Abstract
6Li and 7Li are strategic resources. Because Li+ ions have no outermost electrons and the radii of 6Li and 7Li differ by only one neutron, the separation of the naturally stable isotopes of Li, especially by solvent extraction, is recognized as a difficult problem worldwide. Therefore, in this paper, an advanced β-diketone-driven deep eutectic solvent (DES) extraction system containing 2-thenoyltrifluoroacetone (HTTA) and tri-n-octyl phosphine oxide (TOPO) is introduced to the extraction and separation of 6Li+ and 7Li+ ions. Compared with those of reported HTTA extraction systems and crown ether extraction systems, the separation coefficient () of the β-diketone-driven DES extraction system can reach the best value of 1.068, which is now the highest known β-value reported in the extraction system. From the intramolecular hydrogen bond of HTTA to the intermolecular hydrogen bond of DES, the bond energy increases by 47.8%. Because the active site of the proton in DES provides a higher energy barrier for the separation of 7Li, the is significantly increased. The extractions were characterized by spectrum, using 1H nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared (FT-IR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The mechanism was determined on the basis of the reaction kinetics and density functional theory (DFT). The DES extractant shows excellent cycle performance with regard to stripping and reusability. In conclusion, the highly efficient, economical, and stable β-diketone-driven DES extraction system can be used for the separation of naturally stable Li isotopes, which provides good industrial application prospects.
1. Introduction
Lithium (Li) has two naturally stable isotopes, 6Li and 7Li. The natural abundances of 6Li and 7Li are 7.41% and 92.59%, respectively. 6Li and 7Li play an extremely important role in the development of advanced nuclear energy; therefore, absolutely pure 6Li or 7Li resources need to be prepared at low cost for effective utilization [1]. As a strategic resource, 6Li can be used to produce tritium and resist the thermal neutron shielding of Li hydride [2]. At the same time, when a 7LiF-BeF2-ThF4 molten salt system is used as a fuel and coolant, the purity of 7Li needs to reach more than 99.99% to prevent the generation of tritium [3]. According to current energy development predictions, a fourth-generation molten salt nuclear reactor with a capacity of 1 GW needs 21 to 25 tons of high-purity 7Li [4]. In addition, 6Li and 7Li are the key materials of nuclear fusion reactors [5]. Thus, there is an extremely great demand for 6Li and 7Li. Therefore, the separation of 6Li and 7Li has attracted attention worldwide.
Li+ ions have no outermost electrons, and there is a difference of only one neutron between the radii of 6Li and 7Li. The Zemach radii of 6Li+ and 7Li+ are 2.44 fm and 3.416 fm, respectively, with a difference of 0.976 fm [6]. The Li-O bond lengths differ by 0.5 Å for 6Li and 7Li [7]. The separation of the naturally stable isotopes of Li is recognized as a difficult problem worldwide. Existing separation methods mainly include the lithium amalgam method [8], solvent extraction [9,10], and laser photoactivation [11]. The separation coefficient () in the lithium amalgam method, which is the only method to realize the industrial separation of 6Li and 7Li, is 1.056 [12]. Therefore, the separation coefficient of the lithium amalgam method is taken as the gold standard for industrialization. However, the lithium amalgam method has poor environmental friendliness, as it uses a large amount of heavy metal mercury, which has the potential to cause environmental safety hazards. In addition, among the separation systems involving solvent extraction, the crown ether extractant is the most representative, with a of 1.038~1.049 [13]. However, crown ether is expensive, and high-acid or precious pure-water resources are used in the stripping experiment [14]. Because the stripping experiment is not ideal, industrialization needs further optimization. Therefore, it is necessary to develop a green and low-cost system to separate 6Li and 7Li.
A deep eutectic solvent (DES) extractant is a potential separation extractant. In 2003, a DES extractant was first reported by Abbott et al., and DES can substitute for organic solvents and ionic liquids [15]. The DES extractant is a mixture of hydrogen bond donors and hydrogen bond acceptors in an appropriate proportion [16]. Due to the formation of intermolecular hydrogen bonds between these components, the melting point of such a system is lowered by mixing, resulting in the fluidization of DES extractants [17,18,19]. Therefore, the formation of DES extractants does not require a solvent [20].
According to the literature, DES extraction systems are mostly used in research focused on different metal ion separations [21,22,23], volatile organic acids separation [24,25], and gas separation [26,27,28] and are rarely used in isotope separation [29,30,31,32]. Therefore, the DES extraction system was introduced to achieve the widely desired separation of 6Li and 7Li.
In this paper, we developed the β-diketone-driven DES extractant system composed of 2-thenoyltrifluoroacetone (HTTA), which has a β-diketone structure, and tri-n-octyl phosphine oxide (TOPO). The was systematically studied, and the highest β-value was obtained. The extractions were characterized by their spectra using 1H nuclear magnetic resonance (NMR) spectroscopy, Fourier-transform infrared (FT-IR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The mechanism was determined on the basis of the reaction kinetics and density functional theory (DFT).
2. Experimental Section
2.1. Reagents and Chemicals
2-thenoyltrifluoroacetone (TTA, 99%) was purchased from Budweiser Technology, Beijing, China. Tri-n-octyl phosphine oxide (TOPO, 98%), lithium chloride (LiCl, 99.99%), lithium tetrafluoroborate (LiBF4, 99.99%), lithium sulfate (Li2SO4, 99.99%), and kerosene were purchased from the Aladdin Reagent Co., Ltd., Shanghai, China. Ammonium chloride (NH4Cl, ≥99.99%), ammonium fluoroborate (NH4BF4, ≥99.99%), and ammonium sulfate ((NH4)2SO4, ≥99.99%) were purchased from the Shanghai MacLean Biochemical Technology Co., Ltd., Shanghai, China. Concentrated hydrochloric acid (HCl, 12 M), concentrated nitric acid (HNO3, 16 M), concentrated sulfuric acid (H2SO4, 18 M), and concentrated ammonia (NH3·H2O, 9.96 M) were purchased from the Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. The other drugs used were of analytical grade.
2.2. Aqueous-Phase Composition and Lithium Testing
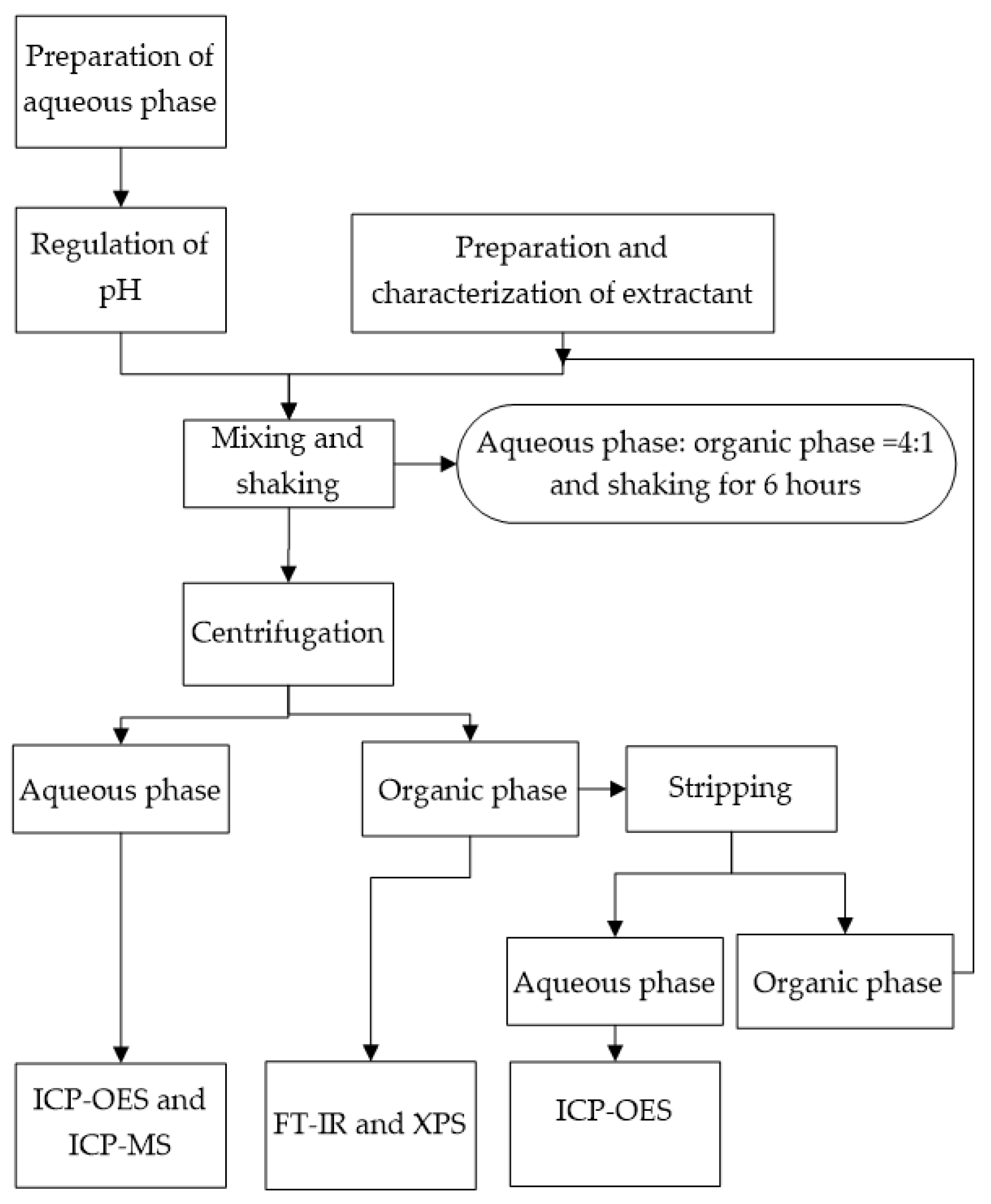
The composition of the aqueous phase was 25 ppm LiCl and 0.1 M NH4Cl. The strong acid and weak alkali salt was NH4Cl, and a conjugate acid–base pair formed in the solution [33,34]. Hydrochloric acid (HCl) was used as the strong acid. NH3·H2O was used as the weak base. The pH values of the aqueous phase were adjusted using 0.1 M HCl and 0.1 M NH3·H2O [35]. The pH value was measured by a pH meter (Five Easy Plus-FE20, Mettler Toledo, Zurich, Switzerland). The aqueous phases of LiBF4 and Li2SO4 regulate pH according to corresponding anionic acid and NH3·H2O. The initial concentration of Li+ in the aqueous phase was determined by ICP-OES (JY-Horiba, Agilent Ltd., Santa Clara, CA, USA) [36]. The experimental procedure is shown in Figure 1.
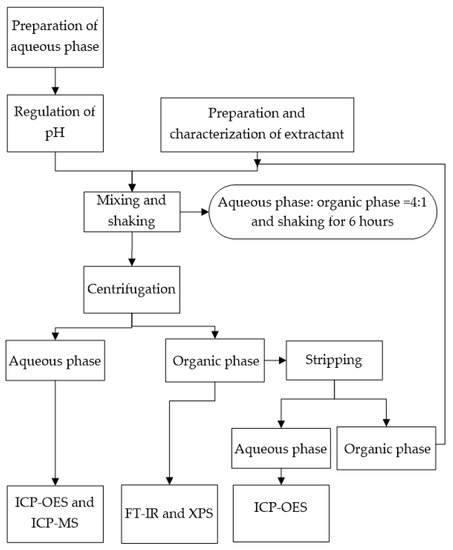
Figure 1.
Experimental procedure.
