Abstract
The subject of this study was to compare the enantioseparation of fourteen racemic esters that are liquid crystals. This study aimed to determine the difference in the enantioseparation of mixtures with protonated and fluorinated aliphatic chains and those with different orders of occurrence of benzene rings (benzoates and biphenylates). This research was carried out on two chiral polysaccharide columns: amylose tris(3-chloro-5-methylphenylcarbamate) (ReproSil Chiral MIG) and cellulose tris(3,5-dichlorophenylcarbamate) (ReproSil Chiral MIC). The columns were evaluated in HPLC separation. The analyses were performed in the normal- and reversed-phase systems. The mobile phase consisted of different solvent systems (acetonitrile/water and n-hexane/2-propanol) in different volume ratios to select optimal separation conditions. The main parameter evaluated in separating racemic mixtures was the resolution—Rs. All measurements were performed at 25 °C. The elution order was also determined. The highest value of resolution (over 11) and selectivity (over 3) was obtained for the ReproSil Chiral MIG column and the volume ratio of ACN:H2O (95:5 v/v).
1. Introduction
Enantioselective organic synthesis is a key element in developing modern chemistry, industry, and technology, providing an opportunity to create highly specific and effective chemical products with precisely controlled properties [1,2,3]. Enantiomerically pure chemical compounds, such as pharmaceuticals, are receiving significant attention for their applications [4,5].
A racemic mixture (racemate) is an equimolar mixture of two optically active enantiomers. Right-handed enantiomers rotate the plane of polarized light by a specific angle to the right. In contrast, left-handed enantiomers rotate the plane of polarized light by a specific angle to the left. Enantiomers are mirror images of each other, which cannot be superimposed [6,7]. Due to the symmetry of enantiomers, a racemic mixture does not exhibit optical activity. This is due to enantiomers with opposite rotations in an equimolar ratio. In a racemic mixture, equal amounts of two enantiomers cancel each other’s optical activity [8]. Enantiomers have the same chemical and physical properties, but the different interactions of enantiomers with polarized light create different applications.
Current developments in chemical synthesis and knowledge of the properties of individual enantiomers have led to the use of selected enantiomers [9,10]. Various methods have been developed for separating racemic mixtures, ranging from traditional chromatographic techniques to more advanced separation methods. Racemic mixtures can be separated by two main methods, i.e., indirect and direct [11,12]. An indirect method for separating enantiomers involves forming a diastereoisomer pair using chiral reagents. Diastereoisomers, in turn, can be separated by classical methods, i.e., crystallization, countercurrent extraction, distillation, or chromatography on non-chiral columns. The direct method of separating enantiomers uses chiral stationary phases or chiral mobile phase additives. The separation by both methods involves the formation of transient diastereomeric complexes of varying persistence between the enantiomer and the chiral selector [13,14].
Chiral stationary phases are usually made of chiral compounds chemically coated on the surface of the chromatographic carrier (silica or polymer beads) or immobilized, i.e., permanently bound to the carrier [15]. Chiral stationary phases coated on silica support restrict the use of the mobile phase. Polar solvents, i.e., dichloromethane, tetrahydrofuran, ethyl acetate, and acetone, are impossible to use because they swell or dissolve polysaccharide derivatives, removing the column fill [16]. Consequently, eluents based on mixtures of alkanes and alcohols or solvents containing acetonitrile or alcohols have been used for coated chiral stationary phases [17]. Considering the disadvantages of coated chiral stationary phases, the advantages of immobilized stationary phases are worth noting. More solvents can be used once the chiral stationary phase is immobilized on the chromatographic substrate [18].
Depending on the choice of chiral stationary phase used, a different selectivity, resolution, and number of theoretical plates can be obtained during the separation of the racemic mixtures. Polysaccharides (cellulose and amylose derivatives) [19,20,21,22,23,24] have gained the most significant recognition as stationary phases due to their optical activity for separating liquid crystalline racemates. Chiral columns based on chiral polysaccharides were selected for their generally remarkable recognition capabilities, enabling efficient chiral resolutions of many different classes of compounds covering a wide range of structures. They have already been used for liquid crystalline three-ring esters with a perfluoroalkoxyalkoxy terminal chain [25,26,27,28,29,30] or diazenes [31,32]. Since the analyzed materials have a similar structure to previously tested mixtures and differ only in the terminal chain and the order of occurrence of benzene rings, polysaccharide columns were selected. Previous research has also proven that immobilized columns in which the modified polysaccharide is covalently bonded to silica allow a better separation of liquid crystalline racemates than coated columns [27,30].
The chiral recognition of racemic solutes on polysaccharide CSPs is achieved through various types of bonding within the chiral helical grooves of the chiral selector, in particular through H-bonding, dipole–dipole, and π–π interactions, as well as through steric effects. Differences in the structure of analytes, the type of polysaccharide selector, and mobile phase composition significantly influence chirality recognition through the interactions indicated above.
This work aimed to achieve the enantiomeric separation of fourteen liquid crystalline racemates. The studied racemates were characterized by varying protonated or fluorinated chain lengths and inverted benzene ring orders (benzoates and biphenylates). For this purpose, two different immobilized chiral columns (ReproSil Chiral MIG and ReproSil Chiral MIC) and two solvent systems were evaluated. The effect of different chromatographic conditions was examined to obtain the best enantioseparation in the shortest analysis time. In this work, we demonstrate that the reversed-phase system can be effectively used for the enantioseparation of liquid crystalline racemates on the chiral stationary phase derived from amylose.
