Abstract
Among the various phytochemicals, which are present in Lamiaceae plants, carnosic acid and carnosol stand out. Carnosic acid is a phenolic diterpene carrying two phenolic hydroxyl groups and a carboxyl group, while carnosol carries a lactone moiety in addition to phenolic hydroxyls. Both these phenolic diterpenes exhibit interesting biological properties, such as antioxidant, anticancer, anti-inflammatory and neuroprotective activities. In this review, we summarize the existing analytical methods for the determination of carnosic acid and carnosol, primarily in plants, but also in foods and biological samples. Due to the biological importance of carnosic acid and carnosol, a variety of analytical methods, including high-performance liquid chromatography–ultra violet (HPLC–UV), liquid chromatography–mass spectrometry (LC–MS) and capillary electrophoresis (CE), were developed for their determination. In addition, we discuss the extraction methods applied for their isolation from plants and in brief the bioactivities of these phytochemicals.
1. Introduction
The family of Lamiaceae or Labiatae plants, which includes rosemary (Salvia rosmarinus, synonym Rosmarinus officinalis), sage (Salvia officinalis), thyme (Thymus vulgaris), lemon balm (Melissa officinalis) or wild marjoram (Origanummajorana), is an enriched source of antioxidant compounds, such as phenolic acids, flavonoids and terpenes [1,2]. Most of these plants are native to the temperate Mediterranean region and are exported worldwide either as extracts or in their dried form [1,2,3]. In rosemary and sage, the major bioactive components are rosmarinic acid, carnosic acid and carnosol (Figure 1) [4]. Carnosic acid is a phenolic diterpene carrying two phenolic hydroxyl groups and a carboxyl group. When oxidized, carnosic acid can be directly converted to carnosol with hydroxyl groups at positions C-11 and C-12 and a lactone moiety. Carnosol can in turn be converted into rosmanol or epirosmanol by hydroxylation at C-7 on its lactone ring (Figure 1) [5]. Carnosic acid can also form methyl carnostate by be methylation of the carboxyl group.
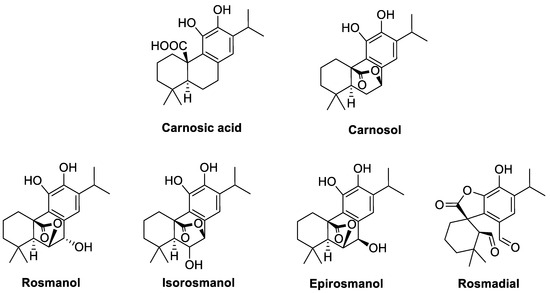
Figure 1.
Structures of carnosic acid, carnosol and their derivatives.
Carnosic acid, carnosol and their derivatives are normally found in photosynthetic green tissues, e.g., sepals, leaves and petals of plants, specifically in the chloroplasts [6]. The contents of these bioactive components in rosemary plants grown in fields display seasonal fluctuations, and in particular, carnosic acid contents tend to decline in response to conditions of environmental stress, i.e., high temperatures and/or low precipitation rates during summer [7].
Simultaneously, an increase in oxidized metabolites was observed, suggesting that cellular oxidative stress is evidently accompanied by the decrease in carnosic acid levels [8,9]. Environmental stress strongly influences the synthesis of bioactive compounds due to the generation of excess ROS free radicals, triggering the biosynthesis of secondary ROS scavenging systems. As a consequence, diterpene derivatives, such as carnosic acid, and their oxidized derivatives can be acknowledged as biomarkers of the environmental stress in plants such as sage and rosemary [10,11].
Table 1 summarizes the presence of carnosic acid, carnosol and their derivatives in various Lamiaceae plants. Specifically, all derivatives were identified in rosemary and sage extracts [12,13]. Carnosic acid, carnosol and methyl carnosate were also identified in oregano extracts [14], while carnosol and methyl carnosate were detected in thyme extracts, where carnosic acid was absent [14]. Finally, in marjoram extracts, both carnosic acid and carnosol were detected [15], while lemon balm was only found to contain carnosic acid [16].

Table 1.
Carnosic acid, carnosol and their derivatives in Lamiaceae plants.
Rosemary extract is employed in food industry as a result of its established high antioxidant activity. In the European Union, rosemary extract is assigned as an antioxidant food additive (E 392), with an acceptable daily intake of 0–0.3 mg/kg body weight, expressed as the sum of carnosic acid and carnosol [17]. Rosemary extracts were added to lipids or foods containing lipids, such as plant seed oils, fish oils, fat-based spreads and meats, to prolong their storage life [18].
Because of the importance of the plant bioactive components carnosic acid and carnosol, a variety of appropriate extraction and analytical methods, resulting in high recovery, sensitivity and reproducibility, were developed. The aim of this review article is to summarize the existing analytical methods, which include high-performance liquid chromatography–ultra violet (HPLC–UV), liquid chromatography–mass spectrometry (LC–MS) as well as capillary electrophoresis (CE) techniques. Furthermore, the extraction procedures and the sample preparation methods and in brief the bioactivities of carnosic acid and carnosol are discussed.
2. Extraction Methods
Extraction is a crucial and essential process for the isolation of bioactive components from plants in concentrated forms of higher purity. The development of new effective extractive procedures with high recovery yields and better selectivity is always an important and popular research topic [19]. Regarding bioactive compounds with antioxidative properties, such as carnosic acid and carnosol, some parameters that must be taken into consideration are pressure, solvent type and temperature because they can easily affect their recovery [20].
2.1. Sample Pretreatment
As in most samples derived from plants, an initial pre-treatment step is commonly performed. Specifically, in the case of rosemary and sage plant materials, such as leaves and stems, there is an initial drying step (ambient drying, convection drying, freeze drying, vacuum–microwave drying, radio frequency drying, etc.) for the reduction in moisture and the preservation of the plant material. In the case of sensitive compounds, such as carnosic acid, the drying time and temperature should be taken into account, though ambient drying, convection drying and freeze drying seem to be appropriate and are widely used [3,20]. The drying step is often followed by a milling or grinding step in order to obtain the sample as a fine homogeneous powder [20].
2.2. Conventional Extraction Methods
Plant extracts can be obtained through already well-established conventional techniques, such as maceration, heat reflux or Soxhlet extraction, steam distillation and hydrodistillation [21]. These techniques suffer from numerous disadvantages that include long extraction times, low selectivity of compounds, decomposition of thermolabile compounds and high solvent consumption, especially of non-green solvents such as methanol or hexane [21]. For example, maceration, a simple extraction technique that is frequently employed in literature [22,23], requires a long extraction time in order to be sufficiently effective [24]. Heat reflux extraction techniques such as Soxhlet extraction are also efficient when maintained for several hours [25,26,27,28,29,30,31,32]. Although extractions by heating can be more efficient, compounds readily affected by heat can undergo decomposition, such as the conversion of carnosic acid to carnosol and other derivatives [32]. Such challenges were taken into consideration for the constant improvement of modern methods.
