Functionalized Carbon-Based Materials for Uranium Extraction: A Review
Abstract
1. Introduction

2. Uranium Extraction by Functionalized Carbon-Based Materials
2.1. Activated Carbon
| Adsorbent | Qmax (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| ACFs-AO (Amidoxime functionalized activated carbon fibers) | 191.6 | Batch experiment conducted in aqueous solutions; pH 5; 25 °C; 48 h equilibrium time; exhibited good selectivity for U(VI) against competing ions (Ni2+, La3+, Sr2+, Eu3+, Ba2+, Zn2+, Nd3+); >95% desorption was achieved using 1.0 M HNO3 with only minor capacity loss after four regeneration cycles. | [31] |
| AO-AC (Amidoxime functionalized activated carbon) | 66.35 | Batch experiment conducted in aqueous solutions; pH 6; 25 °C; 1.3 h equilibrium time; exhibited good selectivity for U(VI) against competing ions (K+, Na+, Ca2+, Mg2+, CO32−, HCO3−, SO42−); desorption was achieved using 0.1 M HCl; 88.32% efficiency after five adsorption. | [73] |
| TOA-AC (Trioctylamine- AC) | 50 | Batch experiment conducted at laboratory scale in synthetic sulfuric acid solutions; pH 3.4; 25 °C; 0.5 h equilibrium time; 94.88% elution was achieved using 0.1 M HCl. | [78] |
| PAF/AC (Polyethyleneimine modified activated carbon/Fe) | 115.3 | Batch experiment conducted in aqueous solutions; pH 5; 20 °C; 1 h equilibrium time; desorption with 0.25–1 M HCl achieved 90–95% uranium recovery, with only a 12–13% performance loss after five cycles. | [79] |
2.2. Graphene Oxide

| Adsorbent | Qmax (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| PAHA-GO (Polyamidoxime-hydroxamic acid-GO) | 178.7 | Batch experiment conducted in aqueous solution containing Na+, K+, Ca2+, Mg2+, V5+, Cu2+, Pb2+, La3+, Ce4+, Ni2+, Co2+, Zn2+, Fe3+ ions; pH 3.6; 25 °C; 10 h equilibrium time; demonstrated efficient U(VI) extraction; efficiency retained >97% under 0.5 M HCl; good performance over four cycles. | [87] |
| GO-DM-AO (GO-diaminomaleonitrile-amidoxime) | 935 | Lab experiment conducted in artificial seawater containing Al3+, Ba2+, Na+, Zn2+, Cu2+, K+, Ni2+, Mg2+, Ca2+, Sr2+, Fe3+ ions; pH 8; 25 °C; 0.5 h equilibrium time; desorption was achieved using 0.2 M citric acid; 90% capacity was retained after five cycles; demonstrated efficient uranium selectivity. | [101] |
| Cu-BDC-NH2@GO-A (Copper-2-aminobenzene-1,4-dicarboxylate MOF/GO-Acid-modified) | 1078.4 | Batch experiment conducted in simulated seawater containing V+, Fe3+, Ni2+, Co2+, Zn2+, Na+, Mg2+, K+ ions; pH 8; 25 °C; demonstrated efficient uranium extraction against the competing ions. | [105] |
| GO-CS (Graphene oxide-chitosan Aerogel) | 384.6 | Batch experiment conducted in simulated seawater containing Sr2+, Ni2+, Co2+, Zn2+, La3+, Nd3+, Sm3+, Gd3+, Yb3+ ions; pH 8.3; 25 °C; 1 h equilibrium time; demonstrated efficient U recovery; 0.1 M HNO3 was used as the desorption agent; capacity retention was 93–87% after three cycles. | [108] |
| CS-GO-DO/ZnO (Chitosan-GO-Amidoximated Diaminomaleonitrile-Zinc Oxide) | 561.09 | Batch experiment conducted in a 96% pure uranium solution containing Na+, K+, Ca2+, Mg2+, Cu2+, Mn2+, Ce3+, La3+, Eu3+ ions; pH 6; 25 °C; 6.6 h equilibrium time; demonstrated efficient U recovery; 0.1 M HCl was used as the desorption agent; capacity retention was 98% after five cycles. | [109] |
| PG (Phosphoryl-functionalized graphene) | 316 | Batch experiment conducted in distilled water containing Na+, Mg2+, Ca2+, K+, Sr2+, Fe3+, VO43−, Cl−, SO42− ions; pH 7; 25 °C; 3 h equilibrium time; 91.76% efficiency after four cycles | [116] |
| rGO-PPy-Fe0 (reduced-GO-polypyrrole-zero-valent iron) | 384.24 | Lab experiment conducted in simulated seawater containing Na+, Ba2+, Mg2+, Zn2+, Cu2+, Cr3+, Ca2+, Al3+, V5+ competing ions; pH 8; 25 °C; 1.6 h equilibrium time; demonstrated efficient uranium recovery against coexisting ions. | [117] |
2.3. Carbon Cloths
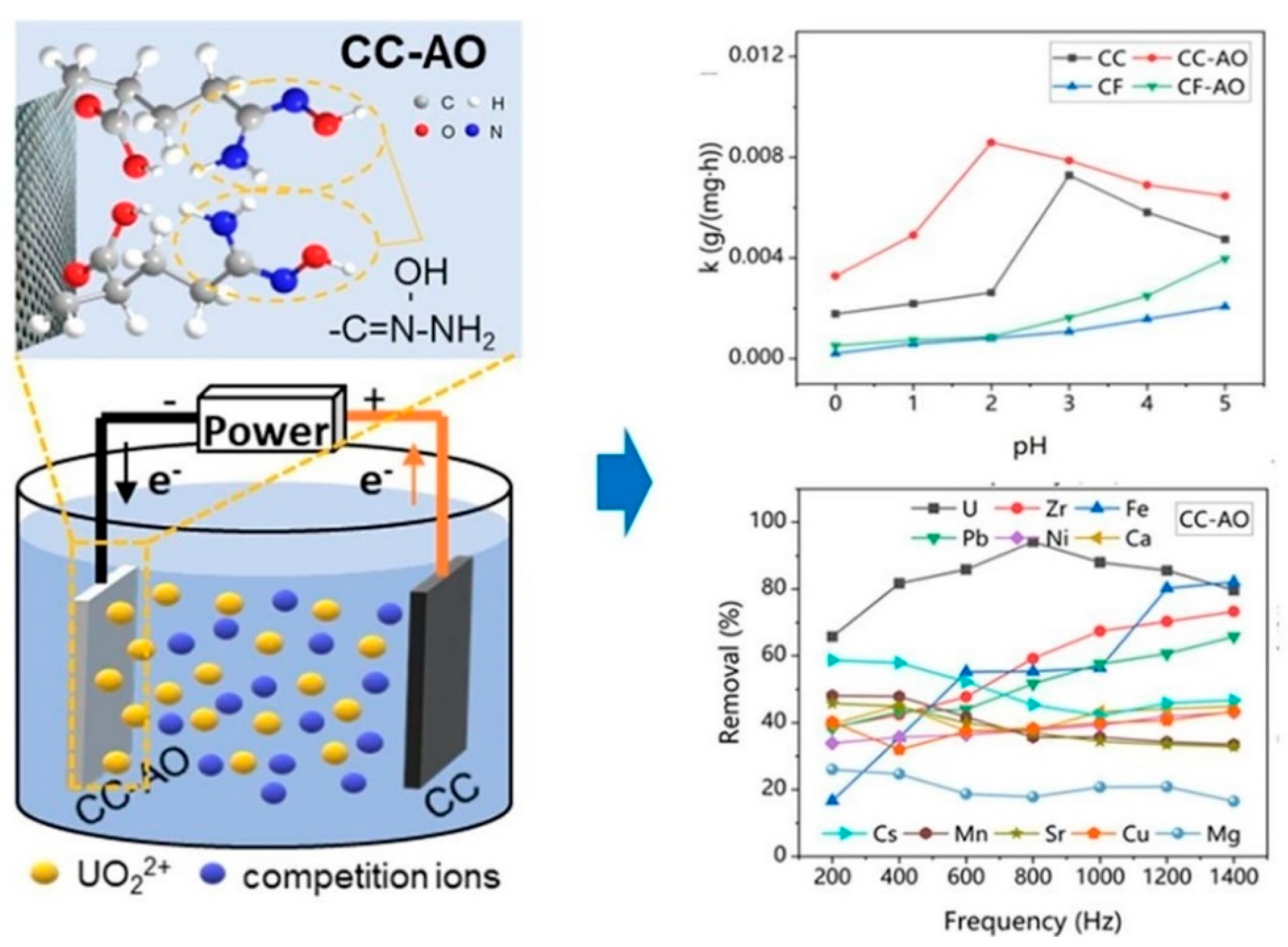
| Adsorbent | Qmax (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| CC-AO (Amidoxime-modified carbon cloth) | 1235 | Lab scale experiment conducted in acidic uranium mine wastewater containing Zr4+, Fe3+, Pb2+, Ni2+, Ca2+, Cs+, Mn2+, Sr2+, Cu2+, Mg2+ competing ions; pH 2; 25 °C; 12.5 h equilibrium time; 0.1 M HNO3 was used as the desorption agent; 68.6% UO22+ uptake retained after seven cycles. | [123] |
| CP-AO (Amidoxime-modified carbon paper) | 1401 | Lab scale experiment conducted in acidic uranium-containing wastewater with Zr4+, Fe3+, Pb2+, Ca2+, Ni2+, Cs+, Mn2+, Sr2+, Cu2+, Mg2+ competing ions; pH 3; 10 h; 0.1 M HNO3 as desorption agent; showed 87.3% desorption after seven cycles. | [126] |
| MoS2-GO/CC (Sulfide-GO- composites-CC) | 74.38 | Lab-scale experiment conducted in simulated uranium-containing wastewater with Fe2+, Na+, Mg2+, Mn2+ competing ions (initial concentration: 5 ppm); pH 4; 25 °C; 12 h; 93.2% capacity retained after 10 cycles; demonstrated efficient uranium recovery against coexisting ions. | [128] |
2.4. Biochar
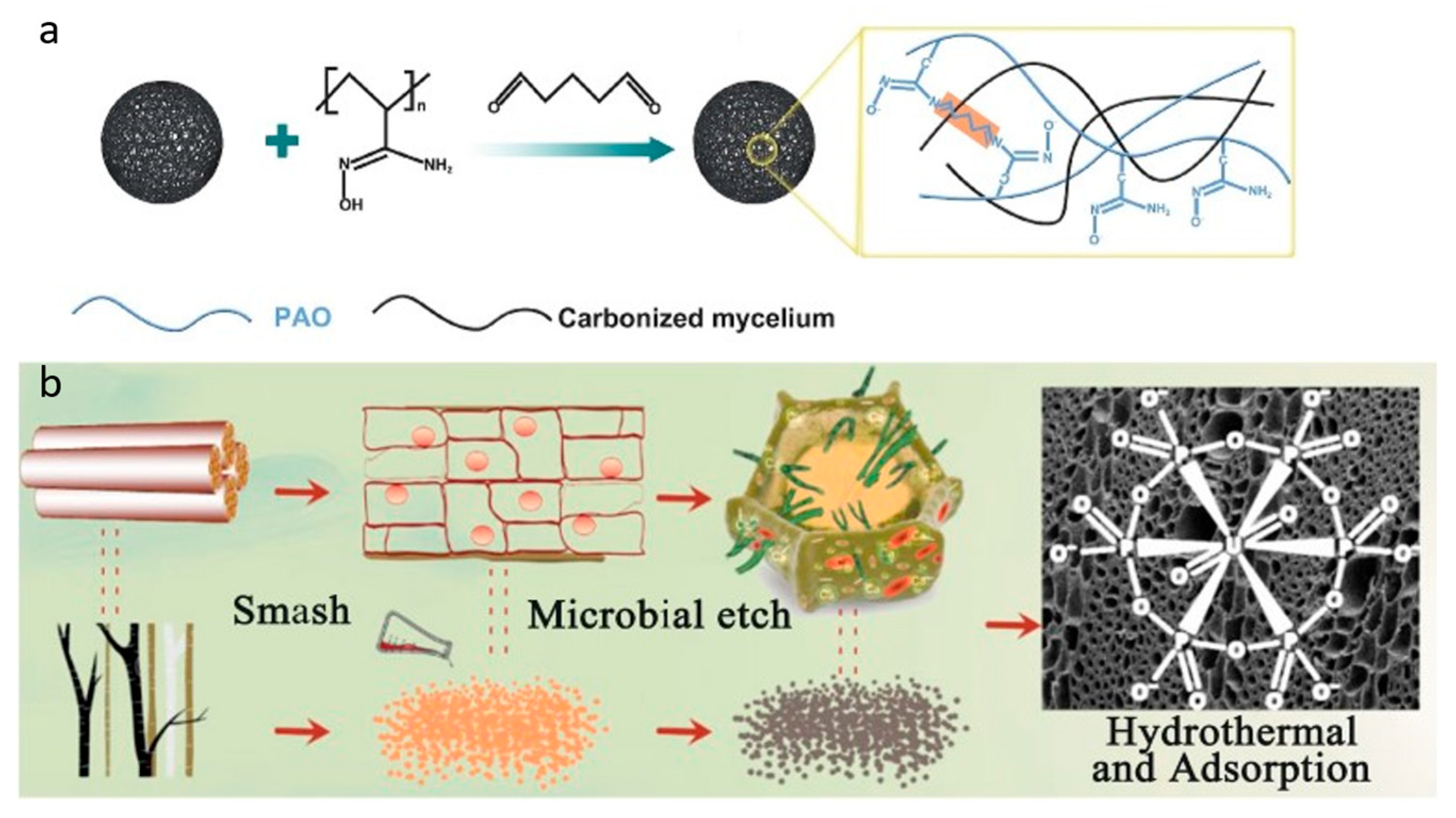
| Adsorbent | Qmax (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| CHMBC-PAO (Polyamidoxime-coated coconut haustorium-derived magnetic biochar) | 810.22 | Lab experiment conducted in nuclear wastewater with Al3+, Ba2+, Co2+, Cr3+, Fe3+, Ni2+, Mg2+, Sr2+, Zn2+, Mn2+, Cd2+ competing ions using coconut haustorium as carbon source; pH 5; 25 °C; 12 h; 1 M Na2CO3 used as desorption agent; after five cycles the adsorption and adsorption capacity decreased, around 3.54% per cycle. | [62] |
| PAO-BS (Polyamidoxime-Loaded Biochar Sphere) | 211.35 | Batch experiment conducted in simulated seawater with V5+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Cr3+, Mn2+, Al3+ ions using fungal mycelium as carbon source; pH 6; 25 °C; 10 h; 1M Na2CO3 used as desorption agent; 22.5% adsorption loss after the fifth cycle. | [132] |
| PEA-CTS@WBC (Polyethanolamine amidoxime modified winter melon and chitosan-derived biochar) | 552.75 | Lab experiment conducted in nuclear wastewater with K+, Cs+, Mg2+, Zn2+, Sr2+, Ca2+, La3+, Gd3+, Sm3+, Eu3+, and Ce3+ ions using winter melon as a carbon source; pH 5; 25 °C; 3 h; 1 M HCl used as a desorption agent; desorption rate decreased by 5.3% after five consecutive cycles. | [133] |
| CCS-P (Microbial etch cotton straw with phosphate doping) | 590.8 | Lab experiment conducted in radioactive wastewater with K+, Cu2+, Mn2+, Cs+, Sr2+, and Fe3+ competing ions using cotton straw as a carbon source; pH 3.5; 1.6 h; 1 M Na2CO3 and 0.1 M H2O2 used as desorption agents; desorption rate decreased by 11% after six consecutive cycles. | [134] |
| PBs (Phosphate-functionalized biochars) | 229.2 | Lab experiment conducted in simulated aqueous solution with Cs+, Sr2+, Co2+, Cu2+, Fe3+, Ni2+, Eu3+ ions using bamboo sawdust as carbon source; pH 4; 25 °C; 8 h; 0.1 M Na2CO3 used as desorption agent; sorption capacity decreased by only 7.47% after six cycles. | [136] |
2.5. Carbon Nanotubes
| Adsorbent | Qmax (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| PAO/CNT (Polyamidoxime-carbon nanotube) | 247 | Lab-scale experiment conducted in uranium-containing solution; pH 4; 20 °C; 2 h equilibrium time; demonstrated efficient uranium recovery. | [63] |
| DGA-MWCNTs (Diglycolamide-multi-walled CNTs) | 133.74 | Lab-scale experiment conducted in uranyl nitrate stock solution; 25 °C; 3 h equilibrium time; demonstrated efficient uranium recovery. | [147] |