2. Materials and Methods
2.1. Racemic Mixtures
This research was conducted on 14 previously synthesized racemic mixtures. Each racemate differed in the length of the protonated or fluorinated chain (n = 1, 2, 4, 5, 7). This study included racemates with identical protonated or fluorinated chains, differing in the inverted order of the benzene rings, with the biphenyls first, followed by the phenyl (Figure 1a) and the reverse order (Figure 1b).
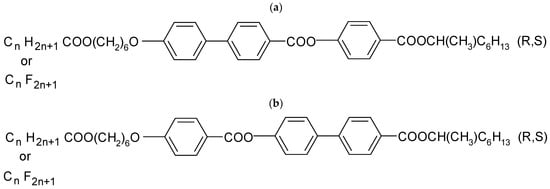
Figure 1.
(a,b) The general formulas of the racemic mixtures used in the studies.
The methods of synthesis of the studied esters and their properties are described in detail in Refs. [33,34,35,36]. Depending on the protonated or fluorinated chain length, each racemic mixture was assigned an appropriate acronym, as presented in Table 1. The chemical purity and compatibility with the molecular structure of the analyzed racemates before starting the enantioseparation were checked by the high-performance liquid chromatography HPLC-PDA-MS (APCI-ESI dual source) Shimadzu LCMS 2010 EV (Kyoto, Japan), which is equipped with a polychromatic UV–VIS detector (Kyoto, Japan). The purity, calculated based on the % of the area and the % of the height, was over 99% (appropriate chromatograms and purity curves for the protonated racemates with the PhBi structure are presented in the Supplementary Materials in Figures S1–S8). As a result of the ionization process of the analyzed mixtures, the strong molecular ion with a captured sodium atom [M + Na]+ or without a proton [M − H]− was observed for the studied racemates, as shown in Table 1. In the Supplementary Materials, the MS spectra for the protonated racemates with the PhBi structure are presented in Figures S9–S12. If we ionize the mixture CH3BiPh (R,S) with a mass of 588, as a result of the loss of a proton, we obtain m/z = (588 − 1)/1, i.e., 587, as shown in Table 1.

Table 1.
The acronyms and quasimolecular ions for the studied racemic mixtures (Ph means phenyl and Bi means biphenyl).
2.2. Chiral HPLC Separation
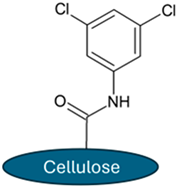
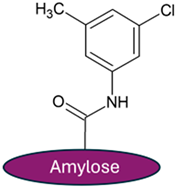
The ReproSil Chiral MIC column with cellulose tris(3,5-dichlorophenylcarbamate), immobilized on a silica gel, and the ReproSil Chiral MIG column with amylose tris(3-chloro-5-methylphenylcarbamate), immobilized on a silica gel, were used, as shown in Table 2.

Table 2.
Chemical structure of chiral selectors employed in chromatographic columns.
The columns used had dimensions of 250 mm × 4.6 mm, a particle size of 5 µm, a pore size of 1000 Å, and a surface area of 30 m2/g (Dr. Maisch GmbH, Ammerbuch, Germany).
The mobile phase consisted of acetonitrile and water (hereafter referred to as ACN:H2O) and n-hexane and 2-propanol (hereafter referred to as HEX:IPA) in different volume ratios (Table 3). The analyses were performed in an isocratic elution with a constant mobile phase composition throughout the analysis. The sample concentrations were 0.5–0.6 mg/mL. The samples were dissolved in ACN or IPA. All solvents were used as purchased (ACN—POCH S.A., Poland; IPA—HONEYWELL, Poland; HEX—CHEMPUR, Poland). In addition, ultrapure water was used. The results obtained were analyzed at a wavelength of 254 nm.

Table 3.
Separation parameters used in the analysis.
The studies were performed at 25 °C. The Shimadzu LC-20AP HPLC system (Kyoto, Japan), consisting of a binary solvent delivery pump, an autosampler (SIL-10AP), a communications bus module (CBM-20A), a diode array detector (SPD-M20A) and a fraction collector (FRC-10A), was used for the separation and detection of analytes. Data acquisition was performed by the Shimadzu software (Labsolutions, 2010–2017 Shimadzu Corporation).
3. Results
3.1. ReproSil Chiral MIG Column, ACN:H2O Solvent System
In the ACN:H2O solvent system, a volume ratio of 99:1 was initially used. A total of 11 of the 14 liquid crystalline racemic mixtures were separated in the system considered. The mixtures C2H5BiPh(R,S), C7F15BiPh(R,S), and C7F15PhBi (R,S) were not separated. The resolution parameter (Rs), which determines the baseline separation of the enantiomers above a value of 1.5, was analyzed.
Nine mixtures were baseline separated, while the remaining two were partially separated. When changing the volume ratio of the solvents used, increasing the amount of water in the mobile phase improved the resolution (Rs) in all the mixtures analyzed. In addition, using the ACN:H2O solvent system in a 95:5 volume ratio separated the C2H5BiPh(R,S) mixture, which did not separate under the previously discussed conditions.
