As-Synthesized Oleic Amido Propyl Betaine Surfactant Mixture and the Effect on the Crude Oil–Seawater Interfacial Tension
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
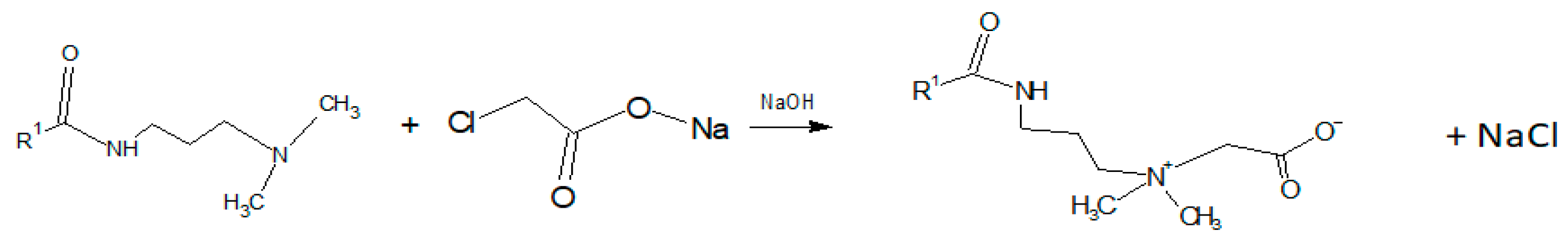
2.2. Synthesis of Surfactant
2.3. High Performance Liquid Chromatography (HPLC)
2.4. Fourier Transform Infra-Red Spectroscopy Analysis
2.5. Hyphenated Thermogravimetry-Infra-Red (TG-IR) Analysis
2.6. Surface Tension Analysis and Critical Micelle Concentration (CMC) Analysis
2.7. Interfacial Tension (IFT) Analysis
3. Results and Discussion
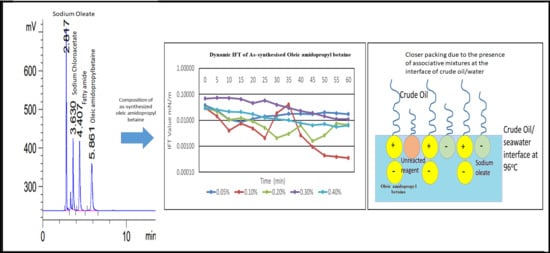
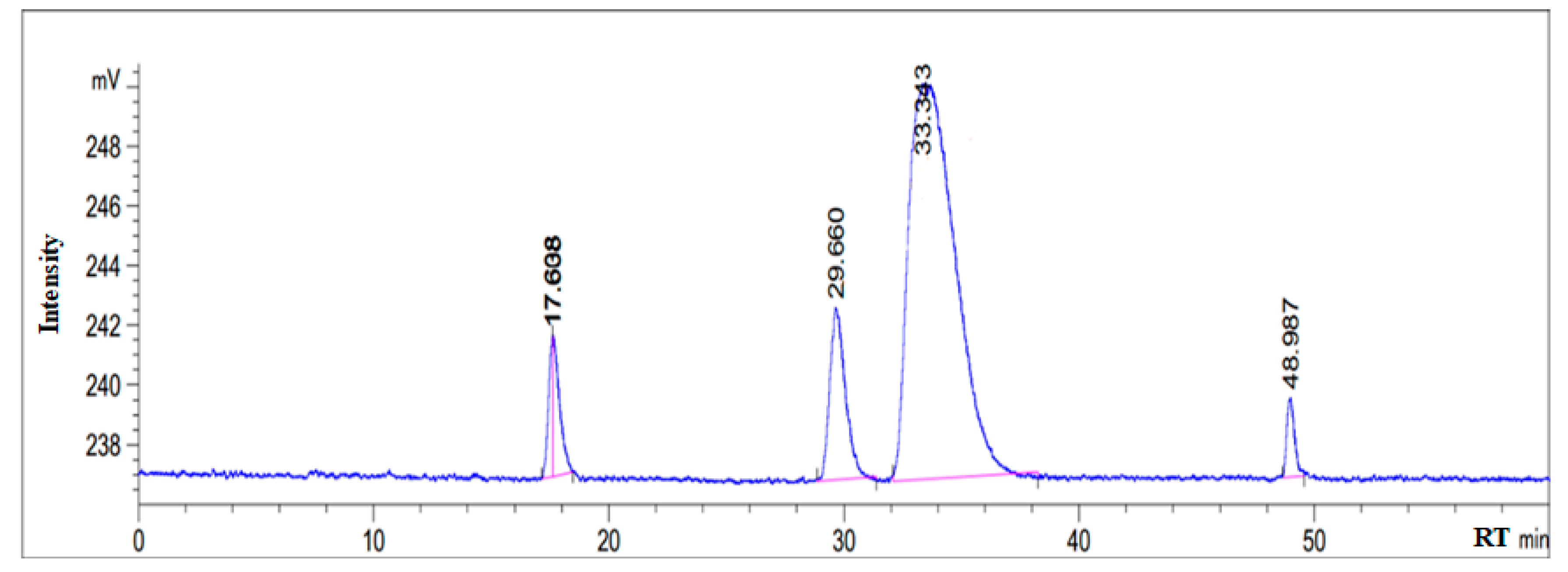
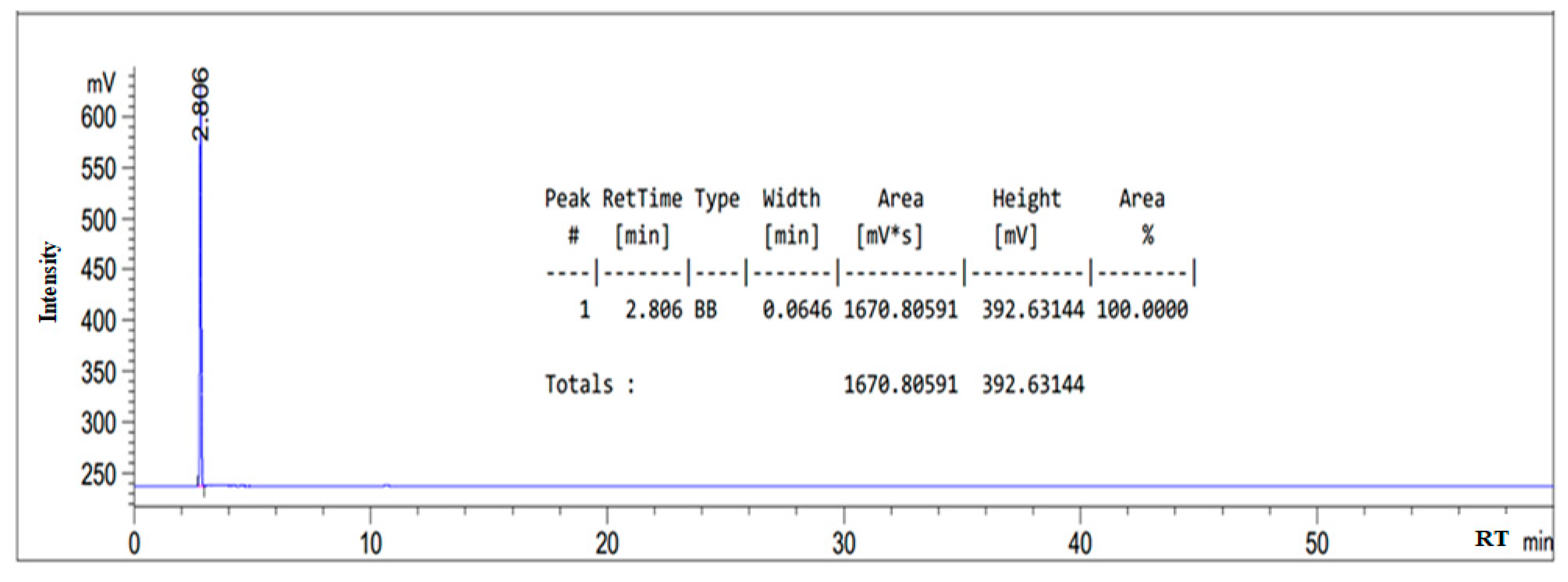
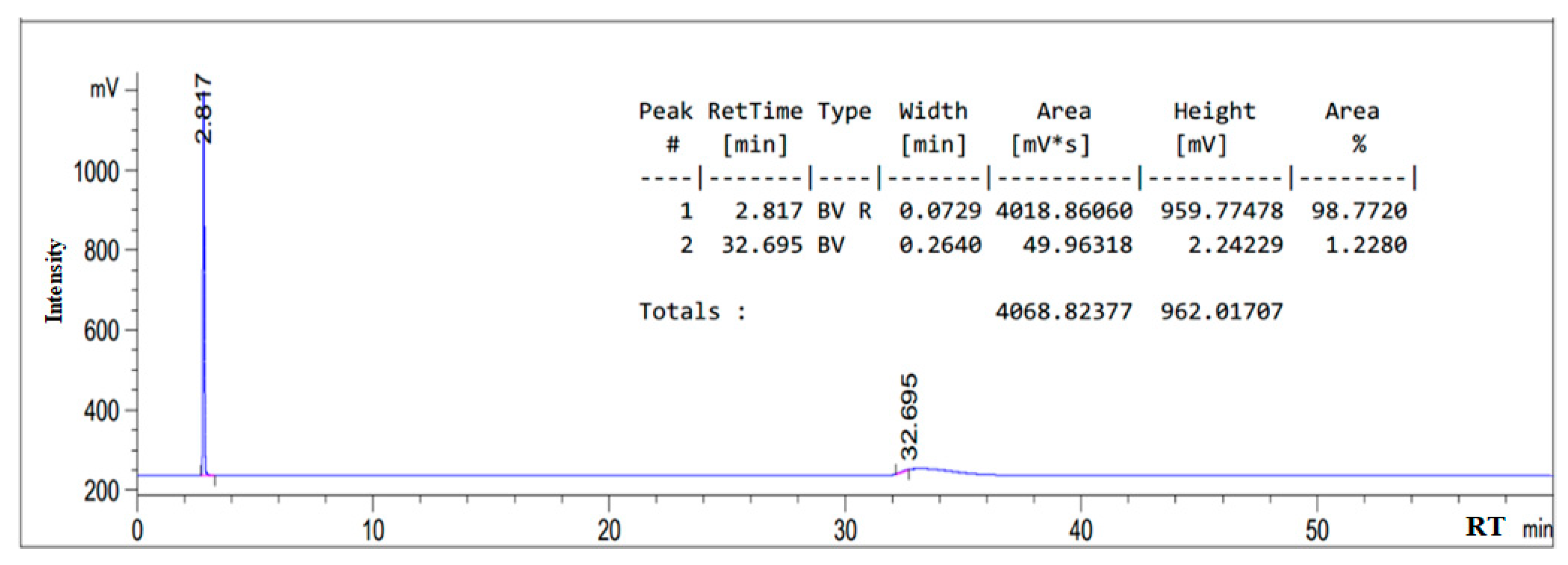
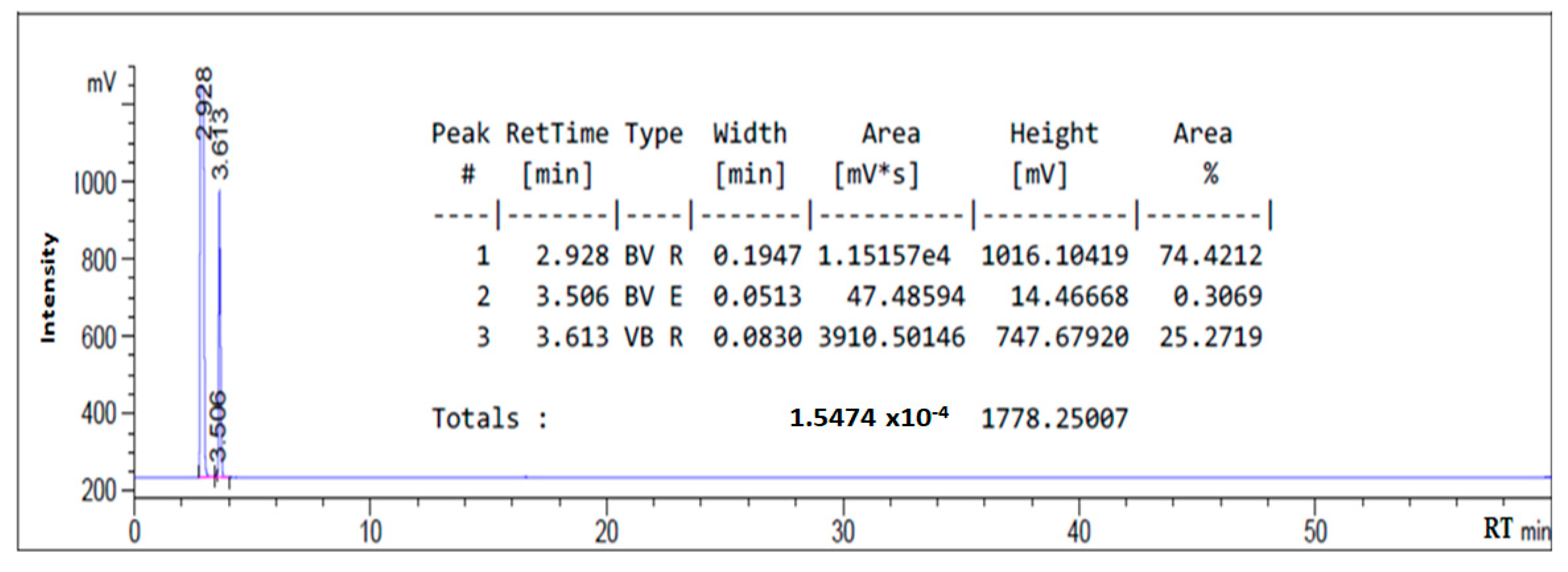
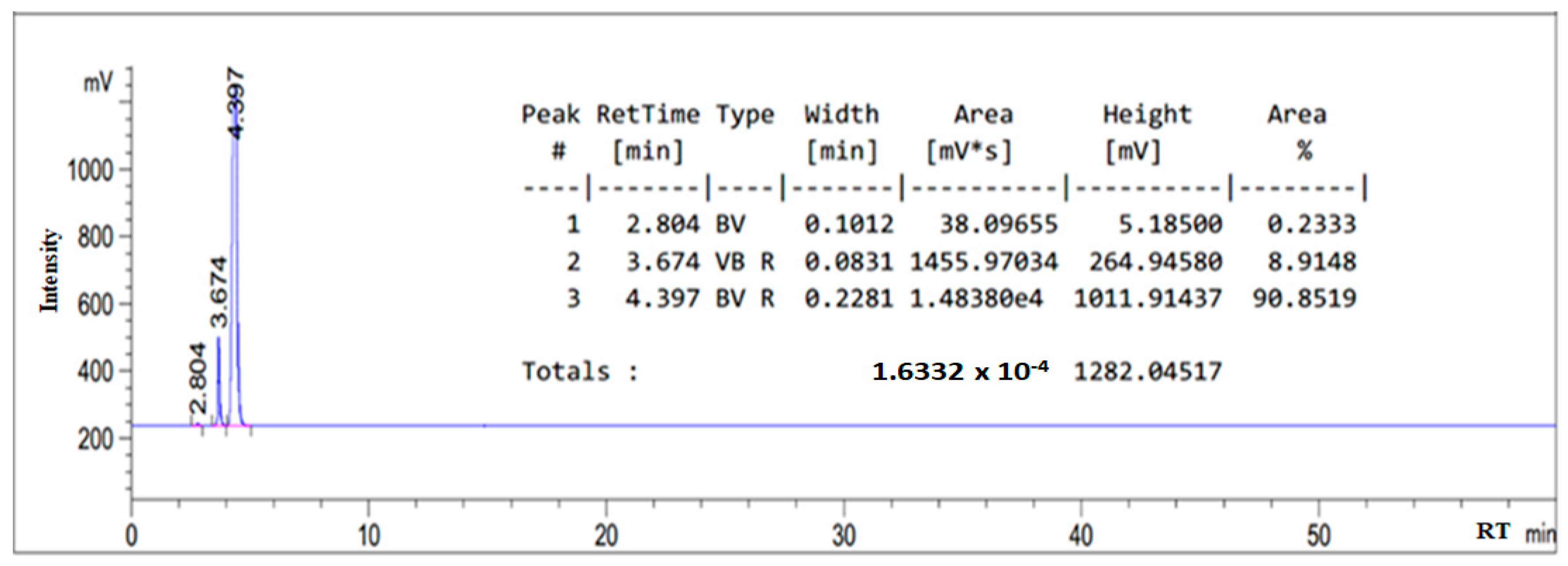
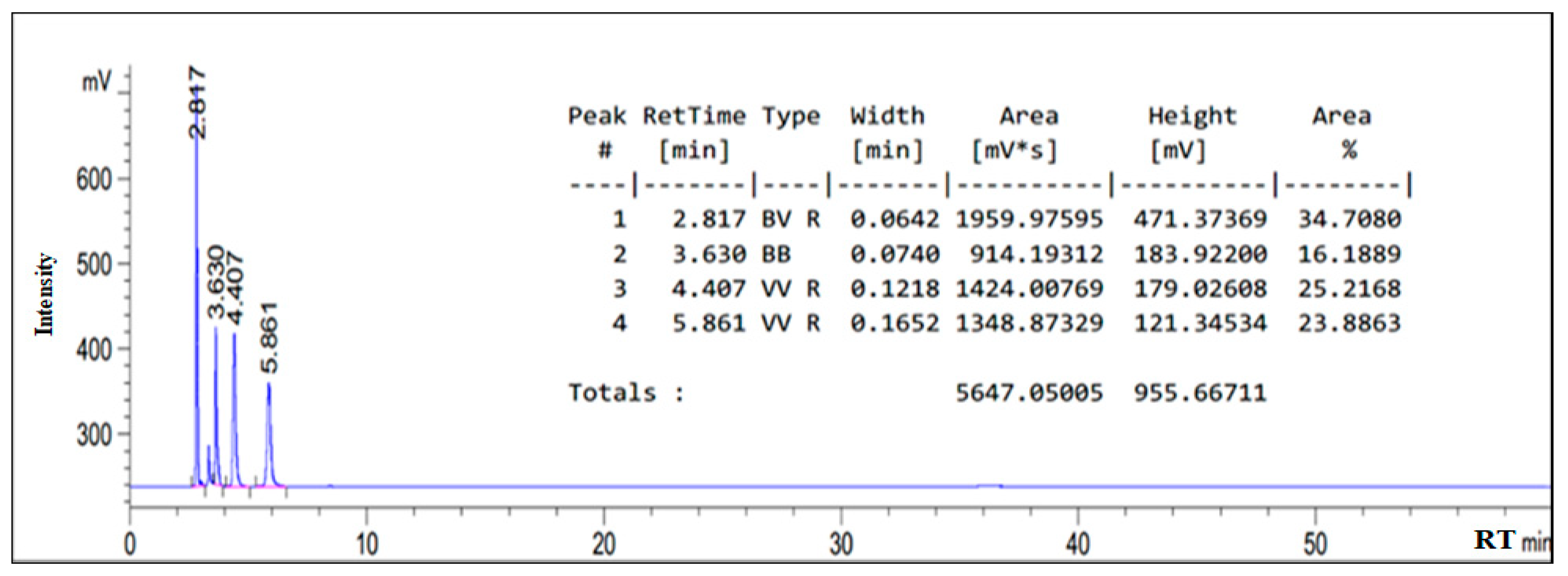
3.1. HPLC Chromatogram of as-Synthesized Surfactant
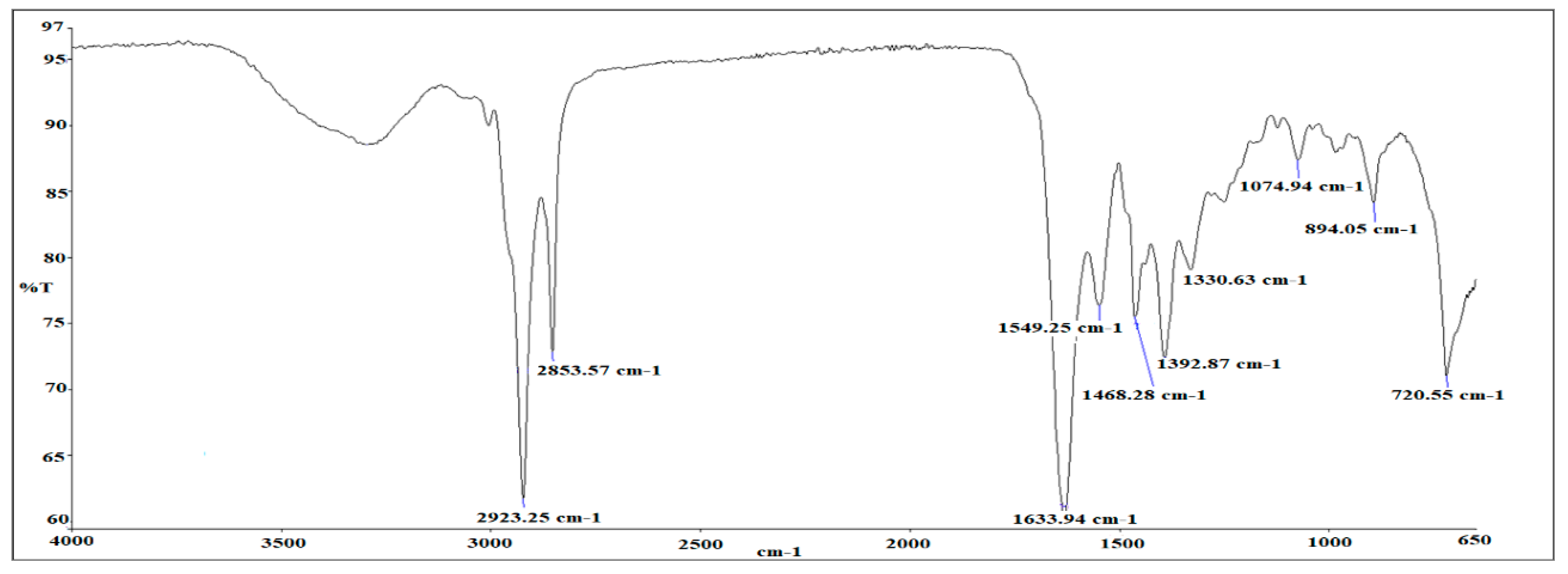
3.2. FTIR Analysis of as-Synthesized Surfactant
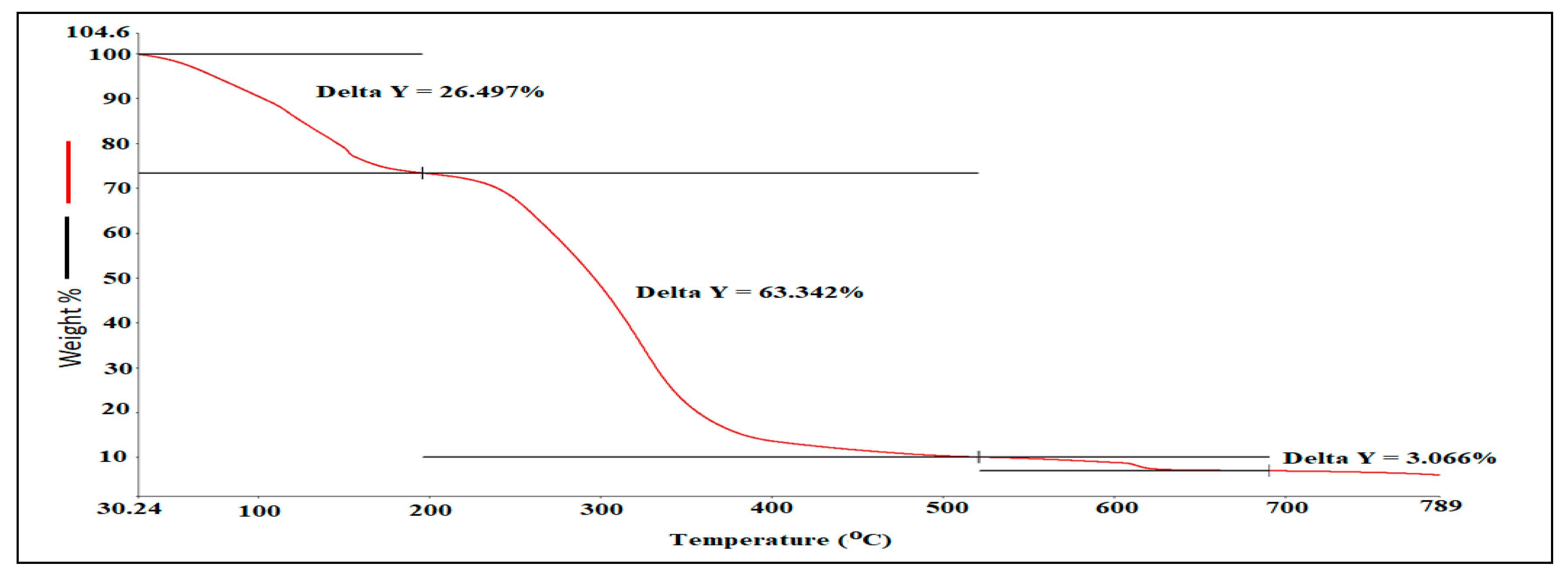
