Durian Waste Husks as an Adsorbent in Improving Soaking Water during the Retting Process of Piper nigrum L. (Pepper Berries)
Abstract
1. Introduction
2. Materials and Methods
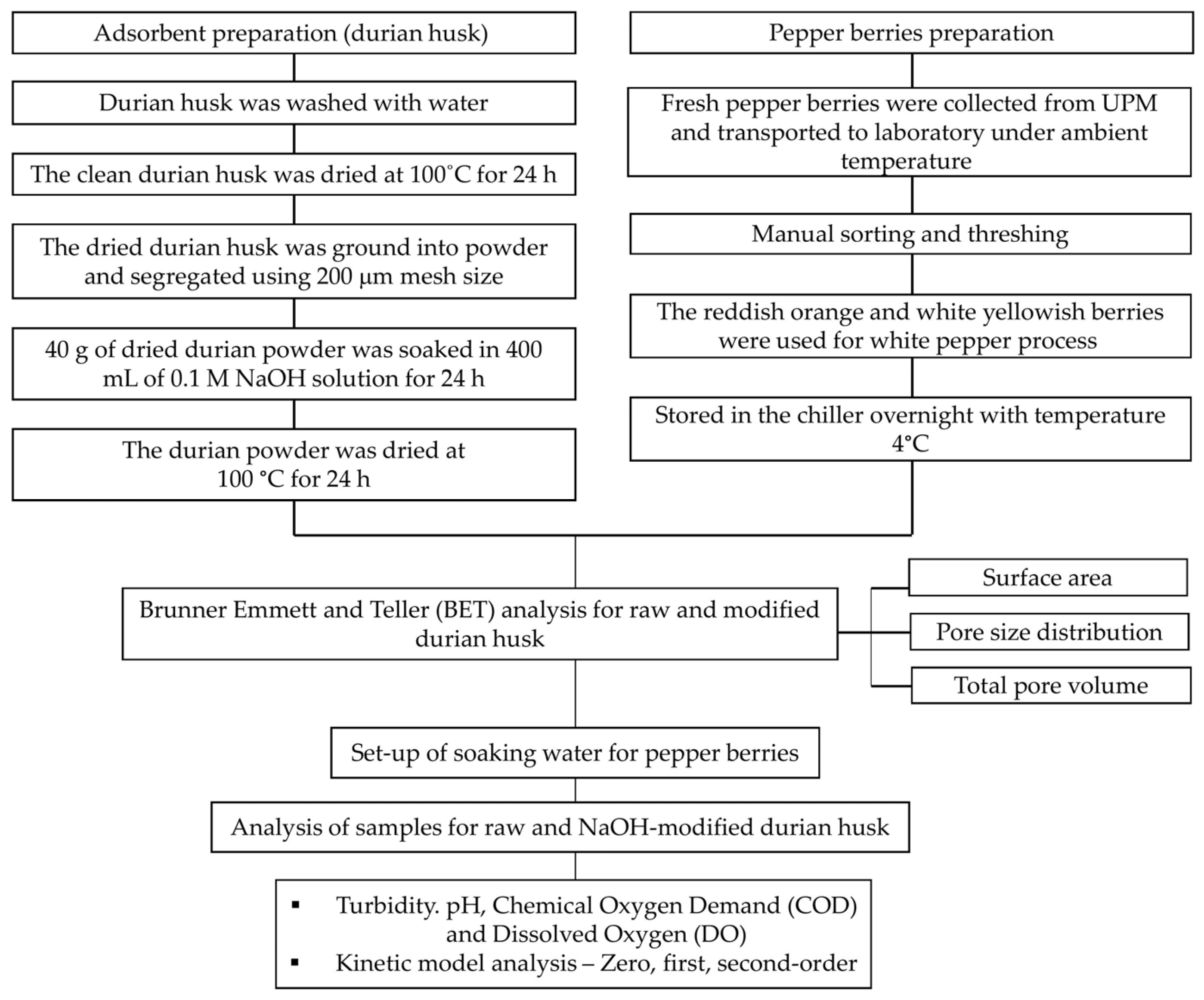
2.1. Flowchart of Methodology
2.2. Preparation of Raw and NaOH-Modified Adsorbents from Durian Husk
2.3. Brunauer–Emmett–Teller (BET) Surface Area Analysis
2.4. Pepper Berries Sample Preparation
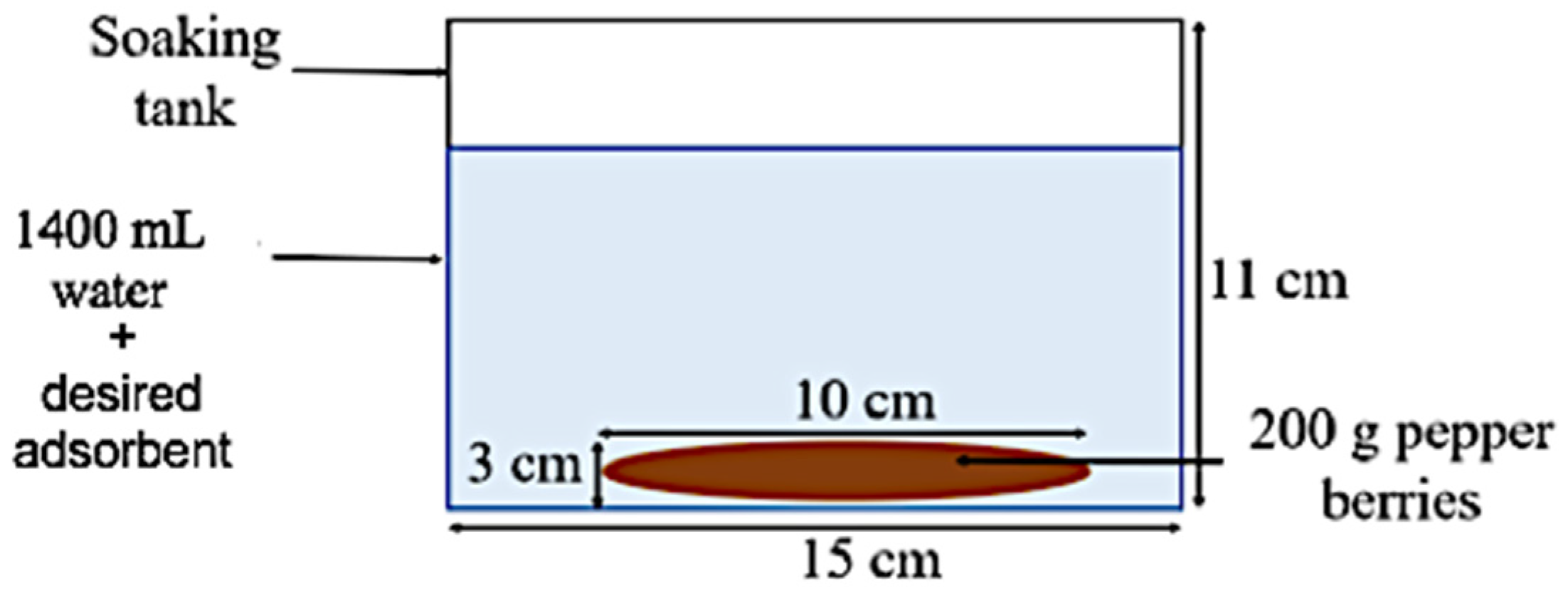
2.5. Experimental Set-Up of Soaking Water for Pepper Berries
2.6. Analysis of Soaking Water Quality
2.6.1. pH
2.6.2. Turbidity
2.6.3. COD
2.6.4. DO
2.7. Kinetic Model Analysis
3. Results and Discussion
3.1. BET Analysis
3.2. Analysis of Soaking Water Quality
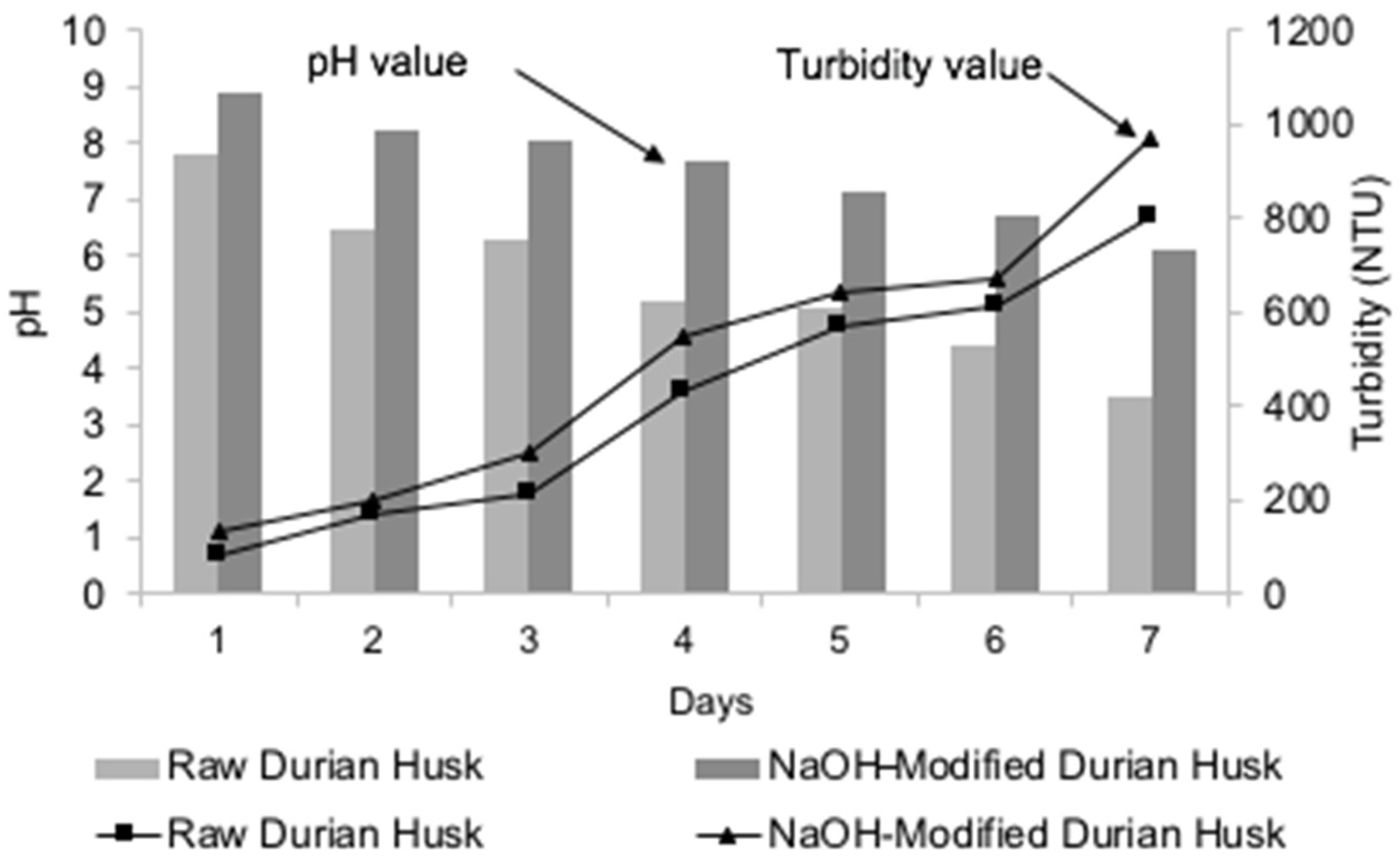
3.2.1. Effect of Adsorbent Dose on pH
3.2.2. Effect of Adsorbent Dose on Turbidity
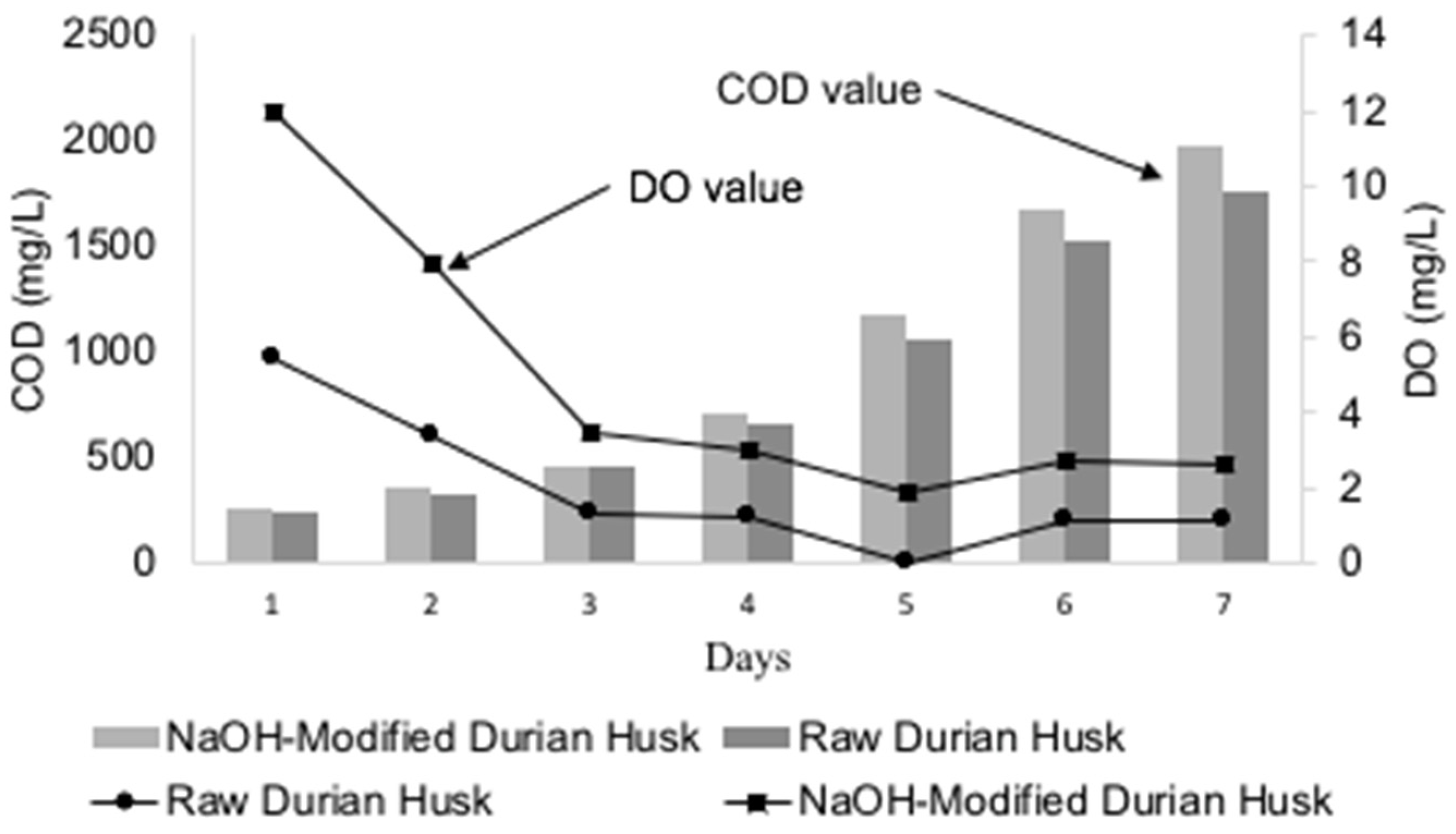
3.2.3. Effect of Adsorbent Dose on COD
3.2.4. Effect of Adsorbent Dose on DO
3.3. Kinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bermawie, N.; Wahyuni, S.; Heryanto, R.; Darwati, I. Morphological Characteristics, Yield and Quality of Black Pepper Ciinten Variety in Three Agro Ecological Conditions. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Food Science and Technology, Semarang, Indonesia, 28–29 November 2018. [Google Scholar]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahoomodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, 210–243. [Google Scholar] [CrossRef] [PubMed]
- Abang Mustafa, D.S.; Zulkharnain, A.; Awang Husaini, A.A.S. Enzymatic retting of Piper nigrum L. using commercial Pectinase (Peelzyme). J. Chem. Pharm. Sci. 2015, 8, 360–364. [Google Scholar]
- Aziz, N.S.; Sofian-Seng, N.S.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. A review on conventional and biotechnological approaches in white pepper production. J. Sci. Food Agric. 2019, 99, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Megat Ahmad Azman, P.N.; Shamsudin, R.; Che Man, H.; Ya’acob, M.E. Kinetics of quality changes in soaking water during the retting process of pepper berries (Piper nigrum L.). Processes 2020, 8, 1255. [Google Scholar] [CrossRef]
