Abstract
Background: Fibromyalgia (FM) is a complex condition marked by increased pain sensitivity and central sensitization. Studies often explore the link between FM and depressive anxiety disorders, but few focus on dysthymia or persistent depressive disorder (PDD), which can be more disabling than major depression (MD). Objective: To identify clinical scales and subscales of the Personality Assessment Inventory (PAI) that effectively describe and differentiate the psychological profile of PDD, with or without comorbid MD, in FM patients with PDD previously dimensionally classified by the Millon Clinical Multiaxial Inventory III (MCMI-III). Method: An observational, cross-sectional study was conducted with 66 women (mean age 49.18, SD = 8.09) from Hospital del Mar. The PAI, the MCMI-III, and the Fibromyalgia Impact Questionnaire (FIQ) were used to assess the sample. Results: The PAI showed strong discriminative ability in detecting PDD, characterized by high scores in cognitive and emotional depression and low scores in identity alteration, dominance, and grandeur. High scores in cognitive, emotional, and physiological depression, identity alteration, cognitive anxiety, and suicidal ideation, along with low scores in dominance and grandeur, were needed to detect MD with PDD. Discriminant analysis could differentiate 69.6–73.9% of the PDD group and 84.6% of the PDD+MD group. Group comparisons showed that 72.2% of patients with an affective disorder by PAI were correctly classified in the MCMI-III affective disorder group, and 70% without affective disorder were correctly classified. Conclusions: The PAI effectively identifies PDD in FM patients and detects concurrent MD episodes, aiding in better prognostic and therapeutic guidance.
1. Introduction
Fibromyalgia (FM) is a complex disease characterized by heightened pain sensitivity with a multifactorial etiopathogenesis (Thieme et al., 2017). It is defined as a central sensitization syndrome (Ablin et al., 2012; Arnold et al., 2016) due to aberrant central pain processing (López-Solà et al., 2017; Pujol et al., 2014; Sluka & Clauw, 2016). Comorbidly, individuals with FM often exhibit psychopathological disorders, affecting 13–80% of cases, which are predominantly categorized within affective spectrum disorders (Arnold et al., 2004; Duque & Fricchione, 2019; Gálvez-Sánchez et al., 2019; Kleykamp et al., 2021). Mental health problems seem to play a significant role in the development, course and maintenance of this condition (Gálvez-Sánchez et al., 2019; Hirsch et al., 2021), with a dysfunctional psychological coping state considered key to poorer physical health and quality of life (Duque & Fricchione, 2019; Gálvez-Sánchez et al., 2019; Martínez et al., 2021a; Garcia-Fontanals et al., 2017).
FM has been associated with biological etiologies, including central hyperactivation, endocrine system dysfunction, and altered sensory processing (Pujol et al., 2014). Additionally, psychological factors play a significant role in the characteristic pain of fibromyalgia (Thieme et al., 2017; Gálvez-Sánchez et al., 2019). Various psychological models provide insights into the interaction between central sensitization, emotional factors, and social stressors. The Biopsychosocial Model illustrates how these elements disproportionately affect affective spectrum disorders in fibromyalgia. Cognitive Behavioral Theory links dysfunctional thought patterns, such as catastrophizing and pain hypervigilance, to increased pain perception and emotional distress. Social Cognitive Theory highlights the impact of low self-efficacy and ineffective coping on functional disability, while the Somatic Symptom Model underscores how psychological factors mediate physical symptom amplification (Gálvez-Sánchez et al., 2019).
FM patients do not form a clinically homogenous group, and not all of them exhibit comorbid mental disorders (Garcia-Fontanals et al., 2017; Fernández-de-Las-Peñas et al., 2023; González et al., 2020, 2021). However, there is a specific psychopathological profile associated with FM that distinguishes it from other chronic pain conditions (González et al., 2019; López-Ruíz et al., 2019). The presence of FM tends to have a more pronounced impact on mood and anxiety disorders (Kleykamp et al., 2021; Garcia-Fontanals et al., 2017; Henao-Pérez et al., 2022; Janssens et al., 2015). Among these, major depressive disorder is the most prevalent, affecting 28–63% of women and 23–42% of men, while dysthymic disorder affects 50–53% of FM patients (Kleykamp et al., 2021; Garcia-Fontanals et al., 2017; Henao-Pérez et al., 2022). Nearly one-third of FM patients may also suffer other mental conditions, including bipolar disorder (26%), panic disorder (33%), post-traumatic stress disorder (16–39%), anxiety disorder (8–30%), obsessive–compulsive disorder (2–4%), and specific phobias (14–17%) (Kleykamp et al., 2021). In addition to these psychological issues, FM symptoms such as cognitive performance problems and somatoform problems (Brooks et al., 2012), general fatigue, and poor sleep quality (Martínez et al., 2021a; Berkol et al., 2017; Gupta & Moldofsky, 1986) are also associated with psychopathological problems like pain catastrophizing, pain hypervigilance, and lower levels of pain self-efficacy and pain acceptance (Martínez et al., 2021b).
Several studies have examined the link between depressive anxiety disorders and FM, although few have focused on dysthymia or persistent depressive disorder (PDD), which is often more disabling than major depression (MD) (Schramm et al., 2020). PDD is a chronic state of depressed mood for most of the days, lasting 2 years or longer. It implies a high risk of developing MD (Schramm et al., 2020; Ventriglio et al., 2020) and particularly showcases a melancholic temperament among those affected patients (Ventriglio et al., 2020). In fact, this affective disorder has often been called “double depression” (Keller et al., 1997), with personality disorders (Garcia-Fontanals et al., 2017; Schramm et al., 2020) and somatoform conditions (Ventriglio et al., 2020) being commonly comorbid (Schramm et al., 2020; Ventriglio et al., 2020). Indeed, following the current Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, FM patients exhibit a higher prevalence of a diagnosis related to somatic disorder and/or PDD in comparison to other chronic pain conditions without central sensitization syndrome (López-Ruíz et al., 2019; Berkol et al., 2017; Ciaramella et al., 2020). In the broader population with chronic pain, the prevalence of MD and PDD falls within ranges between 10 and 30% (Howe et al., 2015). Furthermore, only chronic fatigue, frequently associated with FM, displays a higher prevalence of PDD than MD (Janssens et al., 2015).