2.3. Ultrasound-Assisted Extraction (UAE)
UAE is a commonly used technique for extracting different compounds from natural sources and was successfully used in the extraction of bioactive constituents from rosemary, sage and other plants [11,33,34,35,36,37,38,39,40,41]. It is a simple, low-cost technique with short extraction times and reduced solvent consumption, though it offers limited selectivity. In the case of phenolic diterpenes, it is indicated in literature that UAE with aqueous ethanol or methanol is the most efficient, affording higher yields when the solvent polarity decreases [42]. When compared to maceration, UAE proved to be more effective in the extraction of carnosic, rosmarinic and ursolic acids [43,44].
2.4. Microwave-Assisted Extraction (MAE)
MAE is a faster and more environmentally friendly technique relying on microwave volumetric heating, with short extraction times and lower solvent consumption, in comparison to conventional methods. Furthermore, it can be combined with other extraction techniques, such as UAE, and since there is no specific solvent for this type of extraction, any solvent can be chosen according to the target compound as long as it can absorb microwaves [45]. In a recent study, MAE extraction of total phenolics from rosemary afforded a three-fold increase in yield, in comparison to the conventional maceration technique, in a shorter period of time [46]. On the other hand, temperature should be carefully monitored as it was reported that above 150 °C, the content of carnosol increases and is higher than carnosic acid content [47].
2.5. Supercritical Fluid Extraction (SFE)
SFE employs solvents at a supercritical state, taking advantage of their properties, such as low surface tension and viscosity and high solvating capacity. It is a valuable tool for the extraction of bioactive compounds from natural products in high yields and it is environmentally friendly, though appropriate instrumentation can be quite expensive and complex [3]. In particular, supercritical CO2 offers many advantages in such applications because it facilitates the extraction of sensitive and/or easily oxidized compounds, such as carnosic acid and its derivatives [30,37,48,49,50,51,52]. Pressure is one of the most important parameters regarding this method. Carnosic acid can be extracted using solely supercritical CO2, not requiring the use of a polar co-solvent [53]. Another advantage of SFE is that it can take place at lower temperatures and in the dark, avoiding the decomposition of carnosic acid during the extraction process [54]. Interestingly, a two-step sequential SFE process can lead to the attainment of rosemary extracts that are enriched in carnosic acid and carnosol. Firstly, neat supercritical CO2 is employed in order to remove less active fractions, such as waxes and oleoresins, and as a second step, CO2 is combined with 7% ethanol as a co-solvent. This procedure led to improved recovery for carnosic acid and carnosol in a shortened total extraction time in comparison to the single-step SFE (180 min versus 300 min) [55]. Similarly, semi-preparative supercritical fluid chromatography (SFC) was employed for the fractionation of rosemary extracts, employing an array of SFC-designed columns, operating at different conditions and managing to obtain fractions with carnosic acid concentrations greater than 80% mass [56]. A different method, namely supercritical antisolvent fractionation, is based on the contact between a polar liquid mixture (extract) and a supercritical carbon dioxide current in a pressurized chamber leading to the precipitation of polar constituents, while nonpolar compounds remain in solution [20]. Sánchez-Camargo et al. employed ASE with a mixture of ethanol/water and supercritical antisolvent fractionation in rosemary leaves and reportedly attained highly enriched extracts of carnosic acid and carnosol with potent antiproliferative activity against colon cancer cells HT-29 and HCT116 in vitro [57].
2.6. Accelerated Solvent Extraction (ASE)
The main characteristic of ASE is the use of conventional solvents under high pressure and temperature. Compared to conventional extraction methods, ASE provides rapid extraction and can be used for fractionation of the same extract over time [24]. This type of extraction reportedly afforded rosemary and sage extracts in high yields [30,49,50,51]. For example, the results obtained in a study employing ASE at high temperatures (200 °C), utilizing water and ethanol as solvents, yielded enriched rosemary extracts after 20 min. Furthermore, under these conditions, two rosemary antioxidants with diverse polarities, carnosic acid and rosmarinic acid, were simultaneously extracted, whereas ASE using ethanol proved more effective for the extraction of carnosic acid and carnosol [58]. When employing solely water as the solvent, this technique can be called subcritical water extraction. Published results indicate a high selectivity for the bioactive compounds of rosemary, i.e., carnosic acid, carnosol, rosmanol and methyl carnosate among others, while the antioxidant activity of different fractions obtained at different water temperatures was comparable to that of SFE-obtained fractions [59].
2.7. Green and Sustainable Solvents
In recent years, new renewable alternatives to volatile organic solvents were developed in order to afford safer extracts with low cost and low toxicity. Such alternatives are ionic liquids and deep eutectic solvents (DES). The former are liquid salts with a melting point below 100 °C, comprised of large cations paired with inorganic or organic anions. They are characterized by their low volatility and flammability [60]. In literature, ionic liquids were combined with eco-friendly extraction techniques, such as MAE and UAE for the extraction of bioactive constituents of rosemary, including carnosic acid [60,61]. In 2011, Liu et al. reported the use of [C8mim]Br (1-octyl-3-methylimidazolium bromide) under microwave irradiation, which led to improved extraction yields for carnosic acid and shorter extraction times compared to conventional techniques, such as hydrodistillation [60]. Additionally, Zu et al. utilized the same ionic liquid in UAE of carnosic acid and rosmarinic acid from rosemary while testing an array of anions, demonstrating that the extraction of a particular compound can be dependent on the use of the appropriate anion, influencing the miscibility of the ionic liquid [61].
On the other hand, DESs are liquid mixtures of different compounds formed by a hydrogen bond donor and a hydrogen bond acceptor and exhibiting a melting point that is lower than those of the individual compounds [62]. Natural DESs are specifically composed of naturally derived compounds, e.g., carbohydrates, alcohols, amino acids and organic acids [63]. Regarding the extraction of analytes from rosemary, it was demonstrated that using choline chloride-based DES and UAE or simple stirring with a plethora of hydrogen bond donors can afford higher extraction yields and antioxidant activity in comparison to extraction with ethanol [64,65]. Furthermore, Wang et al. published a study comparing different DESs according to their hydrophobicity, where hydrophobic menthol-based DESs, especially menthol:lactic acid 1:2, were more effective in extracting carnosic acid and carnosol than hydrophilic DESs and organic solvents [66]. Interestingly, the same team later developed a mixture of DES and an ionic liquid with water, which was reportedly effective for the extraction and isolation of carnosic acid from rosemary leaves. In detail, a mixture of choline chloride:laevulinic acid/[BMIM]PF6/water (1/2/1, v/v/v) was employed, which, when heated, is a single-phase system extracting carnosic acid and rosmarinic acid from rosemary, and when cooled, is switched to a two-phase system with carnosic acid being isolated in the lower phase at a high recovery yield [67]. Finally, a study dedicated to the extraction of bioactive compounds from rosemary with biphasic NADES showcased that a biphasic system consisting of lactic acid:glucose (5:1)/menthol:lauric acid (2:1) separated carnosic acid and carnosol (nonpolar phase) from rosmarinic and caffeic acid (polar phase) [68].
