Abstract
Defined by its potential for self-renewal, differentiation and tumorigenicity, cancer stem cells (CSCs) are considered responsible for drug resistance and relapse. To understand the behavior of CSC, the effects of the microenvironment in each tissue are a matter of great concerns for scientists in cancer biology. However, there are many complicated obstacles in the mimicking the microenvironment of CSCs even with current advanced technology. In this context, novel biomaterials have widely been assessed as in vitro platforms for their ability to mimic cancer microenvironment. These efforts should be successful to identify and characterize various CSCs specific in each type of cancer. Therefore, extracellular matrix scaffolds made of biomaterial will modulate the interactions and facilitate the investigation of CSC associated with biological phenomena simplifying the complexity of the microenvironment. In this review, we summarize latest advances in biomaterial scaffolds, which are exploited to mimic CSC microenvironment, and their chemical and biological requirements with discussion. The discussion includes the possible effects on both cells in tumors and microenvironment to propose what the critical factors are in controlling the CSC microenvironment focusing the future investigation. Our insights on their availability in drug screening will also follow the discussion.
1. Introduction
Cancer stem cells (CSCs) refer to subpopulations of cancer cells that have a high ability to construct tumors upon experimental implantation in immunodeficient animal models. CSCs are highly tumorigenic and have self-renewal ability in addition to differentiation capacity, while the majority of cancer cells as non-CSCs are not tumorigenic in small numbers [1]. CSCs divide asymmetrically giving CSCs and cancer daughter cells which eventually resulting in heterogeneous bulk of the tumor [2]. CSCs have been identified in a wide range of cancers in both hematological malignancies and solid tumors such as leukemia, lung, pancreas, liver, brain, ovarian, prostate cancers, and so on [3,4,5,6,7]. CSCs are routinely characterized by CSC markers including both surface and cytoplasmic markers. Several cell-surface markers—such as CD44, CD133, CD24, and Epcam—have been used to isolate CSCs [8,9,10].
The components of the tumor microenvironment (TME), which vary across tumor types allowing overlaps in some features and cell phenotypes, are generally different phenotypes of cells, extracellular matrix, and secreted factors establishing the CSC niche which plays a vital role in maintaining CSC stemness. Fibroblasts, immune cells, adipocytes, and endothelial cells as well are typical non-cancer cells in TME affecting tumor growth. Extracellular vesicles from these cells and cancer cells also play critical roles in cell-to-cell communication in TME (Figure 1) [11,12]. Low nutrient availability, low oxygen and acidic pH are the conditions often found in TME which have critical effects on CSC stemness. CSCs show different status depending on the niche they are residing in. Reciprocal signaling between CSCs and other cells in TME affects the fate of one another and has considerable implications for therapeutic targeting of tumor heterogeneity [13]. Therefore, treatments targeting TME could significantly enhance the effects on conventional treatments. The emerging concept of CSCs has given some new clues on drug resistance for which CSCs are currently believed to be responsible leading to relapse. CSCs with their quiescence exhibit more drug resistance to conventional therapies than non-CSCs do result in cancer relapse [14]. Invasion and migration of CSCs are also affected by TME cells and physical features of TME architecture including extracellular matrix [15,16]. CSCs may directly be responsible for metastasis or differentiate into cancer cell phenotypes that have the metastatic potential [17,18,19]. Characterization of the interaction between CSCs and TME components is particularly important to choose the more effective options for conventional cancer therapy.
In addition to the difficulties of maintaining CSCs in vitro, their isolation from tumor cell populations with cell-surface markers is another challenge in CSC research. The traditional two-dimensional (2D) in vitro culture systems limit cells in a single microenvironment while in vivo models are 3D, which is more complex and more similar to the tumor in a patient. Many efforts have recently been performed to establish the environment in vitro that mimics their TME and niches to model and study cancer cells and CSCs (Figure 2) [20]. Construction of the three-dimensional (3D) culture systems provides new prospects for studying TME and CSCs and paving the way for developing more efficient therapies targeting CSCs according to the context of their niche. Additionally, biomanufacturing methods including biomaterials provide emerging opportunities to investigate signaling pathways and related phenomena that control progression of cancer and drug response. The models engineered with biomaterials offer significant platforms for basic and translational research in cancer [20,21,22].
In this review, we outline the utility and recent advances of biomaterials to mimic the CSC microenvironment. Finally, we also describe specific examples of biomaterials and their applications in capturing figure of cancers and in screening drugs clarifying their future opportunities as quintessence tools in cancer research.
2. Cancer Stem Cell Niche
‘Niche’ means a comfortable or suitable environment. There should be enough food and water for living things, and they can live longer in it since they can keep less starving and stress than those in any other environment. Every living creature has its proper niche depending on its living style: herbivores need plants and carnivores need other animals. Cells are not exceptions, having their niches. Every cell in tissue needs its nutrients to keep its homeostasis. For stem cells, the niche is much more important to maintain their plastic character than other cells. According to the niche, stem cells decide to self-renew and differentiate maintaining themselves and regenerating tissues as well [23]. Therefore, the niche is a specific microenvironment that regulates stem cell fate by providing signals as secreted factors or cell-to-cell contacts. This applies not only to normal stem cells but also to CSCs [24]. Stem cells are rare cell populations contained in almost all tissues. Stem cells reside in a niche that maintains their characters. The niches control stem cell fate by providing signals to maintain them in a dormant state or promote their proliferation and differentiation. Normal stem cells divide symmetrically or asymmetrically producing stem cells/progenitor or differentiated cells. This process is controlled by intracellular and extracellular signals [25]. The normal stem cell niche is usually a space containing a specific number of stem cells controlled by the signals for self-renewal and survival resulting in a balance between self-renewal and differentiation in order. The distance plays a crucial role in the balance of stem cell niches. Namely, the daughters of stem cells that move away from their niches undergo differentiation. Therefore, stem cell place—in addition to their adhesion to either the basement membrane or supporter cells—are the main controllers of their fate [26]. On the other hand, CSCs are proposed to be developed from normal stem cells. Chronic inflammation imbalances the homeostasis of stem cell niche inducing CSCs and promoting cancer initiation. CSCs hijack normal stem cell niche and turn it into a TME. According to the tumor development, the CSC niche begins to mature. Comparing with normal stem cell niche, CSC niche is composed of different phenotypes of cancer cells and cancer-associated cells. This difference could be based on the CSC’s differentiation potentials, which have been reported to construct their niche providing a wide range of cancer cells and cancer-associated cells. CSC niche is connected to the different types of cells and factors depending on the tissues surrounding CSCs although it has not been described as a certain location. The bidirectional interactions between CSCs and TME could provide the heterogeneity of CSC niche maintaining stemness of CSCs [27]. The intratumoral heterogeneity promotes cancer progression and affects therapeutic efficacy where the TME contributes to tumor heterogeneity. CSCs are developed in a specific niche which supports their self-renewal, tumorigenesis, and metastasis abilities (Figure 1).
CSC niche consists of different types of cells including tumor cell and non-cancer cells—such as cancer associated fibroblast, tumor associated macrophage, endothelial cells, and other types of immune cells—sometimes as the result of differentiation potential [11,28,29,30]. These cells secrete various factors acting as communicators, inducing angiogenesis and recruiting other cells such as stromal cells and immune suppressor cells which will promote progression, invasion, and metastasis of tumors [31,32]. The physical features of the CSC niche such as stiffness also profoundly shape the functional status of CSCs. Remodeling of extracellular matrix can cause a shift of stiffness from one status to another resulting in the promotion of tumor progression. The mechanical features of CSC niche affect not only tumor progress but also their metastasis ability controlling the migration of CSC which is significantly affected by matrix stiffness. The CSC niche could also act as a protector of CSCs by preventing toxic substances from reaching themselves and consequently contribute to the drug resistance of CSC. Formerly, all these cells within the CSC niche are thought to be recruited from the peripheral site of the tumor which had been reported by different research groups [33,34,35,36,37,38,39,40,41]. During tumor progression, the CSC fate in the primary tumor relies crucially on the TME and CSC niche. Recently, however, CSCs have shown the ability to differentiate into different types of tumor-associated cells—such as tumor vascular cells [42], cancer-associated fibroblasts [43], and hematopoietic derived cells [30]—and therefore can create their niches by themselves [30,42,43,44,45,46]. The differentiation potential of CSCs and their abilities to provide their niches are still controversial and being investigated further very recently. Since the CSC niche could be more complex with their differentiation potentials, new in vitro models are needed mimicking the CSC niche, which their differentiation potentials and their interactions with other cells being taken into consideration.
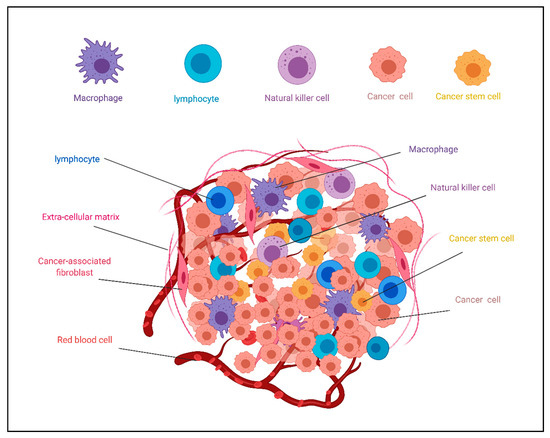
Figure 1.
Schematic illustration of heterogenicity of the tumor microenvironment (TME). TME contains different cell phenotypes in addition to extracellular matrix affecting cancer stem cell fate. This figure is reused from [47] on MDPI.
