Abstract
The widespread use of plastics in our daily life has caused many health problems. Conventional water treatment processes have low efficiency in the removal of microplastics from water. In this work, we investigated the efficiency of ozonation pretreatment followed by flocculation to remove microplastics from water. After the ozonation pretreatment, it was found that microplastic removal could be significantly enhanced by flocculation from 40% to 91%. The characterization results show that the ozonation-pretreated microplastics had rougher surfaces and larger amounts of surface hydroxyl groups and carbonyls, which might be responsible for their increased removal. However, there was still a small amount of microplastics that had not been removed. They floated on the surface of the solution and could not be effectively oxidized by ozone, thus not changing their surface properties. This further confirms the importance of hydroxyl groups.
1. Introduction
The widespread use of plastic products has led to a significant presence of microplastics in water sources. Microplastics, which are defined as plastic particles measuring less than 5 mm in diameter, represent a new category of pollutants [1,2,3,4]. Current research shows that microplastics are commonly found in oceans, freshwater, and soil [5,6,7]. Some reports have confirmed that a wide variety of microplastics are already widely detected in the environment, including polyethylene terephthalate (PET), polyethylene (PE) [8], polyvinyl chloride (PVC), polystyrene (PS), and polypropylene (PP), most of which are found in aquatic environments [9]. The presence of large quantities of microplastics in the environment might cause organisms to inevitably ingest microplastics [10,11,12]. Although some microplastics are excreted after ingestion, most of them can remain and accumulate in organisms, causing adverse effects, such as endocrine disruption, cell damage, and inflammatory reactions [13,14]. Microplastics are also potential carriers of hazardous pollutants, which can be even more serious [15,16].
Huiwen Cai et al. found that the retention rate of microplastics by polycarbonate filters (single-pore type) was only 61.7% [17]. Microplastics are hydrophobic, which can easily lead to surface clogging of filter materials. Frequent backwashing or the replacement of filter materials is required, increasing the usage cost. At the same time, the filter materials themselves may become potential secondary pollution sources of microplastics. Yaliang Duan et al. identified the fungal strain DL-1, capable of degrading polyethylene microplastics (MPs) [18]. After 30 days, the biodegradation rate of polyethylene microplastics by strain DL-1 was 7.65 ± 0.92%. Biological degradation has a long cycle, which can last from several weeks to several months. Most strains are only effective against specific microplastics (such as PE).
Conventional water treatment processes have been proven to have relatively low efficiency in removing microplastics. As a common method, flocculation is extensively used in water treatment [8,19,20,21], in which coagulants play a critical role in coagulating and settling pollutants in water through electric neutralization and adsorption bridging [22,23]. Common flocculants are ferric chloride (FeCl3), poly aluminum chloride (PAC), and aluminum sulfate (Al2(SO4)3), among others, and most studies have shown that although the dosage is large, the efficiency of removing microplastics is very low. Therefore, effective treatment methods or processes need to be developed. Ozonation is an advanced process that can effectively solve the problem of low removal rates of difficult-to-degrade organic pollutants [24,25]. In addition, ozonation can destroy the structure of epoxy resin, rubber, polyethylene, polypropylene, and polyethylene terephthalate [26,27,28,29,30]; therefore, pre-ozonation might be an effective way to improve the efficiency of coagulation.
In this study, ozonation pretreatment was employed to handle water contaminated with microplastics, followed by flocculation to achieve the objective of effectively removing the microplastics. PVC (a polymerized plastic) and PET (a polycondensation plastic) were chosen as microplastic models in this study because these two plastics account for a high percentage of those used daily, and they are the main components of plastic debris detected in the aquatic environment. The specific objective of this study was to investigate the removal efficiency of the process of ozonation followed by flocculation with aluminum sulfate and polyacrylamide for the two microplastics, and to reveal the removal mechanism. Additionally, the surface properties of the unremoved microplastics remained almost unchanged because they floated on the solution surface and could not be effectively oxidized by ozone.
2. Experimental
2.1. Materials and Reagents
Polyvinyl chloride (PVC, about 50 μm) and polyethylene terephthalate (PET, about 50 µm) were purchased from Ruixiang Plasticizing Co., Ltd., Dongguan, China and used directly without further treatment. Aluminum sulfate (Al2(SO4)3) was purchased from Aladdin Chemical Reagent Co., Ltd., Shanghai, China, polyacrylamide (PAM) was provided by Chengjie Environmental Protection Co., Hangzhou, China and oxygen with 99.999% purity for ozone generation was provided by Jinte Gas Co., Hangzhou, China.