| AO-MWCNTs (Amidoxime-MWCNTs) | 67.9 | Lab-scale experiment conducted in aqueous solution; pH 5; 25 °C; 1 h equilibrium time; demonstrated efficient recovery. | [154] |
| Ta-CNTs (Triazine- CNTs) | 74.62 | Lab experiment conducted in aqueous solution; pH 2; 1.5 h equilibrium time; demonstrated efficient uranium recovery. | [160] |
| PS-MWCNTs (Phosphoryl-multiwalled CNTs) | 806.45 | Lab-scale experiment conducted in aqueous solution; pH 5; 25 °C; 0.5 h equilibrium time; 1 M HCl used as desorption agent; adsorbent maintained 90.2% efficiency after eight cycles. | [166] |
| CS-CNTs (Chitosan-based composite -CNTs) | 126.7 | Lab-scale experiment conducted in wastewater with Th4+, Eu3+, La3+, Cs+, Yb3+ ions; pH 4; 25 °C; 1 h equilibrium time; 0.2 M acidified EDTA used as desorption agent; maintained 87.9% efficiency after five cycles. | [169] |
2.6. Carbon Aerogels
| Adsorbent | Qm (mg/g) | Adsorption Conditions and Key Findings | Ref. |
|---|---|---|---|
| KC@C-ZnO-CAs (Konjac glucomannan-derived carbon-encapsulated carbon-doped ZnO carbon aerogels) | 738.4 | Lab-scale experiment conducted in simulated aqueous solution with K+, Mg2+, Sr2+, Na+, Ca2+, Cu2+ competing ions; pH 5; 3 h equilibrium time; 0.1M Na2CO3 used as eluent; adsorbent maintained 92.9% U(VI) removal after five cycles. | [174] |
| nZVI@KGMC (Carbon-doped nano zero-valent iron particles with konjac glucomannan-derived CAs) | 720.8 | Lab-scale experiment conducted in simulated radioactive wastewater with Zn2+, Ca2+, Sr2+, K+, Co2+, Cu2+, Mg2+ competing ions; pH 5; 25 °C; 1 h equilibrium time; demonstrated efficient U recovery. | [175] |
| rGO/CNQDs/ZIF-67(GCZCA) (Reduced graphene oxide-carbon nitride quantum dots-zeolitic imidazolate framework-67-carbon aerogel) | 278.64 | Lab-scale experiment conducted in aqueous solution with Zn2+, Fe3+, Ni2+, Sr2+, Ca2+, K+, Na+ competing ions; pH 3; 35 °C; 0.001 M NaNO3 used as desorption agent; adsorbent maintained >80% removal rate after five cycles. | [176] |
| AO/BB-CAs (Amidoxime/ Biomass Bit-Derived Carbon Aerogel) | 801.2 | Lab-scale experiment conducted in nuclear wastewater with Cu2+, Zn2+, Sr2+, Ni2+, Co2+, Mg2+ ions; pH 5; 25 °C; 0.5 M HNO3 used as desorption agent; maintained 90% adsorbent efficiency after five cycles. | [177] |
| CS-CCN aerogel (Chitosan/carboxylated carbon nanotube composite Aerogels) | 307.5 | Lab-scale experiment conducted in radioactive wastewater with Gd3+, Yb3+, La3+, Nd3+, Sm3+, Co2+, Sr2+, Zn2+, Ni2+ competing ions (initial concentration: 120 ppm); pH 5; 25 °C; 1 h equilibrium time; demonstrated efficient uranium recovery. | [178] |
2.7. Other Carbon-Based Materials
3. Binding Mechanisms of Uranium with Major Chelating Functional Groups Grafted on Carbon Sorbents
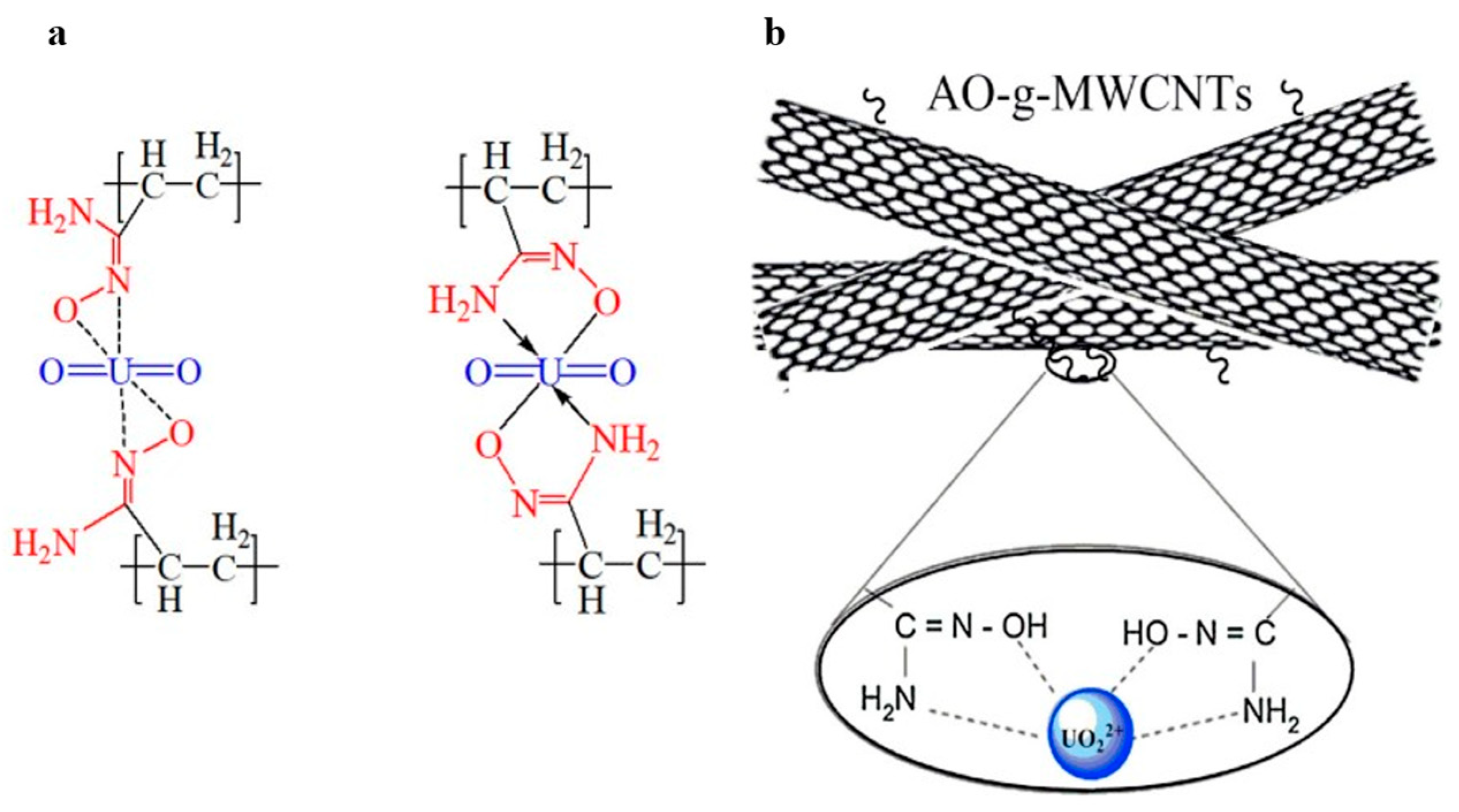
3.1. Amidoxime Functional Groups
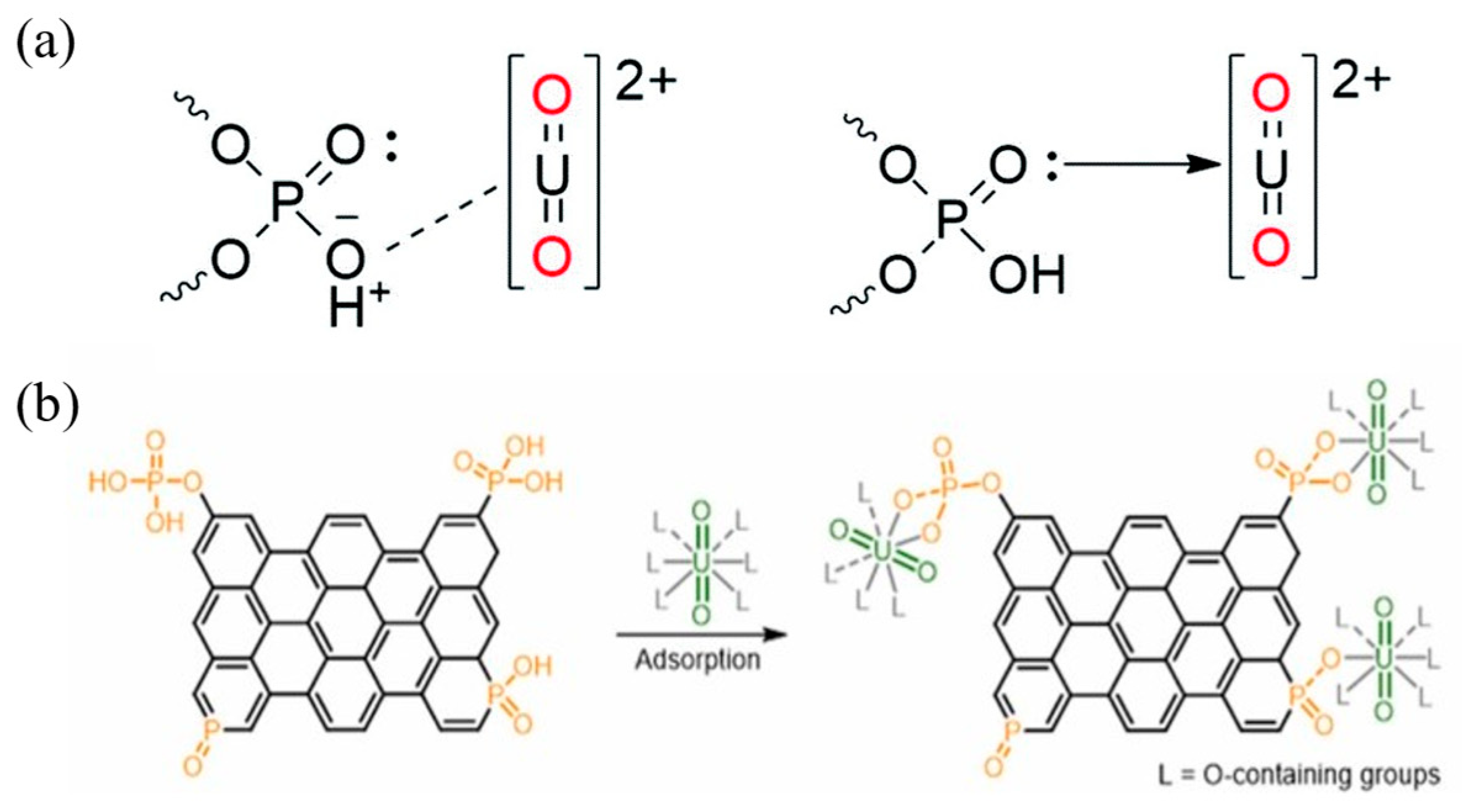
3.2. Phosphoryl Functional Groups
3.3. Amine Functional Groups
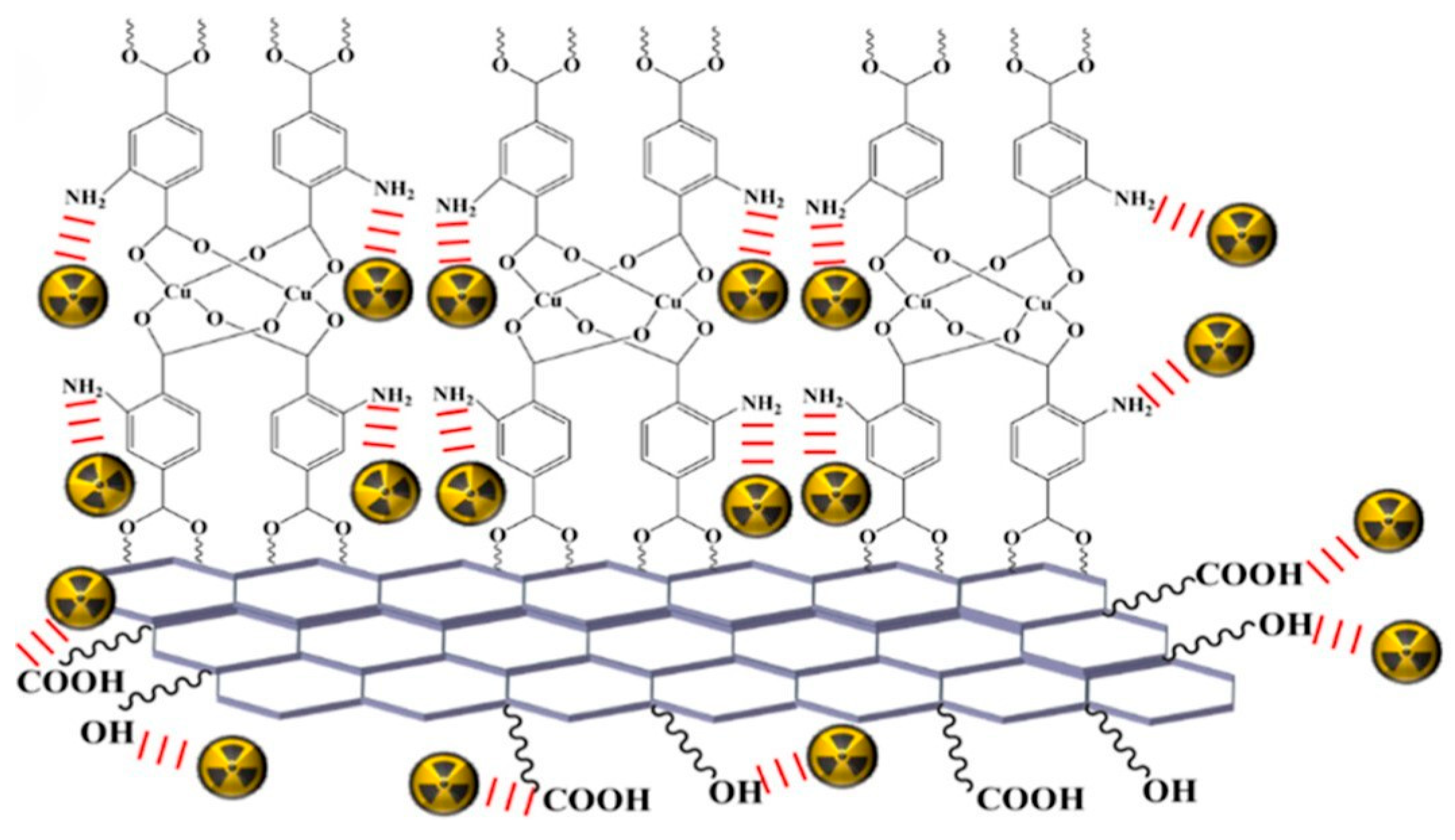
4. Challenges and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Yang, L.; Wang, D.; Tang, C.; Deng, W.; Wang, Z. High-Capacity Amidoxime-Functionalized β-Cyclodextrin/Graphene Aerogel for Selective Uranium Capture. Environ. Sci. Technol. 2021, 55, 9181–9188. [Google Scholar] [CrossRef]
- Rooney, M.; Nuttall, W.J.; Kazantzis, N. A Dynamic Model of the Global Uranium Market and the Nuclear Fuel Cycle. Resour. Policy. 2015, 43, 50–60. [Google Scholar] [CrossRef]
- Wu, F.; Pu, N.; Ye, G.; Sun, T.; Wang, Z.; Song, Y.; Wang, W.; Huo, X.; Lu, Y.; Chen, J. Performance and Mechanism of Uranium Adsorption from Seawater to Poly(Dopamine)-Inspired Sorbents. Environ. Sci. Technol. 2017, 51, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Endrizzi, F.; Rao, L. Chemical Speciation of Uranium(VI) in Marine Environments: Complexation of Calcium and Magnesium Ions with [(UO2)(CO3)3]4− and the Effect on the Extraction of Uranium from Seawater. Chem.—A Eur. J. 2014, 20, 14499–14506. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Liu, S.; Zhou, Y.; Li, B.; Liu, D.; Fu, C.; Ye, L. Polyethylenimine Embellished Multiwalled Carbon Nanotube (MWCNTs) for Efficiently Enhancing Sequestration of Uranium(Ⅵ) from Seawater. J. Environ. Chem. Eng. 2022, 10, 108513. [Google Scholar] [CrossRef]
- Xu, L.; Hu, J.-T.; Ma, H.-J.; Ling, C.-J.; Wang, M.-H.; Shen, R.-F.; Guo, X.-J.; Wang, Y.-N.; Li, J.-Y.; Wu, G.-Z. Amidoxime-Based Adsorbents Prepared by Cografting Acrylic Acid with Acrylonitrile onto HDPE Fiber for the Recovery of Uranium from Seawater. Nucl. Sci. Tech. 2017, 28, 45. [Google Scholar] [CrossRef]
- Parker, B.F.; Zhang, Z.; Rao, L.; Arnold, J. An Overview and Recent Progress in the Chemistry of Uranium Extraction from Seawater. Dalton Trans. 2018, 47, 639–644. [Google Scholar] [CrossRef]
- Bruland, K.W.; Middag, R.; Lohan, M.C. Controls of Trace Metals in Seawater. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 19–51. ISBN 978-0-08-098300-4. [Google Scholar]
- Kim, J.; Tsouris, C.; Mayes, R.T.; Oyola, Y.; Saito, T.; Janke, C.J.; Dai, S.; Schneider, E.; Sachde, D. Recovery of Uranium from Seawater: A Review of Current Status and Future Research Needs. Sep. Sci. Technol. 2013, 48, 367–387. [Google Scholar] [CrossRef]
- Xu, H.; Wang, C.; Liu, Z.; Shi, W. Advances in Adsorption Materials and Coordination Mechanism of Functional Groups for Uranium Extraction from Seawater. Acta Chim. Sin. 2024, 82, 458–470. [Google Scholar] [CrossRef]
- Guo, H.; Mei, P.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Carbon Materials for Extraction of Uranium from Seawater. Chemosphere 2021, 278, 130411. [Google Scholar] [CrossRef]
- Zhang, A.; Uchiyama, G.; Asakura, T. pH Effect on the Uranium Adsorption from Seawater by a Macroporous Fibrous Polymeric Material Containing Amidoxime Chelating Functional Group. React. Funct. Polym. 2005, 63, 143–153. [Google Scholar] [CrossRef]
- Tamada, M.; Seko, N.; Yoshii, F. Application of Radiation-Graft Material for Metal Adsorbent and Crosslinked Natural Polymer for Healthcare Product. Radiat. Phys. Chem. 2004, 71, 223–227. [Google Scholar] [CrossRef]
- Tamada, M. Current Status of Technology for Collection of Uranium from Seawater. In Proceedings of the International Seminar on Nuclear War and Planetary Emergencies—42nd Session, Erice, Italy, 19–24 August 2009; pp. 243–252. [Google Scholar]
- Liu, X.; Li, J.; Wang, X.; Chen, C.; Wang, X. High Performance of Phosphate-Functionalized Graphene Oxide for the Selective Adsorption of U(VI) from Acidic Solution. J. Nucl. Mater. 2015, 466, 56–64. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Xiao, D.; Qiao, Q.; Yin, P.; Yang, Z.; Li, J.; Winchester, W.; Wang, Z.; Hayat, T. Ultra-Thin Iron Phosphate Nanosheets for High Efficient U(VI) Adsorption. J. Hazard. Mater. 2019, 371, 83–93. [Google Scholar] [CrossRef]
- Endrizzi, F.; Leggett, C.J.; Rao, L. Scientific Basis for Efficient Extraction of Uranium from Seawater. I: Understanding the Chemical Speciation of Uranium under Seawater Conditions. Ind. Eng. Chem. Res. 2016, 55, 4249–4256. [Google Scholar] [CrossRef]