2.3. Preparation and Characterization of DES
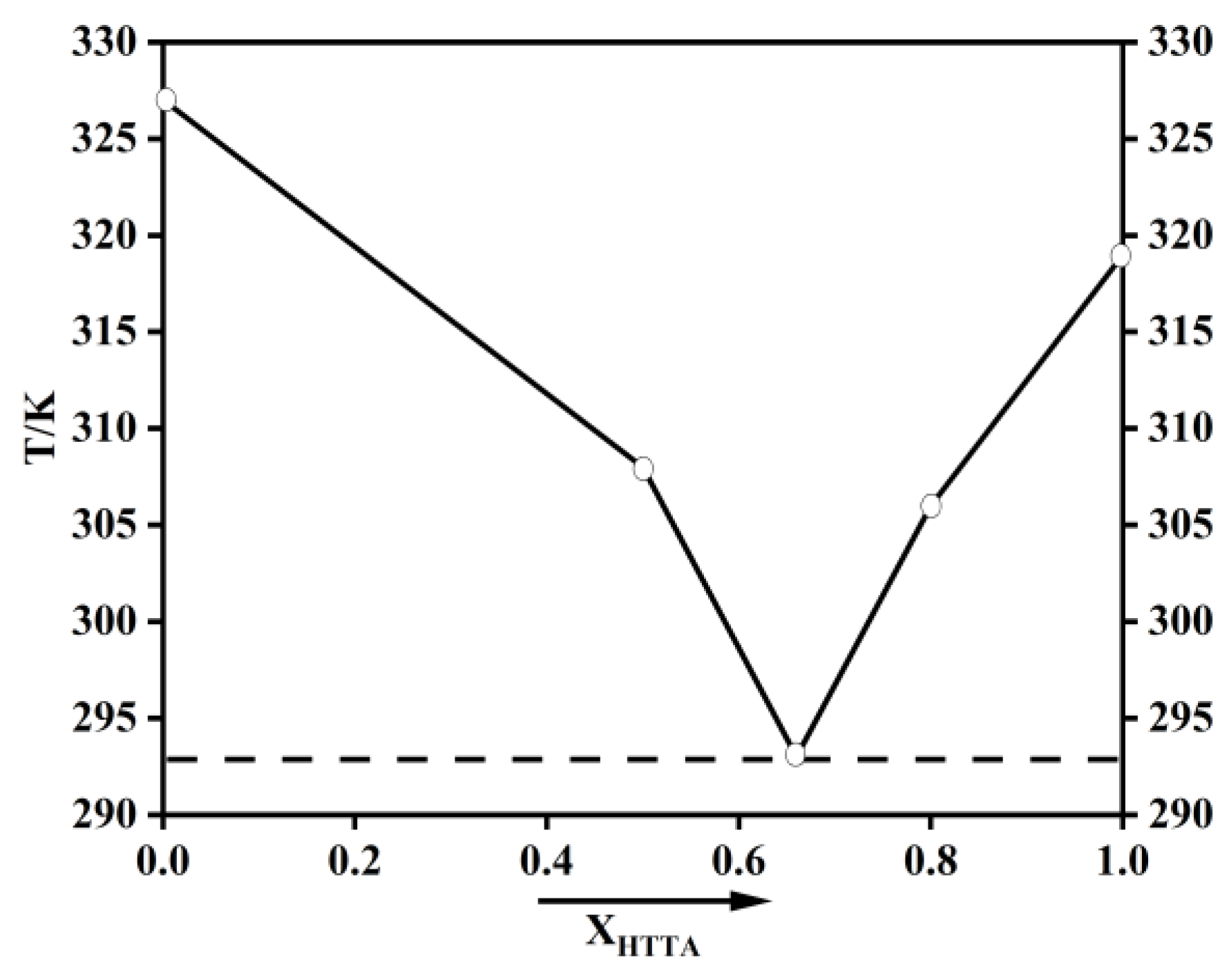
Because of ketone–enol and enol–enol isomerization, the β-diketone structure has the isomers shown in Figure S1. Because most fluoroalkyl-substituted diketones exist in the form of enol isomers, they are suitable for use as hydrogen bond donors [37,38,39]. TTA was selected in this paper. According to a previously reported article, the phosphate group is an excellent electron acceptor [40]. Therefore, TOPO was used in the current study as the hydrogen bond acceptor. After HTTA and TOPO were mixed in known molar ratios in a beaker with a magnetic stirrer, they were heated to 313 K to form the DES [23]. Heating for the dissolution process was achieved by a water bath. The prepared extractant is shown in Figure S2. Gaussian 09 (functional/basis set: b3lyp/6-311G**) was used for the density functional theory (DFT) calculations on extractants. The extractions were characterized by spectrum, using 1H nuclear magnetic resonance spectroscopy (NMR) (Bruker AVANCE III 500 MHz, Fllanden, Switzerland), Fourier-transform infrared spectroscopy (FT-IR) (ThermoNicoletis50, Thermo Fisher Scientific, Waltham, MA, USA), and X-ray photoelectron spectroscopy (XPS) (Scientific K-Alpha+, Thermo Fisher Scientific, Waltham, MA, USA). A rotary viscometer (NDJ-8S, Shanghai Changji Geological Instrument Co., Ltd., Shanghai, China) was used to test viscosity. The phase diagram of HTTA and TOPO are shown in Figure 2. The density and viscosity of HTTA, TOPO, and DES (molar ratio: HTTA/TOPO = 2/1) are shown in Table 1.
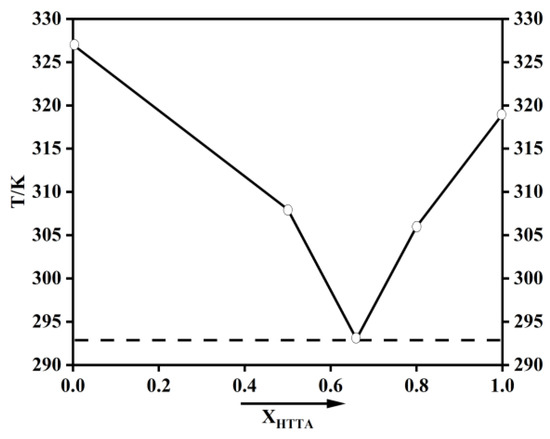
Figure 2.
Phase diagram of HTTA and TOPO.

Table 1.
Density and viscosity, DES (molar ratio: HTTA/TOPO = 2/1).
2.4. Solvent Extraction Experiment
In the solvent extraction experiments, the pH of the aqueous (aq) phase was adjusted, as in Section 2.3. The prepared aqueous phase was mixed with the organic (org) phase in a volume ratio of 4:1 (aqueous phase: organic phase = 4:1) and shaken in a full-temperature oscillation incubator (ZQPW-70, Tianjin Leibo Terry Equipment Co., Ltd., Tianjin, China) at 313 K for 6 h. In order to ensure uniform mixing, the rotating speed was 250 rpm. After shaking, the solution was passed through a centrifuge (CTK80, Xiangyi Centrifuge Co., Ltd., Hunan, China) with a set speed of 3000 rpm and a time of 3 min and taken out of the aqueous phase. Through the ICP-OES (JY-Horiba, Agilent Ltd., Santa Clara, CA, USA) test, the Li+ concentration in the aqueous phase was determined; the method detection limit of ICP-OES is 1 ppm. The concentrations of 7Li and 6Li in the aqueous phase were measured using an ICP-MS 8800 (JY-Horiba, Agilent Ltd., Santa Clara, CA, USA); the method detection limit of ICP-MS 8800 is 20 ppb. The isotope standard solution was 7Li/6Li = 12.17523.
The extraction percentage (E%), distribution ratio () and separation coefficient () were used to determine the separation effect of 7Li and 6Li, and were calculated using the following equations.
In Equations (1) and (2), is the extraction initial concentration of Li+ in the aqueous phase, is the extraction equilibrium concentration of Li+ in the aqueous phase, is the volume of the aqueous phase and is the volume of the organic phase. In Equation (3), () is the extraction initial concentration of 6Li+ (7Li+) in the aqueous phase and () is the extraction equilibrium concentration of 6Li+ (7Li+) in the aqueous phase. The concentration determination of all the solutions was repeated at least 3 times, and the error was within the allowable range of the experiment.
2.5. Stripping Experiment
The extracted organic phase was selected as the stripping phase in the stripping experiment, and HCl and HNO3 at concentrations of 0.1 to 1 M were selected as the stripping agents. An ultrapure water machine (Direct-q3uv, Xiamen Jingyi Xingye Technology Co., Ltd., Xiamen, China) was used to obtain ultrapure water. Ultrapure water was used for the stripping experiment. The volume ratio of the stripping agent to the stripping phase was 4:1 (aqueous phase: organic phase = 4:1). The stripping percentage (S%) was calculated using the following equation.
In Equation (4), is the stripping equilibrium concentration of Li+ in the aqueous phase, is the extraction initial concentration of Li+ in the aqueous phase, and is the extraction equilibrium concentration of Li+ in the aqueous phase. The concentration determination of all the solutions was repeated at least 3 times, and the error was within the allowable range of the experiment.
3. Results and Discussion
3.1. Characterization of the DES Extractant
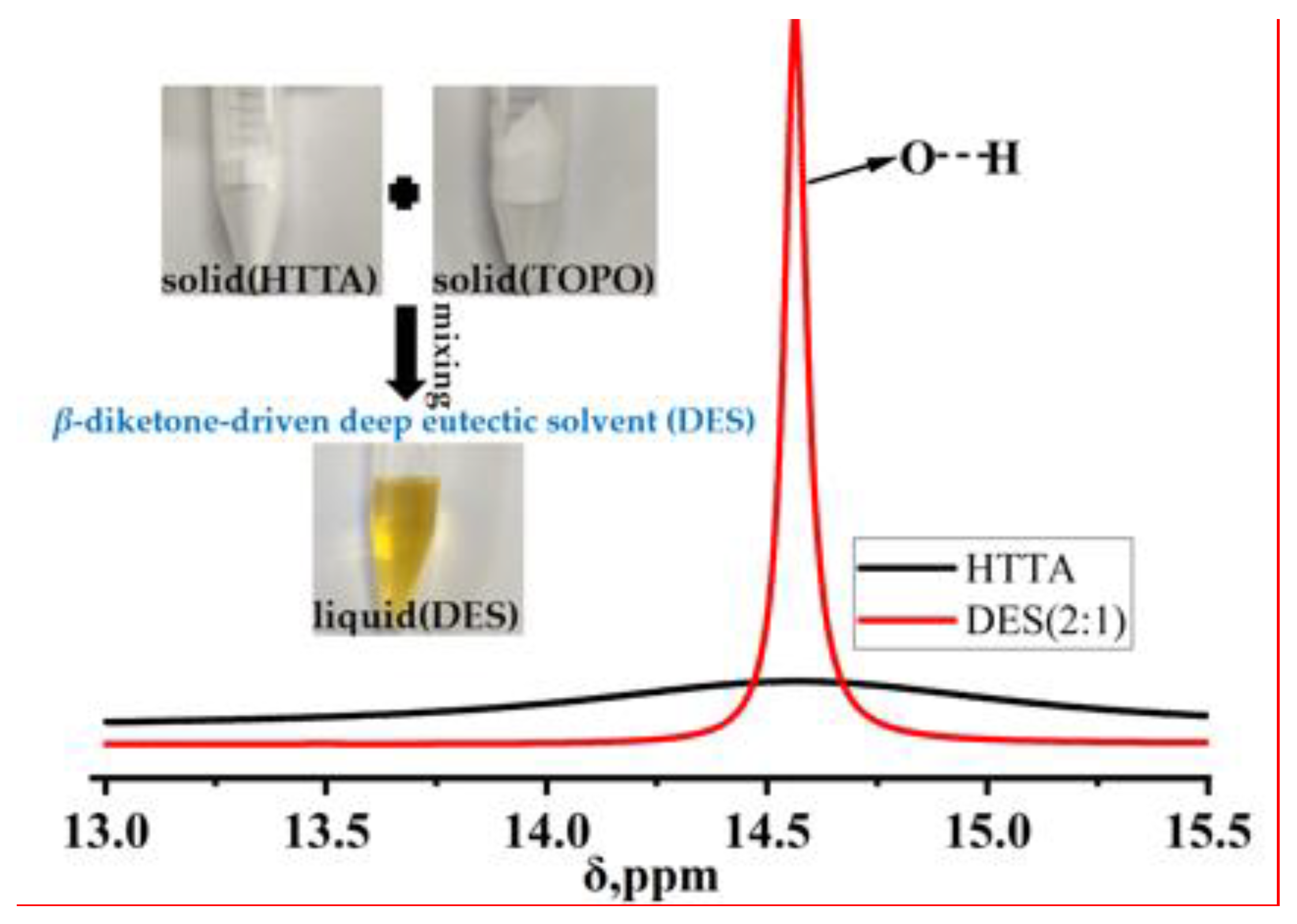
To explore the difference between the DES and HTTA extractants, 1H NMR spectroscopy was performed. The σ value is the chemical shift of the extractant. As shown in Figure 3, a sharp peak occurred in the range of σ values from 14.4 ppm to 14.6 ppm after the formation of the DES containing HTTA and TOPO [37]. Due to the formation of intermolecular hydrogen bonds between HTTA and TOPO in the DES extractant, a sharp peak occurred. Through the above comparison between HTTA and DES (2:1), we can predict the formation of intermolecular hydrogen bonds in DES (2:1). In the case of the formation of intermolecular hydrogen bonds, the DES (2:1) extractant may effectively separate 6Li and 7Li.
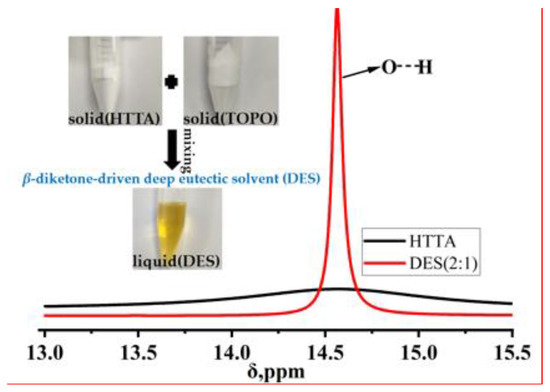
Figure 3.
1H NMR spectra of the HTTA extractant and DES extractant (molar ratio: HTTA/TOPO = 2/1).
