Computational Modeling of Sodium-Ion-Channel-Based Glucose Sensing Biophysics to Study Cardiac Pacemaker Action Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Adaptation
2.2. General Membrane Current Descriptions
2.3. Na Channel with Blood Glucose Sensing Mechanism
3. Results
- Cycle length (CL): The duration between the peaks of two consecutive APs, representing the pacemaker activity cycle.
- Peak potential (PP): The maximum value reached during the AP.
- Action potential amplitude (APA): The difference between the peak potential and the most negative repolarization potential, reflecting the overall strength of the AP.
- Maximum diastolic potential (MDP): The most negative potential reached just before the peak potential, indicating the cell’s readiness for the next depolarization.
- Diastolic depolarization rate (DDR): The rate at which the membrane potential rises during diastole, indicative of the pacemaker cell’s automaticity.
- Diastolic depolarization rate over the first 100 ms (DDR100): A more specific measure of the initial depolarization rate during diastole.
- Action potential duration (APD): The time required for the membrane potential to repolarize to 90% of its peak value, providing insight into the refractory period and overall duration of the AP.
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Vinson, S.; Shofer, F.; Brice, J. The relationship between knowledge and risk for heart attack and stroke. J. Stroke Cerebrovasc. Dis. 2013, 22, 996–1001. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A. Sudden cardiac death. Cardiovasc. Pathol. 2001, 10, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Israel, C.W. Mechanisms of sudden cardiac death. Indian Heart J. 2014, 66, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Kalman, J.M.; Pedagogos, E.; Toussaint, N.; Vohra, J.K.; Sparks, P.B.; Sanders, P.; Kistler, P.M.; Halloran, K.; Lee, G.; et al. Bradycardia and asystole is the predominant mechanism of sudden cardiac death in patients with chronic kidney disease. J. Am. Coll. Cardiol. 2015, 65, 1263–1265. [Google Scholar] [CrossRef]