- Xiong, X.H.; Yu, Z.W.; Gong, L.L.; Tao, Y.; Gao, Z.; Wang, L.; Yin, W.H.; Yang, L.X.; Luo, F. Ammoniating Covalent Organic Framework (COF) for High-Performance and Selective Extraction of Toxic and Radioactive Uranium Ions. Adv. Sci. 2019, 6, 1900547. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Liu, T.; Xiao, J.; Yu, Q.; Feng, L.; Niu, B.; Feng, S.; Zhang, J.; Wang, N. DNA Nano-Pocket for Ultra-Selective Uranyl Extraction from Seawater. Nat. Commun. 2020, 11, 5708. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Wang, Y.; Li, Y.; Liu, W.; Chen, L.; Sheng, D.; Diwu, J.; Chai, Z.; Albrecht-Schmitt, T.E.; Wang, S. First Cationic Uranyl–Organic Framework with Anion-Exchange Capabilities. Inorg. Chem. 2016, 55, 6358–6360. [Google Scholar] [CrossRef]
- Orabi, A.H.; Mohamed, B.T.; Ismaiel, D.A.; Elyan, S.S. Sequential Separation and Selective Extraction of Uranium and Thorium from Monazite Sulfate Leach Liquor Using Dipropylamine Extractant. Miner. Eng. 2021, 172, 107151. [Google Scholar] [CrossRef]
- Liang, P.; Yuan, L.; Deng, H.; Wang, X.; Wang, L.; Li, Z.; Luo, S.; Shi, W. Photocatalytic Reduction of Uranium(VI) by Magnetic ZnFe2O4 under Visible Light. Appl. Catal. B 2020, 267, 118688. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.-C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Chu, S.; Cui, Y. A Half-Wave Rectified Alternating Current Electrochemical Method for Uranium Extraction from Seawater. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Boussouga, Y.-A.; Joseph, J.; Stryhanyuk, H.; Richnow, H.H.; Schäfer, A.I. Adsorption of Uranium (VI) Complexes with Polymer-Based Spherical Activated Carbon. Water Res. 2024, 249, 120825. [Google Scholar] [CrossRef]
- Bachmaf, S.; Merkel, B.J. Sorption of Uranium(VI) at the Clay Mineral–Water Interface. Environ. Earth Sci. 2011, 63, 925–934. [Google Scholar] [CrossRef]
- Yu, J.; Liao, H.; Zhu, W.; Duan, T.; Wang, S.; Kuang, M.; Zhang, Y.; Lin, X.; Luo, X.; Zhou, J. Marinobacter Sp. Stable Hydrous Titanium Oxide-Functionalized Bovine Serum Albumin Nanospheres for Uranium Capture from Spiked Seawater. ACS Appl. Mater. Interfaces 2019, 11, 40898–40908. [Google Scholar] [CrossRef]
- Huang, G.; Li, W.; Liu, Q.; Liu, J.; Zhang, H.; Li, R.; Li, Z.; Jing, X.; Wang, J. Efficient Removal of Uranium(VI) from Simulated Seawater with Hyperbranched Polyethylenimine (HPEI)-Functionalized Polyacrylonitrile Fibers. New J. Chem. 2018, 42, 168–176. [Google Scholar] [CrossRef]
- Yin, Z.; Cui, C.; Chen, H.; Duoni; Yu, X.; Qian, W. The Application of Carbon Nanotube/Graphene-Based Nanomaterials in Wastewater Treatment. Small 2020, 16, 1902301. [Google Scholar] [CrossRef] [PubMed]
- Sellin, R.; Alexandratos, S.D. Polymer-Supported Primary Amines for the Recovery of Uranium from Seawater. Ind. Eng. Chem. Res. 2013, 52, 11792–11797. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Li, Z.; Liu, J.; Zhang, H.; Li, R.; Wang, J. Efficient Extraction of Uranium from Aqueous Solution Using an Amino-Functionalized Magnetic Titanate Nanotubes. J. Hazard. Mater. 2018, 353, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, D.; Tesfay Reda, A.; Liu, C.; Yang, Z.; Guo, S.; Xiao, S.; Ouyang, Y. Synthesis of Amidoxime-Grafted Activated Carbon Fibers for Efficient Recovery of Uranium(VI) from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 11936–11947. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Lan, J.-H.; Wu, Q.-Y.; Luo, Q.; Zhao, Y.-L.; Wang, X.-K.; Chai, Z.-F.; Shi, W.-Q. Theoretical Insights on the Interaction of Uranium with Amidoxime and Carboxyl Groups. Inorg. Chem. 2014, 53, 9466–9476. [Google Scholar] [CrossRef]
- Barber, P.S.; Kelley, S.P.; Rogers, R.D. Highly Selective Extraction of the Uranyl Ion with Hydrophobic Amidoxime-Functionalized Ionic Liquids via H2 Coordination. RSC Adv. 2012, 2, 8526. [Google Scholar] [CrossRef]
- Chen, X.; Wan, C.; Yu, R.; Meng, L.; Wang, D.; Duan, T.; Li, L. Fabrication of Amidoximated Polyacrylonitrile Nanofibrous Membrane by Simultaneously Biaxial Stretching for Uranium Extraction from Seawater. Desalination 2020, 486, 114447. [Google Scholar] [CrossRef]
- Ju, P.; Liu, Q.; Zhang, H.; Chen, R.; Liu, J.; Yu, J.; Liu, P.; Zhang, M.; Wang, J. Hyperbranched Topological Swollen-Layer Constructs of Multi-Active Sites Polyacrylonitrile (PAN) Adsorbent for Uranium(VI) Extraction from Seawater. Chem. Eng. J. 2019, 374, 1204–1213. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.-J.; Ao, J.-X.; Li, R.; Xing, Z.; Liu, X.-Y.; Guo, X.-J.; Wu, G.-Z.; Ma, H.-J.; Zhao, X.-Y. Preparation of Amidoxime-Based PE/PP Fibers for Extraction of Uranium from Aqueous Solution. Nucl. Sci. Tech. 2019, 30, 20. [Google Scholar] [CrossRef]
- Neti, V.S.; Das, S.; Brown, S.; Janke, C.J.; Kuo, L.-J.; Gill, G.A.; Dai, S.; Mayes, R.T. Efficient Functionalization of Polyethylene Fibers for the Uranium Extraction from Seawater through Atom Transfer Radical Polymerization. Ind. Eng. Chem. Res. 2017, 56, 10826–10832. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, X.; Lu, Y.; Chen, W.; Chen, X.; Li, L. Advanced Amidoximated Polyethylene Nanofibrous Membranes for Practical Uranium Extraction from Seawater. ACS Sustain. Chem. Eng. 2022, 10, 12307–12318. [Google Scholar] [CrossRef]
- Hu, J.; Ma, H.; Xing, Z.; Liu, X.; Xu, L.; Li, R.; Lin, C.; Wang, M.; Li, J.; Wu, G. Preparation of Amidoximated Ultrahigh Molecular Weight Polyethylene Fiber by Radiation Grafting and Uranium Adsorption Test. Ind. Eng. Chem. Res. 2016, 55, 4118–4124. [Google Scholar] [CrossRef]
- Seko, N.; Katakai, A.; Hasegawa, S.; Tamada, M.; Kasai, N.; Takeda, H.; Sugo, T.; Saito, K. Aquaculture of Uranium in Seawater by a Fabric-Adsorbent Submerged System. Nucl. Technol. 2003, 144, 274–278. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the Recovery of Uranium from Seawater. Chem. Rev. 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Bai, C.; Li, J.; Liu, S.; Yang, X.; Yang, X.; Tian, Y.; Cao, K.; Huang, Y.; Ma, L.; Li, S. In Situ Preparation of Nitrogen-Rich and Functional Ultramicroporous Carbonaceous COFs by “Segregated” Microwave Irradiation. Microporous Mesoporous Mater. 2014, 197, 148–155. [Google Scholar] [CrossRef]
- Abney, C.W.; Gilhula, J.C.; Lu, K.; Lin, W. Metal-Organic Framework Templated Inorganic Sorbents for Rapid and Efficient Extraction of Heavy Metals. Adv. Mater. 2014, 26, 7993–7997. [Google Scholar] [CrossRef]
- Tang, N.; Liang, J.; Niu, C.; Wang, H.; Luo, Y.; Xing, W.; Ye, S.; Liang, C.; Guo, H.; Guo, J.; et al. Amidoxime-Based Materials for Uranium Recovery and Removal. J. Mater. Chem. A Mater. 2020, 8, 7588–7625. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Geng, Y.; Li, H.; Wang, N.; Song, Y.; Wang, X.; Chen, J.; Wang, J.; Ma, S.; et al. Uranium Extraction from Seawater: Material Design, Emerging Technologies and Marine Engineering. Chem. Soc. Rev. 2023, 52, 97–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Zhao, L.; Zhang, S.; Huang, Y.; Wu, X.; Wang, X. Synthesis of Amidoxime-Functionalized Fe3O4@SiO2 Core–Shell Magnetic Microspheres for Highly Efficient Sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Yue, Y.; Mayes, R.T.; Kim, J.; Fulvio, P.F.; Sun, X.; Tsouris, C.; Chen, J.; Brown, S.; Dai, S. Seawater Uranium Sorbents: Preparation from a Mesoporous Copolymer Initiator by Atom-Transfer Radical Polymerization. Angew. Chem. 2013, 125, 13700–13704. [Google Scholar] [CrossRef]
- Kim, J.; Tsouris, C.; Oyola, Y.; Janke, C.J.; Mayes, R.T.; Dai, S.; Gill, G.; Kuo, L.-J.; Wood, J.; Choe, K.-Y.; et al. Uptake of Uranium from Seawater by Amidoxime-Based Polymeric Adsorbent: Field Experiments, Modeling, and Updated Economic Assessment. Ind. Eng. Chem. Res. 2014, 53, 6076–6083. [Google Scholar] [CrossRef]