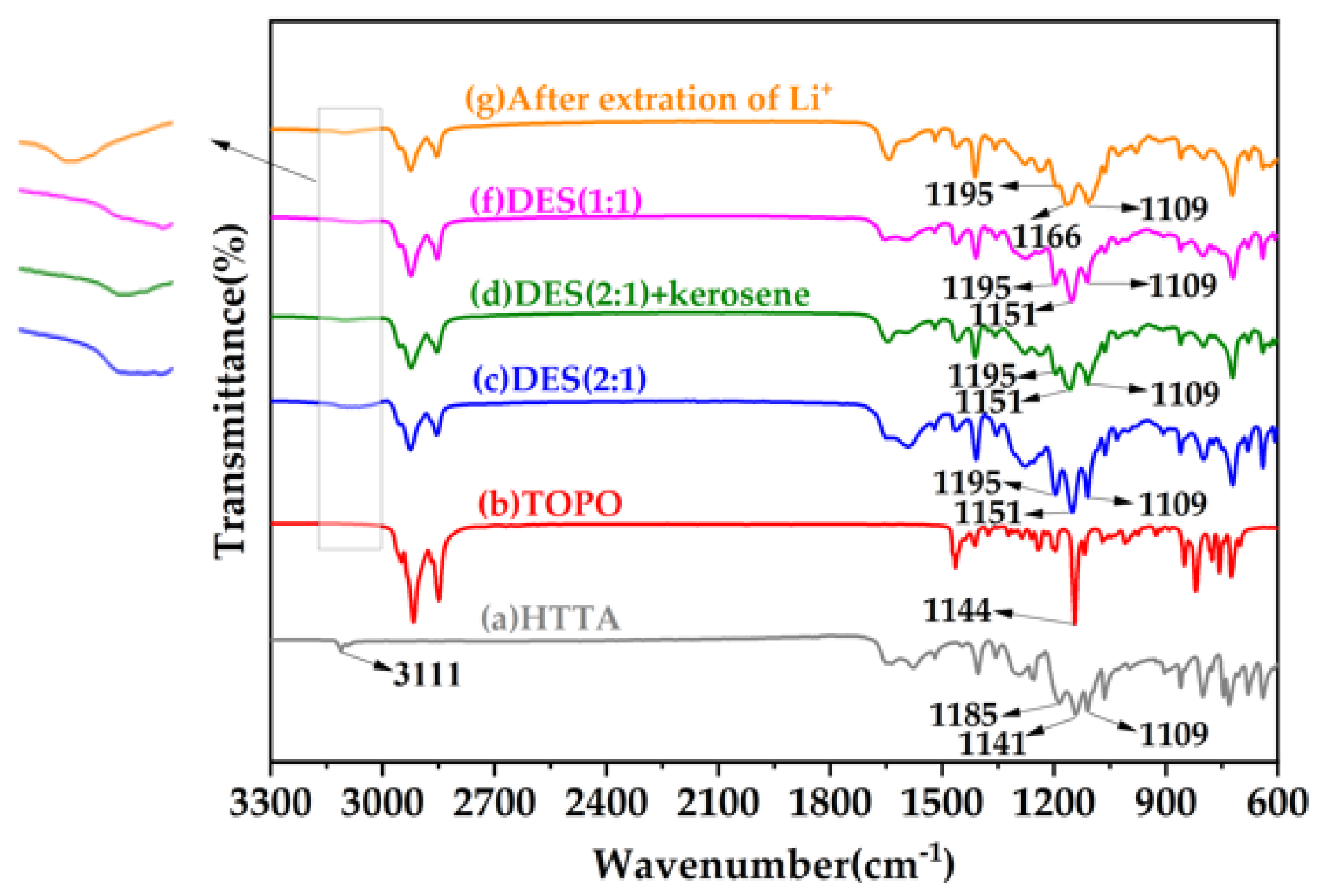
To evaluate the characteristics of the extractants, Figure 4 shows the FT-IR spectra of the HTTA, TOPO, DES (2:1), DES (2:1) + kerosene, DES (1:1) extractants, and after the extraction of Li+, respectively. In the FT-IR spectrum in Figure 4a, the main FT-IR bands of HTTA were observed at 3111, 1185, 1141, and 1109 cm−1 and were indexed to the stretching vibrations of intramolecular hydrogen bonds (ν O┄H), oxygen-hydrogen bonds (ν O-H), carbon-oxygen bonds (ν C-O) and carbon–fluorine bonds (ν C-F), respectively [41,42,43]. As shown in Figure 4b, the main FT-IR band of TOPO was observed at 1144 cm−1 and was indexed to the stretching vibrations of phosphorus–oxygen bonds (ν P=O) [44]. As seen in Figure 4c–g compared with Figure 4a, the C-F bonds did not change significantly. As seen by comparing Figure 4a,c, DES (2:1) formed intermolecular hydrogen bonds, and the intramolecular hydrogen bonds were weakened. The peaks of the O-H bond and C-O bond observed at 1195 cm−1 and 1151 cm−1, respectively, correspond to a blue shift of 10 cm−1. As seen by comparing Figure 4c,d, when an equal volume of kerosene was added to DES (2:1), the intermolecular hydrogen bonds were broken, the O-H bond (giving rise to a peak at 1195 cm−1) was weakened, and the C-O bond did not change significantly. As seen by comparing Figure 4c,f, the intermolecular hydrogen bond (O┄H) formed by DES (1:1) was incomplete, and the intramolecular hydrogen bond was weakened. The O-H and C-O bonds did not change significantly. As seen by comparing Figure 4c,g, after the extraction of Li+, the O-Li bond, which gives rise to the peak at 1195 cm−1, weakened, and the peak at 1166 cm−1, attributed to the C-O bond, blue-shifted by 15 cm−1. Intramolecular hydrogen bonds were replaced by weaker intramolecular coordination bonds (O→Li).
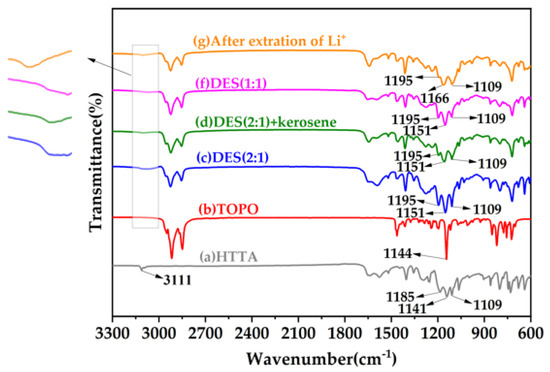
Figure 4.
FT-IR spectra of different extractants. The subfigure is an enlarged infrared spectrum of 3150~3000 cm−1.
Taken together with the fact that changes in the bonds of the DES (2:1) were observed compared to the other components, these results allow us to conclude that a unique intermolecular hydrogen bond structure is present in DES. It is also obvious that the fluidization of DES is related to intermolecular hydrogen bonds.
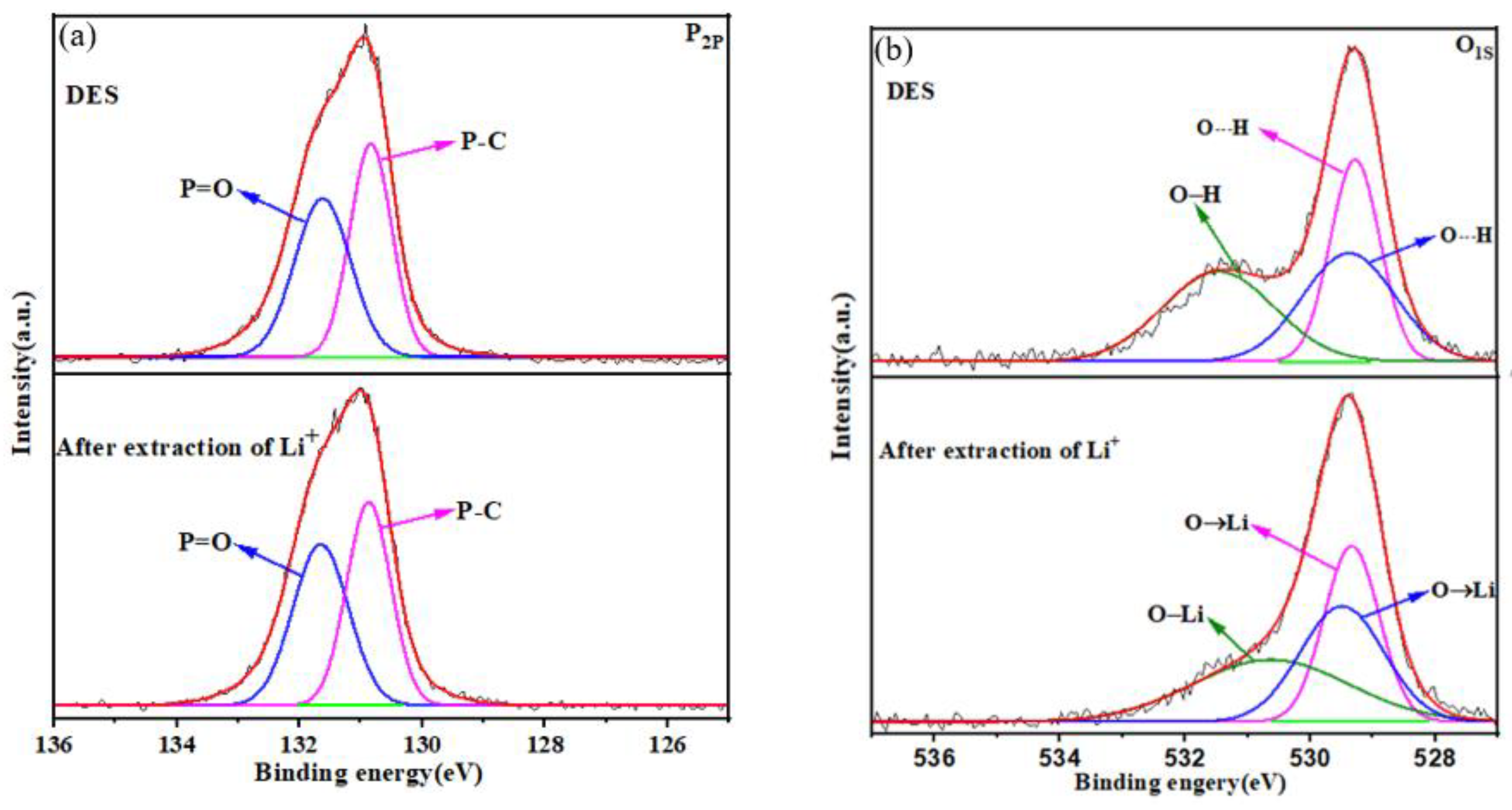
To further understand the exchange of Li+ ions with DES, XPS spectra of the DES extractant were used to evaluate the exchange of ions. Compared with that in TOPO, the 2p orbital of P (P2p) in DES has a higher binding energy due to the formation of intermolecular hydrogen bonds in DES [45]. In Figure 5a, the XPS spectra before and after the extraction of P show that the P2p orbital does not change. In the extraction system, HTTA played a major role in the extraction of Li+ ions. In Figure 5b, the XPS spectra before and after the extraction of O show that the O-H bond was converted to an O-Li bond, and its binding energy decreased by 0.82 eV. This change was consistent with the theory that a decrease in electron binding energy indicates an increase in electron cloud density [46]. O┄H bonds were converted to O→Li bonds due to the replacement of H+ by Li+. In conclusion, Li+ ions exchange with the proton hydrogen ions of the HTTA of DES.
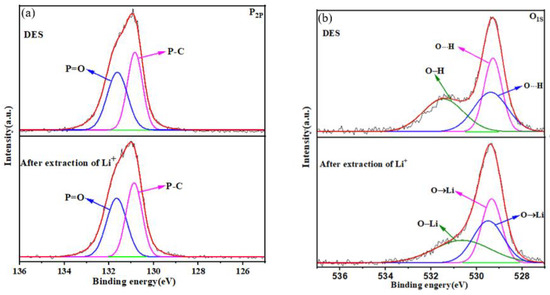
Figure 5.
(a) P2p XPS spectra of the DES extractant (molar ratio: HTTA/TOPO = 2/1). (b) O1s XPS spectra of the DES extractant.