2.2. Microplastic Removal Experiments and Measurements
The experiment was carried out in a beaker, as shown in Figure 1. The microplastic concentration used in each experiment was 10 ppm [31,32], and the volume of the solution was 500 mL. The coagulation test was performed by rapid stirring for 1 min, followed by slow stirring for 5–10 min, and then the solution was left to stand for 30 min [33]. An unspecified ozone flux of 20 mg/min was applied, where ozone was introduced to the water in the form of bubbles, accompanied by stirring. A 20% aluminum sulfate aqueous solution and a 5 ‰ PAM solution were used in the experiment [34]. At the end of the experiment, the microplastics floating in the solution were collected, filtered, dried, and weighed. The operation was repeated twice to obtain the average value.
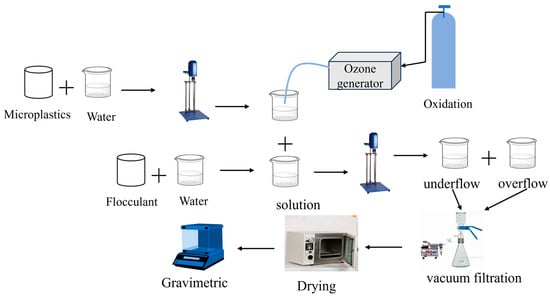
Figure 1.
Procedures for microplastic removal experiments.
Measurement of microplastics: First, the filter membranes required for the experiment were numbered and dried at 60 °C until a constant mass was obtained, and then their mass was measured as M1 (g). After flocculation, the microplastics in the water were collected for filtration. The filter membrane containing microplastics was dried at 60 °C until the mass was constant, and then the mass at this time was recorded as M2 (g). The starting concentration of microplastics was 10 ppm. We conducted three independent and repeated experiments. The relative standard error was usually less than 5%. The microplastic removal rate α was calculated according to the following equation:
where W0 is the weight of microplastics before processing (g).
All data were statistically analyzed by SPSS software (IBM SPSS Statistics 27). The Pearson correlation coefficient was used to analyze the normal distribution relationship between the dosage and removal rate. A correlation coefficient greater than 0.7 indicates a strong correlation, between 0.4 and 0.7 indicates a moderate correlation, and less than 0.4 indicates a low correlation [35,36].
2.3. Characterization
Both the removed and the unremoved microplastics were collected and dried in a dryer. The dried samples were characterized using optical microscopy (SAGA/SG30, Suzhou Shenying Optics Co., Ltd., Suzhou, China), electron microscopy (SEM, ZEISS Sigma 300, Oberkochen, Germany), and Fourier transform infrared spectroscopy (FT-IR, Nicolet 6700, Thermo, Waltham, MA, USA). Ultraviolet-visible spectrophotometry (CARY 100 Conc, Thermo, Waltham, MA, USA) and LC/MS (Agilent 6530 Q-TOF LC/MS, San Diego, CA, USA) were used to determine the removal mechanism of the microplastics.
3. Results and Discussion
3.1. Factors Affecting Flocculation Experiments
3.1.1. Microplastic Removal Performance
When the Al2(SO4)3 solution was used alone, the removal of PET microplastics by flocculation increased from 22.28% to 38.0% as the dosage ranged from 100 ppm to 1000 ppm (20% Al2(SO4)3). This result is consistent with previous reports showing that conventional flocculation cannot effectively remove microplastics. As shown in Figure 2, the removal rates of PVC and PET microplastics by 500 ppm of 20% aluminum sulfate solution alone were 40.8% and 30.8%, respectively. However, the removal rates of PVC and PET microplastics by adding 500 ppm of 20% Al2(SO4)3 solution and 1 ppm of 5 ‰ PAM solution were 73.8% and 68.8%, respectively. The removal rates of PVC and PET in the ozonation treatment (10 min) followed by flocculation with 500 ppm of 20% Al2(SO4)3 solution and 1 ppm of 5 ‰ PAM solution (O3_coagulation of Al2(SO4)3/PAM) reached 96.23% and 91.00%, respectively. Compared to coagulation with Al2(SO4)3/PAM, the removal rates of PVC and PET by the O3_coagulation of Al2(SO4)3/PAM were significantly enhanced. When the ozonation pretreatment time extended to 30 min, the removal rate of PVC went up to 99.84%, and that of PET went up to 92.24% by the O3_coagulation of Al2(SO4)3/PAM. The removal rate did not increase further with the extension of the ozonation time. The efficiency of the O3_coagulation of Al2(SO4)3/PAM in removing PVC was slightly higher than that of PET, which might be due to the different performances of PVC and PET. Additionally, about 10% mass loss was also observed for microplastics treated with ozone alone. This might be because some microplastic particles reacted and dissolved in the water following ozone oxidation.
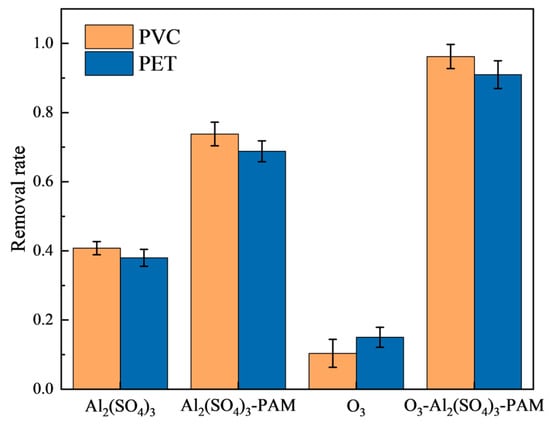
Figure 2.