The assessment of affective disorders in FM presents challenges due to inconsistent findings (Løge-Hagen et al., 2019), attributed to overlapping somatic symptoms (Duque & Fricchione, 2019) and varying study criteria (Torres et al., 2013). Nevertheless, identifying depressive disorders, especially PDD, is essential as they cause intense suffering for these patients with particular needs and deficits (Brinkmann et al., 2019). For this purpose, a variety of psychopathological multidimensional screening tools have been utilized, including the Symptom Checklist-90-Revised (SCL-90-R) (Keller et al., 2011; Glazer et al., 2009; Salgueiro et al., 2012), the Minnesota Multiphasic Personality Inventory (MMPI) (González et al., 2020, 2021, 2019; Novo et al., 2017; Naylor et al., 2017), the Millon Clinical Multiaxial Inventory (MCMI) (Garcia-Fontanals et al., 2017; López-Ruíz et al., 2019; Brooks et al., 2012; Naylor et al., 2017), and recently the Personality Assessment Inventory (PAI) (Doreste et al., 2024). The MCMI shows appealing measurement characteristics for routine clinical practice, particularly its brief administration and robust theoretical focus based on dimensional criteria and classification approximation to the DSM, facilitating the assessment of psychopathological and personality issues (Brooks et al., 2012; Rossi & Derksen, 2015; Teixidó-Abiol et al., 2023). Specifically, it demonstrates diagnosis efficiency for both categorical and dimensional assessment of PDD and melancholic personality traits (Saulsman, 2011), distinguishing itself from other screening or multidimensional tests.
The PAI is effective in uncovering a range of psychopathological and personality concerns, boasting robust psychometric properties, particularly among individuals with chronic pain (Karlin et al., 2005), including those with FM (Doreste et al., 2024). Furthermore, PAI allows for more specific identification of affective disorder subtypes and other clinical disorders, better assessment of personality disorders, and a reduced emphasis on psychosomatic issues. However, the PAI may not fully capture the psychopathological profile associated with PDD, a limitation that is addressed by using the MCMI. This study aims to examine which clinical scales and subscales scores of the PAI can accurately describe and effectively discriminate the psychological profile of PDD, with or without MD, in a sample of FM patients previously classified based on the MCMI criterion for the PDD profile.
2. Method
2.1. Eligibility Criteria
The research included females aged 18 to 65 diagnosed with FM according to the criteria established by the American College of Rheumatology (Wolfe et al., 1997). Inclusion criteria involved having a stable pharmacological treatment, understanding the study requirements, and a commitment to compliance. Exclusion criteria encompassed the presence of other conditions explaining pain; inflammatory or rheumatic diseases; severe or unstable medical, endocrine, or neurological conditions; a history of neuropathic pain; acute psychotic disorders; substance abuse; and invalidating scores on the MCMI and PAI validity scales, which could compromise the interpretation of results.
2.2. Participants
Patients were enlisted from the Fibromyalgia Unit at Barcelona’s Hospital del Mar by senior rheumatologists (FO or JM) and a senior psychologist (JD) between January 2021 and June 2022. During the study period, 136 female patients were diagnosed with FM, and 110 underwent eligibility assessments during consecutive clinical visits. Forty-four either did not meet the study criteria or declined participation, resulting in a final sample of sixty-six participants who completed both the MCMI-III and PAI questionnaires. Detailed sociodemographic and clinical characteristics can be found in Table 1.

Table 1.
Clinical and sociodemographic characteristics of the female sample [N = 66).
2.3. Study Design and Procedure
We used a non-randomized, purposeful sampling method to include all eligible participants from the study population. This was an observational, cross-sectional study. Female patients initially attended their regular rheumatology appointments (FO and JM). After thorough screening for inclusion/exclusion criteria and willingness to participate, they were enrolled in the study, and informed consent was obtained. Psychological assessments, conducted by another senior clinical psychologist (AD), occurred within the same week and lasted up to an hour and a half to prevent response fatigue.
2.4. Instruments
Millon Clinical Multiaxial Inventory III (MCMI-III), we administered the Spanish version of the MCMI-III (Cardenal & Sánchez, 2007). The MCMI-III (Millon et al., 2009) is a self-administered questionnaire of 175 true–false items that provide insights into personality and psychopathology patterns aligned with the disorders outlined in the DSM (-IV, -IV-R and -V). It comprises a total of 28 scales: 11 basic personality scales and 3 severe personality pathology scales, 7 clinical syndromes and 3 severe clinical syndromes, 3 modifying indices, and a validation scale. The MCMI-III uses “Base Rate” scores for reporting and interpretation. The Base Rate scores (BR) are classified as follows: general population (0–34), low range (35–59), likelihood of presenting the syndrome/trait (60–74), presence of the syndrome/trait (75–84), severe presence of syndrome/trait (85–115). The last two categories are the cut-off points for the instrument. The Spanish adaptation [44] has shown internal consistency > 0.80 with coefficients in personality scales (0.66–0.89), clinical syndromes (0.71–0.90), test-retest values (0.84–0.96), and sensitivity (0.44–0.92) (Millon, 2006; Ortiz-Tallo et al., 2011). The MCMI-III’s dimensional and classificatory approach to the DSM makes it a widely used multidimensional inventory for clinicians, serving as a tool to check the reliability and validity of other test scores (Rossi & Derksen, 2015). Specifically, the Depressive Personality, Major Depression, and Dysthymia scale scores capture depressive pathology and show large convergent correlations with the depression symptom measure by the Beck Depression Inventory (r = 0.48; r = 0.69; r = 0.67 with p < 0.001, respectively). Also, Dysthymia and Major Depression scale scores (at a cut-off of 75) showed an acceptable diagnosis accuracy (Specificity = 0.64; Specificity = 0.87, respectively; both p < 0.001) (Saulsman, 2011).