3.2. The Separation of 6Li and 7Li by DES Extractant
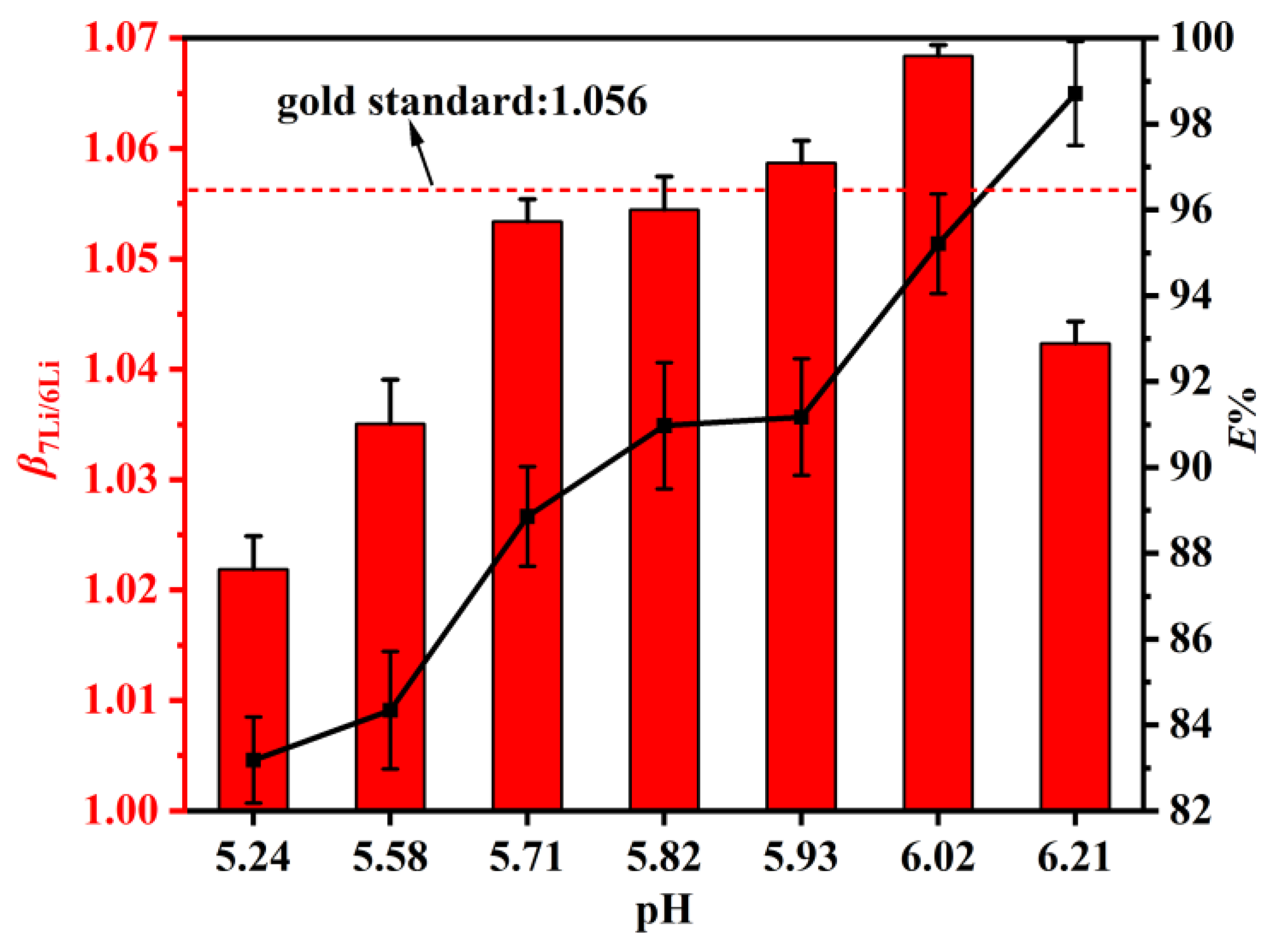
The aqueous phase acidity was an important factor for many extraction systems and could not be ignored. Therefore, it was of interest to study the effect of the pH value of the aqueous phase on the extraction of 6Li and 7Li using DES. Figure 6 shows that and E% increased significantly when the equilibrium pH increased. A high and a high E% are desirable. Therefore, the point of equilibrium pH = 6.02 was chosen, at which reached a maximum value of 1.068, and E% reached 95%. The reason for this phenomenon may be that after the DES extractant extracts Li+, the hydrogen ions that participate in intermolecular hydrogen bonding with the DES extractant enter the aqueous phase, providing extractable binding sites and improving the extraction percentage. We can conclude that the E% increases with the increased equilibrium pH and the reaches the maximum value of 1.068 at pH = 6.02.
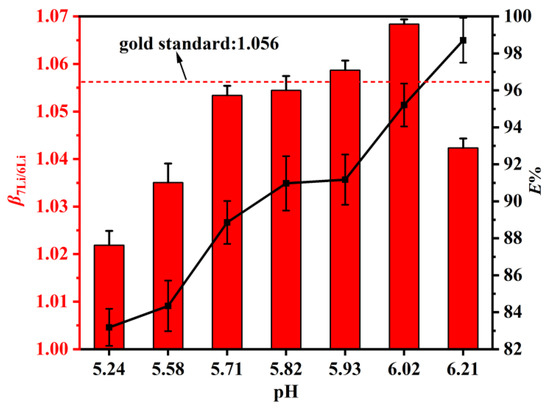
Figure 6.
The DES extractant (molar ratio: HTTA/TOPO = 2/1) was used. The composition of the aqueous phase was 25 ppm LiCl and 0.1 M NH4Cl. Aqueous phase: organic phase = 4:1.
3.3. Explanation of the Mechanism
3.3.1. DES and DES Solvation
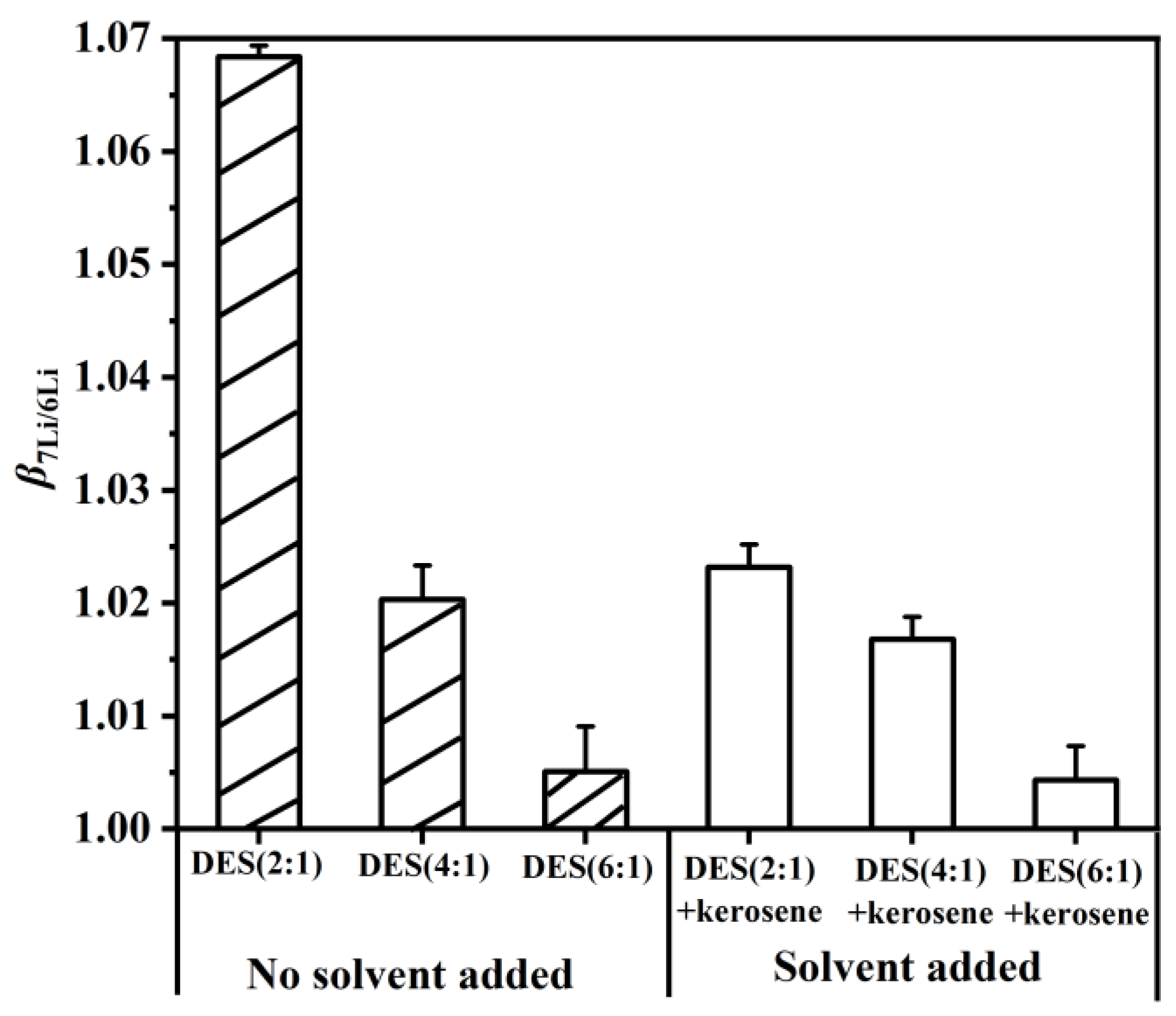
In order to explore the effect of DES without added solvent and with added solvent on the , extractants were prepared, containing DES (2:1), DES (4:1), DES (6:1), DES (2:1) + kerosene, DES (4:1) + kerosene and DES (6:1) + kerosene. As shown in Figure 7, the of the DES extractant with a molar ratio of HTTA/TOPO = 2/1 was higher than those of the DES extractants with molar ratios of 4:1 and 6:1. This finding was attributed to the stabilization of intermolecular hydrogen bonds in the structure of the DES extractant with a molar ratio of HTTA/TOPO = 2/1, which stabilizes the high value. When an equal volume of kerosene was added to the DES extractants, their structures were destroyed, resulting in a decrease in . This means that the more serious the cleavage of the intermolecular hydrogen bonds is, the lower the . On the basis of the obtained results, the of DES without added solvent is better than that with added solvent. Among them, the of DES (2:1) is the highest.
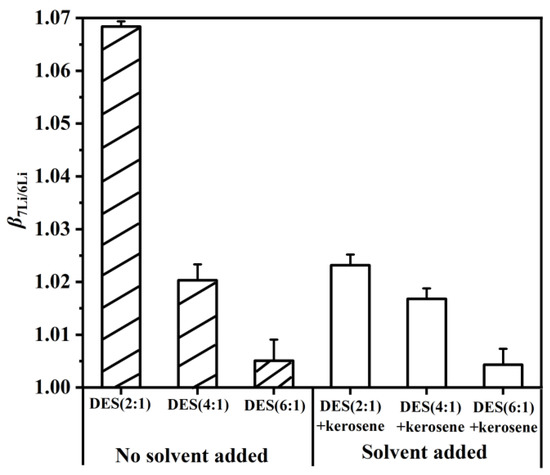
Figure 7.
The molar ratio of the extractant was HTTA: TOPO = 2:1 (4:1 or 6:1). The solvent was added in equal volume to kerosene to form an extractant to separate 6Li and 7Li. The composition of the aqueous phase was 25 ppm LiCl and 0.1 M NH4Cl. Aqueous phase: organic phase = 4:1.
