Abstract
Biobutanol can be produced by Clostridia via an acetone–butanol–ethanol (ABE) fermentation under strictly anaerobic conditions. Oxygen-free nitrogen (OFN) gas is typically used to create anaerobic conditions for ABE fermentations. However, this method is not appropriate for large-scale fermentations as it is quite costly. The aim of this work was to study the feasibility of butanol production from sweet sorghum juice (SSJ) by Clostridium beijerinckii TISTR 1461 using various methods to create anaerobic conditions, i.e., growth of a strictly aerobic bacterium, an Arthrobacter sp., under different conditions and a chemical method using sodium dithionite (SDTN) to consume residual oxygen. SSJ containing 60 g/L of total sugar supplemented with 1.27 g/L of (NH4)2SO4 was used as a substrate for butanol production. The results showed that 0.25 mM SDTN could create anaerobic conditions, but in this case, C. beijerinckii TISTR 1461 could produce butanol at a concentration (PB) of only 8.51 g/L with a butanol productivity (QB) of 0.10 g/L·h. Arthrobacter sp. BCC 72131 could also be used to create anaerobic conditions. Mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 created anaerobic conditions by inoculating the C. beijerinckii 4 h after Arthrobacter. This gave a PB of 10.39 g/L with a QB of 0.20 g/L·h. Comparing butanol production with the control treatment (using OFN gas to create anaerobic conditions, yielding a PB of 9.88 g/L and QB of 0.21 g/L·h) indicated that using Arthrobacter sp. BCC 72131 was an appropriate procedure for creating anaerobic conditions for high levels of butanol production by C. beijerinckii TISTR 1461 from a SSJ medium.
1. Introduction
Butanol or butyl alcohol (C4H9OH) is a linear 4-carbon aliphatic saturated alcohol. It is widely used as a chemical starting material for production of plastics, polymers, paints, lubricants, brake fluids and synthetic rubber [1]. Furthermore, it is a superior renewable fuel that is considered better as a transportation fuel than bioethanol in future fuel systems [2]. This is mainly due to its greater number of carbon atoms and consequently higher energy content, miscibility with gasoline, lower corrosivity and greater blending capacity [3]. Butanol has a high energy density of 29.2 MJ/L and can be substituted for gasoline (energy density 32 MJ/L) without alteration of the current internal combustion engines [4]. Presently, butanol is primarily obtained from crude oil [5]. Nevertheless, another alternative for butanol production is from a fermentation process called the acetone–butanol–ethanol (ABE) fermentation. Biphasic metabolism is observed in batch ABE fermentations. Clostridium spp. produces hydrogen, carbon dioxide, acetate, and butyrate in an acidogenic phase. The metabolism of the cells is then shifted to solvent production including the formation of acetone, butanol and ethanol in a solventogenic phase [6].
Biobutanol can be produced by Clostridia strains, Clostridium acetobutylicum, C. beijerinckii, C. sporogenes, C. carbodivorans, C. saccharoperbutylacetonicum and C. saccharoacetobutylicum, via ABE fermentation under strictly anaerobic conditions. Oxygen-free nitrogen (OFN) gas flushing is generally used to create anaerobic condition for ABE fermentations at a laboratory scale [7,8,9]. However, this is impractical in large-scale bioreactors due to the high operational costs and complexity of application. Hence, the use of a chemical that can strongly react with oxygen may be an alternative way to create anaerobic conditions for ABE fermentations. It was reported that 0.005–0.20 M sodium dithionite (SDTN) can reduce the oxygen level in a stirred-tank bioreactor. Reactions between oxygen and dithionite likely play an important role in consuming oxygen [10,11,12]. Consequently, use of SDTN is one choice to chemically create anaerobic conditions. Additionally, the use of an obligate aerobic microorganism to consume residual oxygen is an alternative method to create anaerobic conditions. According to literature reviews, Arthrobacter spp. are obligate aerobic bacteria that are Gram-positive, rod-shaped non-spore formers. They can utilize a wide range of sugars and consume oxygen under appropriate conditions [13,14,15]. Until now, there were no reports of using SDTN or an Arthrobacter sp. to create anaerobic conditions for ABE fermentations. It is, therefore, a challenge of this work to explore the utility of SDTN and an Arthrobacter sp. to produce anaerobic conditions prior to Clostridium sp. inoculation for butanol production.
