a gastight syringe. These samples were injected into vacuum vials for later quantification. Each gas phase extraction was gently replaced with 15 mL of ultrapure helium with agitation to maintain a gas phase volume of 45 mL in the flask.
In a second set of experiments, triplicate experiments were performed for each strain in nitrate broth (50 mL), inoculated with each strain (2.5 mL) growing in liquid medium adjusted to a McFarland standard of 0.5 (600 nm). The amber flasks were sealed with butyl stoppers and metal caps. Subsequently, in triplicate flasks, ultrapure helium (45 mL) was added to create anoxic conditions, in another three flasks, ultrapure helium (35 mL) plus acetylene (15% v/v) was added to inhibit nitrous oxide reductase activity [15], and in another three flasks, air (45 mL) was injected to induce aerobic denitrification. Additionally, 9 flasks of the same treatments without bacterial inoculum were prepared as controls. All flasks were incubated at 28 °C with constant shaking (300 rpm), reducing the incubation time to 24 h since the first set of experiments showed high N2O production within this time frame. Samples of 15 mL from the gas phase of each amber flask were taken at 0, 6, and 24 h using a gastight syringe, and these samples were injected into vacuum vials for subsequent quantification. Each gas phase extraction was replaced with ultrapure helium or air to maintain a gas phase volume of 45 mL in the experimental flasks. At the end of this experiment, 0.5 mL of 50% ZnCl was added to inactivate the bacteria. The cultures were then centrifuged at 6000 rpm for 10 min to quantify ammonium, nitrite, and nitrate in the supernatants using standard spectrophotometric techniques [16].
2.3. Quantification of Nitrous Oxide
Nitrous oxide quantification was performed using a gas chromatograph (Shimadzu GC) equipped with a 63Ni electron capture detector at 325 °C (N2O), with ultrapure N2 as the mobile phase and a detection limit of 0.1 ppb. N2O standards balanced in N with a precision of ±5% were used. The concentration of dissolved N2O (ppb) was calculated using the Weiss & Price solubility formula [17].
With these data, the N2O production rate (nM/h) was estimated through linear regression analysis of all data obtained from the three replicates over the 24 or 48 h of the experiment, and the rate was estimated from the slope obtained in this analysis. The error in the rate was estimated from the standard error of the slope. The normal distribution of the data was verified with the Shapiro–Wilk test after data transformation with Box–Cox. The comparison of N2O production between experiments and bacteria was performed using analysis of variance and Tukey’s test with a significance level of 5% (p < 0.05). Statistical analyses were conducted with the R 4.0.2 Windows package [18].
2.4. DNA Extraction and Amplification of 16S rDNA and Functional Denitrification Genes
DNA extraction was performed using the heat lysis method, where a sweep over the pure colony with a sterile loop was dissolved in 100 µL of sterile water and incubated at 97 °C for 5 min to induce cell lysis. It was then centrifuged at 15,000 rpm for 10 min, the supernatant was taken, and the presence of DNA was verified by electrophoresis on 1% agarose gel with ethidium bromide. The DNA was stored at −20 °C until amplification.
Bacterial isolates were identified after Sanger sequencing of purified amplicons (1400 bp) obtained by polymerase chain reaction from the V1–V9 region of the 16S rDNA gene using primers 1492R-Y and 27F [19]. Denaturation was performed at 95 °C (1 min) followed by 35 cycles of 95 °C (60 s), 50 °C (60 s), and 72 °C (3 min), with a final extension at 72 °C (4 min). The reaction mix (25 µL) included 0.5 µL of One Taq Hot Start DNA polymerase (5 U/µL), 0.4 µL of 20 mM dNTPs, 1 µL of 10 pM of each primer, 4 µL of 5X enzyme buffer, and 1 µL DNA. Bioinformatics analysis was performed using Geneious Prime 2020.1 (https://www.geneious.com) (accessed on 15 May 2025). Approximate taxonomic identification of the sequences obtained from 16S rDNA of the bacterial isolates were achieved through the BlastN 2.16.0 software (http://www.ncbi.nlm.nih.gov/BLAST/) (accessed on 15 May 2025).
To characterize and verify the strains, analysis of the presence of functional denitrification genes for nitrite reductase (nirS) and nitrous oxide reductase (nosZ), commonly used as molecular markers of this community, was conducted. For the nirS gene, denaturation was performed at 94 °C (1 min) followed by 10 cycles of 94 °C (30 s), 58 °C (30 s) decreasing by 0.5 °C per cycle, and 72 °C (40 s), followed by 30 cycles of 94 °C (30 s), 55 °C (30 s), and 72 °C (40 s), with a final extension at 72 °C (1 min). The primers used were cd3aF-R3cd. For the nosZ gene, denaturation was at 94 °C (1 min) followed by 10 cycles of 94 °C (1 min), 57 °C (1 min) decreasing by 0.5 °C per cycle, and 72 °C (1 min). Then, 25 cycles of 94 °C (1 min), 56 °C (1 min), 72 °C (1 min), and a final extension at 72 °C (1 min) were performed. The primers used were nosZ1188F-nosZ1869R [20]. The reaction mix (15 µL) included 200 ng/µL of bovine serum albumin (Sigma), 0.75 U Taq polymerase (ABM), 60 pM dNTPs, 10 pM of each primer, and 1.5 µL of 10X enzyme buffer. Amplification products for nirS (450 bp) and nosZ (700 bp) were verified by electrophoresis on 2% agarose gel with ethidium bromide, using amplicons of Pseudomonas fluorescens as a positive control.
