Abstract
Lichen Planus Pigmentosus (LPP) is an uncommon variant of lichen planus characterized by the development of dark greyish-brown macules and patches primarily affecting sun-exposed areas. Histologically, it presents with lichenoid interface dermatitis with many melanophages. In select cases, the presence of melanocytic nests or pseudomelanocytic nests within LPP lesions has been documented, posing a diagnostic challenge. We present a detailed case report of a 32-year-old Eritrean woman with a longstanding history of hyperpigmented macules, alongside an in-depth review of the existing literature on lichenoid dermatoses featuring melanocytic or pseudomelanocytic nests. This paper delves into the clinical presentation, histopathological features, differential diagnosis, and potential mechanisms underlying this intriguing phenomenon.
1. Introduction
Lichen Planus Pigmentosus (LPP) is a distinctive subtype of lichen planus that predominantly impacts individuals with darker pigmented skin. Lichenoid interface dermatitis with many melanophages is the typical histopathological presentation [1]. The microscopic identification of pseudomelanocytic nests or true melanocytic nests within LPP lesions introduces complexity to the diagnostic process and stimulates inquiries into the intricate interplay between inflammatory processes and melanocyte biology.
2. Case Report
A 32-year-old Eritrean woman exhibited hyperpigmented macules that manifested between the ages of eight and ten. Initially, small, grey-brown macules emerged mainly on sun-exposed areas and were, at times, associated with mild pruritus. The condition progressively worsened after the age of 30, extensively involving her limbs and trunk. Notably, she had a history of autoimmune thyroiditis, and her familial background featured similar skin lesions on her father and brother. On examination, oval greyish-brown patches and macules located on the trunk, axillary flexures, and upper and lower extremities were seen (Figure 1A,B). The oral mucosa and nails were not involved. The dermatoscopy of a patch on the abdomen revealed a diffuse, blotchy pattern (Figure 1C).
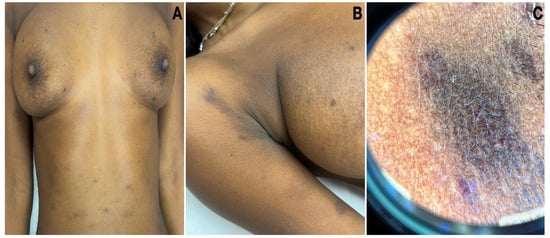
Figure 1.
Clinical pictures of LPP. (A) Oval grey-brown patches and macules located on the trunk and upper extremities. (B) Hyperpigmentation involving the flexure. (C) Dermatoscopy of a hyperpigmented patch on the abdomen revealed a diffuse, blotchy pattern.
The routine laboratory data were within normal limits or negative. The histopathological examination of a hyperpigmented lesion on the hip showed a band-like lymphohistiocytic inflammatory infiltrate with numerous melanophages within the papillary dermis (Figure 2A,B). Additionally, the vacuolar degeneration of the basal cell layer with keratinocyte apoptosis was evident. The overlying epidermis showed irregular acanthosis with flattening in places, orthohyperkeratosis, and hypergranulosis. Most remarkably, junctional aggregates of melanocytes were present at the dermo-epidermal junction (Figure 2C,D).
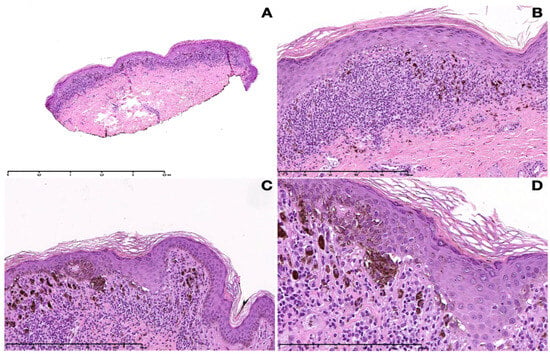
Figure 2.