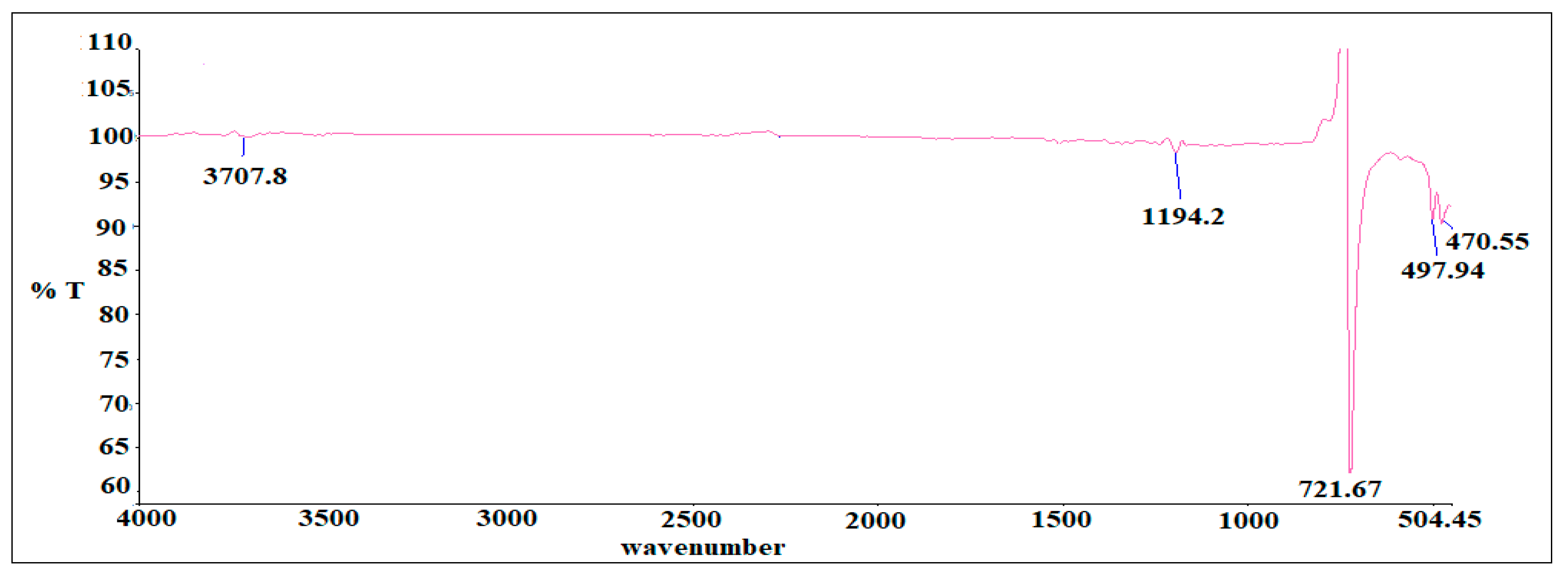
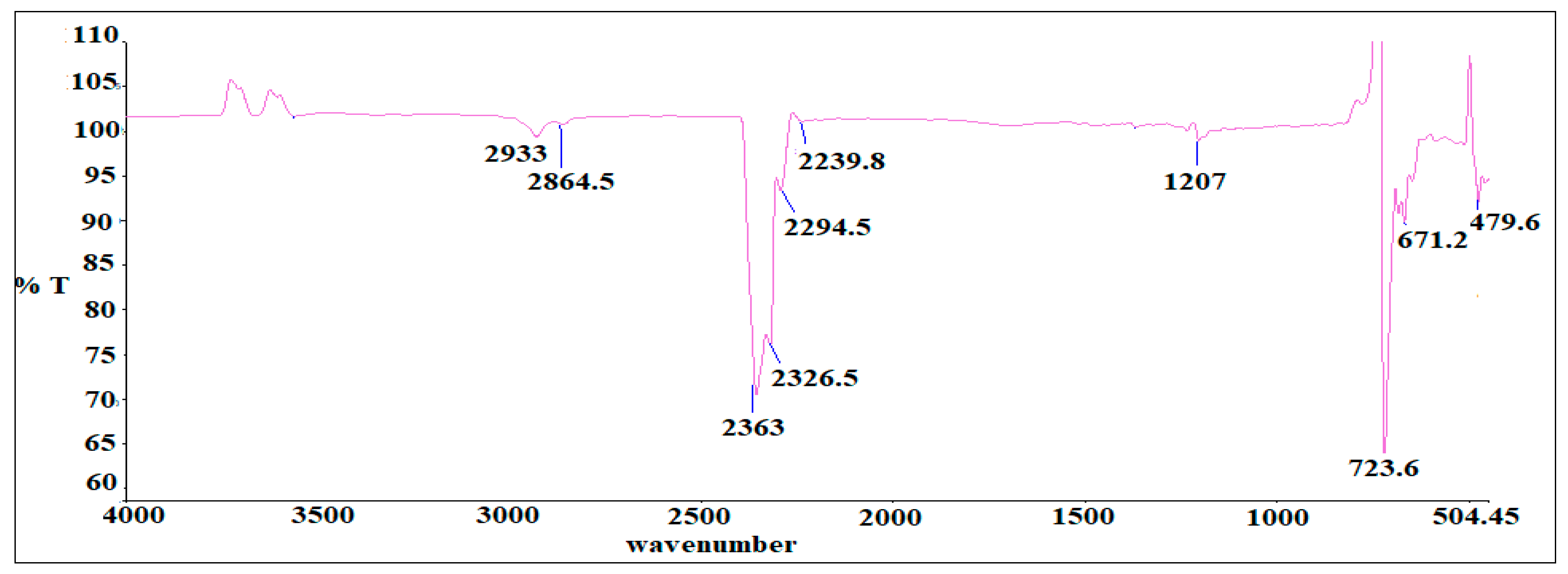
3.3. Hyphenated TGA-IR-Method
3.4. Determination of Surface Tension
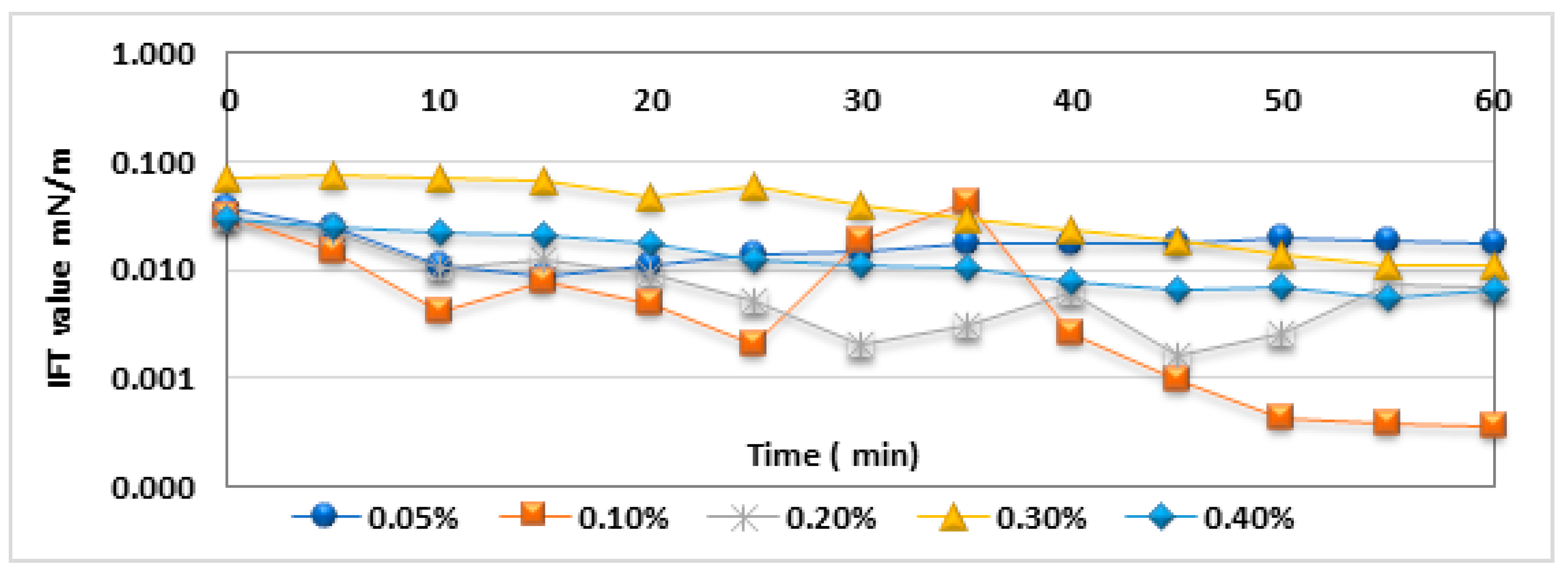
3.5. Dynamic Interfacial Tension (IFT) Analysis in Seawater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Babu, K.; Maurya, N.K.; Mandal, A.; Saxena, V.K. Synthesis and characterization of sodium methyl ester sulfonate for chemically-enhanced oil recovery. Braz. J. Chem. Eng. 2015, 32, 795–803. [Google Scholar] [CrossRef]
- Bataweel, A. Enhanced Oil Recovery in High Salinity High Temperature Reservoir by Chemical Flooding Enhanced Oil Recovery in High Salinity High; Texas A&M University Libraries: Killeen, TX, USA, 2011. [Google Scholar]
- Shuyan, C.; Hongjuan, L.; Hong, S.; Xiang, Y.; Gehua, W.; Yujie, Z.; Jianan, Z. Synthesis and physiochemical performance evaluation of novel sulphobetaine zwitterionic surfactants from lignin for enhanced oil recovery. J. Mol. Liq. 2018, 249, 73–82. [Google Scholar] [CrossRef]