2.5. Sequencing and Metagenomics Analysis
This analysis was carried out to detect the presence of functional genes in genomes of organisms present in two sediments samples (S1 and S2). For this the DNA extraction was carried out with the Invisorb spin soil DNA kit (Invitek Inc., Hayward, CA, USA) following the manufacturer’s instructions. Subsequently, the DNA samples (50 ng/µL) were sent to Novogen Corporation Inc. (USA) for library preparation and shotgun metagenomics (WOBI) analysis using the NovaSeq X Plus Series (PE150) platform with a 150-bp paired-end sequencing protocol, and 10 Gb of data was obtained per sample.
Raw sequence reads were assessed for quality using FastQC v0.12.1 [21] and MultiQC v1.25.1 [22]. Quality filtering was performed with Trimmomatic v0.39 [23], removing reads shorter than 45 bp, those with an average quality score below 20, or a Phred score under 33, ensuring the retention of high-quality sequences. Short reads were assembled using MEGAHIT v1.2.9 [24] (–min-contig-len 1500), generating 7031 and 22,367 contigs.
To characterize the genomic composition of nitrogen cycle–related genes, functional annotation of the filtered contigs was performed using DIAMOND v2.1.10 [25] BLASTP (–max-target-seqs 1, –max-hsps 1), enabling high-speed homology searches against reference databases. Assembled contigs were taxonomically classified using Kraken2 v2.1.3 [26] with the Standard-16 database, and the resulting taxonomic profiles were visualized using the Krona v2.7.1.
Raw metagenomic sequencing data were submitted as forward and reverse fastq compressed files to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1134729 (accessed on 11 July 2024); BioSample numbers are SAMN42435546 and SAMN42435547 for S1 and S2 raw reads, respectively.
3. Results
3.1. Microscopic Characterization of the Strains
The results showed that 53% of the strains were Gram-positive and 47% were Gram-negative, with morphological variation between bacilli and coccobacilli (Table 1).

Table 1.
Microscopic characteristics, N2O production rates, taxonomic determination, and detection of functional genes of nitrous oxide reductase-nosZ or nitrite reductase-nirS in denitrifying strains isolated from SML wetland sediments. * Strains with initial inoculum in culture equal to a McFarland standard of 0.5. nd: not determined. (x) = presence of the gene, (−) = absence of the gene.
3.2. N2O Production in Aerobic and Anaerobic Conditions
On the other hand, N2O production under anoxia showed that most of the isolated strains produced this GHG at different rates. As shown in Table 1, production levels reached up to 3865.5 nM/h in the first set of experiments where the microbial biomass was not standardized in the initial inoculum. Significant differences were found between the strains (p < 0.001 F = 20.7) and between incubation time (p < 0.001 F = 35.46) especially to 24 h where the isolated M43, M63, and M51 were different from M41 and M61 (p < 0.001 F = 29.44) by the highest N2O production in the first ones; however, the strains M64, M53, M21, M34, and M13 generated high N2O but did not present significant differences between them (p = 0.05 F = 3.46). In contrast, in the second set of experiments where the initial inoculum was standardized to a McFarland standard of 0.5, N2O production rates were lower (up to 163.6 nM/h). Statistical analysis showed significant differences in N2O production over time, particularly up to 24 h (p < 0.001 F = 8.44), and the interaction between time and bacteria had a significant but smaller effect on gas production (p = 0.01 F = 3.1).
When studying the effect of acetylene on the inhibition of N2O reductase in five strains under anoxic conditions (Table 2), it was observed that production increased 2.5 times for strain 4 only. Contrary to expectations, N2O production decreased by 91% for strain 5, 78.7% for strain 2, 29% for strain M31, and even showed consumption for strain 1 compared to the control under anoxia alone (Table 1). From this experiment, it was observed that some strains did not show N2O reductase inhibition by acetylene that would cause the accumulation of this greenhouse gas, except for strain 4. From this, it was estimated that although this strain reduced N2O to N2 at a rate of 12.9 nM/h, it did not completely reduce it under an anoxic environment and emitted approximately 7.8 nM/h to the environment.

Table 2.
Nitrous oxide production rates (nM/h) with acetylene addition as an N2O reductase inhibitor and the air addition for aerobic denitrification detection.
The addition of air (Table 2) showed that all strains can maintain N2O production at different rates. Strains M31 and 5, for instance, decreased their N2O production by 27% and 49%, respectively, compared to the anoxic experiments. However, strain 4 increased its N2O production by 23% in the presence of air, corresponding to an estimated rate of 1.8 nM/h for N2O to N2 reduction. For strain 1, it was evident that the N2O reductase was inhibited by oxygen and was quantified as N2O production of 0.07 ± 0.05 nM/h. The statistical analysis carried out for each isolated showed significant differences between experiments for strain 2 (p < 0.001 F = 7.52) between anoxia and acetylene addition only.