The cost of substrate is one of the main problems for industrial production of butanol from ABE fermentation. The three major substrate classes for butanol production include starchy feedstocks (potato, corn, cassava, etc.), sugary feedstocks (sugarcane, sugar beets and molasses) and hydrolysates of lignocellulosic materials [16]. This research focuses on using sugary feedstocks because substrate pretreatment is not required. Sweet sorghum (Sorghum bicolor (L.) Moench) is a non-competitive substrate. It can be cultivated with lower water requirements with tolerance to salinity and drought. In one year, it can be harvested 3–4 times. Sweet sorghum stalks contain up to 78% juice. The juice contains between 14% and 23% of sugars, depending on factors such as the season of growth, soil composition, and the plant strain grown [17]. Sweet sorghum juice (SSJ) consists of fermentable sugars, such as sucrose, glucose and fructose. These sugars can be directly fermented to produce acetone, butanol and ethanol by Clostridium spp. [18] in an optimized a SSJ medium for butanol production. They found that sweet sorghum juice containing 60 g/L of total sugar supplemented with 7.14 g/L of CaCO3, 1.27 g/L of (NH4)2SO4 and 5.18 g/L of butyric acid, gave the highest butanol titer (PB), 16.91 g/L. CaCO3 was used as a buffer, whereas (NH4)2SO4 was used as a supplemental nitrogen source. Butyric acid was directly used as a substrate (precursor) for butanol production in an ABE fermentation. However, to reduce the costs of the SSJ medium for butanol production, only (NH4)2SO4 was added into the SSJ in the current study.
The aim of this research was to investigate new methods to create anaerobic conditions for high levels of biobutanol production by C. beijerinckii TISTR 1461. First, the feasibility of butanol production from a simplified SSJ medium [SSJ supplemented with (NH4)2SO4] was studied. Then, it was used for butanol production by C. beijerinckii TISTR 1461 using two different methods to create anaerobic conditions. The first method was addition of SDTN to remove oxygen through chemical reactions, while the second was growth of a strictly aerobic bacterium, Arthrobacter sp. BCC 72131, in the medium to consume oxygen prior inoculation with C. beijerinckii TISTR 1461 and a subsequent ABE fermentation.
2. Materials and Methods
2.1. Raw Materials
Sweet sorghum juice or SSJ (cv. KKU 40) was extracted from its stalks using a sugarcane juice extractor (Faculty of Agriculture, Khon Kaen University, Thailand). Then, it was concentrated by heating at 80–85 °C to reduce its volume followed by storage at −20 °C to protect it from bacterial growth before use [19]. The composition of raw SSJ is shown in Table 1.

Table 1.
Composition of sweet sorghum juice KKU 40.
2.2. Butanol Production Medium
Concentrated SSJ was thawed at room temperature and diluted with distilled water to obtain a fermentation broth with 60 g/L of total sugar. The broth was supplemented with 1.27 g/L of (NH4)2SO4 (BDH, Poole, UK) and autoclaved at 110 °C for 28 min before use as a butanol production medium called SSJ medium (modified from [18]). A typical synthetic butanol production medium, P2 medium, containing 60 g/L of glucose (BDH, Leuvn, Belgium) was also used as a control medium for butanol production. P2 medium contained 1 g/L of yeast extract (Oxoid, Basingstoke, Hants, UK), as well as 10 mL/L each of stock solutions A, B and C. Stock solution A is composed of K2HPO4 (BDH, Leuvn, Belgium), 50 g/L; KH2PO4 (BDH, Leuvn, Belgium), 50 g/L and ammonium acetate (BDH, Leuvn, Belgium), 220 g/L. Stock solution B consists of para-amino-benzoic acid (Fluka, Steinheim, Sweden), 0.1 g/L; thiamin (Labchem, New South Wales, Australia), 0.1 g/L and biotin (Sigma-Aldrich, MO, USA), 1 mg/L. Stock solution C is composed of MgSO4·7H2O (BDH, Poole, UK), 20 g/L; MnSO4·H2O (BDH, Poole, UK), 1 g/L; FeSO4·7H2O (Riedel-de Haën, Seelze, Germany), 1 g/L and NaCl (Ajax, Auckland, New Zealand), 1 g/L [20,21]. Stock solutions A and C were sterilized in an autoclave at 110 °C for 28 min, whereas stock solution B was sterilized by membrane filtration using a 0.2 µm cellulose acetate membrane [22,23]. Afterwards, each stock solution (1%, v/v) was aseptically added into the sterile P2 medium.
