Abstract
Non-alcoholic fatty liver disease (NAFLD) is a disease associated with metabolic syndromes such as diabetes and obesity, regardless of alcohol consumption, and refers to the accumulation of triacylglycerols in the liver. Thymol (THY) is a vegetable essential oil that is naturally contained in the Zingiberaceae and Lamiaceae families. THY was isolated from Curcuma longa L. The rhizomes of Curcuma longa L. were dried, sliced and extracted with 50% ethanol and then isolated through repeated column chromatography. This study was conducted to investigate the inhibitory effect of THY, even in non-alcoholic fatty liver disease, in relation to the inhibiting hyperlipidemia effect of THY, which was demonstrated in previous studies. Hepatocytes were treated with oleate (OA) containing THY to observe lipid accumulation by Oil Red O staining (ORO). We also tested the effect of THY on triacylglycerols (TG) and total cholesterol (TC) in HepG2 cells. Western blot and real-time RT-PCR using sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS), acetyl-CoA carboxylase (ACC), CCAAT-enhancer-binding protein (C/EBP), proliferator-activated receptor γ (PPARγ), and adenosine monophosphate (AMP)-activated protein kinase (AMPK) expressions were carried out. Consequently, inhibition of lipogenesis by THY (100 μM or 200 μM) in NAFLD treated with OA in HepG2 cells was confirmed. The results of TG and TC experiments confirmed a decrease in the degree of fat accumulation in the liver. Furthermore, inhibition of the SREBP-1c, FAS, ACC, C/EBP and PPARγ expressions that mediated fat accumulation and increased AMPK phosphorylation was observed. Taken together, THY is proposed as a potential natural constituent for the treatment of NAFLD.
1. Introduction
Fatty liver disease is caused by the buildup of excess fat in the liver. In general, fatty liver disease is defined as the accumulation of triacylglycerols in more than 5% of hepatocytes [1]. Fatty liver disease can be divided into two categories: alcoholic and non-alcoholic. Alcoholic fatty liver disease occurs under the condition of heavy alcohol drinking, as the name reveals. In contrast, fatty liver disease associated with diabetes, obesity, and hyperlipidemia is generalized under the term non-alcoholic fatty liver disease (NAFLD), regardless of alcohol consumption [2]. NAFLD appears in relation to metabolic syndrome [3]. NAFLD tends to impact not only simple fatty liver disease, but also chronic hepatitis and cirrhosis [4]. Recently, the incidence of NAFLD has been reported to be higher than alcoholic fatty liver disease, and its occurrence has been related to westernized eating habits [5].
Curcuma longa L. (C. longa) belongs to the Gingeraceae family. It is a perennial plant that mainly grows in Southeast Asia. Its roots are ginger-shaped and yellow in color [6]. It is well known as a raw material for curry and has traditionally been used as a spice [7]. The main active component that makes up C. longa is curcumin, and other phytochemicals are curcumenol, curcumol, zingibere, curcumeene, and p-cymene [8,9]. According to reports, curcumin has anti-hyperlipidemic, anti-inflammatory, and liver-protective effects [10]. Thymol (THY), a type of monoterpene, has been reported to have various biological activities, including anti-inflammatory, anti-hyperlipidemic [11], and antioxidant activities [12]. Furthermore, it has been widely used as a preservative ingredient in cosmetics.
In this study, we hypothesized that THY isolated from C. longa would be effective in NAFLD treatment based on its effect. To verify this hypothesis, HepG2 cells were treated with OA, and then Oil Red O staining, western blot, and real-time RT-PCR experiments were performed to determine the degree of lipolysis caused by varying concentrations of THY. As a result, it was confirmed that THY is effective at reducing NAFLD. Our study proposes the possible use of THY as a natural remedy that could replace other NAFLD drugs. Additionally, an HPLC method was established for quantification of the effective compound of the C. longa extracts.
2. Results
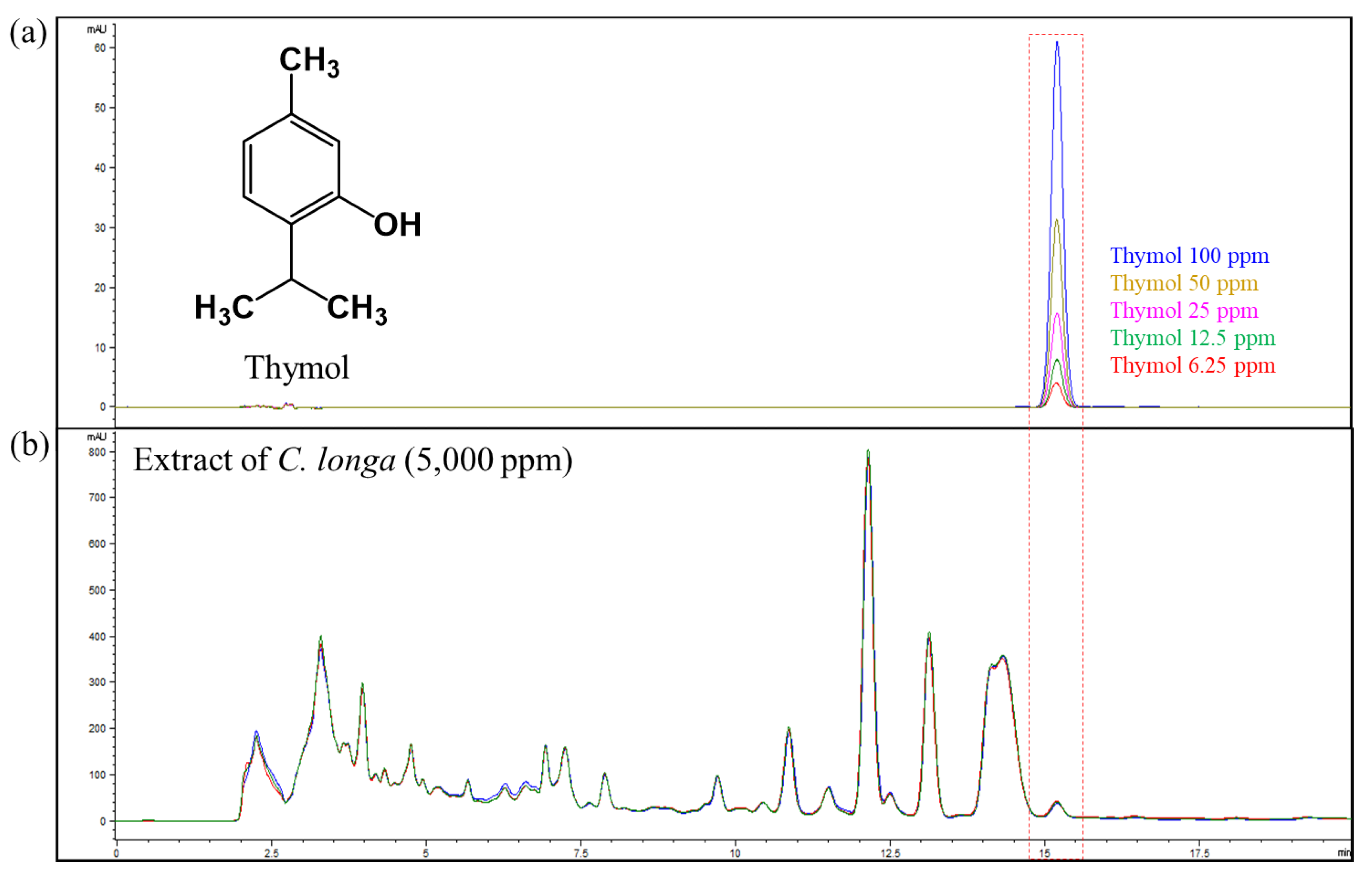
2.1. Identification and Quantification of Thymol in Curcuma Longa
For investigating bioactive compounds in C. longa, extraction of the root was conducted with 50% fermented EtOH (CE), and fractionation was conducted with EtOAc and water, extracted by repeated chromatographies with the silica gel (SiO2) and the octadecyl silica gel (ODS) column. On the basis of extensive spectroscopic data (Figure S1) and the spectroscopic data reported by Khan et al., the structure of this compound was determined [13]. The contents of the THY of the C. longa extract were analyzed by HPLC-UV detection. In order to obtain the chromatograms with a high resolution and separate adjacent peaks rapidly, the conditions of the chromatography were optimized (Figure 1). The calibration curve was generated from the peak areas in the chromatogram of HPLC-UV detection. The linearity of the calibration curve covering the tested range described above was excellent, with 0.9999 of the correlation coefficients for THY (Figure S2). It was determined that the contents of THY in the 50% fermented EtOH extract were 10.67 mg/g, respectively (NIHHS-18-42458).