The nutrient analysis of the supernatants from the second set of experiments showed that the studied bacteria could generate ammonium from nitrate reduction between 0.004 and 0.29 mg/L (Table 3). Strains M31 and 2 showed the highest production of this nutrient, reducing 99% of the nitrate present in the medium, producing little nitrite, and emitting similar N2O concentrations under anoxia. Conversely, strain 1 did not produce ammonium and actively reduced nitrate without accumulating nitrite. Strain 4 produced very little ammonium and reduced 97% of the nitrate without accumulating nitrite, which remained at low levels, but produced N2O. Strain 5 was unique in completely reducing the nitrate, making it undetectable after 24 h. This led to a nitrite accumulation of 0.42 mg/L and the highest N2O accumulation (163 nM/h) recorded in the second set of experiments under anoxia, with its production inhibited by 90% with acetylene and 50% with air.

Table 3.
Concentration (mg/L) of ammonium, nitrate, and nitrite in the supernatants of each strain below anoxic experiments.
As mentioned earlier, nitrite levels remained low in anoxic experiments (<0.02 mg/L). However, in all strains except strain 5, nitrite levels were quantified between 0.7 and 0.8 mg/L in experiments with acetylene addition, with nitrite production rates estimated at up to 0.017 mg/h (data do not show). This suggests that acetylene somehow affects the accumulation of this nutrient.
3.3. Presence of Functional Denitrification Genes in Bacterial Isolates and Sediment Samples
Given that we wanted to investigate whether there was any relationship between the absence of the nitrous oxide reductase gene (nosZ) and the production of this GHG through partial denitrification in the analyzed strains, amplification of nosZ was performed along with the nirS gene, which is also a marker for denitrifying microorganisms that reduce nitrite to nitric oxide. The results in Table 1 show that 7 out of 10 strains possess the nitrous oxide reductase gene, but its presence did not correlate with low or negligible N2O production under anoxic conditions, except for strain M41, which produced N2O at low rates (3.1 nM/h). In fact, high rates of N2O production were observed in strains with the nosZ gene under anoxic conditions, suggesting that these bacteria are not undergoing complete denitrification to N2 or that this gene, although present, is not functional under the experimental conditions. It was also observed in some strains that the nirS or nosZ genes were not detected, suggesting they may possess other types of genes associated with denitrification processes that should be detected using different primers.
Sequencing of some of the isolates that showed greater N2O production under anoxia indicated that they belonged to the Bacillus genus such as Bacillus sp., Bacillus mojavensis, and Bacillus pumilus and to Lelliottia amnigena strain Br-5, all with 98% sequence similarity.
The functional gene searching within metagenomes showed that the nosZ gene was similar (>70%) to this present in Ignavibacterium album atypical while the nirS gene was related to Sulfurifustis variabilis and Thiobacillus denitrificans and the nirK gene was related to Ralstonia pickettii, Noviherbaspirillum autotrophicum (autotrophic denitrifier), Nitrospira japónica y Nevskia soli (strict aerobic). It is interesting to note that the results showed that the last two organisms are nitrifiers that carry the nirK gene. The shotgun analysis also detected through the amoA gene the presence of Nitrospira sp. SG-bi and with the nitrite oxidoreductase alpha subunit (nXRA) gene to Nitrospira lenta, Candidatus Nitrospira nitrosa y Candidatus Nitrospira inopinata, the latter two belonging to the Comammox type (Figure 1).
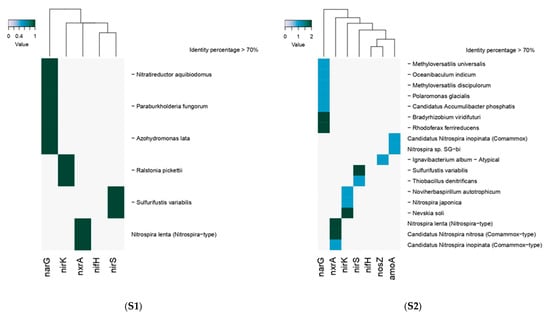
Figure 1.
Detection of denitrification and nitrification functional genes in sediment samples S1 and S2.
The metagenomic analysis showed a bacterial abundance between 54% (S1) and 63% (S2) in the sediments and the other sequences did not present similarity with the genomic region studied (Figure 2). The Pseudomonadota phylum predominated in both sediments with 71–75% abundance. Within this phylum the Beta proteobacteria was dominant (51%—S2, 38%—S1) followed by Alpha proteobacteria (38%—S1, 17%—S2) and Gamma proteobacteria (23%—S2, 17%—S1). Terrabacteria (10–12%), other bacteria (7–10%), and Acidobacteria (3%) were also detected in both sediments. On the other hand, Archaea was found in both samples (0.3–1%) with a dominance of Euryarchaeota (84%) followed by Nitrososphaeria (11%) and Methanomassilliicoccales (5%) at S1 and Nitrososphaerota (78%) followed by Euryarchaeota (21%) at Figure 2 (S2).