- Megat Ahmad Azman, P.N.; Shamsudin, R.; Che Man, H.; Ya’acob, M.E. Effects of flowing water on soaking water quality during the retting process of pepper berries (Piper nigrum L.). Adv. Agric. Food Res. J. 2021, 3, 1–10. [Google Scholar] [CrossRef]
- Rosnah, S.; Chan, S.C. Enzymatic rettings of green pepper berries for white pepper production. Int. Food Res. J. 2014, 21, 237–245. [Google Scholar]
- Sukasih, E.; Hernani; Sasmitaloka, K.S. The Improvement of White Pepper Quality using Ozone Application. In Proceedings of the IOP Conference Series: Earth and Environmental Science, The 3rd International Conference on Food and Agriculture, Jember, Indonesia, 7–8 November 2020. [Google Scholar]
- Sasmitaloka, K.S.; Hidayat, T.; Hernani. The Improvement of White Pepper Quality through Fermentation Process by Acetobacter sp. In Proceedings of the IOP Conference Series: Earth and Environmental Science, International Conference on Green Agro-industry and Bioeconomy, Malang, Indonesia, 25 August 2020. [Google Scholar]
- Sasmitaloka, K.S.; Hernani. Quality improvement of white pepper with processing through optimizing the ratio of starter culture from Acetobacter sp., Bacillus subtilis, and Bacillus cereus. HAYATI J. Biosci. 2022, 29, 87–96. [Google Scholar] [CrossRef]
- Sreekala, G.S.; Meenakumari, K.S.; Vigi, S. Microbial isolate for the production of quality white pepper (Piper nigrum L.). J. Trop. Agric. 2019, 57, 114–121. [Google Scholar]
- William Kajjumba, G.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. Modelling of adsorption kinetic processes—Errors, theory and application. In Advanced Sorption Process Applications; Edebali, S., Ed.; InTech Open Limited: London, UK, 2019; pp. 1–19. [Google Scholar]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review. J. Clean. Prod. 2020, 255, 1–13. [Google Scholar] [CrossRef]
- Kusrini, E.; Usman, A.; Sani, F.A.; Wilson, L.D.; Abdullah, M.A.A. Simultaneous adsorption of lanthanum and yttrium from aqueous solution by durian rind biosorbent. Environ. Monit. Assess. 2019, 191, 1–8. [Google Scholar] [CrossRef]
- Bhat, R.; Paliyath, G. Fruits of Tropical Climates: Biodiversity and Dietary Importance. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2015; Volume 3, pp. 138–143. [Google Scholar]