- Mittal, H.; Alfantazi, A.; Alhassan, S.M. Recent Developments in the Adsorption of Uranium Ions from Wastewater/Seawater Using Carbon-Based Adsorbents. J. Environ. Chem. Eng. 2024, 12, 111705. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Z.-Y.; Wang, X.; Ding, C.; Cheng, W.; Yu, S.-H.; Wang, X. Macroscopic and Microscopic Investigation of U(VI) and Eu(III) Adsorption on Carbonaceous Nanofibers. Environ. Sci. Technol. 2016, 50, 4459–4467. [Google Scholar] [CrossRef]
- Liu, F.; Xiong, W.; Liu, J.; Cheng, Q.; Cheng, G.; Shi, L.; Zhang, Y. Novel Amino-Functionalized Carbon Material Derived from Metal Organic Framework: A Characteristic Adsorbent for U(VI) Removal from Aqueous Environment. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 72–80. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Goel, J.; Rajagopal, C. Sorption of Lead, Mercury and Cadmium Ions in Multi-Component System Using Carbon Aerogel as Adsorbent. J. Hazard. Mater. 2008, 153, 502–507. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Sun, H.; Zhang, J. Compressible and Conductive Carbon Aerogels from Waste Paper with Exceptional Performance for Oil/Water Separation. J. Mater. Chem. A Mater. 2017, 5, 14858–14864. [Google Scholar] [CrossRef]
- Mellah, A.; Chegrouche, S.; Barkat, M. The Removal of Uranium(VI) from Aqueous Solutions onto Activated Carbon: Kinetic and Thermodynamic Investigations. J. Colloid. Interface Sci. 2006, 296, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, R.; Chen, M.; Liu, Y.; Xie, Z.; Tang, S.; Yuan, Y.; Wang, N. Vertically Aligned Polyamidoxime/Graphene Oxide Hybrid Sheets’ Membrane for Ultrafast and Selective Extraction of Uranium from Seawater. Adv. Funct. Mater. 2022, 32, 2111049. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Feng, L.; Zhang, J.; Tian, X.; Huang, S.; Yuan, Y.; Wang, N. Biochar Decorated 2D/2D Heterojunction with Enhanced Photocatalytic Activity for Uranium Extraction. Sep. Purif. Technol. 2025, 360, 131026. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of Magnetic Biochar Composites for Enhanced Uranium(VI) Adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, X.; Yu, J.; Zhang, H.; Tawfik Alali, K.; Liu, Q.; Zhu, J.; Yu, J.; Liu, J.; Li, R.; et al. Facile Synthesis of TiO2-PAN Photocatalytic Membrane with Excellent Photocatalytic Performance for Uranium Extraction from Seawater. Sep. Purif. Technol. 2024, 328, 125026. [Google Scholar] [CrossRef]
- Khamirchi, R.; Hosseini-Bandegharaei, A.; Alahabadi, A.; Sivamani, S.; Rahmani-Sani, A.; Shahryari, T.; Anastopoulos, I.; Miri, M.; Tran, H.N. Adsorption Property of Br-PADAP-Impregnated Multiwall Carbon Nanotubes towards Uranium and Its Performance in the Selective Separation and Determination of Uranium in Different Environmental Samples. Ecotoxicol. Environ. Saf. 2018, 150, 136–143. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ren, Q.; Feng, Z.; Li, Y.; Du, Y.; Li, X.; Liu, Y.; Yuan, D.; Lan, T. Unlocking the Potential of Cotton-Derived Carbon Aerogel for Uranium Extraction from Real Radioactive Wastewater: A Path to Amidoxime and Polyguanidine Modification. Chem. Eng. J. 2025, 519, 165635. [Google Scholar] [CrossRef]
- Fasfous, I.I.; Dawoud, J.N. Uranium (VI) Sorption by Multiwalled Carbon Nanotubes from Aqueous Solution. Appl. Surf. Sci. 2012, 259, 433–440. [Google Scholar] [CrossRef]
- Zhou, G.; Gao, F.; Liu, T.; Shi, S.; Wang, H.; Yuan, Y.; Wang, N. Polyamidoxime-Coated Coconut Haustorium Derived Magnetic Biochar Adsorbent with Photothermal Conversion for Highly Efficient Uranium Recovery from Nuclear Wastewater. Adv. Funct. Mater. 2024, 34, 2406329. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, J. Poly Amidoxime Functionalized Carbon Nanotube as an Efficient Adsorbent for Removal of Uranium from Aqueous Solution. J. Mol. Liq. 2020, 319, 114288. [Google Scholar] [CrossRef]
- Ioannidou, O.; Zabaniotou, A. Agricultural Residues as Precursors for Activated Carbon Production—A Review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Nabais, J.M.V.; Laginhas, C.E.C.; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Production of Activated Carbons from Almond Shell. Fuel Process. Technol. 2011, 92, 234–240. [Google Scholar] [CrossRef]
- Ribas, M.C.; Adebayo, M.A.; Prola, L.D.T.; Lima, E.C.; Cataluña, R.; Feris, L.A.; Puchana-Rosero, M.J.; Machado, F.M.; Pavan, F.A.; Calvete, T. Comparison of a Homemade Cocoa Shell Activated Carbon with Commercial Activated Carbon for the Removal of Reactive Violet 5 Dye from Aqueous Solutions. Chem. Eng. J. 2014, 248, 315–326. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Feng, M.; Chen, Z.; Li, S.; Tian, G.; Wang, L.; Huang, J.; Li, S. Solid Phase Extraction of Uranium(VI) onto Benzoylthiourea-Anchored Activated Carbon. J. Hazard. Mater. 2010, 176, 119–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Dai, Y.; Xiao, S.; Cao, X.; Liu, Y.; Guo, W.; Luo, M.; Le, Z. Amidoxime-Functionalized Hydrothermal Carbon Materials for Uranium Removal from Aqueous Solution. RSC Adv. 2016, 6, 102462–102471. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Zhang, S.; Ding, C.; Sheng, G.; Alsaedi, A.; Hayat, T.; Li, J.; Song, Y. Amidoxime-Functionalized Hollow Carbon Spheres for Efficient Removal of Uranium from Wastewater. ACS Sustain. Chem. Eng. 2019, 7, 10800–10807. [Google Scholar] [CrossRef]
- Yin, Z.; Xiong, J.; Chen, M.; Hu, S.; Cheng, H. Recovery of Uranium(VI) from Aqueous Solution by Amidoxime Functionalized Wool Fibers. J. Radioanal. Nucl. Chem. 2016, 307, 1471–1479. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, S.; Wang, X.; Xu, C.; Li, J.; Chen, C.; Chen, J.; Hayat, T.; Alsaedi, A.; Alharbi, N.S.; et al. Plasma-Facilitated Synthesis of Amidoxime/Carbon Nanofiber Hybrids for Effective Enrichment of 238 U(VI) and 241 Am(III). Environ. Sci. Technol. 2017, 51, 12274–12282. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Serafin, J.; Dziejarski, B.; Moreno-Piraján, J.C. Activated Carbon from Biomass Waste as Potential Materials for Uranium Removal. Chem. Eng. Sci. 2025, 306, 121222. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Liu, S.; Zhou, Y.; Liu, D.; Fu, C.; Ye, L.; Fu, Y. UO22+ Capture Using Amidoxime Grafting Low-Cost Activated Carbon (AO-AC) from Solution: Adsorption Kinetic, Isotherms and Interaction Mechanism. Inorg. Chim. Acta 2023, 544, 121226. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Dong, Z.; Wang, Y.; Liu, Y.; Cao, X.; Zhang, Z.; Xu, C.; Wang, N.; Liu, Y. Boosting Uranium Extraction from Seawater by Micro-Redox Reactors Anchored in a Seaweed-like Adsorbent. Nat. Commun. 2024, 15, 9124. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, B.; Zhang, M.; Wen, R.; Guo, X.; Li, X.; Zhang, J.; Li, S.; Ma, L. Pore-Free Matrix with Cooperative Chelating of Hyperbranched Ligands for High-Performance Separation of Uranium. ACS Appl. Mater. Interfaces 2016, 8, 28853–28861. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Liu, L.; Yan, B. Adsorption of Uranium (VI) by Metal-Organic Frameworks and Covalent-Organic Frameworks from Water. Coord. Chem. Rev. 2023, 475, 214917. [Google Scholar] [CrossRef]