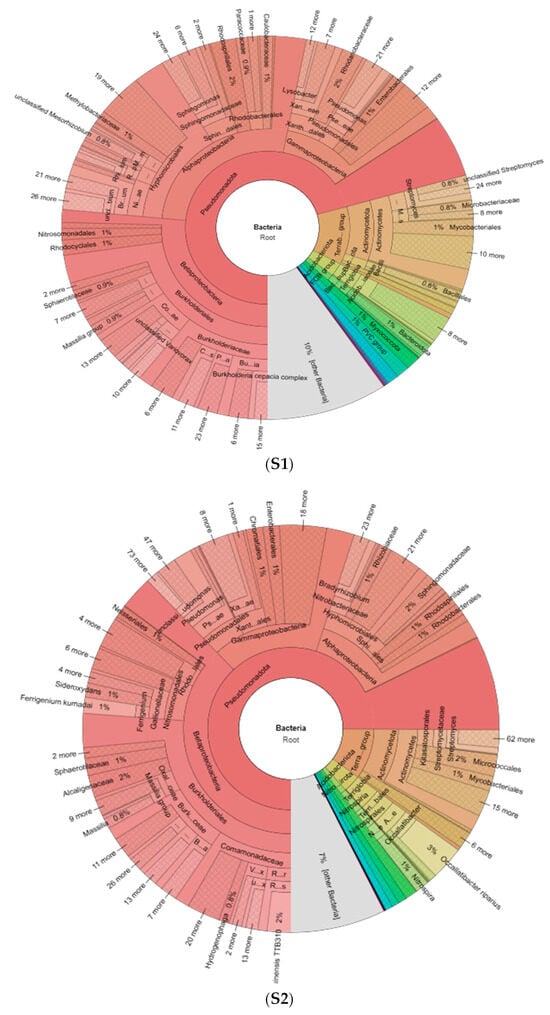
Figure 2.
Metagenomic analysis of wetland sediment samples S1 and S2.
4. Discussion
In this research, we evaluated the N2O production capacity of denitrifying strains isolated from sediments of the urban wetland Santa María del Lago under anoxic conditions, in the presence of air, and in the presence of acetylene (an inhibitor of nitrous oxide reductase enzyme) to determine if they could contribute to greenhouse gas emissions, and if these emissions could be related to the absence of the nitrous oxide reductase gene nosZ.
According to our results, we observed that all strains produced N2O at different rates under anoxic and oxic conditions. Regarding N2O production under anoxia, rates as high as 570 nM/h have been observed in strains of P. denitrificans [4], indicating that some of our strains have a higher capacity for N2O production under anoxia. Although there is substantial evidence supporting the denitrification capabilities of various Bacillus species [27], only one study reports that B. pumilus presented capabilities to reduce nitrate, nitrite, and ammonium even under aerobic conditions [28]; however, along with B. mojavensis, this is the first report about its role in the N2O production. Our results should be interpreted cautiously when compared to previous reports because the highest N2O production rates were obtained in the first set of experiments, with strains where microbial biomass was not standardized at the start of the assay and nitrite levels in the culture were not determined. This is important, because nitrite, which is in equilibrium with free nitrous acid, can inhibit nitrous oxide reduction as it is toxic to bacteria [29] and could be overestimating our results.
In the second set of experiments, the strain 5, which was like Lelliottia amnigena Br-5 (98%), exhibited the highest N2O production under anoxia (163 nM/h), suggesting lack or inactivity of the N2O reductase. In line with our findings, a study conducted on Lelliottia amnigena PTJIIT1005 revealed that assimilatory and respiratory nitrate reductase, nitrite reductase, and nitric oxide reductase are highly conserved among strains of this species. However, the genome assembly indicated the absence of nitrous oxide reductase (nosZ) [30], suggesting that, like our isolates, this bacterium may produce N2O as a by-product of an incomplete denitrification pathway. This phenomenon has also been reported for Accumulibacter clade IC [31] and other taxa lacking nosZ isolated from soils [32] as well as a nitrate ammonifier such as Escherichia coli or S. Typhimurium where N2O tends to accumulate due to the inability to reduce it to N2 [33].
The N2O production under aerobic conditions (28–38 µM O2) has been quantified in P. stutzeri and P. denitrificans at rates of 372 and 1680 nM/h, respectively [4], which are much higher compared to the production rates presented by our strains, not exceeding 83.0 nM/h (strain 5); however, our rate is similar to the rate of 58.2 nM/h reported under hypoxic conditions (5.3 µM O2) for the strain P. stutzeri TR2 [4]. However, it is noted that aerobic denitrification depends on the culture conditions and substrate carbon source. It has also been reported that up to 38 µM O2 there are two strains (P. stutzeri TR2 and Pseudomonas sp. K50) that can reduce nitrate to N2, identified as aerobic denitrifiers [4]. These results are like those obtained in this study with strain 2, which showed 99% nitrate reduction and a N2O consumption rate of 0.2 nM/h under aerobic conditions. From this study, we can also highlight that strain 2 is promising, as it may be a good candidate for use in aerobic bioremediation systems that require microorganisms with a high affinity for N2O to reduce GHG into the atmosphere, as has been reported previously with other strains possessing this capability such as P. denitrificans [34], M. aerodenitrificans [35], T. mechernichensis [36], Azospira [7], Pseudomona JM5, Acinetobacter, and Marinobacter [6].
The experiments with acetylene did not inhibit the N2O reductase enzyme in most strains, except for strain 4. It has been reported that acetylene can catalyze the oxidation of nitric oxide in samples with high nitrate levels, reducing its effectiveness [37], or in other cases, acetylene can be decomposed by heterotrophic denitrifiers that degrade it as a carbon source associated with high CO2 production during prolonged exposure periods [38,39,40]. Although this was not the objective of this research, it was quantified that strains 4 and M31 reached CO2 levels of up to 87.6 ppm and 21.9 ppm, respectively, after 24 h of experimentation in the presence of acetylene (data do not show). However, this assertion needs to be verified with new experiments. It is also possible that there was incomplete inhibition of the reductase, as previously reported by some researchers who showed that the denitrifying bacterium Flexibacter canadensis reduced N2O even in the presence of inhibitory concentrations of acetylene [41,42].
