Hydrodynamic and Mass Transfer in the Desorption Process of CO2 Gas in a Packed-Bed Stripper
Abstract
1. Introduction
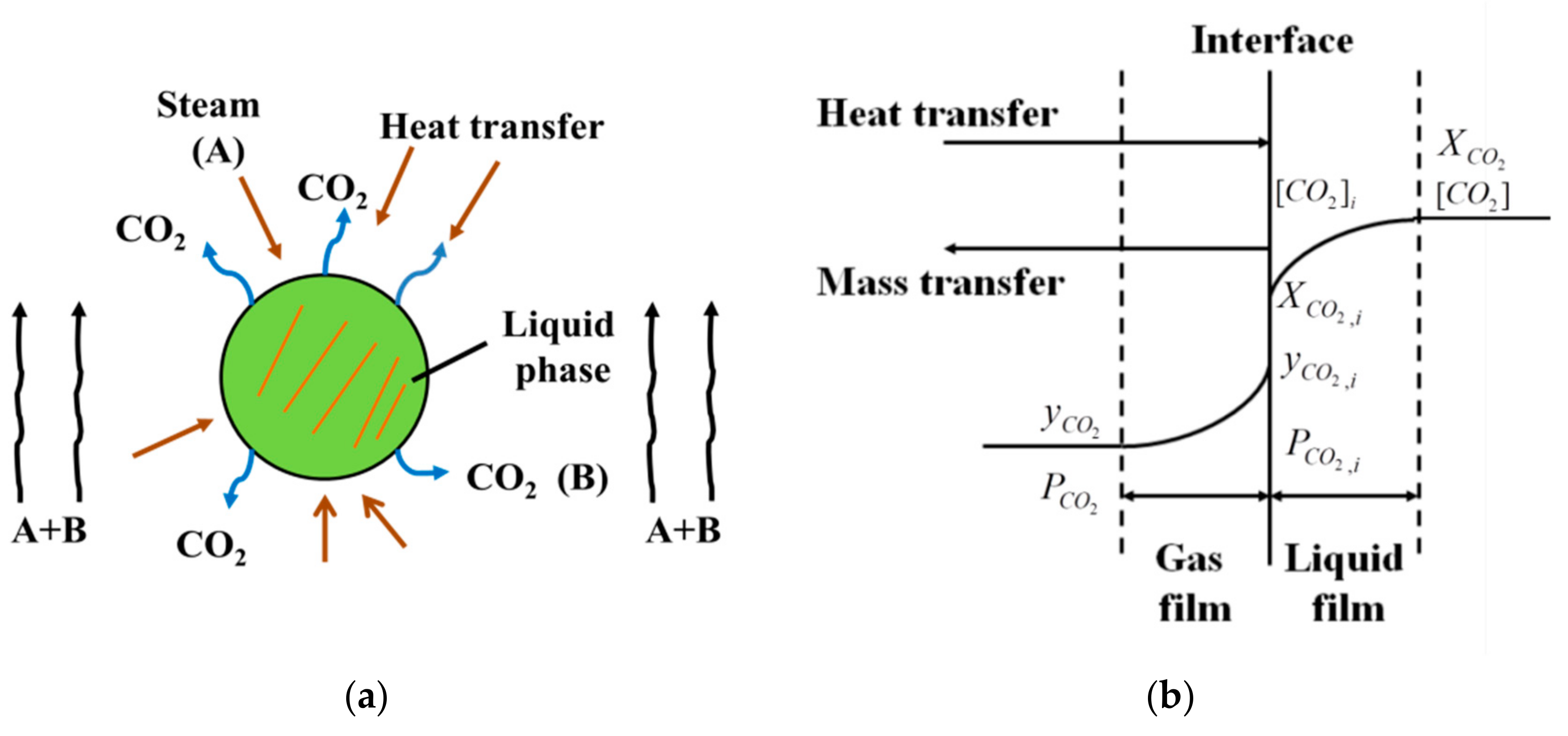
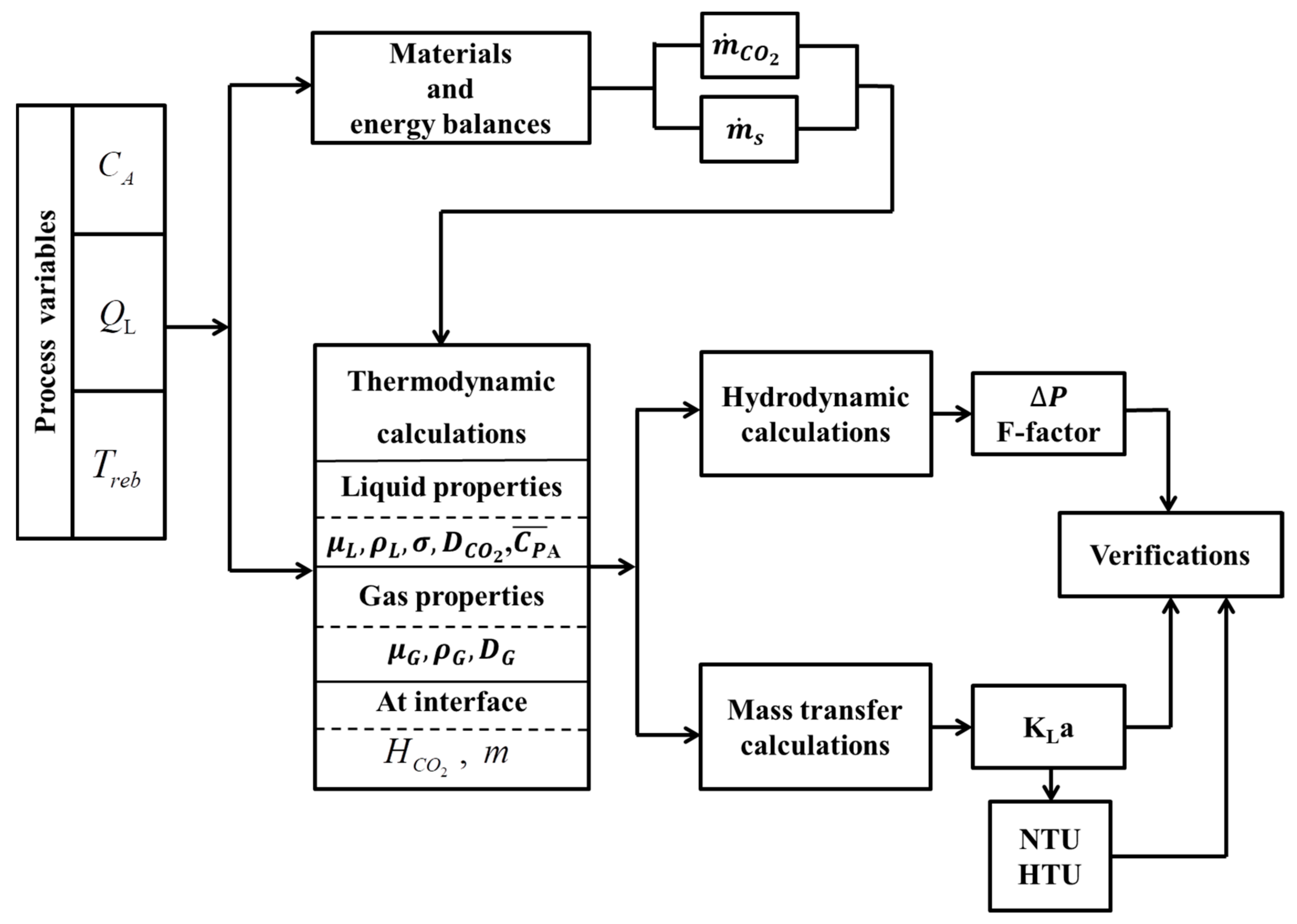
2. Models
3. Experiment
3.1. Experimental Design
3.2. Experimental Device and Operating Procedure
4. Results and Discussion
4.1. Dynamic and Steady State of the CO2 Stripper
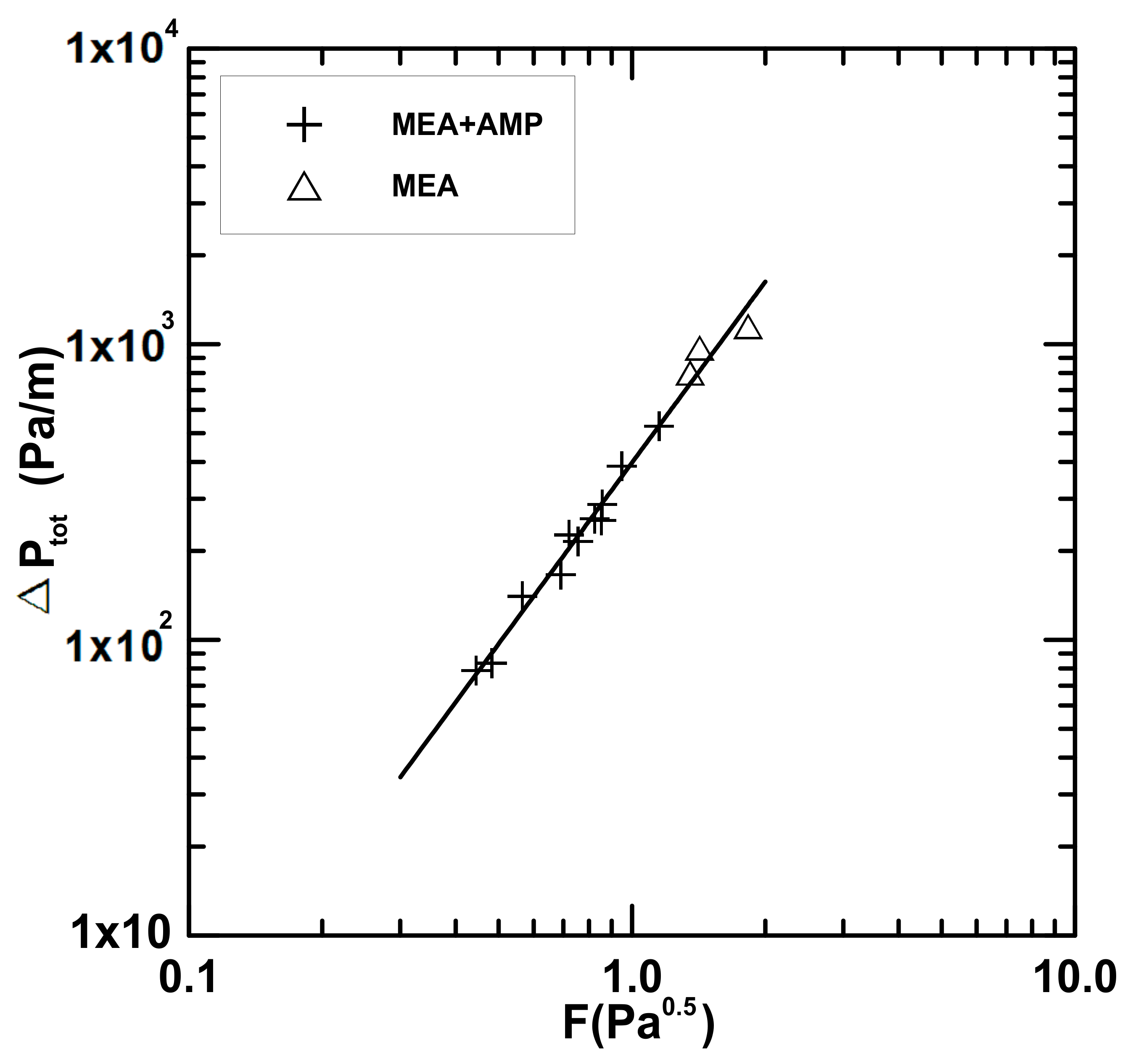
4.2. Hydrodynamic in a Packed Column
4.3. Evaluation Using Mass-Transfer Model
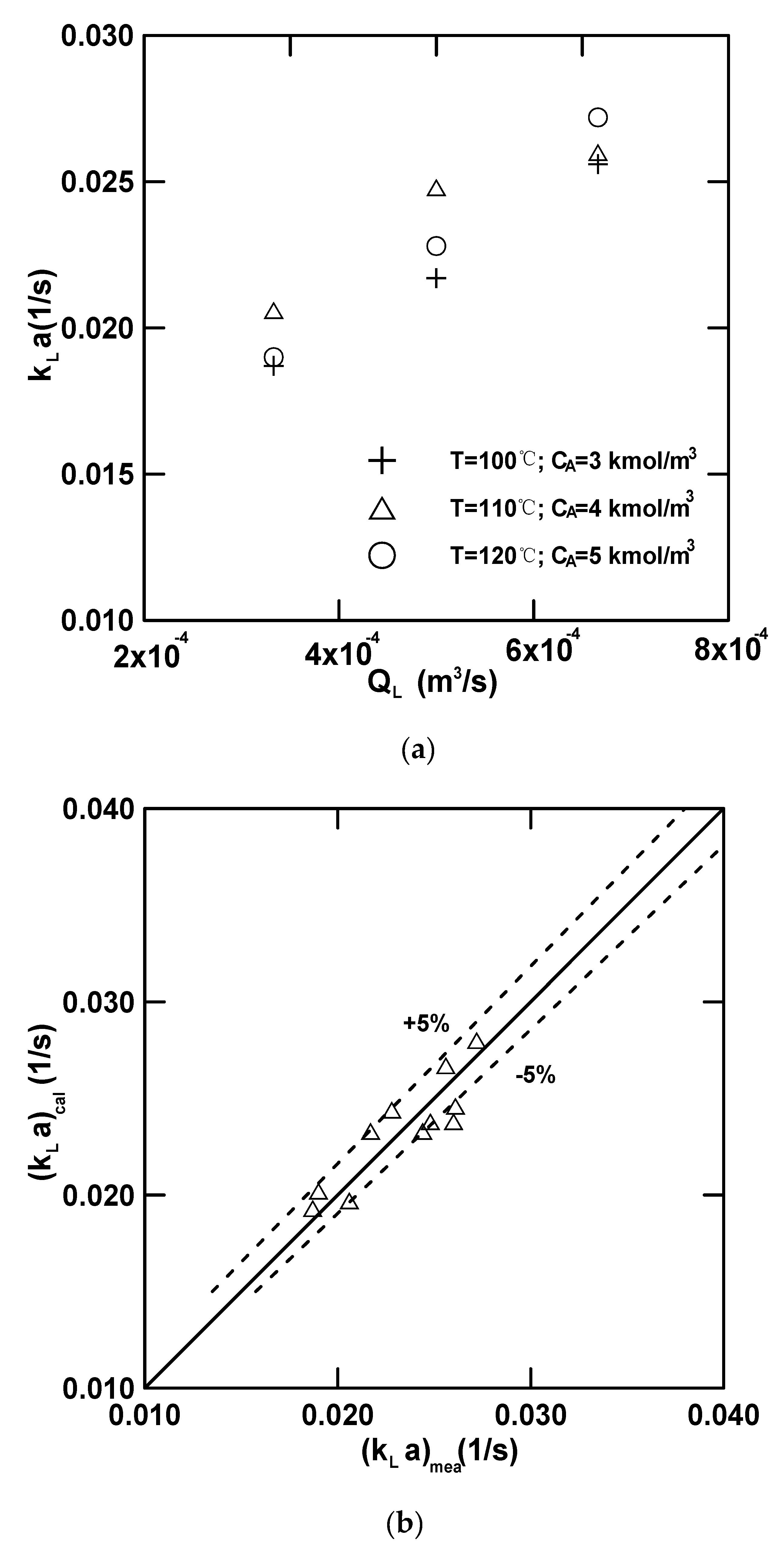
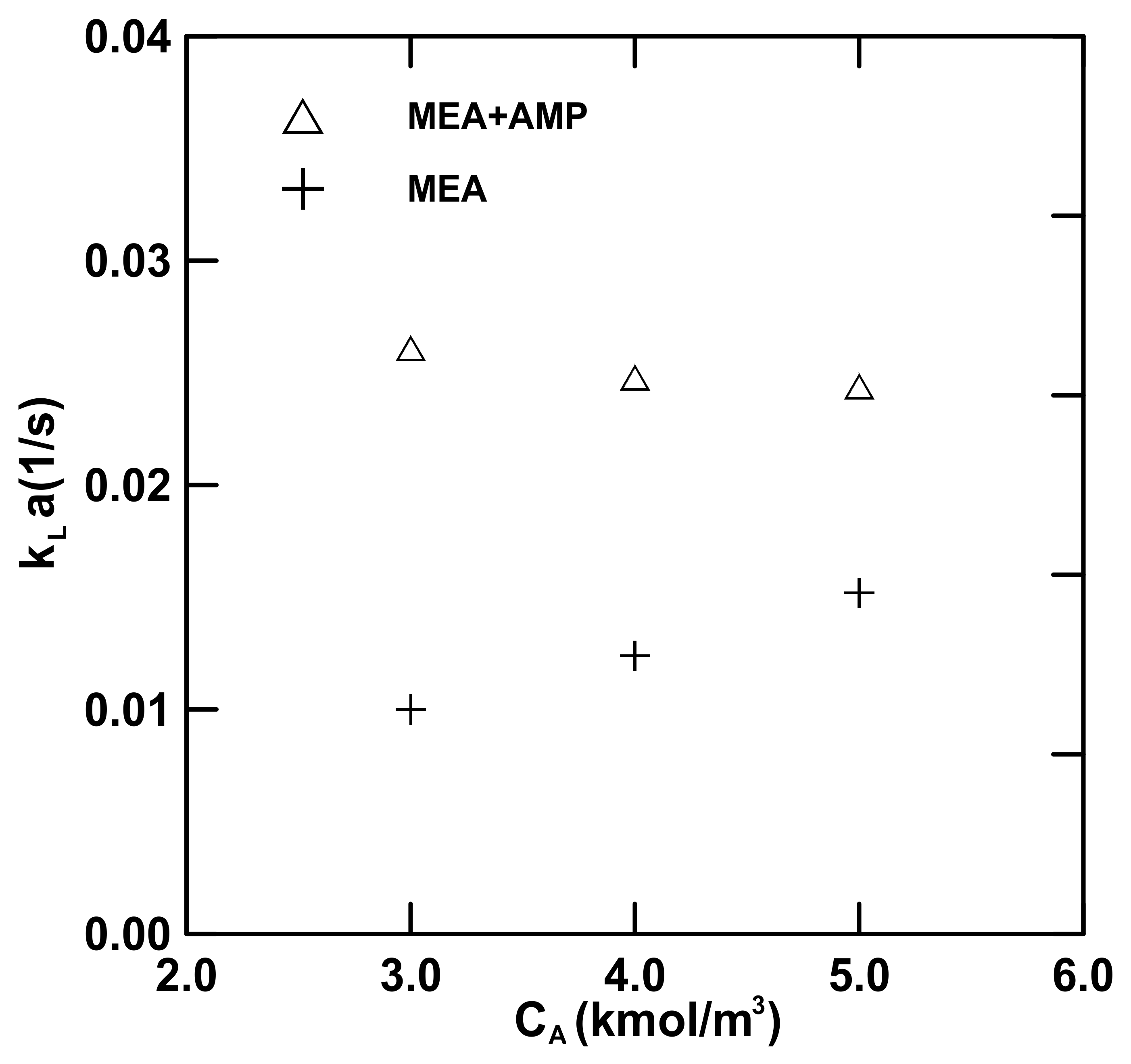
4.3.1. Mass Transfer Data
4.3.2. Effect of Parameter on the Overall Mass-Transfer Coefficient
4.3.3. NTU and HTU
4.4. Comparison with Base-Line
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| specific surface area of liquid (m2 m−3) | |
| effective specific surface area (m2 m−3) | |
| specific surface area of packings (m2 m−3) | |
| C | total concentration (kmolm−3) |
| heat capacity of mixed amine (kJ kg−1 K−1) | |
| CA | concentration of amine (kmol m−3) |
| diameter of packings (m) | |
| diameter of liquid (m) | |
| DG | diffusivity of gas (m2 s−1) |
| DL | diffusivity of liquid (m2 s−1) |
| F | F-factor defined in Equation (24) (pa0.5) |