- de la Torre-Miranda, N.; Eloy, P.; de la Torre, E.; Steenhaut, T.; Poleunis, C.; Hermans, S. Activated Carbon Functionalized with Amine Sites as an Efficient Alternative for Gold Thiosulfate Recovery. Mater. Chem. Phys. 2024, 312, 128657. [Google Scholar] [CrossRef]
- Ahmed, S.H.; Sharaby, C.M.; El Gammal, E.M. Uranium Extraction from Sulfuric Acid Medium Using Trioctylamine Impregnated Activated Carbon. Hydrometallurgy 2013, 134–135, 150–157. [Google Scholar] [CrossRef]
- Saleh, T.A.; Naeemullah; Tuzen, M.; Sarı, A. Polyethylenimine Modified Activated Carbon as Novel Magnetic Adsorbent for the Removal of Uranium from Aqueous Solution. Chem. Eng. Res. Des. 2017, 117, 218–227. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of Graphene Oxide Formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Romanchuk, A.Y.; Slesarev, A.S.; Kalmykov, S.N.; Kosynkin, D.V.; Tour, J.M. Graphene Oxide for Effective Radionuclide Removal. Phys. Chem. Chem. Phys. 2013, 15, 2321. [Google Scholar] [CrossRef]
- Ren, H.; Cao, Z.-F.; Chen, Y.-Y.; Jiang, X.-Y.; Yu, J.-G. Graphene Oxide-Bicine Composite as a Novel Adsorbent for Removal of Various Contaminants from Aqueous Solutions. J. Environ. Chem. Eng. 2021, 9, 106769. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lan, X.-W.; Ren, H.; Li, W.-J.; Chen, J.; Jiang, X.-Y.; Yu, J.-G. Three-Dimensional Hybrid Nitrogen/Oxygen-Containing Components Modified Graphene Oxide as a Recyclable Adsorbent for Rapid Adsorption of REEs. J. Environ. Chem. Eng. 2021, 9, 106500. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gao, P.; Fan, Y.; Jin, Q.; Chen, Z.; Guo, Z.; Liu, B. Efficient Removal of U(VI) from Aqueous Solution Using Poly(Amidoxime-Hydroxamic Acid) Functionalized Graphene Oxide. Environ. Sci. Pollut. Res. 2024, 31, 24064–24076. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, J.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Song, D.; Li, R.; Wang, J. Amidoxime-Functionalized MXene/Graphene Oxide Aerogel for Sunlight Enhanced Uranium Adsorption. J. Environ. Chem. Eng. 2025, 13, 116254. [Google Scholar] [CrossRef]
- Wang, F.; Li, H.; Liu, Q.; Li, Z.; Li, R.; Zhang, H.; Liu, L.; Emelchenko, G.A.; Wang, J. A Graphene Oxide/Amidoxime Hydrogel for Enhanced Uranium Capture. Sci. Rep. 2016, 6, 19367. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Zhang, S.; Wang, X. Amidoxime-Functionalized Magnetic Mesoporous Silica for Selective Sorption of U(VI). RSC Adv. 2014, 4, 32710. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Chai, Y.; Hua, Z.; Xiao, Y.; Yang, Y. Adsorption Performances and Mechanisms of Amidoxime Resin toward Gallium(III) and Vanadium(V) from Bayer Liquor. ACS Sustain. Chem. Eng. 2016, 4, 53–59. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Huang, G.; Peng, W.; Guo, C.; Shi, J. Preparation of Amidoxime-Functionalized Biopolymer/Graphene Oxide Gels and Their Application in Selective Adsorption Separation of U(VI) from Aqueous Solution. J. Radioanal. Nucl. Chem. 2020, 324, 847–855. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Wang, C.-Z.; Wu, Q.-Y.; Lan, J.-H.; Nie, C.-M.; Chen, S.-S.; Song, Y.; Li, H.; Shi, W.-Q. Theoretical Exploration of the Synergistic Effect of Phosphate and Amidoxime for Uranium Recovery from Seawater. Inorg. Chem. 2024, 63, 24685–24696. [Google Scholar] [CrossRef]
- Wang, J.; Deng, S.; Liu, Z.; Liu, Z. The Rare Two-Dimensional Materials with Dirac Cones. Natl. Sci. Rev. 2015, 2, 22–39. [Google Scholar] [CrossRef]
- Sarkar, S.; Bekyarova, E.; Haddon, R.C. Chemistry at the Dirac Point: Diels–Alder Reactivity of Graphene. Acc. Chem. Res. 2012, 45, 673–682. [Google Scholar] [CrossRef]
- Nandivada, H.; Jiang, X.; Lahann, J. Click Chemistry: Versatility and Control in the Hands of Materials Scientists. Adv. Mater. 2007, 19, 2197–2208. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.-C.; Guibal, E. Uranium and Europium Sorption on Amidoxime-Functionalized Magnetic Chitosan Micro-Particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Wang, X.; Ying, D.; Niu, F.; Cao, X.; Wang, Y.; Hua, R.; Liu, Y.; Wang, X. Ordered Mesoporous Polymer–Carbon Composites Containing Amidoxime Groups for Uranium Removal from Aqueous Solutions. Chem. Eng. J. 2018, 341, 208–217. [Google Scholar] [CrossRef]
- Ladshaw, A.P.; Ivanov, A.S.; Das, S.; Bryantsev, V.S.; Tsouris, C.; Yiacoumi, S. First-Principles Integrated Adsorption Modeling for Selective Capture of Uranium from Seawater by Polyamidoxime Sorbent Materials. ACS Appl. Mater. Interfaces 2018, 10, 12580–12593. [Google Scholar] [CrossRef]
- Husnain, S.M.; Kim, H.J.; Um, W.; Chang, Y.-Y.; Chang, Y.-S. Superparamagnetic Adsorbent Based on Phosphonate Grafted Mesoporous Carbon for Uranium Removal. Ind. Eng. Chem. Res. 2017, 56, 9821–9830. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, H.; Liu, Q.; Liu, J.; Chen, R.; Yu, J.; Hou, J.; Bai, X.; Wang, J. Nano-Sized Architectural Design of Multi-Activity Graphene Oxide (GO) by Chemical Post-Decoration for Efficient Uranium(VI) Extraction. J. Hazard. Mater. 2019, 375, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, S.D. Recovery of Uranium from Seawater: Modified Polyacrylonitrile Fibers as Selective Extractants. Technical Report for Nuclear Energy University Programs (DOE-CUNY-0000729), Department of Energy, Washington, DC, USA, 2017.
- Franczyk, T.S.; Czerwinski, K.R.; Raymond, K.N. Stereognostic Coordination Chemistry. 1. The Design and Synthesis of Chelators for the Uranyl Ion. J. Am. Chem. Soc. 1992, 114, 8138–8146. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Ivanov, A.S.; Bryantsev, V.S.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Ma, S. Bio-Inspired Nano-Traps for Uranium Extraction from Seawater and Recovery from Nuclear Waste. Nat. Commun. 2018, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Fang, Z.; Hu, Y.; Ye, Z.; Peng, X. Anodic Electrodepositing Bioinspired Cu-BDC-NH2 @ Graphene Oxide Membrane for Efficient Uranium Extraction. Langmuir 2024, 40, 5348–5359. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-Based Biosorbents: Modification and Application for Biosorption of Heavy Metals and Radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene Oxide–Chitosan Composite Hydrogels as Broad-Spectrum Adsorbents for Water Purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zheng, L.; Zhou, L.; Chai, Z.; Wang, X.; Shi, W. Interaction Mechanism of Uranium(VI) with Three-Dimensional Graphene Oxide-Chitosan Composite: Insights from Batch Experiments, IR, XPS, and EXAFS Spectroscopy. Chem. Eng. J. 2017, 328, 1066–1074. [Google Scholar] [CrossRef]
- Liu, N.; Liang, H.; Tian, W.; Li, C.; Gao, Q.; Wang, N.; Guo, R.; Mo, Z. An Antibacterial and Antifouling Amidoxime-Functionalized Graphene Oxide Aerogel for Selective Uranium Adsorption in Salt Lake Water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129367. [Google Scholar] [CrossRef]
- Wei, D.; Liu, X.; Lv, S.; Liu, L.; Wu, L.; Li, Z.; Hou, Y. Fabrication, Structure, Performance, and Application of Graphene-Based Composite Aerogel. Materials 2021, 15, 299. [Google Scholar] [CrossRef]
- Gan, J.; Zhang, L.; Wang, Q.; Xin, Q.; Xiong, Y.; Hu, E.; Lei, Z.; Wang, H.; Wang, H. Phosphorylation Improved the Competitive U/V Adsorption on Chitosan-Based Adsorbent Containing Amidoxime for Rapid Uranium Extraction from Seawater. Int. J. Biol. Macromol. 2023, 238, 124074. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Extraction and Adsorption of U(VI) from Aqueous Solution Using Affinity Ligand-Based Technologies: An Overview. Rev. Environ. Sci. Biotechnol. 2019, 18, 437–452. [Google Scholar] [CrossRef]