2.3. Microorganisms and Inoculum Preparation
C. beijerinckii TISTR 1461 (from the Thailand Institute of Scientific and Technology Research, Thailand) was maintained as a spore suspension and stored at 4 °C in sterile distilled water. One mL of the spore suspension (~1 × 106 spores/mL) was activated by heat shocking it in a water bath at 80 °C for 1 min, then quickly cooling it in an iced water bath for 1 min. Thereafter, the spore suspension was immediately transferred into a sterile cooked meat medium (CMM) (Oxoid, Basingstoke, Hants, UK). It was then incubated at 37 °C for 16–19 h under static conditions. CMM is comprised of 1 g of CMM powder and 0.08 g of glucose in 10 mL distilled water. The CMM was autoclaved at 110 °C for 28 min and sparged with OFN gas to create strictly anaerobic conditions before use. Vegetative cells at a level of 5% (v/v) from the CMM medium were inoculated into a sterile tryptone-glucose-yeast extract (TGY) medium and incubated at 37 °C for 4–6 h to obtain actively growing cells in the log phase with an OD of 0.5 (optical density at 600 nm) or 0.97 g/L of dry cell weight before use as an inoculum for the ABE fermentation [23,24]. TGY medium is comprised of 5 g tryptone (Oxoid, Basingstoke, Hants, UK), 1 g glucose (BDH, Leuvn, Belgium), 5 g yeast extract (Oxoid, Basingstoke, Hants, UK) and K2HPO4 (BDH, Leuvn, Belgium) in 1 L distilled water. TGY medium was autoclaved and purged with OFN gas in the same manner as the CMM medium before use.
Arthrobacter sp. BCC 72131 was purchased from the Thailand Bioresource Research Center (TBRC), Khlong Luang, Pathum thani, Thailand. A loopful of Arthrobacter sp. BCC 72131 was inoculated into a sterile nutrient broth (NB) medium and incubated in a shaking incubator at 200 rpm, 30 °C for 6–7 h. Then, they were transferred into fresh NB medium under the same conditions to obtain active cells with approximately 0.8 g/L of dry cell weight. To avoid effects of the culture medium on butanol production, the active cells (35 mL) were centrifuged at 12,000 rpm for 15 min. Then, the cell pellet was washed with sterile normal saline and resuspended in the SSJ medium before use as an inoculum for creation of anaerobic conditions. The NB medium consists of peptone, 10 g/L (Himedia, Nashik, India); beef extract, 10 g/L (Bacto, Le Pont de Claix, France) and NaCl, 5 g/L (modified from [15]).
2.4. Experimental Procedures
2.4.1. Use of SSJ Medium for Butanol Production
SSJ medium supplemented with 1.27 g/L of (NH4)2SO4 (modified from [18]) was used in this study. P2 and SSJ media with no (NH4)2SO4 addition were used as positive and negative control media. Sterile fermentation media in 1-L screw-capped bottles with working volumes of 700 mL were purged with OFN gas to create anaerobic conditions [22]. Then, 5% (v/v) of actively growing cells of C. beijerinckii TISTR 1461 in TGY (Section 2.3) was transferred into the media. The fermentation was operated at 37 °C with an agitation rate of 150 rpm until the end of the fermentation. Samples were withdrawn at regular time internals for analyses.
2.4.2. Use of SDTN to Create Anaerobic Conditions for Butanol Production
SDTN (BKKchemi, Thailand) powder was added into sterile butanol production media in 1-L screw-capped bottles to produce concentrations of 0.125 mM–1.0 mM. It was then allowed to react for 4 h to consume residual oxygen (modified from [25]). Afterwards, 5% of active C. beijerinckii TISTR 1461 cells was inoculated into the media to start butanol production with incubation at 37 °C and agitation at 150 rpm until the end of fermentation.
