Abstract
(1) Background: Endocrine Mucin-Producing Sweat Gland Carcinoma (EMPSGC) is a rare, low-grade, neuroendocrine-differentiated, cutaneous adnexal tumor, officially recognized by the World Health Organization (WHO) Skin Tumors Classification in 2018 as a separate entity and homologue of endocrine ductal carcinoma in situ (eDCIS)/solid papillary carcinoma of the breast. Although it is more frequent in the female sex, between 60 and 70 years old, in the peri-orbital region, EMPSGC has also been described in the male sex, in subjects under 60 and over 80, and in extra-eyelid localizations (cheek, temple, scalp), but also in extra-facial localizations (chest and scrotum). (2) Methods: We present the clinical case of a 71-year-old woman with an undated lesion of the scalp, which presented as a nodule, skin-colored, and 2.5 cm in maximum diameter. We also conduct a comprehensive literature review from 1997 to the end of 2022, consulting PubMed, Scopus, Web of Science (WoS), and Google Scholar using the following keywords: “Endocrine mucin-producing sweat gland carcinoma” and/or “EMPSGC” and/or “skin” and “cutaneous neoplasms”. In addition, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A total of 253 patients were recorded; 146 were females (57.7%) and 107 were males (42.2%). The vast majority of the lesions were in the eyelids (peri-ocular region), and only a minority of cases involved the cheeks, supra-auricular, retro-auricular, and occipital region, with very rare cases in the scalp, to which the present is also added. (4) Conclusions: The morphological and immunophenotypical features are essential both for the correct diagnosis and to be able to classify this lesion among the corresponding eDCIS/solid papillary carcinoma of the breast, with neuroendocrine differentiation. Recent papers have attempted to shed light on the molecular features of EMPSGC, and much remains to be conducted in the attempt to subtype the molecular profiles of these entities. Future studies with large case series, and especially with molecular biology techniques, will be needed to further add information about EMPSGC and its relationship in the PCMC spectrum.
1. Introduction
Endocrine Mucin-Producing Sweat Gland Carcinoma (EMPSGC) is an entity described recently, in 1997, by Flieder A. et al. [1], and is considered a low-grade neuroendocrine neoplasm, homologous to solid papillary/endocrine ductal carcinoma in situ (eDCIS) of the breast in the World Health Organization (WHO) 2018 classification [2]. Epidemiologically, EMPSGC is more common in women than men, and the median age at presentation is 70 years (range 36–84 years) [3]. For a long time, EMPSGC was considered to pertain exclusively to the eyelid region (around the eye, peri-ocular) but, albeit rarely, cases in extra-eyelid localizations have been reported, for example, by Tsai J.H. et al. [4] and Raquena L. and Sangueza O. [3] in their book “Cutaneous Adnexal Neoplasms”; in particular, while the former case was discussed as it was localized in the areolar region [4], the case presented in [3] was localized at the level of a man’s scalp, casting doubt on the possible extra-eyelid localization. Various authors, and even the WHO classification, recognize that EMPSGC may be a precursor of primary mucinous carcinoma of the skin (PCMC), and, therefore, it should be discussed in the differential diagnosis [5]. Histologically, EMPSGCs appear as rather well-circumscribed uni/multi-nodular tumors with solid, cystic, and papillary areas. The nodular areas usually show a solid growth pattern with small, scattered cysts, and, in addition, in some areas, a cribriform arrangement is present, in which the tumor cells grow on a lacelike network and/or a pseudo-rosette pattern [3,4].
From an immunohistochemical point of view, EMPSGC is usually positive for Cytokeratin 7 (CK7), CK8, CK8/18, CKAE1/AE3, CK-CAM5.2, Epithelial Membrane Antigen (EMA), Gross Cystic Disease Fluid Protein-15 (GCDFP-15), WT1, Estrogen Receptor (ER), and Progesterone Receptor (PgR), but it is very important to underline the positivity for Synaptophysin (Syn) and/or Chromogranin A (CgA), Neuron-Specific Enolase (NSE), and CD56 [3,4,5], which are markers of neuroendocrine differentiation.
In this paper, we present a new extra-eyelid localization of EMPSGC in a 71-year-old female, discuss the morphological and immunohistochemical features with a brief mention of the molecular biology information, and, finally, we conduct an extensive and exhaustive review of the world literature with a focus on future perspectives.
2. Case Presentation
A 71-year-old woman presented to the Complex Plastic Surgery Unit with an unspecified history of a non-itchy, tan-to-pink nodule, 2.5 cm, on her scalp. The woman denied the presence of any symptoms and reported only high blood pressure that had been treated with Telmisartan for many years without other disease or concomitant treatment. After physical examination, it was decided to remove the nodule which, after fixation in 10% buffered formaldehyde, was sent to the Complex Operative Unit of Pathological Anatomy.
Histologically, the neoplasm was multinodular, with solid and sometimes cystic areas (Figure 1A) containing mucin (Figure 1C). Usually, the epithelial aggregates of neoplastic cells were well circumscribed and separated by scant stroma (Figure 1A,B), and there was also the possibility to appreciate areas of cribriform arrangement (Figure 1C) with lacelike network and/or pseudo-rosette pattern of growth (Figure 1D).