- Fozzard, H.A. Cardiac muscle: Excitability and passive electrical properties. Prog. Cardiovasc. Dis. 1977, 19, 343–359. [Google Scholar] [CrossRef]
- Reilly, J.P.; Antoni, H. Electrical properties of the heart. In Applied Bioelectricity: From Electrical Stimulation to Electropathology; Springer: New York, NY, USA, 1998; pp. 148–193. [Google Scholar]
- Varró, A.; Tomek, J.; Nagy, N.; Virág, L.; Passini, E.; Rodriguez, B.; Baczkó, I. Cardiac transmembrane ion channels and action potentials: Cellular physiology and arrhythmogenic behavior. Physiol. Rev. 2021, 101, 1083–1176. [Google Scholar] [CrossRef]
- Bers, D.M.; Bers, D.M. Cardiac action potential and ion channels. In Excitation-Contraction Coupling and Cardiac Contractile Force; Springer: New York, NY, USA, 2001; pp. 63–100. [Google Scholar]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Ion channels and the electrical properties of membranes. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Kass, R.S. Ionic basis of electrical activity in the heart. In Physiology and Pathophysiology of the Heart; Springer: Boston, MA, USA, 1989; pp. 81–93. [Google Scholar]
- Johnson, E.A.; Lieberman, M. Heart: Excitation and contraction. Annu. Rev. Physiol. 1971, 33, 479–530. [Google Scholar] [CrossRef]
- Santana, L.F.; Cheng, E.P.; Lederer, W.J. How does the shape of the cardiac action potential control calcium signaling and contraction in the heart? J. Mol. Cell. Cardiol. 2010, 49, 901. [Google Scholar] [CrossRef]
- Grant, A.O. Cardiac ion channels. Circ. Arrhythmia Electrophysiol. 2009, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bartos, D.C.; Eleonora, G.; Ripplinger, C.M. Ion channels in the heart. Compr. Physiol. 2015, 5, 1423. [Google Scholar] [PubMed]
- Morad, M.; Leslie, T. Ionic events responsible for the cardiac resting and action potential. Am. J. Cardiol. 1982, 49, 584–594. [Google Scholar] [CrossRef]