Microplastic removal rates.
Reaction conditions: 500 ppm of 20% Al2(SO4)3 solution; ozone flow rate of 20 mg/min; 1 ppm of 5‰ PAM solution; ozonation pre-treatment time of 10 min; pH 7.
3.1.2. Effects of Different pH Values
The effect of the initial pH on removal was investigated. As shown in Figure 3, the effects of pH on the removal rates of PET and PVC were similar, and both removal rates were the highest when the pH was neutral. When the pH increased to 9, no significant increase was observed. So, the pH value in the following experiment was set at 7. The pH of the water was found to decrease owing to the addition of Al2(SO4)3. Ozonation pretreatment could enhance microplastic removal at all studied pH levels of water, as shown in Figure 3a. The pH value showed a trend of increasing first and then decreasing (see Figure 3b). The increases in pH could be attributed to hydrogen ions being consumed by aluminum sulfate hydrolysis. The decreases in pH may have been due to the formation of acidic substances caused by the ozone degrading the microplastics.
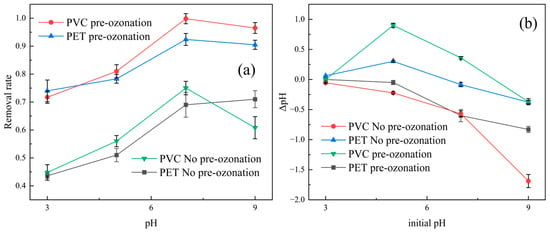
Figure 3.
Effects of pH on removal rate of PVC and PET microplastics (a), changes in pH of solution (b).
Reaction conditions: 500 ppm of 20% Al2(SO4)3 solution; ozone flow rate of 20 mg/min; 1 ppm of 5‰ PAM solution; ozonation pre-treatment time of 10 min.
3.1.3. Effects of Ozonation Pre-Treatment Time and Flux
The removal rates of both PVC and PET increased from 76.91% to 99.84% and from 71.31% to 92.40% when the ozonized time increased from 2 min to 30 min, respectively. As shown in Figure 4a,b, although the removal rates of PVC and PET showed a tendency to increase with increasing ozone flux, the removal rates of PVC and PET were already relatively high when the ozone flux was 15 mg/min, and further increases in ozone flux had a negligible effect on microplastic removal.
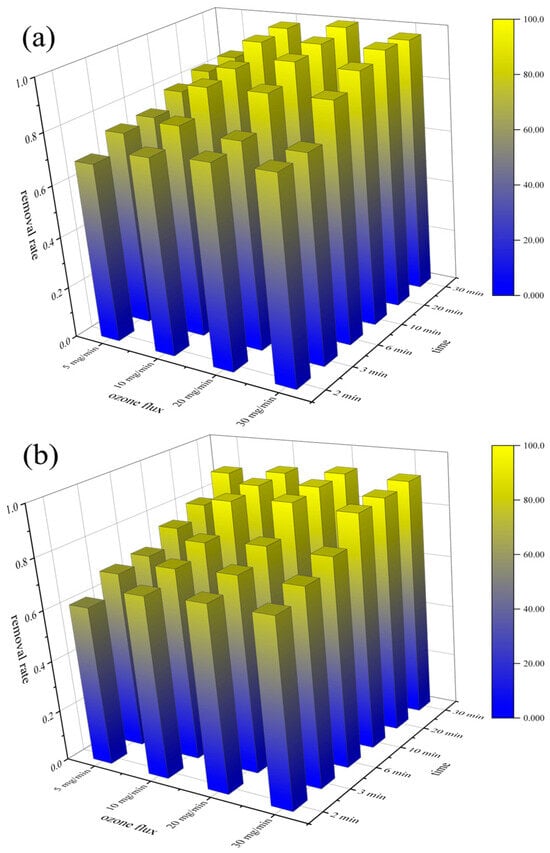
Figure 4.
Effects of ozonation pre-treatment time and ozone flux on the removal of microplastics: PVC (a); PET (b).
Reaction conditions: 500 ppm of 20% Al2(SO4)3 solution; 1 ppm of 5‰ PAM solution.
The Pearson correlation coefficient between the dosages of Al2 (SO4) 3 and PAM and the removal efficiency of microplastics was relatively low, indicating that the influence of the coagulant concentration on the removal efficiency of microplastics was relatively small. The ozonation pretreatment time, ozone flux, and the removal rates of PVC and PET all showed correlations greater than 0.7. Statistical analysis revealed distinct correlation patterns between the ozone dosage parameters and the microplastic removal efficiency (Table 1).