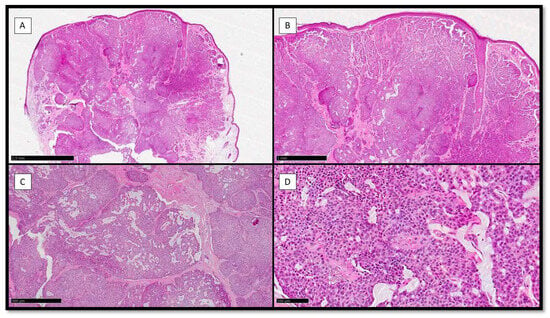
Figure 1.
(A) Histological preparation for Hematoxylin-Eosin (H&E) showing a polypoid, dome-shaped, well-circumscribed, multinodular neoplasm with solid, cystic, and papillary areas (H&E, Original Magnification 4×). (B) Scanning magnification of the previous picture, which shows solid aggregates with a cribriform arrangement (H&E, Original Magnification 10×). (C) Histological photomicrograph showing mucin secretion in solid aggregates, with resulting cribriform pattern of growth (H&E, Original Magnification 20×). (D) Scanning magnification of the previous picture, showing tumor cells growing on a lacelike network and/or pseudo-rosette pattern with monomorphous, round to oval, and medium size features, and their nuclei with a “salt and pepper” appearance and ample and eosinophilic cytoplasm (H&E, Original Magnification 40×).
From an immunohistochemical point of view, neoplastic cells were positive for CK8/18 and CK7, with higher positivity for the first one (Figure 2A,B); Synaptophisin was almost positive in a cytoplasmatic pattern, while Chromogranin A was partially positive (Figure 2C,D). Estrogen Receptor (ER) and Progesterone Receptor (PgR) were almost totally positive in the nuclei of the neoplastic cells (Figure 2E,F).
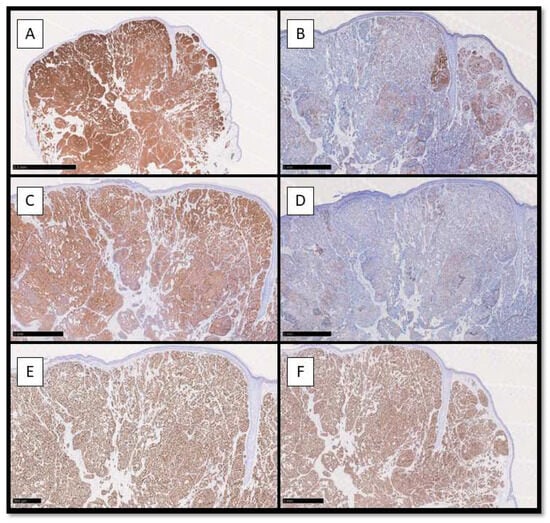
Figure 2.
(A) Immunohistochemical preparation for anti-CK8/18 antibody: note the diffuse cytoplasmatic positivity of the neoplastic cells constituting EMPSGC (Immunohistochemistry for CK8/18, Original Magnification 4×); (B) Immunohistochemical preparation for CK7 antibody: note the partial positivity for CK7 of the neoplastic cells (Immunohistochemistry for CK7, Original Magnification 10×); (C) Immunohistochemical photomicrograph showing diffuse positivity for Synaptophysin, marker of endocrine differentiation (Immunohistochemistry for Syn, Original Magnification 10×); (D) Photomicrograph showing weak positivity for Chromogranin A (Immunohistochemistry for CgA, Original Magnification 10×). (E) Immunohistochemical preparation for Estrogen Receptor: note the diffuse nuclear positivity of the neoplastic cells (Immunohistochemistry for ER, Original Magnification 10×); (F) Immunohistochemical preparation for Progesterone Receptor: note that this picture is almost similar to the previous (ER). (Immunohistochemistry for PgR, Original Magnification 10×).
Follow-up data up to 7 months after removal were negative for disease recurrence/metastasis.
In order to recognize and summarize all data present in literature, we also performed a comprehensive review using the following keywords: “Endocrine mucin-producing sweat gland carcinoma” and/or “EMPSGC” and/or “skin” AND “cutaneous neoplasms”, on the PubMed, Scopus, Web of Sciences (WoS), and Google Scholar databases with particular attention to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Only articles in the English language were selected, and entity discussion articles have been eliminated, favoring case reports and case series, limiting the discussion of other types of information to our ‘discussion’ section.
Figure 3 presents the features of our review process.
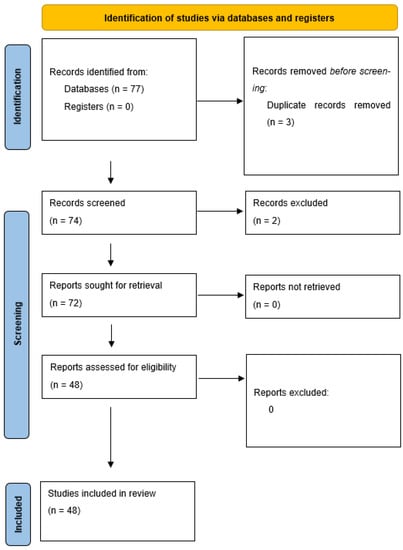
Figure 3.