- Diamant, H.; David, A. Kinetics of surfactant adsorption at fluid-fluid interfaces. J. Phys. Chem. 1996, 100, 13732–13742. [Google Scholar] [CrossRef]
- Eastoe, J.; Rico, F.T. Colloidal foundations of nanoscience. In Surfactants and Nanoscience; Elsevier, B.V.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Abdul, H.Y. Investigation on some properties of SDS solutions. Aust. J. Basic Appl. Sci. 2011, 5, 1221–1227. [Google Scholar]
- Feng, D. Synthesis and surface activities of amidobetaine surfactants with ultra-long unsaturated hydrophobic chains. J. Surfactants Deterg. 2012, 15, 657–661. [Google Scholar] [CrossRef]
- Salager, J.L.; Forgiarini, A.M.; Márquez, L.; Manchego, L.; Bullón, J. How to attain an ultralow interfacial tension and a three-phase behavior with a surfactant formulation for enhanced oil recovery: A review. Part 2. Performance improvement trends from winsor’s premise to currently proposed inter- and intra-molecular. J. Surfactants Detergents 2013, 16, 631–663. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Li, X.; Zhang, L. Surface properties, micellar molecular interaction, and physical properties for binary systems of sodium oleate with three anionic surfactants. J. Dispers. Sci. Technol. 2017, 38, 712–720. [Google Scholar] [CrossRef]
- Lomax, E.G. Amphoteric Surfactants, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1996. [Google Scholar]
- Gogoi, S.B. Adsorption-desorption of surfactant for enhanced oil recovery. Transp. Porous Media 2011, 90, 589–604. [Google Scholar] [CrossRef]
- Hutin, A. Dynamic Adsorption of Surfactants at Air/Water Interface; Linkedin: Sunnyvale, CA, USA, 2019. [Google Scholar]
- Ping, J.; Na, L.; Jijiang, G.; Guicai, Z.; Yang, W.; Lifeng, C.; Lei, Z. Efficiency of a sulfobetaine-type surfactant on lowering IFT at crude oil-formation water interface. Colloids Surf. A: Physicochem. Eng. Asp. 2014, 443, 141–148. [Google Scholar]
- Kedar, V.; Sunil, S.B. Effect of salinity on the ift between aqueous surfactant solution and crude oil. Pet. Sci. Technol. 2018, 36, 835–842. [Google Scholar] [CrossRef]