- Payus, C.M.; Refdin, M.A.; Zahari, N.Z.; Rimba, A.B.; Geetha, M.; Saroj, C.; Gasparatos, A.; Fukushi, K.; Alvin Oliver, P. Durian Husk Wastes as Low-Cost Adsorbent for Physical Pollutants Removal: Groundwater Supply. In Proceedings of the International Conference of Chemical Engineering & Industrial Biotechnology, Kuala Lumpur, Malaysia, 9–11 August 2020. [Google Scholar]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Sudrajat, H.; Susanti, A.; Putri, D.K.Y.; Hartuti, S. Mechanistic insights into the adsorption of methylene blue by particulate durian peel waste in water. Water Sci. Technol. 2021, 84, 1774–1792. [Google Scholar] [CrossRef] [PubMed]
- Abood, M.M.; Rajendiran, J.; Azhari, N.N. Agricultural waste as low-cost adsorbent for the removal of Fe (II) ions from aqueous solution. Infrastruct. Univ. Kuala Lumpur Res. J. 2015, 3, 29–39. [Google Scholar]
- Mat Lazim, Z.; Hadibarata, T.; Puteh, M.H.; Yusop, Z.; Wirasnita, R.; Mohd Nor, N. Utilization of durian peel as potential adsorbent for bisphenol a removal in aquoeus solution. J. Teknol. 2015, 74, 109–115. [Google Scholar] [CrossRef]
- Gan, J.L.; Cheok, Y. Progress in energy and environment enhanced removal efficiency of methylene blue and water hardness using NaOH-modified durian and passion fruit peel adsorbents. Prog. Energy Environ. 2021, 16, 36–44. [Google Scholar]
- Hamzah, M.H.; Ahmad Asri, M.F.; Che Man, H.; Mohammed, A. Prospective application of palm oil mill boiler ash as a biosorbent: Effect of microwave irradiation and palm oil mill effluent decolorization by adsorption. Int. J. Environ. Res. Public Health. 2019, 16, 3453. [Google Scholar] [CrossRef]
- HACH Company. DR/890 Colorimeter Procedures Manual; HACH Company: Loveland, CO, USA, 2013; pp. 13–51. [Google Scholar]
- Laidler, K.J. The development of the arrhenius equation. J. Chem. Educ. 1984, 61, 494–498. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Sultana, N.; Sayem, A.S.M.; Smriti, S.A. Sustainable adsorbents from plant-derived agricultural wastes for anionic dye removal: A review. Sustainability 2022, 14, 11098. [Google Scholar] [CrossRef]
- Yu, H.; Wang, J.; Xia Yu, J.; Wang, Y.; An Chi, R. Adsorption performance and stability of the modified straws and their extracts of cellulose, lignin, and hemicellulose for Pb2+: pH effect. Arab. J. Chem. 2020, 13, 9019–9033. [Google Scholar] [CrossRef]
- Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Shigidi, I.; Elhag, A.B.; Karama, B. Evaluation of the adsorption efficiency on the removal of lead(II) ions from aqueous solutions using Azadirachta indica leaves as an adsorbent. Processes 2021, 9, 559. [Google Scholar] [CrossRef]
- Wulan, P.; Kusumastuti, Y.; Prasetya, A. Adsorption of Fe (II) ion into chitosan/activated carbon composite: Isotherm and kinetics studies. J. Eng. Sci. Technol. 2022, 17, 2218–2233. [Google Scholar]
- Okhovat, A.; Ahmadpour, A.; Ahmadpour, F.; Khaki Yadegar, Z. Pore size distribution analysis of coal-based activated carbons: Investigating the effects of activating agent and chemical ratio. ISRN Chem. Eng. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Hamzah, M.H.; Bowra, S.; Cox, P. Organosolv lignin aggregation behaviour of soluble lignin extract from Miscanthus x giganteus at different ethanol concentrations and its influence on the lignin esterification. Chem. Biol. Technol. Agric. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Li, T.; Wu, J.J.; Wang, X.G.; Huang, H. Particle size effect and temperature effect on the pore structure of low-rank coal. ACS Omega. 2021, 6, 5865–5877. [Google Scholar] [CrossRef]
- Horvat, G.; Pantić, Ž.; Knez, M.; Novak, Z. A brief evaluation of pore structure determination for bioaerogels. Gels 2022, 8, 438. [Google Scholar] [CrossRef]
- Aziz, N.S.; Sofian-Seng, N.S.; Wan Mustapha, W.A. Functional properties of oleoresin extracted from white pepper (Piper nigrum L.) retting wastewater. Sains Malaysiana 2018, 47, 2009–2015. [Google Scholar] [CrossRef]
- Agboola, O.D.; Benson, N.U. Physisorption and chemisorption mechanisms influencing micro(nano) plastics-organic chemical contaminants interactions: A review. Front. Environ. Sci. 2021, 9, 678574. [Google Scholar] [CrossRef]
- Sims, R.A.; Harmer, S.L.; Quinton, J.S. The role of physisorption and chemisorption in the oscillatory adsorption of organosilanes on aluminium oxide. Polymers 2019, 11, 410. [Google Scholar] [CrossRef]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Tharmalingam, M.A.; Gunawardana, M.; Mowjood, M.I.M.; Dharmasena, D.A.N. Coagulation-flocculation treatment of white pepper (Piper nigrum L.) processing wastewater. Trop. Agric. Res. 2017, 28, 435–446. [Google Scholar] [CrossRef]