PRISMA guidelines flow-chart followed to perform this review.
Table 1 summarizes all studies reviewed and analyzed in this paper, with particular attention to year of publication of the papers, number of patients, gender, age of presentation of EMPSGC, localization, and immunohistochemical features. A total of 253 patients were recorded; 146 were females (57.7%), and 107 were males (42.2%). The vast majority of the lesions were in the eyelids (peri-ocular region) and only a minority of cases involved the cheeks, supra-auricular, retro-auricular, and occipital region, with very rare cases in the scalp, to which the present is also added. The average age was reported to be between the sixth and seventh decade, with some cases present before the age of 60 and some present after the age of 80. The most representative immunohistochemistry stains were CK7, CK AE1/AE3, CAM5.2, EMA and, in terms of neuroendocrine markers, Syn, CgA, and NSE, with some cases tested with CD56 (less specific). Furthermore, markers such as GCDFP-15 and Mammoglobin were positive in a good percentage of published cases. With regard to hormone receptors, almost all EMPSGC lesions were positive for at least one of ER, PgR, and AgR. In almost all published cases, immunostaining for p63 and/or SMA was performed to allow the study of the myoepithelium and to understand whether the lesion was in situ or invasive [6,7]. In a paper [8], the diagnostic usefulness of immunohistochemical staining for MYB in 11 cases was reported, while a very recent paper [9] analyzed the use of a new neuroendocrine differentiation marker, Insulinoma-associated protein 1 (INSM1), which was extremely specific and more sensitive than the routinely used neuroendocrine markers, such as Syn and CgA. Finally, in a paper by Mathew et al. [10], immunostaining data were presented on five cases of EMPSGC in which INSM1, AR, BCL2, MUC2, MUC4, RB, Beta-Catenin and MCPyV were tested, of which INSM1 and MUC2 were positive with 4+ intensity, and MUC4 showed 2+/3+ staining mainly at the periphery of the tumor.

Table 1.
Detailed features of the papers constituting this review.
Table 2 summarizes the follow-up data of the patients and the clinical outcomes. The vast majority of the lesions were without any recurrence/metastasis, with only a few cases with metastatic or recurrence setting.

Table 2.
Summary of the follow-up data and clinical outcomes of the patients included in the review.
3. Discussion
EMPSGC represents a very peculiar entity in dermatopathology and ophthalmopathology, and only in the last decade has there been an increasing number of scientific papers studying and shedding light on its histopathological, immunohistochemical, and, moreover, molecular features. Since its initial description [1] up to the latest version of the WHO Skin Tumors [2], the histological and immunophenotypical similarity to its counterpart, referred to as endocrine ductal carcinoma in situ carcinoma (eDCIS) of the breast/solid papillary breast carcinoma, was emphasized, assuming that the very similar embryological nature of the mammary and eccrine gland was the basis for these similarities [3,4,5,6,11,12,13,14,15,16,17,18,19,20,21,22].
Epidemiologically, our review of the literature confirms that EMPSGC is more frequent in females, between 60 and 70 years old, with more frequent localization in the peri-ocular region, although there are also reports of other skin sites, such as the cheeks, the scalp, and other very rare localizations such as the skin of the scrotal region, reported in the paper by Shah et al. [54], in which the course of EMPSGC was aggressive, with lymph node, visceral, and bone metastases. A careful analysis of the literature seems to indicate that, although, initially, the published cases of EMPSGC were of the female sex, in the last 10 years, numerous cases have also been published in the male sex, almost in contrast to previous years.
Clinically, it is important to emphasize that EMPSGC does not have a clear profile that allows it to be easily recognized, so much so that, in the papers in the literature, the main suspected clinical diagnoses range from basal cell carcinoma (BCC) to squamous cell carcinoma (SCC), but also Merkel cell carcinoma (MCC) or an innocent epidermal inclusion cyst, hidroadenoma, chalazion, or dermatofibroma (DF). In this regard, Hasegawa-Murakami et al. [35] analyzed the dermoscopic pattern of the case presented in their paper, pointing out that the lesion presented an aggregation of pink to reddish globules (pink ovoid nests), with each globule separated by white to pink meshes of bands (pink network). Furthermore, the red/blue globules were seen in pink ovoid nests of the tumor and, also, some very fine linear–irregular disrupted vessels were recognized. Usually, EMPSGC presents such as a slowly growing skin-colored nodule that can be cystic, multiple, or pigmented [3,42].
In any case, it seems plausible that the clinical diagnosis of EMPSGC does not yet have standardized dermoscopic criteria and that histological examination is always mandatory for the diagnosis.