Further increases in the water content of the mobile phase composition resulted in a successively higher resolution of the mixtures, increasing the analysis time significantly. Due to the long analysis time, studies for the volume ratio ACN:H2O (90:10 v/v) were not continued for mixtures with a protonated aliphatic chain. In the case of mixtures with the fluorinated chain, the analyses lasted more than twice as long and did not increase as significantly with an increasing chain length (the analysis time for the 90:10 ACN:H2O volume ratio increased by a maximum of two times for the 99:1 ACN:H2O separation conditions). The collected retention times for the analyzed racemic mixtures are summarized in Table 4. The designations used in this table and subsequent tables in the article are explained below Table 4.

Table 4.
Retention times of the studied racemic mixtures on the ReproSil Chiral MIG column.
Table 5 summarizes the results for the resolution parameter (Rs) values for the studied racemic mixtures. However, because not all racemic mixtures were separated, a detailed illustration of the resolution parameter for the separated racemic mixtures is shown in Figure 2. It can be seen that the highest value of this parameter was obtained for the mixture C7H15BiPh (R,S). Furthermore, the resolution parameter increased with the elongation of the protonated chain. The resolution parameter was much lower for the mixtures with the fluorinated chain, and its value decreased with an increasing fluorinated chain length.

Table 5.
Resolution values for the studied racemic mixtures, ACN:H2O (99:1 v/v, 95:5 v/v, and 90:10 v/v).
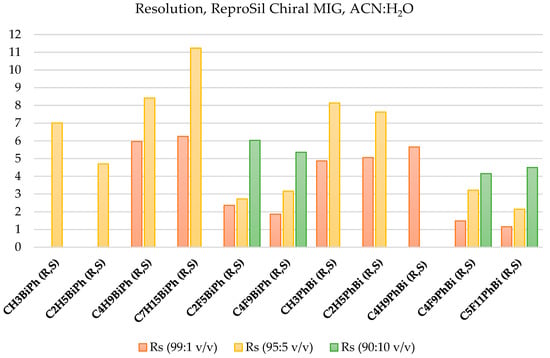
Figure 2.
Resolution summary of the racemic mixtures, ACN:H2O (99:1 v/v, 95:5 v/v, and 90:10 v/v).
Furthermore, by comparing the resolution parameter with retention time, it can be observed that as the retention time increases, the resolution also increases. The resolution is described by Formula (1), where tr1 and tr2 are retention times of the first and the second peaks, and Wb1 and Wb2 are baseline widths [37].
Rs = tr2 − tr1/0.5(Wb1 + Wb2)
Analyzing racemic mixtures in non-equimolar ratios enabled the determination of the elution order of individual enantiomers. For the presented mixture, in Figure 3, the chromatogram of the racemic mixture is depicted, while in Figure 4, the chromatogram of the non-equimolar mixture is shown. The chromatographic analysis indicates that the enantiomer (S) elutes first, followed by the enantiomer (R). This same trend was observed for all analyzed mixtures on this column.
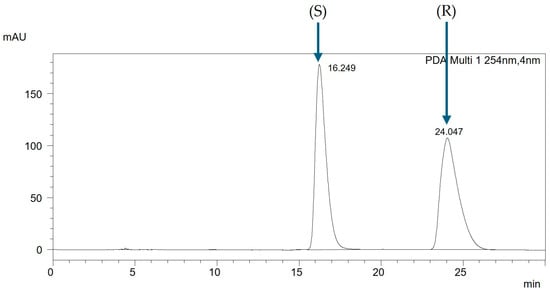
Figure 3.
The chromatogram of the racemic mixture CH3BiPh (R,S), ACN:H2O (99:1 v/v), with a flow rate of 1 mL/min.
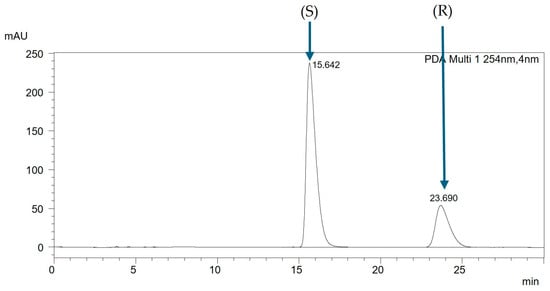
Figure 4.
The chromatogram of the non-equimolar mixture CH3BiPh (R,S) + (S), ACN:H2O (99:1 v/v), with a flow rate of 1 mL/min.
A selectivity parameter is shown for all studied mixtures. The selectivity values are in the range of 1.000 to 3.526. The value of this parameter for the mixtures that have not separated is 1.000.
An enantiomer forming a less stable complex with the chiral selector, i.e., with a lower value of the retention factor k1, will elute faster than an enantiomer with a higher retention factor k2, i.e., that is more stable. Formula (2) shows the relationship between the enantiomers’ k2 and k1 retention factors, which defines selectivity [37].
α = k2/k1
The number of theoretical plates also influences the effective separation of the racemic mixtures. This number defines the quantity of sites where separated substances temporarily halt during the separation process. An increase in the number of theoretical plates often leads to a better column resolution. Moreover, the number of theoretical plates affects the width and shape of chromatographic peaks; for higher values, the peaks are narrower, while for lower values, they are blurred.