Personality Assessment Inventory (PAI) (Morey, 1991), in its Spanish version (Burneo-Garcés et al., 2020), is a widely utilized self-administered tool consisting of 344 items designed to assess various psychopathological features and personality disorders. The PAI includes 27 scales encompassing 4 validity scales, 5 complementary validity scales, 11 clinical scales, 5 treatment consideration scales, and 2 interpersonal scales, along with 30 conceptually driven clinical subscales. This comprehensive set of scales and subscales allows for the identification of diverse psychopathological profiles, including 15 clinical syndromes and 11 personality disorders (Ortiz-Tallo et al., 2011). Participants rate the 344 items on a Likert-type scale (ranging from 1—not at all true—to 4—very true), and raw scores are converted to T-scores based on Spanish norms. Generally, a T-score > 61 suggests a moderate to marked tendency of a prominent psychopathological trait (Karlin et al., 2005; Burneo-Garcés et al., 2020; Morey & Alarcón, 2013). Nevertheless, it is important to highlight that certain scales may require distinct cut-off points to achieve discriminative capacity, as outlined in the PAI implementation and interpretation manual (Morey & Alarcón, 2013) (Figure 1). The Spanish adaptation exhibited appropriate reliability (0.82), internal consistency (0.78 in the healthy sample and 0.83 in the clinical sample), and satisfactory content and convergent validity when evaluating personality and psychopathology across normative, college, and clinical samples (Burneo-Garcés et al., 2020). In chronic pain patients, the internal consistency reliability coefficients for the PAI full scales and subscales scores are acceptable, consistent with previous reports in chronic pain patients. Consequently, the PAI demonstrated crucial psychometric properties when applied to chronic pain settings (Karlin et al., 2005).
Fibromyalgia Impact Questionnaire (FIQ) (Burckhardt et al., 1991), administered in its Spanish version (Rivera & González, 2004), is a self-reported instrument designed to assess the overall functional impact of FM on an individual’s daily life. It includes a range of aspects, such as physical functioning, work-related difficulties, and psychological distress, providing a comprehensive evaluation of the condition’s effects. Scores range from 0 to 100, where higher values indicate a more pronounced impact. The Spanish version of FIQ exhibits adequate internal consistency (α of 0.81), test-retest reliability after 7 days (significant correlations ranging from 0.52 for fatigue and 0.53 for pain to 0.91 for depression), and internal validity, as well as sensitivity to change (Monterde et al., 2004). By capturing the multidimensional impact of FM, we consider that the FIQ serves as a valuable tool for clinicians and researchers to understand the extent of impairment and guide treatment decisions.
2.5. Data Analysis
A descriptive analysis of sociodemographic and clinical features was conducted to delineate the characteristics of the entire study sample, utilizing IBM SPSS software (Version 21.0, IBM Corp, Armonk, NY, USA) for all analyses. Also, the study sample was divided into three groups based on the dimensional psychological assessment of the MCMI-III regarding the absence, presence, and type of affective disorder, defining: (1) a group with only PDD (group PDD) characterized by BR scores above 75 on that scale; (2) a group with both PDD and MD (group PDD+MD) with scores exceeding 75 BR scores on both scales; and (3) a group without affective disorder (group NAD), with BR scores below 75 on the dysthymia and major depression scale. Furthermore, we obtained a detailed sociodemographic and clinical characteristic for each group and compared them through the non-parametric Kruskal–Wallis test and Chi-square. Finner’s correction was applied. Statistical significance was set at 5%. So, the data analysis involved three main steps:
PAI group comparison. A comparison between the three groups of patients (NAD vs. PDD vs. PDD+MD) was carried out to study the psychopathological profile of the PAI clinical scales and subscales using a non-parametric Kruskal–Wallis test. Finner’s correction was applied. All reported p-values were two-tailed.
Predictors selection. Pairwise comparisons between each pair of groups NAD, PDD, and PDD+MD were conducted using Dunn’s non-parametric pairwise test. Cohen’s d (Cohen, 1988) was calculated for each comparison to assess the effect size, with values of 0.20 indicating a small effect, 0.50 medium, and 0.80 large. This analytical approach allows for a more comprehensive exploration of which clinical scales and subscales of the PAI enable the definition of a psychopathological profile consistent with PDD, with and without MD.
Multiple models. Two discriminant analyses, using the Wilks’ lambda, were performed to assess whether the set of clinical scales and subscales and complementary items of the PAI adequately discriminated which patients exhibited symptomatology of affective disturbance consistent with PDD, with or without MD (PDD vs. NAD and PDD vs. PDD+MD), using a cut-off point (the centroid). The matrices of homogeneity were tested using Box’s M test of equality of covariance. Canonical correlation was applied to measure the association between the discriminant function and the group of PAI variables. Following this, classificatory analysis and cross-validation demonstrated the allocation accuracy for each discriminant analysis. Once the PAI scales were determined in each group, the PDD, NAD, and PDD+MD groups were reformed using these scales and subscales with a cut-off point of 60, which determines the limit of scores within normality. This allowed for observing the percentage of patients in each group, corroborating the discriminant analysis, and conducting a Kappa analysis to compare the 3 groups according to MCMI-III criteria with those based on the PAI scales.
3. Results
After descriptive analysis, comparison analysis was applied to the groups with or without affective disorder, such as PDD without MD (PDD; 34.8%; n = 23), with MD (PPD+MD; 19.7%; n = 13), and NAD (NAD; 45.5%; n = 30). Patients in the PDD+MD group showed significantly higher severity of fatigue and stiffness after resting (p = 0.034 and p = 0.017, respectively) and mood symptoms (depression p = 0.012 and anxiety p < 0.005). Additionally, these patients exhibited a significantly worse overall Fibromyalgia Impact Questionnaire (FIQ) score (p = 0.028), with increased morning tiredness and depression (p = 0.034 both) (Table 2).

Table 2.
Clinical and sociodemographic characteristics of the three groups of fibromyalgia patients based on the presence or absence and type of affective disorder.
PAI group comparison. We compared the psychopathological profile on the PAI among FM groups as per the MCMI-III criteria (Figure 1A,B). All three groups exhibited significantly high T-scores, with mean scores exceeding the defined cut-off point, on the clinical scales of somatization, anxiety, and depression, but only the PDD+MD group had high scores on the anxiety-related disorders scale, schizophrenia scale, and on a single treatment scale, corresponding to suicidal ideation. Moreover, the PDD+MD group exhibited a statistically significant greater elevation on three clinical scales and one treatment scale: anxiety (M = 73.85, SD = 0.38), depression (M = 81.92, SD = 6.30), schizophrenia (M = 65.31, SD = 8.10), and increased suicidal ideation (M = 70.00, SD = 16.88). The PDD group also showed high scores in anxiety (M = 66.09, SD = 10.48) and depression (M = 73.78, SD = 6.37). Additionally, the PDD+MD group exhibited values within PAI criteria normality but statistically significantly higher when compared to NAD patients in potential suicide index (M = 73.62, SD = 6.66), potential violence index (M = 58.62, SD = 14.22), and treatment difficulty index (M = 61.23, SD = 10.75); and PDD patients showed statistically significant lower scores in interpersonal scale dominance (M = 44.09, SD = 11.14) (Figure 1A).