From a histopathological point of view, the morphological features of EMPSGC are well delineated, with many scientific papers having clearly and comprehensively described the peculiarities of this neoplasm, focusing, in particular, on the well-circumscribed growth, uninodular or even multinodular, with the possibility of having a solid, cystic, and papillary component. In various case reports, it is emphasized that it is possible to find scattered small cysts, and also cribriform aspects, in which the cells are arranged in a pseudo-rosette and/or lacelike patterns. Cytologically, the cells constituting the tumor are monomorphic, round to oval, and of medium/small size. The nuclei have fine granular or stippled chromatin imparting a “salt-and-pepper” appearance, and their cytoplasm is large and eosinophilic. In addition, it is important to emphasize the possibility of secreting mucins, both intracellularly and into the extracellular environment, a characteristic that, over time, has suggested a possible placement of EMPSGC in a spectrum of neoplasms that, at the extreme, would have primitive cutaneous mucinous carcinoma (PCMC) [1,3,4,5,6,8,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,62,63].
Although morphology is very important, immunohistochemistry plays a paramount role in the correct diagnostic framing of EMPSGC. All papers published so far show that, with exceptions due to pre-analytical variables, EMPSGC expresses with high concordance Cytokeratin AE1/AE3, CAM 5.2, CK7, and EMA, but also GCDFP-15 and E-cadherin, together with at least one neuroendocrine differentiation marker such as Synaptophysin, Chromogranin A, Neuron-specific Enolase, and/or CD56. Furthermore, it is important to emphasize that this entity expresses Estrogen Receptors (ER) and Progesterone Receptors (PgR) in the vast majority of cases [1,3,4,5,6,8,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Our case presented immunohistochemical expression for CK8/18 and partially for CK7; it also presented diffuse and strong immuno-expression for Syn and, focally, for CgA. Finally, it had nuclear expression for ER and PgR.
Molecular data concerning EMPSGC are still limited, although more and more evidence has been published in recent years. For example, Murshed et al. [45] performed Next Generation Sequencing (NGS) analysis on their case of a 78-year-old man with an EMPSGC of the right inferior eyelid. The analysis, conducted on genomic DNA of a tumor extracted from formalin-fixed paraffin-embedded (FFPE) tissue, targeting frequently mutated 59 genes (including EGFR, KRAS, ALK, ROS1, BRAF, HRAS, NRAS, NTRK, AKT1, PIK3CA, KIT, and PDGFRA), disclosed no gene mutations or fusions. On two cases of EMPSGC, Cornejo et al. used NGS with a focused panel of 50 frequently altered genes [27]. The genes EGFR, KRAS, and GNAS, which are frequently involved in mucinous neoplasms, were not found to have alterations in that study. AKT1 and PIK3CA gene alterations, which are typically found in papillary carcinomas of the breast, were also not found. In another two cases of EMPSGC, Qin et al. [34] used array comparative genomic hybridization (aCGH), which revealed a 6p11.2 to 6q16.1 deletion in one of the cases. Held et al. [8] performed an MYB antibody staining of 10 cases of EMPSGC, revealing that all of the cases displayed significant nuclear MYB expression; furthermore, the expression of MYB was found negative in primary mucinous cutaneous carcinomas and mucin-rich basal cell carcinomas, and, also, they found that MYB might be a helpful surrogate measure, particularly in EMPSGC cases with low mucin levels. However, the authors underscore that fluorescent in situ hybridization (FISH) testing on each case in that study came out negative for MYB gene translocation, and amplification is preferable.
In the terms of differential diagnosis, EMPSGC should be carefully distinguished from adnexal lesions such as hydroroadenoma, hydrocystoma with papillary ductal hyperplasia, apocrine adenoma, and apocrine adenocarcinoma. The main modality lies in the correct morphologic framing of the listed lesions, with special attention to the potential recognition of a PCM component, in which case it is mandated to define the lesion as mucinous adenocarcinoma of the skin.
Our comprehensive review of the literature confirms the already widely established data regarding the almost non-existent recurrence and/or distant metastasis of EMPSGC, with very few cases of this one. Interestingly, Froehlich et al. [49] reported a case of EMPSGC of the right eyelid in a patient who had not wanted to undergo surgery after an initial diagnosis and who, upon recurrence, developed a metastasis in 2/9 excised intra-parotid lymph nodes. It is reasonable to state that this result was not due to the (in itself low) potential of the neoplasm, but to the failure to undergo surgery for therapeutic purposes. In another paper by Hadi et al. [52], a case is presented of a 66-year-old subject who developed several recurrences of EMPSGC over a period of nine years, with a metastatic lesion on the ipsilateral parotid gland and a rib. This anecdotal case is of extraordinary importance, as the authors emphasize that the histological and immunohistochemical evaluation of EMPSGC must be very detailed and careful, as potential foci of invasion can easily be overlooked, and not correctly framed within the rarer but aggressive PCMC. Precisely in this regard, several papers analyzed in the literature have proposed to always perform an immunolabelling with Smooth Actin muscle (SMA), or p63 p CK5/6, to study the continuity of the myoepithelium, similar to what happens in the evaluation of breast cancer. Although the data presented are in agreement with this solution, in order to understand whether there is reduction/disappearance of the myoepithelium with consequent potential invasiveness of EMPSGC, a paper by Saggini et al. [64] critically addresses the use of IHC markers for the myoepithelium, as they are prone to error. In particular, referring to the breast, the authors caution against defining as ‘invasive’ an EMPSGC that, while losing myoepithelial IHC markers, always grows in an expansive pattern in the absence of clear tongues of infiltration.