Histopathological pictures of LPP. (A) Lichenoid band-like lymphohistiocytic inflammatory infiltrate with numerous melanophages within the papillary dermis (10×). (B) Acanthosis with flattening in places, orthohyperkeratosis, and hypergranulosis. Vacuolar degeneration of the basal cell layer with keratinocyte apoptosis and a band-like lymphohistiocytic infiltrate with numerous melanophages (100×). (C) Nesting of melanocytes at the dermo-epidermal junction (100×). (D) Close-up of junctional nests (200×).
Immunohistochemically, the cells in the junctional aggregates were found to be Melan-A-, S-100-, HMB-45-, and SOX-10-positive, confirming that the aggregates were “true melanocytic nests” (Figure 3A–C); CD68 and CD3 were considered negative in the same aggregates.
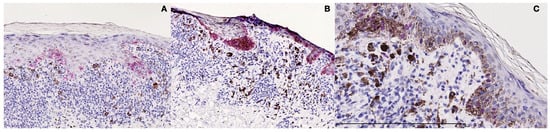
Figure 3.
Immunohistochemical studies. (A) The cells in the junctional aggregates were found to be HMB-45 positive. (B) The cells in the junctional aggregates were found to be Melan-A positive. (C) The cells in the junctional aggregates were found to be SOX-10-positive, confirming that the aggregates were “true melanocytic nests”.
The differential diagnosis included lichenoid dermatitis versus atypical melanocytic proliferation. Ultimately, a conclusive diagnosis of LPP with true melanocytic nests was established based upon the clinico-pathological correlation. Therapy with acitretin associated with extreme sun protection was started with a slight improvement after 6 weeks.
3. Discussion
LPP predominantly emerges in adulthood, typically manifesting insidiously after the age of 30. Small black or greyish-brown macules on sun-exposed areas tend to evolve into larger hyperpigmented patches. While the face, trunk, flexures, and upper extremities are commonly affected, the oral mucosa, palms, soles, and nails remain unaffected. The precise etiology of LPP remains elusive; however, the potential triggers include sunlight exposure, hepatitis C virus, mustard oil, and cosmetic agents. Abnormalities in T-lymphocyte functions have been implicated, and an intriguing association with thyroid disorders, such as in our case, has been noted [2].
Our case is peculiar for the discovery of junctional “true melanocytic nests” on histopathologic grounds. The phenomenon of junctional nesting or pseudonesting in the setting of microscopic lichenoid/interface changes, although infrequently and regardless of the terms used, has been previously reported [3,4,5,6,7,8,9]. The current state of the art seem to categorize this rare phenomenon into three different microscopic groups: “pseudomelanocytic nests” that are composed of macrophages, keratinocytes, and melanocytic debris without melanocytes; “melanocytic pseudonests” or “mixed nests” that are composed of both keratinocytes, inflammatory cells, and melanocytes; and “true melanocytic nests” made exclusively of melanocytes. The phenomena of junctional nesting and pseudonesting have been observed in various interface lichenoid dermatoses. Thirty-one cases have been identified in the literature, including ours, with a prevalence of 20 males (Table 1) [3,4,5,6,7,8,9,10,11,12,13,14,15,16]. The main diagnoses were cutaneous LE in six cases, lichen planus in four cases, fixed drug eruption in four cases, and a range of conditions with a lichenoid pattern including LPP and lichenoid keratoses, and even graft-vs.-host-disease (Table 1). Most of the cases exhibited a “pseudomelanocytic nests” pattern on histopathological grounds, while the presence of “true melanocytic nests” confirmed by the positivity of nuclear panmelanocytic markers, such as SOX-10 or MITF-1, were found in only a few cases, including ours. Specifically, four cases of LPP have been previously described, but all the patients presented with a “pseudomelanocytic nest” or “melanocytic pseudonest” pattern [6,10,12], which makes them different from our case that seems to be the only one with a “true melanocytic” pattern. However, most provided studies in Table 1 consistently reveal the positive reactivity of these nests to at least one melanocytic marker, often with multiple markers. It is noteworthy that the studies wherein nests exhibited negativity with melanocytic markers pertain to the oral mucosa, potentially lacking relevance to the cutaneous manifestation of LPP.