- Jun, L.; Pathma, J.; Liyanage, S.S.; Stephanie, A.; Gayani, P.; Arachchilage, D.H.K.; Christopher, B.; Upali, W.; Gary, A.P. New surfactant developments for chemical enhanced oil recovery. J. Pet. Sci. Eng. 2014, 120, 94–101. [Google Scholar] [CrossRef]
- Ouellette, R.J.; David, R.J. Amines and amides. Princ. Org. Chem. 2015, 315–342. [Google Scholar]
- Rosen, M.J. Ultralow interfacial tension for enhanced oil recovery at very low surfactant concentrations. Langmuir 2015, 21, 3749–3756. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, V.; Georgios, P.; Konstantinos, S. A mild alkaline hydrolysis of N- and N, N-substituted amides and nitriles. Arch. Org. Chem. 2015, 2015, 101–112. [Google Scholar]
- Wang, D.; Liu, C.; Wu, W.; Wang, G. Novel surfactants that attain ultra-low interfacial tension between oil and high salinity formation water without adding alkali, salts, co-surfactants, alcohols and solvents. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 11–13 April 2010. [Google Scholar]
- Shi-Fa, W.; Takeshi, F.; Zhi, C. Synthesis of new betaine-type amphoteric surfactants from tall oil fatty acid. J. Wood Sci. 2002, 48, 419–424. [Google Scholar]
- Garing, C.; Voltolini, M.; Ajo-Franklin, J.B.; Benson, S.M. Pore-scale Evolution of Trapped CO2 at Early Stages Following Imbibition Using Micro-CT Imaging. Energy Procedia 2017, 114, 4872–4878. [Google Scholar] [CrossRef]
- Weers, J.G.; James, F.R.; Frank, U.A.; Charles, C. Effect of the intramolecular charge separation distance the solution properties of betaines and sulfobetaines. Langmuir 1991, 7, 854–867. [Google Scholar] [CrossRef]
- Yeming, X.; Xiaoxu, Z.; Huanxia, Z.; Wen, C. Synthesis, characterization, and surface-active properties of carboxylbetaine and sulfobetaine surfactants based on vegetable oil. J. Surfactants Deterg. 2018, 22, 103–114. [Google Scholar]
- Yarveicy, H.; Ali, H. Effect of amphoteric surfactant on phase behavior of hydrocarbon-electrolyte-water system-an application in enhanced oil recovery. J. Dispers. Sci. Technol. 2018, 39, 522–530. [Google Scholar] [CrossRef]