More recently, an interesting paper by Parra et al. discussed a novel immunohistochemical marker called Insulinoma-associated protein 1 (INSM1) that is a transcriptional repressor that plays an essential role in neuroendocrine differentiation [9]. The authors demonstrated that its nuclear expression is stronger and more diffuse than traditional neuroendocrine markers, such as Syn and CgA, and its staining is cleaner and more non-specific than the aforementioned markers.
As far as the etiopathogenesis is concerned, in the current state of knowledge, it is not possible to characterize the carcinogenesis exactly, although a paper by Nishimoto et al. [53] reports a very rare case of multiple EMPSGC/MCS in the same 71-year-old patient, who simultaneously developed a primary mucinous carcinoma of the breast and had a history of unspecified carcinoma of the uterus body some 24 years earlier. The authors correctly hypothesize that the multiple occurrence of hormone-responsive carcinomas (estrogen and progesterone) could be the explanation for why EMPSGC occurs more in the female sex, through a genetic alteration of the receptors.
4. Conclusions
In conclusion, EMPSGC represent a rare, low-grade cutaneous adnexal tumor, recognized for the first time with such nomenclature and entity specificity in the 2018 WHO classification. Although, in the early years after the initial description by Flieder et al., there had been few cases reported in the literature, mainly on the eyelids of older women, over time, they have also been described in male subjects, in not only extra-eyelid localizations (such as the case we present here) but also extra-facial topography. The morphological and immunophenotypical features are essential both for the correct diagnosis and to be able to classify this lesion among the corresponding eDCIS/solid papillary carcinoma of the breast, with neuroendocrine differentiation. Recent papers have attempted to shed light on the molecular features of EMPSGC, and much remains to be performed in the attempt to subtype the molecular profiles of these entities. Future studies with large case series, and, especially, with molecular biology techniques, will be needed to further add information about EMPSGC and its relationship in the PCMC spectrum.
Author Contributions
Conceptualization, G.C. and D.P.; methodology, G.C. and E.B.; software, G.C.; validation, G.C., I.T., and A.C.; formal analysis, G.C. and N.S.; investigation, V.S.S.; resources, D.P. and L.R.; data curation, G.C. and G.I.; writing—original draft preparation, G.C.; writing—review and editing, G.C. and I.T.; visualization, T.L.; supervision, L.R. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Flieder, A.; Koerner, F.C.; Pilch, B.Z.; Maluf, H.M. Endocrine mucin-producing sweat gland carcinoma: A cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am. J. Surg. Pathol. 1997, 21, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.; Massi, D.; Scolyer, A.; Willemze, R. WHO Classification of Skin Tumours; IARC: Lyon, France, 2018; pp. 168–169. [Google Scholar]
- Requena, L.; Sangueza, O. Cutaneous Adnexal Neoplasms; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Tsai, J.H.; Hsiao, T.L.; Chen, Y.Y.; Hsiao, C.H.; Liau, J.Y. Endocrine mucin-producing sweat gland carcinoma occurring on extra-facial site: A case report. J. Cutan. Pathol. 2014, 41, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Tannous, Z.S.; Avram, M.M.; Zembowicz, A.; Mihm, M.C.; Liteplo, M.; Kwan, T.; Olbricht, S.M. Treatment of synchronous mucinous carcinoma and endocrine mucin-producing sweat gland carcinoma with Mohs’ micrographic surgery. Dermatol. Surg. 2005, 31, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Zembowicz, A.; Garcia, C.F.; Tannous, Z.S.; Mihm, M.C.; Koerner, F.; Pilch, B.Z. Endocrine mucin-producing sweat gland carcinoma: Twelve new cases suggest that it is a precursor of some invasive mucinous carcinomas. Am. J. Surg. Pathol. 2005, 29, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, M.H.; Conti, K.R.; Norris, I.I.; Allensworth, J.J.; Ufkes, N.A.; Nguyen, S.A.; Bruner, E.T.; Cook, J.; Day, T.A. Endocrine mucin-producing sweat gland carcinoma: A systematic review and meta-analysis. J. Dermatol. Treat. 2022, 33, 2182–2191. [Google Scholar] [CrossRef]