Table 1.
Review of all cases of melanocytic nests in lichenoid dermatitis [3,4,5,6,7,8,9,10,11,12,13,14,15,16].
As for pathogenesis, the development of true melanocytic nests within LPP lesions may stem from a reorganization of the “epidermo-melanocytic” unit, which is triggered by inflammatory changes in the dermo-epidermal junction. The disruption of the basement membrane zone may activate cytokines or signaling pathways that contribute to the recruitment and proliferation of residual epidermal melanocytes. The identification of true melanocytic nests in LPP requires differentiation from atypical junctional melanocytic proliferations, especially in cases involving sun-damaged skin. In our case, the absence of pagetoid spreading, the lack of confluence of single melanocytes at the dermo-epidermal junction, and significant cytologic atypia associated with typical lichen planus-like features on histopathological grounds along with the clinical features were not consistent with an atypical melanocytic proliferation and favored a diagnosis of LPP with “true melanocytic nests” [6]. Clinicopathological correlation plays a pivotal role in achieving an accurate diagnosis. The collision of lichen planus with a melanocytic nevus (pseudomelanoma-like changes) in conditions like lichen sclerosus, epidermolysis bullosa (“GABEB nevus”), and post-inflammatory states following toxic epidermal necrolysis, burns, and other inflammatory disorders affecting the junctional area of melanocytic nevi further highlights the complexity of histopathological differential diagnosis that can be easily resolved via clinicopathological correlation.
Our patient reported that some identical hyperpigmented lesions were present in her father and brother, but unfortunately, the histopathological analysis of her relatives is not possible for confirmation. Consequently, we are unable to definitively ascertain the potential occurrence of the familial manifestation of LPP, an exceedingly rare phenomenon. While the influence of genetic factors remains uncertain, instances of Lichen Planus (LP) affecting multiple family members have been reported. Notably, a case involving two monozygotic twin sisters with oral LP has been documented, and one of them additionally exhibited LPP [17].
Therapy is difficult and consists of the avoidance of triggers and topical and systemic medications to stop the inflammatory reaction and reduce pigmentation. Drugs including potent topical corticosteroids, oral steroids, topical calcineurin inhibitors, antimalarials, topical and systemic retinoids, and skin-lightening agents were recommended for LPP [2].
4. Conclusions
The presence of true melanocytic nests in LPP lesions highlights the intricate nature of cutaneous inflammatory disorders and their potential to mimic melanocytic proliferations. Accurate diagnosis necessitates rigorous clinicopathological correlation and heightened awareness of this infrequent phenomenon among dermatologists and dermatopathologists. A multifaceted understanding of LPP, underscored by the occurrence of true melanocytic nests, contributes to a more nuanced comprehension of the complex interplay between inflammation and melanocyte behavior.