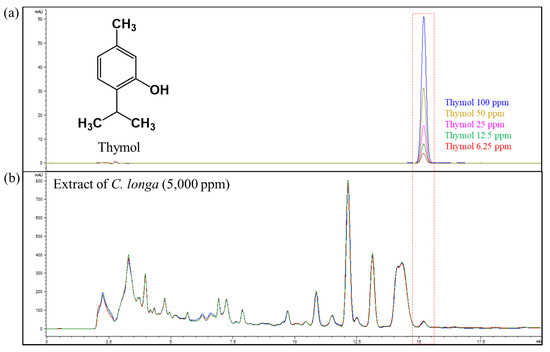
Figure 1.
(a) Chemical structure and quantitation range of thymol. (b) HPLC chromatogram of C. longa extract at 280 nm.
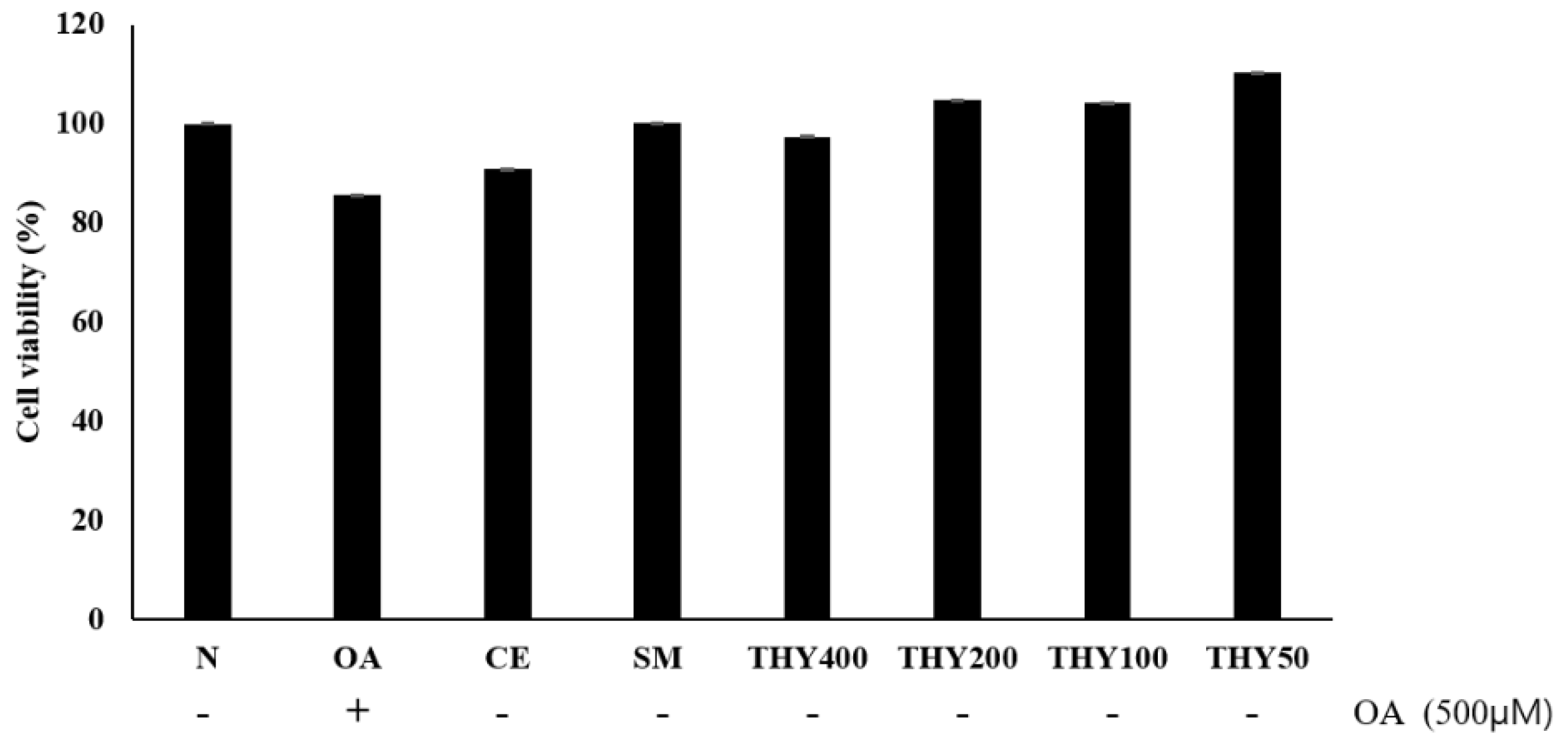
2.2. HepG2 Cells’ Viability Based on Thymol (THY)
The MTS assay was performed to carry out cytotoxicity tests in the presence of a varying concentration of oleate (OA), CE, silymarin (SM), and THY. As shown in the Figure 2, toxicity could not be confirmed in the presence of CE (200 μg), SM (20 μg), or THY (50, 100, 200, and 400 μM). In particular, an increase in cellular activity was observed when the cells were treated with drugs after OA (500 μM) treatment.