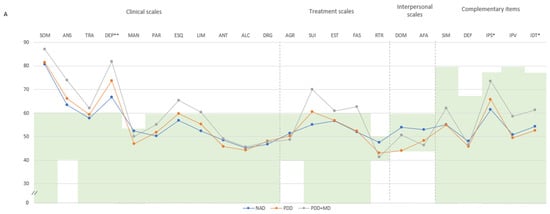
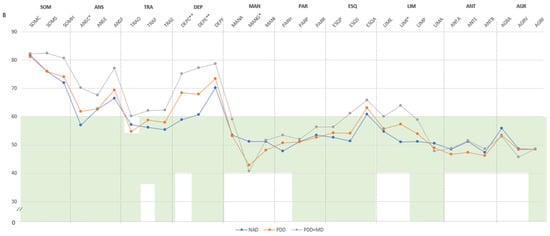


Figure 1.
(A) Comparison between groups in PAI scales and complementary items; (B) Comparison between groups in PAI subscales. (A) The green zone indicates the ranges of normality, according to the psychometric criteria of the PAI. SOM: Somatic Complaints; ANS: Anxiety; TRA: Disorders Related to Anxiety; DEP: Depression; MAN: Mania; PAR: Paranoia; ESQ: Schizophrenia; LIM: Limit Traits; ANT: Antisocial Traits; ALC: Problems with alcohol; DRG: Problems with drugs; AGR: Aggression: SUI: Suicidal Ideation; EST: Stress; FAS: Lack of social support; RTR: Refusal to treatment; DOM: Dominance; AFA: Affability; SIM: Simulation Index; DEF: Defensiveness Index; IPS: Potential Suicide Index; IPV: Potential index of violence; IDT: Treatment Difficulty Index; FM: fibromyalgia group; GC: theoretical control group; (B). Note: SOM-C: Conversion; SOM-S: Somatization; SOM-H: Hypochondria; ANS-C: Cognitive; ANS-E: Emotional; ANS-F: Physiological; TRA-O: Obsessive-compulsive; TRA-F: Phobias; TRA-E: Posttraumatic Stress; DEP-C: Cognitive; DEP-E: Emotional; DEP-F: Physiological; MAN-A: Activity level; MAN-G: Grandeur; MAN-I: Irritability; PAR-H: Hypervigilance; PAR-P: Persecution; PAR-R: Resentment; ESQ-P: Experiences. Psychotics; ESQ-S: Social Indifference; ESQ-A: Alteration of the Thought; LIM-E: Emotional instability; LIM-I: Alteration of identity; LIM-P: Problematic Interpersonal Relationships; LIM-A: Self-aggression; ANT-A: Antisocial Behaviours; ANT-E: Egocentrism; ANT-B: Search for sensations; AGR-A: Attitude aggressive; AGR-V: Verbal aggression; AGR-F: Physical assaults; * statistically significant differences between groups; * p < 0.05; ** p < 0.01.
According to the clinical subscales (Figure 1B), all three groups of FM patients exhibited high T-scores in illness-health concern, cognitive anxiety, physiological anxiety, cognitive depression, emotional depression, physiological depression, and thought alteration. Nevertheless, the PDD+MD group showed statistically significant elevations compared to those with NAD and PDD in illness-health concern (M = 80.62, SD = 7.84), cognitive anxiety (M = 70.15, SD = 10.89), physiological anxiety (M = 77.08, SD = 12.12), cognitive depression (M = 75.16, SD = 8.10), emotional depression (M = 77.23, SD = 11.60), and physiological depression (M = 78.69, SD = 6.67). The PDD group also scored high in illness-health concern (M = 74.04, SD = 10.68), cognitive anxiety (M = 61.83, SD = 10.59), physiological anxiety (M = 69.35, SD = 12.87), cognitive depression (M = 68.35, SD = 9.57), emotional depression (M = 68.00, SD = 8.37), and physiological depression (M = 73.48, SD = 7.61), and the NAD group also scored high in illness-health concern (M = 71.97, SD = 11.06), physiological anxiety (M = 66.57, SD = 9.76), and physiological depression (M = 70.13, SD = 8.32). Additionally, the PDD group exhibited a score within PAI criteria normality but statistically significantly lower in grandeur (M = 42.78, SD = 7.17) and PDD+MD in grandeur (M = 40.77, SD = 8.32); this last group also scored higher in social indifference (M = 61.15, SD = 14.34), thought alteration (M = 65.92, SD = 8.60), and identity alteration (M = 63.92, SD = 7.48) (Figure 1B).
Predictors selection. Pairwise comparisons among the three groups were conducted on the statistically significant and most relevant scale scores of PAI identified in the first step, obtaining the Cohen’s d for a further explanation of the data (Table 3). Based on these results, we found a psychopathological profile of the PDD group derived from the PAI scores that is presumed to hinge on two stringent conditions, supported by a moderate to large effect size according to Cohen’s d: (a) large effect size (d ≥ 0.80) for scales showing mean scores exceeding the defined cut-off point (i.e., depression and cognitive depression) and for scales showing mean scores below the cut-off point (dominance); (b) medium effect size (d = 0.50–0.79) for the scales with mean scores surpassing the defined cut-off point (emotional depression), as well as for scales with mean scores below the cut-off point (grandeur and identity alteration). Moreover, from these findings, we identified a psychopathological profile of the PDD+MD group that appears to be contingent on the same procedure but different scales according to Cohen’s d: (a) large effect size (d ≥ 0.80) for those scales showing mean scores exceeding the defined cut-off point (depression and emotional depression) and for those showing mean scores below the cut-off point (suicidal potential index, violence potential index, and treatment difficulty index); (b) medium effect size (d = 0.50–0.79) for the scales with mean scores surpassing the defined cut-off point (anxiety, schizophrenia, suicidal ideation, illness-health concern, cognitive anxiety, physiological anxiety, cognitive depression, physiological depression, and identity alteration).

Table 3.
Pairwise comparison in PAI scales between groups of study by Kruskal–Wallis and Dunn’s non-parametric comparison.