In addition, polyethylene glycols (PEGs) and short-chain alkyl polyethylene glycol ethers were explored as green solvents for the extraction of carnosic acid from rosemary leaves [69,70]. Alkyl polyethylene glycol ethers act as non-ionic hydrotropes, and those with linear alkyl chains and a small molecular volume proved to be suitable for the extraction of carnosic acid from rosemary and provided good yields compared to conventional ionic hydrotropes and an aqueous solution of ethanol [69]. Recently, the use of PEG-400 in MAE extraction of carnosic and rosmarinic acid from rosemary leaves was reported. This solvent was compared to the ionic liquid [C8mim]Br and ethanol, exhibiting the highest extraction efficiency and fastest extraction rate for the desired compounds [70].
3. Analysis of Carnosic Acid and Carnosol
High-performance liquid chromatography (HPLC) and ultra-high performance liquid chromatography (UHPLC) serve as the most common analytical methods for the separation, identification and quantification of non-volatile compounds from rosemary extracts, such as polyphenols, diterpenes and flavonoids. Characterization of analytes is normally achieved with a suitable detection system, such as a UV diode array detector (DAD) or photodiode array detector (PDA), and in the last two decades, coupled with mass spectrometry (MS) systems [24].
3.1. High-Performance Liquid Chromatography–UV Detection (HPLC–UV)
Chromatographic methods that are discussed below are summarized in Table 2. In literature, there are numerous studies on the characterization of analytes (in most cases carnosic acid, rosmarinic acid and carnosol) from rosemary or sage extracts and their antioxidant activities. The most common methods for the determination of diterpenes, as well as other non-volatile compounds from such extracts, usually employ reverse-phase LC (RPLC) with octadecyl-bonded stationary phases, using both isocratic and gradient mobile phases consisting of different mixtures of water, acetonitrile (ACN) and/or methanol with the addition of acids, with acetic, formic, trifluoroacetic and phosphoric acid being the most prominent [4,5,18,22,25,26,27,28,33,34,35,36,48,71,72,73,74,75,76,77,78,79,80,81].

Table 2.
Summary of reported HPLC–UV analytical methods for the determination of carnosic acid and carnosol.
Apart from rosemary, sage and other commonly studied species of the Lamiaceae family from the Mediterranean area, more uncommon species were additionally studied through the years. In 2010, the antioxidant and anti-inflammatory activities of the methanol/chloroform (1:1) extracts derived from 16 Salvia species from South Africa were evaluated, indicating good antioxidant activity. Rosmarinic acid, carnosic acid and carnosol were detected as main compounds in the chromatographic profiles, with carnosol being abundant in Salvia namaensis and 7-O-methyl-epirosmanol being detected solely in species S. namaensis and S. chamelaeagnea [76]. Furthermore, different extracts of Dorystoechas hastata, a plant endemic in Turkey, consumed as herbal tea by local inhabitants, were investigated using an HPLC–DAD method, which revealed the presence of carnosic acid and carnosol in the plant. The petroleum ether extract exhibited the most potent antioxidant activity containing the highest amount of carnosic acid and carnosol [77].
Carnosic acid and carnosol are often utilized as food additives in the form of rosemary extracts. Analytical methods for the identification of rosemary extract residues in edible products can be used to verify the safety of such products, for example, a HPLC–PDA quantitative method for the identification of rosemary extract in processed meat products, edible oils and dressings was established, though none of the tested samples contained rosemary extract residues [18]. It is worth noting that recently, an analytical method for the detection of carnosol in human plasma was reported for the first time. In the previous years, there was a lack of data for the pharmacokinetic parameters of carnosol. This HPLC–DAD study provided a sensitive, selective and cost-reduced assay for the evaluation of the clinical effects and safety of carnosol in human plasma [81].
3.2. Liquid Chromatography–Mass Spectrometry (LC–MS)
LC–MS is a highly important analytical technique particularly useful in the analysis of plant extracts, which consists of semi-polar compounds such as key secondary metabolites that can be easily separated and detected by employing LC–MS approaches (Table 3) [23,29,30,31,36,37,38,39,40,45,49,50,51,82,83,84,85,86,87,88,89,90,91,92].

Table 3.
Summary of reported LC–MS analytical methods for the determination of carnosic acid and carnosol.
In the majority of cases, extracts from fresh or dried rosemary and sage are studied in literature. In the case of commercially available products, the relevant studies are limited. In 2011, a UHPLC methodology with MS/MS and UV detection for the identification and quantification of the main phenolic components in sage tea was described. An Acquity BEH Shield RP18 column was used and the total analysis time was 34 min. A total of 16 commercial brands of sage tea were characterized, and three isomers of rosmanol, in addition to carnosol and carnosic acid, were found and quantified in all samples [85]. In a different study, separation of phenolic diterpenes was attained in 10 min, using a fused-core column. Such columns consist of stationary phases made from high-purity silica that contain a solid core covered with a porous thin layer. This method was applied to five commercial samples consisting of sage leaves, rosemary leaves, a mixture of herbs (rosemary, sage, thyme and oregano), a mixture for chimichurri sauce and oregano leaves. The use of fused-core technology led to good peak shapes, and carnosic acid, rosmanol, carnosol and methyl carnosate were detected and quantified in all samples except oregano leaves [37]. Various analytical methods, including HPLC and UHPLC–MS/MS, were employed for the analysis of rosemary extracts in vitro and fewer in vivo. For example, one of the first studies to investigate the metabolism of carnosic acid in vitro and in vivo employed an HPLC–Q-trap-MS method, resulting in a multiple-ion monitoring information-dependent acquisition-enhanced product ion (MIM-IDA-EPI) mode for the detection of metabolites as traces in biological samples treated with carnosic acid. Different metabolites and degradation products (carnosol, carnosic acid quinone, rosmanol, epirosmanol, rosmadial and 7-oxo rosmanol) were identified from in vitro metabolism models, while glucuronidation, oxidation and methylation were the main in vivo metabolic pathways observed [92]. In a later study, a UHPLC–ESI-MS/MS method was developed, to simultaneously determine carnosic acid, rosmanol, and carnosol in rat plasma after oral administration of rosemary extract to rats. The quantification for this pharmacokinetic study was attained with the use of multiple reaction monitoring (MRM) mode with electrospray ionization (ESI) [88].
3.3. Capillary Electrophoresis (CE) and Other Techniques
CE with UV or MS detection was also used for the separation of diterpenes from rosemary and sage extracts [58,93,94,95,96] (Table 4). Silica capillaries of 50 cm length were the most commonly used and the pH was maintained between 9 and 10, in favor of the anionic phenolic compounds. When coupled to MS, ammonium acetate was used as a buffer replacing sodium borate or tetraborate, due to their incompatibility with ESI-MS detection as a result of low volatility [58].