2.4.3. Use of Arthrobacter sp. BCC 72131 to Create Anaerobic Conditions for Butanol Production
For creation of anaerobic conditions using an obligate aerobic bacterium, Arthrobacter sp. BCC 72131, two conditions were tested. In Condition I, 5% (v/v) of Arthrobacter culture (resuspended in the SSJ medium from Section 2.3) was inoculated in the butanol production medium in 1-L screw-capped bottles to create anaerobic conditions. Then, 5% of C. beijerinckii TISTR 1461 was immediately inoculated to start butanol production at 37 °C. Under Condition II, the same amount of Arthrobacter sp. BCC 72131 was inoculated into the butanol production medium, followed by incubation at 30 °C, 150 rpm for 4 h (modified from [26]). After that, actively growing cells of C. beijerinckii TISTR 1461 were transferred into the butanol production medium. The fermentation was performed at 37 °C with 150 rpm agitation until the end of the fermentation.
2.5. Analytical Methods
Samples were centrifuged and the supernatant was filtered by passing it through a 0.45 µm cellulose acetate membrane (Whatman, Maidstone, England) before analyses. Fermentation products including acetone, butanol, ethanol, acetic acid and butyric acid were analyzed with gas chromatography (Shimadzu, GC-2014, Japan) using a Porapak Q column (80/100 mesh, 3 mm × 2 mm, Resteck, PA, USA). Nitrogen was used as a carrier gas at a pressure of 150 kPa. The oven temperature was programmed as follows: (1) maintain at 160 °C for 9 min, (2) increase from 160 to 220 °C at 8 °C/min, and (3) maintain at 220 °C for 12 min. The temperatures of injector and flame ionization detector (FID) were 220 °C and 230 °C, respectively. An internal standard of 8 g/L of 2-butanol was used to accurately measure the fermentation products (modified from [27]). The pH values were measured with a pH meter. Total sugar concentration was determined using a phenol-sulphuric acid method [28]. Under suitable anaerobic conditions, a quantitative analysis of fructose, glucose and sucrose in samples was done by employing a high performance liquid chromatography (HPLC) with a refractive index (RI) detector (Waters, MA, USA) using an Inertsil® NH2 column (5 µm, 250 mm × 4.6 mm, GL Sciences, Tokyo, Japan). The analyses were done at 35 °C at a flow rate of 0.8 mL/min using an isocratic system with a mixture of 75 parts acetonitrile: 25 parts water (v/v) as a mobile phase (modified from [29]). Butanol yield (YB/S), ABE yield (YABE/S), volumetric butanol productivity (QB) and sugar consumption (SC) were calculated as follows:
All experiments were carried out in triplicate, and the results are expressed as mean values ± SD.
3. Results and Discussion
3.1. Butanol Production from SSJ Medium
The SSJ medium for butanol production reported by Sirisantimethakom et al. (2018) was simplified by adding only 1.27 g/L of (NH4)2SO4 into the SSJ containing 60 g/L of total sugar. This simplified SSJ medium was evaluated to ascertain that it could be efficiently used for butanol production by C. beijerinckii TISTR 1461. Butanol production from this SSJ medium, a synthetic medium (P2 medium as positive control) and SSJ with no (NH4)2SO4 addition (negative control) were compared. The batch ABE profiles from these three media are shown in Figure 1. The results showed that the profiles in the SSJ medium with (NH4)2SO4 and P2 medium were similar (Figure 1A,B). The pH values decreased sharply in the first 12 h of fermentation due to high production of acetic and butyric acids. The results suggested that phosphotransacetylase and acetate kinase were active in the acetate pathway, and phosphotransbutylase and butyrate kinase operated well in the butyrate pathway [4]. This phase is called acidogenesis. After 12 h of fermentation, the acids were converted into solvents and pH values increased due to this transformation. In the case of the SSJ medium with no (NH4)2SO4, the pH of the medium decreased sharply in 24 h and then slightly decreased until the end of the fermentation (Figure 1C). This might have been due to a lack of buffering agents in the SSJ medium as well as low solvent production. Additionally, most acids could not be transformed to solvents under this condition. However, microscopic observations found that C. beijerinckii TISTR 1461 could grow in the negative control medium to a lesser extent than in the SSJ medium with (NH4)2SO4 and in the P2 medium. Additionally, the cells in the SSJ medium with no supplementation were shorter and thinner than in other two media (data not shown). This is consistent with significantly lower sugar consumption (10.22 g/L corresponding to 28.25%) in the SSJ medium with no (NH4)2SO4 (Table 2).