3. Biomaterials as Scaffolds of CSCs in Culture
3.1. Requirement of Biomaterials in CSC Culture
Generally, cells conventionally require attachment to the surfaces of cell culture vessels in the presence of a culture medium. However, the attachment is not always necessary for the survival and proliferation of CSCs forming spheres [48,49]. Accordingly, inert biological materials lacking cell attachment could provide a 3D environment. For example, natural polymers such as alginate lack cell adhesion property and could enrich and maintain the stemness properties of CSCs [50,51]. In addition, since it is well known that the stemness maintenance of CSCs is closely associated with the hypoxic environment, the 3D cancer models are expected to replicate the unique characteristics of tumor hypoxic environments, differentiation and niche structure including differentiated cell phenotypes and extracellular matrix [52]. It is also expected that 3D cancer models can replicate sufficient mechanical properties for the growth of CSCs [53].
In this context, the biomaterials help CSC maintain stemness characters, mimic the hypoxic environment, and may provide proper mechanical property to allow exhibiting tumor heterogeneity.
3.2. Characteristics of Cells Cultured In Vitro (Cell–Cell and Cell–Matrix Interactions)
Based on the basic study of cancer cell culture during the last decade, 2D cell culture cannot provide tumor heterogeneity in the same way as in vivo models of cancer cells. The heterogeneity will not be provided in 2D cell culture while 3D-environment will be [54]. The interaction between cells and the surrounding extracellular matrix (ECM) have pivotal roles affecting most of the behavior of cancer cells in a tissue-specific manner (Figure 2) [55]. Since 3D cell culture can replicate the in vivo microenvironment by mimicking cell–ECM interactions in 3D, the development of artificial 3D cell culture could be helpful in cancer research.
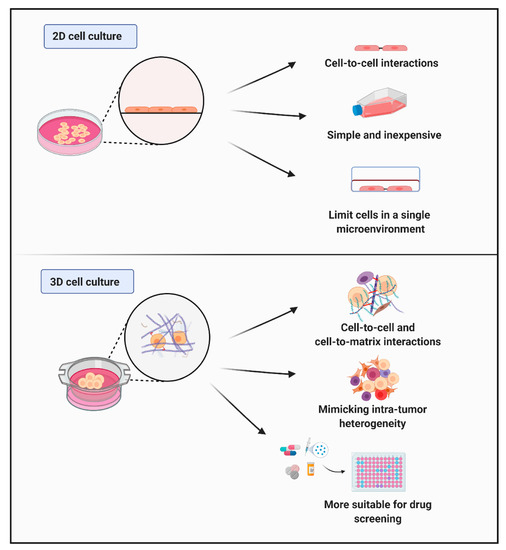
Figure 2.
Schematic illustration of the between 2D and 3D culture models. The 2D culture systems, where cells are cultured in adherent conditions, limit cells in a single microenvironment. The 2D cell culture models are simple and inexpensive providing only cell–cell interactions. On the other hand, the 3D models mimic the tumor microenvironment better than 2D models since they enable cell–cell interactions and cell–matrix interactions in a 3D environment. The 3D models can culture several cell types in one environment mimicking intra-tumor heterogeneity. These features of 3D models make them more suitable for drug screening than 2D models.
3.3. Different Types of Scaffolds
The engineering of 3D scaffold is currently important to apply for the research of CSCs, tumor microenvironments, and drug screening. In this section, therefore, we summarize the recent research discussing the development of various biomaterial-based culture systems and their applications in the CSC field (Figure 3).
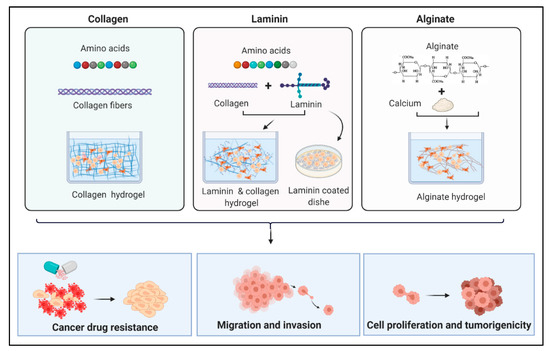
Figure 3.
Schematic illustration of some examples of natural biomaterials and their applications in cancer research. Collagen and laminin are two natural proteins consisting of amino acids that form hydrogels used to construct 3D cell culture scaffolds. Laminin can be used with collagen to form hydrogels or used alone to coat cell culture dishes. Alginate is a polymer that makes hydrogel in presence of calcium ions. Biomaterial hydrogels provide valid tools to investigate drug resistance of cancer cells and their ability for migration and invasion which influence their ability to form metastases. In addition to their usage to explore cancer cell proliferation and growth in different 3D environments.
3.3.1. Collagen
Collagen is the most abundant protein in the human body while types I–V are usually used as biomaterials [56,57]. For example, type I collagen (Col I) is a major constituent of the extracellular matrix (ECM) [58]. Many studies have described the effects of abnormal expression, proteolysis, and structure on tumor progression [59,60]. In 1988, Klebe constructed a 2D tissue of cell culture using collagen, and first applied the idea of this method to the 3D culture [61]. Subsequently, this methodology has been expanded to further applications. Grzesiak et al. investigated suspected integrins that could be responsible for mediating the malignant phenotype of pancreatic cancer in a 3D tumor microenvironment. In his study, he used four types of pancreatic cancer cells, adhered them to a Col I scaffold in a specific manner, and found that integrin-specific adhesion is required for subsequent cancer cell proliferation [62].
Chen et al. studied the proliferation and differentiation of neural cancer stem cells (NCSCs) on a 3D porous collagen scaffold with an 80 μm pore size [63]. The NCSCs, grown on the 3D porous collagen scaffold, significantly enhanced neurite outgrowth due to the collagen sponge supporting the healing process in the tissue. In tumor growth, especially in breast cancer, the microenvironment is a dynamic 3D structure with physicochemical and histopathological properties that are critical for molecular signaling, morphology, motility, differentiation, and proliferation [64]. For example, breast cancer-derived MCF-7 cells in 3D collagen scaffolds increased the time required for cell proliferation (doubling time), produced angiogenic growth factors and matrix metalloproteinase (MMPs), and maintained properties of CSCs [65].
Recently, a novel type of collagen scaffold composites of collagen was devised to contain either glycosaminoglycan or nanohydroxyapatite. This model was applied for the evaluation of metastatic behavior of prostate cancer cells and for the screening of the candidates of therapeutic drugs. This model demonstrated that the 3D collagen scaffold helped clarify the pathogenesis of prostate cancer [66]. We recently reported a unique approach called ‘sedimentary culture’ using a collagen microfiber (CMF) to fabricate large-scale engineered tissues. This method may provide a new platform for CSC research [67,68].
3.3.2. Laminin
Laminin (LM) is considered significantly related to metastasis. Different types of LM receptors were isolated from mammalian cells. For example, Malinoff et al. reported the discovery of the LM receptor known as 67KD, through which cancer cells bind to the LM adhering to the collagen matrix resulting in the promotion of metastasis [69,70]. Due to the binding capabilities and affinity to cells, LM is often used in the construction of 3D scaffolds to improve the artificial matrices [71]. When used in cell culture as a scaffold, LM could maintain stemness or support cell differentiation, growth, and migration depending on the subtypes [72,73].
In order to study the effects of colorectal cancer microenvironment on the invasion, a collagen-laminin scaffold was employed to obtain a compartmentalized and biomimetic 3D cancer model consisting of colorectal cancer cells surrounded by a vascular network [74]. The laminin-derived bioactive peptides and agarose matrix were also used as a convenient biomaterial in cell culture. The LM and other chemical factors have a synergistic effect on the promotion of cell adhesion. Neuronal cells have been reported to have neurite outgrowth while endothelial cells formed capillary-like networks when cultured in 3D cell culture scaffolds containing laminin/agarose materials [75].
In CSC culture, the primary tumor-derived and metastatic tumor-derived cell lines were also tested on the laminin-coated 3D culture plates. The laminin-coated 3D culture plates along with xeno-free media were found to be a useful condition promoting cell viability and maintaining CSC phenotypes of renal cancer stem cells (Figure 3) [76]. Additionally, the culture of glioblastoma cells on a 3D scaffold with seven isoforms of LM in a 3D model showed elevation of stem cell-related genes accompanied with clonogenicity enhancement of cells grown on the scaffolds with LM 411, 421, 511, and 521 [77].
3.3.3. Agar
Since human tumor samples or cells are not always tumorigenic in animal models, it is difficult to evaluate tumor growth in vivo. Therefore, methods to assess cell proliferation in a semi-solid matrix have conventionally been employed to assess malignancy through the ability to form colonies on soft agar matrix [78]. This method is considered to mimic the cancer cell environment in vivo. Nanofiber scaffold is sometimes covered by a layer of soft agar to mimic the reproduction of spermatogonial stem cells (SSCs) in vivo. These studies have shown an improvement of the colonization rate of SSCs, and elevation of the SSCs gene expression as markers of differentiated spermatogonia [79]. When supplemented in a culture medium, low-molecular-weight agar, so-called LA717, allows cells to form spheres in a 3D environment keeping the culture medium transparent for microscopic observation. The cancer spheres generated in this culture medium were available to evoke high sensitivity to the candidates of anti-cancer drugs, demonstrating more efficacy in the assessment of drug screening than the conventional methods [80].