Multiple models. The homogeneity values of the variance-covariance matrices were p = 0.428 for the NAD vs. PDD analysis and p = 0.571 for the PDD vs. PDD+MD analysis. The standardized coefficients and canonical correlation values of each discriminant analysis are reported in Table 4. First, the centroid values for classifying the PDD group were 0.689 and −0.529 for the NAD group. Subsequently, the centroid values for classifying the groups were −0.535 and 0.946 for the PDD and PDD+MD groups, respectively. The classification accuracy was tested, revealing that 73.9% of the cases were correctly classified into the original PDD group and 66.7% into the NAD group. Finally, cross-validation classification resulted in 69.6% and 84.6% for PDD and PDD+MD groups, respectively. Subsequently, the PDD, NAD, and PDD+MD groups were redefined using these scales and subscales, resulting in 28.8% classified as dysthymia by PAI (PDD2), 47% no-affective disorder by PAI (NAD2), and 24.2% dysthymia with major depression by PAI (PDD+MD2). The scales and subscales extracted from this outcome discriminated between 84.2 and 94.7% of PDD2 and 100% of PDD+MD2 cases. Additionally, a Kappa analysis comparing the groups defined by MCMI-III and the groups derived from the PAI scales and subscales showed a match of 43.5% in PDD vs. PDD2 and 53.8% in PDD+MD vs. PDD+MD2, with an overall agreement of 72.2% for affective disorders and 70% for NAD vs. NAD2.

Table 4.
Standardized coefficients and canonical correlation values of discriminant analysis.
4. Discussion
The primary aim of this research was to delineate two psychological profiles based on the PAI that will facilitate the detection of persistent depressive disorder (PDD) or dysthymia and its co-occurrence with major depression (MD) in FM patients. Comparative analysis between groups allowed us to define a psychological profile by PAI of pure PDD, characterized by normal or low scores in dominance, grandeur, and identity alteration and high scores in cognitive and emotional depression. Additionally, we defined a psychological profile of PDD concurrent with MD, along with higher scores in cognitive, emotional, and physiological depression, cognitive anxiety, identity alteration, and suicide potential index. Discriminant analysis has allowed discrimination between 69.6 and 73.9% of the PDD group and 84.6% of the PDD+MD group, indicating robust discriminatory power of the PAI scales and subscales. It also discriminates between 84.2 and 94.7% of PDD according to the criteria that we established in PAI scales and subscales and 100% in PDD+MD, also showing a high level of precision according to the proposed criteria based on the analyses. We have been able to propose a psychometric profile using a set of PAI scales that enables the diagnosis of PDD with and without MD.
It is well established that FM patients present heterogeneous symptomatology not only in moods but in physical symptoms (Martínez et al., 2021b; Wan et al., 2019), as we can see in our sample, where fatigue, headache, morning tiredness, and stiffness are significantly higher in groups with affective disorders than in NAD; this is unlike pain that remains equally severe across the three FM groups. These findings suggest that affective disorders might not directly cause pain but could potentially regulate it, as there may be a type of fibromyalgia where pain and fatigue are significant entities, with the latter potentially independent of the pain. Notably, the somatization scale has shown high scores in PAI across all three groups, reinforcing the hypothesis of somatic involvement in FM, as FM patients exhibit a higher prevalence of somatic complaints compared to other chronic pain patients. In other words, the pain and somatization problems are the disease themselves, and only somatosensory amplification can predict FM, suggesting that somatization disorder and FM are distinct entities (Gálvez-Sánchez et al., 2019; Cohen-Biton et al., 2022).
Dysthymia, characterized by persistent depressive symptoms lasting for at least two years, is often overlooked in medical settings and involves 53–54.5% of our sample, like 50% found in (Garcia-Fontanals et al., 2017). Accurately distinguishing between depressed mood and clinical depression is crucial, especially in chronic pain treatment, where PDD is often dismissed. In this study, PAI has demonstrated strong consistency in detecting PDD with high scores in cognitive and emotional depression. We also found low scores in identity alteration, dominance, and grandeur associated with low self-esteem and the belief that one is incapable, dependent on others, and has a profile of low dominance and self-control. Lower self-esteem reduces neurocognitive performance in FM, particularly affecting attention, memory, and planning, which are symptoms of FM. Proper diagnostic methods and criteria help differentiate PDD subtypes, such as ’anxious dysthymia’, characterized by low self-efficacy and anxiety, and ’anergic dysthymia’, characterized by low energy and anhedonia, adding variability to FM groups (Ventriglio et al., 2020; Howe et al., 2015; Niculescu & Akiskal, 2001).
Affective disorders are prevalent in FM and significantly impact quality of life and disability (Duque & Fricchione, 2019), often co-occurring with PDD. FM patients usually feel isolated, misunderstood, or rejected by relatives, friends, health workers, and society in general. This may contribute to the high prevalence of depression that is associated with increased pain intensity, irritability, physical and mental strain, functional limitations, the number of tender points, non-restorative sleep, neurocognitive deficits, and fatigue (Gálvez-Sánchez et al., 2019). In this context, our analysis using PAI scores indicated that detecting major depression requires high scores in cognitive, emotional, and physiological depression; identity alteration; cognitive anxiety; and suicidal ideation; as well as low scores in dominance and grandeur. Additionally, chronic stressors, particularly somatic conditions, can worsen the condition and increase suicide rates. Furthermore, studies indicate a higher prevalence of suicidality in FM patients than in the general population, where their risk of suicide is similar to that observed in other chronic diseases and correlates with depression, anxiety, sleep quality, and overall mental health (Schramm et al., 2020).
The findings underscore the relevance of psychological models (e.g., Biopsychosocial, Cognitive Behavioral, and Social Cognitive) in understanding the interaction between chronic pain, mental health, and personality traits in fibromyalgia. These frameworks support the development of tailored interventions, such as cognitive–behavioral strategies, self-efficacy enhancement, acceptance and commitment therapy, and somatic symptom management. Incorporating a biopsychosocial approach in primary care can promote multidisciplinary collaboration, improve patient outcomes, and inform health policy.