Author Contributions
Conceptualization, F.R.; methodology, S.G.; software, G.D.B.; validation, F.R.; formal analysis, A.P.B. and G.D.B.; investigation, A.P.B. and G.D.B.; resources, V.C. and L.B.; data curation, S.G.; writing—original draft preparation, A.P.B. and G.D.B.; writing—review and editing, S.G. and F.R.; visualization, G.S.; supervision, F.R.; project administration, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
No additional data relating to this study is available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rieder, E.; Kaplan, J.; Kamino, H.; Sanchez, M.; Pomeranz, M.K. Lichen planus pigmentosus. Dermatol. Online J. 2013, 19, 20713. [Google Scholar] [CrossRef]
- Ghosh, A.; Coondoo, A. Lichen Planus Pigmentosus: The Controversial Consensus. Indian J. Dermatol. 2016, 61, 482–486. [Google Scholar] [CrossRef]
- Maize, J.C., Jr.; Resneck JSJr Shapiro, P.E.; McCalmont, T.H.; LeBoit, P.E. Ducking stray “magic bullets”: A Melan-A alert. Am. J. Dermatopathol. 2003, 25, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Venturini, M.; Manganoni, A.M.; Zanca, A.; Bassissi, S.; Pavoni, L.; Gonzales, S.; Cesinaro, A.; Calzavara-Pinton, P. Pigmented actinic lichen planus (PALP) mimicking lentigo maligna melanoma: Usefulness of in vivo reflectance confocal microscopy in diagnosis and follow-up. JAAD Case Rep. 2018, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.L.; Macedo, R.M.; Almeida, L.Y.; Silva, R.N.; Ribeiro-Silva, A.; León, J.E. Gingival melanoacanthoma associated with pseudomelanocytic nests: Expanding the clinicopathological spectrum of a recently described oral lesion. J. Cutan. Pathol. 2012, 45, 725–727. [Google Scholar] [CrossRef]
- McClanahan, D.; Choudhary, S.; Zahniser, J.; Ho, J. Diagnostic Pitfalls: Pseudomelanocytic Nests in the Setting of Lichenoid Inflammation. Actas Dermosifiliogr. 2019, 110, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Bradamante, M.; Broglia, I.; Petrillo, G.; Stefanato, C.M. Melanocytic and pseudomelanocytic nests coexist in interface dermatitis from head-neck sun-exposed skin: A report of three cases. J. Cutan. Pathol. 2020, 47, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Panse, G.; McNiff, J.M. Lichenoid dermatoses with pseudomelanocytic nests vs inflamed melanoma in situ: A comparative study. J. Cutan. Pathol. 2021, 48, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, I.; Hartschuh, W.; Fink, C.; Haenssle, H.; Enk, A.; Toberer, F. Sudden reticular pigmentation of the face. J. Cutan. Pathol. 2022, 49, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Beltraminelli, H.; Shabrawi-Caelen, L.E.; Kerl, H.; Cerroni, L. Melan-a-positive “pseudomelanocytic nests”: A pitfall in the histopathologic and immunohistochemical diagnosis of pigmented lesions on sun-damaged skin. Am. J. Dermatopathol. 2009, 31, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, K.M.; Gerami, P. An immunohistochemical analysis of pseudomelanocytic nests mimicking melanoma in situ: Report of 2 cases. Am. J. Dermatopathol. 2010, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.Y.; Goldberg, L.J.; Mahalingam, M.; Bhawan, J.; Wolpowitz, D. Nests with numerous SOX10 and MiTF-positive cells in lichenoid inflammation: Pseudomelanocytic nests or authentic melanocytic proliferation? J. Cutan. Pathol. 2011, 38, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Boros, A.L.; Handlers, J.P.; Melrose, R.J. Pseudomelanocytic nests mimicking atypical melanocytic proliferations: First reported cases in the oral cavity. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 118, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Simkin, A.D.; Bhawan, J.; Wolpowitz, D. “Melanocytic Nests Arising in Lichenoid Inflammation”: Reappraisal of the Terminology “Melanocytic Pseudonests”. Am. J. Dermatopathol. 2015, 37, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.D.; Bodendorf, M.O.; Najarian, D.J.; Ferringer, T.; Elston, D. Soluble adenylyl cyclase (sAC) immunostaining distinguishes pseudomelanocytic nests in lichenoid tissue reaction. J. Cutan. Pathol. 2015, 42, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Pantaleão, L.; Orofino-Costa, R.; Torres, M.C.; Lazova, R.; Rochael, M.C. Report of two cases of vacuolar interface dermatitis initially suspected as melanomain situand review of the literature. J. Bras. Patol. Med. Lab. 2016, 52, 116–119. [Google Scholar] [CrossRef]
- Marques, L.C.; Santos, L.R.; da Silva, N.C.; Cunha, K.S.; Junior, A.S.; Conde, D.C. Oral Lichen Planus Associated with Lichen Planus Pigmentosus and Lichen Sclerosus in Monozygotic Twins. Am. J. Dermatopathol. 2021, 43, 368–372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).