3.3.4. Gelatin, Fibrin, Alginate, and Agarose
Although fibrin, alginate, and collagen are known to be hard to fabricate as 3D structures, a bath of gelatin particles allows them to form a 3D structure. The 3D tissue model containing gelatin hydrogels with other artificial materials are promising tools for cancer research in the future. Gelatin, with its good hydration and diffusion properties, can be mixed with methacrylate (GelMA) to make a 3D biomimetic model simulating invasion and metastasis of breast cancer. Breast cancer cells cultured on GelMA scaffold demonstrated the enhancement of both invasiveness in vitro and tumorigenicity in vivo when injected into the tail vein of mice [81,82,83].
The fibrin contributes to the wound healing process as an extracellular matrix protein. The fibrin is easy to degrade, promotes angiogenesis and has high compatibility, therefore it is ideal as 3D scaffold material [84,85,86]. The fibrin 3D hydrogel was found efficient in isolation and enrichment of colon cancer cell colonies, which were highly tumorigenic and potent to self-renew with stemness markers upregulated [87]. Fibrin was independently found to enhance the colony-forming potential of primary kidney tumor cells embedded in a fibrin matrix. Metastasis of these cells in vivo was reduced when the production of fibrin was inhibited suggesting the importance of fibrin in the adhesive interactions with renal cancer cells in cancer progression and metastasis [88].
In the presence of calcium ions, the solution of alginate extracted from brown seaweed forms a gel that supports cell viability in long-term culture [71,89]. Moreover, alginate is used as a common bio-ink material in 3D bioprinting and has many applications in drug screening and regenerative medicine [90,91,92,93]. Breast cancer cells cultured in alginate 3D scaffolds have shown more chemoresistance than those cultured in 2D conditions suggesting that alginate scaffolds have efficiency and possibility as the models for in vitro drug screening which mimic the in vivo 3D tumor microenvironments [94]. The expression levels of CSC-markers in alginate 3D porous scaffolds were found higher than those in 2D culture conditions. The increase of metastatic ability and drug resistance was also observed in these scaffolds indicating that alginate could be a candidate biomaterial as the 3D scaffolds for CSC research (Figure 3) [95,96].
Recently, agarose has been used in cell cultures. In some of its applications, agarose was used as scaffolds-free systems where 3D systems do not rely on solid gels. Plates coated with agarose allow cells to make tumorspheres, which could replate in a method called 3D reverts (3DRs). The 3DRs are used to study cytokine expression, and investigation of invasion, migration, and metastasis of cancer cells [97]. Silica-based materials were also considered in cell culture due to their unique characters such as the possibility of being modified providing a wide range of materials that differ by function, porosity, and wettability. Silica fibers coated with different types of collagen were used to culture different types of cancer cells in the 3D environment where cells showed enhancement of invasiveness and proliferation when the fibers were coated with collagen IV. This method was called “tissueoid cell culture system” and suggested for its advantages of combability with different cell staining methods [98]. Cell culture vessels were also modified into hydrophobic fluoro-silica (FS) surface resulting in low adherence and transient aggregation of breast cancer cells followed by disaggregation. This method was suggested as a tool for study metastatic events where cancer cells disassociate from the tumor, increase their mobility and settle in new tissues [99]. Silica and cell culture media were also mixed to make hydrogels which were tested as a 3D platform to culture different types of mammalian cells including cancer cells. The cells in these hydrogels stayed viable up to 7 weeks of culturing and exhibited a variation in the sensitivity towards toxic drugs [100]. Silica-based materials are now being tested and their applications being wider than before. Although silica-based materials show many advantages in culturing cancer cells in the 3D environment, they have still not been evaluated for CSC culture.
3.3.5. Synthetic 3D Structures and 3D Bioprinting Technology
In addition to the natural materials mentioned above, synthetic polymers are often used in the manufacture of 3D scaffolds. There are many synthetic polymers that have advantages over natural polymers including physical, chemical, and mechanical features, which can be adapted for various medical and biological applications. Their advantages are the low pathogenicity due to the biological inertia and the low production cost [101,102].
Synthetic polymers can also be mixed with natural copolymers to enhance cell affinity [103]. Synthetic polymers for 3D scaffolds include polyethylene glycol (PEG), polycaprolactone (PCL), poly (lactic-co-glycolic acid) (PLGA), poly (2-hydroxyethylmethacrylate) (pHEMA), poloxamer 407 (Pluronic® F127), and others. PEG gel and its derivatives have been used in a variety of 3D cell cultures and as scaffolds to study cell migration, angiogenesis and stem cell differentiation characters [104,105]. Different cell lines can be cultured on a PEG bioactive scaffold after modification of PEG to confer with bioactivity [106,107,108,109]. The PEG scaffold has variable elasticity which could affect the morphology of cancer cells. The PEG scaffold provides good models for understanding the tumor extracellular matrix environment affecting metastasis [110].
The porous PEG/PLA 3D scaffold can be prepared by using sodium chloride and PEG as water-soluble porogen. This scaffold can be used to evaluate the viability and adhesive character of human hepatoma cells where PEG molecular weight has an effect on scaffold morphology, cell viability, and cell adhesion [111,112,113,114,115].
PCL has good ductility and viscoelasticity, no isomers, and low manufacturing cost. Modification of PCL is also easy because of its melting point and solubility in benzene, acetone, chloroform, and other solvents [116]. Therefore, it is widely used as a polymer to provide a high load-bearing mechanical function for 3D tissue scaffolds [101,117,118]. Generally, the 2D culture systems induce CSC differentiation, which may obstruct the development of therapeutic strategies targeting CSCs. The PCL 3D scaffold enhanced triple-negative breast cancer (TNBC) cell proliferation and increased aldehyde dehydrogenase activity when compared with other scaffolds which indicated enrichment of breast CSCs [117,119,120,121].
PLGA polymer has been widely used in drug delivery systems (DDS) and tissue engineering scaffolds. PLGA is approved by the US food and drug administration (FDA) as biodegradable polymers being subjected to the metabolism in vivo [122,123,124,125,126]. The hydroxyapatite (HAp) particles could be incorporated into PLGA nanofibers to construct a biomimetic 3D nanofiber scaffold used to culture breast cancer cells. Cancer cells cultured on the PLGA/HAp scaffold exhibited higher viability and proliferation ability than PLGA scaffolds [127].
The pHEMA has a long history as an implanted biomaterial being shaped into a porous spherical scaffold. pHEMA scaffolds, mimicking the complex microenvironment in vivo, have been used with endothelial cells, fibroblasts, and cancer cells [128,129]. The prostate cancer cell line M12 cells were implanted into a pHEMA porous scaffold where dormant cancer cells became active and formed tumoroids on the scaffold. This discovery is beneficial for the study of the microenvironment and cancer dormancy [128].
Pluronic® F127 is a kind of poloxamer ABA triblock copolymer with 30 wt % of hydrophobic polypropylene oxide (PPO) and 70 wt % of hydrophilic polyethylene oxide (PEO) [130]. Due to the biocompatibility, low toxicity, and ability to form micelles, F127 is widely used as a drug delivery carrier [131]. The blend of F127 with PCL improves the hydrophilicity of 3D scaffolds. The PCL/F127 scaffold could be used as an attractive scaffold to mimic the microenvironment of CSCs and enrichment of CSCs [132]. Many other synthetic polymers are also available for future investigation and synthetic polymer scaffolds have perspective applications as a 3D scaffold for CSC culture. Finally, the 3D bioprinting technology provides an additional advantage for mimicking cancer microenvironment by incorporate multiple cell types in a complex 3D architecture and controlling their organization. The 3D bioprinting can handle cells, biomaterials and extracellular matrix components as bio-inks to create 3D platforms in a spatially defined manner. This technology offers the opportunity of high-throughput screening of cancer drugs where the metabolism and toxicity of these drugs could be evaluated in a better way than other platforms. Also, these platforms could be automated to run thousands of tests in a short time. This method was applied to different types of cancers such as glioblastoma, breast, pancreatic, and ovarian cancers [133]. The glioma stem cells and glioma cells were incorporated within hydrogel microfibers to create 3D printed model to assess drug resistance of glioblastoma cells. Both invasion potential and drug resistance of cells increased in this 3D approach and linked to the elevation of the expression of different genes such as matrix metalloproteinases and vascular endothelial growth factor receptor-2 (VEGFR2) [134]. A high throughput 3D printing approach was also applied to construct a 3D model for ovarian cancer that mimicked their microenvironment. This 3D model contained both fibroblasts and cancer cells. The cell density and 3D structure were automatically controlled. Cells maintained their viability during printing and proliferated in this microenvironment. This model was proposed as a physiologically relevant model of ovarian cancer and as a tool of high-throughput anti-cancer drug screening [135].
4. Polymeric Biomaterials for In Vitro Drug Testing Applications
For the development of anti-cancer drugs, effective high-throughput methods are anticipated to save time and screen numerous drug candidates. Recently, many models of drug screenings have been developed. Since CSCs play essential roles in cancer metastasis and recurrence, drug screening models for targeting CSC is also been established in order to find effective drugs and combinations. Drug screening for CSC comprises many difficulties in terms of technology and cost since CSCs are present as a small subpopulation of tumor cells and it is difficult to isolate and maintain them in vitro. Therefore, CSC research is requiring specific culture models for CSC to maintain and mimic their microenvironments with more efficacy in the drug screening tests [136,137].