Table 6 summarizes the selectivity parameter values and the number of theoretical plates for each enantiomer (if the mixture was separated). The highest selectivity value was obtained for the mixture C2F5BiPh (R,S) in a 95:5 volume ratio of ACN:H2O. The highest ability to produce narrow peaks was demonstrated by the mixture C7H15BiPh (R,S). The number of theoretical plates is described by Formula (3), where tr is the retention time and Wt is the peak width at the base [37].
N = 16(tr/Wt)2

Table 6.
Selectivity factor values and the number of theoretical plates for successive enantiomers of the separated racemic mixtures, ACN:H2O (99:1, 95:5, and 90:10 [v/v]).
3.2. ReproSil Chiral MIC Column, ACN:H2O Solvent System
The separation of the racemic mixtures was also conducted on the second chiral column, ReproSil Chiral MIC. The racemic mixtures prepared earlier were separated under the same chromatographic conditions using isocratic elution for comparison purposes. All chromatographic parameters and results were obtained under the same conditions as for the ReproSil Chiral MIG column, as presented in Table 3. Only when determining the elution order of enantiomers in non-equimolar mixtures in this solvent system was the flow rate reduced to 0.3 mL/min. Initially, the ACN:H2O solvent system was used with a volume ratio of 99:1. In this system, only six mixtures were separated, of which only four were baseline separated.
The separation performed under these mobile phase conditions resulted in changes in the resolution parameter. It was observed that increasing the proportion of water in the eluent volume ratio to 95:5 (v/v) for short-chain protonated mixtures causes a decrease in resolution, but as the aliphatic chain lengthens, this parameter improves, achieving better results than with a lower proportion of water in the eluent. A further modification of the mobile phase composition to 90:10 ACN:H2O (v/v) resulted in a significant decrease in the resolution parameter. Under these conditions, the mixture CH3PhBi (R,S) does not baseline separate (Rs < 1.5). The mobile phase composition partially separated the other two racemic mixtures with fluorinated chains. Table 7 summarizes the results of the resolution parameter (Rs), the selectivity factor values, and the theoretical plate number values for the separated racemic mixtures on this column. Only nine mixtures were separated on the ReproSil Chiral MIC column; therefore, the results are illustrated as a graph in Figure 5. The highest selectivity values were obtained when the mobile phase consisted of 99:1 ACN:H2O (v/v). This parameter successively decreases as the proportion of water in the mobile phase increases. The values were sometimes higher than the results obtained on the ReproSil Chiral MIG column.

Table 7.
Chromatographic parameters obtained for the studied racemic mixtures, ACN:H2O (99:1 v/v, 95:5 v/v, and 90:10 v/v).
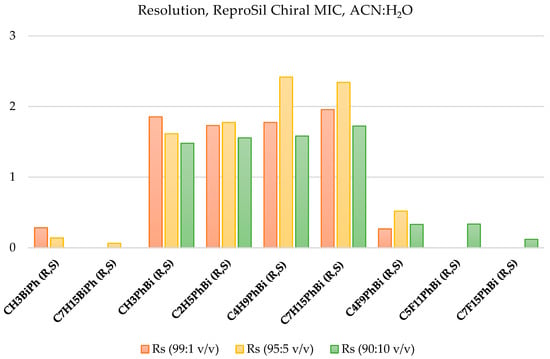
Figure 5.
Resolution summary for the studied racemic mixtures, ACN:H2O (99:1 v/v, 95:5 v/v, and 90:10 v/v).
Increasing the amount of water in the mobile phase for the mixtures with the PhBi structure causes the number of theoretical plates to increase almost 4 times.
In the case of the ReproSil Chiral MIC column, the retention times of the analyzed racemic mixtures were significantly shorter (below 25 min). The influence of the retention time on the resolution parameter can be observed. The longer the retention times, the better the resolution of the racemic mixture. Table 8 compares the retention times of the analyzed mixtures on the ReproSil Chiral MIC column.

Table 8.
Retention times of the studied racemic mixtures on the ReproSil Chiral MIC column.
The analysis of the racemic mixtures in non-equimolar ratios enabled the determination of the elution order of individual enantiomers. The separations were performed using isocratic elution with a mobile phase composition of acetonitrile–water in a volume ratio of 99:1 and at a flow rate of 0.3 mL/min. For the presented mixture CH3BiPh (R,S), the chromatogram of the racemic mixture is shown in Figure 6. In contrast, the chromatogram of the non-equimolar mixture CH3BiPh (R,S) + (S) is depicted in Figure 7. The chromatographic analysis indicates that enantiomer (R) elutes first, followed by enantiomer (S). The same trend is observed for all mixtures.
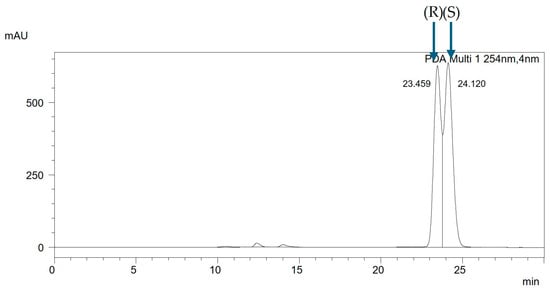
Figure 6.
The chromatogram of the racemic mixture CH3BiPh (R,S), ACN:H2O (99:1 v/v), with a flow rate of 0.3 mL/min.