Table 4.
Summary of capillary electrophoresis and other reported analytical methods for the determination of carnosic acid and carnosol.
It is worth noting that an analytical method was also reported involving HPLC with evaporative light scattering detection (HPLC–ELSD), for the determination of rosmarinic acid, carnosol and carnosic acid, among other analytes, in rosemary, in a cost- and time-effective manner. The drift tube temperature of ELSD was set at 70 °C, and the pressure of the nebulizer nitrogen gas was set at 40 Psi. This method showcased satisfactory sensitivity (limits of detection from 1.3 to 8.6 µg/mL), good repeatability and high accuracy (recovery between 95.5% and 100.8%) [41].
Two less-explored techniques that were applied for the analysis of diterpenes such as carnosic acid are UV spectrophotometry and square-wave voltammetry (SWV) [98,99]. The former was utilized for the quantitative determination of the diterpenes extracted from garden sage leaves. The measured concentrations of diterpenes at 285 nm, ranged from 2.1 to 3.6% in terms of carnosic acid [98]. Yilmaz et al. studied the electrochemical behavior of carnosic acid and based on their findings, developed a square-wave voltammetric method for the determination of carnosic acid in rosemary extracts. This method displayed good linear responses and the results are in good agreement with an HPLC–UV method [99].
4. Biological Activities of Carnosic Acid and Carnosol
4.1. Antioxidant Activity
The in vitro antioxidant activity of rosemary and sage extracts was extensively studied in literature through the use of spectrophotometric methods, including 2,2-diphenyl-1-picrylhydrazil (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays [32]. The specific antioxidant activities of carnosic acid and carnosol were demonstrated via oxidation reactions and by protecting cells from oxidative cell death. For example, carnosic acid can provide protection of neuronal cells against oxidative stress caused by the presence of hydrogen peroxide and lipid hydroperoxides in those circumstances [100].
4.2. Anticancer Activity
The anticancer activity of rosemary extracts and their major bioactive constituents were widely studied in the last decades [101,102,103,104,105,106]. Numerous studies employing in vitro assays regarding different types of cancer, such as leukemia, breast, lung, liver, brain, prostate and colon cancer were conducted. The anticancer activity of rosemary extracts is characterized as chemopreventive, antiproliferative and anti-invasive according to its effect against the different stages in the development of cancer [101,102,103,104,105,106].
Carnosic acid was demonstrated to inhibit angiogenesis, proliferation and migration of cancer cells [107,108]. Moreover, it induced cell apoptosis and DNA damage and was able to inhibit the mitogen-activated protein kinase (MAPK) signaling pathways [109]. Additionally, carnosic acid can inhibit the growth, cell migration and invasion of human non-small cell lung carcinoma cells (A549) via apoptosis and suppression of the PI3K/AKT/m-TOR signaling pathway [110]. Importantly, studies on carnosic acid reported its ability to enhance the effects of different drugs; for example, trastuzumab [111] and temozolomide [112]. A new study reported that carnosic acid displays cytotoxic activity against human gastric cancer cells [113]. Finally, carnosic acid inhibited the tumor growth in BALB/c nude mice transplanted with oral squamous cell carcinoma (OSCC) cells [114].
Carnosol was reported to inhibit prostate and breast cancers by binding to estrogenic as well as androgenic receptors [115] and to exert its effect against breast cancer through downregulation of matrix metallopeptidase 9 (MMP-9) and inhibition of the signal transducer and activator of transcription 3 (STAT3) signaling pathway [116].
4.3. Anti-Inflammatory Activity
Rosemary extracts, in particular, their components carnosic acid and carnosol, exhibited a plethora of anti-inflammatory properties against lung, skin, cardiac, gut, renal, neuronal, endothelial diseases as well as diabetes- and obesity-associated inflammatory diseases [117]. Carnosic acid and carnosol displayed significant in vivo anti-nociceptive and anti-inflammatory effects dose-dependently in carrageenan-induced mouse hyperalgesia and induced inhibition of the analgesic response in the late phase of the formalin test [118]. Xia et al. reported that the administration of carnosic acid to db/db mice led to a reduction in the risk of systemic inflammatory conditions [119], while carnosol and rosmanol alleviated rheumatoid arthritis in a synergistic manner by inhibiting inflammation through regulation of the TLR4/NF-κB/MAPK pathway [120]. Carnosic acid showed osteoarthritis prevention due to its ability to reduce cartilage degeneration in articular chondrocytes [121]. In a bleomycin-induced lung damage animal model, carnosol reduced the levels of oxidative markers and pro-inflammatory cytokines [122].
4.4. Neuroprotective Activity
Accumulating evidence shed light on the relevance of carnosic acid as a neuroprotective agent that exhibits therapeutic efficacy against neurodegenerative disorders [100,123]. A recent review article by Satoh et al. summarizes the ability of carnosic acid to act as a nuclear factor erythroid 2-related factor 2 (NRF2) activator and to inhibit the NLR family pyrin domain containing 3 (NLRP3) inflammasome, which was linked to neurological diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [84]. In vivo protection by carnosic acid was explored in PD models employing 6-hydroxydopamine (6-OHDA) to cause injuries to the dopaminergic neurons in the substantia nigra. Carnosic acid treatment ameliorated the locomotor activity of rats exposed to 6-OHDA and protected them against lipid peroxidation [124].
5. Conclusions
Carnosic acid and carnosol are two very important natural products, which are found in plants belonging to the Lamiaceae family. Both are phenolic diterpenes, exhibiting very attractive biological properties, namely antioxidant, anticancer, anti-inflammatory and neuroprotective activities. Due to their bioactivities and their applications as antioxidant food additives, a variety of analytical methods were developed for their determination. These methods, which include HPLC–UV, LC–MS and CE techniques, are summarized in the present review article. In addition, the various extraction methods of these bioactive phytochemicals from the plant sources are discussed. Future research should consider further focus on the development of robust analytical methodologies for the determination of carnosic acid, carnosol as well as their derivatives on a broader spectrum of samples (foods, plants and biological samples) and to take advantage of their pleiotropic biological activities as individual compounds and as constituents of rosemary and sage extracts.