3.3.2. Different Kinds of Aqueous-Phase Anions
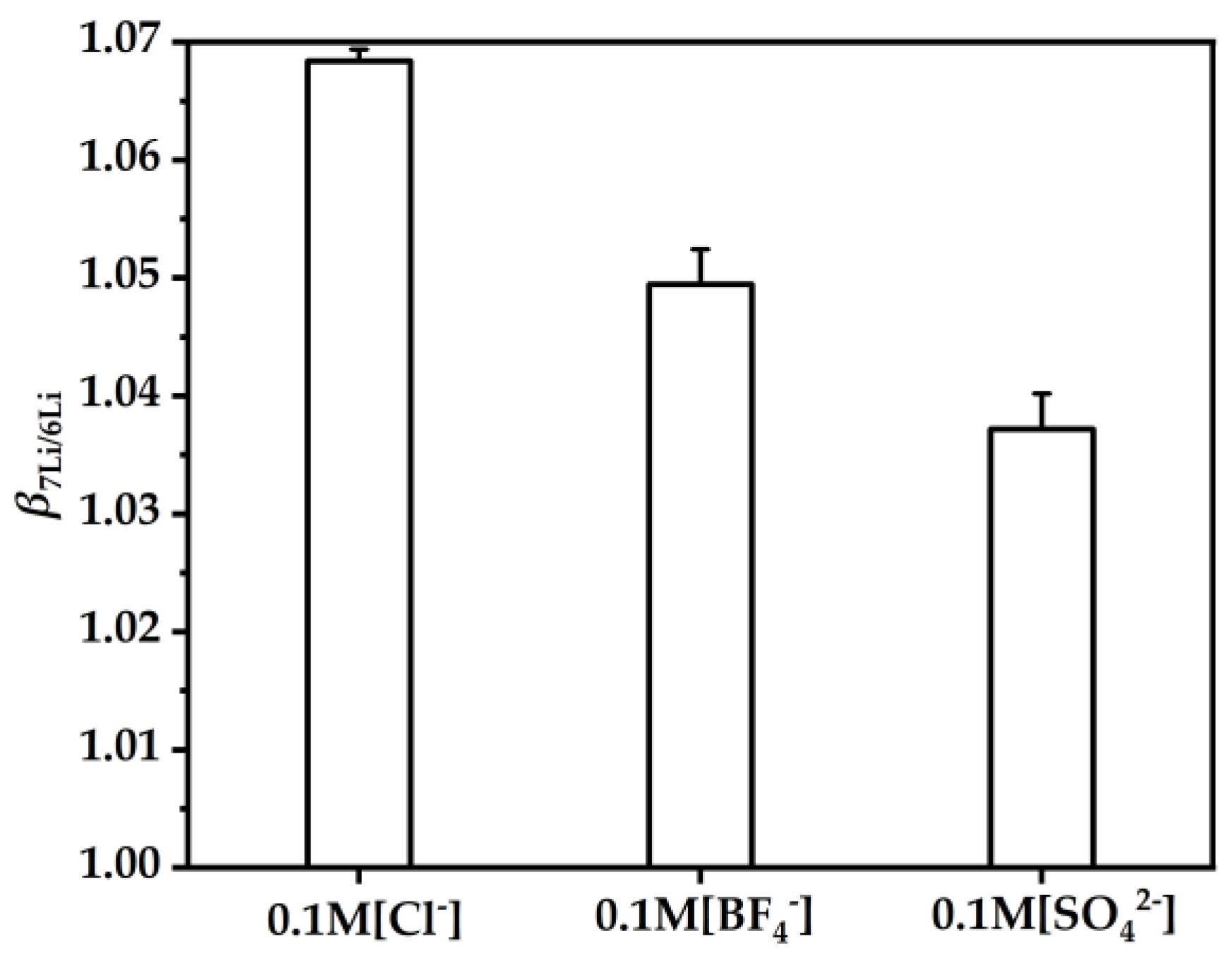
To investigate the effect of the anions in the aqueous phase on the , 0.1 M Cl−, BF4− and SO42− in the aqueous phase were prepared. The synergistic coupling extraction of β-diketone HTTA and neutral TOPO anions in the aqueous phase are related to extraction [47]. As shown in Figure 8, when the anion radius increased (i.e., in the order Cl− < BF4− < SO42−) [48], this was unfavorable for the enrichment of 7Li but favorable for the enrichment of 6Li [49]. Therefore, to enrich 7Li, an anion with a small ionic radius should be selected if possible so that the change in favors the enrichment of 7Li.
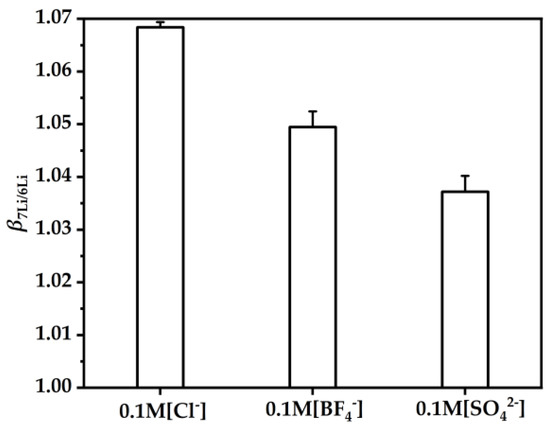
Figure 8.
The DES extractant (molar ratio: HTTA/TOPO = 2/1) was used. The initial concentrations of Cl−, BF4− and SO42− were 0.1 M. The initial concentration of Li+ was 25 ppm. Aqueous phase: organic phase = 4:1.
3.3.3. Reaction Kinetics
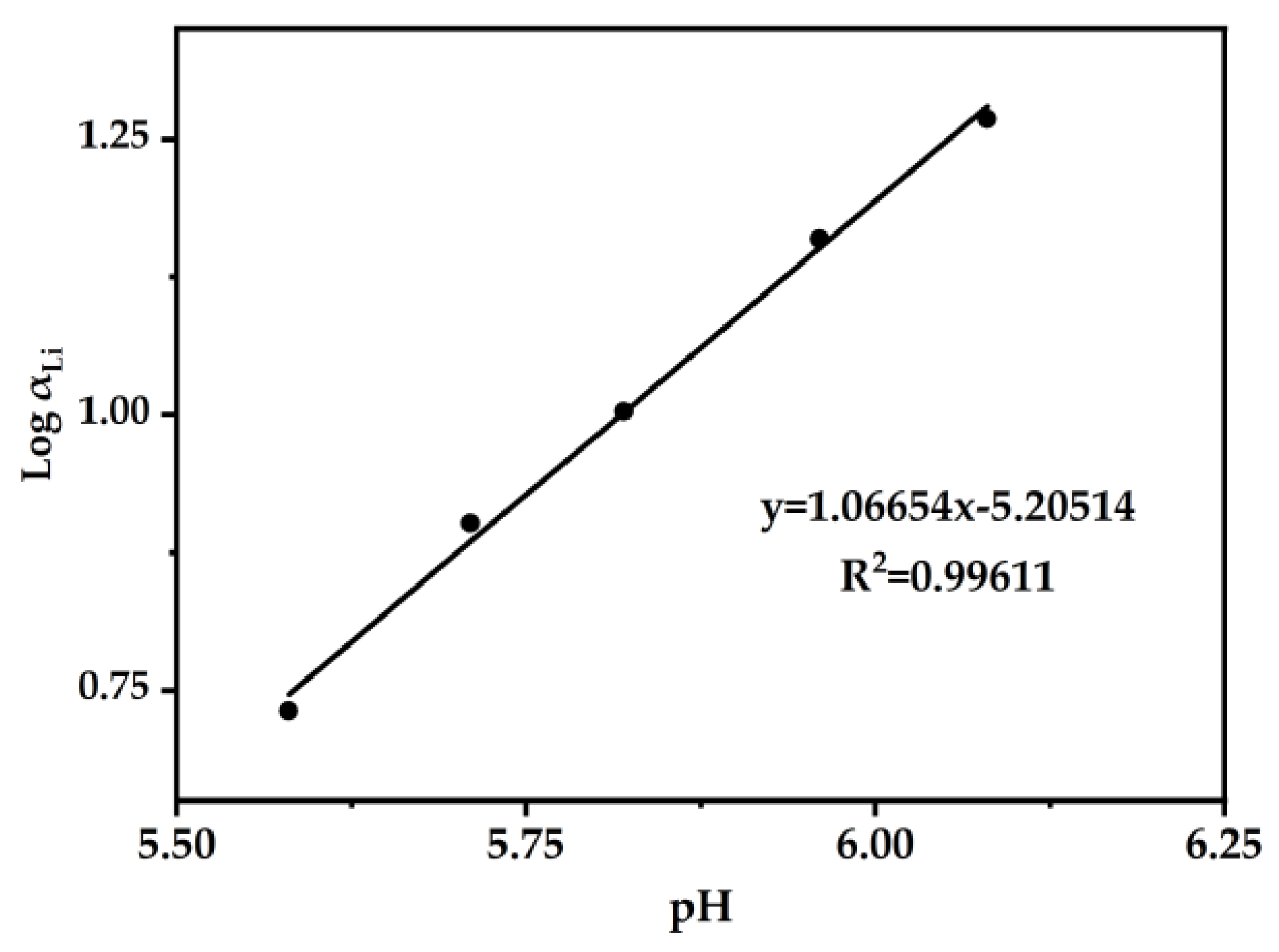
In order to further demonstrate the exchange–quantity relationship between Li+ and H+ in DES (2:1), Figure 9 is presented. Log αLi was plotted versus the equilibrium pH. Through slope analysis and linear fitting, the slope k was determined to be 1.06654. Therefore, the exchange of Li+ ions with H+ ions was 1:1.
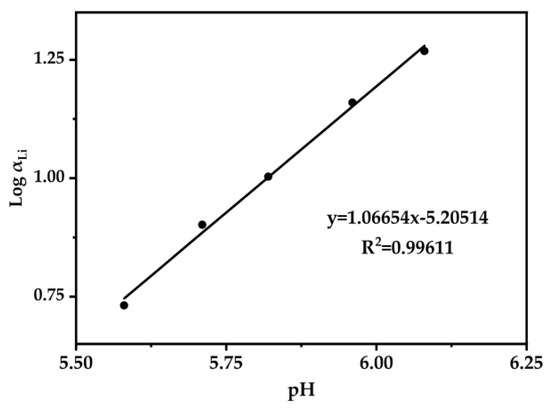
Figure 9.
Linear graphs of Log αLi versus pH were plotted. The DES extractant (molar ratio: HTTA/TOPO = 2/1) was used, and the initial concentration of Li+ was 25 ppm. Aqueous phase: organic phase = 4:1.
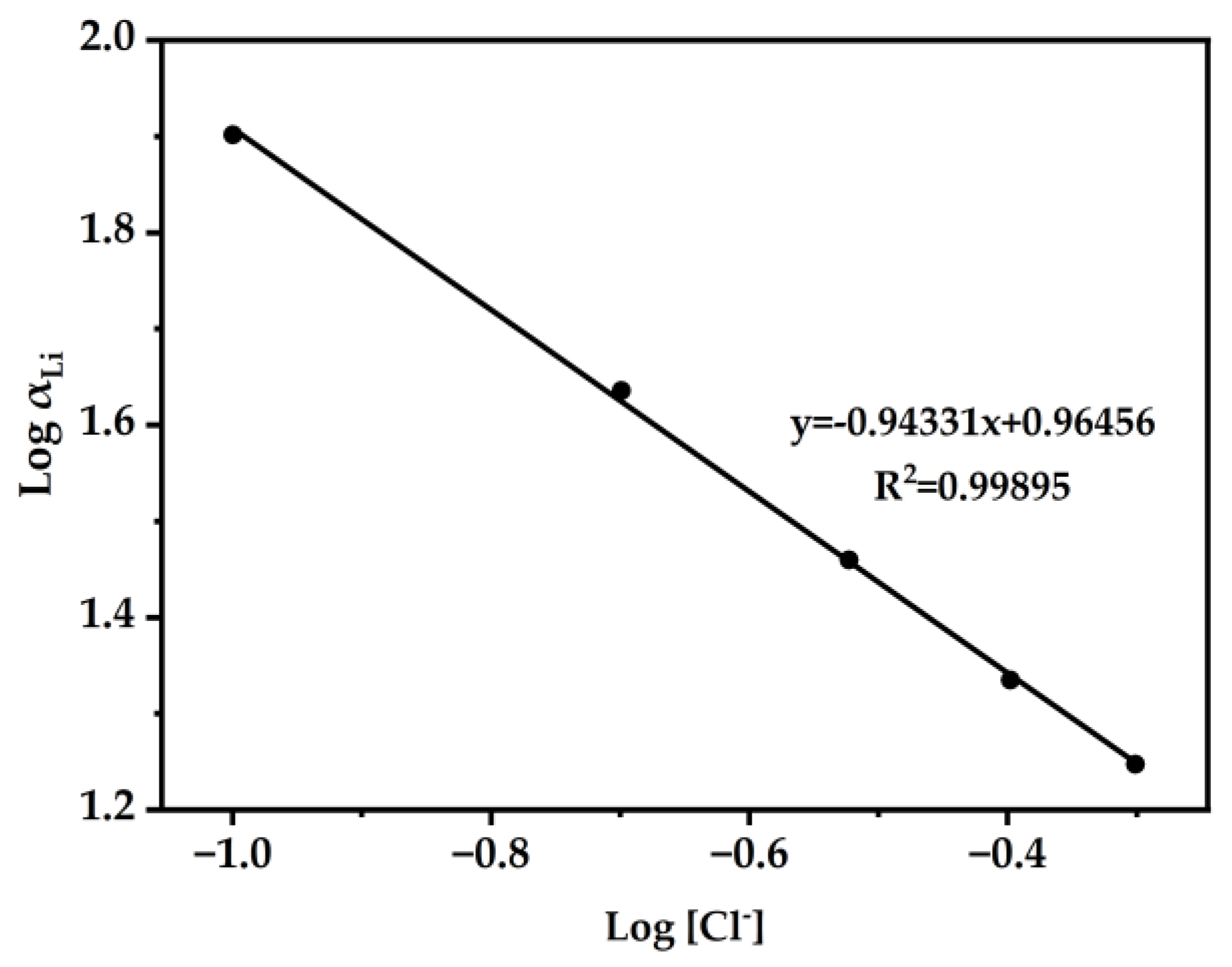
After finding the results in Figure 8, we then explored the influence of Cl− ions on the extraction system [30,50]. As shown in Figure 10, Log αLi L was plotted versus the equilibrium Log [Cl−]. Through slope analysis and linear fitting, the slope k was determined to be −0.94331. According to the experimental results and Hofmeister bias theory [51], the concentration of Cl− in the feeding aqueous phase directly impacts the extraction. A study of the influence of Cl− ions on the extraction system of Li+ may explain the presence of Cl− ions in the extracted species of Li+.