It was interesting to observe the ammonium production from nitrate with the M31 and 2 strains in anoxic experiments. Other studies have also reported the dissimilatory nitrate reduction to ammonium performed by bacteria such as Bacillus, Wollinella, and Anaeromixobacter with a contribution of 0.3–3% of N2O which is subsequently reduced to N2 [43,44]. In this study the M31 strain identified as Bacterium Bac1-35 (98% similarity with one isolated previously [45] also produced N2O with and without oxygen; however, we do not analyze how much of the N2O produced could come from this reaction, suggesting a need for further investigation in this regard.
These results demonstrate that the studied strains possess denitrification enzymes active under both aerobic and anaerobic conditions, leading to N2O accumulation even in the presence of the nosZ gene. This observation suggests a behavior consistent with previous studies, which have shown that the activity of nitrous oxide reductase is influenced by multiple factors, including the carbon-to-nitrogen (C/N) ratio, pH, concentrations of nitrogen oxides, oxygen availability [46], and even transcriptional regulation in response to extracellular copper concentration [47,48]
The data also indicate that the nitrate concentration used (0.5 g/L) in the experiments was sufficient for its reduction and for N2O generation, corroborating previous results with Burkholderia sp. SF-E2 [49], which indicate that higher nitrate proportions in the C/N ratio increase the N2O production, and with Pseudomonas mandelii, which indicate that higher nitrate levels (1000 mg/L) resulted in increased transcription of nitrite and nitric oxide reduction genes, with higher nitrite, N2O production, and denitrification rates of up to 98 µM [50]. In fact, our data support this information; although we estimated a lower denitrification rate for strain 4 (0.3 µM/d) compared to that reported previously [50], our rate is higher than these estimates from in situ experiments from sediment–water samples that were <0.001 µM/d [10].
These results are important when extrapolating to the ecosystem, because high nitrate or carbon concentrations have not been reported in sediment or water in the SML wetland, despite the temporal and spatial variation determined there [10,13], associated with a low N:P ratio indicative of nitrogen limitation during dry and rainy seasons [14]. These aspects suggest that bacteria, although present, depend on or are limited by nitrate availability for N2O generation, which can occur under anaerobic or aerobic conditions by denitrification or DNRA, as we demonstrated in the experiments, indicating the importance of maintaining low levels of this nutrient in the water body to prevent GHG emissions.
Alternatively, it is possible that the bacteria do not possess the nosZ gene, as reported for Burkholderia sp. SF-E2 [49]. However, to definitively identify strains lacking this gene and potentially emitting this GHG, we need to complement the information on the presence of the nosZ-clade I gene in the strains of the second set of experiments to determine if they lack it or not. Furthermore, it would be necessary to confirm that strains lacking this gene also do not possess the nosZ-clade II gene recently identified in some denitrifying and non-denitrifying microorganisms belonging to different phyla [51], which even dominate in experiments of N2O production by denitrification under high acetylene concentrations [52] and may dominate over clade I nosZ denitrifiers in a variety of terrestrial environments [53]. In fact, the metagenomic analysis showed that the nitrous oxide reductase was associated only with Ignavibacterium album which has been suggested to function as a N2O sink harboring the clade II nosZ gene [54,55]. It is possible that this microorganism corresponds to some of the isolated strains in which, although the clade I nosZ gene was not detected, they did reduce N2O; however, to corroborate this statement, sequencing is required. Likewise, it would be necessary to corroborate with new and specific isolates the presence of Sulfurifustis, Thiobacillus, or Noviherbaspirillum whose functional genes nirS and nirK also showed some relationship with these microorganisms which have been previously reported in different types of soils for their abundance, NIR activity, and capacity to reduce N2O [56,57,58].
Finally, sediment metagenomic analysis also revealed the presence of bacteria associated with N2O production, such as Nitrospira lenta, involved in nitrite oxidation to nitrate, and Comammox-type bacteria (Nitrospira nitrosa and Nitrospira inopinata), capable of complete ammonia oxidation. However, this microbial group has been reported to produce significantly lower amounts of N2O compared to canonical ammonia oxidizers [59,60]. Although they do not perform denitrification, their presence complements the microbial diversity identified in this urban wetland and opens new avenues for future research.
5. Conclusions
The denitrifying bacteria isolated from the SML wetland can generate N2O in high concentrations under both aerobic and anaerobic conditions if sufficient nitrate is present in the medium, which may have ecological implications for the SML wetland if it receives input of this nutrient. It was also observed that some bacteria can reduce N2O in the presence of oxygen, which may be advantageous for their potential use in aerobic wastewater treatments and to expand studies on the role of aerobic denitrification in ecosystems. Furthermore, it was determined that although the clade I nosZ gene is present in some strains, N2O is not completely reduced to N2 under anoxia as expected in a complete denitrification process. These results highlight the functional versatility and molecular diversity of this microbial group involved in N2O production. However, advanced technologies are needed to further analyze the effects of climate change on these aspects and on the net flux of microbially driven greenhouse gas emissions from tropical wetlands that could lead to innovative strategies to mitigate N2O emissions.