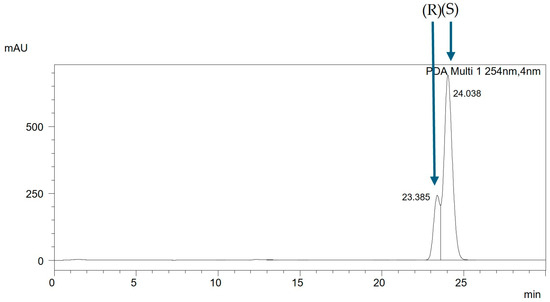
Figure 7.
The chromatogram of the non-equimolar mixture CH3BiPh (R,S) + (S), ACN:H2O (99:1 v/v), with a flow rate of 0.3 mL/min.
3.3. Both Columns, HEX:IPA Solvent System
In the case of the ReproSil Chiral MIG column and the n-hexane-2-propanol solvent system, partial separation was achieved only for four mixtures with a 15-volume ratio of 2-propanol (see Figure 8). The increase in the 2-propanol volume ratio resulted in a deterioration of the separation of the mixtures; the resolution parameter decreased two or three times for the best-separated mixture (CH3BiPh (R,S)), as shown in Figure 9, and, therefore, further analyses in this condition were not conducted. The elution order of enantiomers was the same as that of the ACN:H2O solvent system.
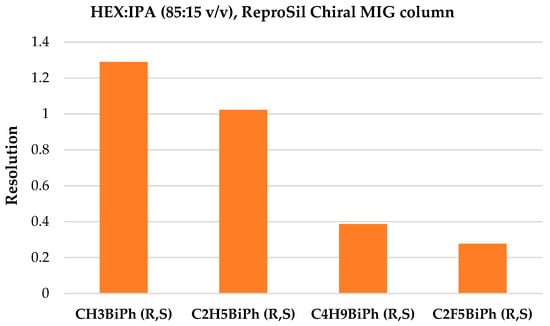
Figure 8.
Resolution summary on the ReproSil Chiral MIG column for four racemic mixtures, HEX:IPA (85:15 v/v).
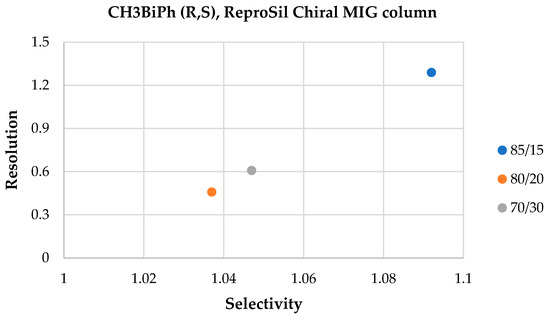
Figure 9.
Resolution and selectivity factor values obtained on the ReproSil Chiral MIG column for the mixture CH3BiPh (R,S), HEX:IPA (85:15 v/v, 80:20 v/v, and 70:30 v/v).
In the case of the ReproSil Chiral MIC column, baseline separation was achieved for four mixtures and the mobile phase composition of HEX:IPA (85:15 v/v), as shown in Figure 10. Increasing the 2-propanol volume ratio, similar to the ReproSil Chiral MIG column, resulted in a resolution deterioration (see Figure 11). The elution order of enantiomers was the same as that of the ACN:H2O solvent system.
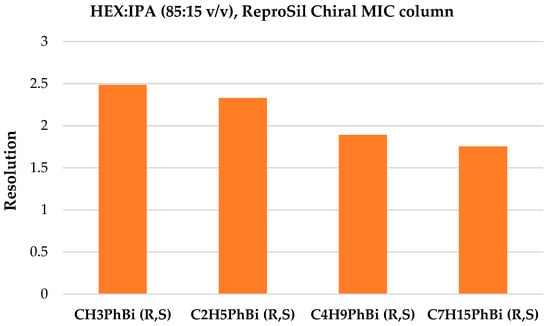
Figure 10.
Resolution summary on the ReproSil Chiral MIC column for four racemic mixtures, HEX:IPA (85:15 v/v).
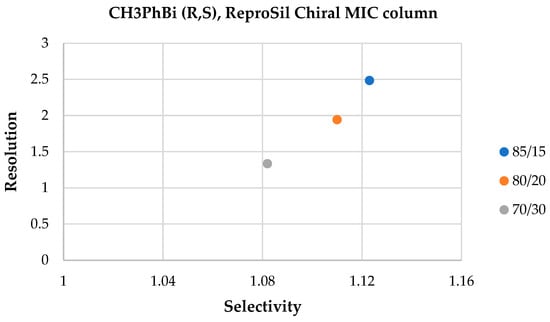
Figure 11.
Resolution and selectivity factor values obtained on the ReproSil Chiral MIC column for the mixture CH3PhBi (R,S), HEX:IPA (85:15 v/v, 80:20 v/v, and 70:30 v/v).
After analyzing the results, it can be concluded that only protonated mixtures were separated in this solvent system. This indicates that the interactions between the fluorinated mixtures and the stationary phases are weak in this solvent system due to the fluorinated chain, which cannot provide additional π–π interactions with CSPs. The ReproSil Chiral MIC column separates mixtures in which phenyl occurs first, and then biphenyl occurs. In the ReproSil Chiral MIG column, the situation is reversed. Interestingly, higher resolutions were obtained for the ReproSil Chiral MIC column. Table 9 lists the chromatographic parameters for the best-separated mixture for each column.