Furthermore, the study highlights FM’s complexity, demanding multidimensional assessment and treatment. While both the MCMI-III and the PAI are crucial in diagnosing psychopathology, including chronic pain patients, they differ in their approach. The MCMI-III aids in clinical and personality disorder assessment, providing a categorical dysthymia diagnosis cut-off, while the PAI offers more comprehensive evaluation using dimensional functions to approach MCMI-III diagnoses, potentially enhancing symptom understanding. Among these analyses with PAI scores, results for PDD diagnosis and co-occurrence with major depression episodes entails a cut-off score of 60 in the mentioned scales and subscales. Following group comparison, it was found that 72.2% of patients with affective disorder by PAI were correctly classified in the MCMI-III group with affective disorder, along with 70% of NAD patients, aiding in reducing false positives and improving classification accuracy.
The study has limitations to consider for future research improvements. In this study, a cut-off score of 60 has been used to determine a high score, but using a cut-off of 70 for cognitive and emotional depression will improve the detection of dysthymia with major depression disorder, where we have observed potential accuracy enhancement. A general study limitation may be that despite strict patient recruitment criteria, our cohort might exclude very vulnerable individuals unwilling or unable to participate in tests on a specific day, limiting our sample despite thorough selection. Additionally, we do not have a control group, and we have not conducted a diagnostic interview for dysthymia; instead, we form the groups based on the MCMI-III scores. In future research we want to replicate the study with a PDD group with and without FM. Another limitation is the lack of exploration of normal personality traits, such as those assessed by the Big Five, which could offer further insights into fibromyalgia and related disorders (Kang et al., 2024). Lastly, medication may interfere with present symptomatology, as significant group differences existed and remained unchanged during evaluation, thus not altering the established pattern in patients.
In conclusion, this study provides a theoretical contribution by offering a psychometric profile by PAI that allows for the detection of persistent depressive disorder (PDD), with or without major depression. While the PAI can already assess 25 DSM-based mental disorders, it did not previously include a specific profile for PDD. Our findings help define when a patient may present with dysthymia, contributing to its clinical utility. It is interesting to note that there are few questionnaires that work properly with FM pathology, and it is of clinical interest to be able to conduct a correct assessment that facilitates the detection of affective disorder types and allows for improving prognostic and therapeutic guidance.
Author Contributions
Conceptualization, J.D. and J.P.; methodology, E.P.; validation, V.P.; formal analysis, A.D.; investigation, F.O., J.M., G.M.-V., and H.P.-T.; writing—original draft preparation, A.D and J.D.; writing—review and editing, L.B.-H. and J.P.; All authors have read and agreed to the published version of the manuscript.
Funding
This study received partial support from the Ministry of Science and Innovation of Spain (Grant PID2021-127703OB-I00, MICIU/AEI/10.130391/501100011033 and FEDER/UE) and the Agency of University and Research Funding Management of the Catalonia Government within the Research Groups SGR 2017/00717 framework.
Institutional Review Board Statement
This research followed the guidelines set forth in the Declaration of Helsinki and obtained approval from the Ethical Committees: Parc de Salut Mar of Barcelona (reference 6932/I) and the Commission on Ethics in Animal and Human Experimentation (CEEAH) at the Autonomous University of Barcelona (UAB) (reference 6496).
Informed Consent Statement
Written informed consent was acquired from all participating patients.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ablin, J. N., Buskila, D., Van Houdenhove, B., Luyten, P., Atzeni, F., & Sarzi-Puttini, P. (2012). Is fibromyalgia a discrete entity? Autoimmunity Reviews, 11(8), 585–588. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L. M., Gebke, K. B., & Choy, E. H. (2016). Fibromyalgia: Management strategies for primary care providers. International Journal of Clinical Practice, 70(2), 99–112. [Google Scholar] [CrossRef]
- Arnold, L. M., Hudson, J. I., Hess, E. V., Ware, A. E., Fritz, D. A., Auchenbach, M. B., Starck, L. O., & Keck, P. E. (2004). Family study of fibromyalgia. Arthritis & Rheumatism, 50(3), 944–952. [Google Scholar] [CrossRef]
- Berkol, T. D., Balcioglu, Y. H., Kirlioglu, S. S., Erensoy, H., & Vural, M. (2017). Dissociative features of fibromyalgia syndrome. Neurosciences (Riyadh), 22(3), 198–204. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, E., Glanert, S., Hüppe, M., Moncada Garay, A. S., Tschepe, S., Schweiger, U., Klein, J. P., & Härter, M. (2019). Psychometric evaluation of a screening question for persistent depressive disorder. BMC Psychiatry, 19(1), 119. [Google Scholar] [CrossRef]
- Brooks, L., Johnson-Greeme, D., Lattie, E., & Ference, T. (2012). The relationship between performance on neuropsychological symptom validity testing and the MCMI-III in patients with fibromyalgia. Clinical Neuropsychologist, 26(5), 816–831. [Google Scholar] [CrossRef]
- Burckhardt, C. S., Clark, S. R., & Bennett, R. M. (1991). The Fibromyalgia Impact Questionnaire: Development and validation. Journal of Rheumatology, 18(5), 728–733. [Google Scholar] [PubMed]
- Burneo-Garcés, C., Fernández-Alcántara, M., Aguayo-Estremera, R., & Pérez-García, M. (2020). Psychometric properties of the Spanish adaptation of the Personality Assessment Inventory in correctional settings: An ESEM study. Journal of Personality Assessment, 102(1), 75–87. [Google Scholar] [CrossRef] [PubMed]
- Cardenal, V., & Sánchez, M. P. (2007). Adaptación y baremación al español del inventario clínico multiaxial de millon-III (MCMI-III). TEA Ediciones, S.A. [Google Scholar]