| G | gas molar flow rate (kmols−1) |
| dynamic hold up below the loading point (-) | |
| dynamic hold up (-) | |
| Henry’s law constant (kpa·m3 kmol−1) | |
| liquid side mass-transfer coefficient (ms−1) | |
| gas side mass-transfer coefficient (ms−1) | |
| overall mass-transfer coefficient (ms−1) | |
| L | liquid molar flow rate (kmol s−1) |
| m | equilibrium ratio (mole fraction/mole fraction) |
| stripping rate (kgs−1) | |
| steam flow rate (kgs−1) | |
| P | total pressure (pa) |
| specific dry pressure drop (pa m−1) | |
| specific pressure drop (pa m−1) | |
| specific dry pressure drop at flooding (pa m−1) | |
| QL | volumetric flow rate of liquid (m3 s−1) |
| Reynolds number for gas (-) | |
| S | stripping factor (-) |
| Treb | temperature in the rebolier (K) |
| T’1 | temperature at the column top (K) |
| uG | gas linear flow rate (ms−1) |
| uL | liquid linear flow rate (ms−1) |
| uGe | effective gas velocity (ms−1) |
| uLe | effective liquid velocity (ms−1) |
| xin | mole fraction of liquid at inlet (-) |
| xout | mole fraction of liquid at outlet (-) |
| X | parameter in Equation (14)(-) |
| Greek symbols | |
| α0 | rich loading (mol-CO2 mol-amine−1) |
| α | lean loading (mol-CO2 mol-amine−1) |