- Yuliusman; Ayu, M.P.; Hanafi, A.; Nafisah, A.R. Activated Carbon Preparation from Durian Peel Wastes using Chemical and Physical Activation. In Proceedings of the International Symposium on Sustainable and Clean Energy (ISSCE): Quality in Research, Padang, Indonesia, 22–24 July 2019. [Google Scholar]
- Aniyikaiye, T.E.; Oluseyi, T.; Odiyo, J.O.; Edokpayi, J.N. Physico-chemical analysis of wastewater discharge from selected paint industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ajala, L.O.; Ali, E.E.; Obasi, N.A.; Fasuan, T.O.; Odewale, I.O.; Igidi, J.O.; Singh, J. Insights into purification of contaminated water with activated charcoal derived from hamburger seed coat. Int. J. Environ. Sci. Technol. 2022, 19, 6541–6554. [Google Scholar] [CrossRef] [PubMed]
- Neshankine, C.; Kannan, N. Comprehensive evaluation of aerated soaking for paddy parboiling in an eco-friendly manner. Trop. Agric. Res. Ext. 2021, 24, 301–317. [Google Scholar] [CrossRef]
- Ong, T.H.; Hamzah, M.H.; Che Man, H. Optimization of palm oil extraction from decanter cake using soxhlet extraction and effects of microwaves pre-treatment on extraction yield and physicochemical properties of palm oil. Food Res. 2021, 5, 25–32. [Google Scholar] [CrossRef]
- Grigoras, C.G.; Andrei-Ionut, S.; Lidia, F.; Cătălin, D.; Lucian, G. Performance of dye removal from single and binary component systems by adsorption on composite hydrogel polymeric matrix. Gels 2022, 8, 795. [Google Scholar] [CrossRef]
- Anako, O.L.; Mohammed Inuwa, I.; Wong, S.; Ngadi, N.; Amirah Razmi, F. Errors and inconsistencies in scientific reporting of aqueous phase adsorption of contaminants: A bibliometric study. Clean. Mater. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Younas, F.; Mustafa, A.; Zia Ur, R.F.; Wang, X.; Younas, S.; Mohy-ud-din, W.; Muhammad Ashir, H.; Abrar, M.; Mohsin, A.; Ali Akbar, M.; et al. Removal of carcinogenic hexavalent chromium from aqueous solutions using newly synthesized and characterized polypyrrole–titanium (IV) phosphate nanocomposite. Water 2021, 13, 215. [Google Scholar]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. Adsorptive removal of reactive black 5 from wastewater using bentonite clay: Isotherms, kinetics and thermodynamics. Sustainability 2015, 7, 15302–15318. [Google Scholar] [CrossRef]