Table 9.
Chromatographic parameters obtained for the protonated racemic mixtures on both columns, HEX:IPA (70:30 v/v, 80:20 v/v, and 85:15 v/v).
The retention times of the separated racemic mixtures were short (below 15 min). The longest retention times were observed for the solvent system’s highest volume ratio of n-hexane. Table 10 compares the retention times of the best-separated mixtures on both columns. The highest ability to produce narrow peaks was demonstrated by the mixture CH3PhBi (R,S). The highest selectivity value is also observed for this mixture.

Table 10.
Retention times of the protonated racemic mixtures on both columns.
4. Conclusions
This study proved that the chemical structure of a racemic mixture strongly influences the separation of liquid crystalline racemic mixtures on both columns. On the ReproSil Chiral MIC column, only mixtures in which phenyl was present first, followed by biphenyl, had separated. This implies that the interactions between these analytes and CSPs were the strongest. The reverse molecular structure inhibited the separation. The highest resolution was obtained on this column for the mixture with the shortest protonated chain in the n-hexane–2-propanol solvent system (85:15 v/v).
Only four protonated mixtures in the HEX:IPA solvent system were separated on the ReproSil Chiral MIG column but without a baseline separation. On this column in the ACN:H2O solvent system, both protonated and fluorinated mixtures were separated, which means that the mobile phase significantly impacted the resolution of the studied materials. It is possible that this solvent system altered the supramolecular structure of the amylose chiral selector by modifying the size of the interaction cavity between the polysaccharide chains and making them more suitable for interactions with the liquid crystalline racemates. The length of the protonated or fluorinated chain also strongly influences the separation process. The elongation of the protonated chain increases the resolution of the analyzed racemic mixtures, which is related to increased retention times. Replacing the protonated chain with the fluorinated chain significantly reduced retention times on this column.
The order of elution of the enantiomer depends on the filling of the column. In the case of an amylose-based column, the enantiomer (S) elutes first, followed by the enantiomer (R). In contrast, when using a cellulose-based column, the elution order of the enantiomers is reversed, eluting the enantiomer (R) first and then the enantiomer (S). The composition of the mobile phase does not affect the elution order.
The studies showed that the chiral stationary phase based on amylose tris(3-chloro-5-methylphenylcarbamate) possesses a higher ability for the chiral recognition of stereoisomers of the studied analytes, and research will be continued using other amylose derivatives [38,39] and other solvent systems. An amylose-based CSP is considered to be more helical than cellulose-based CSPs. Consequently, the difference in helical structures between amylose and cellulose resulted in different enantiorecognition behaviors [40]. The electronegative nature of chlorine atoms causes a deficiency of electrons in the phenyl ring, consequently leading to weak π–π interactions and low chiral recognition capabilities, as was the case on the MIC column.
In summary, the separation process of liquid crystalline racemic mixtures is influenced by many factors, starting with the structure of the separated mixtures, the stationary phases used, the composition of the eluent, and the flow rate. Optimization of the separation parameters of mixtures belonging to the same homologous series provides an opportunity to find a trend in the separation process, which can be used in preparative chiral chromatography [41,42].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11070214/s1, Figure S1. Chromatogram of the racemate CH3PhBi (R,S); Figure S2. Purity curve of the racemate CH3PhBi (R,S); Figure S3. Chromatogram of the racemate C2H5PhBi (R,S); Figure S4. Purity curve of the racemate C2H5PhBi (R,S); Figure S5. Chromatogram of the racemate C4H9PhBi (R,S); Figure S6. Purity curve of the racemate C4H9PhBi (R,S); Figure S7. Chromatogram of the racemate C7H15PhBi (R,S); Figure S8. Purity curve of the racemate C7H15PhBi (R,S); Figure S9. Mass spectrum of the racemate CH3PhBi (R,S); Figure S10. Mass spectrum of the racemate C2H5PhBi (R,S); Figure S11. Mass spectrum of the racemate C4H9PhBi (R,S); Figure S12. Mass spectrum of the racemate C7H15PhBi (R,S).