| γ | contact angel between the liquid and solid(deg) |
| ε | void fraction (-) |
| viscosity of gas (mpa·s) | |
| viscosity of liquid (mpa·s) | |
| density of gas phase (kgm−3) | |
| density of liquid phase (kgm−3) | |
| surface tension (Nm−1) |
Abbreviations
| APM 2 | amino-2-methyl-1-propanol |
| HETP | height equivalent to a theoretical plate |
| HTU | height of transfer unit |
| MEA | monoethanolamine |
| NTU | number of transfer unit |
References
- Stichlmair, J.; Bravo, J.; Fair, J. General model for prediction of pressure drop and capacity of countercurrent gas/liquid packed columns. Gas Sep. Purif. 1989, 3, 19–28. [Google Scholar] [CrossRef]
- Richardson, J.K.; Zaki, W.N. Sedimentation and fluidization. Part1. Trans. Chem. Eng. 1954, 32, 35–53. [Google Scholar]
- Rocha, J.A.; Bravo, J.L.; Fair, J.R. Distillation columns containing structured packings: A comprehensive model for their performance. 1. Hydraulic models. Ind. Eng. Chem. Res. 1993, 32, 641–651. [Google Scholar] [CrossRef]
- Hoffmann, A.; Noeres, C.; Górak, A. Scale-up of reactive distillation columns with catalytic packings. Chem. Eng. Process. Process. Intensif. 2004, 43, 383–395. [Google Scholar] [CrossRef]