Table 1.
Pearson’s correlation analysis was performed between the dosages of Al2(SO4)3, PAM, and ozone and their corresponding removal rates.
3.1.4. Effect of Flocculant Dosage
The effect of flocculation dosage was further investigated (see Figure 5). It was found that the effect of a 20% Al2(SO4)3 solution dosage was similar to the results of flocculation alone. As shown in Figure 5, the removal rate of microplastics increased with increases in PAM dosage at a constant dosage of Al2(SO4)3. When the 5‰ PAM solution dosage was 1 ppm, the removal rates of PVC and PET in the O3_coagulation system of Al2(SO4)3/PAM reached the maximum values of 96.23% and 91%, respectively. As shown in Figure 5, the effects of Al2(SO4)3 and PAM dosage on microplastic removal are similar.
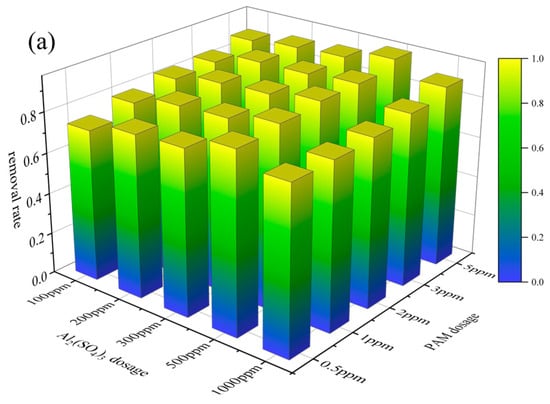
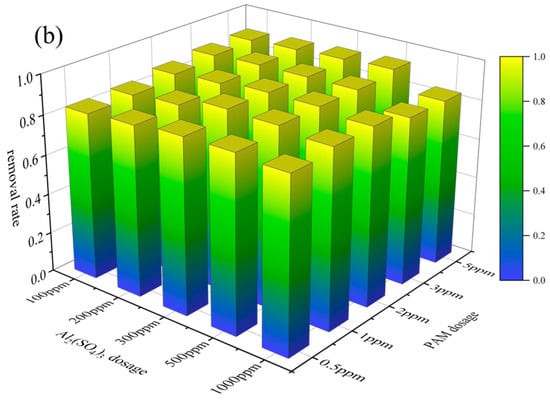
Figure 5.
Effects of Al2(SO4)3 and PAM dosage: PVC (a); PET (b).
Reaction conditions: ozone flow rate of 20 mg/min; ozonation pre-treatment time of 10 min; pH 7.
3.1.5. Effect of Microplastic Particle Size
This study investigated the influence of microplastic particle size on the removal efficiency. The results show (Figure 6) that particle size affected the removal efficiency of microplastics. The removal efficiencies of both PVC and PET microplastics showed a downward trend with decreases in the particle size. The removal efficiency of microplastics with a large particle size (>40 µm) was stable above 90%, while the surface charge density of microplastics with a small particle size increased [37]. Therefore, more Al3+ is needed to complete the charge, which will hinder the entrapment of flocs.
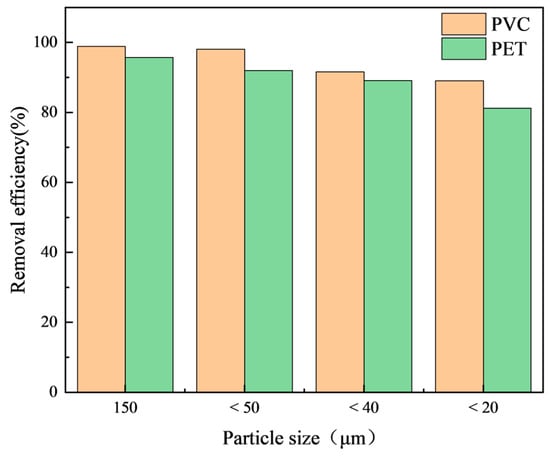
Figure 6.
The influence of microplastic particle size on the removal efficiency.
3.2. Mechanism Study
According to the SEM analysis in Figure 7, the surfaces of both PVC and PET exhibited enhanced roughness following ozonation pretreatment. Notably, SEM imaging of pristine PVC (Figure 7a) revealed the presence of surface pores. These pre-existing pores may have contributed to the observed higher removal rate of PVC compared to PET in this study [8].
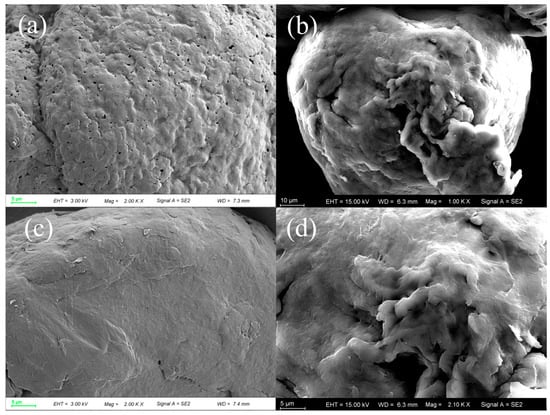
Figure 7.