- Ciaramella, A., Silvestri, S., Pozzolini, V., Federici, M., & Carli, G. (2020). A retrospective observational study comparing somatosensory amplification in fibromyalgia, chronic pain, psychiatric disorders and healthy subjects. Scandinavian Journal of Pain, 21(2), 317–329. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum. [Google Scholar]
- Cohen-Biton, L., Buskila, D., & Nissanholtz-Gannot, R. (2022). Review of fibromyalgia (FM) syndrome treatments. International Journal of Environmental Research and Public Health, 19(12), 12106. [Google Scholar] [CrossRef] [PubMed]
- Doreste, A., Pujol, J., Penelo, E., Pérez, V., Blanco-Hinojo, L., Martínez-Vilavella, G., Pardina-Torner, H., Ojeda, F., Monfort, J., & Deus, J. (2024). Exploring the psychopathological profile of fibromyalgia: Insights from the personality assessment inventory and its association with disease impact. Frontiers in Psychology, 15, 1418644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duque, L., & Fricchione, G. (2019). Fibromyalgia and its new lessons for neuropsychiatry. Medical Science Monitor Basic Research, 25, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C., Valera-Calero, J. A., Arendt-Nielsen, L., Martín-Guerrero, J. D., Cigarán-Méndez, M., Navarro-Pardo, E., Pellicer-Valero, O. J., & Sterling, M. (2023). Clustering analysis identifies two subgroups of women with fibromyalgia with different psychological, cognitive, health-related, and physical features but similar widespread pressure pain sensitivity. Pain Medicine, 24(7), 881–889. [Google Scholar] [CrossRef]
- Gálvez-Sánchez, C. M., Duschek, S., & Reyes Del Paso, G. A. (2019). Psychological impact of fibromyalgia: Current perspectives. Psychology Research and Behavior Management, 12, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fontanals, A., Portell, M., García-Blanco, S., Poca-Dias, V., García-Fructuoso, F., López-Ruiz, M., Gutiérrez-Rosado, T., Gomà-i-Freixanet, M., & Deus, J. (2017). Vulnerability to psychopathology and dimensions of personality in patients with fibromyalgia. Clinical Journal of Pain, 33(11), 991–997. [Google Scholar] [CrossRef] [PubMed]
- Glazer, Y., Cohen, H., Buskila, D., Ebstein, R. P., Glotser, L., & Neumann, L. (2009). Are psychological distress symptoms different in fibromyalgia patients compared to relatives with and without fibromyalgia? Clinical and Experimental Rheumatology, 27(5), S11. [Google Scholar] [PubMed]
- González, B., Novo, R., & Ferreira, A. S. (2020). Fibromyalgia: Heterogeneity in personality and psychopathology and its implications. Psychology, Health & Medicine, 25(6), 703–709. [Google Scholar] [CrossRef]
- González, B., Novo, R., & Peres, R. (2021). Personality and psychopathology heterogeneity in MMPI-2 and health-related features in fibromyalgia patients. Scandinavian Journal of Psychology, 62(2), 203–210. [Google Scholar] [CrossRef] [PubMed]
- González, B., Novo, R., Peres, R., & Baptista, T. (2019). Fibromyalgia and rheumatoid arthritis: Personality and psychopathology differences from the Minnesota Multiphasic Personality Inventory-2. Personality and Individual Differences, 142, 260–269. [Google Scholar] [CrossRef]
- Gupta, M. A., & Moldofsky, H. (1986). Dysthymic disorder and rheumatic pain modulation disorder (fibrositis syndrome): A comparison of symptoms and sleep physiology. Canadian Journal of Psychiatry, 31(7), 608–616. [Google Scholar] [CrossRef] [PubMed]
- Henao-Pérez, M., López-Medina, D. C., Arboleda, A., Monsalve, S. B., & Zea, J. A. (2022). Patients with fibromyalgia, depression, and/or anxiety and sex differences. American Journal of Men’s Health, 16(4), 15579883221110351. [Google Scholar] [CrossRef]
- Hirsch, J. K., Altier, H. R., Offenbächer, M., Toussaint, L., Kohls, N., & Sirois, F. M. (2021). Positive psychological factors and impairment in rheumatic and musculoskeletal disease: Do psychopathology and sleep quality explain the linkage? Arthritis Care & Research, 73(1), 55–64. [Google Scholar] [CrossRef]
- Howe, C. Q., Robinson, J. P., & Sullivan, M. D. (2015). Psychiatric and psychological perspectives on chronic pain. Physical Medicine and Rehabilitation Clinics of North America, 26(2), 283–300. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K. A., Zijlema, W. L., Joustra, M. L., & Rosmalen, J. G. (2015). Mood and anxiety disorders in chronic fatigue syndrome, fibromyalgia, and irritable bowel syndrome: Results from the LifeLines cohort study. Psychosomatic Medicine, 77(4), 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kang, W., Tiego, J., Hellyer, P. J., Trender, W., Grant, J. E., Chamberlain, S. R., Hampshire, A., & Sahakian, B. J. (2024). Validation of an abbreviated Big Five personality inventory at large population scale: Psychometric structure and associations with common psychiatric and neurological disorders. Comprehensive Psychiatry, 134, 152514. [Google Scholar] [CrossRef] [PubMed]
- Karlin, B. E., Creech, S. K., Grimes, J. S., Clark, T. S., Meagher, M. W., & Morey, L. (2005). The personality assessment inventory with chronic pain patients: Psychometric properties and clinical utility. Journal of Clinical Psychology, 61(12), 1571–1585. [Google Scholar] [CrossRef]
- Keller, D., de Gracia, M., & Cladellas, R. (2011). Subtypes of patients with fibromyalgia, psychopathological characteristics and quality of life. Actas Españolas de Psiquiatría, 39(5), 273–279. [Google Scholar] [PubMed]
- Keller, M. B., Hirschfeld, R. M., & Hanks, D. (1997). Double depression: A distinctive subtype of unipolar depression. Journal of Affective Disorders, 45(1–2), 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kleykamp, B. A., Ferguson, M. C., McNicol, E., Bixho, I., Arnold, L. M., Edwards, R. R., Grol-Prokopczyk, H., Ohrbach, R., Dworkin, R. H., & Turk, D. C. (2021). The prevalence of comorbid chronic pain conditions among patients with temporomandibular disorders: A systematic review. Seminars in Arthritis and Rheumatism, 51(1), 166–174. [Google Scholar] [CrossRef]
- Løge-Hagen, J. S., Sæle, A., Juhl, C., Bech, P., Stenager, E., & Mellentin, A. I. (2019). Prevalence of depressive disorder among patients with fibromyalgia: Systematic review and meta-analysis. Journal of Affective Disorders, 245, 1098–1105. [Google Scholar] [CrossRef]
- López-Ruíz, M., Losilla, J. M., Monfort, J., Portell, M., Gutiérrez, T., Poca, V., Garcia-Fructuoso, F., Llorente, J., Garcia-Fontanals, A., & Deus, J. (2019). Central sensitization in knee osteoarthritis and fibromyalgia: Beyond depression and anxiety. PLoS ONE, 14(12), e0225836. [Google Scholar] [CrossRef] [PubMed]