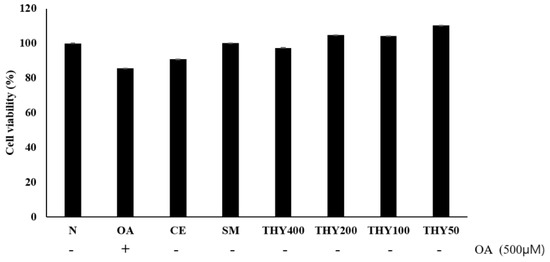
Figure 2.
Effects of silymarin (SM) and thymol (THY) concentrations on cell viability in HepG2 cells. Cell viability was measured in HepG2 cells after treatment with EtOH (CE) (200 μg), SM (20 μg), and THY (50, 100, 200, and 400 μM). Cell viability was evaluated using the MTS assay. N: normal; OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
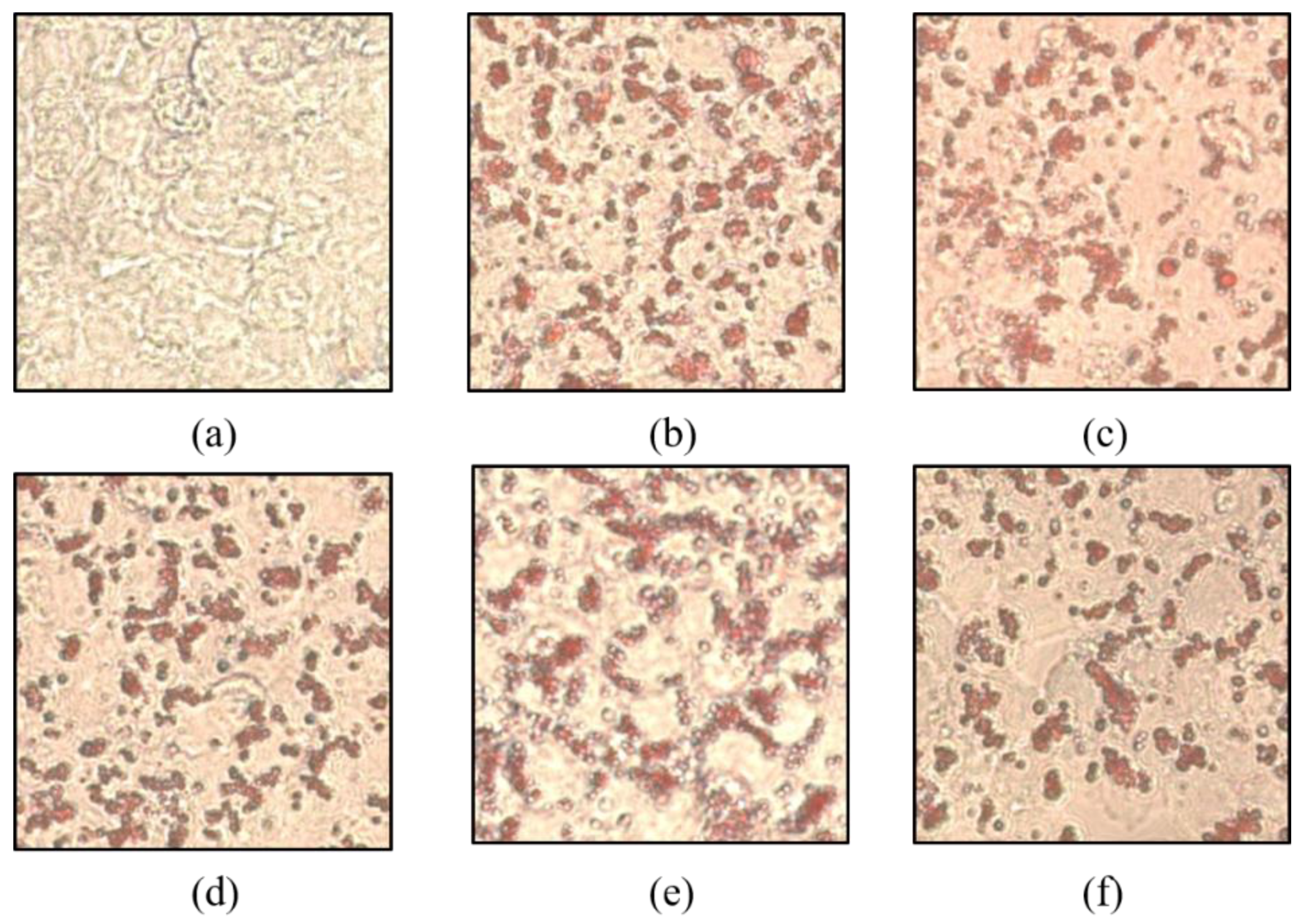
2.3. Effects on Intracellular Lipid Accumulation in HepG2 Cells
Before investigating the inhibitory effect of fat accumulation by THY, the degree of fat pinching was confirmed through Oil Red O staining in CE and tested with THY (CE data not shown). Fat accumulation was induced normally by intracellular OA (500 μM) treatment alone. As shown in Figure 3, suppression of fat accumulation was observed in the THY treatment group and, in particular, THY showed a significant fat suppression effect at 200 μM.
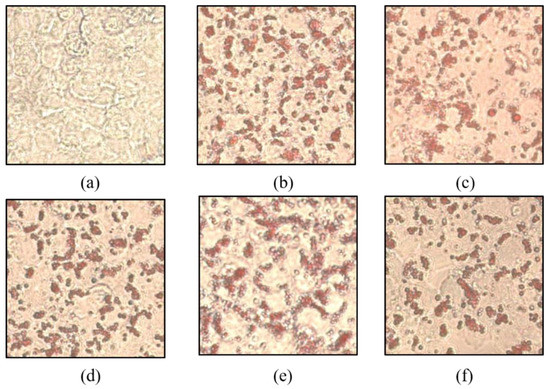
Figure 3.