Author Contributions
Conceptualization, M.G.K.; methodology, C.M. and M.G.K.; writing—original draft preparation, C.M. and M.G.K.; writing—review and editing, P.A.T. and M.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
M.G.K. would like to thank L’Oréal-Unesco for the award “For Women in Science 2023”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jassbi, A.R.; Zare, S.; Firuzi, O.; Xiao, J. Bioactive phytochemicals from shoots and roots of Salvia species. Phytochem. Rev. 2016, 15, 829–867. [Google Scholar] [CrossRef]
- Carović-Stanko, K.; Petek, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Herak Ćustić, M.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef]
- Pizani, R.S.; Viganó, J.; De Souza Mesquita, L.M.; Contieri, L.S.; Sanches, V.L.; Chaves, J.O.; Souza, M.C.; Da Silva, L.C.; Rostagno, M.A. Beyond aroma: A review on advanced extraction processes from rosemary (Rosmarinus officinalis) and sage (Salvia officinalis) to produce phenolic acids and diterpenes. Trends Food Sci. Technol. 2022, 127, 245–262. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S. fruticosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content: Antioxidant activity of sage and oregano extracts. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Schwarz, K.; Ternes, W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis: II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z. Lebensm. Unters. Forschung 1992, 195, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001, 125, 1094–1102. [Google Scholar] [CrossRef]
- Luis, J.C.; Johnson, C.B. Seasonal variations of rosmarinic and carnosic acids in rosemary extracts. Analysis of their in vitro antiradical activity. Span. J. Agric. Res. 2005, 3, 106. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol. 2003, 131, 1816–1825. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent extraction techniques: A Review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Boscaiu, M.; Vicente, O.; Bautista, I.; Ranga, F.; Socaciu, C. HPLC-DAD-ESI+-MS phytochemical profiles of several Rosmarinus officinalis accessions from Spain as influenced by different environmental stress conditions. Stud. UBB Chem. 2019, 64, 163–180. [Google Scholar] [CrossRef]
- Nakatani, N.; Inatani, R. Two antioxidative diterpenes from rosemary (Rosmarinus officinalis L.) and a revised structure for rosmanol. Agric. Biol. Chem. 1984, 48, 2081–2085. [Google Scholar] [CrossRef]
- Cuvelier, M.-E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rai, D.K.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 10576–10581. [Google Scholar] [CrossRef]
- Vági, E.; Rapavi, E.; Hadolin, M.; Vásárhelyiné Perédi, K.; Balázs, A.; Blázovics, A.; Simándi, B. Phenolic and triterpenoid antioxidants from Origanum majorana L. Herb and Extracts Obtained with Different Solvents. J. Agric. Food Chem. 2005, 53, 17–21. [Google Scholar] [CrossRef]
- Herodež, Š.S.; Hadolin, M.; Škerget, M.; Knez, Ž. Solvent extraction study of antioxidants from balm (Melissa officinalis L.) leaves. Food Chem. 2003, 80, 275–282. [Google Scholar] [CrossRef]
- EFSA ANS Panel; Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Refined exposure assessment of extracts of rosemary (E 392) from its use as food Additive. EFS2 2018, 16, e05373. [Google Scholar] [CrossRef]
- Choi, S.-H.; Jang, G.-W.; Choi, S.-I.; Jung, T.-D.; Cho, B.-Y.; Sim, W.-S.; Han, X.; Lee, J.-S.; Kim, D.-Y.; Kim, D.-B.; et al. Development and validation of an analytical method for carnosol, carnosic acid and rosmarinic acid in food matrices and evaluation of the antioxidant activity of rosemary extract as a food additive. Antioxidants 2019, 8, 76. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Ali, A.; Chua, B.L.; Chow, Y.H. An insight into the extraction and fractionation technologies of the essential oils and bioactive compounds in Rosmarinus officinalis L.: Past, present and future. TrAC Trends Anal. Chem. 2019, 118, 338–351. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.Á. CO2 -Supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Fotovvat, M.; Radjabian, T.; Saboora, A. HPLC fingerprint of important phenolic compounds in some Salvia L. species from Iran. Rec. Nat. Prod. 2018, 13, 37–49. [Google Scholar] [CrossRef]
- Lemos, M.F.; Lemos, M.F.; Pacheco, H.P.; Endringer, D.C.; Scherer, R. Seasonality modifies rosemary’s composition and biological activity. Ind. Crops Prod. 2015, 70, 41–47. [Google Scholar] [CrossRef]
- Lesellier, E.; Lefebvre, T.; Destandau, E. Recent developments for the analysis and the extraction of bioactive compounds from Rosmarinus officinalis and medicinal plants of the Lamiaceae family. TrAC Trends Anal. Chem. 2021, 135, 116158. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Jordán, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in essential oil, phenolic compounds, and antioxidant activity of Tunisian cultivated Salvia Officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef]
- Jordán, M.J.; Lax, V.; Rota, M.C.; Lorán, S.; Sotomayor, J.A. Relevance of carnosic Acid, carnosol, and rosmarinic acid concentrations in the in vitro antioxidant and antimicrobial activities of Rosmarinus officinalis (L.) methanolic extracts. J. Agric. Food Chem. 2012, 60, 9603–9608. [Google Scholar] [CrossRef]
- Hcini, K.; Sotomayor, J.A.; Jordan, M.J.; Bouzid, S. Identification and quantification of phenolic compounds of Tunisian Rosmarinus officinalis L. Asian J. Chem. 2013, 25, 9299–9301. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Martínez, C.; Moñino, I.; Sotomayor, J.A. Polyphenolic extract and essential oil quality of Thymus zygis Ssp. gracilis shrubs cultivated under different watering levels. Ind. Crops Prod. 2009, 29, 145–153. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Rodríguez-Solana, R.; Salgado, J.M.; Domínguez, J.M.; Cortés-Diéguez, S. Comparison of soxhlet, accelerated solvent and supercritical fluid extraction techniques for volatile (GC-MS and GC/FID) and phenolic compounds (HPLC-ESI/MS/MS) from Lamiaceae species: Lamiaceae essential oils. Phytochem. Anal. 2015, 26, 61–71. [Google Scholar] [CrossRef]
- Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 2019, 24, 2921. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Ternes, W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis: I. Determination of phenolic diterpenes with antioxidative activity amongst tocochromanols using HPLC. Z. Lebensm. Unters. Forschung 1992, 195, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Sagdic, O.; Ekici, L.; Ozturk, I.; Ozcan, M.M. Phenolic compounds of Origanum sipyleum L. extract and its antioxidant and antibacterial activities. J. Food Lipids 2007, 14, 157–169. [Google Scholar] [CrossRef]