Author Contributions
Conceptualization, M.U.; methodology, M.U.; software, M.U.; validation, M.U.; formal analysis, M.U.; investigation, M.U. and E.W.; resources, M.U.; data curation, M.U.; writing—original draft preparation, M.U. and E.W.; writing—review and editing, M.U.; visualization, E.W.; supervision, M.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University Research Grant 22-720, titled “New mesogens with increased electronic polarizability of the molecular core”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Monika Zając for the mass spectra and purity curves.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ohij, T.; Ohnishi, A.; Ogasawara, M. Application of Polysaccharide-Based Chiral High-Performance Liquid Chromatography Columns for the Separation of Regio E/Z− and Enantio-Isomeric Mixtures of Allylic Compounds. ACS Omega 2022, 7, 5146–5153. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, M.; Enomoto, Y.; Uryu, M.; Yang, X.; Kataoka, A.; Ohnishi, A. Application of Polysaccharide-Based Chiral HPLC Columns for Separation of Nonenantiomeric Isomeric Mixtures of Organometallic Compounds. Organometallics 2019, 38, 512–518. [Google Scholar] [CrossRef]
- Nemeti, G.; Berkecz, R.; Shahmohammadi, S.; Forró, E.; Lindner, W.; Péter, A.; Ilisz, I. Enantioselective high-performance liquid chromatographic separation of fluorinated ß-phenylalanine derivatives utilizing Cinchona alkaloid-based ion-exchanger chiral stationary phases: Enantioselective separation of fluorinated ß-phenylalanine derivatives. J. Chromatogr. A 2022, 1670, 462974. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Ianni, F.; Lisanti, A.; Marinozzi, M.; Scorzoni, S.; Natalini, B. The effect of mobile phase composition in the enantioseparation of pharmaceutically relevant compounds with polysaccharide-based stationary phases. Biomed Chromatogr. 2014, 28, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Francotte, E.R. Enantioselective Chromatography as a Powerful Alternative for the Preparation of Drug Enantiomers. J. Chromatogr. A 2001, 906, 379–397. [Google Scholar] [CrossRef]
- Santos, R.; Pontes, K.V.; Nogueira, I.B.R. Enantiomers and Their Resolution. Encyclopedia 2022, 2, 151–188. [Google Scholar] [CrossRef]
- Clark, A.; Kitson, R.R.A.; Mistry, N.; Taylor, P.; Taylor, M.; Lloyd, M.; Akamune, C. Introduction to Stereochemistry, 1st ed.; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 41–52. [Google Scholar]
- Liu, X. Organic Chemistry I; Kwantlen Polytechnic University: Surrey, BC, Canada, 2021; pp. 161–169. [Google Scholar]
- Maier, N.; Pilar, F.; Lindner, W. Separation of Enantiomers: Needs, Challenges, Perspectives. J. Chromatogr. A 2001, 906, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Waugh, J.; Keating, G.M.; Plosker, G.L.; Easthope, S.; Robinson, D.M. Pioglitazone: A review of its use in type 2 diabetes mellitus. Drugs 2006, 66, 85–109. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.; Tiritan, M.E.; Pinto, M.M.M.; Fernandes, C. Chiral Stationary Phases for Liquid Chromatography: Recent Developments. Molecules 2019, 24, 865. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Momotko, M.; Szarmańska, J.; Boczkaj, G.; Kamiński, M. Review of the types of chiral stationary phases and the possibilities of their applications in liquid chromatography. Cam. Sep. 2015, 7, 99–128. [Google Scholar]
- Fanali, C.; D’Orazio, G.; Fanali, S. Chiral Separations Using Nano-Liquid Chromatography. Sci. Chromatogr. 2016, 8, 161–169. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kawashima, M.; Hatada, K. Useful chiral packing materials for high-performance liquid chromatographic resolution of enantiomers: Phenylcarbamates of polysaccharides coated on silica gel. J. Am. Chem. Soc. 1984, 106, 5357–5359. [Google Scholar] [CrossRef]
- Park, J.H.; Whang, Y.C.; Jung, Y.J.; Okamoto, Y.; Yamamoto, C.; Carr, P.W.; McNeff, C.V. Separation of racemic compounds on amylose and cellulose dimethylphenylcarbamate-coated zirconia in HPLC. J. Sep. Sci. 2003, 26, 1331–1336. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y. Immobilized Polysaccharide CSPs: An Advancement in Enantiomeric Separations. Curr. Pharm. Anal. 2007, 3, 71–82. [Google Scholar] [CrossRef]
- Ikai, T.; Yamamoto, C.; Kamigaito, M.; Okamoto, Y. Immobilized Polysaccharide-Based Chiral Stationary Phases for HPLC. Polym. J. 2006, 38, 91–108. [Google Scholar] [CrossRef]
- Khatiashvili, T.; Matarashvili, I.; Karchkhadze, M.; Farkas, T.; Chankvetadze, B. Comparative Study of Cellulose Tris(3-chloro-5-methylphenylcarbamate) Coated or Covalently Immobilized on Silica for the Separation of Enantiomers in High-Performance Liquid Chromatography. Chromatographia 2024, 87, 27–34. [Google Scholar] [CrossRef]
- Kažoka, H.; Turovska, B.; Upmanis, T. Separation of 4-substituted 5-methylpiracetam stereoisomers on polysaccharide-based chiral stationary phases. J. Chromatogr. Open 2024, 5, 100122. [Google Scholar] [CrossRef]
- Jurin, M.; Kontrec, D.; Roje, M. HPLC and SFC Enantioseparation of (±)-Trans-β-Lactam Ureas on Immobilized Polysaccharide-Based Chiral Stationary Phases—The Introduction of Dimethyl Carbonate as an Organic Modifier in SFC. Separations 2024, 11, 38. [Google Scholar] [CrossRef]