- Senges, J.; Brachmann, J.; Pelzer, D.; Krämer, B.; Kübler, W. Combined effects of glucose and hypoxia on cardiac automaticity and conduction. J. Mol. Cell. Cardiol. 1980, 12, 311–323. [Google Scholar] [CrossRef]
- Jouven, X.; Lemaître, R.N.; Rea, T.D.; Sotoodehnia, N.; Empana, J.P.; Siscovick, D.S. Diabetes, glucose level, and risk of sudden cardiac death. Eur. Heart J. 2005, 26, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Abe, R.A.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Mohammed, L. Type 2 diabetes and causes of sudden cardiac death: A systematic review. Cureus 2021, 13, e18145. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Sukhorukov, V.N.; Orekhov, A.N. Effect of glucose levels on cardiovascular risk. Cells 2022, 11, 3034. [Google Scholar] [CrossRef]
- Tenenbaum, A.; Enrique, Z.F. Impaired glucose metabolism in patients with heart failure. Am. J. Cardiovasc. Drugs 2004, 4, 269–280. [Google Scholar] [CrossRef]
- Gallego, M.; Zayas-Arrabal, J.; Alquiza, A.; Apellaniz, B.; Casis, O. Electrical features of the diabetic myocardium. Arrhythmic and cardiovascular safety considerations in diabetes. Front. Pharmacol. 2021, 12, 687256. [Google Scholar] [CrossRef]
- Grisanti, L.A. Diabetes and arrhythmias: Pathophysiology, mechanisms and therapeutic outcomes. Front. Physiol. 2018, 9, 409132. [Google Scholar] [CrossRef]
- Riise, H.K.R.; Igland, J.; Sulo, G.; Graue, M.; Haltbakk, J.; Tell, G.S.; Iversen, M.M. Casual blood glucose and subsequent cardiovascular disease and all-cause mortality among 159 731 participants in Cohort of Norway (CONOR). BMJ Open Diabetes Res. Care 2021, 9, e001928. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Deng, H.; Lin, W.-D.; He, S.-F.; Liu, F.-Z.; Liu, Y.; Zhan, X.-Z.; Fang, X.-H.; Liao, H.-T.; Wei, W.; et al. Association between elevated blood glucose level and non-valvular atrial fibrillation: A report from the Guangzhou heart study. BMC Cardiovasc. Disord. 2019, 19, 270. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Sotoodehnia, N.; Rea, T.D.; Raghunathan, T.E.; Jouven, X.; Lemaitre, R.N. Type 2 diabetes mellitus and the risk of sudden cardiac arrest in the community. Rev. Endocr. Metab. Disord. 2010, 11, 53–59. [Google Scholar] [CrossRef]