Author Contributions
M.C.-G. designed and supervised the study. M.C.-G. performed most of the experiments. V.M. performed bioinformatics and functional annotation analysis. M.C.-G. and V.M. approved the submitted version and contributed to this article. All authors have read and agreed to the published version of the manuscript.
Funding
This publication is a product of the project INV-CIAS 3752, entitled “Producción de óxido nitroso por bacterias aisladas de sedimentos del humedal Santa María del Lago y caracterización metagenomica de la comunidad nitrificante” funded by the research vice rectory at Universidad Militar Nueva Granada in the year 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw metagenomic sequencing data were submitted to the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1134729 (accessed on 11 July 2024), BioSample numbers are SAMN42435546 and SAMN42435547.
Acknowledgments
The authors would like to thank the Environment Secretary of Bogotá, Colombia for granting sampling permission within the Santa María del Lago wetland. Special thanks go to SML wetland staff member A. Ortiz for sampling assistance, to E. Gómez and G. Rincon for the support in the statistical analysis, P. Aguilar-Muñoz for the bioinformatic analysis, and L. Castiblanco for the laboratory support. The authors have reviewed and edited the output and take full responsibility for the content of this publication. This research was developed following the national regulation on the collection of non-commercial specimens of biological diversity for scientific research (Resolution No. 1198/2014 ANLA.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lan, X.; Thoning, K.W.; Dlugokencky, E.J. Trends in Globally Averaged CH4, N2O, and SF6 Determined from NOAA Global Monitoring Laboratory Measurements; Version 2024-06; NOAA Global Monitoring Laboratory: Boulder, CO, USA, 2024. [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Doschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; pp. 659–740. [Google Scholar] [CrossRef]
- Tian, H.; Pan, N.; Thompson, R.L.; Canadell, J.G.; Suntharalingam, P.; Regnier, P.; Davidson, E.A.; Prather, M.; Ciais, P.; Muntean, M.; et al. Global nitrous oxide budget (1980–2020). Earth Syst. Sci. Data 2024, 16, 2543–2604. [Google Scholar] [CrossRef]
- Takaya, N.; Catalan-Sakairi, M.A.B.; Sakaguchi, Y.; Kato, I.; Zhou, Z.; Shoun, H. Aerobic denitrifying bacteria that produce low levels of Nitrous Oxide. Appl. Environ. Microbiol. 2003, 69, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zheng, Y.; Liu, M.; Gong, J.; Zhang, X.; Yin, G.; You, L. Anaerobic ammonium oxidation (Anammox) bacterial diversity, abundance and activity in marsh sediments of the Yangtze Estuary. J. Geophys. Res. Biogeosciences 2013, 118, 1237–1246. [Google Scholar] [CrossRef]
- Kyoung-In, J.; Amith, A.; Okkyoung, C.h.; Byoung-In, S. Aerobic denitrification by a novel Pseudomonas sp. JN5 in different bioreactor systems. Water-Energy Nexus 2020, 2, 37–45. [Google Scholar]
- Suenaga, T.; Riya, S.; Hosomi, M.; Terada, A. Biokinetic characterization and activities of N2O-reducing bacteria in response to various oxygen levels. Front. Microbiol. 2018, 9, 697. [Google Scholar] [CrossRef]
- Canchala, T. Generación de Gases de Efecto Invernadero en los Sedimentos de un Humedal Natural Eutrofizado: Influencia de Nutrientes (N y P). Master’s Thesis, Universidad del Valle, Cali, Colombia, 2014. [Google Scholar]
- Castro-Gonzalez, M.; Torres Valdés, M. Gases invernadero en aguas con bajo oxígeno en el reservorio eutrófico de Prado (Colombia). Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2015, 39, 399–407. [Google Scholar] [CrossRef]
- Castro-González, M.; González, A. La producción neta de óxido nitroso en un humedal urbano en Colombia es principalmente influenciada por cambios estacionales. Limnetica 2020, 39, 693–709. [Google Scholar] [CrossRef]
- Bharam, M.; Espenberg, M.; Pärn, J.; Lehtovirta-Morley, L.; Anslan, S.; Kasak, K.; Kõljalg, U.; Liira, J.; Maddison, M.; Moora, M.; et al. Structure and function of the soil microbiome underlying N2O emissions from global wetlands. Nat. Commun. 2022, 13, 1430. [Google Scholar] [CrossRef]
- Castro-González, M.; Pacheco, M.; Restrepo, M. Bacterial communities in sediments of an urban wetland in Bogotá, Colombia. Univ. Sci. 2022, 27, 163–185. [Google Scholar] [CrossRef]
- López, R.H. Estado Trófico de un Humedal Urbano Andino Tropical: Santa María del Lago, Bogotá, D.C. Colombia; Periódicas S.A.S; Universidad Militar Nueva Granada: Bogotá, Colombia, 2012. [Google Scholar]
- Guillot, M.; Pinilla, G.A. Estudios Ecológicos en Humedales de Bogotá: Aplicaciones para su Evaluación, Seguimiento y Manejo; Editorial Universidad Nacional de Colombia: Bogotá, Colombia, 2017; p. 314. [Google Scholar]