SEM images of PVC before (a) and after (b), and PET before (c) and after (d) ozonation pretreatment.
FT-IR spectroscopy analysis (Figure 8) revealed a significant enhancement in the intensity of surface hydroxyl (-OH) peaks for ozone-pretreated microplastics compared to pristine samples. Furthermore, FTIR analysis revealed the emergence of a carbonyl (C=O) absorption band at 1620 cm−1 specifically in ozonated polyvinyl chloride (PVC) microplastics [29,38]. After ozone pretreatment, the electronegativity of PVC and PET weakened, and the zeta potential values changed from −15 mV ± 2 mV and −20 mV ± 3 mV to −10 mV ± 3 mV and −11 mV ± 3 mV, respectively, which is conducive to coagulation [39].
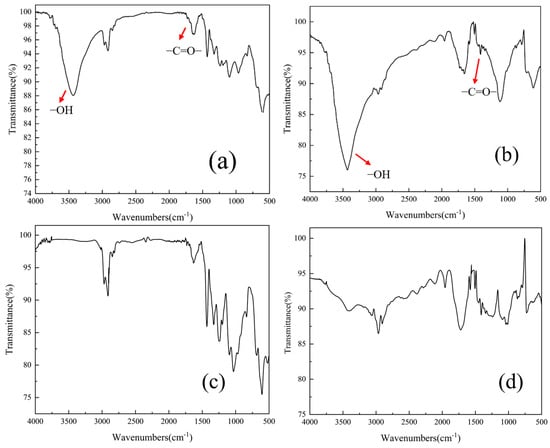
Figure 8.
FTIR of the ozonized (a) and fresh (c) PVC, and the ozonized (b) and fresh (d) PET.
Ozone pretreatment fundamentally modifies microplastic surface properties through three key pathways: amplified roughness (SEM, Figure 7), elevated hydroxyl functionality (FTIR: 3500 cm−1 band intensification, Figure 8), and shifted zeta potential. This surface transformation collectively enhances hydrophilicity and reduces electrostatic repulsion—critical factors governing improved coagulation efficiency.
As shown in Figure 9, the UV-VIS absorption spectrum of the water showed an absorption peak of the carbonyl group near 200 nm, indicating that after ozone treatment, the dissolved microplastics in the water contained C=O. The effects of different pH values on the formation of carbonyl groups in the dissolved products of microplastics after ozone pretreatment were studied. When the pH value was neutral, C=O had a stronger absorption peak, and the intensity of C=O produced by PVC after ozone pretreatment was higher than that of PET.
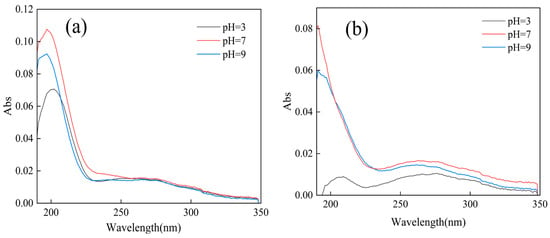
Figure 9.
UV-visible spectra of microplastics after ozonation pretreatment: PVC (a); PET(b).
Following ozone treatment, there was still a small amount of microplastics suspended on the surface of the solution. The SEM image in Figure 10 shows that the surface roughness of these unremoved microplastics did not change a lot, thus resulting in their incomplete removal in the O3_coagulation of Al2(SO4)3/PAM. The unremoved microplastics were attributed to its less contact time with ozone, as they floated on the surface of water, and therefore, they were not oxidized by ozone and their surface chemical properties were not altered.
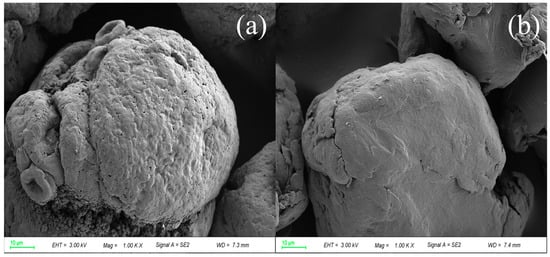
Figure 10.
SEM of unremoved PVC (a) and PET (b).
In this experiment, the particle size of the selected microplastics was about 50 µm, and the particle size of the selected microplastics was not uniform. The particle size of the unremoved microplastics was found to be smaller than that of the microplastics removed through flocculation. The minimum particle size of the precipitated PVC was about 45.26 µm, and the maximum particle size of the unremoved PVC was about 40.55 µm. Similar results were also found for PET, with the minimum particle size of the precipitated PET being about 43.10 µm. The maximum particle size of floating PET was about 38.55 µm, and microplastics with smaller particle sizes were not easy to flocculate and settle.