- Rajan, A.S.; Aguilar-Bryan, L.; A Nelson, D.; Yaney, G.C.; Hsu, W.H.; Kunze, D.L.; Boyd, A.E., III. Ion channels and insulin secretion. Diabetes Care 1990, 13, 340–363. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.E., III. The role of ion channels in insulin secretion. J. Cell. Biochem. 1992, 48, 234–241. [Google Scholar] [CrossRef]
- Ozturk, N.; Uslu, S.; Ozdemir, S. Diabetes-induced changes in cardiac voltage-gated ion channels. World J. Diabetes 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Baenziger, J.E. Ion channels as lipid sensors: From structures to mechanisms. Nat. Chem. Biol. 2020, 16, 1331–1342. [Google Scholar] [CrossRef]
- Zaydman, M.A.; Silva, J.R.; Cui, J. Ion channel associated diseases: Overview of molecular mechanisms. Chem. Rev. 2012, 112, 6319–6333. [Google Scholar] [CrossRef]
- Remme, C.A.; Bezzina, C.R. Sodium channel (dys) function and cardiac arrhythmias. Cardiovasc. Ther. 2010, 28, 287–294. [Google Scholar] [CrossRef]
- Chen, C.; Wang, S.; Hu, Q.; Zeng, L.; Peng, H.; Liu, C.; Huang, L.-P.; Song, H.; Li, Y.; Yao, L.-H.; et al. Voltage-gated Na+ channels are modulated by glucose and involved in regulating cellular insulin content of INS-1 Cells. Cell. Physiol. Biochem. 2018, 45, 446–457. [Google Scholar] [CrossRef]
- Bartocci, E.; Pietro, L. Computational modeling, formal analysis, and tools for systems biology. PLoS Comput. Biol. 2016, 12, e1004591. [Google Scholar] [CrossRef] [PubMed]
- Brodland, G.W. How computational models can help unlock biological systems. In Seminars in Cell & Developmental Biology; Academic Press: New York, NY, USA, 2015; Volume 47, pp. 62–73. [Google Scholar]
- Mahapatra, C.; Samuilik, I. A Mathematical Model of Spontaneous Action Potential Based on Stochastics Synaptic Noise Dynamics in Non-Neural Cells. Mathematics 2024, 12, 1149. [Google Scholar] [CrossRef]