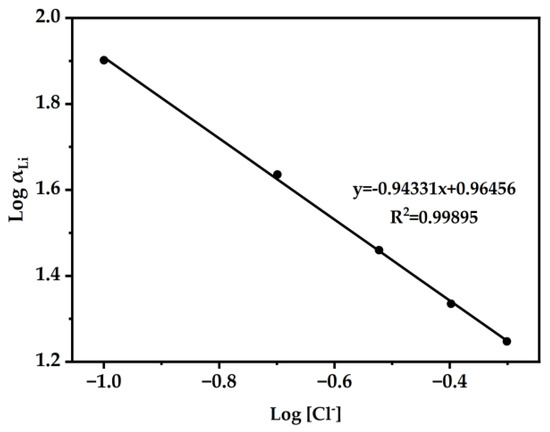
Figure 10.
The DES extractant (molar ratio: HTTA/TOPO = 2/1) was used. The composition of the aqueous phase was 25 ppm LiCl and the concentration of NH4Cl ranged from 0.1 to 0.5 M. Aqueous phase: organic phase = 4:1.
3.3.4. DFT Calculations
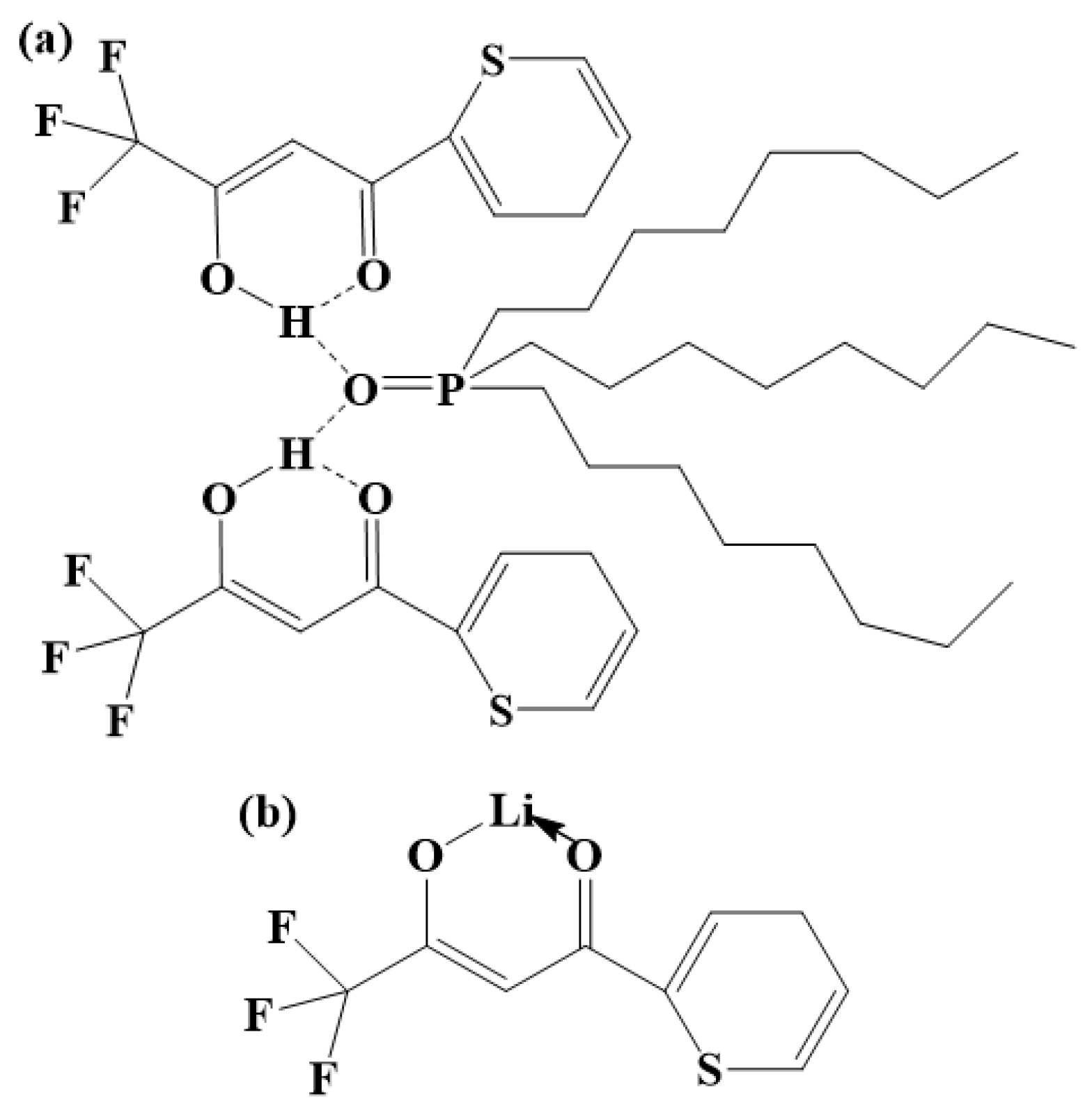
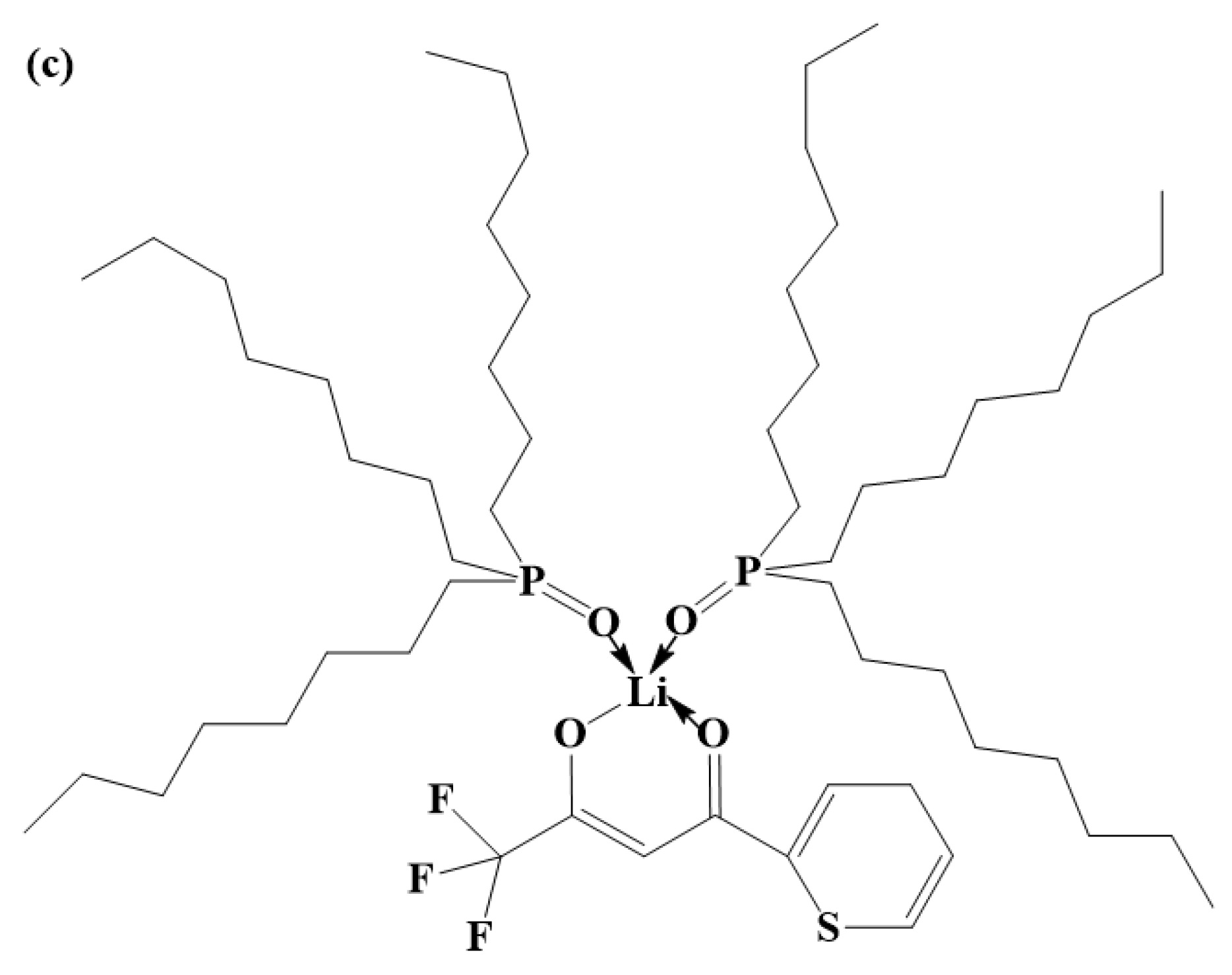
A structural diagram of the DES extractant is shown in Figure 11a. In the DES extractant, HTTA and TOPO form a unique structure of intermolecular hydrogen bonds. We used Gaussian 09 software to calculate the bond dissociation energy of the HTTA extractant, TOPO extractant, and DES extractant. The calculated data are shown in Table 2.
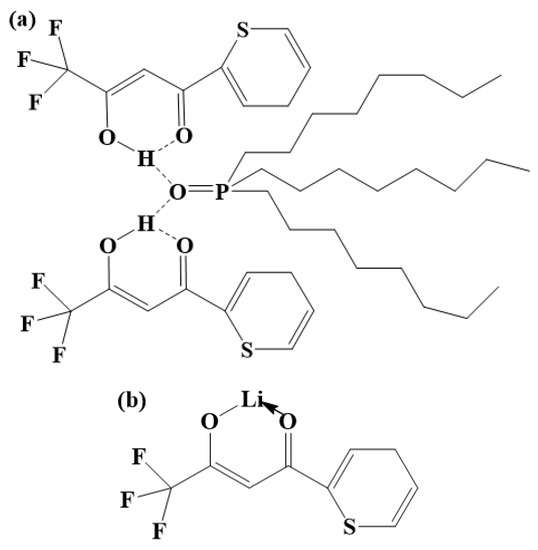
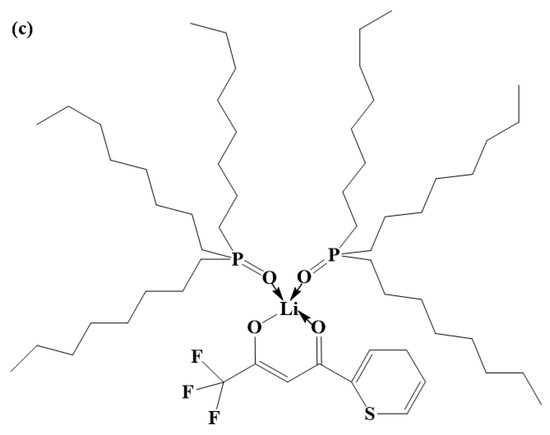
Figure 11.
(a) Structural diagram of the DES extractant. (b) Structural diagram of Li+ ions extracted by the HTTA extractant. (c) Structural diagram of Li+ ions extracted by the DES extractant.

Table 2.
Bond dissociation energy of material. HTTA (1) means no intramolecular hydrogen bond is formed, and HTTA (2) means intramolecular hydrogen bonds are formed.
The energy of the bond was calculated using the following equation.
In Equations (5) and (6), is the energy of the intermolecular hydrogen bond between HTTA and TOPO, is the energy of the intramolecular hydrogen bond of HTTA, is the bond dissociation energy of HTTA (2), is the bond dissociation energy of TOPO, is the bond dissociation energy of DES, and is the bond dissociation energy of HTTA(1). The units of E1 and E2 are kcal/mol.
The energy of the intermolecular hydrogen bond between HTTA and TOPO is 18.4929 kcal/mol, and the energy of the intramolecular hydrogen bond of HTTA is 12.5150 kcal/mol. From the intramolecular hydrogen bond of HTTA to the intermolecular hydrogen bond of DES, the bond energy increases by 47.8%. This indicates that the unique intermolecular hydrogen bond structure is present in the DES extractant. Because the formation of this intermolecular hydrogen bond has a higher energy barrier, the DES extractant can effectively separate 6Li and 7Li. As shown in Figure 11b, Li+ forms a bond with HTTA [52]. As shown in Figure S3, HTTA plays a leading role in extraction. On the basis of this result, we conclude that the site of Li+ in the DES extractant corresponds to the site of the proton hydrogen in the HTTA of DES. At the same time, the force between Li+ and TOPO forms an intermolecular bond. After the extraction of the Li+ ions, the DES extractant retained its structure and remained in the organic phase (liquid). The resulting structure is shown in Figure 11c [53].