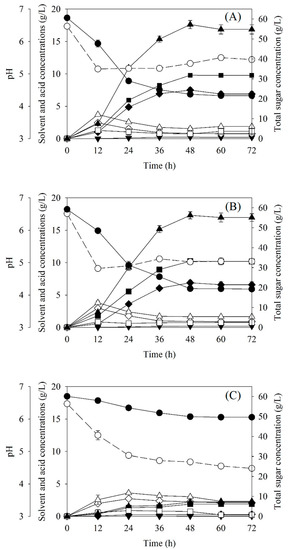
Figure 1.
Batch ABE fermentation profiles on sweet sorghum juice (SSJ) supplemented with 1.27 g/L (NH4)2SO4 (A), P2 medium (B) and SSJ with no nutrient supplementation (C): acetone (♦), butanol (■), ethanol (▼), ABE (▲), acetic acid (◊), butyric acid (□), total acids (∆), pH (○) and total sugar (●).

Table 2.
Fermentation results of batch ABE fermentations by C. beijerinckii TISTR 1461 in various media at an initial sugar concentration of 60 g/L using OFN gas flushing to create anaerobic conditions.
In all media, acetone, butanol and ethanol were clearly observed after 12 h of fermentation, indicating that acetoacetate decarboxylase, butanol dehydrogenase and alcohol dehydrogenase were, respectively, active. This phase is called solventogenesis. At the end of fermentation, the highest titer of butanol (PB) in the SSJ supplemented with (NH4)2SO4 was 9.88 g/L, which was similar to that in P2 medium (10.23 g/L, Table 2). However, it was only 1.90 g/L in SSJ with no (NH4)2SO4. This result indicated that addition of supplemental nitrogen in the SSJ medium promotes butanol production, increasing it by approximately 2–5 folds compared to a medium with no nitrogen supplementation (Table 2). Butanol yield (YB/S, 0.30 g/g) was slightly higher than that in the P2 medium (0.26 g/L), whereas the volumetric butanol productivity (QB, 0.21 g/L·h) and ABE concentrations (PABE, 17.30 and 17.61 g/L) in both media were not different. The obtained results indicate that the SSJ supplemented with only 1.27 g/L of (NH4)2SO4 is a suitable medium for butanol production by C. beijerinckii TISTR 1461. Therefore, the SSJ medium supplemented with (NH4)2SO4 was used as a simplified butanol production medium in subsequent experiments.
3.2. Batch Butanol Production from SSJ Medium by C. beijerinckii TISTR 1461 under Anaerobic Conditions Created Using SDTN
Butanol production by C. beijerinckii TISTR 1461 under anaerobic conditions created using 0.125–1.00 mM SDTN was investigated. The results showed that butanol was not produced when using SDTN concentrations higher than 0.50 mM (Table 3). This might have been due to SDTN toxicity on the bacterial cells [30]. SDTN at 0.125–0.50 mM showed positive effects on butanol production. The PB, PABE, YB/S, QB and YABE/S values under these conditions were higher than those of the negative control (experiment with no adjustments to create anaerobic conditions). The highest PB of 8.51 g/L and PABE of 14.07 g/L were obtained using 0.25 mM SDTN. Under this condition, sugar utilization was 56%. These results indicated that SDTN could create anaerobic conditions in the fermentation broth that were suitable for ABE fermentation. SDTN at 0.125 mM yielded lower PB and PABE values, indicating that SDTN at this condition might not be sufficient to create anaerobic conditions. At 0.50 mM SDTN, low values for PB of 4.26 g/L and PABE of 5.80 g/L were obtained, implying that the SDTN concentration was excessive and toxicity to bacterial cells may have resulted. Unfortunately, the impacts of SDTN on the ABE fermentation under acidic conditions are unclear.

Table 3.
Fermentation results of batch ABE fermentations from the SSJ medium by C. beijerinckii TISTR 1461 using SDTN to create anaerobic conditions.