Effects of SM and THY on intracellular lipid accumulation in HepG2 cells. (a) Normal, (b) OA (500 μM), (c) CE (200 μg), (d) SM (20 μg), (e) THY 100 (100 μM), and (f) THY 200 (200 μM) were treated with OA (500 μM) for 24 h. Cells were stained with Oil Red O and analyzed by a spectraphotometer. OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
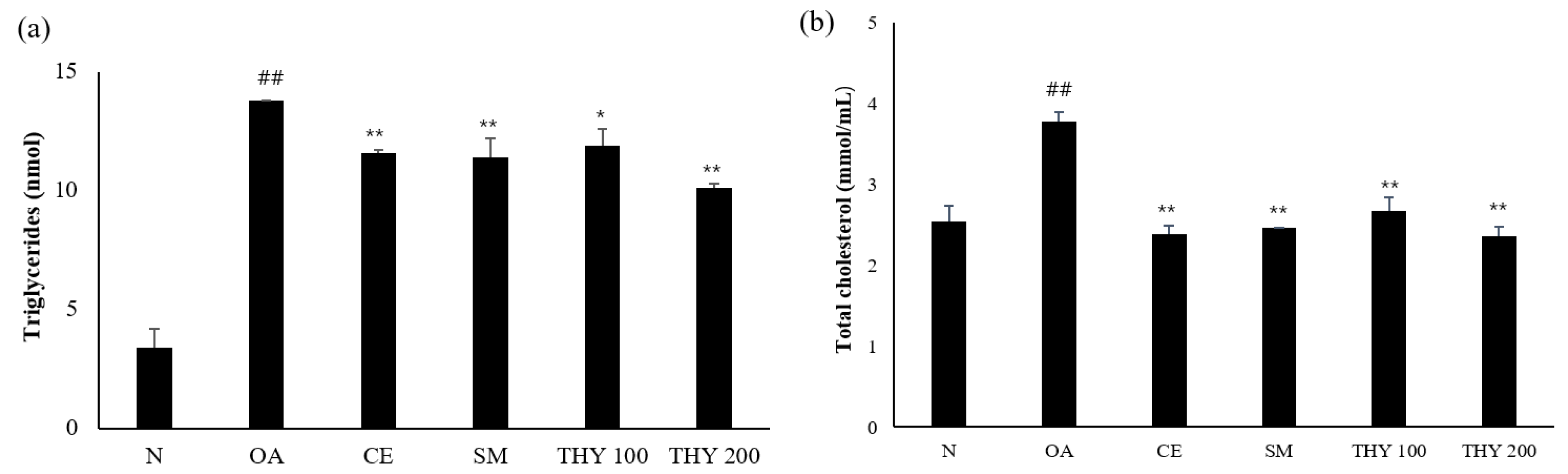
2.4. Effects on TG and TC Levels in HepG2 Cells
To investigate the biochemical changes induced by THY in OA-treated HepG2 cells, the triacylglycerol (TG) and total cholesterol (TC) kit was used. A normal increase in TG and TC levels was observed in the normal and OA-induced groups. Both the TG and TC levels were lower than that of the OA (500 μM) in the presence of THY 100 (100 μM) and THY 200 (200 μM). The THY (200 μM) level was lower than the SM (20 μg) level (Figure 4).
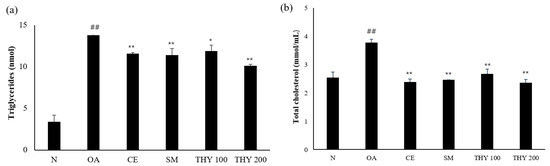
Figure 4.
Effects on TG and TC levels in HepG2 cells. (a) Cellular production of TG and (b) cellular production of TC were induced by 500 μM of OA, and cells were treated with indicated concentrations of CE (200 μg), SM (20 μg), THY 100 (100 μM), and THY 200 (200 μM) for 24 h. Total intracellular triglyceride and total cholesterol were analyzed using the enzymatic colorimetric method. ## p < 0.01, relative to normal; * p < 0.05, ** p < 0.01, relative to OA. N: normal; OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
2.5. Effects on Hepatic Lipid Accumulation and Protein Expression
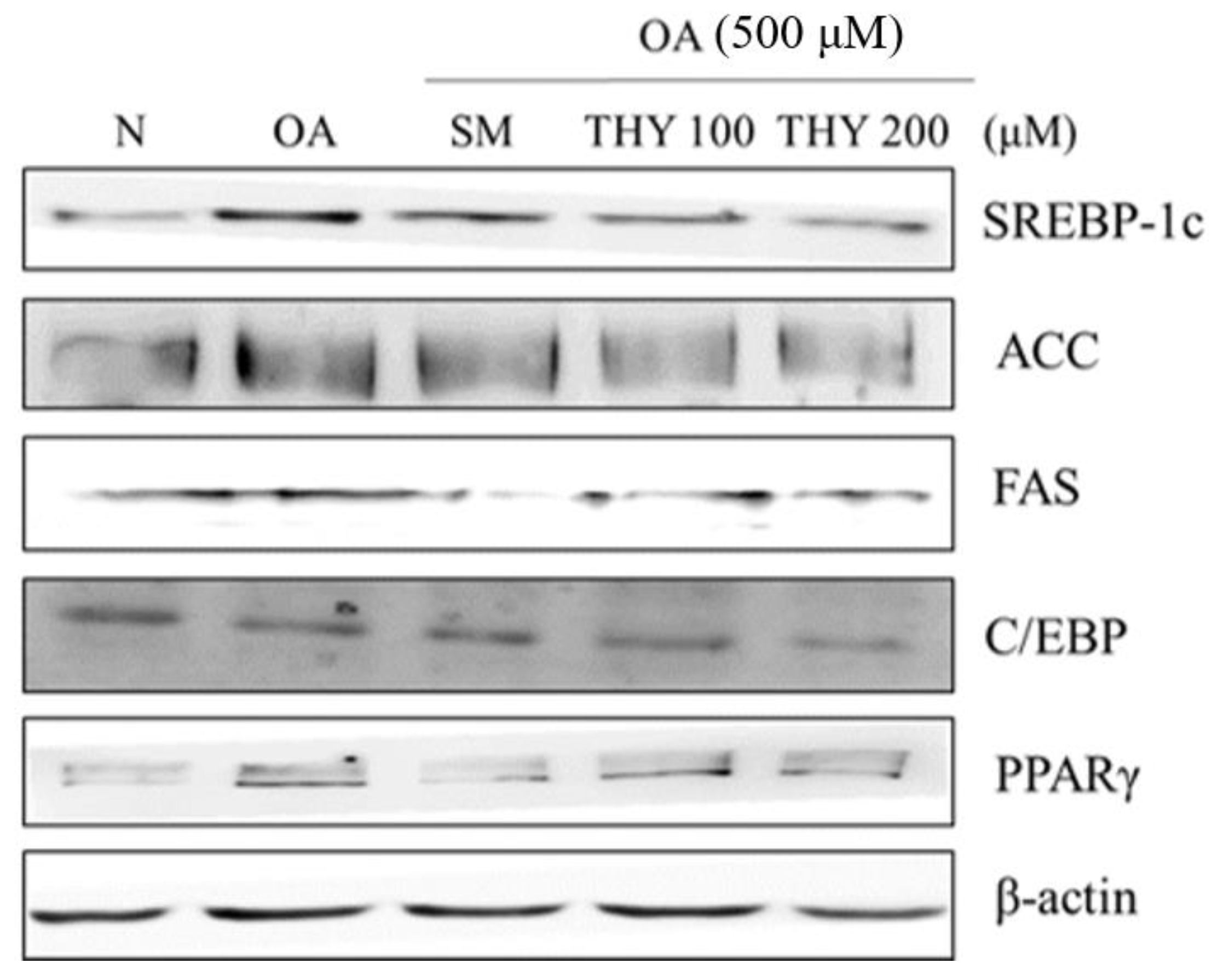
Western blot analysis was performed to determine the expression of adipogenic transcription factors and enzymes in order to demonstrate the effect of THY on the protein level and lipid accumulation. Lipid accumulation induced by OA was treated with SM (20 μg), THY 100 (100 μM), and THY 200 (200 μM). Increased protein expression was observed with OA (500 μM) treatment, and inhibited with SM (20 μg), THY 100 (100 μM), and THY 200 (200 μM) treatment in sterol regulatory element-binding protein-1c (SREBP-1c), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), CCAAT-enhancer-binding protein (C/EBP), and proliferator-activated receptor γ (PPARγ) (Figure 5).