The 2D culture is conventionally used in the drug screening application in vitro where cancer cells are cultured and adhered to the surface of the culture vessels. While tumor tissue in the patient body has a more complex 3D structure making discrepancy between in vitro and in vivo results of cancer drug responses. Current cancer treatments are largely unsuccessful in preventing disease progression, relapse, and overall survival patients since intra-tumoral heterogeneity is usually neglected in vitro models [76,138]. Patient tumor samples transplanted in animal models known as patient-derived xenograft (PDX) models allow invaluable assessment of cancer drugs. However, they are expensive, have high risks of failure and require well-trained experience to make them sufficient as regular drug screening tests [139]. Consequently, various drug screening models have been developed by using 3D culture scaffolds in which cancer cells are proliferated in an environment mimicking in vivo conditions. The features of 3D cell culture models make themselves suitable for drug discovery. Moreover, traditional tests such as proliferation and viability could be combined with a variety of 3D assays. Drugs such as doxorubicin and 5-fluorouracil targeting high proliferated cells could induce higher apoptosis rates in 2D than 3D models, in which dormancy and variable cycle stages exist [20]. Moreover, drug uptake could significantly differ between those two culture models. The density and stiffness of osteosarcoma microenvironment, a bone cancer, are largely affecting cancer progress because of the stiff nature of bones. The dense osteosarcoma spheroid embedded in 3D hydrogels had more drug resistance than cells dissociated from one another and embedded in the same matrix [140]. The 3D matrix containing fibronectin, laminin, and bone granules also altered metabolic activity and responses to doxorubicin of osteosarcoma cells. These findings reflect the importance of tumor mimetic models to screen and develop more appropriate treatments for bone cancers [141]. Alginate hydrogels are used to test hepatotoxicity and drug effects on both liver cancer cells and breast cancer cells at the same time. This model was inexpensive and shown to be capable to analyze cell viability and drug toxicity on multiple-cell types [142]. Also, breast cancer cell lines exhibited drug sensitivity with different IC50 values depending on the culture platform in 2D or 3D models. Cell–cell communications taken into consideration cocultured cancer cells with other types of “cancer-associated cells” such as fibroblasts drastically altered drug response as practical since these models should more physiologically be relevant [143]. The 3D chitosan scaffold was also used to evaluate the cytotoxic effects of tamoxifen on breast cancer cell line MCF-7 cells. Cells were displayed higher resistance in 3D models than in 2D where a higher concentration of the drug was required in 3D to achieve the same actions in 2D [144]. The response of glioblastoma cells towards cytotoxic compounds was also investigated on alginate scaffolds with different stiffness and adhesive characteristics. The drug sensitivity of glioblastoma cells to toxins was found strongly affected by the scaffold stiffness and adhesiveness in accordance with the increase of matrix softness [145]. The hybrid hydrogel of hyaluronic acid-alginate was used to evaluate the effect of 3D matrix on prostate cancer cell behavior and their response to anti-cancer drugs. This method revealed that prostate cancer cells are aggregating and forming spheres in hydrogel and became more resistant to anti-cancer drugs than those in 2D. These matrices are culture enhancing the feasibility of the 3D culture replacing in vivo drug evaluation methods [146].
Finally, hydrogel-based matrices can provide valuable tools for drug screening applications where they can give more feasible data compared to 2D cultures. Moreover, these methods are expected to narrow the gap between in vitro and in vivo results and efficiently reduce failure rates of cancer drug development especially when used for CSCs.
5. Capturing Cancer Cell Behavior Using Biomaterials
The complexity and dynamic nature of cancer are big challenges for cancer investigation and the development of therapeutic strategies. The evolution of biomaterials in cancer research help investigate different cancer phenomena such as metastasis, invasion, tumorigenicity, and so on. Especially, biomaterials have significantly enhanced our understanding of tumor nature by their ability to reflect the mechanical, architectural, and biological aspects of the cancer microenvironment in 3D. Biomaterials sometimes mimic the niche surrounding tumors allowing 3D space to investigate the specific effects of the environment on cancer progression and to test cancer cell behavior [147]. In this context, the behavior of CSCs in metastasis could be understood by biomaterials mimicking the extracellular matrix of specific human organs with biocompatibility, mechanical stiffness, structure, and cell adhesion. Complex interactions between the tumor and its surrounding adjacent normal cells are crucial for carcinogenesis and metastasis.
Scaffolds consisting of different elements of extracellular matrix (ECM) will provide variable stiffness and enable the assessment of the influence on the cellular dissemination and invasion related to metastasis [148]. The alteration of gene expression related to metastasis and invasion usually links to different phases of cancer aggressiveness and relapse which is affected by alteration of microenvironment stiffness. Porous hydrogels affect cancer cell migration and dissemination [149]. Until now, sufficient information of biomaterials required to construct scaffolds has not been available to study the fate and potential of CSCs. Recently, many studies are trying to find effective biomaterials that could be used to study CSC populations to understand their roles in cancer initiation, progresses, metastasis, and resistance to therapy.
The hypoxic niche enhancing metastasis was established with an engineered biomaterial scaffold. Alginate scaffolds for cancer are being investigated to recapitulate the microenvironment and metastasis and evaluate drug efficacy. The alginate-based 3D scaffolds combined with matrigel and gelatin are used in vitro as the models to study metastatic breast cancer cells such as MDA-MB-231 cells. The results from these studies suggested that scaffolds comprised of 50% alginate and 50% matrigel enhanced the characters of cancer cells such as morphology, malignancy, and so on [150,151]. On the other hand, the scaffolds composite of 1% alginate with 7% or 9% gelatin concentrations provided soft matrices with high cell adhesion, proliferation, and aggregation.
Chitosan-based scaffolds also attracted numerous technologists to study cancer metastasis. On the other hand, chondroitin sulfate is contained in tumor tissue as a component of the extracellular matrix which was the reason to design chitosan-chondroitin sulfate-based scaffolds that stimulate epithelial-mesenchymal transition (EMT) of prostate cancer model [152]. Chitosan hydrogel was combined with hydroxyapatite enabled the seeding of human bone marrow mesenchymal stem cells (MSCs) to generate an in vitro model mimicking various features of breast cancer metastasis [153]. In this study, coculture of MSCs and human breast cancer-derived MDA-MB-231 cells in chitosan hydrogels with 10% nanocrystal hydroxyapatite showed that MSCs upregulated the expression of the metastasis-associated gene, metadherin, within the breast cancer cells. This model provided a more biologically relevant microenvironment for studying metastasis of breast cells to the bone. Chitosan-chondroitin sulfate 3D platform supported prostate cancer growth and phenotypic expression with upregulation of metastasis genes, vimentin, MMP-2, and N-cadherin by increasing concentration of chondroitin sulfate in scaffold [154]. The CSC enrichment from different cancer cell lines achieved by 3D porous chitosan-alginate [155].
Also, in another study, tumor cell metastasis was significantly enhanced in chondroitin sulfate-modified alginate hydrogel beads, which lead to the increased expression of metastasis-related genes like MMPs and enhanced invasion ability in vitro, when compared with that in alginate. Consequently, chondroitin sulfate-modified alginate hydrogel beads might be suitable and relevant as the 3D biomimetic scaffold modeling tumor metastasis [156]. The mechanical properties of silk fibroin make it a good choice to develop effective models to study metastasis. The 3D silk scaffolds were used to study metastasis of prostate cancer cells and evaluate their functions where mechanical properties of crosslinked silk scaffolds profoundly influenced the cancer migration rate [157]. The migration of cancer cells was more in 2% silk fibrin hydrogels than in 3%, where no cell migration was observed. The variation in the migration rate was explained by the difference in hydrogel stiffness with different fibrin concentrations. Silk scaffold also showed the ability to support the growth of cancer cells by 3D monoculture and co-culture in silk scaffold. These methods significantly altered ECM production and cell morphology stimulated cancer-associated fibroblast which in turn influenced the metastatic potential [158].
In fact, CSCs not only respond to their microenvironment, but they tune their microenvironment to suit their needs [24]. Hydrogels of biomaterial and other synthetic materials give novel insights into the CSC metastasis and interactions between cancer-associated cells and CSCs. Collectively, the ability of biomaterials to create specific microenvironments made it attractive for cancer research, leading to a critical impact on clinical applications.
6. Conclusions and Future Perspective
Here, we briefly described the concept of CSC niche and tumor microenvironments as 3D platforms. Simultaneously, we summarized the characteristics of biomaterials forming the 3D matrices to mimic tumor microenvironment and introduced both natural and synthetic polymers with their applications in the fields of cancer research and therapy.
Although much progress has been achieved in mimicking tumor microenvironments, where 3D matrices are much more feasible than conventional 2D, still many challenges need to be explored. For the development of 3D matrices, the dissimilarity between cancer types is one of the most problematic obstacles that should be addressed in future research. Since cancers significantly vary regarding their characters of adhesiveness and stiffness, which make the tumor microenvironments more complicated to be mimicked, optimization of 3D matrices is required depending on each type of cancer [159]. The differences in the environmental stiffness in cancer can clearly be distinguished between bone tumors and brain tumors of which stiffness is critically hard or soft [160,161]. Undoubtedly, these differences also affect the obligations of the 3D matrix materials to mimic their microenvironments and to screen drugs for treatment. Meanwhile, the availability of different biomaterials with a wide range of physicochemical properties will assist the development of hydrogels with different characters applicable to different types of cancers. These materials and matrices should be tested in future research to fix the appropriate ones in each type of cancer.