- Mei, X.; Tan, J.; Xiao, N.; Fang, X.; Gu, S.; Li, J.; Wang, J. Simultaneous qualitative and quantitative determination of 17 bioactive components in the fibrous roots of Salvia miltiorrhiza bunge by a combinatorial ultra performance liquid chromatography- ultraviolet characteristic spectra analysis. J. Appl. Res. Med. Aromat. Plants 2021, 20, 100291. [Google Scholar] [CrossRef]
- Xie, L.; Li, Z.; Li, H.; Sun, J.; Liu, X.; Tang, J.; Lin, X.; Xu, L.; Zhu, Y.; Liu, Z.; et al. Fast quantitative determination of principal phenolic anti-oxidants in rosemary using ultrasound-assisted extraction and chemometrics-enhanced HPLC–DAD Method. Food Anal. Methods 2023, 16, 386–400. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Rostagno, M.A.; Meireles, M.A.A. Fast analysis of phenolic terpenes by high-performance liquid chromatography using a fused-core column. Anal. Methods 2014, 6, 7457–7468. [Google Scholar] [CrossRef]
- Ge, X.; Jing, L.; Zhao, K.; Su, C.; Zhang, B.; Zhang, Q.; Han, L.; Yu, X.; Li, W. The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chem. 2021, 335, 127655. [Google Scholar] [CrossRef]
- Duan, H.; Wang, W.; Li, Y.; Jilany Khan, G.; Chen, Y.; Shen, T.; Bao, N.; Hua, J.; Xue, Z.; Zhai, K.; et al. Identification of phytochemicals and antioxidant activity of Premna microphylla turcz. stem through UPLC-LTQ-Orbitrap-MS. Food Chem. 2022, 373, 131482. [Google Scholar] [CrossRef]
- Gkioni, M.D.; Zeliou, K.; Dimaki, V.D.; Trigas, P.; Lamari, F.N. GC-MS and LC-DAD-MS phytochemical profiling for characterization of three native Salvia taxa from eastern Mediterranean with antiglycation properties. Molecules 2022, 28, 93. [Google Scholar] [CrossRef]
- Li, P.; Liu, A.; Li, Y.; Yuan, B.; Xiao, W.; Liu, Z.; Zhang, S.; Lin, H. Development and validation of an analytical method based on HPLC-ELSD for the simultaneous determination of rosmarinic acid, carnosol, carnosic acid, oleanolic acid and ursolic acid in rosemary. Molecules 2019, 24, 323. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, J.P.; Mason, T.J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- Paniwnyk, L.; Cai, H.; Albu, S.; Mason, T.J.; Cole, R. The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason. Sonochem. 2009, 16, 287–292. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Lucas, B.N.; Voss, M.; Santos, D.; Mello, P.A.; Wagner, R.; Cravotto, G.; Barin, J.S. Solvent-free simultaneous extraction of volatile and non-volatile antioxidants from rosemary (Rosmarinus officinalis L.) by microwave hydrodiffusion and gravity. Ind. Crops Prod. 2020, 145, 112094. [Google Scholar] [CrossRef]
- Jacotet-Navarro, M.; Rombaut, N.; Fabiano-Tixier, A.-S.; Danguien, M.; Bily, A.; Chemat, F. Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason. Sonochem. 2015, 27, 102–109. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Martínez, J.R.; Cala, M.P.; Durán, D.C.; Caballero, D. Chromatographic and mass spectrometric characterization of essential oils and extracts from Lippia (Verbenaceae) aromatic plants: Gas chromatography. J. Sep. Sci. 2013, 36, 192–202. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Green processes for the extraction of bioactives from rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J. Chromatogr. A 2010, 1217, 2512–2520. [Google Scholar] [CrossRef]
- Borrás Linares, I.; Arráez-Román, D.; Herrero, M.; Ibáñez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction Methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef]
- Ramírez, P.; García-Risco, M.R.; Santoyo, S.; Señoráns, F.J.; Ibáñez, E.; Reglero, G. Isolation of functional ingredients from rosemary by preparative-supercritical fluid chromatography (Prep-SFC). J. Pharm. Biomed. Anal. 2006, 41, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Bicchi, C.; Binello, A.; Rubiolo, P. Determination of phenolic diterpene antioxidants in rosemary (Rosmarinus officinalis L.) with different methods of extraction and analysis. Phytochem. Anal. 2000, 11, 236–242. [Google Scholar] [CrossRef]
- Kompelly, A.; Kompelly, S.; Vasudha, B.; Narender, B. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. J. Drug Deliv. Ther. 2019, 9, 323–330. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.D.P.; Valdés, A.; Sullini, G.; García-Cañas, V.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Two-step sequential supercritical fluid extracts from rosemary with enhanced anti-proliferative activity. J. Funct. Foods 2014, 11, 293–303. [Google Scholar] [CrossRef]
- Vicente, G.; García-Risco, M.R.; Fornari, T.; Reglero, G. Isolation of carsonic acid from rosemary extracts using semi-preparative supercritical fluid chromatography. J. Chromatogr. A 2013, 1286, 208–215. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.; García-Cañas, V.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Comparative study of green sub- and supercritical processes to obtain carnosic acid and carnosol-enriched rosemary extracts with in vitro anti-proliferative activity on colon cancer cells. Int. J. Mol. Sci. 2016, 17, 2046. [Google Scholar] [CrossRef]
- Herrero, M.; Arráez-Román, D.; Segura, A.; Kenndler, E.; Gius, B.; Raggi, M.A.; Ibáñez, E.; Cifuentes, A. Pressurized liquid extraction–capillary electrophoresis–mass spectrometry for the analysis of polar antioxidants in rosemary extracts. J. Chromatogr. A 2005, 1084, 54–62. [Google Scholar] [CrossRef]
- Ibañez, E.; Kubátová, A.; Señoráns, F.J.; Cavero, S.; Reglero, G.; Hawthorne, S.B. Subcritical water extraction of antioxidant compounds from rosemary plants. J. Agric. Food Chem. 2003, 51, 375–382. [Google Scholar] [CrossRef]
- Liu, T.; Sui, X.; Zhang, R.; Yang, L.; Zu, Y.; Zhang, L.; Zhang, Y.; Zhang, Z. Application of ionic liquids based microwave-assisted simultaneous extraction of carnosic acid, rosmarinic acid and essential oil from rosmarinus officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. [Google Scholar] [CrossRef]