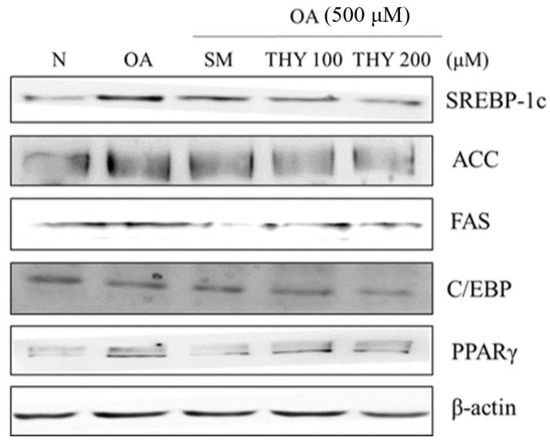
Figure 5.
Effects of SM and THY on hepatic lipogenesis-related protein (sterol regulatory element-binding protein-1c (SREBP-1c), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), CCAAT-enhancer-binding protein (C/EBP), and proliferator-activated receptor γ (PPARγ)) expression levels in OA-treated HepG2 cells. Data are representative of three independent experiments. N: normal; OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
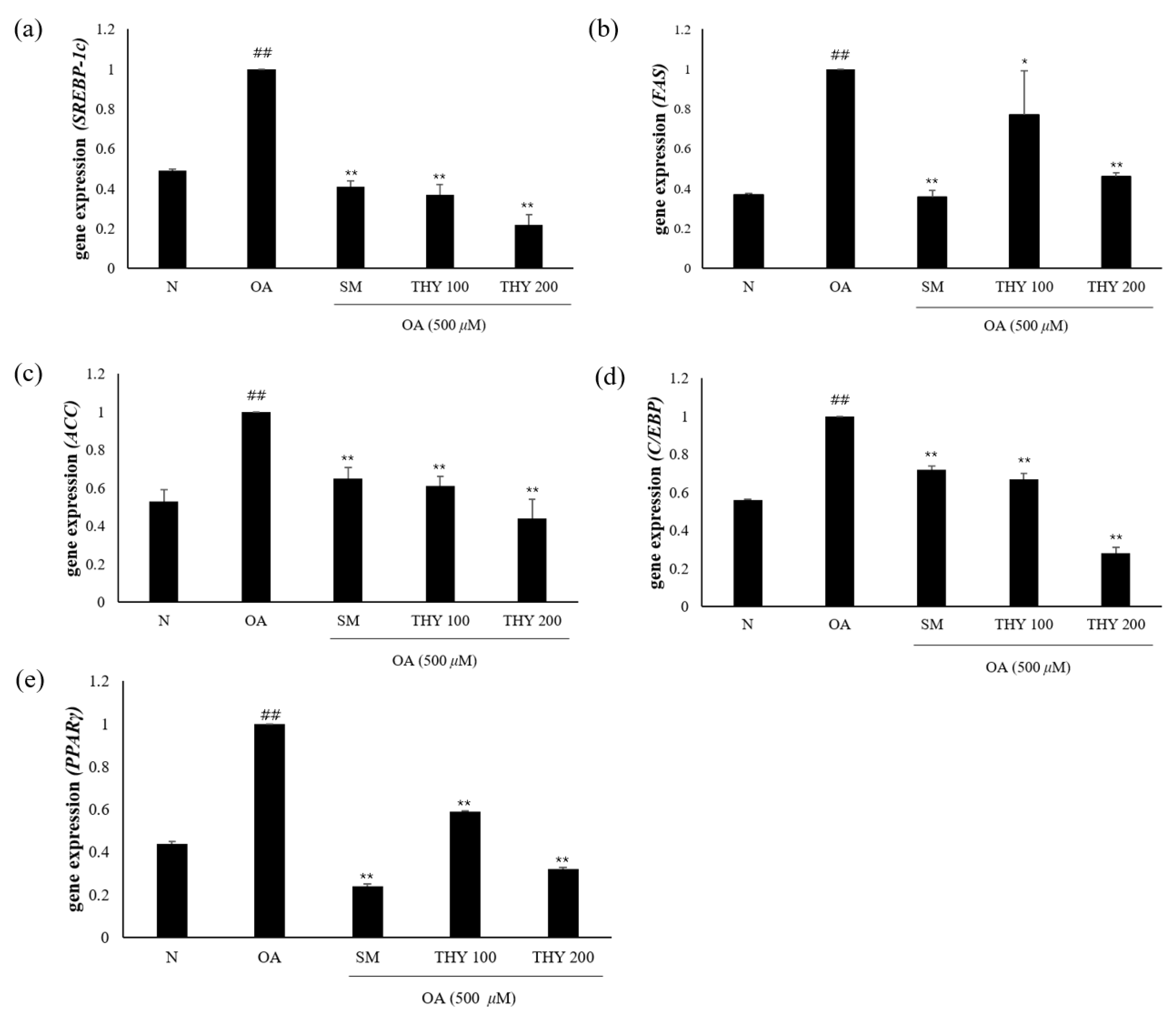
2.6. Effects on Hepatic Lipid Accumulation and mRNA Expression
We determined the underlying mechanisms of SM-, THY 100-, and THY 200-induced expression of genes that are important for lipid metabolism. Genes involved in lipid production (SREBP-1c, FAS, C/EBP, and PPARγ) were significantly increased in OA-treated HepG2 cells. As shown in Figure 6, SM (20 μg), THY 100 (100 μM), and THY 200 (200 μM) reduced the expression of genes involved in lipid production (SREBP-1c, ACC, FAS, C/EBP, and PPARγ). These results suggest that SM (20 μg), THY 100 (100 μM), and THY 200 (200 μM) reduce hepatic lipid accumulation.