On the other hand, the heterogeneity also can be found in tumors corresponding to different cell phenotypes. Tumor bulk contains different populations of cancer cells with variable stages of plasticity and different types of cancer-associated cells which could originate from adjacent normal tissue, be recruited from other sides or be differentiated from CSCs. This heterogeneity plays an essential role in supporting tumor growth at primary sites and after metastasis. From this point of view, tumor heterogeneity becomes critical to be represented in models for the purpose of reflecting their counterparts in vivo [162]. This issue has been challenged to develop in vitro models so that biomaterials should sufficiently become practical in the future. Even though combining all cell types and phenotypes in these models is still difficult to achieve, hybrid culture systems have been successful in culturing several types of cells in some types of 3D scaffolds. These hybrid systems improved the efficiency of 3D scaffold models. The 3D bioprinting technology is arising as a promising and attractive tool to overcome the difficulty of mimicking cancer heterogeneity. By their power of constructing 3D platforms containing a wide range of cancer cells and cancer-associated cells in controlled structures, this technology could improve our knowledge about cancer heterogeneity and assist in developing more effective cancer therapies. This technology also provides a unique tool to design a well-defined 3D cancer microenvironment that could bring new insights into drug development and screening. The 3D bioprinting approaches, by their capacity of mimicking TME in an automated method, will provide a more accurate representation of cancer.
On the other hand, the hypothetical ability of CSCs to construct their own microenvironment CSCs with their differentiation potential should be one of the attractive tools to overcome this challenge. A combination of CSC culture with 3D scaffold could model the plasticity including their differentiation in specific conditions developing heterogeneity in an artificial environment. In fact, isolation of CSCs from patient tissues or cancer cell lines and their maintenance are still considered so difficult that new methods and technologies are demanded [163]. To answer the purpose, our lab has developed a new method to develop CSCs from stem cells from normal tissues, induced pluripotent stem cells prepared from normal cells and embryonic stem cells in a cancerous environment. Currently, we have successfully been developing models for liver, lung, pancreas, and breast CSCs. Our models have given some insights on the differentiation potential of CSCs and their interactions with cancer-associated cells including fibroblasts and immune cells [43,164,165,166]. We believe that these CSC models with other recent models deriving from normal cells could provide unique tools for future applications of biomaterial 3D scaffold mimicking tumor microenvironment. Finally, the applications and usage of biomaterials in cancer research contribute to expand and bring a new style of investigation to clear the cancer mechanisms reducing the cost and animal dependency in the first stages of drug development.
Author Contributions
Conceptualization, G.H. and M.S.; Writing—original draft preparation, G.H., S.M.A., S.K., A.S., H.I., Y.S., M.M., and M.S.; Writing—review and editing, G.H. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank all nanotechnology lab members, Maram H. Zahra, Hend M. Nawara, Hager M. Mansour, Hagar A. Abu Quora, Mona Anas Sheta, and Sadia Monzur for their kind support. Figures created using BioRender.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vinogradova, T.V.; Chernov, I.P.; Monastyrskaya, G.S.; Kondratyeva, L.G.; Sverdlov, E.D. Cancer Stem Cells: Plasticity Works against Therapy. Acta Naturae 2015, 7, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Olmeda, F.; Ben Amar, M. Clonal pattern dynamics in tumor: The concept of cancer stem cells. Sci. Rep. 2019, 9, 15607. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Chen, J.-L. Understanding of leukemic stem cells and their clinical implications. Mol. Cancer 2017, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Alamgeer, M.; Peacock, C.D.; Matsui, W.; Ganju, V.; Watkins, D.N. Cancer stem cells in lung cancer: Evidence and controversies. Respirology 2013, 18, 757–764. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.J.; Hynes, M.; Dosch, J.; Sarkar, B.; Welling, T.H.; Pasca di Magliano, M.; Simeone, D.M. c-Met Is a Marker of Pancreatic Cancer Stem Cells and Therapeutic Target. Gastroenterology 2011, 141, 2218–2227. [Google Scholar] [CrossRef]
- Nio, K.; Yamashita, T.; Kaneko, S. The evolving concept of liver cancer stem cells. Mol. Cancer 2017, 16, 4. [Google Scholar] [CrossRef]
- Moltzahn, F.; Thalmann, G.N. Cancer stem cells in prostate cancer. Transl. Androl. Urol. 2013, 2, 242–253. [Google Scholar]
- Gopalan, V.; Islam, F.; Lam, A.K.-Y. Surface Markers for the Identification of Cancer Stem Cells. Methods Mol. Biol. 2017, 1692, 17–29. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Giraud, J.; Chambonnier, L.; Dubus, P.; Wittkop, L.; Belleannée, G.; Collet, D.; Soubeyran, I.; Evrard, S.; Rousseau, B.; et al. Characterization of Biomarkers of Tumorigenic and Chemoresistant Cancer Stem Cells in Human Gastric Carcinoma. Clin. Cancer Res. 2016, 23, 1586–1597. [Google Scholar] [CrossRef]
- Skoda, J.; Hermanová, M.; Loja, T.; Němec, P.; Neradil, J.; Karasek, P.; Veselska, R. Co-Expression of Cancer Stem Cell Markers Corresponds to a Pro-Tumorigenic Expression Profile in Pancreatic Adenocarcinoma. PLoS ONE 2016, 11, e0159255. [Google Scholar] [CrossRef]
- Osman, A.; Afify, S.M.; Hassan, G.; Fu, X.; Seno, A.; Seno, M. Revisiting Cancer Stem Cells as the Origin of Cancer-Associated Cells in the Tumor Microenvironment: A Hypothetical View from the Potential of iPSCs. Cancers 2020, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Singh, T.; Kumar, P.; Sharma, P.; Kaur, H.; Sharma, S.; Singh, S.; Kumar, S.; Mehta, K. Metabolic Adaptations in Cancer Stem Cells. Front. Oncol. 2020, 10, 1010. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Vila, M.; Takahashi, R.-U.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef]
- Volovetz, J.; Berezovsky, A.D.; Alban, T.; Chen, Y.; Lauko, A.; Aranjuez, G.F.; Burtscher, A.; Shibuya, K.; Silver, D.J.; Peterson, J.; et al. Identifying conserved molecular targets required for cell migration of glioblastoma cancer stem cells. Cell Death Dis. 2020, 11, 152. [Google Scholar] [CrossRef]
- Nia, H.T.; Munn, L.L.; Jain, R.K. Physical traits of cancer. Science 2020, 370, eaaz0868. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Osman, A.; Calle, A.S.; Nawara, H.M.; Zahra, M.H.; El-Ghlaban, D.; Mansour, H.; Alam, M.J.; Abu Quora, H.A.; et al. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering 2019, 6, 73. [Google Scholar] [CrossRef]
- Mansour, H.; Hassan, G.; Afify, S.M.; Yan, T.; Seno, A.; Seno, M. Metastasis Model of Cancer Stem Cell-Derived Tumors. Methods Protoc. 2020, 3, 60. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Nie, B.; Pienta, K.J.; Morgan, T.M.; Taichman, R.S. Cancer stem cells and their role in metastasis. Pharmacol. Ther. 2013, 138, 285–293. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Schutrum, B.E.; Whitman, M.A.; Fischbach, C. 2.5.11—Biomaterials-Based Model Systems to Study Tumor–Microenvironment Interactions. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1217–1236. [Google Scholar] [CrossRef]
- Chen, F.-M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Scadden, D.T. The stem-cell niche as an entity of action. Nat. Cell Biol. 2006, 441, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.; Celso, C.L.; Scadden, D.T. Adult Stem Cels and Their Niches. Adv. Exp. Med. Biol. 2010, 695, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Borovski, T.; De Sousa, E.M.F.; Vermeulen, L.; Medema, J.P. Cancer Stem Cell Niche: The Place to Be. Cancer Res. 2011, 71, 634–639. [Google Scholar] [CrossRef]
- Osman, A.; Oze, M.; Afify, S.M.; Hassan, G.; El-Ghlban, S.; Nawara, H.M.; Fu, X.; Zahra, M.H.; Seno, A.; Winer, I.; et al. Tumor-associated macrophages derived from cancer stem cells. Acta Histochem. 2020, 122, 151628. [Google Scholar] [CrossRef]
- Wang, R.; Chadalavada, K.; A Wilshire, J.; Kowalik, U.; Hovinga, K.E.; Geber, A.; Fligelman, B.; Leversha, M.; Brennan, C.; Tabar, V. Glioblastoma stem-like cells give rise to tumour endothelium. Nat. Cell Biol. 2010, 468, 829–833. [Google Scholar] [CrossRef]