3.3. Dissolved Organic Analysis
The possible dissolved organic compounds in the ozonation process of PVC and PET microplastics were analyzed by LC-MS. It was found that the PVC and PET microplastics may undergo chain breakage and form carbonyl organic compounds after ozonation.
The possible dissolved organic compounds of PVC ozonation process include -CHCl-carbon chains, according to the linear formula of PVC for (CHClCHCl)n. Based on liquid mass analysis, the possible molecular formula of the m/z 163 product is C2HCl3O2, which may contain the C=O; see Table 2. According to the analysis from a previous study [26], under the attack of O3, PVC microplastics form carbon-centered peroxyl radicals at the sites of long-chain breaks ((−CHCl−COO·CHCl−)n). Additionally, the possible intermediate products and the reaction mechanism after ozone oxidation were investigated by infrared spectroscopy.

Table 2.
Possible organic compounds in water dissolve after ozone oxidation (PVC).
It was confirmed that the transformation of PVC under ozonation includes the breaking of long chains into short chains and the decomposition of the broken chains into a soluble oxygen-containing substance. The toxicity of dissolved species was analyzed, and the results are shown in Figure 11. While dissolved organic matter containing chlorine exhibits developmental toxicity and mutagenicity, non-chlorinated dissolved organic matter is largely non-toxic. The degradation of PVC by ozone reduces the environmental toxicity of its leachates, consequently benefiting aquatic organisms.
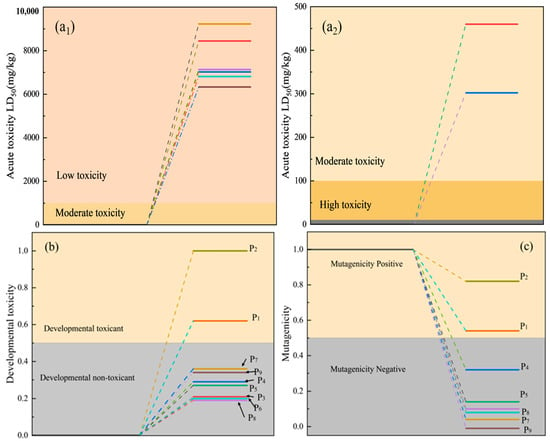
Figure 11.
Toxicity analysis of aqueous extracts: mutagenicity of oral LD50 in rat (a1,a2); developmental toxicity (b); lethal concentration (c).
The potential dissolved organic products of the PET ozonation process include a compound with the possible molecular formula C18H14O12 for the m/z 446.038 product. Combined with infrared spectroscopy, it was speculated that soluble substances appear after ozonation pretreatment; see Table 3. The toxicity of the possible intermediate products was discussed, as shown in Figure 12. Similarly, the conversion process of PET under ozonation includes long chains breaking into short chains in a safe way.

Table 3.
Possible organic compounds in water dissolved after ozone oxidation (PET).
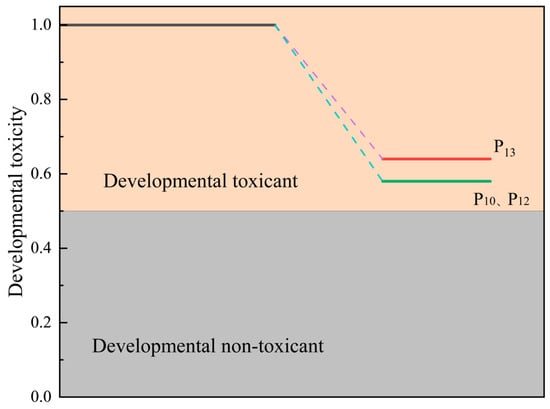
Figure 12.
Toxicity analysis of aqueous extracts: developmental toxicity.
4. Conclusions
In the O3_coagulation system of Al2(SO4)3/PAM, after a 10 min ozone treatment, the removal rates of PVC and PET reached 96.23% and 91.00%, respectively, which were much higher than those of a single flocculant. It can be seen that ozonation pretreatment helped to enhance the removal performance of microplastics. SEM and FT-IR characterization showed that after ozonation pretreatment, the surface structure of the microplastics changed, C=O was formed, and the surface roughness increased. At the same time, the surface hydroxyl group increased, which was conducive to the coagulation and precipitation of microplastics. It was found that O3_coagulation of Al2(SO4)3/PAM had the same performance in removing PVC (a polymerized plastic) and PET (a condensation plastic) microplastics.
The possible dissolved organic matter produced by ozone-pretreated microplastics was deduced by LC-MS analysis. The surface roughness of the unflocculated settling microplastics did not change significantly, which may be due to the fact that some of the microplastics were blown out of the solution surface and did not participate in ozone oxidation. Additionally, it was observed that the small particle size of the microplastics suspended on the liquid surface might have affected the flocculation effect.