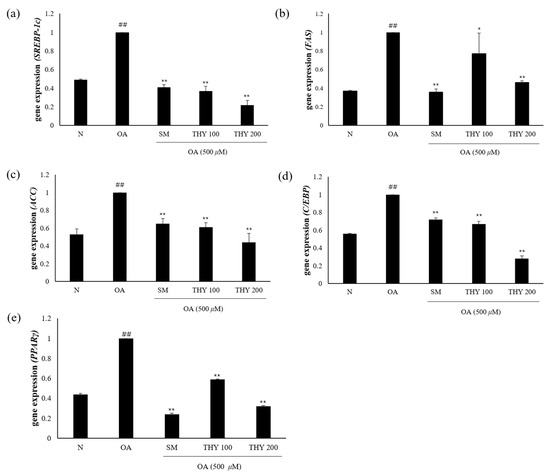
Figure 6.
Effects on hepatic lipogenesis. (a) Gene mRNA expression level of SREBP-1c, (b) gene mRNA expression level of ACC, (c) gene mRNA expression level of FAS, (d) gene mRNA expression level of C/EBP, and (e) gene mRNA expression level of PPARγ. Data are representative of three independent experiments. Expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. ## p < 0.01 relative to normal; * p < 0.05, ** p < 0.01, relative to the OA. N: normal; OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
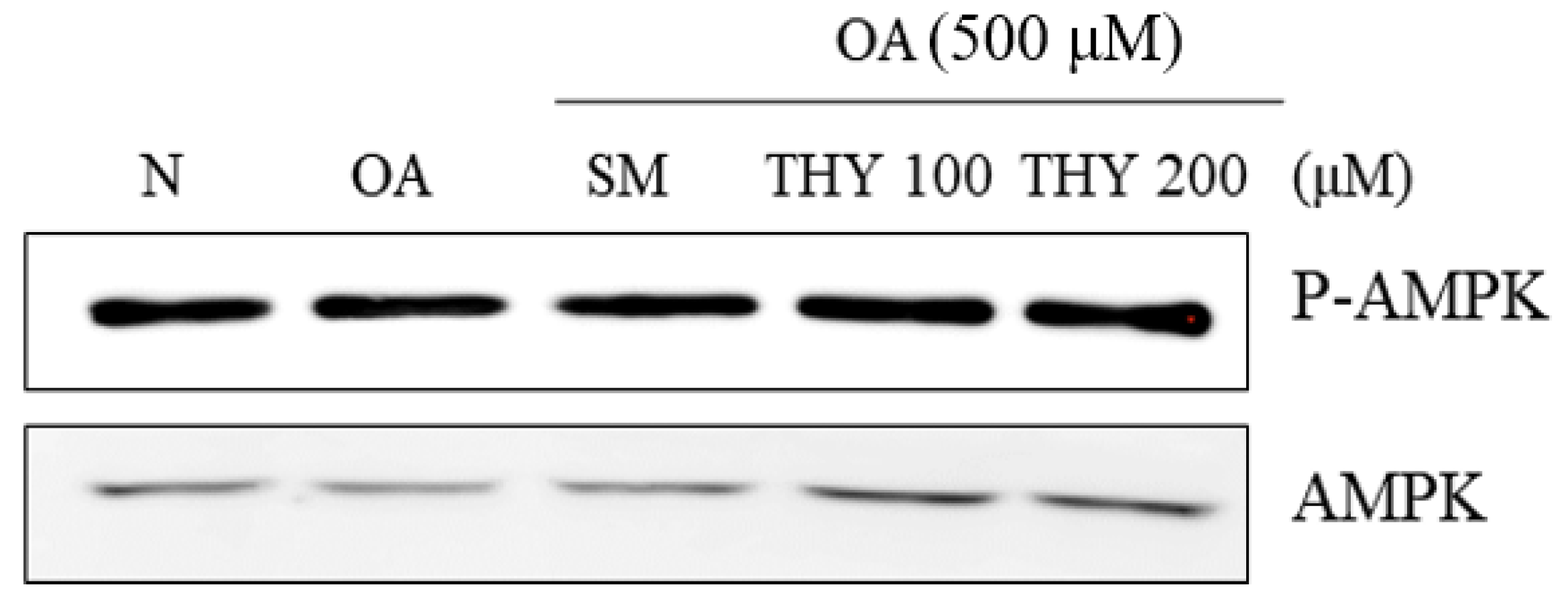
2.7. Effects on AMPK Expression in HepG2 Cells
AMP-activated protein kinase (AMPK) is a major regulator of fat production and fatty acid oxidation in metabolic tissues. Changes in AMPK expression in HepG2 cells are strongly linked to intracellular lipid metabolism. Therefore, HepG2 cells were treated with OA (500 μM), followed by treatment with SM (20 μg) and THY (200 μM) for 24 h for investigating the effect of THY on the phosphorylation of AMPK. As can be seen in Figure 7, THY stimulated AMPK threonine 172 phosphorylation.

Figure 7.
Effects on AMP-activated protein kinase (AMPK) phosphorylation in HepG2 Cells. Protein expression of AMPK was detected by western blot analysis. N: normal; OA: oleate; CE: C. longa 50% EtOH extract; SM: silymarin; THY: thymol.
3. Discussion
Usually, most of the C. longa studies have focused on the components of curcumin derivatives. The aim of this study is to investigate the effects of fatty liver inhibition on NAFLD in relation to the anti-hyperlipidemic efficacy of THY, which is a minor component in C. longa. HepG2 cells derived from human liver cancer cells were used as an NAFLD model by inducing lipid accumulation with OA. When compared with the normal group, an increase in lipid accumulation was observed in the treated group. Various concentrations of THY were tested for cytotoxicity, and the results were negative. In order to directly identify the decrease in the fat content, the Oil Red O experiment was conducted, and the fat significantly decreased in the presence of THY 200. In addition, triglyceride levels and total cholesterol were inhibited. Western blot and real-time RT-PCR were conducted to verify changes in the accumulation of lipid-related factors in HepG2 cells induced by OA (Table 1). SREBP-1c is a necessary component for controlling cholesterol metabolism by protein decomposition and [14] increases gene expression associated with fat acid biosynthesis and TG maturation, such as FAS and ACC [15,16]. FAS is an enzyme that catalyzes fatty acid synthesis and converts nutrients from the liver to fat for nutrient over urban energy storage [17]. ACC is synthesized by malonyl-CoA to bind acetyl coenzymes and produce fatty acids. SREBP-1c, FAS, and ACC levels were significantly decreased by THY treatment. These results show that THY is related to the reduced extent of fat formation in OA-induced fat production. PPARγ is a transcription factor mainly expressed in adipose tissue and is an important factor in adipose tissue differentiation, and it is also involved in glucose constancy and placenta development [18]. Activation of PPARγ led to an increase in adipose tissues, and THY reduced the activation of PPARγ. C/EBP is a gene associated with cell proliferation and adipose tissue differentiation. Inhibition of C/EBP was observed in the THY treatment group. AMPK inhibits the expression of factors such as SREBP-1c, and cholesterol and fatty acid synthesis, through the oxidation of glucose and fatty acids [19,20]. The fact that THY also increased AMPK phosphoric acidification in our results can be seen as THY inhibiting lipid accumulation through increased AMPK phosphorylation. In summary, the underlying mechanism of THY-induced inhibition of non-alcoholic fatty liver disease involves AMPK activation, and activated AMPK further inhibits the synthesis of SREBP-1c and ACC. Inhibited SREBP-1c restrains FAS, and inhibited proteins further inhibit non-alcoholic fatty liver disease by reducing fatty acid, cholesterol, and triglyceride synthesis. In addition, it can be used for the quantitative and qualitative determination of THY, which is useful in improving the quality control of ethanolic extract from C. longa and its pharmaceutical preparations.