- Hassan, G.; Afify, S.M.; Nair, N.; Kumon, K.; Osman, A.; Du, J.; Mansour, H.; Abu Quora, H.A.; Nawara, H.M.; Satoh, A.; et al. Hematopoietic Cells Derived from Cancer Stem Cells Generated from Mouse Induced Pluripotent Stem Cells. Cancers 2019, 12, 82. [Google Scholar] [CrossRef]
- Oskarsson, T.; Batlle, E.; Massagué, J. Metastatic Stem Cells: Sources, Niches, and Vital Pathways. Cell Stem Cell 2014, 14, 306–321. [Google Scholar] [CrossRef]
- Ye, J.; Wu, D.; Wu, P.; Chen, Z.; Huang, J. The cancer stem cell niche: Cross talk between cancer stem cells and their microenvironment. Tumor Biol. 2014, 35, 3945–3951. [Google Scholar] [CrossRef]
- Lyden, D.; Hattori, K.; Dias, S.; Costa, C.; Blaikie, P.; Butros, L.; Chadburn, A.; Heissig, B.; Marks, W.; Witte, L.; et al. Impaired recruitment of bone-marrow–derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001, 7, 1194–1201. [Google Scholar] [CrossRef]
- Conejo-Garcia, J.R.; Benencia, F.; Courreges, M.-C.; Kang, E.; Mohamed-Hadley, A.; Buckanovich, R.J.; O Holtz, D.; Jenkins, A.; Na, H.; Zhang, L.; et al. Tumor-infiltrating dendritic cell precursors recruited by a β-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat. Med. 2004, 10, 950–958. [Google Scholar] [CrossRef]
- Gao, D.; Nolan, D.J.; Mellick, A.S.; Bambino, K.; McDonnell, K.; Mittal, V. Endothelial Progenitor Cells Control the Angiogenic Switch in Mouse Lung Metastasis. Science 2008, 319, 195–198. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Mitsunaga, S.; Kinoshita, T.; Konishi, M.; Takahashi, S.; Gotohda, N.; Kato, Y.; Aizawa, M.; Ochiai, A. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer Sci. 2012, 103, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bernabé, A.M.; Ferjančič, Š.; Tlalka, M.; Zhao, L.; Allen, P.D.; Im, J.H.; Watson, K.; Hill, S.A.; Amirkhosravi, A.; Francis, J.L.; et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 2012, 119, 3164–3175. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A.; et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Yan, T.; Calle, A.S.; Nair, N.; Hurley, L.; Kasai, T.; Kakuta, H.; Masuda, J.; Murakami, H.; Mizutani, A.; et al. iPSC-derived cancer stem cells provide a model of tumor vasculature. Am. J. Cancer Res. 2016, 6, 1906–1921. [Google Scholar]
- Nair, N.; Calle, A.S.; Zahra, M.H.; Prieto-Vila, M.; Oo, A.K.K.; Hurley, L.; Vaidyanath, A.; Seno, A.; Masuda, J.; Iwasaki, Y.; et al. A cancer stem cell model as the point of origin of cancer-associated fibroblasts in tumor microenvironment. Sci. Rep. 2017, 7, 6838. [Google Scholar] [CrossRef]
- Matsuda, S.; Yan, T.; Mizutani, A.; Sota, T.; Hiramoto, Y.; Prieto-Vila, M.; Chen, L.; Satoh, A.; Kudoh, T.; Kasai, T.; et al. Cancer stem cells maintain a hierarchy of differentiation by creating their niche. Int. J. Cancer 2014, 135, 27–36. [Google Scholar] [CrossRef]
- Kasai, T.; Chen, L.; Mizutani, A.; Kudoh, T.; Murakami, H.; Fu, L.; Seno, M. Cancer Stem Cells Converted from Pluripotent Stem Cells and the Cancerous Niche. J. Stem. Cells Regen. Med. 2014, 10, 2–7. [Google Scholar]
- Yan, T.; Mizutani, A.; Matsuda, S.; Murakami, H.; Kasai, T.; Seno, M. Mutual dependence between cancer stem cells and their progenies: The niche created by the progenies is sustaining cancer stem cells. Cancer Cell Microenviron. 2014, 1, 4. [Google Scholar] [CrossRef][Green Version]
- Hassan, G.; Seno, M. Blood and Cancer: Cancer Stem Cells as Origin of Hematopoietic Cells in Solid Tumor Microenvironments. Cells 2020, 9, 1293. [Google Scholar] [CrossRef]
- Ordikhani, F.; Kim, Y.; Zustiak, S.P. The Role of Biomaterials on Cancer Stem Cell Enrichment and Behavior. JOM 2015, 67, 2543–2549. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Kim, H.; Lin, Q.; Glazer, P.M.; Yun, Z. The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells. Breast Cancer Res. 2018, 20, 16. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Dong, D.-L.; Jang, T.-S.; Knowles, J.C.; Kim, H.-W.; Jin, G.-Z.; Xuan, Y. 3D culture technologies of cancer stem cells: Promising ex vivo tumor models. J. Tissue Eng. 2020, 11, 2041731420933407. [Google Scholar] [CrossRef]
- Ravi, M.; Ramesh, A.; Pattabhi, A. Contributions of 3D Cell Cultures for Cancer Research. J. Cell. Physiol. 2017, 232, 2679–2697. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O.; Naba, A. Overview of the Matrisome—An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Prabhakaran, M.P.; Sireesha, M.; Ramakrishna, S. Collagen in Human Tissues: Structure, Function, and Biomedical Implications from a Tissue Engineering Perspective. In Polymer Composites–Polyolefin Fractionation–Polymeric Peptidomimetics–Collagens; Abe, A., Kausch, H.-H., Möller, M., Pasch, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 173–206. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef]
- Boot-Handford, R.P.; Tuckwell, D.S. Fibrillar collagen: The key to vertebrate evolution? A tale of molecular incest. BioEssays 2003, 25, 142–151. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef]
- Klebe, R.J. Cytoscribing: A method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Exp. Cell Res. 1988, 179, 362–373. [Google Scholar] [CrossRef]
- Grzesiak, J.J.; Bouvet, M. Determination of the Ligand-Binding Specificities of the ??2??1 and ??1??1 Integrins in a Novel 3-Dimensional In Vitro Model of Pancreatic Cancer. Pancreas 2007, 34, 220–228. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kuo, S.M.; Liu, G.-S.; Chen, W.-N.U.; Chuang, C.-W.; Liu, L. Enhancement of neurite outgrowth in neuron cancer stem cells by growth on 3-D collagen scaffolds. Biochem. Biophys. Res. Commun. 2012, 428, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Husmann, A.; Hume, R.D.; Watson, C.J.; Cameron, R.E. Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines. Biomaterials 2017, 114, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Guo, J.; Tierney, E.G.; Curtin, C.M.; Malhotra, M.; Darcy, R.; O’Brien, F.J.; O’Driscoll, C.M. The use of collagen-based scaffolds to simulate prostate cancer bone metastases with potential for evaluating delivery of nanoparticulate gene therapeutics. Biomaterials 2015, 66, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kitano, S.; Irie, S.; Levato, R.; Matsusaki, M. Collagen Microfibers Induce Blood Capillary Orientation and Open Vascular Lumen. Adv. Biosyst. 2020, 4, e2000038. [Google Scholar] [CrossRef] [PubMed]
- Naka, Y.; Kitano, S.; Irie, S.; Matsusaki, M. Wholly vascularized millimeter-sized engineered tissues by cell-sized microscaffolds. Mater. Today Bio 2020, 6, 100054. [Google Scholar] [CrossRef]
- Malinoff, H.L.; Wicha, M.S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J. Cell Biol. 1983, 96, 1475–1479. [Google Scholar] [CrossRef]
- Tandon, N.N.; A Holland, E.; Kralisz, U.; Kleinman, H.K.; A Robey, F.; A Jamieson, G. Interaction of human platelets with laminin and identification of the 67 kDa laminin receptor on platelets. Biochem. J. 1991, 274 Pt 2, 535–542. [Google Scholar] [CrossRef]
- Setiawati, A.; Nguyen, H.T.; Jung, Y.; Shin, K. Future Research Directions in the Design of Versatile Extracellular Matrix in Tissue Engineering. Int. Neurourol. J. 2018, 22, S66–S75. [Google Scholar] [CrossRef]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional Diversity of Laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Neal, R.A.; Lenz, S.M.; Wang, T.; Abebayehu, D.; Brooks, B.P.; Ogle, R.C.; Botchwey, E.A. Laminin- and basement membranepolycaprolactone blend nanofibers as a scaffold for regenerative medicine. Nanomater. Environ. 2014, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pape, J.; Magdeldin, T.; Ali, M.; Walsh, C.; Lythgoe, M.; Emberton, M.; Cheema, U. Cancer invasion regulates vascular complexity in a three-dimensional biomimetic model. Eur. J. Cancer 2019, 119, 179–193. [Google Scholar] [CrossRef]
- Yamada, Y.; Hozumi, K.; Aso, A.; Hotta, A.; Toma, K.; Katagiri, F.; Kikkawa, Y.; Nomizu, M. Laminin active peptide/agarose matrices as multifunctional biomaterials for tissue engineering. Biomaterials 2012, 33, 4118–4125. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Olejniczak, K.; Brodaczewska, K.K.; Bielecka, Z.F.; Solarek, W.; Kornakiewicz, A.; Szczylik, C.A.; Porta, C.; Czarnecka, A.M. Development of extracellular matrix supported 3D culture of renal cancer cells and renal cancer stem cells. Cytotechnology 2019, 71, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.K.; Lim, J.K.; Leong, M.F.; Sandanaraj, E.; Ang, B.T.; Tang, C.; Wan, A.C.A. Collaboration of 3D context and extracellular matrix in the development of glioma stemness in a 3D model. Biomaterials 2016, 78, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Horibata, S.; Vo, T.V.; Subramanian, V.; Thompson, P.R.; Coonrod, S.A. Utilization of the Soft Agar Colony Formation Assay to Identify Inhibitors of Tumorigenicity in Breast Cancer Cells. J. Vis. Exp. 2015, 10, e52727. [Google Scholar] [CrossRef]