The PB value (8.51 g/L) using 0.25 mM SDTN was slightly lower than that of the positive control employing OFN gas flushing (9.88 g/L). However, the QB (0.10 g/L·h) was significantly lower than that of the control (0.21 g/L·h) due to longer fermentation time (84 h). In a laboratory scale, the price of SDTN for anaerobic creation condition (<0.01 USD/L of the fermentation broth) was much lower than using OFN gas (≈0.40 USD/L of the fermentation broth). However, these results suggested that SDTN might not be suitable to create anaerobic conditions for ABE fermentation on a large scale. Hence, a novel technique for creating anaerobic conditions using Arthrobacter sp. BCC 72131, an obligate aerobic bacterium, was used to test its utility in the ABE fermentation in subsequent experiments.
3.3. Batch Butanol Production from SSJ Medium by C. beijerinckii TISTR 1461 under Anaerobic Conditions Created Using Arthrobacter sp. BCC 72131
Two strategies of using Arthrobacter sp. BCC 72131 to create anaerobic conditions for butanol production by C. beijerinckii TISTR 1461 were tested. One was simultaneous inoculation of both bacterial strains. The other was inoculation of C. beijerinckii TISTR 1461 4 h after Arthrobacter sp. BCC 72131. The profiles of ABE fermentation for the two strategies are shown in Figure 2A–D. The PB values at the end of fermentation using the mixed cultures under both strategies were similar, 10.72 g/L for simultaneous inoculation and 10.39 g/L for inoculation of Clostridium after 4 h Arthrobacter (Table 4). However, the QB of the simultaneous inoculation was approximately 11% lower. The YB/S value using the mixed cultures was not significantly different, suggesting that the metabolic pathway for butanol production under both strategies was identical. The changes of sugar consumption (SC), total acids (Pacid), PB and PABE under both strategies were similar (Figure 2), indicating that C. beijerinckii TISTR 1461 cells were energetic enough to produce butanol. In these studies, 32–34 g/L of total sugars were consumed, corresponding to 52–56% substrate utilization (Table 4). Moreover, it was found that the use of mixed cultures slightly promoted butanol production compared to the positive control (using a pure culture of C. beijerinckii TISTR 1461 with OFN gas flushing). The PB of the former (10.39–10.72 g/L) was slightly higher than the latter (9.88 g/L). All obtained results show that the creation of anaerobic conditions through cultivation of Arthrobacter sp. BCC 72131 for 4 h was more suitable for butanol production by C. beijerinckii TISTR 1461 and could be used in place of OFN gas flushing. However, the oxygen concentration in the fermentation medium after Arthrobacter inoculation should be monitored to obtain the optimum time for anaerobic condition creation by the obligate aerobic bacterium.
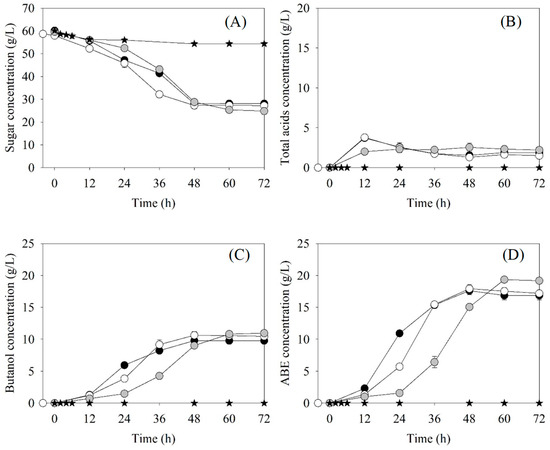
Figure 2.
Batch ABE fermentation profiles using mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 under several strategies. The first is simultaneous inoculation of the two cultures (●), while the others include inoculation of Clostridium 4 h after Arthrobacter (○), ABE fermentation profiles using a pure culture of C. beijerinckii TISTR 1461 employing OFN gas flushing (●) and a pure culture of Arthrobacter sp. BCC 72131 with no gas flushing (★): (A) sugars, (B) total acids, (C) butanol and (D) ABE concentrations.

Table 4.