Table 1.
Real-time RT-PCR primer sequences.
4. Materials and Methods
4.1. Extraction and Isolation Procedure
The dried and sliced C. longa (1 kg) were extracted twice with 80% aqueous MeOH (5 L) at room temperature. The extracts were successively partitioned with water (1 L), ethyl acetate (EtOAc) (1 L × 3), and n-butanol (0.8 L × 3). Then, the EtOAc fraction (CLE) (30 g) was applied to SiO2 (60Å, 70–230 mesh ASTM, Merck, Darmstadt, Germany) column (10 × 60 cm) chromatography, eluted with CHCl3-MeOH (by volume, 100:1→50:1→30:1→10:1, each 3 L), and monitored via thin-layer chromatography (TLC) to provide 10 fractions (CLE-1–CLE-10). Fraction CLE-2 (elution volume/total volume [Ve/Vt], 0.21–0.28; 900 mg) was applied to a SiO2 column (3 × 20 cm) and eluted with CHCl3-MeOH (50:1 by volume, 2 L) to provide five subfractions (CLE-2-1–CLE-2-5). Subfraction CLE-2-2 (190 mg) was subjected to octadecyl silica gel (ODS) (Merck) column (2 × 7 cm) chromatography and eluted with MeOH-H2O (10:1 vol/vol, 1 L) to yield THY (55 mg; TLC (RP-18, F254] ratio of fronts (Rf) = 0.40; MeOH-H2O 1:1 vol/vol).
4.2. High-Performance Liquid Chromatography Analysis
The dried and sliced C. longa roots were homogenized using a ball mill (Retsch MM400, Haan, Germany). Fine powder, which was passed through a 40-mesh sieve, was weighed (1000 mg), extracted in 5 mL of 50% fermented EtOH, and ultrasonically extracted for 1 h at 35 °C. The extract was then evaporated under reduced pressure. The extract solution was filtered through a syringe filter (0.22 µm) and injected directly into the HPLC system. HPLC was carried out on a 1200-RRLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a binary solvent delivery system and an autosampler. Chromatographic separations were carried out a YMC Pack ODS-AM column (4.6 × 250 mm, 5 µm). The column oven was maintained at 35 °C, and the mobile phases were composed of solvent A (water containing 0.1% (vol/vol) formic acid) and solvent B (acetonitrile containing 0.1% (vol/vol) formic acid). Elution conditions of liquid chromatography, which were optimized, were as follows: 0–3 min, 70% B; 3–16 min, 50% B; and 16–20 min, 30% B. The flow rate was 1 mL/min. A 10 µl aliquot was injected into the column using the autosampler.
4.3. Materials and Reagents
Silymarin (SM; S0292, >97.0%) was obtained from Sigma-Aldrich (Merck, St. Louis, MO, USA). Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), TrypLE Express, and Dulbecco’s Phosphate-Buffered Saline (DPBS) were purchased from Gibco (Thermo Fisher Scientific, Inc., Rockville, MD, USA). The lysis buffer was purchased from Intron Bio Technology (Seongnam, Korea). Penicillin/streptomycin was purchased from Hyclone (GE Healthcare Life Sciences, Logan, UT, USA). Dimethyl sulfoxide (DMSO), Oil Red O (#O0625), bovine serum albumin (BSA; #A7030), and oleate (OA, #O7501) were purchased from Sigma-Aldrich (St. Louis, MO, USA). CellTiter 96® AQueous One Solution Reagent (MTS solution) was purchased from Promega (Madison, WI, USA). Fatty acid synthase (FAS, sc-7273), sterol regulatory element-binding protein-1c (SREBP-1c, sc-365513), PPARγ (sc-398394), and β-actin (sc-47778) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Acetyl-CoA carboxylase (ACC, #3676S), phospho-AMP-activated protein kinase (P-AMPK, #2535), and AMP-activated protein kinase (AMPK, #2532) antibodies were purchased from Cell Signaling (Danvers, MA, USA). The Goat Anti-Mouse IgG (H + L) Secondary Antibody, HRP (#31430), Goat Anti-Rabbit IgG (H + L) Secondary Antibody, and HRP (#31460) were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Carlsbad, CA, USA). Human-glyceraldehyde-3-phosphate dehydrogenase (GAPDH), SREBP-1, FAS, ACC, and PPARγ primers were purchased from Bioneer Corp (Daejeon, Korea).
4.4. Cell Culture
The human hepatocellular carcinoma cell line for HepG2 cells was purchased from the American Tissue Culture Collection (ATCC, catalog no. HB-8065; Manassas, VA, USA) and were cultured in DMEM and 10% FBS. The HepG2 cells were incubated at 37 °C under 95% humidified atmosphere and 5% CO2.
4.5. Cell Viability
Cell viability was achieved through the MTS assay. HepG2 cells were seeded at a density of 5 × 105 cells/mL in 24-well cell culture plates (Nunc, A/S, Roskilde, Denmark). A day after incubation, a plate was tested in two parts. Cells were treated with various concentrations of CE (200 μg), SM (20 μg), THY (50, 100, 200, and 400 μM), and OA (500 μM). The plates were incubated for 18 h at 37 °C under 5% CO2. The existing medium was replaced with a new medium supplemented with an MTS solution (5 mg/mL). After 20 min, a microplate reader (BioTek South Korea, Seoul, Korea) was used to automatically read the absorbance of each well at 490 nm.
4.6. Oil Red O Staining
For examination of fat accumulation in HepG2 cells, the cells were treated with CE (200 μg), SM (20 μg), and THY (100, 200 μM), along with OA (500 μM) for 24 h in a 24-well plate at a density of 4 × 105 cells/mL. The HepG2 cells were washed with cold DPBS and fixed in 10% paraformaldehyde for 1 h. Again, the cells were washed with DPBS and finally with 60% isopropanol. The cells were stained for 1 h with freshly prepared diluted Oil Red O solution. After removal of the Oil Red O stain with 60% isopropanol, the images of each group were photographed.
4.7. Measurement of Lipid Levels
HepG2 cells were cultured in a 24-well plate at a density of 4 × 105 cells/mL for a day. Then, the plate was treated with CE (200 μg), SM (20 μg), and THY (100, 200 μM) with OA (500 μM). TC and TG levels were measured according to the kit protocol (BioVision, Mountain View, CA, USA). At the end of the experiment, the absorbance of each well was recorded at 450 nm using a microplate reader.