- Shams, A.; Eslahi, N.; Movahedin, M.; Izadyar, F.; Asgari, H.; Koruji, M. Future of Spermatogonial Stem Cell Culture: Application of Nanofiber Scaffolds. Curr. Stem Cell Res. Ther. 2017, 12, 544–553. [Google Scholar] [CrossRef]
- Abe-Fukasawa, N.; Otsuka, K.; Aihara, A.; Itasaki, N.; Nishino, T. Novel 3D Liquid Cell Culture Method for Anchorage-independent Cell Growth, Cell Imaging and Automated Drug Screening. Sci. Rep. 2018, 8, 3627. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef]
- Meinert, C.; Theodoropoulos, C.; Klein, T.J.; Hutmacher, D.W.; Loessner, D. A Method for Prostate and Breast Cancer Cell Spheroid Cultures Using Gelatin Methacryloyl-Based Hydrogels. Methods Mol. Biol. 2018, 1786, 175–194. [Google Scholar] [CrossRef]
- Peela, N.; Sam, F.S.; Christenson, W.; Truong, D.; Watson, A.W.; Mouneimne, G.; Ros, R.; Nikkhah, M. A three dimensional micropatterned tumor model for breast cancer cell migration studies. Biomaterials 2016, 81, 72–83. [Google Scholar] [CrossRef]
- Dietrich, F.; Lelkes, P.I. Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis 2006, 9, 111–125. [Google Scholar] [CrossRef]
- Falvo, M.R.; Gorkun, O.V.; Lord, S.T. The molecular origins of the mechanical properties of fibrin. Biophys. Chem. 2010, 152, 15–20. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Wang, H.-Z.; Peng, Y.-N.; Li, H.-O.; Zhou, Y.-J.; Liu, S.; Wang, F.; Liu, L.; Chang, Y.; et al. Soft fibrin matrix downregulates DAB2IP to promote Nanog-dependent growth of colon tumor-repopulating cells. Cell Death Dis. 2019, 10, 151. [Google Scholar] [CrossRef]
- Knowles, L.M.; Gurski, L.A.; Maranchie, J.; Pilch, J. Fibronectin Matrix Formation is a Prerequisite for Colonization of Kidney Tumor Cells in Fibrin. J. Cancer 2015, 6, 98–104. [Google Scholar] [CrossRef]
- Wang, L.; Shelton, R.; Cooper, P.; Lawson, M.; Triffitt, J.; Barralet, J.E. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003, 24, 3475–3481. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Chen, M.C.W.; Gupta, M.; Cheung, K.C. Alginate-based microfluidic system for tumor spheroid formation and anticancer agent screening. Biomed. Microdevices 2010, 12, 647–654. [Google Scholar] [CrossRef]
- Estrada, M.F.; Rebelo, S.P.; Davies, E.J.; Pinto, M.T.; Pereira, H.; Santo, V.E.; Smalley, M.J.; Barry, S.T.; Gualda, E.J.; Alves, P.M.; et al. Modelling the tumour microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression. Biomaterials 2016, 78, 50–61. [Google Scholar] [CrossRef]
- Verbridge, S.S.; Choi, N.W.; Zheng, Y.; Brooks, D.J.; Stroock, A.D.; Fischbach, C. Oxygen-Controlled Three-Dimensional Cultures to Analyze Tumor Angiogenesis. Tissue Eng. Part A 2010, 16, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Yu, W.; Xie, Y.; Zhang, X.; Zhang, Y.; Ma, X. Development of an in Vitro Multicellular Tumor Spheroid Model Using Microencapsulation and Its Application in Anticancer Drug Screening and Testing. Biotechnol. Prog. 2005, 21, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.-P.; Zhao, Y.-F.; Li, C.-F.; Yin, Y.-B.; Meng, Q.-Y.; Lin, F.-H.; Liu, Y.; Hou, X.-L.; Guo, K.; Chen, X.-B.; et al. An alginate-based platform for cancer stem cell research. Acta Biomater. 2016, 37, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-X.; Liu, C.; Liu, Y.; Yang, L.; Li, N.; Guo, X.; Sun, G.-W.; Ma, X.-J. Enrichment of cancer stem cell-like cells by culture in alginate gel beads. J. Biotechnol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Subramaniyan, A.; Ravi, M. Agarose hydrogel induced MCF-7 and BMG-1 cell line progressive 3D and 3D revert cultures. J. Cell. Physiol. 2018, 233, 2768–2772. [Google Scholar] [CrossRef]
- Murakami, S.; Mukaisho, K.; Iwasa, T.; Kawabe, M.; Yoshida, S.; Taniura, N.; Nakayama, T.; Noi, M.; Yamamoto, G.; Sugihara, H. Application of “Tissueoid Cell Culture System” Using a Silicate Fiber Scaffold for Cancer Research. Pathobiology 2020, 87, 291–301. [Google Scholar] [CrossRef]
- Nicklin, M.; Rees, R.C.; Pockley, A.G.; Perry, C.C. Development of an hydrophobic fluoro-silica surface for studying homotypic cancer cell aggregation–disaggregation as a single dynamic process in vitro. Biomater. Sci. 2014, 2, 1486–1496. [Google Scholar] [CrossRef]
- Jokinen, M.; Pittois, K.; van den Akker, S.; Gutschoven, I.; Assmuth, T.; Metz, T.; Lehtila, H.; Alanne, P. Multiphase matrix of silica, culture medium and air for 3D mammalian cell culture. Cytotechnology 2020, 72, 271–282. [Google Scholar] [CrossRef]
- Jang, J.; Yi, H.-G.; Cho, D. 3D Printed Tissue Models: Present and Future. ACS Biomater. Sci. Eng. 2016, 2, 1722–1731. [Google Scholar] [CrossRef]
- Albritton, J.L.; Miller, J.S. 3D bioprinting: Improvingin vitromodels of metastasis with heterogeneous tumor microenvironments. Dis. Model. Mech. 2017, 10, 3–14. [Google Scholar] [CrossRef]
- Seol, Y.-J.; Kang, T.-Y.; Kim, Y.K. Solid freeform fabrication technology applied to tissue engineering with various biomaterials. Soft Matter 2012, 8, 1730–1735. [Google Scholar] [CrossRef]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.J.; Saik, J.E.; Poché, R.A.; Leslie-Barbick, J.E.; Lee, S.-H.; Smith, A.A.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31, 3840–3847. [Google Scholar] [CrossRef]
- Cushing, M.C.; Anseth, K.S. Materials Science: Hydrogel Cell Cultures. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef]
- Lutolf, M.P. Biomaterials: Spotlight on hydrogels. Nat. Mater. 2009, 8, 451–453. [Google Scholar] [CrossRef]
- Lutolf, M.P.; A Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Gill, B.J.; Gibbons, D.L.; Roudsari, L.C.; Saik, J.E.; Rizvi, Z.H.; Roybal, J.D.; Kurie, J.M.; West, J.L. A Synthetic Matrix with Independently Tunable Biochemistry and Mechanical Properties to Study Epithelial Morphogenesis and EMT in a Lung Adenocarcinoma Model. Cancer Res. 2012, 72, 6013–6023. [Google Scholar] [CrossRef]
- Nitta, S.K.; Matsumura, S.; Fisher, J.P. Synthesis and characterization of cyclic acetal based degradable hydrogels. Eur. J. Pharm. Biopharm. 2008, 68, 67–73. [Google Scholar] [CrossRef]
- Scaffaro, R.; Re, G.L.; Rigogliuso, S.; Ghersi, G. 3D polylactide-based scaffolds for studying human hepatocarcinoma processesin vitro. Sci. Technol. Adv. Mater. 2012, 13, 045003. [Google Scholar] [CrossRef]
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Soman, P.; Kelber, J.A.; Lee, J.W.; Wright, T.N.; Vecchio, K.S.; Klemke, R.L.; Chen, S. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials 2012, 33, 7064–7070. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Chan, C.K.; Ramakrishna, S. Stem cells and biomimetic materials strategies for tissue engineering. Mater. Sci. Eng. C 2008, 28, 1189–1202. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Rabionet, M.; Yeste, M.; Puig, T.; Ciurana, J. Electrospinning PCL Scaffolds Manufacture for Three-Dimensional Breast Cancer Cell Culture. Polymers 2017, 9, 328. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Feng, S.; Duan, X.; Lo, P.-K.; Liu, S.; Liu, X.; Chen, H.; Wang, Q. Expansion of breast cancer stem cells with fibrous scaffolds. Integr. Biol. 2013, 5, 768–777. [Google Scholar] [CrossRef]
- Palomeras, S.; Rabionet, M.; Ferrer, I.; Sarrats, A.; Garcia-Romeu, M.L.; Puig, T.; Ciurana, J. Breast Cancer Stem Cell Culture and Enrichment Using Poly(ε-Caprolactone) Scaffolds. Molecules 2016, 21, 537. [Google Scholar] [CrossRef]
- Sims-Mourtada, J.; Niamat, A.R.; Samuel, S.; Eskridge, C.; Kmiec, E.B. Enrichment of breast cancer stem-like cells by growth on electrospun polycaprolactone-chitosan nanofiber scaffolds. Int. J. Nanomed. 2014, 9, 995–1003. [Google Scholar] [CrossRef]
- Almajhdi, F.N.; Fouad, H.; Khalil, K.A.; Awad, H.M.; Mohamed, S.H.; Elsarnagawy, T.; Albarrag, A.M.; Al-Jassir, F.F.; Abdo, H.S. In-vitro anticancer and antimicrobial activities of PLGA/silver nanofiber composites prepared by electrospinning. J. Mater. Sci. Mater. Electron. 2014, 25, 1045–1053. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Na Shin, Y.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Santovena, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Llabres, M.; Farina, J.B. Rheological properties of PLGA film-based implants: Correlation with polymer degradation and SPf66 antimalaric synthetic peptide release. Biomaterials 2004, 25, 925–931. [Google Scholar] [CrossRef]
- Cesur, S.; Oktar, F.N.; Ekren, N.; Kilic, O.; Alkaya, D.B.; Seyhan, S.A.; Ege, Z.R.; Lin, C.-C.; Kuruca, S.E.; Erdemir, G.; et al. Preparation and characterization of electrospun polylactic acid/sodium alginate/orange oyster shell composite nanofiber for biomedical application. J. Aust. Ceram. Soc. 2020, 56, 533–543. [Google Scholar] [CrossRef]