| Adsorbent | Pore Volume (cm3/g) | Pore Size (nm) | Surface Area (m2/g) |
|---|---|---|---|
| Raw durian husk | 0.28 | 3660.00 | 1.51 |
| NaOH-modified durian husk | 0.30 | 2587.00 | 2.33 |
| Day | pH | |||||||
| Raw Durian Husk | NaOH-Modified Durian Husk | |||||||
| A | B | C | D | A | B | C | D | |
| 1 | 7.77 ± 0.02 | 7.85 ± 0.03 | 7.85 ± 0.01 | 7.84 ± 0.02 | 8.13 ± 0.02 | 8.57 ± 0.02 | 8.86 ± 0.05 | 8.92 ± 0.03 |
| 2 | 6.52 ± 0.01 | 6.54 ± 0.02 | 6.52 ± 0.01 | 6.49 ± 0.03 | 7.68 ± 0.20 | 8.00 ± 0.04 | 8.12 ± 0.01 | 8.22 ± 0.01 |
| 3 | 6.22 ± 0.01 | 6.25 ± 0.01 | 6.26 ± 0.00 | 6.28 ± 0.02 | 7.29 ± 0.02 | 7.90 ± 0.03 | 7.98 ± 0.01 | 8.03 ± 0.02 |
| 4 | 5.16 ± 0.02 | 5.23 ± 0.02 | 5.23 ± 0.01 | 5.21 ± 0.01 | 6.87 ± 0.02 | 7.81 ± 0.02 | 7.66 ± 0.02 | 7.69 ± 0.01 |
| 5 | 5.05 ± 0.01 | 5.06 ± 0.02 | 5.07 ± 0.02 | 5.09 ± 0.02 | 6.18 ± 0.03 | 6.96 ± 0.01 | 7.03 ± 0.04 | 7.13 ± 0.01 |
| 6 | 4.30 ± 0.02 | 4.36 ± 0.11 | 4.39 ± 0.02 | 4.42 ± 0.01 | 5.87 ± 0.01 | 6.35 ± 0.01 | 6.66 ± 0.03 | 6.73 ± 0.01 |
| 7 | 3.10 ± 0.02 | 3.88 ± 0.01 | 3.77 ± 0.01 | 4.61 ± 0.02 | 5.22 ± 0.01 | 5.85 ± 0.02 | 5.98 ± 0.05 | 6.10 ± 0.02 |
| Day | Turbidity (NTU) | |||||||
| Raw Durian Husk | NaOH-Modified Durian Husk | |||||||
| A | B | C | D | A | B | C | D | |
| 1 | 73.33 ± 1.53 | 87.00 ± 1.00 | 86.33 ± 0.58 | 86.39 ± 1.15 | 93.00 ± 1.00 | 102.33 ± 0.58 | 114.00 ± 1.00 | 132.67 ± 2.08 |
| 2 | 170.33 ± 2.08 | 168.33 ± 2.08 | 165.33 ± 3.21 | 168.67 ± 0.58 | 188.00 ± 0.82 | 197.00 ± 0.82 | 203.33 ± 2.08 | 197.67 ± 2.52 |
| 3 | 288.00 ± 3.61 | 213.00 ± 2.00 | 213.00 ± 1.00 | 217.00 ± 2.65 | 297.67 ± 1.53 | 276.67 ± 1.15 | 291.33 ± 1.00 | 303.33 ± 2.08 |
| 4 | 432.33 ± 1.15 | 429.00 ± 1.00 | 428.33 ± 1.53 | 435.00 ± 3.00 | 491.00 ± 1.00 | 520.00 ± 2.00 | 548.00 ± 4.00 | 552.00 ± 2.08 |
| 5 | 543.00 ± 2.00 | 541.00 ± 1.00 | 541.67 ± 0.58 | 573.00 ± 1.00 | 585.00 ± 1.00 | 610.67 ± 1.53 | 627.67 ± 3.06 | 642.00 ± 2.00 |
| 6 | 607.00 ± 1.00 | 598.00 ± 1.00 | 604.33 ± 1.15 | 612.00 ± 1.00 | 691.00 ± 1.00 | 653.00 ± 2.65 | 664.00 ± 2.65 | 674.00 ± 1.00 |
| 7 | 797.67 ± 0.58 | 799.33 ±1.53 | 873.52 ± 0.01 | 855.00 ± 1.73 | 803.00 ± 2.00 | 855.00 ± 1.00 | 885.98 ± 0.05 | 971.33 ± 1.15 |
| Day | COD (mg/L) | |||||||