Author Contributions
Conceptualization, J.W.; Data curation, J.W. and M.H.; Formal analysis, J.W. and M.H.; Writing—original draft, J.W.; Methodology, J.W.; Validation, M.H. and Z.Z.; Visualization, M.H. and Z.Z.; Supervision, Z.Z. and S.T.; Funding acquisition, S.T.; Writing—review & editing, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (grant number 21176225).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rochman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef]
- Solís-Balbín, C.; Sol, D.; Laca, A.; Laca, A.; Díaz, M. Destruction and entrainment of microplastics in ozonation and wet oxidation processes. J. Water Process Eng. 2022, 51, 103456. [Google Scholar] [CrossRef]
- Schwarzer, M.; Brehm, J.; Vollmer, M.; Jasinski, J.; Xu, C.; Zainuddin, S.; Fröhlich, T.; Schott, M.; Greiner, A.; Scheibel, T.; et al. Shape, size, and polymer dependent effects of microplastics on Daphnia magna. J. Hazard. Mater. 2021, 426, 128136. [Google Scholar] [CrossRef]
- Noumani, A.; Verma, D.; Kaushik, A.; Khosla, A.; Solanki, P.R. Electrochemically microplastic detection using chitosan-magnesium oxide nanosheet. Environ. Res. 2024, 252, 118894. [Google Scholar] [CrossRef]
- Zolotova, N.; Kosyreva, A.; Dzhalilova, D.; Fokichev, N.; Makarova, O. Harmful effects of the microplastic pollution on animal health: A literature review. PeerJ 2022, 10, 13503. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Microplastics in the environment: Occurrence, perils, and eradication. Chem. Eng. J. 2020, 408, 127317. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhu, Z.-R.; Fu, W.; Dai, X.; Ni, B.-J. The atmospheric microplastics deposition contributes to microplastic pollution in urban waters. Water Res. 2022, 225, 119116. [Google Scholar] [CrossRef]
- Hu, J.; Hu, J. Mineralization characteristics and behavior of polyethylene microplastics through ozone-based treatment. Chemosphere 2023, 349, 140839. [Google Scholar] [CrossRef]
- Golwala, H.; Zhang, X.; Iskander, S.M.; Smith, A.L. Solid waste: An overlooked source of microplastics to the environment. Sci. Total Environ. 2021, 769, 144581. [Google Scholar] [CrossRef]
- Taylor, M.L.; Gwinnett, C.; Robinson, L.F.; Woodall, L.C. Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 2016, 6, 33997. [Google Scholar] [CrossRef] [PubMed]
- Kye, H.; Kim, J.; Ju, S.; Lee, J.; Lim, C.; Yoon, Y. Microplastics in water systems: A review of their impacts on the environment and their potential hazards. Heliyon 2023, 9, e14359. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wei, W.; Liu, X.; Ni, B.-J. Emerging electrochemical techniques for identifying and removing micro/nanoplastics in urban waters. Water Res. 2022, 221, 118846. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, K.; Hong, J. Understanding the hazards induced by microplastics in different environmental conditions. J. Hazard. Mater. 2021, 424, 127630. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Hou, Q.; Wang, Z. From natural environment to animal tissues: A review of microplastics(nanoplastics) translocation and hazards studies. Sci. Total Environ. 2022, 855, 158686. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Fajardo, C.; Martín, C.; Costa, G.; Sánchez-Fortún, S.; Rodríguez, C.; de Lucas Burneo, J.J.; Nande, M.; Mengs, G.; Martín, M. Assessing the role of polyethylene microplastics as a vector for organic pollutants in soil: Ecotoxicological and molecular approaches. Chemosphere 2021, 288, 134260. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chen, M.; Chen, Q.; Du, F.; Liu, J.; Shi, H. Microplastic quantification affected by structure and pore size of filters. Chemosphere 2020, 257, 127198. [Google Scholar] [CrossRef]
- Duan, Y.; Yin, Y.; Ni, Z.; Liu, J.; Gui, H.; Wu, D.; Wu, X.; Wang, L. The effective and green biodegradation of polyethylene microplastics by the screening of a strain with its degrading enzymes. Biochem. Eng. J. 2024, 210, 109429. [Google Scholar] [CrossRef]
- Na, S.-H.; Kim, M.-J.; Kim, J.-T.; Jeong, S.; Lee, S.; Chung, J.; Kim, E.-J. Microplastic removal in conventional drinking water treatment processes: Performance, mechanism, and potential risk. Water Res. 2021, 202, 117417. [Google Scholar] [CrossRef]