Fermentation results of batch ABE fermentation from the SSJ medium by mixed cultures, a pure culture of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 using different methods of creating anaerobic conditions.
Butanol production by a pure culture of C. beijerinckii TISTR 1461 with air flushing (negative control) revealed no detectable PB and PABE but the sugar consumption was 3.96 g/L, corresponding to 6.63% substrate utilization (Figure 2 and Table 4). These results suggest that the bacterium consumed sugars for survival but did not produce butanol. They also imply that C. beijerinckii TISTR 1461 is an obligate anaerobe that can generate butanol only under strictly anaerobic conditions. When using a pure culture of Arthrobacter sp. BCC 72131, sugar consumption was 5.77 g/L corresponding to 9.60% substrate utilization (Figure 2 and Table 4). This indicated that the Arthrobacter sp. had little effect on sugar consumption for the ABE fermentation by C. beijerinckii TISTR 1461.
4. Conclusions
This is the first study to report the successful use of Arthrobacter sp. to create anaerobic conditions for butanol production. Sweet sorghum juice supplemented with 1.27 g/L of (NH4)2SO4 can be used as an efficient and simplified substrate for butanol production. SDTN is not suitable for use to create anaerobic conditions for butanol production due to the resulting lower butanol titer and productivity. Use of Arthrobacter sp. BCC 72131 is an efficient method to create anaerobic conditions for butanol production. Mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 by inoculation of Clostridium 4 h after Arthrobacter is an appropriate procedure for high levels of butanol production from a sweet sorghum juice medium.
Author Contributions
Conceptualization, P.L. and L.L.; methodology and formal analysis, C.D., A.K. and L.S.; investigation and writing—original draft preparation, C.D.; writing—review and editing, P.L. and L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Council of Thailand (NRTC), Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This research was supported by the Fermentation Research Center for Value Added Agricultural Products (FerVAAP), Khon Kaen University; Faculty of Technology, Khon Kaen University; and the National Research Council of Thailand (NRTC), Thailand. We would like to thank the members of the Bioalcohol Fermentation Research Laboratory, Faculty of Technology, Khon Kaen University, Thailand for their suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dürre, P. Biobutanol: An attractive biofuel. Biotechnol. J. 2007, 2, 1525–1534. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Luo, Z.; Cui, Y.; Xu, Y.; Lin, L.; Zhao, M.; Guo, Y.; Pang, Z. Dual function of ammonium acetate in acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Bioresour. Technol. 2018, 267, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Cheng, C. Butanol production by Clostridium. In Advances in Bioenergy, 1st ed.; Elsevier Inc.: Oxford, UK, 2019; Volume 4, pp. 35–77. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Tang, I.C.; Zhao, J.; Bai, F.; Yang, S.T. Butanol production in acetone-butanol-ethanol fermentation with in situ product recovery by adsorption. Bioresour. Technol. 2016, 219, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Al-Shorgani, N.K.N.; Ali, E.; Kalil, M.S.; Yusoff, W.M.W. Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. Bioenergy Res. 2012, 5, 287–293. [Google Scholar] [CrossRef]
- Ponthein, W.; Cheirsilp, B. Development of Acetone Butanol Ethanol (ABE) Production from palm pressed fiber by mixed culture of Clostridium sp. and Bacillus sp. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 9, pp. 459–467. [Google Scholar] [CrossRef]
- Oliva-Rodríguez, A.G.; Quintero, J.; Medina-Morales, M.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; Aroca, G.; Rios González, L.J. Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates. Bioresour. Technol. 2019, 275, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Tri, C.L.; Kamei, I. Butanol production from cellulosic material by anaerobic co-culture of white-rot fungus Phlebia and bacterium Clostridium in consolidated bioprocessing. Bioresour. Technol. 2020, 305, 123065. [Google Scholar] [CrossRef] [PubMed]