3.4. Stripping of Li+ from the Metal-Loaded DES
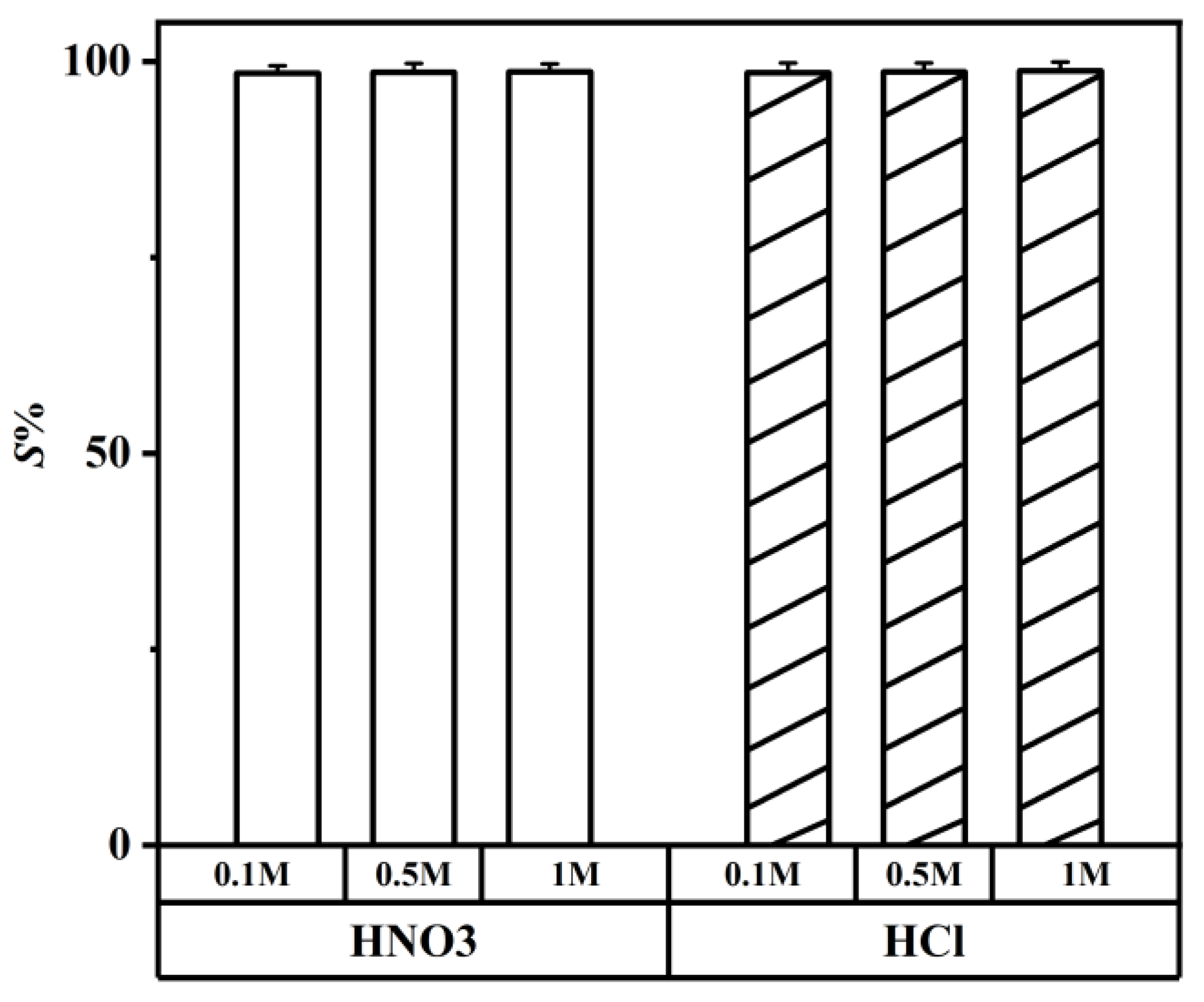
It is essential to carry out the stripping of lithium ions into an aqueous phase and a recovering organic phase after the extraction and separation of lithium isotopes. The acidity required for the stripping experiment of a crown ether is 5 M [54]. However, the concentration of HCl ranged from 0.1 to 1 M in the stripping experiment, and the concentration of HNO3 were the same. Compared with the crown ether extractant, the DES extractant required lower acidity for the stripping experiment. As shown in Figure 12, when the acid concentration reached 0.1 M, the S% of HCl reached a high value; HNO3 followed the same trend. Upon comparing with HNO3, 0.1 M HCl was selected because the S% of 0.1 M HCl was higher.
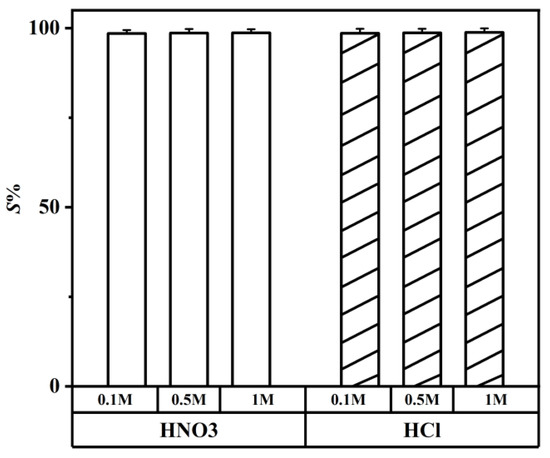
Figure 12.
Different concentrations of HNO3 and HCl for stripping Li+ from the metal-loaded DES extractant (molar ratio: HTTA/TOPO = 2/1). The initial concentration of Li+ was 25 ppm. Aqueous phase: organic phase = 4:1.
3.5. Reusability of the DES Extractant
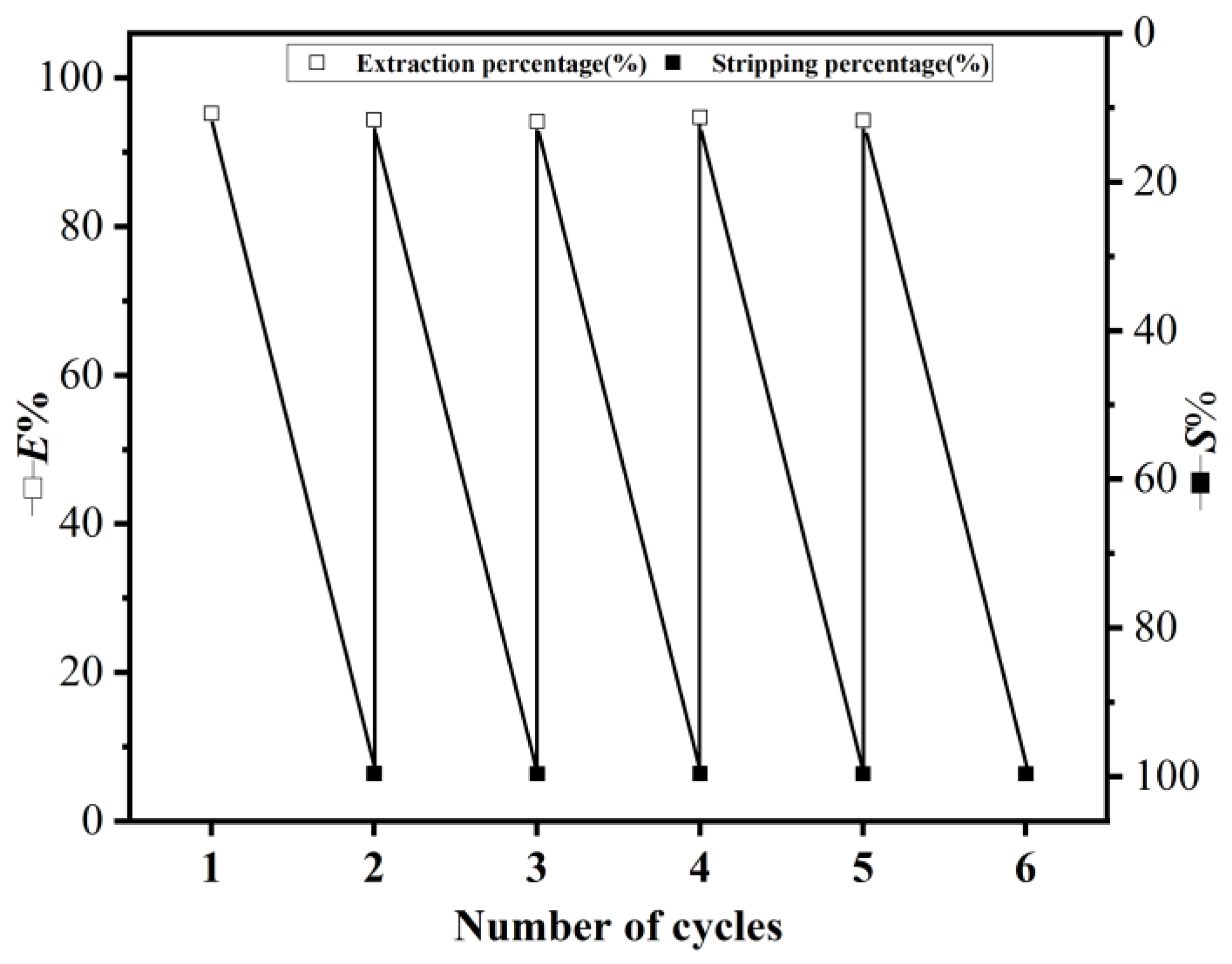
The extractant plays the most important role in extraction, and the reusability of the extractant is particularly critical. As shown in Figure 13, the DES extractant can be reused at least five times [55]. The E% can reach more than 93%. In addition, only a -2.0% loss in E% was observed after five cycles, which ensures the reusability of the extractant. Therefore, DES (2:1) is highly stable and can be used for the long-term separation of lithium natural stable isotopes.
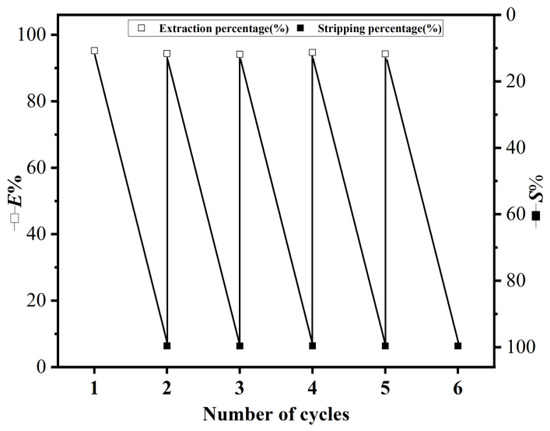
Figure 13.
The reusability of the DES extractant (molar ratio: HTTA/TOPO = 2/1) was explored by determining E% and S%. The initial concentration of Li+ was 25 ppm (volume ratio: aqueous phase: organic phase = 4:1). Stripping step: 0.1 M HCl was used as the stripping agent with aqueous phase: organic phase = 4:1.
4. Conclusions
In this research, an advanced β-diketone-driven DES extraction system was introduced to achieve the widely desired separation of 6Li and 7Li. The point of equilibrium pH = 6.02 was chosen, at which reached a maximum value of 1.068, and the extraction percentage (E%) reached 95%. Compared with the benzo-15-crown-5 extraction system, which can reach the best β-value of 1.049, the β-diketone-driven DES extraction system has great potential to replace expensive benzo-15-crown-5 extraction systems. The DES extraction system has now reported the highest known β-value. The DES extractant can be reused at least five times. Only a -2.0% loss in extraction percentage (E%) was observed after five cycles.
Additionally, from the intramolecular hydrogen bond of HTTA to the intermolecular hydrogen bond of DES, the bond energy increases by 47.8%. A higher energy barrier is the reason why the separation performance is improved. We will deeply study the reaction mechanism and develop a DES-derivative extractant to reduce costs. Furthemore, we will continue to refine the DES extraction system and can make it a closer fit to practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10020111/s1, Figure S1. Structural changes of TTA; Figure S2. Three DES extractants with different ratios were synthesized and three extractants were added to kerosene with the same volume as the extractant; Figure S3. The DES extractant (molar ratio: HTTA/TOPO=2/1) was used. The composition of the aqueous phase was 25ppm LiCl and 0.1M NH4Cl.The dosage of single HTTA or TOPO is consistent with that of DES extractant.
Author Contributions
Conceptualization, Z.X and M.X.; methodology, Z.X. and T.T.; software, Z.X. and L.X.; validation, Z.X., M.X. and T.T.; formal analysis, Z.X.; investigation, Z.X.; resources, F.Y.; data curation, Z.X.; writing—original draft preparation, Z.X.; writing—review and editing, Z.X. and M.X.; visualization, Z.X.; supervision, Z.J.; project administration, F.Y.; funding acquisition, F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2022YFB3504302; the Fund of Science and Technology on Reactor Fuel and Materials Laboratory, grant number STRFM-2021-07; the independent deployment project of the Gan Jiang Innovation Research Institute of the Chinese Academy of Sciences, grant number E055A002.
Data Availability Statement
The data presented in this study are available in the manuscript and Supplementary Materials.