- Zhang, L.; Su, T.; Luo, Z.; Xu, B.; Yao, W.; Zhou, M.; Yang, W.; Xu, H. A Graphene-Based Porous Composite Hydrogel for Efficient Heavy Metal Ions Removal from Wastewater. Sep. Purif. Technol. 2023, 305, 122484. [Google Scholar] [CrossRef]
- Zhang, C.-R.; Cui, W.-R.; Niu, C.-P.; Yi, S.-M.; Liang, R.-P.; Qi, J.-X.; Chen, X.-J.; Jiang, W.; Zhang, L.; Qiu, J.-D. RGO-Based Covalent Organic Framework Hydrogel for Synergistically Enhance Uranium Capture Capacity through Photothermal Desalination. Chem. Eng. J. 2022, 428, 131178. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Qin, S.; Song, Y.; Liu, Z.; Yang, P.; Li, S.; Liu, C.; Shen, C. Zwitterion Functionalized Graphene Oxide/Polyacrylamide/Polyacrylic Acid Hydrogels with Photothermal Conversion and Antibacterial Properties for Highly Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2023, 33, 2301773. [Google Scholar] [CrossRef]
- Pykal, M.; Šedajová, V.; Thakur, A.; Sengupta, S.; Brahmananda Rao, C.V.S.; Zbořil, R.; Sreenivasulu, B.; Otyepka, M.; Jayaramulu, K. Phosphoryl-Graphene for High-Efficiency Uranium Separation and Recycling. ACS Appl. Mater. Interfaces 2025, 17, 17284–17294. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Liu, Q.; Chen, R.; Liu, J.; Yu, J.; Jing, X.; Zhang, M.; Wang, J. Polypyrrole Modified Fe0 -Loaded Graphene Oxide for the Enrichment of Uranium(VI) from Simulated Seawater. Dalton Trans. 2018, 47, 12984–12992. [Google Scholar] [CrossRef]
- Fan, J.; Gao, L.; Li, S.; Chai, N.; Lei, D. Efficient Recovery of Gallium(Ⅲ) from Alkaline Solution by Electrosorption Using Biomass-Derived Carbon Aerogel Electrodes. Sep. Purif. Technol. 2025, 353, 128297. [Google Scholar] [CrossRef]
- León, M.I.; Castañeda, L.F.; Márquez, A.A.; Walsh, F.C.; Nava, J.L. Review—Carbon Cloth as a Versatile Electrode: Manufacture, Properties, Reaction Environment, and Applications. J. Electrochem. Soc. 2022, 169, 053503. [Google Scholar] [CrossRef]
- Xiong, Y.; Cui, X.; Zhang, M.; Wang, Y.; Lou, Z.; Shan, W. Microwave Hydrothermal Synthesis of Gallotannin/Carbon Nanotube Composites for the Recovery of Gallium Ion. Appl. Surf. Sci. 2020, 510, 145414. [Google Scholar] [CrossRef]
- Chi, F.; Zhang, S.; Wen, J.; Xiong, J.; Hu, S. Highly Efficient Recovery of Uranium from Seawater Using an Electrochemical Approach. Ind. Eng. Chem. Res. 2018, 57, 8078–8084. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.-L.; Chai, Z.-F.; Shi, W.-Q. Electroseparation of Uranium from Lanthanides (La, Ce, Pr, Nd and Sm) on Liquid Gallium Electrode. Sep. Purif. Technol. 2021, 265, 118524. [Google Scholar] [CrossRef]
- Pan, M.; Cui, C.; Tang, W.; Guo, Z.; Zhang, D.; Xu, X.; Li, J. Carbon Cloth as an Important Electrode Support for the High Selective Electrosorption of Uranium from Acidic Uranium Mine Wastewater. Sep. Purif. Technol. 2022, 281, 119843. [Google Scholar] [CrossRef]
- Guo, D.; Yan, C.; Huang, B.; Jin, T.; Liu, Z.; Qian, Y. Combining Electrosorption and Electrochemical Reduction Mechanisms for Uranium Removal Using 1,2,3,4-Butane Tetracarboxylic Acid-Modified MIL-101: An In-Depth Exploration of Uranyl–Adsorbent Interactions. Inorg. Chem. 2025, 64, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Oyola, Y.; Dai, S. High Surface-Area Amidoxime-Based Polymer Fibers Co-Grafted with Various Acid Monomers Yielding Increased Adsorption Capacity for the Extraction of Uranium from Seawater. Dalton Trans. 2016, 45, 8824–8834. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, D.; Xu, X.; Reda, A.T.; Li, J. Efficient Electrosorption of Uranyl Ions by a Homemade Amidoxime-modified Carbon Paper-based Electrode in Acidic Aqueous Condition. J. Chem. Technol. Biotechnol. 2021, 96, 2916–2929. [Google Scholar] [CrossRef]
- Gu, X.; Wang, R.; Yang, S.; Shangguan, Y.; Feng, X.; Chen, H. Boosting Capacitive Deionization in MoS2 via Interfacial Coordination Bonding and Intercalation-Induced Spacing Confinement. ACS Nano 2025, 19, 6488–6498. [Google Scholar] [CrossRef]
- Wang, Z.; Kou, J.; Li, M.; Zhang, X.; Hua, Y.; Fang, Q.; Tian, M.; Cao, M.; Shao, Z.; Wu, X. Enhancement and Sustained Uranium Removal of 2D Transition Metal Sulfide-Graphene Oxide Composite/Carbon Cloth Cathodes in Capacitive Deionization System. Desalination 2025, 605, 118745. [Google Scholar] [CrossRef]
- Khan, S.; Irshad, S.; Mehmood, K.; Hasnain, Z.; Nawaz, M.; Rais, A.; Gul, S.; Wahid, M.A.; Hashem, A.; Abd_Allah, E.F.; et al. Biochar Production and Characteristics, Its Impacts on Soil Health, Crop Production, and Yield Enhancement: A Review. Plants 2024, 13, 166. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of Pesticide Sorption Behaviour of Slow Pyrolysis Biochars as Low Cost Adsorbent for Atrazine and Imidacloprid Removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, M.; Peng, Q.; Wang, L.; Cao, X.; Feng, L.; Yuan, Y.; Wang, N. Polyamidoxime-Loaded Biochar Sphere with High Water Permeability for Fast and Effective Recovery of Uranium from Seawater. J. Water Process Eng. 2023, 55, 104205. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, Y.; Feng, Z.; Ren, Q.; Wang, Y.; Chen, X.; Li, Y.; Wang, Y. Immobilization of Novel Bifunctional Polymer with Amide and Amidoxime Groups in Biochar-Chitosan Composite Gels for Enhancing Uranium(VI) Uptake. Sep. Purif. Technol. 2025, 354, 128891. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, X.; Cai, C.; Liang, C.; Dai, L.; He, X.; He, R.; Liu, H.; Zhu, W. Microbial Etch: A Novel Construction Method of Functionalized Biochar for Enhanced Uranium Extraction in Radioactive Wastewater. Chemosphere 2024, 361, 142544. [Google Scholar] [CrossRef]
- Wang, P.; Ye, H.; Yin, Y.; Chen, H.; Bian, Y.; Wang, Z.; Cao, F.; Guo, Y. Fungi-Enabled Synthesis of Ultrahigh-Surface-Area Porous Carbon. Adv. Mater. 2019, 31, 1805134. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Chen, G.; Chen, L.; Tian, X. Engineering of Phosphate-Functionalized Biochars with Highly Developed Surface Area and Porosity for Efficient and Selective Extraction of Uranium. Chem. Eng. J. 2020, 379, 122388. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, J.; Xiao, T.; Lin, K.; Huang, Y.; Deng, P.; Hu, H.; Wang, J. Remediation of Uranium-Contaminated Water and Soil by Biochar-Based Materials: A Review. Biochar 2025, 7, 41. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon. Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Popov, V. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R: Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Dutta, A.K.; Ghorai, U.K.; Chattopadhyay, K.K.; Banerjee, D. Removal of Textile Dyes by Carbon Nanotubes: A Comparison between Adsorption and UV Assisted Photocatalysis. Phys. E Low. Dimens. Syst. Nanostruct. 2018, 99, 6–15. [Google Scholar] [CrossRef]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of Heavy Metals on Conventional and Nanostructured Materials for Wastewater Treatment Purposes: A Review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef]
- Kar, P.; Jain, P.; Gupta, R.K.; Tripathi, K.M. Emerging Carbon-Based Nanocomposites for Remediation of Heavy Metals and Organic Pollutants from Wastewater. In Emerging Carbon-Based Nanocomposites for Environmental Applications; Mishra, A.K., Hussain, C.M., Mishra, S.B., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–29. ISBN 978-1-119-55485-1. [Google Scholar]