- Kilroy, W.P. Anaerobic decomposition of sodium dithionite in alkaline solution. J. Inorg. Nucl. Chem. 1980, 42, 1071–1073. [Google Scholar] [CrossRef]
- Camacho, F.; Páez, M.P.; Jiménez, M.C.; Fernández, M. Application of the sodium dithionite oxidation to measure oxygen transfer parameters. Chem. Eng. Sci. 1997, 52, 1387–1391. [Google Scholar] [CrossRef]
- Holman, D.A.; Bennett, D.W. A multicomponent kinetics study of the anaerobic decomposition of aqueous sodium dithionite. J. Phys. Chem. 1994, 98, 13300–13307. [Google Scholar] [CrossRef]
- Luscombe, B.M.; Gray, T.R.G. Characteristics of Arthrobacter grown in continuous culture. J. Gen. Microbiol. 1974, 82, 213–222. [Google Scholar] [CrossRef]
- Eschbach, M.; Möbitz, H.; Rompf, A.; Jahn, D. Members of the genus Arthrobacter grow anaerobically using nitrate ammonification and fermentative processes: Anaerobic adaptation of aerobic bacteria abundant in soil. FEMS Microbiol. Lett. 2003, 223, 227–230. [Google Scholar] [CrossRef]
- Silva, C.R.; Souza, J.C.; Araújo, L.S.; Kagohara, E.; Garcia, T.P.; Pelizzari, V.H.; Andrade, L.H. Exploiting the enzymatic machinery of Arthrobacter atrocyaneus for oxidative kinetic resolution of secondary alcohols. J. Mol. Catal. B Enzym. 2012, 83, 23–28. [Google Scholar] [CrossRef]
- Cascon, H.R.; Choudhari, S.K.; Nisola, G.M.; Vivas, E.L.; Lee, D.J.; Chung, W.J. Partitioning of butanol and other fermentation broth components in phosphonium and ammonium-based ionic liquids and their toxicity to solventogenic Clostridia. Sep. Purif. Technol. 2011, 78, 164–174. [Google Scholar] [CrossRef]
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet sorghum as biofuel feedstock: Recent advances and available resources. Biotechnol. Biofuels 2017, 10, 1–19. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Enhancement of butanol production efficiency from sweet sorghum stem juice by Clostridium beijerinckii using statistical experimental design. Chiang. Mai. J. Sci. 2018, 45, 1235–1246. [Google Scholar]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Butanol recovery from model solution/fermentation broth by pervaporation: Evaluation of membrane performance. Biomass Bioenergy 1999, 17, 175–184. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Laopaiboon, L.; Sanchanda, P.; Chatleudmongkol, J.; Laopaiboon, P. Improvement of butanol production from sweet sorghum juice by Clostridium beijerinckii using an orthogonal array design. Ind. Crop. Prod. 2016, 79, 287–294. [Google Scholar] [CrossRef]
- Narueworanon, P.; Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Impacts of initial sugar, nitrogen and calcium carbonate on butanol fermentation from sugarcane molasses by Clostridium beijerinckii. Energies 2020, 13, 694. [Google Scholar] [CrossRef]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Enhancement of batch butanol production from sugarcane molasses using nitrogen supplementation integrated with gas stripping for product recovery. Ind. Crop. Prod. 2017, 95, 216–226. [Google Scholar] [CrossRef]
- Camacho Rubio, F.; Paez Dueñas, M.P.; Blazquez Garcia, G.; Garrido Martin, J.M. Oxygen absorption in alkaline sodium dithionite solutions. Chem. Eng. Sci. 1992, 47, 4309–4314. [Google Scholar] [CrossRef]
- Qi, G.; Xiong, L.; Luo, M.; Huang, Q.; Huang, C.; Li, H.; Chen, X.; Chen, X. Solvents production from cassava by co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae. J. Environ. Chem. Eng. 2018, 6, 128–133. [Google Scholar] [CrossRef]
- Areesirisuk, A.; Laopaiboon, L.; Khongsay, N.; Laopaiboon, P. Improvement of gas chromatographic analysis for organic acids and solvents in acetone-butanolethanol fermentation from sweet sorghum juice. Afr. J. Biotechnol. 2010, 9, 6422–6429. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Polsokchuak, N.; Butkun, K.; Laopaiboon, L. Enhancement of ethanol production efficiency in repeated-batch fermentation from sweet sorghum stem juice: Effect of initial sugar, nitrogen and aeration. Electron. J. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Yand, C.Y.; He, L. Neuroprotective effects of sinapine on PC12 cells apoptosis induced by sodium dithionite. Chin. J. Nat. Med. 2008, 6, 205–209. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).