- López-Solà, M., Woo, C. W., Pujol, J., Deus, J., Harrison, B. J., Monfort, J., & Wager, T. D. (2017). Towards a neurophysiological signature for fibromyalgia. Pain, 158(1), 34–47. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M. P., Sánchez, A. I., Cáliz, R., & Miró, E. (2021a). Psychopathology as a moderator of the relationship between physical symptoms and impairment in fibromyalgia patients. Psicothema, 33(2), 214–221. [Google Scholar] [CrossRef]
- Martínez, M. P., Sánchez, A. I., Prados, G., Lami, M. J., Villar, B., & Miró, E. (2021b). Fibromyalgia as a heterogeneous condition: Subgroups of patients based on physical symptoms and cognitive-affective variables related to pain. Spanish Journal of Psychology, 24, e33. [Google Scholar] [CrossRef] [PubMed]
- Millon, T. (2006). Millon clinical multiaxial inventory-III (MCMI-III) (3rd ed.). Pearson Assessments. [Google Scholar]
- Millon, T., Davis, R., & Millon, C. (2009). Inventario clínico multiaxial de millon-III (MCMI-III) (V. Cardenal, & M. P. Sánchez, Trans.; 2nd ed.). TEA Ediciones, S.A. [Google Scholar]
- Monterde, S., Salvat, I., Montull, S., & Fernández-Ballart, J. (2004). Validation of the Spanish version of the Fibromyalgia Impact Questionnaire. Revista Española de Reumatología, 31(9), 507–513. [Google Scholar] [PubMed]
- Morey, L. C. (1991). Personality assessment inventory—Professional manual. Psychological Assessment Resources. [Google Scholar]
- Morey, L. C., & Alarcón, M. O. T. (2013). PAI: Inventario de evaluación de la personalidad. Manual de corrección, aplicación e interpretación. TEA Ediciones. [Google Scholar]
- Naylor, B., Boag, S., & Gustin, S. M. (2017). New evidence for a pain personality? A critical review of the last 120 years of pain and personality. Scandinavian Journal of Pain, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A. B., III, & Akiskal, H. S. (2001). Proposed endophenotypes of dysthymia: Evolutionary, clinical and pharmacogenomic considerations. Molecular Psychiatry, 6(4), 363–366. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Novo, R., González, B., Peres, R., & Aguiar, P. (2017). A meta-analysis of studies with the Minnesota Multiphasic Personality Inventory in fibromyalgia patients. Personality and Individual Differences, 116, 96–108. [Google Scholar] [CrossRef]
- Ortiz-Tallo, M., Cardenal, V., Ferragut, M., & Cerezo, M. V. (2011). Personalidad y síndromes clínicos: Un estudio con el MCMI-III basado en una muestra española. Revista de Psicopatología y Psicología Clínica, 16(1), 49–60. [Google Scholar] [CrossRef]
- Pujol, J., Macià, D., Garcia-Fontanals, A., Blanco-Hinojo, L., López-Solà, M., Garcia-Blanco, S., Poca-Dias, V., Harrison, B. J., Contreras-Rodríguez, O., Monfort, J., Garcia-Fructuoso, F., & Deus, J. (2014). The contribution of sensory system functional connectivity reduction to clinical pain in fibromyalgia. Pain, 155(8), 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J., & González, T. (2004). The Fibromyalgia Impact Questionnaire: A validated Spanish version to assess the health status in women with fibromyalgia. Clinical and Experimental Rheumatology, 22, 554–560. [Google Scholar] [PubMed]
- Rossi, G., & Derksen, J. (2015). International adaptations of the Millon Clinical Multiaxial Inventory: Construct validity and clinical applications. Journal of Personality Assessment, 97(6), 572–590. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M., Aira, Z., Buesa, I., Bilbao, J., & Azkue, J. J. (2012). Is psychological distress intrinsic to fibromyalgia syndrome? Cross-sectional analysis in two clinical presentations. Rheumatology International, 32(11), 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Saulsman, L. M. (2011). Depression, anxiety, and the MCMI-III: Construct validity and diagnostic efficiency. Journal of Personality Assessment, 93(1), 76–93. [Google Scholar] [CrossRef] [PubMed]
- Schramm, E., Klein, D. N., Elsaesser, M., Furukawa, T. A., & Domschke, K. (2020). Review of dysthymia and persistent depressive disorder: History, correlates, and clinical implications. Lancet Psychiatry, 7(9), 801–812. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K. A., & Clauw, D. J. (2016). Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience, 338, 114–129. [Google Scholar] [CrossRef]
- Teixidó-Abiol, L., De Arriba-Arnau, A., Seguí-Montesino, J., Gil-Gallardo, G. H., Sánchez-López, M. J., & De Sanctis Briggs, V. (2023). Psychopathological and personality profile in chronic nononcologic nociceptive and neuropathic pain: Cross-sectional comparative study. International Journal of Psychological Research (Medellín), 15(2), 51–67. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K., Mathys, M., & Turk, D. C. (2017). Evidenced-based guidelines on the treatment of fibromyalgia patients: Are they consistent and if not, why not? Have effective psychological treatments been overlooked? Journal of Pain, 18(7), 747–756. [Google Scholar] [CrossRef] [PubMed]
- Torres, X., Bailles, E., Valdes, M., Gutierrez, F., Peri, J. M., Arias, A., Gomez, E., Collado, A., & Alegre, J. (2013). Personality does not distinguish people with fibromyalgia but identifies subgroups of patients. General Hospital Psychiatry, 35(6), 640–648. [Google Scholar] [CrossRef] [PubMed]
- Ventriglio, A., Bhugra, D., Sampogna, G., Luciano, M., De Berardis, D., Sani, G., & Fiorillo, A. (2020). From dysthymia to treatment-resistant depression: Evolution of a psychopathological construct. International Review of Psychiatry, 32(3–5), 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wan, B., Gebauer, S., Salas, J., Jacobs, C. K., Breeden, M., & Scherrer, J. F. (2019). Gender-stratified prevalence of psychiatric and pain diagnoses in a primary care patient sample with fibromyalgia. Pain Medicine, 20(11), 2129–2133. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F., Anderson, J., Harkness, D., Bennett, R. M., Caro, X. J., Goldenberg, D. L., Russell, I. J., Yunus, M. B., & Clark, P. (1997). A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis & Rheumatism, 40(9), 1560–1570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the University Association of Education and Psychology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).