| Raw Durian Husk | NaOH-Modified Durian Husk | |||||||
| A | B | C | D | A | B | C | D | |
| 1 | 253.67 ± 3.21 | 255.00 ± 1.00 | 254.00 ± 1.00 | 252.67 ± 1.53 | 250.00 ± 3.00 | 251.00 ± 1.73 | 256.33 ± 1.53 | 265.33 ± 2.08 |
| 2 | 334.67 ± 2.52 | 335.00 ± 1.00 | 334.00 ± 2.00 | 335.00 ± 3.61 | 334.00 ± 2.94 | 335.00 ± 0.82 | 348.67 ± 3.06 | 357.00 ± 1.00 |
| 3 | 453.67 ± 4.73 | 457.33 ± 1.15 | 458.00 ± 1.00 | 464.67 ± 2.52 | 450.33 ± 1.53 | 451.00 ± 1.00 | 454.67 ± 1.53 | 463.33 ± 1.53 |
| 4 | 656.00 ± 1.00 | 659.00 ± 1.00 | 656.00 ± 1.00 | 664.00 ± 1.00 | 653.67 ± 1.53 | 697.00 ± 1.00 | 705.00 ± 1.00 | 714.00 ± 1.00 |
| 5 | 1061.00 ± 1.00 | 1058.33 ± 1.53 | 1067.00 ± 1.00 | 1068.67 ± 1.53 | 1056.33 ± 1.53 | 1157.00 ± 1.00 | 1166.67 ± 4.73 | 1170.33 ± 2.08 |
| 6 | 1462.67 ± 2.08 | 1476.00 ± 1.00 | 1466.00 ± 1.00 | 1523.33 ± 5.77 | 1457.00 ± 1.00 | 1486.00 ± 1.00 | 1496.33 ± 1.15 | 1672.33 ± 2.52 |
| 7 | 1747.67 ± 26.58 | 1748.00 ± 1.00 | 1750.00 ± 19.47 | 1760.67 ± 1.53 | 1770.00 ± 17.32 | 1777.00 ± 1.73 | 1895.33 ± 1.53 | 1984.67 ± 3.21 |
| Day | DO (mg/L) | |||||||
| Raw Durian Husk | NaOH-Modified Durian Husk | |||||||
| A | B | C | D | A | B | C | D | |
| 1 | 5.62 ± 0.01 | 5.42 ± 0.01 | 5.41 ± 0.01 | 5.42 ± 0.01 | 5.69 ± 0.01 | 6.46 ± 0.02 | 6.48 ± 0.01 | 6.58 ± 0.02 |
| 2 | 3.45 ± 0.01 | 3.44 ± 0.01 | 3.42 ± 0.05 | 3.42 ± 0.02 | 3.57 ± 0.00 | 4.37 ± 0.00 | 4.47 ± 0.01 | 4.57 ± 0.08 |
| 3 | 1.32 ± 0.02 | 1.32 ± 0.02 | 1.31 ± 0.03 | 1.32 ± 0.02 | 1.41 ± 0.01 | 1.95 ± 0.02 | 1.97 ± 0.02 | 2.13 ± 0.02 |
| 4 | 1.27 ± 0.02 | 1.25 ± 0.01 | 1.25 ± 0.04 | 1.24 ± 0.01 | 1.30 ± 0.01 | 1.58 ± 0.02 | 1.67 ± 0.02 | 1.78 ± 0.01 |
| 5 | 1.24 ± 0.02 | 1.23 ± 0.01 | 1.21 ± 0.01 | 1..23 ± 0.01 | 1.26 ± 0.01 | 1.45 ± 0.01 | 1.65 ± 0.01 | 1.86 ± 0.02 |
| 6 | 1.18 ± 0.01 | 1.13 ± 0.02 | 1.12 ± 0.05 | 1.13 ± 0.01 | 1.25 ± 0.02 | 1.34 ± 0.01 | 1.42 ± 0.01 | 1.59 ± 0.01 |
| 7 | 1.13 ± 0.01 | 1.11 ± 0.01 | 1.12 ± 0.09 | 1.13 ± 0.02 | 1.20 ± 0.01 | 1.25 ± 0.01 | 1.34 ± 0.01 | 1.49 ± 0.03 |
| Parameter | Condition | Zero-Order Model | First-Order Model | Second-Order Model | |||
|---|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | ||
| pH | A | −0.645 | 0.9706 | −0.1118 | 0.9692 | 0.0228 | 0.9537 |
| B | −0.6589 | 0.9711 | −0.1215 | 0.9732 | 0.0233 | 0.9415 | |