Acknowledgments
The authors would like to thank the use of ICP-MS and ICP-OES analytical facilities of Xiamen Institute of Rare Earth Materials, Hai Xi Institute, Chinese Academy of Sciences.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ault, T.; Brozek, K.; Fan, L.; Folsom, M. Lithium Isotope Enrichment: Feasible Domestic Enrichment Alternatives; University of California: Berkeley, CA, USA, 2012; pp. 3–4. [Google Scholar]
- Symons, E.A. Lithium isotope-separation-a review of possible techniques. Sep. Sci. Technol. 1985, 20, 633–651. [Google Scholar]
- Ho, M.K.M.; Yeoh, G.H.; Braoudakis, G. Molten salt reactors. In Materials and Processes for Energy: Communicating Current Research and Technological Developments; Formatex: Badajoz, Spain, 2013; pp. 761–768. [Google Scholar]
- Zhang, Z.; Murali, A.M.; Sarswat, P.K.; Free, M.L. High-efficiency lithium isotope separation in an electrochemical system with 1-butyl-3-methylimidazolium dicyanamide, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, and diethyl carbonate as the solvents. Sep. Purif. Technol. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Alam, T.M.; Conzone, S.; Brow, R.K.; Boyle, T.J. 6Li, 7Li nuclear magnetic resonance investigation of lithium coordination in binary phosphate glasses. J. Non-Cryst. Solids 1999, 258, 140–154. [Google Scholar] [CrossRef]
- Qi, X.Q.; Zhang, P.P.; Yan, Z.C.; Drake, G.W.F.; Zhong, Z.X.; Shi, T.Y.; Chen, S.L.; Huang, Y.; Guan, H.; Gao, K.L. Precision calculation of hyperfine structure and the zemach radii of 6,7Li+ ions. Phys. Rev. Lett. 2020, 125, 183002. [Google Scholar] [CrossRef]
- Cui, L.; Yang, X.; Wang, J.; He, H.; Guo, Y.; Cheng, F.; Zhang, S. Theoretical prediction of 6Li/7Li separation in solvent extraction system using Urey model. Chem. Eng. J. 2019, 358, 435–445. [Google Scholar] [CrossRef]
- Lewis, G.N.; Macdonald, R.T. The separation of lithium isotopes. J. Am. Chem. Soc. 2002, 58, 2519–2524. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Jia, Y.; Zhu, W.; Zhang, Q.; Wang, X.; Yao, Y.; Jing, Y. Lithium isotope separation by crown ethers of different nitrogen-containing derivatives in the ionic liquid-anisole system. J. Mol. Liq. 2018, 272, 548–553. [Google Scholar] [CrossRef]
- Xiao, J.; Jia, Y.; Shi, C.; Wang, X.; Ying, Y.; Yan, J. Liquid-liquid extraction separation of lithium isotopes by using room-temperature ionic liquids-chloroform mixed solvent system contained benzo-15-crown-5. J. Mol. Liq. 2016, 223, 1032–1038. [Google Scholar] [CrossRef]
- Saleem, M.; Hussain, S.; Zia, M.A.; Baig, M.A. An efficient pathway for 6 Li isotope enrichment. Appl. Phys. B Lasers Opt. 2007, B87, 723–726. [Google Scholar] [CrossRef]
- Cui, L.; Fan, Y.; Li, S.; Bai, R.; Guo, Y.; Cheng, F. Research progress on the theory and new technology for separation of lithium isotopes by chemical exchange. CIESC J. 2021, 72, 3215–3227. [Google Scholar]
- Cui, L.; Li, S.; Kang, J.; Yin, C.; Guo, Y.; He, H.; Cheng, F. A novel ion-pair strategy for efficient separation of lithium isotopes using crown ethers. Sep. Purif. Technol. 2021, 274, 118989. [Google Scholar] [CrossRef]
- He, L.; Weng, X.; Yang, D.; Song, C. Extraction of stronium from high—Level active waste with crown—Ether. J. Nucl. Radiochem. 1994, 16, 22. [Google Scholar]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: The quest for type V DES. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for green-solvent design: From hydrophilic to hydrophobic (deep) eutectic solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the nature of eutectic and deep eutectic mixtures. J. Solut. Chem. 2018, 48, 962–982. [Google Scholar] [CrossRef]
- Osch, D.; Dietz, C.; Warrag, S.; Kroon, M.C. The curious case of hydrophobic deep eutectic solvents: A story on the discovery, design, and applications. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar]
- Wang, T.; Luo, H.; Bai, Y.; Li, J.; Belharouak, I.; Dai, S. Direct recycling of spent NCM cathodes through ionothermal lithiation. Adv. Energy Mater. 2020, 10, 2001204. [Google Scholar] [CrossRef]
- Geng, S.; Dong, H.; Lu, Y.; Wang, S.; Huang, Y.; Zou, X.; Zhang, Y.; Xu, Q.; Lu, X. Electrolytic production of Cu-Ni alloy from nickel matte through chlorination and deep eutectic solvent leaching-electrodeposition. Sep. Purif. Technol. 2020, 242, 116779. [Google Scholar] [CrossRef]
- Hanada, T.; Goto, M. Synergistic deep eutectic solvents for lithium extraction. ACS Sustain. Chem. Eng. 2021, 9, 2152–2160. [Google Scholar] [CrossRef]
- van den Bruinhorst, A.; Raes, S.; Maesara, S.A.; Kroon, M.C.; Esteves, A.C.C.; Meuldijk, J. Hydrophobic eutectic mixtures as volatile fatty acid extractants. Sep. Purif. Technol. 2019, 216, 147–157. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Lin, H.; Gong, K.; Hykys, P.; Chen, D.; Ying, W.; Sofer, Z.; Yan, Y.; Li, Z.; Peng, X. Nanoconfined deep eutectic solvent in laminated MXene for efficient CO2 separation. Chem. Eng. J. 2021, 405, 126961. [Google Scholar] [CrossRef]
- Lian, S.; Li, R.; Zhang, Z.; Liu, Q.; Song, C.; Lu, S. Improved CO2 separation performance and interfacial affinity of composite membranes by incorporating amino acid-based deep eutectic solvents. Sep. Purif. Technol. 2021, 272, 118953. [Google Scholar] [CrossRef]
- Haider, M.B.; Kumar, R. Solubility of CO2 and CH4 in sterically hindered amine-based deep eutectic solvents. Sep. Purif. Technol. 2020, 248, 117055. [Google Scholar] [CrossRef]
- Yadav, N.; Venkatesu, P. Current understanding and insights towards protein stabilization and activation in deep eutectic solvents as sustainable solvent media. Phys. Chem. Chem. Phys. 2022, 24, 13474–13509. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Kist, J.A.; Zhao, H.; Mitchell-Koch, K.R.; Baker, G.A. The study and application of biomolecules in deep eutectic solvents. J. Mater. Chem. B 2021, 9, 536–566. [Google Scholar] [CrossRef]
- Villar, L.; Martínez-Rico, Ó.; Asla, A.; Domínguez, Á.; González, B. Testing thymol-based DES for the elimination of 11 textile dyes from water. Separations 2022, 9, 442. [Google Scholar] [CrossRef]
- Lin, W.; Zhao, Z.; Yang, F.; Liu, Z.; Tan, F.; Xie, M.; Ma, Y.; Meng, L. Promising priority separation of europium from lanthanide by novel DGA-functionalized metal organic frameworks. Miner. Eng. 2021, 164, 106831. [Google Scholar] [CrossRef]
- Tang, T.; Yang, F.; Xie, M.; Xue, L.; Jiang, Z.; Xie, Z.; Wang, K.; Li, Z.; Geng, L.; Hu, T. Highly efficient separation and enrichment of hafnium from zirconium oxychloride solutions by advanced ion-imprinted membrane separation technology. J. Membr. Sci. 2023, 668, 121237. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, F.; Zhao, Z.; Liao, Q.; Zhang, Y.; Chen, P.; Guo, W.; Bai, R. A simple preparation method for rare-earth phosphate nano materials using an ionic liquid-driven supported liquid membrane system. J. Ind. Eng. Chem. 2017, 54, 369–376. [Google Scholar] [CrossRef]
- Cai, C.; Yang, F.; Zhao, Z.; Liao, Q.; Bai, R.; Guo, W.; Chen, P.; Zhang, Y.; Zhang, H. Promising transport and high-selective separation of Li(I) from Na(I) and K(I) by a functional polymer inclusion membrane (PIM) system. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- Sloop, J.C.; Bumgardner, C.L.; Washington, G.; Loehle, W.D.; Sankar, S.S.; Lewis, A.B. Keto-enol and enol-enol tautomerism in trifluoromethyl-β-diketones. J. Fluor. Chem. 2006, 127, 780–786. [Google Scholar] [CrossRef]
- Darugar, V.R.; Vakili, M.; Nekoei, A.R.; Tayyari, S.F.; Afzali, R. Tautomerism, molecular structure, intramolecular hydrogen bond, and enol-enol equilibrium of para halo substituted 4,4,4-trifluoro-1-phenyl-1,3-butanedione; Experimental and theoretical studies. J. Mol. Struct. 2017, 1150, 427–437. [Google Scholar] [CrossRef]
- Nekoei, A.-R.; Tayyari, S.F.; Vakili, M.; Holakoei, S.; Hamidian, A.H.; Sammelson, R.E. Conformation and vibrational spectra and assignment of 2-thenoyltrifluoroacetone. J. Mol. Struct. 2009, 932, 112–122. [Google Scholar] [CrossRef]
- Gilmore, M.; McCourt, E.N.; Connolly, F.; Nockemann, P.; Swadzba-Kwasny, M.; Holbrey, J.D. Hydrophobic deep eutectic solvents incorporating trioctylphosphine oxide: Advanced liquid extractants. ACS Sustain. Chem. Eng. 2018, 6, 17323–17332. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Y.; Huang, F.; Gu, D.; Gan, F. Synthesis, spectral, and thermal characterizations of Ni(II) and Cu(II) beta-diketone complexes with thenoyltrifluoroacetone ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 66, 1024–1029. [Google Scholar] [CrossRef]
- Eshaghi Malekshah, R.; Salehi, M.; Kubicki, M.; Khaleghian, A. Crystal structure, molecular docking, and biological activity of the zinc complexes with 2-thenoyltrifluoroacetone and N-donor heterocyclic ligands. J. Mol. Struct. 2017, 1150, 155–165. [Google Scholar] [CrossRef]
- Thamilarasan, V.; Jayamani, A.; Sengottuvelan, N. Synthesis, molecular structure, biological properties and molecular docking studies on Mn(II), Co(II) and Zn(II) complexes containing bipyridine-azide ligands. Eur. J. Med. Chem. 2015, 89, 266–278. [Google Scholar] [CrossRef]
- Ma, J. Preparation and characterization of ZrO2 nanoparticles capped by trioctylphosphine oxide (TOPO). J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 611–614. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Ho, J.C.; Fan, Z.; Javey, A. Phosphine oxide monolayers on SiO2 surfaces. Angew. Chem. Int. Ed. Engl. 2008, 47, 4440–4442. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Ni, S.; Bie, C.; Zhi, H.; Sun, X. A clean process for selective recovery of copper from industrial wastewater by extraction-precipitation with p-tert-octyl phenoxy acetic acid. J. Environ. Manag. 2022, 304, 114164. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, K.; Liu, H. The nature of salt effect in enhancing the extraction of rare earths by non-functional ionic liquids: Synergism of salt anion complexation and hofmeister bias. J. Colloid Interface Sci. 2019, 539, 214–222. [Google Scholar] [CrossRef]
- He, M.; Liu, Y. Design for PVC mombrane ion-selective electrodes based on ion-pairsof triphenylmethane dye-anions as electroactive materials-the relationship between the ratio of charge to thermochemical radius of anions and the potentiometric selectivity coefficient. Chin. J. Anal. Chem. 1983, 11, 81–83. [Google Scholar]
- Sun, X.-L.; Zhou, W.; Gu, L.; Qiu, D.; Ren, D.-H.; Gu, Z.-G.; Li, Z. Liquid–liquid extraction to lithium isotope separation based on room-temperature ionic liquids containing 2,2′-binaphthyldiyl-17-crown-5. J. Nucl. Sci. Technol. 2014, 52, 332–341. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Doidge, E.D.; Carson, I.; Love, J.B.; Morrison, C.A.; Tasker, P.A. The influence of the hofmeister bias and the stability and speciation of chloridolanthanates on their extraction from chloride media. Solvent Extr. Ion Exch. 2016, 34, 579–593. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, D.; Li, L.; Peng, X.; Song, F.; Rui, H. Solvent extraction of lithium from ammoniacal solution using thenoyltrifluoroacetone and neutral ligands. J. Mol. Liq. 2019, 274, 746–751. [Google Scholar] [CrossRef]
- Swain, B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: A review. J. Chem. Technol. Biotechnol. 2016, 91, 2549–2562. [Google Scholar] [CrossRef]
- Ananyev, A.V.; Tsarenko, N.A.; Strelnikova, A.M.; Koshcheev, A.M.; Tsivadze, A.Y. Extraction of cesium by crown ethers in the presence of activating additives. Russ. Chem. Bull. 2014, 63, 1308–1311. [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.; Hou, L.; Shi, C.; Li, K.; Hang, F.; Xie, C. Amine-functionalized MOF-derived carbon materials for efficient removal of congo red dye from aqueous solutions: Simulation and adsorption studies. RSC Adv. 2023, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).