- Hong, K.H.; Woo, S.H.; Kang, T.J. In vitro degradation and drug-release behavior of electrospun, fibrous webs of poly(lactic-co-glycolic acid). J. Appl. Polym. Sci. 2012, 124, 209–214. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Y.; Gan, D.; Yang, Z.; Ao, H.; Zhang, Q.; Fanglian, Y.; Wan, Y. Incorporation of hydroxyapatite into nanofibrous PLGA scaffold towards improved breast cancer cell behavior. Mater. Chem. Phys. 2019, 226, 177–183. [Google Scholar] [CrossRef]
- Long, T.J.; Sprenger, C.C.; Plymate, S.R.; Ratner, B.D. Prostate cancer xenografts engineered from 3D precision-porous poly(2-hydroxyethyl methacrylate) hydrogels as models for tumorigenesis and dormancy escape. Biomaterials 2014, 35, 8164–8174. [Google Scholar] [CrossRef]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef]
- Escobar-Chavez, J.J.; Lopez-Cervantes, M.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applica-tions of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Sheu, M.-T.; Jhan, H.-J.; Su, C.-Y.; Chen, L.-C.; Chang, C.-E.; Liu, D.-Z.; Ho, H.-O. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf. B Biointerfaces 2016, 143, 260–270. [Google Scholar] [CrossRef]
- Wu, B.; Takeshita, N.; Wu, Y.; VijayaVenkataRaman, S.; Ho, K.Y.; Lu, W.F.; Fuhb, J.Y.H. Pluronic F127 blended polycaprolactone scaffolds via e-jetting for esophageal tissue engineering. J. Mater. Sci. Mater. Electron. 2018, 29, 140. [Google Scholar] [CrossRef]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. NPJ Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Datta, P.; Shanmughapriya, S.; Ozbolat, I.T. 3D Bioprinting of Tumor Models for Cancer Research. ACS Appl. Bio Mater. 2020, 3, 5552–5573. [Google Scholar] [CrossRef]
- Xu, F.; Celli, J.; Rizvi, I.; Moon, S.; Hasan, T.; Demirci, U. A three-dimensional in vitro ovarian cancer coculture model using a high-throughput cell patterning platform. Biotechnol. J. 2011, 6, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. In vitro assays and techniques utilized in anticancer drug discovery. J. Appl. Toxicol. 2019, 39, 38–71. [Google Scholar] [CrossRef]
- Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Cell Culture Based in vitro Test Systems for Anticancer Drug Screening. Front. Bioeng. Biotechnol. 2020, 8, 322. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT–mTOR–S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Risbridger, G.; Lawrence, M.G.; A Taylor, R. PDX: Moving Beyond Drug Screening to Versatile Models for Research Discovery. J. Endocr. Soc. 2020, 4, bvaa132. [Google Scholar] [CrossRef]
- Monteiro, M.V.; Gaspar, V.; Ferreira, L.P.; Mano, J.F. Hydrogel 3D in vitro tumor models for screening cell aggregation mediated drug response. Biomater. Sci. 2020, 8, 1855–1864. [Google Scholar] [CrossRef]
- Pavlou, M.; Shah, M.; Gikas, P.; Briggs, T.; Roberts, S.; Cheema, U. Osteomimetic matrix components alter cell migration and drug response in a 3D tumour-engineered osteosarcoma model. Acta Biomater. 2019, 96, 247–257. [Google Scholar] [CrossRef]
- Lan, S.-F.; Starly, B. Alginate based 3D hydrogels as an in vitro co-culture model platform for the toxicity screening of new chemical entities. Toxicol. Appl. Pharmacol. 2011, 256, 62–72. [Google Scholar] [CrossRef]
- Brooks, E.A.; Galarza, S.; Gencoglu, M.F.; Cornelison, R.C.; Munson, J.M.; Peyton, S.R. Applicability of drug response metrics for cancer studies using biomaterials. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180226. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, H.K.; Ray, A.R.; Panda, A.K. Three-dimensional chitosan scaffold-based MCF-7 cell culture for the determination of the cytotoxicity of tamoxifen. Biomaterials 2005, 26, 979–986. [Google Scholar] [CrossRef]
- Zustiak, S.P.; Dadhwal, S.; Medina, C.; Steczina, S.; Chehreghanianzabi, Y.; Ashraf, A.; Asuri, P. Three-dimensional matrix stiffness and adhesive ligands affect cancer cell response to toxins. Biotechnol. Bioeng. 2016, 113, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, B.; Dong, Y.; Wang, W.; Zheng, X.; Zhou, W.; Zhang, K.; Du, Z. Three-dimensional prostate tumor model based on a hyaluronic acid-alginate hydrogel for evaluation of anti-cancer drug efficacy. J. Biomater. Sci. Polym. Ed. 2017, 28, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Mooney, D.J. Biomaterials and emerging anticancer therapeutics: Engineering the microenvironment. Nat. Rev. Cancer 2016, 16, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef] [PubMed]
- Beri, P.; Matte, B.F.; Fattet, L.; Kim, D.; Yang, J.; Engler, A.J. Biomaterials to model and measure epithelial cancers. Nat. Rev. Mater. 2018, 3, 418–430. [Google Scholar] [CrossRef]
- Cavo, M.; Caria, M.; Pulsoni, I.; Beltrame, F.; Fato, M.; Scaglione, S. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci. Rep. 2018, 8, 5333. [Google Scholar] [CrossRef]
- Jiang, T.; Munguia-Lopez, J.G.; Gu, K.; Bavoux, M.M.; Flores-Torres, S.; Kort-Mascort, J.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Ehrlicher, A.J.; et al. Engineering bioprintable alginate/gelatin composite hydrogels with tunable mechanical and cell adhesive properties to modulate tumor spheroid growth kinetics. Biofabrication 2019, 12, 015024. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S. 3D porous chitosan-chondroitin sulfate scaffolds promote epithelial to mesenchymal transition in prostate cancer cells. Biomaterials 2020, 254, 120126. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, M.; Fu, Y.; Castro, N.J.; Fu, S.W.; Zhang, L.G. Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomater. 2015, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Ganapathy, K.; Andl, T.; Wang, Z.; Copland, J.A.; Chakrabarti, R.; Florczyk, S. 3D porous chitosan-alginate scaffold stiffness promotes differential responses in prostate cancer cell lines. Biomaterials 2019, 217, 119311. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, S.J.; Kievit, F.M.; Wang, K.; Erickson, A.E.; Ellenbogen, R.G.; Zhang, M. 3D porous chitosan—Alginate scaffolds promote proliferation and enrichment of cancer stem-like cells. J. Mater. Chem. B 2016, 4, 6326–6334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Sun, D.; Liu, Y.; Wang, Y.; Liu, C.; Wu, H.; Lv, Y.; Ren, Y.; Guo, X.; et al. Development of a Biomimetic Chondroitin Sulfate-modified Hydrogel to Enhance the Metastasis of Tumor Cells. Sci. Rep. 2016, 6, 29858. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.R.; Maia, F.R.; Vieira, S.; Reis, R.L.; Oliveira, I.M. Tuning Enzymatically Crosslinked Silk Fibroin Hydrogel Properties for the Development of a Colorectal Cancer Extravasation 3D Model on a Chip. Glob. Chall. 2018, 2, 1700100. [Google Scholar] [CrossRef]
- Dondajewska, E.; Juzwa, W.; Mackiewicz, A.; Dams-Kozlowska, H. Heterotypic breast cancer model based on a silk fibroin scaffold to study the tumor microenvironment. Oncotarget 2018, 9, 4935–4950. [Google Scholar] [CrossRef]
- Malandrino, A.; Mak, M.; Kamm, R.D.; Moeendarbary, E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extreme Mech. Lett. 2018, 21, 25–34. [Google Scholar] [CrossRef]
- Barney, L.E.; Jansen, L.E.; Polio, S.R.; Galarza, S.; Lynch, M.E.; Peyton, S.R. The predictive link between matrix and metastasis. Curr. Opin. Chem. Eng. 2016, 11, 85–93. [Google Scholar] [CrossRef]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695–724. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour heterogeneity and cancer cell plasticity. Nat. Cell Biol. 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Duan, J.-J.; Qiu, W.; Xu, S.-L.; Wang, B.; Ye, X.-Z.; Ping, Y.-F.; Zhang, X.; Bian, X.-W.; Yu, S.-C. Strategies for Isolating and Enriching Cancer Stem Cells: Well Begun Is Half Done. Stem Cells Dev. 2013, 22, 2221–2239. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Calle, A.S.; Hassan, G.; Kumon, K.; Nawara, H.M.; Zahra, M.H.; Mansour, H.M.; Khayrani, A.C.; Alam, J.; Du, J.; et al. A novel model of liver cancer stem cells developed from induced pluripotent stem cells. Br. J. Cancer 2020, 122, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Calle, A.S.; Nair, N.; Oo, A.K.; Prieto-Vila, M.; Koga, M.; Khayrani, A.C.; Hussein, M.; Hurley, L.; Vaidyanath, A.; Seno, A.; et al. A new PDAC mouse model originated from iPSCs-converted pancreatic cancer stem cells (CSCcm). Am. J. Cancer Res. 2016, 6, 2799–2815. [Google Scholar] [PubMed]
- Chen, L.; Kasai, T.; Li, Y.; Sugii, Y.; Jin, G.; Okada, M.; Vaidyanath, A.; Mizutani, A.; Satoh, A.; Kudoh, T.; et al. A Model of Cancer Stem Cells Derived from Mouse Induced Pluripotent Stem Cells. PLoS ONE 2012, 7, e33544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).