- Rocha, J.A.; Bravo, J.L.; Fair, J.R. Distillation Columns Containing Structured Packings: A Comprehensive Model for Their Performance. 2. Mass-Transfer Model. Ind. Eng. Chem. Res. 1996, 35, 1660–1667. [Google Scholar] [CrossRef]
- Gualito, J.J.; Cerino-Córdova, F.; Cardenas, J.C.; Rocha, J.A. Design Method for Distillation Columns Filled with Metallic, Ceramic, or Plastic Structured Packings. Ind. Eng. Chem. Res. 1997, 36, 1747–1757. [Google Scholar] [CrossRef]
- Ortiz-Del-Castillo, J.R.; Guerrero-Medina, G.; Lopez-Toledo, J.; Rocha, J.A. Design of Steam-Stripping Columns for Removal of Volatile Organic Compounds from Water Using Random and Structured Packings. Ind. Eng. Chem. Res. 2000, 39, 731–739. [Google Scholar] [CrossRef]
- Li, M.-H.; Lie, Y.-C. Densities and Viscosities of Solutions of Monoethanolamine + N-methyldiethanolamine + Water and Monoethanolamine + 2-Amino-2-methyl-1-propanol + Water. J. Chem. Eng. Data 1994, 39, 444–447. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Unit Operations, 2nd ed.; Allyn and Bacon Inc.: Boston, MA, USA, 1983; pp. 384–387. [Google Scholar]