- Held, L.; Ruetten, A.; Kutzner, H.; Palmedo, G.; John, R.; Mentzel, T. Endocrine mucin-producing sweat gland carcinoma: Clinicopathologic, immunohistochemical, and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J. Cutan. Pathol. 2018, 45, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Parra, O.; Linos, K.; Yan, S.; Lilo, M.; LeBlanc, R.E. Comparative performance of insulinoma-associated protein 1 (INSM1) and routine immunohistochemical markers of neuroendocrine differentiation in the diagnosis of endocrine mucin-producing sweat gland carcinoma. J. Cutan. Pathol. 2021, 48, 41–46. [Google Scholar] [CrossRef]
- Mathew, J.G.; Bowman, A.S.; Saab, J.; Busam, K.J.; Nehal, K.; Pulitzer, M. Next-generation sequencing analysis suggests varied multistep mutational pathogenesis for endocrine mucin-producing sweat gland carcinoma with comments on INSM1 and MUC2 suggesting a conjunctival origin. J. Am. Acad. Dermatol. 2022, 86, 1072–1079. [Google Scholar] [CrossRef]
- Bulliard, C.; Murali, R.; Maloof, A.; Adams, S. Endocrine mucin-producing sweat gland carcinoma: Report of a case and review of the literature. J. Cutan. Pathol. 2006, 33, 812–816. [Google Scholar] [CrossRef]
- Emanuel, P.O.; de Vinck, D.; Waldorf, H.A.; Phelps, R.G. Recurrent endocrine mucin-producing sweat gland carcinoma. Ann. Diagn. Pathol. 2007, 11, 448–452. [Google Scholar] [CrossRef]
- Mehta, S.; Thiagalinga, S.; Zembowicz, A.; Hatton, M.P. Endocrine mucin-producing sweat gland carcinoma of the eyelid. Ophthalmic. Plast. Reconstr. Surg. 2008, 24, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Shim, S.H.; Joo, M.; Kim, H.; Kim, Y.K. A Case of Endocrine Mucin-Producing Sweat. Gland Carcinoma Co-existing with Mucinous Carcinoma: A Case Report. J. Pathol. Transl. Med. 2010, 44, 97–100. [Google Scholar] [CrossRef]
- Inozume, T.; Kawasaki, T.; Harada, K.; Tanaka, K.; Kawamura, T.; Shibagaki, N.; Katoh, R.; Shimada, S. A case of endocrine mucin-producing sweat gland carcinoma. Pathol. Int. 2012, 62, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Karim, R.Z.; McCarthy, S.W.; Scolyer, R.A. Endocrine mucin producing sweat gland carcinoma: A clinicopathological analysis of three cases. Pathology 2012, 44, 568–571. [Google Scholar] [CrossRef]
- Koike, T.; Mikami, T.; Maegawa, J.; Iwai, T.; Wada, H.; Yamanaka, S. Recurrent endocrine mucin-producing sweat gland carcinoma in the eyelid. Australas. J. Dermatol. 2013, 54, e46–e49. [Google Scholar] [CrossRef]
- Dhaliwal, C.A.; Torgersen, A.; Ross, J.J.; Ironside, J.W.; Biswas, A. Endocrine mucin-producing sweat gland carcinoma: Report of two cases of an under-recognized malignant neoplasm and review of the literature. Am. J. Dermatopathol. 2013, 35, 117–124. [Google Scholar] [CrossRef]
- Hoguet, A.; Warrow, D.; Milite, J.; McCormick, S.A.; Maher, E.; Della Rocca, R.; Della Rocca, D.; Goldbaum, A.; Milman, T. Mucin-producing sweat gland carcinoma of the eyelid: Diagnostic and prognostic considerations. Am. J. Ophthalmol. 2013, 155, 585–592.e2. [Google Scholar] [CrossRef]
- Shimizu, I.; Dufresne, R.; Robinson-Bostom, L. Endocrine mucin-producing sweat gland carcinoma. Cutis 2014, 93, 47–49. [Google Scholar]
- Shon, W.; Salomão, D.R. WT1 expression in endocrine mucin-producing sweat gland carcinoma: A study of 13 cases. Int. J. Dermatol. 2014, 53, 1228–1234. [Google Scholar] [CrossRef]
- Collinson, A.C.; Sun, M.T.; James, C.; Huilgol, S.C.; Selva, D. Endocrine mucin-producing sweat gland carcinoma of the eyelid. Int. Ophthalmol. 2015, 35, 883–886. [Google Scholar] [CrossRef]
- Fernandez-Flores, A.; Cassarino, D.S. Endocrine mucin-producing sweat gland carcinoma: A study of three cases and CK8, CK18 and CD5/6 immunoexpression. J. Cutan. Pathol. 2015, 42, 578–586. [Google Scholar] [CrossRef]
- Jedrych, J.; Jones, M.; Seethala, R.; Ho, J. Primary cutaneous endocrine mucin-producing sweat gland carcinoma co-occurring simultaneously with low-grade ductal mucinous breast cancer: A clinicopathologic conundrum. Am. J. Dermatopathol. 2015, 37, 425–427. [Google Scholar] [CrossRef]