- Mahapatra, C.; Kaur, A. Abstract 2221 In silico electrophysiological study reveals Ibrutinib, an important therapeutic agent for B-Cell lymphoma causes cardiac toxicity by inhibiting sodium current. J. Biol. Chem. 2024, 300, 106784. [Google Scholar] [CrossRef]
- Mahapatra, C.; Keith, B.; Rohit, M. Biophysically Realistic Models of Detrusor Ion Channels: Role in shaping spike and excitability. In Urinary Bladder Physiology: Computational Insights; Narosa Publishing House: New Delhi, India, 2024. [Google Scholar]
- Mahapatra, C.; Brain, K.L.; Manchanda, R. A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PLoS ONE 2018, 13, e0200712. [Google Scholar] [CrossRef] [PubMed]
- Amanfu, R.K.; Saucerman, J.J. Cardiac models in drug discovery and development: A review. Crit. Rev. Biomed. Eng. 2011, 39, 379–395. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, A.D. Systems biophysics: Multiscale biophysical modeling of organ systems. Biophys. J. 2016, 110, 1023–1027. [Google Scholar] [CrossRef]
- Mayourian, J.; Sobie, E.A.; Costa, K.D. An introduction to computational modeling of cardiac electrophysiology and arrhythmogenicity. In Experimental Models of Cardiovascular Diseases; Humana Press: New York, NY, USA, 2018; pp. 17–35. [Google Scholar]
- Puertas-Martín, S.; Banegas-Luna, A.J.; Paredes-Ramos, M.; Redondo, J.L.; Ortigosa, P.M.; Brovarets’, O.O.; Pérez-Sánchez, H. Is high performance computing a requirement for novel drug discovery and how will this impact academic efforts? Expert Opin. Drug Discov. 2020, 15, 981–985. [Google Scholar] [CrossRef]
- Southern, J.; Pitt-Francis, J.; Whiteley, J.; Stokeley, D.; Kobashi, H.; Nobes, R.; Kadooka, Y.; Gavaghan, D. Multi-scale computational modelling in biology and physiology. Prog. Biophys. Mol. Biol. 2008, 96, 60–89. [Google Scholar] [CrossRef]
- Noble, D.; Garny, A.; Noble, P.J. How the Hodgkin–Huxley equations inspired the cardiac physiome project. J. Physiol. 2012, 590, 2613–2628. [Google Scholar] [CrossRef]