| C | −0.6586 | 0.9689 | −0.1217 | 0.9686 | 0.0234 | 0.933 | |
| D | −0.6532 | 0.9646 | −0.1207 | 0.9636 | 0.0233 | 0.927 | |
| Turbidity | A | 117.91 | 0.9912 | 0.3691 | 0.9107 | −0.0017 | 0.6829 |
| B | 114.223 | 0.9605 | 0.3169 | 0.9686 | 0.0288 | 0.6463 | |
| C | 118.73 | 0.9726 | 0.3615 | 0.9465 | 0.0015 | 0.786 | |
| D | 121.17 | 0.9727 | 0.3657 | 0.9417 | −0.0015 | 0.7797 | |
| DO | A | −0.6461 | 0.6493 | −0.2507 | 0.713 | 0.1173 | 0.7683 |
| B | −0.63 | 0.6607 | −0.2519 | 0.7254 | 0.1212 | 0.7853 | |
| C | −0.6275 | 0.7209 | −0.2513 | 0.7209 | 0.121 | 0.7776 | |
| D | −0.6264 | 0.6561 | −0.2496 | 0.7174 | 0.1194 | 0.7718 | |
| COD | A | 262.33 | 0.9439 | 0.3425 | 0.9905 | −0.0006 | 0.9384 |
| B | 262.93 | 0.9436 | 0.3421 | 0.9905 | −0.0005 | 0.9384 | |
| C | 262.89 | 0.9444 | 0.3426 | 0.9904 | −0.0016 | 0.9375 | |
| D | 268.02 | 0.9427 | 0.3459 | 0.9898 | −0.00083 | 0.9844 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, M.H.; Amran, A.S.; Aili Hamzah, A.F.; Mohd Nor, M.Z.; Shamsudin, R.; Che Man, H.; Wan Razali, W.A. Durian Waste Husks as an Adsorbent in Improving Soaking Water during the Retting Process of Piper nigrum L. (Pepper Berries). Separations 2023, 10, 96. https://doi.org/10.3390/separations10020096
Hamzah MH, Amran AS, Aili Hamzah AF, Mohd Nor MZ, Shamsudin R, Che Man H, Wan Razali WA. Durian Waste Husks as an Adsorbent in Improving Soaking Water during the Retting Process of Piper nigrum L. (Pepper Berries). Separations. 2023; 10(2):96. https://doi.org/10.3390/separations10020096
Chicago/Turabian StyleHamzah, Muhammad Hazwan, Ainaa Syaheera Amran, Adila Fazliyana Aili Hamzah, Mohd Zuhair Mohd Nor, Rosnah Shamsudin, Hasfalina Che Man, and Wan Aizuddin Wan Razali. 2023. "Durian Waste Husks as an Adsorbent in Improving Soaking Water during the Retting Process of Piper nigrum L. (Pepper Berries)" Separations 10, no. 2: 96. https://doi.org/10.3390/separations10020096
APA StyleHamzah, M. H., Amran, A. S., Aili Hamzah, A. F., Mohd Nor, M. Z., Shamsudin, R., Che Man, H., & Wan Razali, W. A. (2023). Durian Waste Husks as an Adsorbent in Improving Soaking Water during the Retting Process of Piper nigrum L. (Pepper Berries). Separations, 10(2), 96. https://doi.org/10.3390/separations10020096