- Turnbull, L.I.; Puchalsky, D.R.; Xu, Y.G.; Bennett, D.D. A cystic growth on the lower eyelid: A presentation of two cases. Int. J. Dermatol. 2015, 54, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, M.; Medline, P.; Cullen, J.B.; Gill, H. Histopathology of endocrine mucin-producing sweat gland carcinoma of the eyelid. Can. J. Ophthalmol. 2016, 51, e72–e75. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, K.M.; Hutchinson, L.; Meng, X.; O’Donnell, P.; Deng, A. Endocrine Mucin-Producing Sweat Gland Carcinoma of the Eyelid: A Report of a Case with Molecular Analysis. Am. J. Dermatopathol. 2016, 38, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Abdulkader, M.; Kuhar, M.; Hattab, E.; Linos, K. GATA3 Positivity in Endocrine Mucin-Producing Sweat Gland Carcinoma and Invasive Mucinous Carcinoma of the Eyelid: Report of 2 Cases. Am. J. Dermatopathol. 2016, 38, 789–791. [Google Scholar] [CrossRef]
- Ross, A.G.; Chan, A.A.; Mihm, M.C., Jr.; Yu, J.Y. Endocrine Mucin-Producing Sweat Gland Carcinoma: An Uncommon Presentation. Semin. Ophthalmol. 2017, 32, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Brett, M.A.; Salama, S.; Gohla, G.; Alowami, S. Endocrine Mucin-Producing Sweat Gland Carcinoma, a Histological Challenge. Case Rep. Pathol. 2017, 2017, 6343709. [Google Scholar] [CrossRef]
- Scott, B.L.; Anyanwu, C.O.; Vandergriff, T.; Nijhawan, R.I. Endocrine Mucin-Producing Sweat Gland Carcinoma Treated With Mohs Micrographic Surgery. Dermatol. Surg. 2017, 43, 1498–1500. [Google Scholar] [CrossRef]
- Chou, Y.H.; Chang, Y.C.; Huang, Y.L.; Wu, C.T. Endocrine mucin-producing sweat gland carcinoma with GATA3 expression: Report of two cases. Pathology 2017, 49, 805–808. [Google Scholar] [CrossRef]
- Charles, N.C.; Proia, A.D.; Lo, C. Endocrine Mucin-Producing Sweat Gland Carcinoma of the Eyelid Associated With Mucinous Adenocarcinoma. Ophthalmic Plast. Reconstr. Surg. 2018, 34, e37–e38. [Google Scholar] [CrossRef]
- Qin, H.; Moore, R.F.; Ho, C.Y.; Eshleman, J.; Eberhart, C.G.; Cuda, J. Endocrine mucin-producing sweat gland carcinoma: A study of 11 cases with molecular analysis. J. Cutan. Pathol. 2018, 45, 681–687. [Google Scholar] [CrossRef]
- Hasegawa-Murakami, Y.; Kono, M.; Yokota, K.; Inaba, H.; Fukumoto, T.; Akiyama, M. Dermoscopic features of endocrine mucin-producing sweat gland carcinoma. J. Dermatol. 2018, 45, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.G.; Bhargava, R.; Furniturewala, A.U. Endocrine mucin-producing sweat gland carcinoma of the eyelid: A clinical and histological conundrum. Indian J. Ophthalmol. 2018, 66, 1042–1044. [Google Scholar] [CrossRef] [PubMed]
- Navrazhina, K.; Petukhova, T.; Wildman, H.F.; Magro, C.M.; Minkis, K. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018, 4, 887–889. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kubota, T.; Ichihara, S.; Horibe, K.; Hasebe, T. Neuroendocrine cells associated with endocrine mucin-producing sweat gland carcinoma: A potential precursor lesion? Pathology 2018, 50, 573–575. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Wang, S.-H.; Chang, Y.-J. Endocrine mucin-producing sweat gland carcinoma: Report of two cases. Dermatol. Sin. 2018, 36, 262–263. [Google Scholar] [CrossRef]
- Mulay, K.; Menon, V.; Lahane, S.; Sharma, M.; Honavar, S.G. Endocrine mucin-producing sweat gland carcinoma (EMPSGC) of the eyelid: Clinicopathologic features, immunohistochemical findings and review of literature. Indian J. Ophthalmol. 2019, 67, 1374–1377. [Google Scholar] [CrossRef]
- Meltzer, O.A.; Joseph, J.M. Delayed treatment of endocrine mucin-producing sweat gland carcinoma initially diagnosed as a chalazion. JAAD Case Rep. 2019, 5, 789–791. [Google Scholar] [CrossRef]
- Ansari, A.N.; Bobos, M.; Shih, S.; Chen, M.C.; Ardakani, N.M.; Rosales, C.M.; Chen, C.J.; Savage, C.; Bracey, T.; McKee, P.H.; et al. Renal Cell Carcinoma Antigen Expression in Primary Cutaneous Endocrine Mucinous Carcinomas: A Case Series of 14 Patients and Review of the Literature. Am. J. Dermatopathol. 2019, 41, 571–577. [Google Scholar] [CrossRef]
- Nathan, N.R.; Emerick, K.S.; Hoang, M.P.; Smith, G.P.; Yancovitz, M. Perineural and Vascular Invasion in an Endocrine Mucin-Producing Sweat Gland Carcinoma of the Ear with Associated Mucinous Carcinoma. Dermatopathology 2020, 6, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Nasser, H.; Siddiqui, S. Endocrine Mucin-Producing Sweat Gland Carcinoma: Two New Eyelid Cases and Review of the Literature. Int. J. Surg. Pathol. 2020, 28, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Murshed, K.A.; Ben-Gashir, M. A Case of Endocrine Mucin-Producing Sweat Gland Carcinoma: Is it Still an Under-Recognized Entity? Case Rep. Dermatol. 2020, 12, 255–261. [Google Scholar] [CrossRef]