- Xiao, J.; Li, C.-W.; Li, M.-H. Kinetics of absorption of carbon dioxide into aqueous solutions of 2-amino-2-methyl-1-propanol+monoethanolamine. Chem. Eng. Sci. 2000, 55, 161–175. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Li, M.-H. Viscosities of Aqueous Blended Amines. J. Chem. Eng. Data 1997, 42, 714–720. [Google Scholar] [CrossRef]
- Jayarathna, S.A.; Weerasooriya, A.; Dayarathna, S.; Eimer, D.A.; Melaaen, M.C. Densities and Surface Tensions of CO2 Loaded Aqueous Monoethanolamine Solutions with r = (0.2 to 0.7) at T = (303.15 to 333.15) K. J. Chem. Eng. Data 2013, 58, 986–992. [Google Scholar] [CrossRef]
- Vázquez, G.; Álvarez, E.; Navaza, J.M.; Rendo, R.; Romero, E. Surface Tension of Binary Mixtures of Water + Monoethanolamine and Water + 2-Amino-2-methyl-1-propanol and Tertiary Mixtures of These Amines with Water from 25 °C to 50 °C. J. Chem. Eng. Data 1997, 42, 57–59. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Otto, F.; Mather, A. Solubility of N2O in alkanolamines and in mixed solvents. Chem. Eng. J. 1992, 48, 31–40. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Watanasiri, S. Representing Vapor–Liquid Equilibrium for an Aqueous MEA–CO2System Using the Electrolyte Nonrandom-Two-Liquid Model. Ind. Eng. Chem. Res. 1999, 38, 2080–2090. [Google Scholar] [CrossRef]
- Chiu, L.-F.; Li, M.-H. Heat Capacity of Alkanolamine Aqueous Solutions. J. Chem. Eng. Data 1999, 44, 1396–1401. [Google Scholar] [CrossRef]