- Lu, C.; Chiu, H. Chemical Modification of Multiwalled Carbon Nanotubes for Sorption of Zn2+ from Aqueous Solution. Chem. Eng. J. 2008, 139, 462–468. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Luo, Y.; Sun, G.; Cui, Y. UO22+ Extraction and Mechanism by Diglycolamide Extractants with Different Ether-Oxygen Chain Skeletons. J. Radioanal. Nucl. Chem. 2024, 333, 2421–2431. [Google Scholar] [CrossRef]
- Sharma, J.N.; Ruhela, R.; Harindaran, K.N.; Mishra, S.L.; Tangri, S.K.; Suri, A.K. Separation Studies of Uranium and Thorium Using Tetra(2-Ethylhexyl) Diglycolamide (TEHDGA) as an Extractant. J. Radioanal. Nucl. Chem. 2008, 278, 173–177. [Google Scholar] [CrossRef]
- Ravi, J.; Venkatesan, K.A.; Antony, M.P.; Srinivasan, T.G.; Vasudeva Rao, P.R. Unsymmetrical Diglycolamide for the Safe Management of Nuclear Waste. J. Environ. Chem. Eng. 2013, 1, 690–695. [Google Scholar] [CrossRef]
- Deb, A.K.S.; Ilaiyaraja, P.; Ponraju, D.; Venkatraman, B. Diglycolamide Functionalized Multi-Walled Carbon Nanotubes for Removal of Uranium from Aqueous Solution by Adsorption. J. Radioanal. Nucl. Chem. 2012, 291, 877–883. [Google Scholar] [CrossRef]
- Misra, R.K.; Jain, S.K.; Abraham, T.N.; Khatri, P.K. Amide Functionalization of Multiwalled Carbon Nanotubes and Their Evaluation for Hg(II) Removal from Water. Int. J. Nanosci. 2011, 10, 205–208. [Google Scholar] [CrossRef]
- Dubey, R.; Dutta, D.; Sarkar, A.; Chattopadhyay, P. Functionalized Carbon Nanotubes: Synthesis, Properties and Applications in Water Purification, Drug Delivery, and Material and Biomedical Sciences. Nanoscale Adv. 2021, 3, 5722–5744. [Google Scholar] [CrossRef]
- Gopalan, A.; Philips, M.F.; Jeong, J.-H.; Lee, K.-P. Synthesis of Novel Poly(Amidoxime) Grafted Multiwall Carbon Nanotube Gel and Uranium Adsorption. J. Nanosci. Nanotechnol. 2014, 14, 2451–2458. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Tang, J. Amidoxime-Grafted Multiwalled Carbon Nanotubes by Plasma Techniques for Efficient Removal of Uranium(VI). Appl. Surf. Sci. 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Rengarajan, R.; Vicic, M.; Lee, S. Solid Phase Graft Copolymerization. I. Effect of Initiator and Catalyst. J. Appl. Polym. Sci. 1990, 39, 1783–1791. [Google Scholar] [CrossRef]
- Seko, N.; Katakai, A.; Tamada, M.; Sugo, T.; Yoshii, F. Fine Fibrous Amidoxime Adsorbent Synthesized by Grafting and Uranium Adsorption–Elution Cyclic Test with Seawater. Sep. Sci. Technol. 2004, 39, 3753–3767. [Google Scholar] [CrossRef]
- Wu, J.; Tian, K.; Wang, J. Adsorption of Uranium (VI) by Amidoxime Modified Multiwalled Carbon Nanotubes. Progress. Nucl. Energy 2018, 106, 79–86. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhang, Z.-B.; Liu, Y.-H.; Cao, X.-H.; Liu, Y.-T.; Li, Q. Adsorption of U(VI) from Aqueous Solution by the Carboxyl-Mesoporous Carbon. Chem. Eng. J. 2012, 198–199, 246–253. [Google Scholar] [CrossRef]
- Parab, H.; Joshi, S.; Shenoy, N.; Verma, R.; Lali, A.; Sudersanan, M. Uranium Removal from Aqueous Solution by Coir Pith: Equilibrium and Kinetic Studies. Bioresour. Technol. 2005, 96, 1241–1248. [Google Scholar] [CrossRef]
- Liu, S.; Luo, J.; Ma, J.; Li, J.; Li, S.; Meng, L.; Liu, S. Removal of Uranium from Aqueous Solutions Using Amine-Functionalized Magnetic Platelet Large-Pore SBA-15. J. Nucl. Sci. Technol. 2021, 58, 29–39. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Ma, K.-Q.; Yan, H.; Luo, Y.; Wu, F.-C.; Yang, C.-T.; Hu, S.; Peng, S.-M. Highly Selective Extraction of Uranium from Wastewater Using Amine-Bridged Diacetamide-Functionalized Silica. J. Hazard. Mater. 2022, 435, 129022. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Zhao, J.; Wei, P.; Wang, C.; Liu, P.; Zhao, X.; Zeng, K.; Wu, F.; Liu, Z. Unexpected Ultrafast and Highly Efficient Removal of Uranium from Aqueous Solutions by a Phosphonic Acid and Amine Functionalized Polymer Adsorbent. New J. Chem. 2021, 45, 10777–10787. [Google Scholar] [CrossRef]
- Alijani, H.; Beyki, M.H.; Mirzababaei, S.N. Adsorption of UO22+ Ions from Aqueous Solution Using Amine Functionalized MWCNT: Kinetic, Thermodynamic and Isotherm Study. J. Radioanal. Nucl. Chem. 2015, 306, 165–173. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Iliopoulou, M.; Tzimopoulos, D.I.; Perlepes, S.P. Synthetic and Structural Chemistry of Uranyl-Amidoxime Complexes: Technological Implications. Chemistry 2023, 5, 1419–1453. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Lada, Z.G.; Skiadas, S.G.; Tzimopoulos, D.I.; Raptopoulou, C.P.; Psycharis, V.; Perlepes, S.P. Understanding the Selective Extraction of the Uranyl Ion from Seawater with Amidoxime-Functionalized Materials: Uranyl Complexes of Pyrimidine-2-Amidoxime. Inorganics 2024, 12, 82. [Google Scholar] [CrossRef]
- Xie, X.; Qin, Z.; He, Y.; Xiong, P.; Huang, Z.; Mao, Y.; Wei, H.; Zhuo, L. Significant Enhanced Uranyl Ions Extraction Efficiency with Phosphoramidate-Functionalized Ionic Liquids via Synergistic Effect of Coordination and Hydrogen Bond. Sci. Rep. 2017, 7, 15735. [Google Scholar] [CrossRef]
- Shao, D.; Li, Y.; Wang, X.; Hu, S.; Wen, J.; Xiong, J.; Asiri, A.M.; Marwani, H.M. Phosphate-Functionalized Polyethylene with High Adsorption of Uranium(VI). ACS Omega 2017, 2, 3267–3275. [Google Scholar] [CrossRef]
- Xue, G.; Yurun, F.; Li, M.; Dezhi, G.; Jie, J.; Jincheng, Y.; Haibin, S.; Hongyu, G.; Yujun, Z. Phosphoryl Functionalized Mesoporous Silica for Uranium Adsorption. Appl. Surf. Sci. 2017, 402, 53–60. [Google Scholar] [CrossRef]
- Guo, X.; Feng, Y.; Ma, L.; Yu, J.; Jing, J.; Gao, D.; Khan, A.S.; Gong, H.; Zhang, Y. Uranyl Ion Adsorption Studies on Synthesized Phosphoryl Functionalised MWCNTs: A Mechanistic Approach. J. Radioanal. Nucl. Chem. 2018, 316, 397–409. [Google Scholar] [CrossRef]
- Barnett, M.O.; Jardine, P.M.; Brooks, S.C.; Selim, H.M. Adsorption and Transport of Uranium(VI) in Subsurface Media. Soil. Sci. Soc. Am. J. 2000, 64, 908–917. [Google Scholar] [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Preparation of a Novel Electrospun Polyvinyl Alcohol/Titanium Oxide Nanofiber Adsorbent Modified with Mercapto Groups for Uranium(VI) and Thorium(IV) Removal from Aqueous Solution. Chem. Eng. J. 2013, 220, 161–171. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, Y.; Li, T.; Zhou, L.; Liu, Z. Immobilization of Carboxyl-Modified Multiwalled Carbon Nanotubes in Chitosan-Based Composite Membranes for U(VI) Sorption. J. Radioanal. Nucl. Chem. 2018, 317, 1419–1428. [Google Scholar] [CrossRef]
- Li, W.-C.; Lu, A.-H.; Guo, S.-C. Control of Mesoporous Structure of Aerogels Derived from Cresol–Formaldehyde. J. Colloid. Interface Sci. 2002, 254, 153–157. [Google Scholar] [CrossRef]
- Szczurek, A.; Jurewicz, K.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. Structure and Electrochemical Capacitance of Carbon Cryogels Derived from Phenol–Formaldehyde Resins. Carbon. N. Y. 2010, 48, 3874–3883. [Google Scholar] [CrossRef]
- Korbutowicz, R.; Prazmowsk, J. Wet Thermal Oxidation of GaAs and GaN. In Semiconductor Technologies; Jan, G., Ed.; InTech: Rijeka, Croatia, 2010; ISBN 978-953-307-080-3. Available online: http://www.intechopen.com/books/semiconductor-technologies/wet-thermal-oxidation-of-gaas-and-gan (accessed on 15 July 2025).