- Yoshinari, T.; Knowles, R. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 1976, 69, 705–710. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Weiss, R.; Price, B.A. Nitrous oxide solubility in water and seawater. Mar. Chem. 1980, 8, 347–359. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Heuer, H.; Krsek, M.; Baker, P.; Smalla, K.; Wellington, E. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997, 63, 3233–3241. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, A.; Hallin, S. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. Babraham Bioinformatics—FastQC A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 5 September 2019).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, D.H.; Luo, R.; Liu, C.-M.; Leung, C.-M.; Ting, H.-F.; Sadakane, K.; Yamashita, H.; Lam, T.-W. MEGAHIT v1.0: A fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 2016, 102, 3–11. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2014, 12, 59–60. [Google Scholar] [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Verbaendert, I.; Boon, N.; De Vos, P.; Heylen, K. Denitrification is a common feature among members of the genus Bacillus. Syst. Appl. Microbiol. 2011, 34, 385–391. [Google Scholar] [CrossRef]
- Yang, T.; Xin, Y.; Zhang, L.; Gu, Z.; Li, Y.; Ding, Z.; Shi, G. Characterization on the aerobic denitrification process of Bacillus strains. Biomass Bioenergy 2020, 140, 105677. [Google Scholar] [CrossRef]
- Cao, S.; Cheng, Z.; Koch, K.; Fang, J.; Du, R.; Peng, Y. Municipal wastewater driven partial- denitrification (PD) aggravated nitrous oxide production. J. Clean. Prod. 2024, 434, 139916. [Google Scholar] [CrossRef]
- Thakur, P.; Gauba, P. Identification and examination of nitrogen metabolic genes in Lelliottia amnigena PTJIIT1005 for their ability to perform nitrate remediation. BMC Genom. 2023, 24, 104. [Google Scholar] [CrossRef]
- Gao, H.; Mao, Y.; Zhao, X.; Liu, W.-T.; Zhang, T.; Wells, G. Genome-centric metagenomics resolves microbial diversity and prevalent truncated denitrification pathways in a denitrifying PAO-enriched bioprocess. Water Res. 2019, 155, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Roco, C.A.; Bergaust, L.L.; Bakken, L.R.; Yavitt, J.B.; Shapleigh, J.P. Modularity of nitrogen-oxide reducing soil bacteria: Linking phenotype to genotype. Environ. Microbiol. 2017, 19, 2507–2519. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Simon, J.; Rowley, G.; Bedmar, E.J.; Richardson, D.J.; Gates, A.J.; Delgado, M.J. Nitrous Oxide Metabolism in Nitrate-Reducing Bacteria: Physiology and Regulatory Mechanisms, chapter seven. Adv. Microb. Physiol. 2016, 68, 353–432. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.A.; Dalsgaard, T.; Revsbech, N.P.; Kuenen, J.G. Confirmation of aerobic denitrification’ in batch cultures, using gas chromatography and 15N mass spectrometry. FEMS Microbiol. Ecol. 1995, 18, 113–120. [Google Scholar] [CrossRef]
- Patureau, D.; Bernet, N.; Moletta, R. Effect of oxygen on denitrification in continuous chemostat culture with Comamonas sp. strain SGLY2. J. Indian Microbiol. 1995, 16, 124–128. [Google Scholar] [CrossRef]
- Scholten, E.; Lukow, T.; Auling, G.; Kroppenstedt, R.M.; Rainey, F.A.; Dielmann, H. Thaurea mecharnichensis sp. nov., an aerobic denitrifier from a leachate treatment Plant. Int. J. Syst. Bacteriol. 1999, 49, 1045–1051. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Z.; Qin, S.; Zhou, S.; Hu, C.; Clough, T.; Wrage-Mönnig, N.; Luo, J.; Conrad, R. Effects of nitrate and water content on acetylene inhibition technique bias when analyzing soil denitrification rates under an aerobic atmosphere. Geoderma 2019, 334, 33–36. [Google Scholar] [CrossRef]
- Yeomans, J.C.; Beauchamp, E.G. Acetylene as a possible substrate in the denitrification process. Can. J. Soil Sci. 1982, 62, 137–144. [Google Scholar] [CrossRef]
- Terry, R.E.; Duxbury, J.M. Acetylene decomposition in soils. Soil Sci. Soc. Am. J. 1985, 49, 90–94. [Google Scholar] [CrossRef]
- Topp, E.; Germon, J.C. Acetylene metabolism and stimulation of denitrification in an agricultural soil. Appl. Environ. Microbiol. 1986, 52, 802–806. [Google Scholar] [CrossRef]
- Yu, K.; Seo, D.-C.h.; DeLaune, R.D. Incomplete acetylene inhibition of Nitrous Oxide reduction in potential denitrification assay as revealed by using 15N-Nitrate tracer. Commun. Soil Sci. Plant Anal. 2010, 41, 2201–2210. [Google Scholar] [CrossRef]
- Qin, S.; Hu, C.h.; Oenema, O. Quantifying the underestimation of soil denitrification potential as determined by the acetylene inhibition method. Soil Biol. Biochem. 2012, 47, 14–17. [Google Scholar] [CrossRef]