- Amuzescu, B.; Airini, R.; Epureanu, F.B.; Mann, S.A.; Knott, T.; Radu, B.M. Evolution of mathematical models of cardiomyocyte electrophysiology. Math. Biosci. 2021, 334, 108567. [Google Scholar] [CrossRef]
- Earm, Y.E.; Noble, D. A model of the single atrial cell: Relation between calcium current and calcium release. Proc. R. Soc. London. B. Biol. Sci. 1990, 240, 83–96. [Google Scholar]
- Lindblad, D.S.; Murphey, C.R.; Clark, J.J.W.; Giles, W.R.; Muñoz, M.A.; Kaur, J.; Vigmond, E.J.; Fink, M.; Noble, P.J.; Noble, D.; et al. A model of the action potential and underlying membrane currents in a rabbit atrial cell. Am. J. Physiol. Heart Circ. Physiol. 1996, 271, H1666–H1696. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol. Heart Circ. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef] [PubMed]
- Nygren, A.; Fiset, C.; Firek, L.; Clark, J.W.; Lindblad, D.S.; Clark, R.B.; Giles, W.R. Mathematical model of an adult human atrial cell: The role of K+ currents in repolarization. Circ. Res. 1998, 82, 63–81. [Google Scholar] [CrossRef]
- Ramirez, R.J.; Nattel, S.; Courtemanche, M. Mathematical analysis of canine atrial action potentials: Rate, regional factors, and electrical remodeling. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1767–H1785. [Google Scholar] [CrossRef]
- Grandi, E.; Pandit, S.V.; Voigt, N.; Workman, A.J.; Dobrev, D.; Jalife, J.; Bers, D.M. Human atrial action potential and Ca2+ model: Sinus rhythm and chronic atrial fibrillation. Circ. Res. 2011, 109, 1055–1066. [Google Scholar] [CrossRef]
- Davies, M.R.; Wang, K.; Mirams, G.R.; Caruso, A.; Noble, D.; Walz, A.; Lavé, T.; Schuler, F.; Singer, T.; Polonchuk, L. Recent developments in using mechanistic cardiac modelling for drug safety evaluation. Drug Discov. Today 2016, 21, 924–938. [Google Scholar] [CrossRef][Green Version]
- Di Francesco, D.; Noble, D. A model of cardiac electrical activity incorporating ionic pumps and concentration changes. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 307, 353–398. [Google Scholar]