- Toribio, L.; Magdaleno, I.; Martín-Gómez, B.; Martín, M.T.; Valverde, S.; Ares, A.M. Study of Different Chiral Columns for the Enantiomeric Separation of Azoles Using Supercritical Fluid Chromatography. Separations 2023, 10, 9. [Google Scholar] [CrossRef]
- Jurin, M.; Kontrec, D.; Dražić, T.; Roje, M. Enantioseparation of syn- and anti-3,5-Disubstituted Hydantoins by HPLC and SFC on Immobilized Polysaccharides-Based Chiral Stationary Phases. Separations 2022, 9, 157. [Google Scholar] [CrossRef]
- Ibrahim, D.; Ghanem, A. On the Enantioselective HPLC Separation Ability of Sub-2 µm Columns: Chiralpak IG-U and ID-U. Molecules 2019, 24, 1287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, B.; Bhadury, P.S.; Hu, D.; Yang, S.; Shi, X.; Liu, D.; Jin, L. Analytical and semi-preparative enantioseparation of organic phosphonates on a new immobilized amylose based chiral stationary phase. J. Sep. Sci. 2008, 31, 2946–2952. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M.; Vaňkátová, P.; Kubíčková, A.; Kalíková, K. Synthesis, characterisation and supercritical fluid chromatography enantioseparation of new liquid crystalline materials. Liq. Cryst. 2020, 47, 1832–1843. [Google Scholar] [CrossRef]
- Vojtylová-Jurkovičová, T.; Vaňkátová, P.; Urbańska, M.; Hamplová, V.; Sýkora, D.; Bubnov, A. Effective control of optical purity by chiral HPLC separation for ester-based liquid crystalline materials forming anticlinic smectic phases. Liq. Cryst. 2008, 48, 43–53. [Google Scholar] [CrossRef]
- Urbańska, M. Separation of liquid crystalline racemic mixtures obtained on the basis of (R,S)-2-hexanol on amylose tris(3-chloro-5-methylphenylcarbamate) covalently immobilised on silica in high-performance liquid chromatography. Liq. Cryst. 2023, 50, 1893–1901. [Google Scholar] [CrossRef]
- Vojtylová, T.; Kašpar, M.; Hamplová, V.; Novotná, V.; Sýkora, D. Chiral HPLC for a study of the optical purity of new liquid crystalline materials derived from lactic acid. Phase Transit. 2014, 87, 758–769. [Google Scholar] [CrossRef]
- Vaňkátová, P.; Kalíková, K.; Kubíčková, A. Advantages of polar organic solvent chromatography for enantioseparation of chiral liquid crystals. J. Chromatogr. A 2023, 1709, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, M. Optimization of Liquid Crystalline Mixtures Enantioseparation on Polysaccharide-Based Chiral Stationary Phases by Reversed-Phase Chiral Liquid Chromatography. Int. J. Mol. Sci. 2024, 25, 6477. [Google Scholar] [CrossRef]
- Vojtylová, T.; Niezgoda, I.; Galewski, Z.; Hamplová, V.; Sýkora, D. A new approach to the chiral separation of novel diazenes. J. Sep. Sci. 2015, 38, 4211–4215. [Google Scholar] [CrossRef]
- Vojtylová, T.; Hamplová, V.; Galewski, Z.; Korbecka, I.; Sýkora, D. Chiral separation of novel diazenes on a polysaccharide-based stationary phase in the reversed-phase mode. J. Sep. Sci. 2017, 40, 1465–1469. [Google Scholar] [CrossRef]
- Gąsowska, J.; Dąbrowski, R.; Drzewiński, W.; Filipowicz, M.; Przedmojski, J.; Kenig, K. Comparison of Mesomorphic Properties in Chiral and Achiral Homologous Series of High Tilted Ferroelectrics and Antiferroelectrics. Ferroelectrics 2004, 309, 83–93. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Gąsowska, J.; Filipowicz, M.; Przedmojski, J.; Tykarska, M.; Otón, J.M.; Castillo, P.L.; Bennis, N. Comparison of phase situation in recently prepared chiral, racemic and achiral anticlinic high tilted compounds. Phase Trans. 2005, 78, 927–942. [Google Scholar] [CrossRef]
- Gąsowska, J.; Dziaduszek, J.; Drzewiński, W.; Filipowicz, M.; Dąbrowski, R.; Przedmojski, J.; Kenig, K. Influence of rigid core structure on layer tilt and mesomorphic properties in homologous series of three ring antiferroelectric esters. Proc. SPIE 2004, 5565, 72–78. [Google Scholar]
- Dąbrowski, R.; Gąsowska, J.; Otón, J.M.; Piecek, W.; Przedmojski, J.; Tykarska, M. High tilted antiferroelectric liquid crystalline materials. Displays 2004, 25, 9–19. [Google Scholar] [CrossRef]
- Ravisankar, P.; Anusha, S.; Supriya, K.; Kumar, U.A. Fundamental Chromatographic Parameters. Inter. J. Pharm. Sci. Rev. Res. 2019, 55, 46–50. [Google Scholar]
- Tang, S.; Jin, Z.; Sun, B.; Wang, F.; Tang, W. Preparation and evaluation of regioselectively substituted amylose derivatives for chiral separations. Chirality 2017, 29, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ikai, T.; Okamoto, Y. Synthesis and application of immobilized polysaccharide-based chiral stationary phases for enantioseparation by high-performance liquid chromatography. J. Chromatogr. A 2014, 1363, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Pelusoa, P.; Mashiko, V.; Aubert, E.; Cossu, S. High-performance liquid chromatography enantioseparation of atropisomeric 4,4′-bipyridines on polysaccharide-type chiral stationary phases: Impact of substituents and electronic properties. J. Chromatogr. A. 2012, 1251, 91–100. [Google Scholar] [CrossRef]
- Majors, R.E. Developments in preparative-scale chromatography: Columns and accessories. LC-GC Eur. 2004, 17, 630–638. [Google Scholar]
- Speybrouck, D.; Lipka, E. Preparative supercritical fluid chromatography: A powerful tool for chiral separations. J. Chromatogr. A 2016, 1467, 33–55. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).