- Li, J.; Wang, J.; Wang, W.; Zhang, X. Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction. Molecules 2019, 24, 1821. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dong, H.; Li, X.; Zhou, L.; Zhu, W.; Chen, T. Biomass Encapsulated ZIF-8-Derived ZnO Carbon Aerogels for Efficient Uranium Extraction by Synergistic Adsorption-Photoreduction. Chem. Eng. J. 2023, 478, 147331. [Google Scholar] [CrossRef]
- Wang, R.; Li, M.; Liu, T.; Li, X.; Zhou, L.; Tang, L.; Gong, C.; Gong, X.; Yu, K.; Li, N.; et al. Encapsulating Carbon-Coated Nano Zero-Valent Iron Particles with Biomass-Derived Carbon Aerogel for Efficient Uranium Extraction from Uranium-Containing Wastewater. J. Clean. Prod. 2022, 364, 132654. [Google Scholar] [CrossRef]
- Zhou, Q.; Jin, B.; Zhao, P.; Chu, S.; Peng, R. RGO/CNQDs/ZIF-67 Composite Aerogel for Efficient Extraction of Uranium in Wastewater. Chem. Eng. J. 2021, 419, 129622. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Tan, G.; Ju, Y.; Chen, Y.; Huo, Y.; Li, H.; Zhou, T.; Song, J.; Fan, Z.; et al. Scalable and Sustainable Zinc (II) Ions-Glue-Assisted Conversion of Biomass Waste Bits into Carbon Aerogels for Efficient Uranium Extraction. Angew. Chem. Int. Ed. 2024, 63, e202409629. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, L.; Le, Z.; Wang, Y.; Liu, Z.; Huang, G.; Adesina, A.A. Preparation of Porous Chitosan/Carboxylated Carbon Nanotube Composite Aerogels for the Efficient Removal of Uranium(VI) from Aqueous Solution. Int. J. Biol. Macromol. 2020, 160, 1000–1008. [Google Scholar] [CrossRef]
- Zhao, W.-W.; Li, L.-H.; Liao, J.-Z.; Liang, R.-P.; Song, A.-M.; Zhang, F.-D.; Ke, H.; Qiu, J.-D. Regenerable and Stable Biomimetic Hydroxyl-Modified Metal-Organic Frameworks for Targeted Uranium Capture. Chem. Eng. J. 2022, 433, 133787. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Liu, F.; Hu, B. Effective Separation and Immobilization of Uranium(VI) from Wastewater Using Amino and Carboxyl Groups Functionalized Covalent Organic Frameworks. Sep. Purif. Technol. 2024, 338, 126501. [Google Scholar] [CrossRef]
- Alali, K.T.; Zhu, J.; Liu, Q.; Liu, J.; Yu, J.; Tan, S.; Wang, J. Hydrogel Polyamidoxime Shell Layer Constructed on Cyclized Polyacrylonitrile Nanofibrous Mats for Efficient Uranium Extraction from Seawater. Desalination 2024, 586, 117856. [Google Scholar] [CrossRef]
- He, Y.; Yu, S.; Shaban, M.; Ren, X.; Chen, S.; Li, Z.; Li, H.; Chen, C. Plasma-Assisted Preparation of Amidoxime-Carbon Nanotubes Hybrids for Effective Uranium Extraction. J. Environ. Chem. Eng. 2024, 12, 114495. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Wang, X. Poly(Amidoxime)-Reduced Graphene Oxide Composites as Adsorbents for the Enrichment of Uranium from Seawater. Sci. China Chem. 2014, 57, 1449–1458. [Google Scholar] [CrossRef]
- Carboni, M.; Abney, C.W.; Taylor-Pashow, K.M.L.; Vivero-Escoto, J.L.; Lin, W. Uranium Sorption with Functionalized Mesoporous Carbon Materials. Ind. Eng. Chem. Res. 2013, 52, 15187–15197. [Google Scholar] [CrossRef]
- Raychaudhuri, D.; Gopakumar, G.; Nagarajan, S.; Brahmmananda Rao, C.V.S. On the Nature of the Carbonyl versus Phosphoryl Binding in Uranyl Nitrate Complexes. J. Phys. Chem. A 2020, 124, 7805–7815. [Google Scholar] [CrossRef]
- Pinaeva, U.; Ollier, N.; Cavani, O.; Balanzat, E.; Al-Sheikhly, M.; Wade, T.L.; Clochard, M.-C. An Uranyl Sorption Study inside Functionalised Nanopores. Sci. Rep. 2020, 10, 5776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, R.; Wang, H.; Liu, L.; Yue, C. Fabrication of Phosphate-Containing Mesoporous Carbon for Fast and Efficient Uranium (VI) Extraction. Colloids Surf. A Physicochem. Eng. Asp. 2023, 662, 130994. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, R.; Zhou, Z.; Xu, Y.; Yu, P. 3D-Porous Carbon Nitride Through Proton Regulation and Photocatalytic Synergy for Efficient Uranium Extraction from Seawater. Small 2025, 21, e2408650. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, Y.; Yuan, D.; Wang, Y.; Liu, J.; Chew, J.W. Investigation of the High U(VI) Adsorption Properties of Phosphoric Acid-Functionalized Heteroatoms-Doped Carbon Materials. Solid. State Sci. 2020, 104, 106248. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, K.; Lu, Y.; Liu, S.; Wang, Z.; Wang, X. Photoinduction of Pt Single Atoms Decorated on UiO-66-NH2 for Photocatalytic Extraction of Uranium under Open System. J. Water Process Eng. 2024, 67, 106210. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhang, N.; Zhang, L.; Fang, Y.; Deng, W.; Yu, M.; Wang, Z.; Li, L.; Liu, X.; Li, J. Adsorption of Uranyl Ions on Amine-Functionalization of MIL-101(Cr) Nanoparticles by a Facile Coordination-Based Post-Synthetic Strategy and X-Ray Absorption Spectroscopy Studies. Sci. Rep. 2015, 5, 13514. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Tian, B.; Li, T.; Zhang, J.; Zhao, H. The Preparation of Amino-Reinforced Phosphorylated Biochar for Efficient Uranium Adsorption. J. Radioanal. Nucl. Chem. 2023, 332, 3305–3315. [Google Scholar] [CrossRef]
- Nezhad, M.M.; Semnani, A.; Tavakkoli, N.; Shirani, M. Selective and Highly Efficient Removal of Uranium from Radioactive Effluents by Activated Carbon Functionalized with 2-Aminobenzoic Acid as a New Sorbent. J. Environ. Manag. 2021, 299, 113587. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, F.; Chen, D.; Gao, J.; Ju, H.; Tang, J.; Jiang, Q.; Wang, X. Synergistic and Efficient Sorption of Uranium by Amidoxime-Based Chitosan with Multiple Functional Groups. J. Environ. Chem. Eng. 2024, 12, 111955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Zhao, L.; Zhang, X.; Yang, C.; Cui, Y.; Yu, Z.; Zheng, J. Functionalized Carbon-Based Materials for Uranium Extraction: A Review. Separations 2025, 12, 283. https://doi.org/10.3390/separations12100283
Hussain M, Zhao L, Zhang X, Yang C, Cui Y, Yu Z, Zheng J. Functionalized Carbon-Based Materials for Uranium Extraction: A Review. Separations. 2025; 12(10):283. https://doi.org/10.3390/separations12100283
Chicago/Turabian StyleHussain, Maqbool, Liang Zhao, Xusheng Zhang, Chen Yang, Yi Cui, Zhisheng Yu, and Jianzhong Zheng. 2025. "Functionalized Carbon-Based Materials for Uranium Extraction: A Review" Separations 12, no. 10: 283. https://doi.org/10.3390/separations12100283
APA StyleHussain, M., Zhao, L., Zhang, X., Yang, C., Cui, Y., Yu, Z., & Zheng, J. (2025). Functionalized Carbon-Based Materials for Uranium Extraction: A Review. Separations, 12(10), 283. https://doi.org/10.3390/separations12100283