| Time | A% | B% | C% | D% | Flow (mL/min) | Max Pressure Limit (Bar) |
|---|---|---|---|---|---|---|
| 0 | 0.00 | 65.00 | 35.00 | 0.00 | 1.00 | 400.00 |
| 2 | 0.00 | 52.00 | 48.00 | 0.00 | 1.00 | 400.00 |
| 6 | 0.00 | 55.00 | 45.00 | 0.00 | 1.00 | 400.00 |
| 10 | 0.00 | 50.00 | 50.00 | 0.00 | 1.00 | 400.00 |
| 30 | 0.00 | 45.00 | 55.00 | 0.00 | 1.00 | 400.00 |
| 40 | 0.00 | 55.00 | 45.00 | 0.00 | 1.00 | 400.00 |
| 60 | 0.00 | 65.00 | 35.00 | 0.00 | 1.00 | 400.00 |
| Fatty Acids | Retention Time | % Area |
|---|---|---|
| C18 & below | 17.638 | 3.968 |
| C18:1 | 33.343 | 79.25 |
| C18:2 | 29.660 | 11.498 |
| others | 48.987 | 2.63 |
| Composition | Retention Time (min) |
|---|---|
| Oleic amido betaine | 5.669 |
| Fatty amide | 4.407 |
| Sodium oleate | 2.817 |
| Sodium chloroacetate | 3.630 |
| Mass Loss (%) | Temperature (°C) |
|---|---|
| 26.46 | 30–201.2 |
| 63.43 | 210–520.5 |