- Bakrin, I.H.; Rajaintharan, P.S.; Tawil, Z.; Mahayidin, H. Endocrine mucin-producing sweat gland carcinoma—Newly described skin appendageal tumours. Malays. J. Pathol. 2020, 42, 111–114. [Google Scholar]
- Shah, S.; Parekh, P.; Rodriguez, M. Endocrine Mucin-Producing Sweat Gland Carcinoma in an Elderly Man. Am. J. Dermatopathol. 2020, 42, 153–155. [Google Scholar] [CrossRef]
- Agni, M.; Raven, M.L.D.; Bowen, R.C.; Laver, N.V.; Chevez-Barrios, P.; Milman, T.; Eberhart, C.G.; Couch, S.; Bennett, D.D.; Albert, D.M.; et al. An Update on Endocrine Mucin-producing Sweat Gland Carcinoma: Clinicopathologic Study of 63 Cases and Comparative Analysis. Am. J. Surg. Pathol. 2020, 44, 1005–1016. [Google Scholar] [CrossRef]
- Froehlich, M.; Cook, J.; Bruner, E.; Stalcup, S.; Patel, K.; Day, T. Endocrine Mucin-Producing Sweat Gland Carcinoma of the Eyelid with Locoregional Metastasis to the Parotid Gland. Dermatol. Surg. 2020, 46, 1116–1118. [Google Scholar] [CrossRef]
- Nakamura, M.; Sakurai, M.; Ozawa, H.; Morita, A. Four male cases of endocrine mucin-producing sweat gland carcinoma: Specific gender differences in East Asia. Kaohsiung J. Med. Sci. 2020, 36, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Katsura, Y.; Sasaki, A.; Minagawa, D.; Kato, I.; Ueda, T. Endocrine mucin-producing sweat gland carcinoma of the cheek. Dermatol. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Hadi, R.; Xu, H.; Barber, B.R.; Shinohara, M.M.; Moshiri, A.S. A case of endocrine mucin-producing sweat gland carcinoma with distant metastasis. J. Cutan. Pathol. 2021, 48, 937–942. [Google Scholar] [CrossRef]
- Nishimoto, A.; Kuwahara, H.; Ohashi, R.; Ansai, S.I. Multicentric endocrine mucin-producing sweat gland carcinoma and mucinous carcinoma of the skin: A case report. J. Cutan. Pathol. 2021, 48, 165–170. [Google Scholar] [CrossRef]
- Shah, M.; Aman, A.; Srinivaas, K.; Gudipati, A.; Chavali, P. Endocrine mucin-producing sweat gland carcinoma of the peno-scrotum with systemic metastases: A rare case report. Indian J. Pathol. Microbiol. 2021, 64, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Homer, N.A.; Hoesly, P.M.; Durairaj, V.D. Atypical presentation of an endocrine mucin-producing sweat gland carcinoma of the eyelid. Orbit 2021, 40, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Schafer, C.N.; Hurst, E.A.; Rosman, I.S.; Council, M.L. Endocrine Mucin-Producing Sweat Gland Carcinoma Treated with Mohs Micrographic Surgery. Dermatol. Surg. 2022, 48, 362–364. [Google Scholar] [CrossRef]
- Chuang, I.C.; Jang, C.S. Endocrine mucin-producing sweat gland carcinoma: Reappraisal of patient demographics and tumour immunophenotypes. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.Y.; Walsh, N.M.; Archibald, C.; Pasternak, S. Endocrine Mucin-Producing Sweat Gland Carcinoma: Emerging Evidence of Multicentric Cutaneous Origin and Occasional Concurrence with Analogous Breast Tumors. Am. J. Dermatopathol. 2022, 44, 321–326. [Google Scholar] [CrossRef]
- Sarangi, J.; Konkimalla, A.; Kaur, K.; Sikka, K.; Sen, S.; Kakkar, A. Endocrine Mucin Producing Sweat Gland Carcinoma with Metastasis to Parotid Gland: Not as Indolent as Perceived? Head Neck Pathol. 2022, 16, 331–337. [Google Scholar] [CrossRef]
- Shah, D.S.; Homer, N.A.; Epstein, A.; Durairaj, V.D. Simultaneous presentation of orbital mantle cell lymphoma and endocrine mucin-producing sweat gland carcinoma. Orbit 2022, 41, 509–513. [Google Scholar] [CrossRef]
- Wang, D.; Deng, W.; LoBue, S.; Giovinazzo, K.; Rosen, M.; Heilman, E.; Hodgson, N.M. Bilateral Concurrent Endocrine Mucin-Producing Sweat Gland Carcinoma and Mucinous Carcinoma of the Eyelids. Ophthalmic Plast. Reconstr. Surg. 2022, 38, e96–e99. [Google Scholar] [CrossRef]
- Au, R.T.M.; Bundele, M.M. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: Review of the literature. J. Cutan. Pathol. 2021, 48, 1156–1165. [Google Scholar] [CrossRef]
- Quattrochi, B.; Russell-Goldman, E. Utility of Insulinoma-Associated Protein 1 (INSM1) and Mucin 2 (MUC2) Immunohistochemistry in the Distinction of Endocrine Mucin-Producing Sweat Gland Carcinoma from Morphologic Mimics. Am. J. Dermatopathol. 2022, 44, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Saggini, A.; Cota, C. Local invasion in endocrine mucin-producing sweat gland carcinoma: Still an open issue. J. Cutan. Pathol. 2020, 47, 192–194. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).