- Sun, Y.; De Vos, P.; Heylen, K. Nitrous oxide emission by the non-denitrifying, nitrate ammonifier Bacillus licheniformis. BMC Genom. 2016, 17, 68. [Google Scholar] [CrossRef]
- Yoon, S.; Heo, H.; Han, H.; Song, D.; Bakken, L.R.; Frostegård, Å.; Yoon, S. Suggested role of NosZ in preventing N2O inhibition of dissimilatory nitrite reduction to ammonium. mBio 2023, 14, e01540-23. [Google Scholar] [CrossRef]
- Lucas, J.A.; García-Villaraco, A.; Ramos, B.; García-Cristobal, J.; Algar, E.; Gutierrez-Mañero, J. Structural and functional study in the rhizosphere of Oryza sativa L. plants growing under biotic and abiotic stress. J. Appl. Microbiol. 2013, 115, 218–235. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, L.J.; Wang, R.; He, Z.; Chen, X. New insight on the regulation of N2O production in aerobic condition: An N2O metabolic perspective based on enzymatic analysis of nitrous oxide reductase. J. Water Process Eng. 2021, 41, 102090. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Gates, A.J.; Appia-Ayme, C.; Rowley, G.; Richardson, D.J. Copper control of bacterial nitrous oxide emission and its impact on vitamin B12-dependent metabolism. Proc. Nat. Acad. Sci. USA 2013, 110, 19926–19931. [Google Scholar] [CrossRef] [PubMed]
- Gaimster, H.; Alston, M.; Richardson, D.J.; Gates, A.J.; Rowley, G. Transcriptional and environmental control of bacterial denitrification and N2O emissions. FEMS Microbiol. Lett. 2018, 365, fnx277. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wang, M.; Tahvanainen, T.; Hashidoko, Y. Nitrous oxide emission potentials of Burkholderia species isolated from the leaves of a boreal peat moss Sphagnum fuscum. Biosci. Biotechnol. Biochem. 2015, 79, 2086–2095. [Google Scholar] [CrossRef]
- Saleh-Lakha, S.; Shannon, K.E.; Henderson, S.-L.; Zebarth, B.J.; Burton, D.L.; Goyer, C.; Trevors, J.T. Effect of nitrate and acetylene on nirS, cnorB, and nosZ expression and denitrification activity in Pseudomonas mandelii. Appl. Environ. Microbiol. 2009, 75, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Graf, D.R.H.; Bru, D.; Philippot, L.; Hallin, S. The unaccounted yet abundant nitrous oxide- reducing microbial community: A potential nitrous oxide sink. ISME J. 2013, 7, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhuge, Y.; Liu, Y.; Shapleigh, J.; Li, W. Long-term effects of acetylene on denitrifying N2O production: Biomass performance and microbial community. J. Water Process Eng. 2021, 42, 102137. [Google Scholar] [CrossRef]
- Coyotzi, S.; Doxey, A.C.; Clark, I.D.; Lapen, D.R.; Van Cappellen, P.; Neufeld, J.D. Agricultural soil denitrifiers possess extensive nitrite reductase gene diversity. Environ. Microbiol. 2017, 19, 1189–1208. [Google Scholar] [CrossRef]
- Zhenfeng, L.; Niels-Ulrik, F.; Kajetan, V.; Takao, I.; Moriya, O.; Joerg, O.; Donald, B. Complete Genome of Ignavibacterium album, a metabolically versatile, flagellated, facultative anaerobe from the Phylum Chlorobi. Front. Microbiol. 2012, 3, 185. [Google Scholar] [CrossRef]
- Oba, K.; Suenaga, T.; Yasuda, S.; Kuroiwa, M.; Hori, T.; Lackner, S.; Terada, A. Quest for Nitrous Oxide-reducing bacteria present in an Anammox biofilm fed with Nitrous Oxide. Microbes Environ. 2023, 39, ME23106. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, K.; Liu, Y.; Zhao, J.; Ma, J.; Yu, Q.; Zou, P.; Lin, H.; Wang, Q. Differential responses of soil nirS- and nirK-type denitrifying microbial communities to long-term application of biogas slurry in a paddy soil. Appl. Soil Ecol. 2023, 182, 104711. [Google Scholar] [CrossRef]
- Maheshwari, A.; Jones, C.M.; Tiemann, M.; Hallin, S. Carbon substrate selects for different lineages of N2O reducing communities in soils under anoxic conditions. Soil Biol. Biochem. 2023, 177, 108909. [Google Scholar] [CrossRef]
- Ishii, S.; Ashida, N.; Ohno, H.; Segawa, T.; Yabe, S.; Otsuka, S.; Yokota, A.; Senoo, K. Noviherbaspirillum denitrificans sp. nov., a denitrifying bacterium isolated from rice paddy soil and Noviherbaspirillum autotrophicum sp. nov., a denitrifing, facultatively autotrophic bacterium isolated from rice paddy soil and proposal to reclassify Herbaspirillum massiliense as Noviherbaspirillum massiliense comb. nov. Int. J. Syst. Evol. Microbiol. 2017, 7, 1841–1848. [Google Scholar] [CrossRef]
- Han, P.; Wu, D.; Sun, D.; Zhao, M.; Wang, M.; Wen, T.; Zhang, J.; Hou, L.; Liu, M.; Klümper, U.; et al. N2O and NOy production by the comammox bacterium Nitrospira inopinata in comparison with canonical ammonia oxidizers. Water Res. 2021, 190, 116728. [Google Scholar] [CrossRef]
- Kits, K.D.; Jung, M.Y.; Vierheilig, J.; Pjevac, P.; Sedlacek, C.J.; Liu, S.; Herbold, C.; Stein, L.Y.; Richter, A.; Wissel, H.; et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 2019, 10, 1836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).