- Chen, P.C.; Lai, Y.-L. Optimization in the Stripping Process of CO2 Gas Using Mixed Amines. Energies 2019, 12, 2202. [Google Scholar] [CrossRef]
- Brunazzi, E.; Macías-Salinas, R.; Viva, A. Calculation Procedure for Flooding in Packed Columns Using a Channel Model. Chem. Eng. Commun. 2008, 196, 330–341. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Notz, R.; Hoch, S.; Asprion, N.; Sieder, G.; García, H.; Hasse, H. Pilot plant experimental studies of post combustion CO2 capture by reactive absorption with MEA and new solvents. Energy Procedia 2009, 1, 963–970. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operations of Chemical Engineering, 5th ed.; McGraw-Hill Inc.: New York, NY, USA, 1993; pp. 722–724. [Google Scholar]


| No | (°C) | CA (kmol/m3) | |
|---|---|---|---|
| 1 | 100 | 0.2 | 3 |
| 2 | 100 | 0.3 | 3 |
| 3 | 100 | 0.4 | 3 |
| 4 | 110 | 0.2 | 4 |
| 5 | 110 | 0.3 | 4 |
| 6 | 110 | 0.4 | 4 |
| 7 | 120 | 0.2 | 5 |
| 8 | 120 | 0.3 | 5 |
| 9 | 120 | 0.4 | 5 |
| 10 | 110 | 0.3 | 3 |
| 11 | 110 | 0.3 | 4 |
| 12 | 110 | 0.3 | 5 |
| 13 | 110 | 0.3 | 3 |
| 14 | 110 | 0.3 | 4 |
| 15 | 110 | 0.3 | 5 |