- Fabbri, A.; Fantini, M.; Wilders, R.; Severi, S. Computational analysis of the human sinus node action potential: Model development and effects of mutations. J. Physiol. 2017, 595, 2365–2396. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952, 117, 500. [Google Scholar] [CrossRef]
- Hines, M.L.; Carnevale, N.T. The NEURON simulation environment. Neural Comput. 1997, 9, 1179–1209. [Google Scholar] [CrossRef] [PubMed]
- Spach, M.S.; Kootsey, J.M. The nature of electrical propagation in cardiac muscle. Am. J. Physiol. Heart Circ. Physiol. 1983, 244, H3–H22. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.; Oesterlein, T.; Loewe, A.; Luik, A.; Schmitt, C.; Dössel, O. Regional conduction velocity calculation from clinical multichannel electrograms in human atria. Comput. Biol. Med. 2018, 92, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Jæger, K.H.; Edwards, A.G.; Giles, W.R.; Tveito, A. Arrhythmogenic influence of mutations in a myocyte-based computational model of the pulmonary vein sleeve. Sci. Rep. 2022, 12, 7040. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.A.; Ghovanloo, M.R.; Ruben, P.C. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br. J. Pharmacol. 2020, 177, 2932–2946. [Google Scholar] [CrossRef]
- Nieves-Cintrón, M.; Flores-Tamez, V.A.; Le, T.; Baudel, M.M.-A.; Navedo, M.F. Cellular and molecular effects of hyperglycemia on ion channels in vascular smooth muscle. Cell. Mol. Life Sci. 2021, 78, 31–61. [Google Scholar] [CrossRef]
- Yoshida, M.; Dezaki, K.; Yamato, S.; Aoki, A.; Sugawara, H.; Toyoshima, H.; Ishikawa, S.E.; Kawakami, M.; Nakata, M.; Yada, T.; et al. Regulation of voltage-gated K+ channels by glucose metabolism in pancreatic β-cells. FEBS Lett. 2009, 583, 2225–2230. [Google Scholar] [CrossRef]
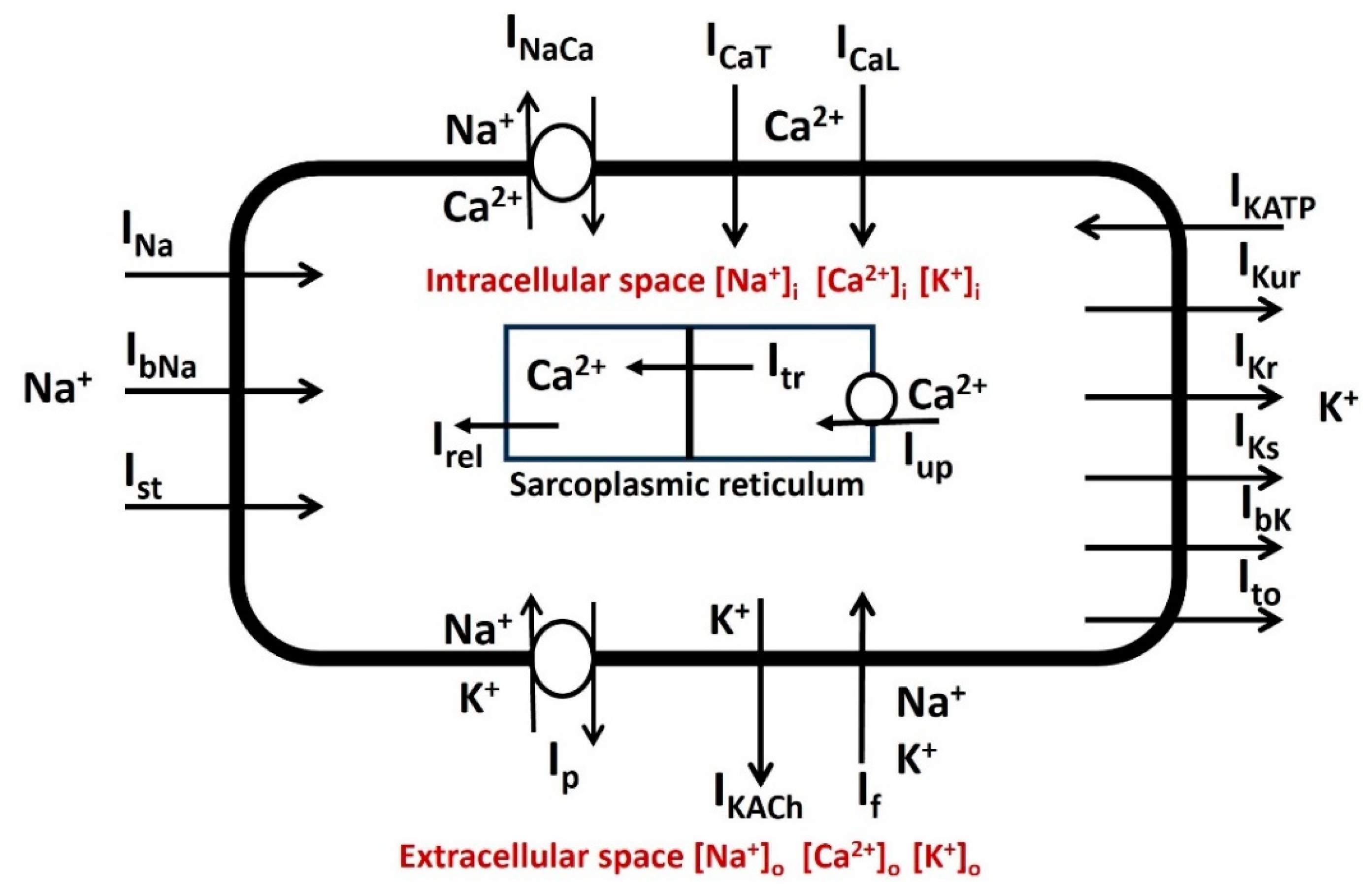
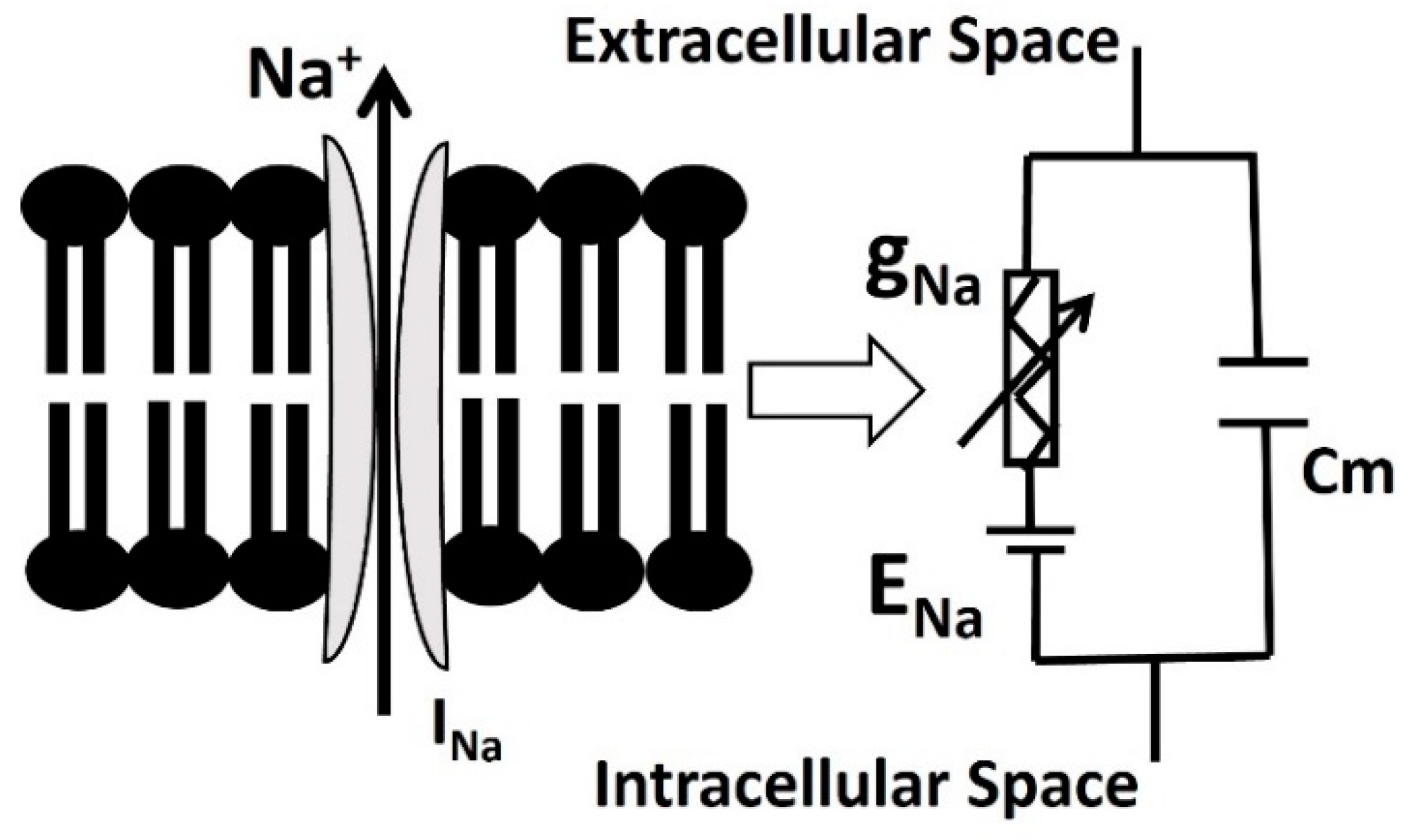
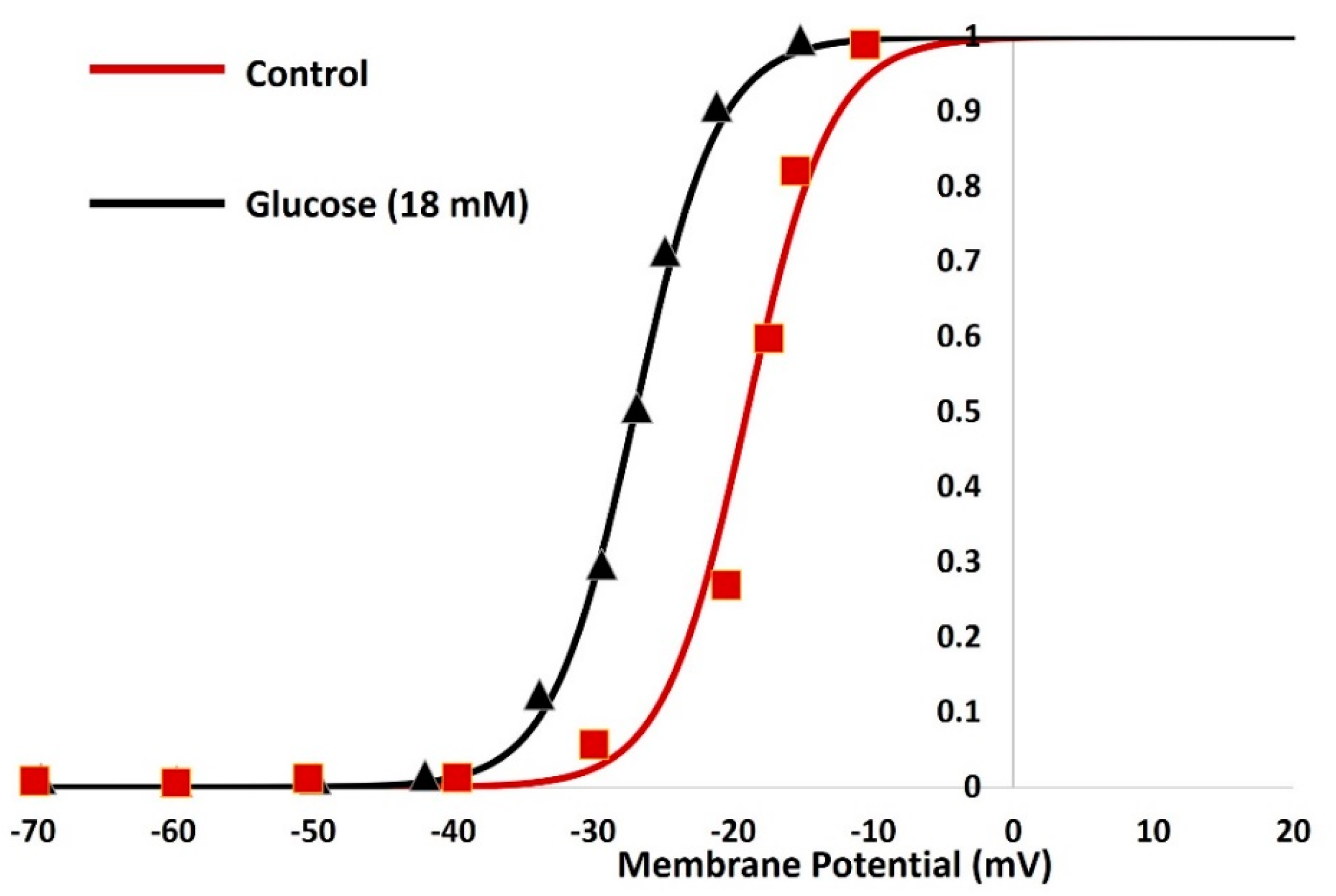
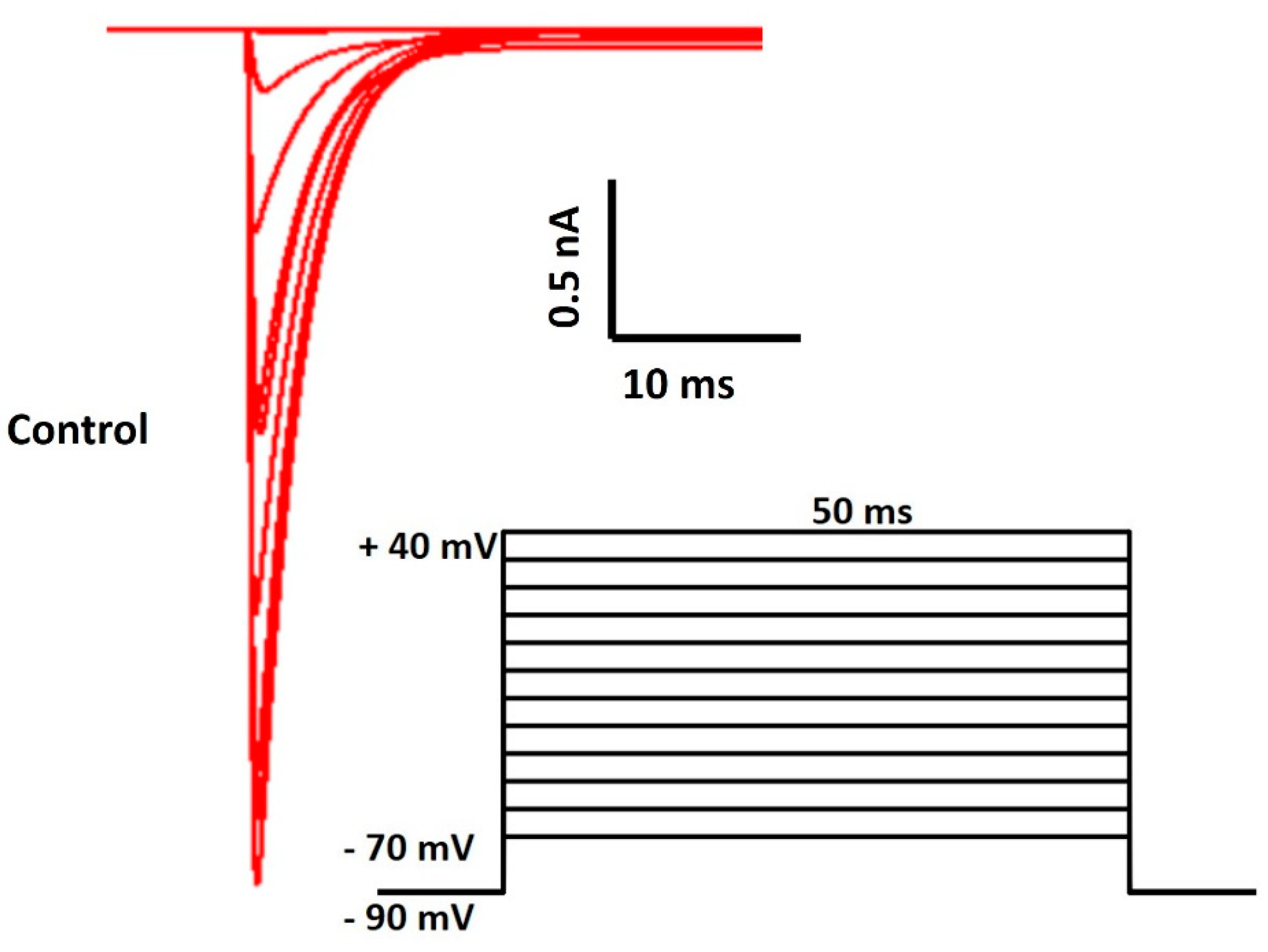