| 3.066 | 520–690 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Wazir, N.; Ramli, A.; Yusof, N.M.; Saphanuchart, W.; S. Majanun, E. As-Synthesized Oleic Amido Propyl Betaine Surfactant Mixture and the Effect on the Crude Oil–Seawater Interfacial Tension. Processes 2020, 8, 965. https://doi.org/10.3390/pr8080965
Ahmad Wazir N, Ramli A, Yusof NM, Saphanuchart W, S. Majanun E. As-Synthesized Oleic Amido Propyl Betaine Surfactant Mixture and the Effect on the Crude Oil–Seawater Interfacial Tension. Processes. 2020; 8(8):965. https://doi.org/10.3390/pr8080965
Chicago/Turabian StyleAhmad Wazir, Norhidayah, Anita Ramli, Nurida M. Yusof, Wasan Saphanuchart, and Emily S. Majanun. 2020. "As-Synthesized Oleic Amido Propyl Betaine Surfactant Mixture and the Effect on the Crude Oil–Seawater Interfacial Tension" Processes 8, no. 8: 965. https://doi.org/10.3390/pr8080965
APA StyleAhmad Wazir, N., Ramli, A., Yusof, N. M., Saphanuchart, W., & S. Majanun, E. (2020). As-Synthesized Oleic Amido Propyl Betaine Surfactant Mixture and the Effect on the Crude Oil–Seawater Interfacial Tension. Processes, 8(8), 965. https://doi.org/10.3390/pr8080965