4.8. Western Blot Analysis
The HepG2 cells were harvested in a six-well plate at a density of 5 × 105 cells/mL. Subsequently, the cells were treated with SM (20 μg) and THY (100, 200 μM). One hour later, OA (500 μM) was added for 24 h in a 37 °C incubator with a 95% humidified atmosphere and 5% CO2. After 24 h of incubation, the cells were washed twice with DPBS. Next, the HepG2 cells were lysed with a lysis buffer for 20 min. For protein extraction, centrifugation was performed at 16,000× g at 4 °C for 5 min. Protein samples were quantified according to the quantification protocol, and the quantified samples were SDS gel electrophoresed. The running gel was transferred to polyvinylidene difluoride (PVDF; Millipore, Bedford, MA, USA) membranes at 15 V for 45 min. The membranes were blocked with 3% BSA for 1 h and left overnight at 4 °C with primary antibodies against SREBP-1c (1:1000), FAS (1:1000), ACC (1:1000), C/EBP (1:1000), PPARγ (1:1000), pAMPK(1:1000), and AMPK(1:1000). Subsequently, the membrane was washed several times in TBST and treated with secondary antibodies (1:5000) at room temperature. Washed membranes were detected using Atto ECL plus (Tokyo, Japan). An Image Quant LAS 4000 Mini Bio molecular Imager (GE Healthcare, UK) was used for analyzing the bands.
4.9. Real-Time RT-PCR
Total RNA was isolated using an E.Z.N.A.® Total RNA Kit (Omega Bio-tek, Inc.; Norcross, GA, USA) following the kit protocol. Reverse transcription was performed using a QuantiTect® Reverse Transcription Kit (Qiagen, Hilden, Germany) and quantitative real-time RT-PCR was performed using a Power SYBR® Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc. Foster City, CA, USA) according to protocol.
4.10. Statistical Analysis
All data were collected through statistical analysis, followed by Duncan’s test for various comparisons. The results are presented as a mean ± standard deviation of three independent experiments. All calculations were performed using SPSS Statistics 23 software (SPSS Inc., Chicago, IL, USA). Comparison with * p < 0.05 or ** p < 0.01 was considered statistically significant.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/9/1191/s1, Figure S1: 1H (a) and 13C (b) NMR data of thymol isolated from Curcuma longa. Figure S2: Quantification and calibration curve of thymol isolated from Curcuma longa.
Author Contributions
Conceptualization, O.-H.K., and D.Y.L.; methodology, O.-H.K., D.J.C., and D.Y.L.; analysis, D.-H.K., S.M.O., D.Y.; resources, Y.-S.L. and D.Y.L.; data curation, Y.-S.L., D.Y.L., and O.-H.K.; writing—original draft preparation, D.-H.K., Y.-S.L., and D.Y.L.; writing—review and editing, O.-H.K., and D.Y.L.; supervision, D.-Y.K.; project administration, Y.-S.L.; funding acquisition, Y.-S.L. and O.-H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Next Generation Bio-Green 21 (PJ01327501) Project from the Rural Development Administration, Korea.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; or the decision to publish the results.
References
- Sanyal, A.J.; Brunt, E.M.; Kleiner, D.E.; Kowdley, K.V.; Chalasani, N.; Lavine, J.E.; Ratziu, V.; McCullough, A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011, 54, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Kim, S.B.; Seo, Y.S.; Joung, D.K.; Mun, S.H.; Choi, J.G.; Lee, Y.M.; Kang, D.G.; Lee, H.S.; Kwon, D.Y. Curcumin decreases oleic acid-induced lipid accumulation via AMPK phosphorylation in hepatocarcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2578–2586. [Google Scholar] [PubMed]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Makhlouf, H.R. Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in Adults and Children. Clin. Liver Dis. 2016, 20, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Kong, Y.; Han, H.S.; Kang, D.H.; Lee, S.J.; Lee, C.H.; Wang, S.; Kwon, D.Y.; Kang, O.H. Non-alcoholic fatty liver protective effects, and studies on the mechanism of action of Crataegi Fructus. Korea J. Herbol. 2018, 33, 61–70. [Google Scholar]
- Karlowicz-Bodalska, K.; Han, S.; Freier, J.; Smolenski, M.; Bodalska, A. Curcuma Longa as Medicinal Herb in the Treatment of Diabet- IC Complications. Acta Pol. Pharm. 2017, 74, 605–610. [Google Scholar] [PubMed]
- Chen, I.N.; Chang, C.C.; Ng, C.C.; Wang, C.Y.; Shyu, Y.T.; Chang, T.L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum. Nutr. 2008, 63, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.P.; Mishra, R.K.; Kamran, A.; Mishra, P.; Bajaj, A.K.; Dikshit, A. Studies on antidermatophytic activity of waste leaves of Curcuma longa L. Physiol. Mol. Biol. Plants 2010, 16, 177–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lestari, M.L.; Indrayanto, G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [PubMed]
- Yu, Y.M.; Chao, T.Y.; Chang, W.C.; Chang, M.J.; Lee, M.F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016, 24, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, B.G.; Abbaspour-Ravasjani, S.; Jamal Musavinejad, S.; Ahmad Salehzadeh, S.; Abdolhosseinzadeh, A.; Hamishehkar, H.; Ghahremanzadeh, K.; Shokouhi, B. In vivo Evaluation of Anti-Hyperglycemic, Anti-hyperlipidemic and Anti-Oxidant Status of Liver and Kidney of Thymol in STZ-Induced Diabetic Rats. Drug Res. 2019, 69, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, B.; Abd-Elkader, O.; Musarrat, H.; Alkhathlan, H.; Al-kedhairy, A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. The SREBP pathway: Regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 1997, 89, 331–340. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Mingrone, G.; Rosa, G.; Greco, A.V.; Manco, M.; Vega, N.; Nanni, G.; Castagneto, M.; Vidal, H. Intramyocitic lipid accumulation and SREBP-1c expression are related to insulin resistance and cardiovascular risk in morbid obesity. Atherosclerosis 2003, 170, 155–161. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.; Sememenkovich, C.F. Fatty acid synthase and liver triglyceride metabolism: Housekeeper or messenger? Biochim. Biophys. Acta 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Olson, P.; Evans, R.M. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Goldstein, J.L.; Hammer, R.E.; Moon, Y.A.; Brown, M.S.; Horton, J.D. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. USA 2001, 98, 13607–13612. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lee, W.S.; Hwang, J.T.; Kwon, D.Y.; Surh, Y.J.; Park, O.J. Curcumin exerts antidifferentiation effect through AMPKalpha-PPAR-gamma in 3T3-L1 adipocytes and antiproliferatory effect through AMPKalpha-COX-2 in cancer cells. J. Agric. Food Chem. 2009, 57, 305–310. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).