- Zhang, Y.; Diehl, A.; Lewandowski, A.; Gopalakrishnan, K.; Baker, T. Removal efficiency of micro- and nanoplastics (180 nm–125 μm) during drinking water treatment. Sci. Total Environ. 2020, 720, 137383. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Sarmiento-Sánchez, J.I.; Ruelas-Leyva, J.P.; Crini, G.; Hermosillo-Ochoa, E.; Gutierrez-Montes, J.A. Environment-Friendly Approach toward the Treatment of Raw Agricultural Wastewater and River Water via Flocculation Using Chitosan and Bean Straw Flour as Bioflocculants. ACS Omega 2020, 5, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Sun, J.; Liu, Y.; Wang, H.; Ding, J.; Chen, L.; Tian, X.; Yuan, Y. Physicochemical characterization and flocculation performance evaluation of PAC/PMAPTAC composite flocculant. J. Appl. Polym. Sci. 2021, 139, e51653. [Google Scholar] [CrossRef]
- Huang, L.; He, W.; Zhang, Y.; Wang, X.; Wu, K.; Yang, Z.; Zhang, J. Chitosan enhances poly aluminum chloride flocculation system removal of microplastics: Effective, stable, and pollution free. J. Water Process Eng. 2023, 54, 103929. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Xiang, F.Y.; Mao, J.Q.; Ding, Y.L.; Tong, S.P. Oxidative Efficiency of Ozonation Coupled with Electrolysis for Treatment of Acid Wastewater. J. Electrochem. 2022, 28, 42–48. [Google Scholar]
- Li, Y.; Zhang, Z.; Zhang, L.; Li, Y.; Lin, K.-Y.A.; Tong, S. Preparation of metal organic frame derived MgO-porous carbon composite and its high catalytic activity in ozonation with excellent stability. J. Taiwan Inst. Chem. Eng. 2024, 156, 105339. [Google Scholar] [CrossRef]
- Fitri, A.N.; Amelia, D.; Karamah, E.F. The effect of Ozonation on the chemical structure of microplastics. IOP Conf. Ser. Mater. Sci. Eng. A 2021, 1173, 012017. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. The effect of water ozonation in the presence of microplastics on water quality and microplastics degradation. Sci. Total Environ. 2024, 929, 172595. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, T.; Wang, X. Investigation of microplastics release behavior from ozone-exposed plastic pipe materials. Environ. Pollut. 2021, 296, 118758. [Google Scholar] [CrossRef]
- Homin, K.; Yeojoon, Y.; Tae-Mun, H. Changes in physical and chemical properties of microplastics by ozonation. Process Saf. Environ. Prot. 2024, 192, 1062–1072. [Google Scholar]
- Mohamad Yusof, M.S.; Othman, M.H.D.; Abdul Wahab, R.; Abu Samah, R.; Kurniawan, T.A.; Mustafa, A.; Abdul Rahman, M.; Jaafar, J.; Ismail, A.F. Effects of pre and post-ozonation on POFA hollow fibre ceramic adsorptive membrane for arsenic removal in water. J. Taiwan Inst. Chem. Eng. 2020, 110, 100–111. [Google Scholar] [CrossRef]
- Yutao, Z.; Jianhai, Z.; Zhaoyang, L.; Sufeng, T.; Jingfang, L.; Rong, M.; Hongying, Y. Coagulation removal of microplastics from wastewater by magnetic magnesium hydroxide and PAM. Water Process. Eng. 2021, 43, 102250. [Google Scholar]
- Wang, W.; Yang, M.; Ma, H.; Liu, Z.; Gai, L.; Zheng, Z.; Ma, H. Removal behaviors and mechanism of polystyrene microplastics by coagulation/ultrafiltration process: Co-effects of humic acid. Sci. Total Environ. 2023, 881, 163408. [Google Scholar] [CrossRef]
- Awan, M.M.A.; Malkoske, T.; Almuhtaram, H.; Andrews, R.C. Microplastic removal in batch and dynamic coagulation-flocculation-sedimentation systems is controlled by floc size. Sci. Total Environ. 2023, 909, 168631. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, Q.-S.; Luo, T.-Y.; Wu, R.-L.; Wei, W.; Ni, B.-J. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chem. Eng. J. 2021, 416, 129123. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef]
- Leece, J.; Parker, F. Use and misuse of SPSS. Softw. Pract. Exper. 1978, 8, 301–311. [Google Scholar] [CrossRef]
- Tang, S.; Gao, L.; Tian, A.; Zhao, T.; Zou, D. The coagulation behavior and removal efficiency of microplastics in drinking water treatment. Water Process. Eng. 2023, 53, 103885. [Google Scholar] [CrossRef]
- Nieto-Sandoval, J.; Ammar, R.; Sans, C. Enhancing nanoplastics removal by metal ion-catalyzed ozonation. Chem. Eng. J. Adv. 2024, 19, 100621. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, H.; Ou, H.; Liu, R.; Chen, Y.; Wu, X.; Fu, J. Optimizing Microplastic Removal Through Coagulation-Sedimentation with Permanganate Pre-oxidation and Pre-chlorination. Water Air Soil Pollut. 2024, 235, 1–13. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).