- Zu, G.; Zhang, R.; Yang, L.; Ma, C.; Zu, Y.; Wang, W.; Zhao, C. Ultrasound-assisted extraction of carnosic acid and rosmarinic acid using ionic liquid solution from rosmarinus officinalis. Int. J. Mol. Sci. 2012, 13, 11027–11043. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.B.; Goltz, C.; Batistão Cavalheiro, F.; Theodoro Toci, A.; Igarashi-Mafra, L.; Mafra, M.R. Deep eutectic solvents applied in the extraction and stabilization of rosemary (Rosmarinus officinalis L.) phenolic compounds. Ind. Crops Prod. 2020, 144, 112049. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Extraction of phenolic compounds from rosemary using choline chloride–based deep eutectic solvents. Sep. Purif. Technol. 2021, 258, 117975. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Guo, Q.; Li, P.; Yang, H. A hydrophobic deep eutectic solvents-based integrated method for efficient and green extraction and recovery of natural products from rosmarinus officinalis leaves, ginkgo biloba leaves and salvia miltiorrhiza roots. Food Chem. 2021, 363, 130282. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Q.; Li, P.; Yang, H. Deep-eutectic solvents/ionic liquids/water mixture as a novel type of green thermo-switchable solvent system for selective extraction and separation of natural products from Rosmarinus officinalis leaves. Food Chem. 2022, 390, 133225. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Rebocho, S.; Craveiro, R.; Paiva, A.; Duarte, A.R.C. Selective extraction and stabilization of bioactive compounds from rosemary leaves using a biphasic NADES. Front. Chem. 2022, 10, 954835. [Google Scholar] [CrossRef]
- Mazaud, A.; Lebeuf, R.; Laguerre, M.; Nardello-Rataj, V. Hydrotropic extraction of carnosic acid from rosemary with short-chain alkyl polyethylene glycol ethers. ACS Sustain. Chem. Eng. 2020, 8, 15268–15277. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, Y.; Bai, X. A Green and effective polyethylene glycols-based microwave-assisted extraction of carnosic and rosmarinic acids from Rosmarinus officinalis leaves. Foods 2023, 12, 1761. [Google Scholar] [CrossRef]
- Thorsen, M.A.; Hildebrandt, K.S. Quantitative determination of phenolic diterpenes in rosemary extracts. J. Chromatogr. A 2003, 995, 119–125. [Google Scholar] [CrossRef]
- Del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Del Río, J.A.; Ortuño, A.; Quirin, K.-W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Wellwood, C.R.L.; Cole, R.A. Relevance of carnosic acid concentrations to the selection of rosemary, Rosmarinus officinalis (L.), accessions for optimization of antioxidant yield. J. Agric. Food Chem. 2004, 52, 6101–6107. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, N.; Sierra, H.; Carvajal, L.; Delpiano, P.; Günther, G. Fast high performance liquid chromatography and ultraviolet–visible quantification of principal phenolic antioxidants in fresh rosemary. J. Chromatogr. A 2005, 1100, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rau, O.; Wurglics, M.; Paulke, A.; Zitzkowski, J.; Meindl, N.; Bock, A.; Dingermann, T.; Abdel-Tawab, M.; Schubert-Zsilavecz, M. Carnosic acid and carnosol, phenolic diterpene compounds of the Labiate herbs rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med. 2006, 72, 881–887. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Steenkamp, P. Antioxidant, antiinflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010, 119, 684–688. [Google Scholar] [CrossRef]
- Erkan, N.; Akgonen, S.; Ovat, S.; Goksel, G.; Ayranci, E. Phenolic compounds profile and antioxidant activity of Dorystoechas hastata L. Boiss et Heldr. Food Res. Int. 2011, 44, 3013–3020. [Google Scholar] [CrossRef]
- Zhang, Y.; Smuts, J.P.; Dodbiba, E.; Rangarajan, R.; Lang, J.C.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar] [CrossRef]
- Meziane-Assami, D.; Tomao, V.; Ruiz, K.; Meklati, B.Y.; Chemat, F. Geographical differentiation of rosemary based on GC/MS and fast HPLC analyses. Food Anal. Methods 2013, 6, 282–288. [Google Scholar] [CrossRef]
- Mira-Sánchez, M.D.; Castillo-Sánchez, J.; Morillas-Ruiz, J.M. Comparative study of rosemary extracts and several synthetic and natural food antioxidants. Relevance of carnosic acid/carnosol ratio. Food Chem. 2020, 309, 125688. [Google Scholar] [CrossRef]
- Ceylan, B.; Tırıs, G.; Tekkeli, S.E.K. A New HPLC method with UV detection for the determination of carnosol in human plasma and application to a pharmacokinetic study. Chromatographia 2021, 84, 855–860. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction techniques, analytical methods and health-promoting biological effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- Almela, L.; Sánchez-Muñoz, B.; Fernández-López, J.A.; Roca, M.J.; Rabe, V. Liquid chromatograpic–mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chromatogr. A 2006, 1120, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef]
- Zimmermann, B.F.; Walch, S.G.; Tinzoh, L.N.; Stühlinger, W.; Lachenmeier, D.W. Rapid UHPLC determination of polyphenols in aqueous infusions of Salvia officinalis L. (sage tea). J. Chromatogr. B 2011, 879, 2459–2464. [Google Scholar] [CrossRef]
- Napoli, E.M.; Siracusa, L.; Saija, A.; Speciale, A.; Trombetta, D.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leone, R.; et al. Wild Sicilian rosemary: Phytochemical and morphological screening and antioxidant activity evaluation of extracts and essential oils. Chem. Biodivers. 2015, 12, 1075–1094. [Google Scholar] [CrossRef] [PubMed]
- Rajauria, G.; Foley, B.; Abu-Ghannam, N. Identification and characterization of phenolic antioxidant compounds from brown Irish seaweed Himanthalia elongata using LC-DAD–ESI-MS/MS. Innov. Food Sci. Emerg. Technol. 2016, 37, 261–268. [Google Scholar] [CrossRef]
- Wang, L.; Gan, C.; Wang, Z.; Liu, L.; Gao, M.; Li, Q.; Yang, C. Determination and pharmacokinetic study of three diterpenes in rat plasma by UHPLC-ESI-MS/MS after oral administration of Rosmarinus officinalis L. extract. Molecules 2017, 22, 934. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, X.; Zhou, N.; Li, J.; Liu, J.; Yue, J.; Hao, X.; Gan, M.; Lin, P.; Shang, X. Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 2021, 344, 128674. [Google Scholar] [CrossRef]
- Paloukopoulou, C.; Karioti, A. A validated method for the determination of carnosic acid and carnosol in the fresh foliage of Salvia rosmarinus and Salvia officinalis from Greece. Plants 2022, 11, 3106. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; Polissiou, M.; Izquierdo-Melero, M.E.; Astraka, K.; Tarantilis, P.A.; Herraiz-Peñalver, D.; Sánchez-Vioque, R. Polyphenol composition, antioxidant and bioplaguicide activities of the solid residue from hydrodistillation of Rosmarinus officinalis L. Ind. Crops Prod. 2014, 59, 125–134. [Google Scholar] [CrossRef]
- Song, Y.; Yan, H.; Chen, J.; Wang, Y.; Jiang, Y.; Tu, P. Characterization of in vitro and in vivo metabolites of carnosic acid, a natural antioxidant, by high performance liquid chromatography coupled with tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Bonoli, M.; Pelillo, M.; Lercker, G. Fast separation and determination of carnosic acid and rosmarinic acid in different rosemary (Rosmarinus officinalis) extracts by capillary zone electrophoresis with ultra violet-diode array detection. Chromatographia 2003, 57, 505–512. [Google Scholar] [CrossRef]
- Baskan, S.; Oztekin, N.; Erim, F. Determination of carnosic acid and rosmarinic acid in sage by capillary electrophoresis. Food Chem. 2007, 101, 1748–1752. [Google Scholar] [CrossRef]