| Experimental Number | (g/min) | (pa/m) | (pa/m) | (1/s) | HTU (m) | |
|---|---|---|---|---|---|---|
| No.13–15 (MEA) | 8.4–11.6 | 119.8–147.7 | 799.9–1150 | 1441–1492 | 0.01–0.0152 | 0.1678–0.2535 |
| No.10–12 (MEA + AMP) | 7.82–11.7 | 66.1–107.6 | 257.1–528.9 | 1514–1562 | 0.0244–0.0261 | 0.0976–0.1045 |
| No.1–12 (MEA + AMP) | 3.77–15.3 | 34.8–107.6 | 83.4–528.9 | 1360–1725 | 0.0187–0.0272 | 0.0823–0.1329 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.C.; Yang, M.-W.; Lai, Y.-L. Hydrodynamic and Mass Transfer in the Desorption Process of CO2 Gas in a Packed-Bed Stripper. Processes 2021, 9, 46. https://doi.org/10.3390/pr9010046
Chen PC, Yang M-W, Lai Y-L. Hydrodynamic and Mass Transfer in the Desorption Process of CO2 Gas in a Packed-Bed Stripper. Processes. 2021; 9(1):46. https://doi.org/10.3390/pr9010046
Chicago/Turabian StyleChen, Pao Chi, Ming-Wei Yang, and Yan-Lin Lai. 2021. "Hydrodynamic and Mass Transfer in the Desorption Process of CO2 Gas in a Packed-Bed Stripper" Processes 9, no. 1: 46. https://doi.org/10.3390/pr9010046
APA StyleChen, P. C., Yang, M.-W., & Lai, Y.-L. (2021). Hydrodynamic and Mass Transfer in the Desorption Process of CO2 Gas in a Packed-Bed Stripper. Processes, 9(1), 46. https://doi.org/10.3390/pr9010046