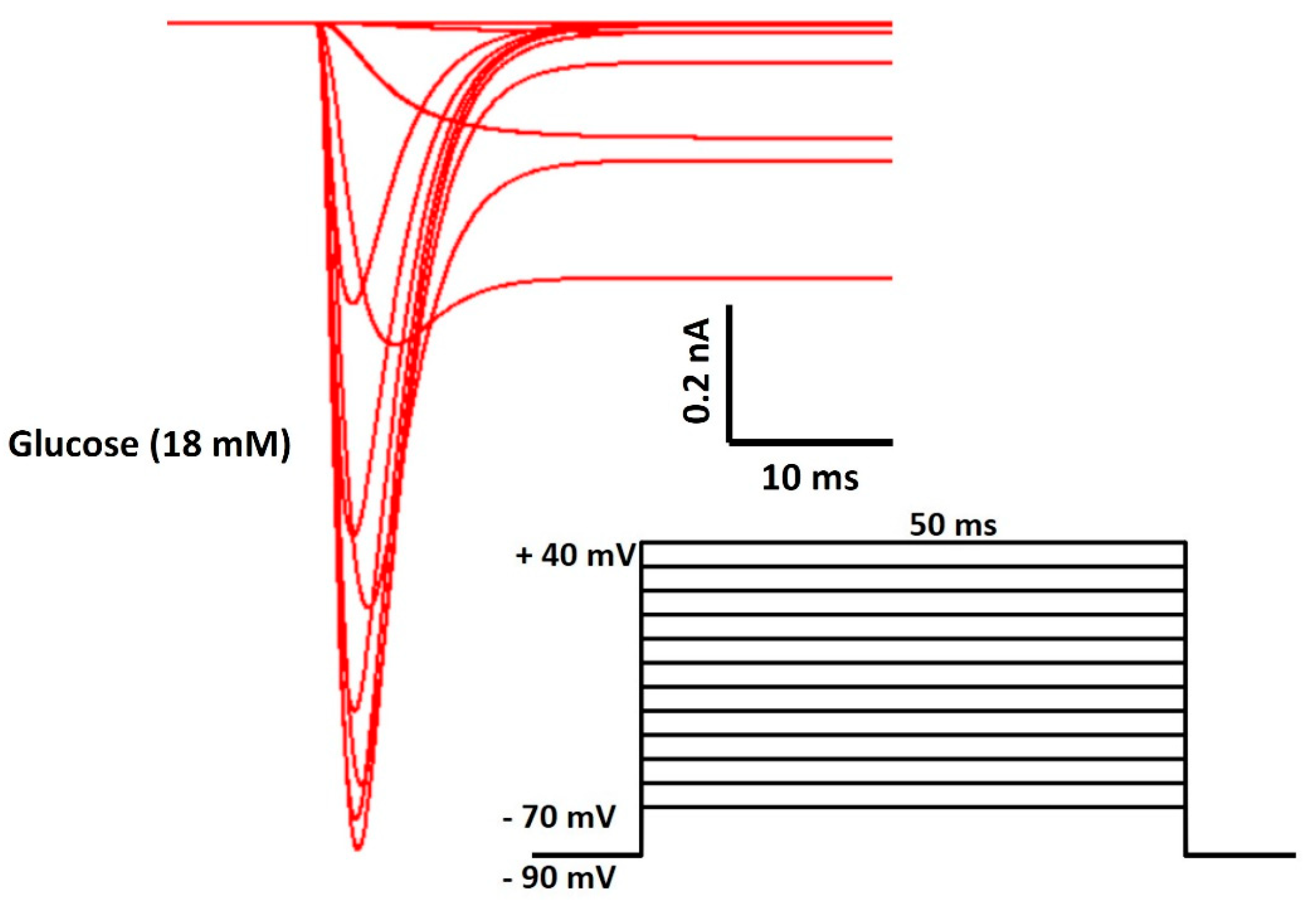
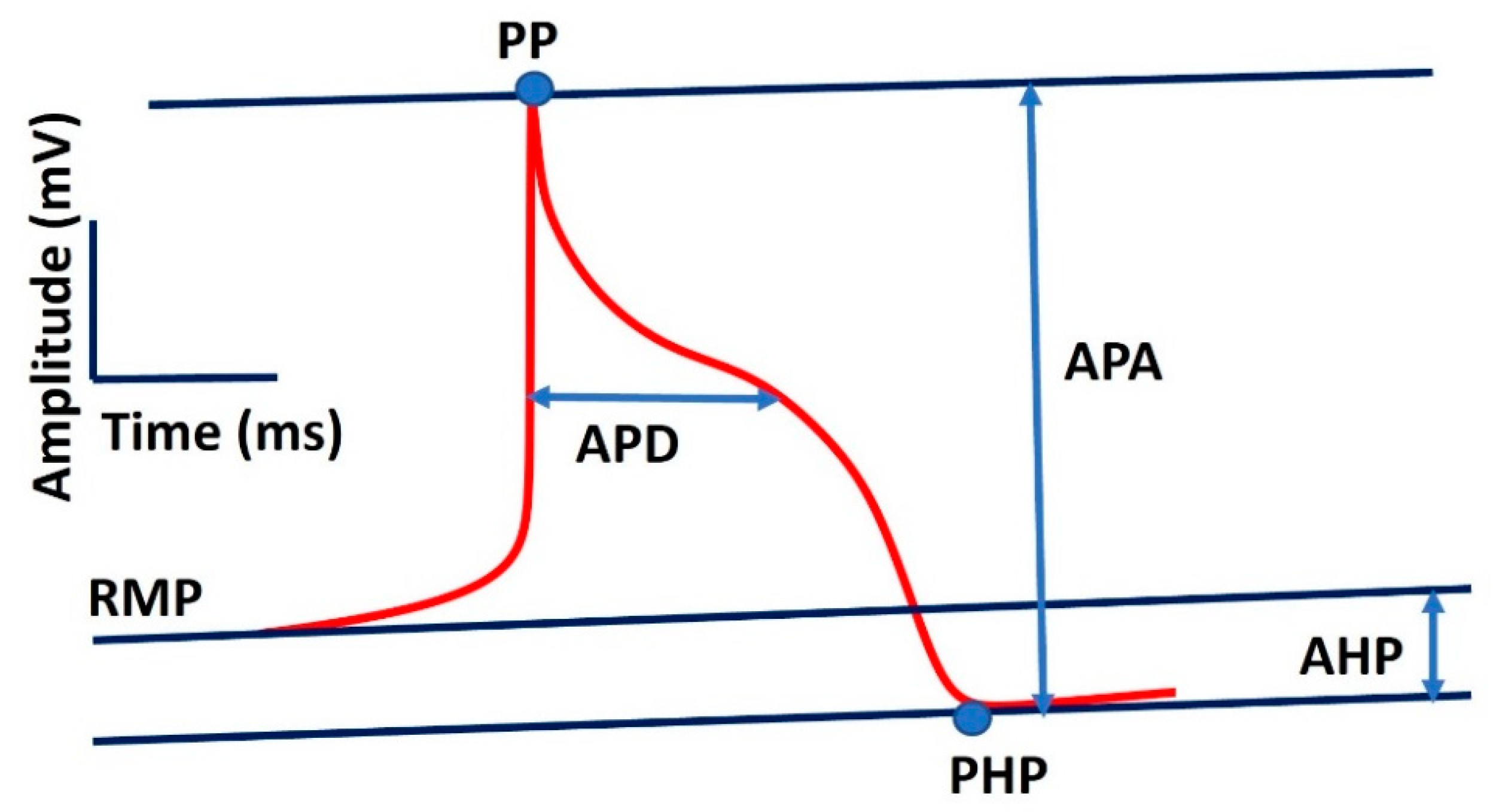

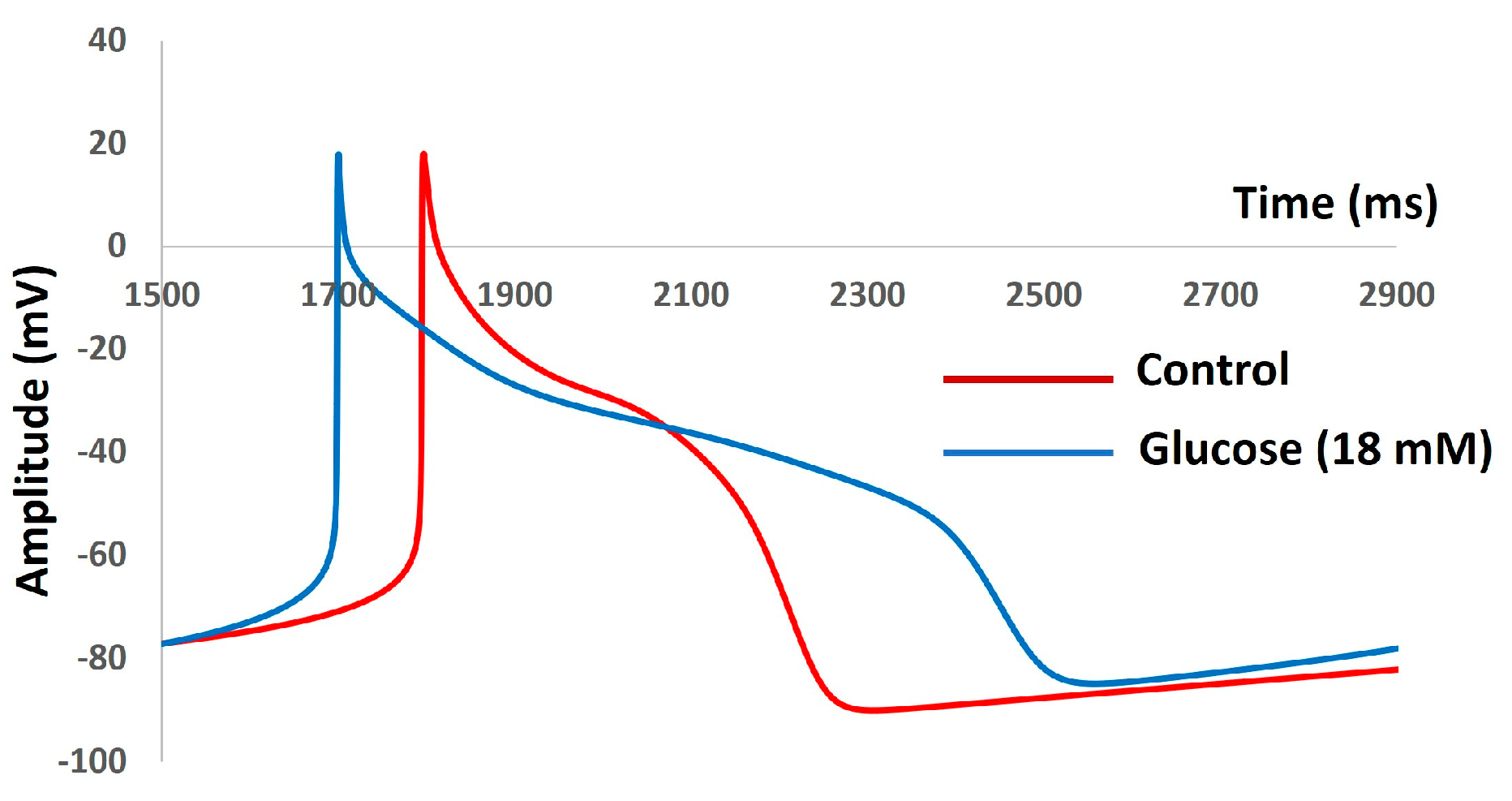
| Parameter | Definition | Value |
|---|---|---|
| R | Gas constant | 8.3143 J K−1 mol−1 |
| T | Temperature | 310 K |
| Cm | Membrane capacitance | 100 pF |
| F | Faraday constant | 96.4867 C/mmol |
| Vcell | Cell volume | 20,100 μm3 |
| Vi | Intracellular volume | 13,668 μm3 |
| Vup | SR uptake compartment volume | 1109.52 μm3 |
| Vrel | SR release compartment volume | 96.48 μm3 |
| [K+]o | Extracellular K1 concentration | 5.4 mM |
| [Na+]o | Extracellular Na1 concentration | 140 mM |
| [Ca2+]o | Extracellular Ca21 concentration | 1.8 mM |
| Parameter | Control | Glucose |
|---|---|---|
| RMP (mV) | −79 | −80 |
| AP Peak (mV) | 17 | 5 |
| AHP peak (mV) | −83 | −82 |
| AP Duration (ms) | 38 | 35 |
| CV (m/s) | 0.85 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahapatra, C.; Shanmugam, K.; Rusho, M.A. Computational Modeling of Sodium-Ion-Channel-Based Glucose Sensing Biophysics to Study Cardiac Pacemaker Action Potential. Math. Comput. Appl. 2024, 29, 84. https://doi.org/10.3390/mca29050084
Mahapatra C, Shanmugam K, Rusho MA. Computational Modeling of Sodium-Ion-Channel-Based Glucose Sensing Biophysics to Study Cardiac Pacemaker Action Potential. Mathematical and Computational Applications. 2024; 29(5):84. https://doi.org/10.3390/mca29050084
Chicago/Turabian StyleMahapatra, Chitaranjan, Kirubanandan Shanmugam, and Maher Ali Rusho. 2024. "Computational Modeling of Sodium-Ion-Channel-Based Glucose Sensing Biophysics to Study Cardiac Pacemaker Action Potential" Mathematical and Computational Applications 29, no. 5: 84. https://doi.org/10.3390/mca29050084
APA StyleMahapatra, C., Shanmugam, K., & Rusho, M. A. (2024). Computational Modeling of Sodium-Ion-Channel-Based Glucose Sensing Biophysics to Study Cardiac Pacemaker Action Potential. Mathematical and Computational Applications, 29(5), 84. https://doi.org/10.3390/mca29050084