- Lu, P.; Ma, J.; Li, P.; Zhou, H.; Xu, X.; Guo, W. Determination of carnosic acid and carnosol in Rosmarinus officinalis L. by high-performance capillary electrophoresis. Instrum. Sci. Technol. 2017, 45, 268–275. [Google Scholar] [CrossRef]
- Adımcılar, V.; Kalaycıoğlu, Z.; Aydoğdu, N.; Dirmenci, T.; Kahraman, A.; Erim, F.B. Rosmarinic and carnosic acid contents and correlated antioxidant and antidiabetic activities of 14 salvia species from Anatolia. J. Pharm. Biomed. Anal. 2019, 175, 112763. [Google Scholar] [CrossRef]
- Pachura, N.; Zimmer, A.; Grzywna, K.; Figiel, A.; Szumny, A.; Łyczko, J. Chemical investigation on Salvia officinalis L. affected by multiple drying techniques—The comprehensive analytical approach (HS-SPME, GC–MS, LC-MS/MS, GC-O and NMR). Food Chem. 2022, 397, 133802. [Google Scholar] [CrossRef]
- Khaliullina, A.S.; Khaziev, R.S.; Salamatin, A.A. Quantitative determination of diterpene acids in garden sage leaves. J. Anal. Chem. 2017, 72, 810–814. [Google Scholar] [CrossRef]
- Yilmaz, Ü.T.; Calik, E.; Akdulum, B.; Yilmaz, H. Determination of carnosic acid in Rosmarinus officinalis L. using square wave voltammetry and electrochemical behavior. J. Food Drug Anal. 2018, 26, 300–308. [Google Scholar] [CrossRef]
- Satoh, T.; Trudler, D.; Oh, C.-K.; Lipton, S.A. Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer’s disease, Parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome. Antioxidants 2022, 11, 124. [Google Scholar] [CrossRef]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Diterpenes and their derivatives as potential anticancer agents: Diterpenes in cancer. Phytother. Res. 2017, 31, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Anticancer activity of Rosmarinus officinalis L.: Mechanisms of action and therapeutic potentials. Nutrients 2020, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. An overview of the chemistry and anticancer properties of rosemary extract and its diterpenes. J. Herbmed Pharmacol. 2021, 11, 10–19. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef]
- Kakouri, E.; Nikola, O.; Kanakis, C.; Hatziagapiou, K.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic effect of Rosmarinus officinalis extract on glioblastoma and rhabdomyosarcoma cell lines. Molecules 2022, 27, 6348. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, A.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur. J. Nutr. 2013, 52, 85–95. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Den Hartogh, D.J.; Azizi, K.; Tsiani, E. Anticancer properties of carnosol: A summary of in vitro and in vivo evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, Y.; Wang, Z.; Ji, Y.; Zhang, X.; Yan, X.; Zhan, Z. Carnosic acid induces antiproliferation and anti-metastatic property of esophageal cancer cells via MAPK signaling pathways. J. Oncol. 2021, 2021, 4451533. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Fan, Y.; Li, Y. Antiproliferative activity of carnosic acid is mediated via inhibition of cell migration and invasion, and suppression of phosphatidylinositol 3-kinases (PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway. Med. Sci. Monit. 2019, 25, 7864–7871. [Google Scholar] [CrossRef]
- D’Alesio, C.; Bellese, G.; Gagliani, M.C.; Aiello, C.; Grasselli, E.; Marcocci, G.; Bisio, A.; Tavella, S.; Daniele, T.; Cortese, K.; et al. Cooperative antitumor activities of carnosic acid and trastuzumab in ERBB2+ breast cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 154. [Google Scholar] [CrossRef]
- Shao, N.; Mao, J.; Xue, L.; Wang, R.; Zhi, F.; Lan, Q. Carnosic acid potentiates the anticancer effect of temozolomide by inducing apoptosis and autophagy in glioma. J. Neurooncol. 2019, 141, 277–288. [Google Scholar] [CrossRef] [PubMed]
- El-Huneidi, W.; Bajbouj, K.; Muhammad, J.S.; Vinod, A.; Shafarin, J.; Khoder, G.; Saleh, M.A.; Taneera, J.; Abu-Gharbieh, E. Carnosic acid induces apoptosis and inhibits Akt/mTOR signaling in human gastric cancer cell lines. Pharmaceuticals 2021, 14, 230. [Google Scholar] [CrossRef]
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic acid suppresses the development of oral squamous cell carcinoma via mitochondrial-mediated apoptosis. Front. Oncol. 2021, 11, 760861. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Syed, D.N.; Suh, Y.; Heren, C.R.; Saleem, M.; Siddiqui, I.A.; Mukhtar, H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: Implications for chemoprevention. Cancer Prev. Res. 2010, 3, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Alsamri, H.; El Hasasna, H.; Al Dhaheri, Y.; Eid, A.H.; Attoub, S.; Iratni, R. Carnosol, a natural polyphenol, inhibits migration, metastasis, and tumor growth of breast cancer via a ROS-dependent proteasome degradation of STAT3. Front. Oncol. 2019, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Anti-inflammatory therapeutic mechanisms of natural products: Insight from rosemary diterpenes, carnosic acid and carnosol. Biomedicines 2023, 11, 545. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N.; et al. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions: Anti-inflammatory response of CA and CS. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- Xia, G.; Wang, X.; Sun, H.; Qin, Y.; Fu, M. Carnosic acid (CA) attenuates collagen-induced arthritis in db/db mice via inflammation suppression by regulating ROS-dependent P38 pathway. Free Radic. Biol. Med. 2017, 108, 418–432. [Google Scholar] [CrossRef]
- Li, L.; Pan, Z.; Ning, D.; Fu, Y. Rosmanol and carnosol synergistically alleviate rheumatoid arthritis through inhibiting TLR4/NF-κB/MAPK pathway. Molecules 2021, 27, 78. [Google Scholar] [CrossRef]
- Ishitobi, H.; Sanada, Y.; Kato, Y.; Ikuta, Y.; Shibata, S.; Yamasaki, S.; Lotz, M.K.; Matsubara, K.; Miyaki, S.; Adachi, N. Carnosic acid attenuates cartilage degeneration through induction of heme oxygenase-1 in human articular chondrocytes. Eur. J. Pharmacol. 2018, 830, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kalantar, H.; Sadeghi, E.; Abolnezhadian, F.; Goudarzi, M.; Hemmati, A.A.; Basir, Z.; Kalantar, M. Carnosol attenuates bleomycin-induced lung damage via suppressing fibrosis, oxidative stress and inflammation in rats. Life Sci. 2021, 287, 120059. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.J.; Zahid, S.; Holsinger, R.M.D. Neuroprotective effects of carnosic acid: Insight into its mechanisms of action. Molecules 2023, 28, 2306. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-R.; Tsai, C.-W.; Chang, S.-W.; Lin, C.-Y.; Huang, L.-C.; Tsai, C.-W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem.-Biol. Interact. 2015, 225, 40–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).